ABSTRACT
This study reports the prevalences of qnrVC genes in 74 ciprofloxacin-resistant Vibrio sp. isolates. Two novel functional qnrVC alleles, qnrVC8 and qnrVC9, sharing 98% and 99% nucleotide similarity with qnrVC6 and qnrVC7, respectively, were identified. Our findings suggested that carriage of qnrVC alleles, together with target mutations in gyrA and parC genes, may contribute to the development of fluoroquinolone resistance in Vibrio species, posing a serious threat to public health.
KEYWORDS: qnrVC, Vibrio, ciprofloxacin resistance
TEXT
Vibrio spp. are some of the most important pathogens that cause foodborne illnesses worldwide. Ciprofloxacin is the main choice for the treatment of infections caused by Vibrio species. Previous studies have shown that quinolone resistance arises as a result of mutational changes in genes encoding the target bacterial enzymes of fluoroquinolones, namely, DNA gyrase and DNA topoisomerase IV (1–3), and by changes in the levels of expression of efflux pumps and porins that control the accumulation of these agents inside the bacterial cell (4). In 1998, plasmid-mediated quinolone resistance (PMQR) was reported in a clinical isolate of Klebsiella pneumoniae, from which low-level quinolone resistance could be transferred to other Gram-negative bacteria (5). The PMQR gene in such an isolate, subsequently named qnr, was found to encode a protein that could bind and protect DNA gyrase and topoisomerase IV from inhibition by ciprofloxacin (6). Qnr proteins belong to the pentapeptide repeat family. Until now, six families of Qnr proteins have been described, including QnrA, QnrB, QnrC, QnrD, QnrE, QnrS, and QnrVC (2, 7–11). The qnrVC genes, first described in V. cholerae in 2008, encodes a pentapeptide repeat protein (PRP) which consists of 218 amino acids (8). Vibrio spp. were considered a possible source of qnr-like quinolone resistance determinants (12). To date, seven qnrVC alleles (qnrVC1 to qnrVC7) have been reported in Vibrionaceae (8, 13–15), but the prevalences of qnrVC alleles in foodborne Vibrio spp. are not well documented.
In this study, a total of 589 nonduplicated isolates of the Vibrio spp. were recovered from 801 food samples purchased from open markets and supermarkets in Shenzhen, China, during 2015 and 2016 (Supplemental Materials and Methods). The rates of positivity of various food samples were 89% (shrimp), 24% (pork), 23% (chicken), and 12% (beef), with 379, 149, 51, and 10 Vibrio strains isolated from each sample type, respectively. V. parahaemolyticus was the most dominant species, accounting for 66% (n = 386) of the Vibrio sp. isolates tested, followed by V. alginolyticus (30% [n = 175]), V. cholerae (3% [n = 20]), and V. vulnificus (1% [n = 8]) (see Table S1 in the supplemental material).
Antimicrobial susceptibilities were determined for these Vibrio isolates as described in the Supplemental Materials and Methods. Resistance to ampicillin was the most common among the Vibrio spp., with rates of 96%, 77%, 35%, and 38% in V. parahaemolyticus, V. alginolyticus, V. cholerae, and V. vulnificus, respectively (Table S2). The rates of resistance to ciprofloxacin among the four species were 9.3%, 17.1%, 40.0%, and 0%, respectively. However, the resistance rates for ofloxacin, at 5.4%, 9.7%, 5.0%, and 0.0%, respectively, for the four species, did not align well with those for ciprofloxacin, suggesting that the mechanisms of resistance to different fluoroquinolones were not identical. Around 20% of the V. parahaemolyticus and V. alginolyticus isolates exhibited resistance to cephalosporins (ceftriaxone and cefotaxime), whereas the resistance rates were lower (5% and 10%, respectively) in V. cholerae but higher in V. vulnificus (37.5% and 12.5%, respectively). Resistance to other antibiotics was not high compared to that described in other reports (Table S2). All 74 ciprofloxacin-resistant Vibrio sp. isolates were selected for further analysis, among which 37, 30, and 7 isolates were V. parahaemolyticus, V. alginolyticus, and V. cholerae, respectively.
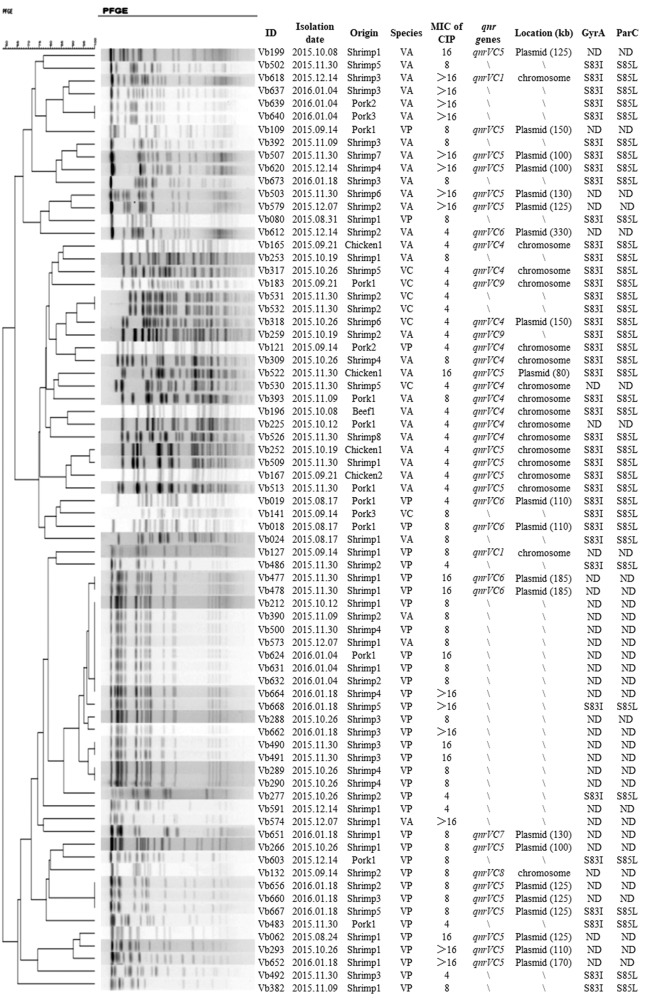
Pulsed-field gel electrophoresis (PFGE) was conducted to study the genetic relationships of the ciprofloxacin-resistant Vibrio sp. isolates (Supplemental Materials and Methods). Although signs of clonal dissemination were detectable among organisms collected from different food types on different dates, remarkable genetic diversity can be seen (Fig. 1). Strain Vb624, a V. parahaemolyticus strain isolated from a pork sample, was found to exhibit a PFGE pattern identical to those of seven other V. parahaemolyticus strains isolated from shrimp samples purchased on different dates. Most of the Vibrio strains that exhibited identical PFGE profiles were isolated from either the same sample or different samples on the same date. However, in view of the differences in the antimicrobial susceptibility profiles of these strains, we still consider them different strains.
FIG 1.
Genetic and phenotypic characteristics of 74 ciprofloxacin (CIP)-resistant foodborne isolates of Vibrio species. The format of the isolation date is year.month.date. VP, V. parahaemolyticus; VA, V. alginolyticus; VC, V. cholerae; VV, V. vulnificus; ND, not done; ID, identification.
The 74 ciprofloxacin-resistant Vibrio sp. isolates were screened for the presence of qnrVC genes (Supplemental Materials and Methods). Thirty-nine of the ciprofloxacin-resistant Vibrio sp. isolates tested (53%) were found to harbor the qnrVC genes. The rates of positivity among V. alginolyticus, V. parahaemolyticus, and V. cholerae were 63% (n = 19), 43% (n = 16), and 57% (n = 4), respectively. A total of five different qnrVC alleles were detected in these isolates, with qnrVC5 (n = 18 [46%]) being the most dominant type, followed by qnrVC4 (n = 10 [26%]), qnrVC6 (n = 5 [13%]), qnrVC1 (n = 2 [5%]), and qnrVC7 (n = 1 [3%]). Additionally, three isolates (Vb132, Vb183, and Vb259) carrying novel variants of qnrVC were detected. Strain Vb132, a V. parahaemolyticus isolate obtained from shrimp sample, carried an allele that shared 98% nucleotide identity with qnrVC6. This allele encodes a protein with the D166A and K185N amino acid substitutions compared with qnrVC6 and was designated qnrVC8 (accession no. MH181806). Strain Vb183, a V. alginolyticus isolate obtained from a shrimp sample, and strain Vb259, a V. cholerae isolate obtained from a pork sample, carried a qnrVC variant designated qnrVC9 (accession no. MH181807). Such a variant exhibited 99% nucleotide similarity to qnrVC7, with an A100V substitution (Fig. S1). Both qnrVC8 and qnrVC9 were cloned into the pET-15b vector, as described in Supplemental Materials and Methods. Transformants of Escherichia coli BL21(DE3) carrying pET15b-qnrVC8 or pET15b-qnrVC9 exhibited a 16-fold elevated MIC of ciprofloxacin compared with that for strain E. coli BL21(DE3), suggesting that these two qnrVC variants may contribute to quinolone resistance (Table 1).
TABLE 1.
Susceptibilities of Vibrio sp. isolates and the corresponding E. coli transformants and transconjugants to fluoroquinolones
| Bacterial strain (variant carried)a | Bacterial species | MIC (μg/ml) |
|
|---|---|---|---|
| Ciprofloxacin | Nalidixic acid | ||
| BL21(DE3) | E. coli | 0.0075 | 1 |
| BL21(DE3)/pET15b | E. coli | 0.0075 | 1 |
| BL21(DE3)/pET15b-qnrVC8 | E. coli | 0.12 | 16 |
| BL21(DE3)/pET15b-qnrVC9 | E. coli | 0.12 | 16 |
| Vb132 (qnrVC8) | V. parahaemolyticus | 8 | >64 |
| Vb183 (qnrVC9) | V. cholerae | 4 | >64 |
| Vb259 (qnrVC9) | V. parahaemolyticus | 4 | >64 |
| J53 AZR | E. coli | 0.015 | 2 |
| Vb266 | V. parahaemolyticus | 8 | 32 |
| TC-Vb266 | E. coli | 0.5 | 16 |
| Vb507 | V. alginolyticus | >16 | >64 |
| TC-Vb507 | E. coli | 0.5 | 16 |
| Vb620 | V. alginolyticus | >16 | >64 |
| TC-Vb620 | E. coli | 0.5 | 16 |
TC, transconjugant.
S1-PFGE and hybridization were performed to determine the genetic locations of the qnrVC alleles (Supplemental Materials and Methods) and showed that all qnrVC1 (n = 2) genes were located in the chromosome, while all qnrVC6 genes (n = 5) and the qnrVC7 gene (n = 1) were plasmid borne. The qnrVC4 and qnrVC5 genes were detected in both chromosomes and plasmids; among them, 1 out of the 10 qnrVC4 genes was located in the chromosome, whereas 13 out the 18 qnrVC5 genes were located in plasmids. The novel qnrVC alleles, qnrVC8 (n = 1) and qnrVC9 (n = 2), were all found to reside in the chromosome. Plasmids carrying different qnrVC genes varied from ∼80 kb to 330 kb in size (Fig. 1). Conjugation experiments were performed on Vibrio sp. isolates which carried both chromosome- and plasmid-borne qnrVC genes (Supplemental Materials and Methods). Three strains, Vb266 (a V. parahaemolyticus strain isolated from shrimp sample and that carried qnrVC5), Vb507 (a V. alginolyticus strain isolated from shrimp sample and that carried qnrVC5), and Vb620 (a V. alginolyticus strain isolated from shrimp sample and that carried qnrVC5), were able to transfer the quinolone resistance phenotype to E. coli strain J53 AZR. All transconjugants exhibited a 32-fold reduction in susceptibility to ciprofloxacin compared with E. coli J53 AZR (Table 1).
Forty randomly selected ciprofloxacin-resistant Vibrio sp. isolates which harbored the qnrVC gene were subjected to screening of mutations in the quinolone resistance-determining region (QRDR) of known resistance genes (Supplemental Materials and Methods). As shown in Fig. 1, no mutations were found in the gyrB and parE genes of the 40 isolates tested. A mutation at codon 83 of the gyrA gene that resulted in a Ser-to-Ile substitution and the Ser-to-Leu change at residue 85 of the parC gene were identified in all 40 test isolates.
In summary, we screened the qnrVC gene and determined the mutations in the quinolone resistance-determining regions of target genes in ciprofloxacin-resistant foodborne isolates of Vibrio species. Two novel qnrVC alleles (qnrVC8 and qnrVC9) were identified. The findings in this work expand our knowledge on the molecular basis of quinolone resistance among foodborne Vibrio species.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Collaborative Research Fund of Hong Kong Research Grant Council (C5026-16G) and Health and Medical Research Fund from Hong Kong Food and Health Bureau (grant 15141322).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00529-18.
REFERENCES
- 1.Kim ES, Hooper DC. 2014. Clinical importance and epidemiology of quinolone resistance. Infect Chemother 46:226–238. doi: 10.3947/ic.2014.46.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tran JH, Jacoby GA. 2002. Mechanism of plasmid-mediated quinolone resistance. Proc Natl Acad Sci U S A 99:5638–5642. doi: 10.1073/pnas.082092899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodríguez-Martínez JM, Machuca J, Cano ME, Calvo J, Martínez-Martínez L, Pascual A. 2016. Plasmid-mediated quinolone resistance: two decades on. Drug Resist Updat 29:13–29. doi: 10.1016/j.drup.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Poole K. 2000. Efflux-mediated resistance to fluoroquinolones in Gram-negative bacteria. Antimicrob Agents Chemother 44:2233–2241. doi: 10.1128/AAC.44.9.2233-2241.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez-Martínez L, Pascual A, Jacoby GA. 1998. Quinolone resistance from a transferable plasmid. Lancet 351:797–799. doi: 10.1016/S0140-6736(97)07322-4. [DOI] [PubMed] [Google Scholar]
- 6.Jacoby GA. 2005. Mechanisms of resistance to quinolones. Clin Infect Dis 41(Suppl 2):S120–S126. doi: 10.1086/428052. [DOI] [PubMed] [Google Scholar]
- 7.Cavaco LM, Hasman H, Xia S, Aarestrup FM. 2009. qnrD, a novel gene conferring transferable quinolone resistance in Salmonella enterica serovar Kentucky and Bovismorbificans strains of human origin. Antimicrob Agents Chemother 53:603–608. doi: 10.1128/AAC.00997-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonseca ÉL, dos Santos Freitas F, Vieira VV, Vicente ACP. 2008. New qnr gene cassettes associated with superintegron repeats in Vibrio cholerae O1. Emerg Infect Dis 14:1129–1131. doi: 10.3201/eid1407.080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hata M, Suzuki M, Matsumoto M, Takahashi M, Sato K, Ibe S, Sakae K. 2005. Cloning of a novel gene for quinolone resistance from a transferable plasmid in Shigella flexneri 2b. Antimicrob Agents Chemother 49:801–803. doi: 10.1128/AAC.49.2.801-803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacoby GA, Walsh KE, Mills DM, Walker VJ, Oh H, Robicsek A, Hooper DC. 2006. qnrB, another plasmid-mediated gene for quinolone resistance. Antimicrob Agents Chemother 50:1178–1182. doi: 10.1128/AAC.50.4.1178-1182.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M, Guo Q, Xu X, Wang X, Ye X, Wu S, Hooper DC, Wang M. 2009. New plasmid-mediated quinolone resistance gene, qnrC, found in a clinical isolate of Proteus mirabilis. Antimicrob Agents Chemother 53:1892–1897. doi: 10.1128/AAC.01400-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poirel L, Liard A, Rodriguez-Martinez JM, Nordmann P. 2005. Vibrionaceae as a possible source of Qnr-like quinolone resistance determinants. J Antimicrob Chemother 56:1118–1121. doi: 10.1093/jac/dki371. [DOI] [PubMed] [Google Scholar]
- 13.Kumar P, Thomas S. 2011. Presence of qnrVC3 gene cassette in SXT and class 1 integrons of Vibrio cholerae. Int J Antimicrob Agents 37:280–281. doi: 10.1016/j.ijantimicag.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Liu M, Wong MHY, Chen S. 2013. Molecular characterisation of a multidrug resistance conjugative plasmid from Vibrio parahaemolyticus. Int J Antimicrobial Agents 42:575–579. doi: 10.1016/j.ijantimicag.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Po KH, Wong MH, Chen S. 2015. Identification and characterisation of a novel plasmid-mediated quinolone resistance gene, qnrVC7, in Vibrio cholerae of seafood origin. Int J Antimicrob Agents 45:667–668. doi: 10.1016/j.ijantimicag.2015.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.