ABSTRACT
Borrelia miyamotoi is an emerging relapsing fever (RF) Borrelia species that is reported to cause human disease in regions in which Lyme borreliosis is endemic. We recently showed that B. miyamotoi tick isolates are resistant to amoxicillin in vitro; however, clinical isolates have not been studied. Therefore, our aim was to show the antimicrobial susceptibility of recently obtained clinical isolates of B. miyamotoi. A dilution series of various antibiotics was made in modified Kelly-Pettenkofer medium with 10% fetal calf serum. The susceptibilities of different B. miyamotoi clinical, B. miyamotoi tick, RF Borrelia, and Borrelia burgdorferi sensu lato isolates were tested by measuring MICs through colorimetric changes and by counting motile spirochetes by dark-field microscopy after 72 h of incubation. The ceftriaxone and azithromycin MIC ranges of the six B. miyamotoi clinical isolates tested were 0.03 to 0.06 mg/liter and 0.0016 to 0.0032 mg/liter, respectively. These values are similar to MICs for RF Borrelia strains and B. miyamotoi tick isolates. All tested RF Borrelia strains were susceptible to doxycycline (microscopic MIC range, 0.0625 to 0.25 mg/liter). In contrast to the MICs of the tested B. burgdorferi sensu lato strains and in line with our previous findings, the amoxicillin MICs (range, 8 to 32 mg/liter) of all RF Borrelia strains, including B. miyamotoi clinical isolates, were above the clinical breakpoint for resistance (≤4 mg/liter). Clinical isolates of B. miyamotoi are highly susceptible to doxycycline, azithromycin, and ceftriaxone in vitro. Interestingly, as described previously for tick isolates, amoxicillin shows poor in vitro activity against B. miyamotoi clinical isolates.
KEYWORDS: hard-tick-borne relapsing fever, relapsing fever Borrelia, Borrelia miyamotoi disease, Borrelia miyamotoi, antibiotic susceptibility, antimicrobials
INTRODUCTION
Hard-bodied Ixodes ticks are found across the Northern Hemisphere and are important vectors of pathogenic viruses, bacteria, and parasites (1, 2). They can lead to well-established tick-borne diseases, such as anaplasmosis, tick-borne encephalitis, and Lyme borreliosis (caused by Borrelia burgdorferi sensu lato), and also diseases caused by newly discovered pathogens such as Borrelia miyamotoi (3–8). Phylogenetically, B. miyamotoi clusters with relapsing fever (RF) Borrelia species, such as Borrelia hermsii and Borrelia recurrentis, which, unlike B. miyamotoi, are commonly transmitted by soft ticks or body lice (9).
Human infection with B. miyamotoi has recently been observed in Russia, the United States, and Japan, causing fever accompanied by nonspecific symptoms in immunocompetent patients (3, 4). Three immunocompromised patients, from the Netherlands, Germany, and the United States, were diagnosed with meningoencephalitis caused by B. miyamotoi (5, 6, 8). Due to lack of experimental data, B. miyamotoi disease (BMD) is empirically treated based on experiences with treatment of other RF Borrelia species and guidelines for treatment of Lyme borreliosis, and only minor treatment failures have been reported (3–6, 8, 10). Of interest, a recent case of BMD in the United States was described as showing full recovery without antibiotics (11).
We recently described the in vitro susceptibility of two tick isolates of B. miyamotoi (12). We found that B. miyamotoi tick isolates were susceptible to azithromycin, doxycycline, and ceftriaxone but, unlike the tested B. burgdorferi sensu lato strains, were resistant to amoxicillin in vitro (12). Our aim was to improve the clinical importance of these findings by using six recently obtained clinical isolates of B. miyamotoi (13). We used two well-established and reproducible methods for determination of antimicrobial susceptibility (12, 14, 15), by measuring the MICs of these isolates for the antibiotics most commonly used in the treatment of Lyme and RF borreliosis (10). This study offers further insights in options for antimicrobial therapy in BMD.
RESULTS
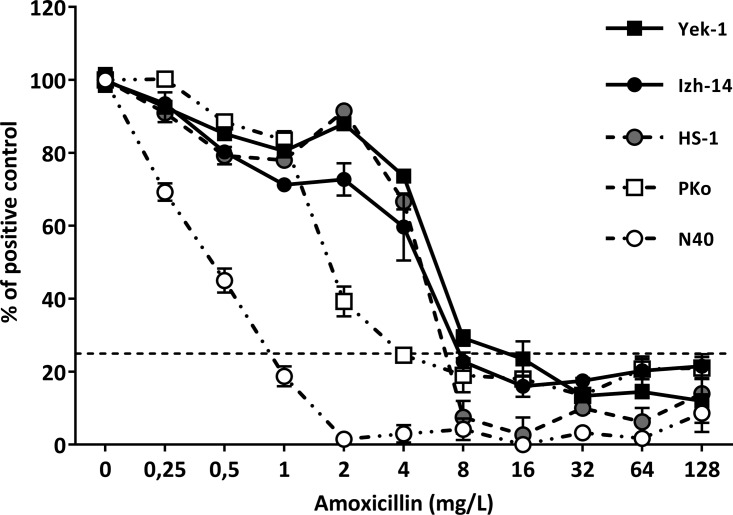
Antimicrobial susceptibility was determined as the colorimetric MIC (MICc) and microscopic MIC (MICm) of each Borrelia strain for all tested antibiotics (Table 1). MICc values were equal to or within 2 dilutions (2-fold) of the MICm values. Clinical isolates of B. miyamotoi showed similar susceptibilities to ceftriaxone and azithromycin, compared to B. burgdorferi sensu lato, RF Borrelia, and B. miyamotoi control strains (Table 1). Susceptibilities to doxycycline were similar (within 2 dilutions [2-fold]) for RF Borrelia strains, B. miyamotoi tick isolates, and B. miyamotoi clinical isolates (MICm range, 0.0625 to 0.25 mg/liter). However, the MICm values of all tested RF Borrelia strains were significantly lower (P = 0.029) than those of the B. burgdorferi sensu lato control (PKo) (Table 1). Interestingly, the amoxicillin MICm values of both B. miyamotoi tick isolates, all B. miyamotoi clinical isolates, and the B. hermsii control strain were significantly higher than the clinical breakpoint for resistance (≤4 mg/liter, according to the Clinical and Laboratory Standards Institute [CLSI]) (15, 16). In line with our previous findings, the MICm value of Borrelia afzelii strain PKo was 4 mg/liter, thus significantly lower (P = 0.029) than the MICm values of all tested RF Borrelia strains (Table 1). This difference was also observed in colorimetric assays testing the antibiotic susceptibility of B. burgdorferi strain N40 and B. afzelii strain PKo versus B. hermsii strain HS-1 and two B. miyamotoi clinical isolates (Fig. 1). In line with these findings, the MICm value of B. burgdorferi strain N40 was 1 mg/ml, significantly lower (P = 0.029) than those of all tested RF Borrelia strains (data not shown).
TABLE 1.
MICs of all tested antibiotics for each Borrelia strain
| Antimicrobial agent and MIC type | MIC range (mg/liter)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| PKo | HS1 | HT31 | Izh-4 | Izh-5 | Izh-14 | Izh-16 | Yek-1 | Yek-6 | |
| Amoxicillin | |||||||||
| MICc | 4 | 8 | 8 | 8 | 16 | 8 | 8 | 16 | 16 |
| MICm | 4 | 8–16b | 16b | 8–16b | 16–32b | 8b | 8–16b | 16b | 16b |
| Doxycycline | |||||||||
| MICc | 4 | 0.25 | 0.125 | 0.25 | 0.125 | 0.0625 | 0.125 | 0.125 | 0.125 |
| MICm | 4 | 0.25c | 0.0625c | 0.25c | 0.125–0.25c | 0.125–0.25c | 0.25c | 0.25c | 0.25c |
| Ceftriaxone | |||||||||
| MICc | 0.125 | 0.06 | 0.03 | 0.06 | 0.06 | 0.03 | 0.03 | 0.06 | 0.06 |
| MICm | 0.125 | 0.125 | 0.03 | 0.03–0.06 | 0.06–0.125 | 0.03–0.06 | 0.03 | 0.03–0.06 | 0.03–0.06 |
| Azithromycin | |||||||||
| MICc | 0.0032 | 0.0032 | NR | 0.0016 | 0.0016 | 0.0016 | 0.0016 | 0.0032 | 0.0032 |
| MICm | 0.0064 | 0.0064 | 0.0032–0.0064 | 0.0016 | 0.0016–0.0032 | 0.0016 | 0.0016–0.0032 | 0.0032 | 0.0032 |
MIC ranges are the results of tests conducted in quadruplicate. PKo, B. afzelii (B. burgdorferi sensu lato control); HS1, B. hermsii (RF Borrelia control); HT31, B. miyamotoi laboratory strain (B. miyamotoi control); Izh-4, Izh-5, Izh-14, Izh-16, Yek-1, and Yek-6 (B. miyamotoi clinical isolates). NR, not reached.
Significantly (P < 0.05) higher from the amoxicillin MICm found for PKo.
Significantly (P < 0.05) lower from the doxycycline MICm found for PKo.
FIG 1.
Decrease in absorbance after incubation with amoxicillin. Absorbance was measured at 562 (corrected for absorbance at 630 nm), and the decrease in absorbance was measured after 72 h of incubation with amoxicillin and compared with the decrease in absorbance of the positive-control sample ([Et0 − Et72] of <25% of [EPOS,t0 − EPOS,t72]). Shown are representative results of two individual experiments with five representative Borrelia strains, i.e., PKo (B. afzelii), N40 (B. burgdorferi sensu stricto), HS1 (B. hermsii from the United States), and Izh-14 and Yek-1 (B. miyamotoi clinical isolates). Symbols and error bars represent the mean ± standard error of the mean of quadruplicate results. If no error bars are visible, then the error bars were smaller than the symbol.
DISCUSSION
BMD is an emerging infectious disease and, since cultivation is cumbersome and has only recently been described (17), experimental studies on B. miyamotoi are scarce. We combined previous experience with cultivation and antimicrobial susceptibility testing of Borrelia with a unique set of recently isolated B. miyamotoi clinical strains (12, 13, 17). By measuring acidification as a result of Borrelia growth, using colorimetry, we were able to simultaneously test MICc values for several clinical isolates and control strains in single experiments and thus reliably compare results. Colorimetry is a reproducible method to approximate the antimicrobial susceptibility of Borrelia spirochetes (12). We subsequently confirmed susceptibilities by enumerating motile spirochetes near the MICc by dark-field microscopy (MICm).
We were able to confirm our previous findings on the antibiotic susceptibility of B. miyamotoi tick isolates and demonstrated in vitro susceptibility of B. miyamotoi clinical isolates to azithromycin, doxycycline, and ceftriaxone. Importantly, in vitro resistance to amoxicillin was observed for B. miyamotoi clinical isolates, confirming the previously observed resistance of tick isolates (12). It is known that the efficacy of β-lactam antibiotics, including amoxicillin, is temperature dependent (18). Therefore, the fact that the amoxicillin MICm found for RF Borrelia is over the clinical breakpoint for resistance could be partially attributed to experimental conditions. The experimental conditions, however, cannot explain the relative difference in susceptibilities between RF Borrelia and the tested B. burgdorferi sensu lato strains.
Current treatment of BMD is based mainly on guidelines for treatment of Lyme borreliosis. Supported by successful treatment described in the literature (3, 5, 7, 19), our results confirm doxycycline, ceftriaxone, and azithromycin as treatment options for BMD. The doxycycline MICc values we report for B. burgdorferi sensu lato and RF Borrelia are comparable to MICs found in the literature (12). Interestingly, the significantly lower doxycycline MICs of B. miyamotoi clinical isolates, compared to B. burgdorferi sensu lato isolates (Table 1), might suggest that the therapeutic dose of doxycycline for BMD could be decreased, to avoid side effects; however, this would require additional in vivo studies and clinical trials.
The low in vitro activity of amoxicillin against clinical isolates of B. miyamotoi, which is in line with our previous findings (12), might suggest that amoxicillin is not a preferred antibiotic for the treatment of BMD. While in vitro results by themselves do not allow us to draw conclusions regarding the clinical efficacy of the tested antimicrobial agents, they reveal the need for further investigation in animal models and clinical settings.
MATERIALS AND METHODS
Borrelia strains.
The Borrelia control strains included were PKo (B. afzelii; a skin isolate from Germany), N40 (B. burgdorferi sensu stricto; a tick isolate from the United States), HT31 (B. miyamotoi; a tick isolate from Japan), and HS1 (B. hermsii; a tick isolate from the United States). The B. miyamotoi clinical isolates used in this study were obtained through cultivation of pelleted plasma from patients with PCR-proven B. miyamotoi disease, in Izhevsk, Russia, and Yekaterinburg, Russia. Isolation of these strains was described previously (13), and they were designated Izh-4, Izh-5, Izh-14, Izh-16, Yek-1, and Yek-6.
Borrelia cultures.
Borrelia glycerol stocks (<5 passages) were thawed from −80°C and cultured at 33°C in modified Kelly-Pettenkofer (MKP) medium (for B. burgdorferi sensu lato) or MKP medium with the addition of 10% fetal calf serum (MKP-F medium) (for RF Borrelia), in a regular incubator (Memmert, Schwabach, Germany), as described previously (17). Spirochete concentrations were determined using a Petroff-Hausser counting chamber and dark-field microscopy.
Antimicrobial agents.
The antibiotics tested were the antibiotics suggested for the treatment of Lyme borreliosis, i.e., doxycycline, amoxicillin, azithromycin, and ceftriaxone (10). Detailed information on each antibiotic is shown in Table 2.
TABLE 2.
Detailed information on tested antimicrobial agents
| Antimicrobial | Suppliera | Product no. | Dilution range (mg/liter) | Breakpoint (mg/liter)b | Stock concn (mg/ml) (solvent)c |
|---|---|---|---|---|---|
| Amoxicillin | Centrafarm | 14029596 | 0.25–128 | ≤4 | 8.89 (PBS) |
| Doxycycline | Sigma | D3447 | 0.016–8 | ≤4 | 44.4 (DMSO) |
| Ceftriaxone | Sigma | PHR1382 | 0.016–8 | ≤8 | 8.89 (PBS) |
| Azithromycin | Sigma | PHR1088 | 0.0004–0.2048 | ≤2 | 5.56 (DMSO) |
The suppliers were Centrafarm (Etten-Leur, The Netherlands) and Sigma-Aldrich (St. Louis, MO, USA).
Breakpoints are according CLSI guidelines (16).
PBS, phosphate-buffered saline; DMSO, dimethyl sulfoxide.
Experimental design.
The experimental design was described previously (12). Spirochetes were cultured for 4 days to reach the mid-log phase of growth. For all Borrelia isolates (including B. burgdorferi sensu lato), MKP-F medium containing phenol red (25 mg/liter) was preincubated for 96 h at 33°C in 50-ml Falcon tubes with 30% air, to circumvent nonspecific color shifts (12). Medium containing spirochetes was centrifuged at 4,500 × g for 45 min at room temperature, and spirochetes were suspended in the preincubated MKP-F medium. Spirochetes were enumerated and adjusted to 5 × 107 spirochetes/ml. A 10-step, 2-fold dilution series of each antimicrobial agent was made in MKP-F medium containing phenol red; 180 μl of each antibiotic concentration was added to 96-well, flat-bottom, polystyrene microtiter trays (product no. M0687; Greiner), in quadruplicate, and 20 μl of spirochete suspension was added to each well for a final concentration of 5 × 106 spirochetes/ml. The microtiter plates were sealed with adhesive plastic and incubated at 33°C for 72 h.
MIC measurements.
Colorimetric changes through acidification of MKP-F medium by expansion of viable Borrelia spirochetes were used to determine antimicrobial susceptibility, as described previously (12). In short, absorbance values at 562 nm (corrected for absorbance at 630 nm) were measured at 0 and 72 h using a commercially available enzyme-linked immunosorbent assay (ELISA) reader (PowerWave 200; BioTek Instruments). Colorimetric changes were calculated by comparing the absorbance at 72 h (Et72) and the initial absorbance (Et0) for each well (Et0 − Et72), corrected for the change in absorbance of the negative-control sample (MKP-F medium without Borrelia). The colorimetric MIC (MICc) was calculated by comparing the decrease in absorbance to the decrease in absorbance of the positive-control sample (no antibiotics). MICc was defined as the lowest concentration of antibiotic at which the average decrease in absorbance (of four replicates) was <25% of the decrease in absorbance found for the positive-control sample ([Et0 − Et72] of <25% of [EPOS,t0 − EPOS,t72]). Findings were confirmed by dark-field microscopy; well contents were resuspended, and 5 μl of each well sample at the MICc was transferred to a microscopy slide and covered by a coverslip. The microscopic MIC (MICm) was defined as the lowest concentration of antimicrobial agent at which no motile spirochetes were observed by dark-field microscopy, as described previously (12). In cases in which motile spirochetes were observed, one antibiotic concentration above the MICc was also tested, until no motile spirochetes were observed in any of the four replicate wells. The lower detection limit using this method was 4 × 104 spirochetes/ml (12).
Statistical analyses.
Data analyses were performed using PASW Statistics 19.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 7.0.2 (GraphPad Software Inc., La Jolla, CA, USA). Nonparametric Mann-Whitney tests were used to calculate the significance between MICm results from different tested strains.
ACKNOWLEDGMENTS
We thank Barbara Johnson and Volker Fingerle for providing B. miyamotoi strain HT31.
The Ticking on Pandora's Box project by J.W.H. and D.H. is funded by ZonMW (project 50-52200-98-313). This study was also supported by a grant from the Russian Science Foundation (project 15-15-00072) to N.M.K., D.S.S., M.G.T., and A.E.P.
We have no conflicts of interest to disclose.
REFERENCES
- 1.Michelet L, Delannoy S, Devillers E, Umhang G, Aspan A, Juremalm M, Chirico J, van der Wal FJ, Sprong H, Boye Pihl TP, Klitgaard K, Bodker R, Fach P, Moutailler S. 2014. High-throughput screening of tick-borne pathogens in Europe. Front Cell Infect Microbiol 4:103. doi: 10.3389/fcimb.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, Li X, Mead PS. 2016. Lyme borreliosis. Nat Rev Dis Primers 2:16090. doi: 10.1038/nrdp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, Maleev VV, Fish D, Krause PJ. 2011. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis 17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molloy PJ, Telford SR III, Chowdri HR, Lepore TJ, Gugliotta JL, Weeks KE, Hewins ME, Goethert HK, Berardi VP. 2015. Borrelia miyamotoi disease in the northeastern United States: a case series. Ann Intern Med 163:91–98. doi: 10.7326/M15-0333. [DOI] [PubMed] [Google Scholar]
- 5.Hovius JW, de Wever B, Sohne M, Brouwer MC, Coumou J, Wagemakers A, Oei A, Knol H, Narasimhan S, Hodiamont CJ, Jahfari S, Pals ST, Horlings HM, Fikrig E, Sprong H, van Oers MH. 2013. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet 382:658. doi: 10.1016/S0140-6736(13)61644-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gugliotta JL, Goethert HK, Berardi VP, Telford SR III. 2013. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med 368:240–245. doi: 10.1056/NEJMoa1209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krause PJ, Narasimhan S, Wormser GP, Rollend L, Fikrig E, Lepore T, Barbour A, Fish D. 2013. Human Borrelia miyamotoi infection in the United States. N Engl J Med 368:291–293. doi: 10.1056/NEJMc1215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boden K, Lobenstein S, Hermann B, Margos G, Fingerle V. 2016. Borrelia miyamotoi-associated neuroborreliosis in immunocompromised person. Emerg Infect Dis 22:1617–1620. doi: 10.3201/eid2209.152034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutler SJ. 2015. Relapsing fever borreliae: a global review. Clin Lab Med 35:847–865. doi: 10.1016/j.cll.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. 2006. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 11.Sudhindra P, Wang G, Schriefer ME, McKenna D, Zhuge J, Krause PJ, Marques AR, Wormser GP. 2016. Insights into Borrelia miyamotoi infection from an untreated case demonstrating relapsing fever, monocytosis and a positive C6 Lyme serology. Diagn Microbiol Infect Dis 86:93–96. doi: 10.1016/j.diagmicrobio.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koetsveld J, Draga ROP, Wagemakers A, Manger A, Oei A, Visser CE, Hovius JW. 2017. In vitro susceptibility of the relapsing-fever spirochete Borrelia miyamotoi to antimicrobial agents. Antimicrob Agents Chemother 61:e00535-17. doi: 10.1128/AAC.00535-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koetsveld J, Kolyasnikova NM, Wagemakers A, Toporkova MG, Sarksyan DS, Oei A, Platonov AE, Hovius JW. 2017. Development and optimization of an in vitro cultivation protocol allows for isolation of Borrelia miyamotoi from patients with hard tick-borne relapsing fever. Clin Microbiol Infect 23:480–484. doi: 10.1016/j.cmi.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Hunfeld KP, Kraiczy P, Wichelhaus TA, Schafer V, Brade V. 2000. New colorimetric microdilution method for in vitro susceptibility testing of Borrelia burgdorferi against antimicrobial substances. Eur J Clin Microbiol Infect Dis 19:27–32. doi: 10.1007/s100960050005. [DOI] [PubMed] [Google Scholar]
- 15.Veinovic G, Cerar T, Strle F, Lotric-Furlan S, Maraspin V, Cimperman J, Ruzic-Sabljic E. 2013. In vitro susceptibility of European human Borrelia burgdorferi sensu stricto strains to antimicrobial agents. Int J Antimicrob Agents 41:288–291. doi: 10.1016/j.ijantimicag.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2010. Performance standards for antimicrobial susceptibility testing; 20th informational supplement. Document M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Wagemakers A, Oei A, Fikrig MM, Miellet WR, Hovius JW. 2014. The relapsing fever spirochete Borrelia miyamotoi is cultivable in a modified Kelly-Pettenkofer medium, and is resistant to human complement. Parasit Vectors 7:418. doi: 10.1186/1756-3305-7-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reisinger E, Wendelin I, Gasser R, Halwachs G, Wilders-Truschnig M, Krejs G. 1996. Antibiotics and increased temperature against Borrelia burgdorferi in vitro. Scand J Infect Dis 28:155–157. doi: 10.3109/00365549609049067. [DOI] [PubMed] [Google Scholar]
- 19.Sarksyan DS, Platonov AE, Karan LS, Malinin IE, Khalitova LI, Shakhov VI, Dudarev MV, Malinin OV, Maleev VV. 2012. Clinical presentation of “new” tick-borne borreliosis caused by Borrelia miyamotoi. Ter Arkh 84:34–41. (In Russian.) [PubMed] [Google Scholar]