ABSTRACT
The increasing prevalence of multidrug-resistant Gram-negative pathogens has generated a requirement for new treatment options. Avibactam, a novel non-β-lactam–β-lactamase inhibitor, restores the activity of ceftazidime against Ambler class A, C, and some class D β-lactamase-producing strains of Enterobacteriaceae and Pseudomonas aeruginosa. The in vitro activities of ceftazidime-avibactam versus comparators were evaluated against 1,440 clinical isolates obtained in a phase 3 clinical trial in patients with complicated intra-abdominal infections (cIAI; ClinicalTrials.gov identifier NCT01499290). Overall, in vitro activities were determined for 803 Enterobacteriaceae, 70 P. aeruginosa, 304 Gram-positive aerobic, and 255 anaerobic isolates obtained from 1,066 randomized patients at baseline. Susceptibility was determined by broth microdilution. The most commonly isolated Gram-negative, Gram-positive, and anaerobic pathogens were Escherichia coli (n = 549), Streptococcus anginosus (n = 130), and Bacteroides fragilis (n = 96), respectively. Ceftazidime-avibactam was highly active against isolates of Enterobacteriaceae, with an overall MIC90 of 0.25 mg/liter. In contrast, the MIC90 for ceftazidime alone was 32 mg/liter. The MIC90 value for ceftazidime-avibactam (4 mg/liter) was one dilution lower than that of ceftazidime alone (8 mg/liter) against isolates of Pseudomonas aeruginosa. The ceftazidime-avibactam MIC90 for 109 ceftazidime-nonsusceptible Enterobacteriaceae isolates was 2 mg/liter, and the MIC range for 6 ceftazidime-nonsusceptible P. aeruginosa isolates was 8 to 32 mg/liter. The MIC90 values were within the range of susceptibility for the study drugs permitted per the protocol in the phase 3 study to provide coverage for aerobic Gram-positive and anaerobic pathogens. These findings demonstrate the in vitro activity of ceftazidime-avibactam against bacterial pathogens commonly observed in cIAI patients, including ceftazidime-nonsusceptible Enterobacteriaceae. (This study has been registered at ClinicalTrials.gov under identifier NCT01499290.)
KEYWORDS: ceftazidime-avibactam, complicated intra-abdominal infection, in vitro activity, ceftazidime-nonsusceptible
INTRODUCTION
The increasing prevalence of β-lactamase-mediated antibiotic resistance has generated a need for the development of new treatment options (1). Avibactam is a novel non-β-lactam–β-lactamase inhibitor with in vitro activity against Ambler class A and C β-lactamases (including Klebsiella pneumoniae carbapenemase [KPC] and the carbapenem-hydrolyzing oxacillinase OXA-48), as well as some class D enzymes (2, 3). Ceftazidime is an established antipseudomonal cephalosporin; since its introduction, a number of new extended-spectrum β-lactamases have been identified, eroding the effectiveness of ceftazidime and other cephalosporins (1). When combined with avibactam, the in vitro spectrum of activity of ceftazidime is extended to include isolates producing extended-spectrum β-lactamases (ESBLs) and AmpC-producing Enterobacteriaceae and Pseudomonas aeruginosa (4–7).
Ceftazidime-avibactam has been approved in Europe, the United States, and several other countries for the treatment of adults with complicated intra-abdominal infection (cIAI) in combination with metronidazole and complicated urinary tract infection (cUTI) (8, 9). In addition, in Europe, ceftazidime-avibactam has been approved for the treatment of hospital-acquired pneumonia (HAP), including ventilator-associated pneumonia (VAP) and other aerobic Gram-negative infections for which there are limited treatment options (8).
Ceftazidime-avibactam has been investigated in two identical prospective randomized double-blind comparative phase 3 noninferiority studies in patients with cIAI (RECLAIM; ClinicalTrials.gov identifier NCT01499290) (10). These were combined and analyzed as one study and demonstrated the efficacy, safety, and tolerability of ceftazidime-avibactam plus metronidazole in comparison with meropenem in patients with cIAI. As part of this study, bacterial cultures were isolated from abdominal and blood specimens in patients with confirmed cIAI and submitted to a central reference laboratory for identification and susceptibility testing. This report describes the in vitro activities of ceftazidime-avibactam and relevant comparator agents against these clinical isolates.
RESULTS
In total, 1,440 isolates from 1,066 randomized patients in the phase 3 cIAI clinical trial RECLAIM (ClinicalTrials.gov identifier NCT01499290) were sent to the central laboratory for identification and susceptibility testing. Of these isolates, 803 isolates were Enterobacteriaceae, and 70 isolates were P. aeruginosa. Escherichia coli was the most common member of the Enterobacteriaceae to be identified (isolated in 549 of the randomized patients [51.5%]), followed by K. pneumoniae (100 patients [9.4%]). Citrobacter freundii complex and Klebsiella oxytoca were the third most commonly isolated Enterobacteriaceae (isolated in 32 patients each [3.0%]).
In total, 304 Gram-positive aerobes and 255 anaerobes were isolated at baseline. Of the Gram-positive aerobes, baseline isolates most frequently belonged to the Streptococcus anginosus group (130 isolates [12.2%]), followed by Enterococcus faecalis (59 isolates [5.5%]) and Enterococcus faecium (39 isolates [3.7%]). Bacteroides fragilis was the most frequently isolated anaerobe (96 isolates [9.0%]).
In vitro activity against Gram-negative isolates.
The in vitro activities of ceftazidime-avibactam, ceftazidime alone, and comparator agents against Enterobacteriaceae and P. aeruginosa are summarized in Table 1. The MIC50 and MIC90 values for ceftazidime alone against all Enterobacteriaceae isolates were 0.12 mg/liter and 32 mg/liter, respectively (Table 1). In contrast, ceftazidime-avibactam was highly active against Enterobacteriaceae isolates, with overall MIC50 and MIC90 values of 0.12 mg/liter and 0.25 mg/liter, respectively, confirming a 128-fold reduction in MIC90 for ceftazidime-avibactam compared with ceftazidime alone (Table 1).
TABLE 1.
In vitro activities of ceftazidime-avibactam and comparative agents against baseline Enterobacteriaceae and Pseudomonas aeruginosa clinical isolates for all randomized patientsa
| Baseline pathogen and agent (no. of pathogens tested) | MIC (mg/liter) |
||
|---|---|---|---|
| Range | MIC50 | MIC90 | |
| Enterobacteriaceae | |||
| All Enterobacteriaceae (803) | |||
| Ceftazidime-avibactam | ≤0.008 to >256 | 0.12 | 0.25 |
| Ceftazidime | ≤0.03 to >64 | 0.12 | 32 |
| Amikacin | 0.5 to >64 | 2 | 4 |
| Aztreonam | ≤0.03 to >64 | 0.06 | 64 |
| Cefepime | ≤0.008 to >16 | 0.03 | >16 |
| Ceftaroline | ≤0.008 to >256 | 0.12 | >256 |
| Ceftriaxone | ≤0.015 to >32 | 0.06 | >32 |
| Gentamicin | ≤0.12 to >16 | 0.5 | >16 |
| Imipenem | 0.06 to 16 | 0.12 | 0.5 |
| Levofloxacin | 0.008 to >8 | 0.06 | >8 |
| Meropenem | 0.008 to >8 | 0.015 | 0.06 |
| Piperacillin-tazobactam | ≤0.06 to >128 | 2 | 16 |
| Tigecycline | 0.06 to 9 | 0.25 | 1 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to >8 | ≤0.25 | >8 |
| Citrobacter freundii complex (32) | |||
| Ceftazidime-avibactam | 0.03 to 0.5 | 0.12 | 0.25 |
| Ceftazidime | 0.12 to >64 | 0.25 | 2 |
| Amikacin | 0.5 to 4 | 1 | 2 |
| Aztreonam | ≤0.03 to >64 | 0.12 | 16 |
| Cefepime | 0.015 to >16 | 0.03 | 1 |
| Ceftaroline | 0.06 to >256 | 0.12 | 128 |
| Ceftriaxone | 0.03 to >32 | 0.12 | >32 |
| Gentamicin | 0.25 to >16 | 0.5 | 0.5 |
| Imipenem | 0.12 to 1 | 0.25 | 0.5 |
| Levofloxacin | 0.015 to 8 | 0.06 | 0.5 |
| Meropenem | 0.008 to 0.06 | 0.015 | 0.03 |
| Piperacillin-tazobactam | 0.5 to >128 | 2 | 128 |
| Tigecycline | 0.12 to 2 | 0.25 | 0.5 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to >8 | ≤0.25 | ≤0.25 |
| Enterobacter aerogenes (10) | |||
| Ceftazidime-avibactam | 0.12 to 2 | 0.12 | 0.5 |
| Ceftazidime | 0.12 to >64 | 0.12 | 0.5 |
| Amikacin | 1 to >64 | 1 | 2 |
| Aztreonam | ≤0.03 to >64 | ≤0.03 | 0.25 |
| Cefepime | 0.03 to >16 | 0.03 | 0.06 |
| Ceftaroline | 0.06 to >256 | 0.12 | 0.25 |
| Ceftriaxone | 0.06 to >32 | 0.06 | 0.25 |
| Gentamicin | 0.25 to >16 | 0.5 | 0.5 |
| Imipenem | 0.12 to 1 | 0.5 | 1 |
| Levofloxacin | 0.03 to 0.12 | 0.06 | 0.12 |
| Meropenem | 0.015 to 0.06 | 0.03 | 0.03 |
| Piperacillin-tazobactam | 1 to >128 | 4 | 4 |
| Tigecycline | 0.25 to 1 | 0.5 | 0.5 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to >8 | ≤0.25 | 0.5 |
| Enterobacter cloacae (29) | |||
| Ceftazidime-avibactam | 0.06 to >256 | 0.25 | 2 |
| Ceftazidime | 0.06 to >64 | 0.5 | >64 |
| Amikacin | 1 to >64 | 1 | 8 |
| Aztreonam | ≤0.03 to >64 | 0.12 | >64 |
| Cefepime | 0.015 to >16 | 0.06 | >16 |
| Ceftaroline | 0.03 to >256 | 0.25 | >256 |
| Ceftriaxone | ≤0.015 to >32 | 0.25 | >32 |
| Gentamicin | 0.25 to >16 | 0.5 | >16 |
| Imipenem | 0.12 to 8 | 0.25 | 0.5 |
| Levofloxacin | 0.03 to >8 | 0.06 | 8 |
| Meropenem | 0.015 to >8 | 0.03 | 0.5 |
| Piperacillin-tazobactam | 0.5 to >128 | 4 | >128 |
| Tigecycline | 0.5 to 8 | 0.5 | 1 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to >8 | ≤0.25 | >8 |
| Escherichia coli (549) | |||
| Ceftazidime-avibactam | ≤0.008 to 4 | 0.06 | 0.12 |
| Ceftazidime | ≤0.03 to >64 | 0.12 | 8 |
| Amikacin | 0.5 to >64 | 2 | 4 |
| Aztreonam | ≤0.03 to >64 | 0.06 | 16 |
| Cefepime | ≤0.008 to >16 | 0.03 | 16 |
| Ceftaroline | ≤0.008 to >256 | 0.06 | >256 |
| Ceftriaxone | ≤0.015 to >32 | 0.06 | >32 |
| Gentamicin | 0.25 to >16 | 0.5 | >16 |
| Imipenem | 0.06 to 0.5 | 0.12 | 0.12 |
| Levofloxacin | 0.008 to >8 | 0.03 | >8 |
| Meropenem | 0.008 to 0.5 | 0.015 | 0.03 |
| Piperacillin-tazobactam | ≤0.06 to >128 | 2 | 8 |
| Tigecycline | 0.06 to 2 | 0.25 | 0.5 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to >8 | ≤0.25 | >8 |
| Klebsiella oxytoca (32) | |||
| Ceftazidime-avibactam | 0.03 to 0.25 | 0.06 | 0.12 |
| Ceftazidime | ≤0.03 to 0.25 | 0.12 | 0.12 |
| Amikacin | 0.5 to 4 | 1 | 1 |
| Aztreonam | ≤0.03 to 1 | 0.06 | 0.25 |
| Cefepime | 0.015 to 0.06 | 0.03 | 0.03 |
| Ceftaroline | 0.03 to 0.5 | 0.12 | 0.25 |
| Ceftriaxone | ≤0.015 to 0.12 | 0.03 | 0.06 |
| Gentamicin | ≤0.12 to 1 | 0.25 | 0.5 |
| Imipenem | 0.06 to 0.25 | 0.12 | 0.25 |
| Levofloxacin | 0.03 to 1 | 0.06 | 0.06 |
| Meropenem | 0.015 to 0.03 | 0.03 | 0.03 |
| Piperacillin-tazobactam | 0.5 to 4 | 2 | 2 |
| Tigecycline | 0.25 to 0.5 | 0.25 | 0.5 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to 0.5 | ≤0.25 | ≤0.25 |
| Klebsiella pneumoniae (100) | |||
| Ceftazidime-avibactam | ≤0.008 to >256 | 0.12 | 0.5 |
| Ceftazidime | ≤0.03 to >64 | 0.12 | >64 |
| Amikacin | 0.5 to >64 | 1 | 2 |
| Aztreonam | ≤0.03 to >64 | ≤0.03 | >64 |
| Cefepime | 0.015 to >16 | 0.03 | >16 |
| Ceftaroline | 0.015 to >256 | 0.12 | >256 |
| Ceftriaxone | ≤0.015 to >32 | 0.06 | >32 |
| Gentamicin | ≤0.12 to >16 | 0.25 | >16 |
| Imipenem | 0.06 to 16 | 0.12 | 0.25 |
| Levofloxacin | 0.015 to >8 | 0.06 | >8 |
| Meropenem | 0.015 to >8 | 0.03 | 0.12 |
| Piperacillin-tazobactam | 0.5 to >128 | 2 | >128 |
| Tigecycline | 0.25 to 4 | 0.5 | 2 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to >8 | ≤0.25 | >8 |
| Proteus mirabilis (17) | |||
| Ceftazidime-avibactam | 0.015 to 2 | 0.03 | 0.5 |
| Ceftazidime | ≤0.03 to >64 | 0.06 | 32 |
| Amikacin | 1 to 16 | 4 | 8 |
| Aztreonam | ≤0.03 to 2 | ≤0.03 | 0.5 |
| Cefepime | 0.03 to 2 | 0.06 | 1 |
| Ceftaroline | 0.03 to >256 | 0.06 | 256 |
| Ceftriaxone | ≤0.015 to >32 | ≤0.015 | >32 |
| Gentamicin | 0.5 to >16 | 1 | >16 |
| Imipenem | 0.12 to 4 | 1 | 4 |
| Levofloxacin | 0.03 to >8 | 0.12 | >8 |
| Meropenem | 0.03 to 0.12 | 0.06 | 0.12 |
| Piperacillin-tazobactam | ≤0.06 to 16 | 0.25 | 4 |
| Tigecycline | 2 to 8 | 4 | 4 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to >8 | ≤0.25 | >8 |
| Pseudomonas aeruginosa (70) | |||
| Ceftazidime-avibactam | 0.5 to 32 | 2 | 4 |
| Ceftazidime | 1 to >64 | 2 | 8 |
| Amikacin | ≤0.12 to >64 | 4 | 8 |
| Cefepime | 0.5 to >16 | 2 | 8 |
| Ceftriaxone | 4 to >32 | 32 | >32 |
| Gentamicin | ≤0.12 to >16 | 1 | 2 |
| Imipenem | 0.5 to 16 | 1 | 2 |
| Levofloxacin | 0.03 to >8 | 0.5 | >8 |
| Meropenem | 0.03 to >8 | 0.12 | 2 |
| Piperacillin-tazobactam | 0.5 to >128 | 4 | 16 |
Total of 1,066 randomized patients. Some patients had more than one pathogen isolated. Multiple isolates of the same species from the same patient were counted only once using the isolate with the highest MIC to the study drug received. For bacteremic patients, multiple isolates of the same species from the same patient were counted only once using the isolate with the highest MIC to the study drug received across culture source (intra-abdominal site or blood).
With respect to the individual members of the Enterobacteriaceae family, the ceftazidime-avibactam MIC90 values for E. coli and K. pneumoniae (the most commonly isolated Gram-negative pathogens in this study) were 0.12 mg/liter and 0.5 mg/liter, respectively. In addition, the MIC90 values were ≤2 mg/liter for all the other members of the Enterobacteriaceae family where there were 10 or more isolates (Table 1). A group of nine other members of the Enterobacteriaceae family (where there were fewer individual isolates), including Citrobacter farmeri (1 isolate), Citrobacter koseri (5 isolates), Hafnia alvei (3 isolates), Morganella morganii (9 isolates), Proteus vulgaris group species (7 isolates), Providencia rettgeri (2 isolates), Raoultella planticola (2 isolates), Salmonella species (1 isolate), and Serratia marcescens (4 isolates) tested with a ceftazidime-avibactam MIC range of 0.015 to 1 mg/liter.
For the 70 P. aeruginosa isolates, the ceftazidime-avibactam MIC50 and MIC90 values were 2 mg/liter and 4 mg/liter, respectively (Table 1). Ceftazidime-avibactam was one dilution more active than ceftazidime alone, based on MIC90 values (Table 1).
The ceftazidime-avibactam MIC values for other baseline non-Enterobacteriaceae Gram-negative pathogens with <10 isolates were as follows: Aeromonas spp. (n = 2), with an MIC range of 0.12 to 0.25 mg/liter; and other Pseudomonas spp. (n = 6), with an MIC range of 0.25 to 8 mg/liter.
In vitro activity against Gram-positive and anaerobic isolates.
The in vitro activities of ceftazidime-avibactam and comparators against Gram-positive baseline isolates are summarized in Table 2. The MIC90 values for vancomycin, linezolid, and daptomycin against the Gram-positive isolates characterized in this study were typically ≤2 mg/liter, with E. faecium and other enterococci having a daptomycin MIC90 of 4 mg/liter (Table 2).
TABLE 2.
In vitro activities of ceftazidime-avibactam and comparative agents against baseline Gram-positive isolates for all randomized patientsa
| Baseline pathogen and agent (no. of tested pathogens) | MIC (mg/liter) |
||
|---|---|---|---|
| Range | MIC50 | MIC90 | |
| Enterococcus faecalis (59) | |||
| Ceftazidime-avibactam | 32 to >256 | >256 | >256 |
| Ceftazidime | 32 to >64 | >64 | >64 |
| Ceftaroline | 0.25 to 256 | 1 | 64 |
| Clindamycin | 4 to >16 | >16 | >16 |
| Daptomycin | 0.06 to 4 | 1 | 2 |
| Levofloxacin | 0.5 to >8 | 1 | >8 |
| Linezolid | 1 to 2 | 2 | 2 |
| Meropenem | 1 to >8 | 4 | >8 |
| Teicoplanin | 0.25 to 2 | 0.5 | 1 |
| Ticarcillin-clavulanate | 32 to >128 | 64 | >128 |
| Tigecycline | 0.06 to 0.25 | 0.12 | 0.12 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to >8 | ≤0.25 | >8 |
| Vancomycin | 0.5 to 2 | 1 | 2 |
| Enterococcus faecium (39) | |||
| Ceftazidime-avibactam | 32 to >256 | >256 | >256 |
| Ceftazidime | 16 to >64 | >64 | >64 |
| Ceftaroline | 0.12 >256 | 0.5 | >256 |
| Clindamycin | 0.06 to >16 | 8 | >16 |
| Daptomycin | 0.5 to 8 | 4 | 4 |
| Levofloxacin | 0.5 to >8 | 2 | >8 |
| Linezolid | 1 to 4 | 2 | 2 |
| Meropenem | 0.25 to >8 | >8 | >8 |
| Teicoplanin | 0.25 to >32 | 1 | 1 |
| Tigecycline | 0.03 to 0.12 | 0.06 | 0.12 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to >8 | ≤0.25 | >8 |
| Vancomycin | 0.25 to >32 | 0.5 | 1 |
| Other Enterococcus spp. (37)b | |||
| Ceftazidime-avibactam | 1 to >256 | 128 | >256 |
| Ceftazidime | 2 to >64 | >64 | >64 |
| Ceftaroline | ≤0.008 to 4 | 0.25 | 1 |
| Clindamycin | 0.5 to >16 | 4 | >16 |
| Daptomycin | 0.12 to 4 | 0.5 | 4 |
| Levofloxacin | 0.25 to >8 | 1 | 2 |
| Linezolid | 1 to 4 | 2 | 2 |
| Meropenem | 0.03 to >8 | 4 | 8 |
| Teicoplanin | ≤0.12 to 2 | 0.5 | 1 |
| Tigecycline | ≤0.015 to 0.12 | 0.03 | 0.06 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to >8 | ≤0.25 | ≤0.25 |
| Vancomycin | 0.25 to 8 | 0.5 | 2 |
| Streptococcus anginosus group (131)c | |||
| Ceftazidime-avibactam | ≤0.06 to >4 | 4 | >4 |
| Ceftazidime | 0.5 to >4 | 4 | >4 |
| Clindamycin | ≤0.015 to >1 | 0.03 | 0.06 |
| Daptomycin | 0.06 to 1 | 0.5 | 1 |
| Levofloxacin | ≤0.12 to 1 | 0.5 | 1 |
| Linezolid | ≤0.12 to 2 | 2 | 2 |
| Meropenem | ≤0.015 to 0.25 | 0.06 | 0.12 |
| Tigecycline | ≤0.008 to 0.25 | ≤0.008 | 0.03 |
| Trimethoprim-sulfamethoxazole | ≤0.06 to 0.5 | ≤0.06 | ≤0.06 |
| Vancomycin | 0.5 to 1 | 0.5 | 1 |
| Other streptococci (46)d | |||
| Ceftazidime-avibactam | 0.12 to >4 | 1 | >4 |
| Ceftazidime | 0.12 to >4 | 1 | >4 |
| Clindamycin | ≤0.015 to >1 | 0.03 | 0.06 |
| Daptomycin | ≤0.03 to 1 | 0.5 | 1 |
| Levofloxacin | 0.5 to 4 | 1 | 2 |
| Linezolid | 0.5 to 2 | 1 | 2 |
| Meropenem | ≤0.015 to 0.5 | 0.03 | 0.25 |
| Tigecycline | ≤0.008 to 0.5 | 0.06 | 0.25 |
| Trimethoprim-sulfamethoxazole | ≤0.06 to 2 | 0.12 | 1 |
| Vancomycin | 0.25 to 1 | 0.5 | 0.5 |
| Staphylococcus aureus (33) | |||
| Ceftazidime-avibactam | 4 to >256 | 8 | 128 |
| Ceftazidime | 4 to >64 | 8 | 64 |
| Ceftaroline | 0.12 to 32 | 0.25 | 1 |
| Clindamycin | 0.12 to >16 | 0.12 | >16 |
| Daptomycin | 0.25 to 0.5 | 0.5 | 0.5 |
| Levofloxacin | 0.06 to >8 | 0.25 | 4 |
| Linezolid | 1 to 4 | 2 | 2 |
| Meropenem | 0.03 to >8 | 0.06 | 2 |
| Teicoplanin | 0.5 to 4 | 1 | 1 |
| Tigecycline | 0.06 to 1 | 0.12 | 0.25 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to ≤0.25 | ≤0.25 | ≤0.25 |
| Vancomycin | 0.5 to 1 | 0.5 | 1 |
Total of 1,066 randomized patients. Data are provided for pathogens identified in at least 10 patients. A patient could have more than one pathogen isolated. Multiple isolates of the same species from the same patient were counted only once using the isolate with the highest MIC to the study drug received. For bacteremic patients, multiple isolates of the same species from the same patient were counted only once using the isolate with the highest MIC to the study drug received across culture source (intra-abdominal site or blood).
Other Enterococcus spp. include Enterococcus avium (n = 23), Enterococcus casseliflavus (n = 3), Enterococcus durans (n = 1), Enterococcus gallinarum (n = 3), Enterococcus hirae (n = 3), Enterococcus raffinosus (n = 1), and Enterococcus thailandicus (n = 3).
Streptococcus anginosus group includes Streptococcus anginosus group (n = 130) and Streptococcus constellatus (n = 1).
Other streptococci include Streptococcus bovis group (n = 10), Streptococcus dysgalactiae (n = 3), Streptococcus mitis group (n = 26), Streptococcus pyogenes (n = 2), and Streptococcus salivarius group (n = 5).
The MIC90 values for metronidazole against baseline anaerobe species with ≥10 isolates were 1 to 4 mg/liter, and the MIC90 values for meropenem against baseline anaerobes were 0.03 to 4 mg/liter, indicating that both drugs were active against these isolates (Table 3).
TABLE 3.
In vitro activities of ceftazidime-avibactam and comparative agents against anaerobic species isolated at baseline for all randomized patientsa
| Baseline pathogen and agent (no. of pathogens tested) | MIC (mg/liter) |
||
|---|---|---|---|
| Range | MIC50 | MIC90 | |
| Bacteroides fragilis group (96) | |||
| Ceftazidime-avibactam | 1 to 32 | 4 | 8 |
| Ceftazidime | 4 to >128 | 32 | >128 |
| Amoxicillin-clavulanate | 0.25 to 16 | 0.5 | 4 |
| Ampicillin | 1 to >128 | 32 | >128 |
| Clindamycin | ≤0.015 to >32 | 1 | >32 |
| Meropenem | 0.06 to 8 | 0.12 | 4 |
| Metronidazole | 0.25 to 8 | 1 | 2 |
| Other Bacteroides fragilis group (163)b | |||
| Ceftazidime-avibactam | 2 to >128 | 64 | 128 |
| Ceftazidime | 8 to >128 | >128 | >128 |
| Amoxicillin-clavulanate | 0.25 to >128 | 1 | 8 |
| Ampicillin | 1 to >128 | 32 | >128 |
| Clindamycin | 0.03 to >32 | 8 | >32 |
| Meropenem | 0.06 to 2 | 0.25 | 1 |
| Metronidazole | 0.25 to 8 | 2 | 4 |
| Other Bacteroides spp. (21)c | |||
| Ceftazidime-avibactam | ≤0.06 to 64 | 16 | 64 |
| Ceftazidime | 0.12 to >128 | 32 | >128 |
| Amoxicillin-clavulanate | ≤0.06 to 8 | 0.5 | 2 |
| Ampicillin | ≤0.06 to >128 | 16 | >128 |
| Clindamycin | ≤0.015 to >32 | 2 | >32 |
| Meropenem | ≤0.015 to 2 | 0.25 | 1 |
| Metronidazole | 0.12 to 8 | 1 | 2 |
| Clostridium perfringens (14) | |||
| Ceftazidime-avibactam | ≤0.06 to ≤0.06 | ≤0.06 | ≤0.06 |
| Ceftazidime | 0.25 to 8 | 1 | 4 |
| Amoxicillin-clavulanate | ≤0.06 to 0.12 | ≤0.06 | ≤0.06 |
| Ampicillin | ≤0.06 to 0.25 | ≤0.06 | 0.12 |
| Clindamycin | 0.03 to >32 | 1 | 4 |
| Meropenem | ≤0.015 to 0.25 | ≤0.015 | 0.03 |
| Metronidazole | 0.25 to 4 | 1 | 4 |
| Other Clostridium spp. (28)d | |||
| Ceftazidime-avibactam | ≤0.06 to >128 | 16 | >128 |
| Ceftazidime | 2 to >128 | 32 | >128 |
| Amoxicillin-clavulanate | ≤0.06 to 2 | 0.5 | 1 |
| Ampicillin | ≤0.06 to 64 | 0.5 | 8 |
| Clindamycin | 0.06 to >32 | 1 | >32 |
| Meropenem | ≤0.015 to 4 | 1 | 4 |
| Metronidazole | ≤0.06 to 4 | 0.5 | 2 |
| Eggerthella lenta (13) | |||
| Ceftazidime-avibactam | >128 to >128 | >128 | >128 |
| Ceftazidime | >128 to >128 | >128 | >128 |
| Amoxicillin-clavulanate | 0.25 to 2 | 1 | 1 |
| Ampicillin | 1 to 4 | 2 | 2 |
| Clindamycin | 0.06 to >32 | 0.5 | >32 |
| Meropenem | 0.25 to 1 | 0.25 | 0.5 |
| Metronidazole | 0.5 to 16 | 2 | 4 |
| Parvimonas micra (16) | |||
| Ceftazidime-avibactam | ≤0.06 to 4 | ≤0.06 | 0.5 |
| Ceftazidime | 0.12 to 8 | 0.5 | 2 |
| Amoxicillin-clavulanate | ≤0.06 to 0.5 | ≤0.06 | 0.25 |
| Ampicillin | ≤0.06 to 8 | ≤0.06 | 0.25 |
| Clindamycin | 0.06 to 4 | 0.25 | 0.5 |
| Meropenem | ≤0.015 to 0.25 | 0.03 | 0.12 |
| Metronidazole | 0.12 to 1 | 0.5 | 1 |
| Prevotella spp. (21)e | |||
| Ceftazidime-avibactam | ≤0.06 to 8 | 0.5 | 4 |
| Ceftazidime | 0.12 to 128 | 2 | 16 |
| Amoxicillin-clavulanate | ≤0.06 to 1 | 0.12 | 0.5 |
| Ampicillin | ≤0.06 to 16 | 0.25 | 8 |
| Clindamycin | ≤0.015 to >32 | ≤0.015 | >32 |
| Meropenem | ≤0.015 to 0.25 | 0.03 | 0.12 |
| Metronidazole | 0.12 to 8 | 1 | 4 |
Total of 1,066 randomized patients. Data are provided for pathogens identified in at least 10 patients.
Other Bacteroides fragilis group includes Bacteroides caccae (n = 3), Bacteroides ovatus (n = 41), Bacteroides stercoris (n = 11), Bacteroides thetaiotaomicron (n = 47), Bacteroides uniformis (n = 15), Bacteroides vulgatus (n = 16), Parabacteroides distasonis (n = 29), and Parabacteroides merdae (n = 1).
Other Bacteroides spp. includes Bacteroides (n = 5), Bacteroides dorei (n = 4), Bacteroides faecis (n = 4), Bacteroides nordii (n = 3), Bacteroides salyersiae (n = 1), Bacteroides splanchnicus (n = 3), and Bacteroides xylanisolvens (n = 1).
Other Clostridium spp. includes Clostridium aldenense (n = 1), Clostridium bolteae (n = 1), Clostridium citroniae (n = 1), Clostridium clostridioforme (n = 3), Clostridium hathewayi (n = 3), Clostridium innocuum (n = 9), Clostridium ramosum (n = 6), Clostridium septicum (n = 1), Clostridium sporogenes (n = 1), and Clostridium symbiosum (n = 2).
Prevotella spp. includes Prevotella (n = 1), Prevotella bivia (n = 2), Prevotella buccae (n = 6), Prevotella denticola (n = 2), Prevotella heparinolytica (1), Prevotella intermedia (n = 4), Prevotella melaninogenica (n = 2), Prevotella nigrescens (2), and Prevotella oralis (n = 1).
In vitro activity against ceftazidime-nonsusceptible Gram-negative isolates.
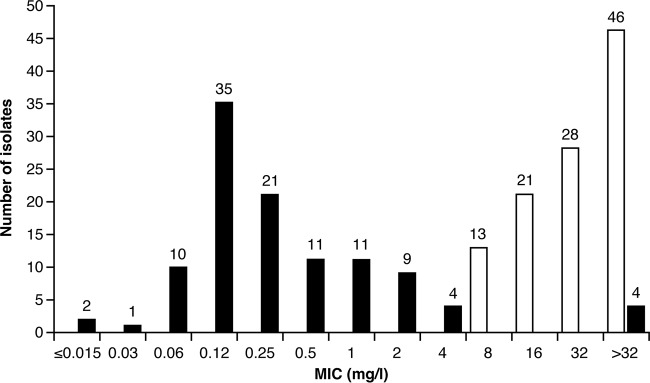
The overall ceftazidime-avibactam MIC90 value for 109 ceftazidime-nonsusceptible Enterobacteriaceae isolates was 2 mg/liter (Table 4). The MIC frequency distributions for ceftazidime-avibactam and ceftazidime against ceftazidime-nonsusceptible Enterobacteriaceae are shown in Fig. 1. Most isolates tested at ≤4 mg/liter for ceftazidime-avibactam, and there was a left shift in MIC distribution versus ceftazidime alone. Four (3.7%) of the 109 ceftazidime-nonsusceptible isolates were also found to be nonsusceptible to ceftazidime-avibactam (Fig. 1). These isolates (two Enterobacter cloacae from India and two K. pneumoniae isolates, with one from Romania and one from India) had previously been determined to express the NDM-1 or NDM-4 metallo-β-lactamase (11).
TABLE 4.
In vitro activity of ceftazidime-avibactam and comparative agents against ceftazidime-nonsusceptible Gram-negative isolates for all randomized patientsa
| Baseline pathogen and agent (no. of pathogens tested) | MIC (mg/liter) |
||
|---|---|---|---|
| Range | MIC50 | MIC90 | |
| All Enterobacteriaceae (109) | |||
| Ceftazidime-avibactam | ≤0.008 to >256 | 0.25 | 2 |
| Ceftazidime | 8 to >64 | 32 | >64 |
| Amikacin | 0.5 to >64 | 4 | >64 |
| Aztreonam | 0.12 to >64 | 64 | >64 |
| Cefepime | 0.06 to >16 | >16 | >16 |
| Ceftaroline | 0.25 to >256 | >256 | >256 |
| Ceftriaxone | 1 to >32 | >32 | >32 |
| Gentamicin | ≤0.12 to >16 | >16 | >16 |
| Imipenem | 0.06 to 16 | 0.12 | 2 |
| Levofloxacin | 0.03 to >8 | >8 | >8 |
| Meropenem | 0.015 to >8 | 0.03 | 0.5 |
| Piperacillin-tazobactam | 0.25 to >128 | 16 | >128 |
| Tigecycline | 0.12 to 8 | 0.5 | 2 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to >8 | >8 | >8 |
| Enterobacter cloacae (10) | |||
| Ceftazidime-avibactam | 0.25 to >256 | 1 | >256 |
| Ceftazidime | 16 to >64 | >64 | >64 |
| Amikacin | 2 to >64 | 4 | 16 |
| Aztreonam | 8 to >64 | >64 | >64 |
| Cefepime | 2 to >16 | >16 | >16 |
| Ceftaroline | 32 to >256 | >256 | >256 |
| Ceftriaxone | 32 to >32 | >32 | >32 |
| Gentamicin | 0.25 to >16 | >16 | >16 |
| Imipenem | 0.25 to 8 | 0.5 | 8 |
| Levofloxacin | 0.5 to >8 | 8 | >8 |
| Meropenem | 0.03 to >8 | 0.06 | 8 |
| Piperacillin-tazobactam | 4 to >128 | 64 | >128 |
| Tigecycline | 0.5 to 8 | 0.5 | 4 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to >8 | >8 | >8 |
| Escherichia coli (59) | |||
| Ceftazidime-avibactam | ≤0.008 to 4 | 0.12 | 2 |
| Ceftazidime | 8 to >64 | 32 | >64 |
| Amikacin | 0.5 to >64 | 4 | 8 |
| Aztreonam | 8 to >64 | 64 | >64 |
| Cefepime | 0.06 to >16 | >16 | >16 |
| Ceftaroline | 0.25 to >256 | >256 | >256 |
| Ceftriaxone | 1 to >32 | >32 | >32 |
| Gentamicin | 0.25 to >16 | 2 | >16 |
| Imipenem | 0.06 to 0.5 | 0.12 | 0.25 |
| Levofloxacin | 0.03 to >8 | >8 | >8 |
| Meropenem | 0.015 to 0.5 | 0.015 | 0.03 |
| Piperacillin-tazobactam | 1 to >128 | 8 | >128 |
| Tigecycline | 0.12 to 1 | 0.25 | 0.5 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to >8 | >8 | >8 |
| Klebsiella pneumoniae (27) | |||
| Ceftazidime-avibactam | 0.12 to >256 | 0.5 | 2 |
| Ceftazidime | 8 to >64 | >64 | >64 |
| Amikacin | 0.5 to >64 | 2 | >64 |
| Aztreonam | 2 to >64 | >64 | >64 |
| Cefepime | 0.25 to >16 | >16 | >16 |
| Ceftaroline | 8 to >256 | >256 | >256 |
| Ceftriaxone | 8 to >32 | >32 | >32 |
| Gentamicin | ≤0.12 to >16 | >16 | >16 |
| Imipenem | 0.12 to 16 | 0.12 | 8 |
| Levofloxacin | 0.06 to >8 | >8 | >8 |
| Meropenem | 0.015 to >8 | 0.03 | 8 |
| Piperacillin-tazobactam | 4 to >128 | 128 | >128 |
| Tigecycline | 0.5 to 4 | 1 | 4 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to >8 | >8 | >8 |
| Pseudomonas aeruginosa (6) | |||
| Ceftazidime-avibactam | 8 to 32 | NA | NA |
| Ceftazidime | 32 to >64 | NA | NA |
| Amikacin | 8 to >64 | NA | NA |
| Cefepime | 16 to >16 | NA | NA |
| Ceftriaxone | >32 to >32 | NA | NA |
| Ciprofloxacin | >4 to >4 | NA | NA |
| Gentamicin | 2 to >16 | NA | NA |
| Imipenem | 1 to 16 | NA | NA |
| Levofloxacin | >8 to >8 | NA | NA |
| Meropenem | 2 to >8 | NA | NA |
| Piperacillin-tazobactam | 32 to >128 | NA | NA |
Total of 1,066 randomized patients. NA, not applicable (MIC50 and MIC90 were not calculated for pathogens identified in <10 patients). Data are provided for pathogens identified in at least 10 patients, with the exception of Pseudomonas aeruginosa, n = 6; ceftazidime-avibactam MIC values for other pathogens are as follows: Citrobacter freundii complex (n = 3), MIC range, 0.25 to 0.5 mg/liter; Enterobacter aerogenes (n = 1), MIC, 2 mg/liter, Proteus mirabilis (n = 5), MIC range, 0.015 to 2 mg/liter; other Enterobacteriaceae (n = 4), MIC range, 0.03 to 0.12 mg/liter; Alcaligenes faecalis (n = 3), MIC range, 4 to 4 mg/liter; and Comamonas testosteroni (n = 1), MIC, >256 mg/liter. A patient could have more than one pathogen isolated. Multiple isolates of the same species from the same patient were counted only once using the isolate with the highest MIC to the study drug received. For bacteremic patients, multiple isolates of the same species from the same patient were counted only once using the isolate with the highest MIC to the study drug received across culture source (intra-abdominal site or blood).
FIG 1.
Activities of ceftazidime-avibactam (black bars) and ceftazidime (white bars) against 108 ceftazidime-nonsusceptible Enterobacteriaceae (determined for the microbiologically modified intent-to-treat [mMITT] patient analysis set). For Enterobacteriaceae, ceftazidime-nonsusceptible isolates were defined as those having a ceftazidime MIC of ≥8 mg/liter. mMITT includes 108 of the 109 ceftazidime-nonsusceptible Enterobacteriaceae isolates obtained from all randomized patients.
The most common ceftazidime-nonsusceptible isolates were E. coli (59/109 [54.1%] isolates; ceftazidime-avibactam MIC50, 0.12 mg/liter; MIC90, 2 mg/liter), K. pneumoniae (27/109 [24.8%] isolates; ceftazidime-avibactam MIC50, 0.5 mg/liter; MIC90, 2 mg/liter), and Enterobacter cloacae (10/109 [9.2%] isolates; ceftazidime-avibactam MIC50, 1 mg/liter; MIC90, >256 mg/liter).
The ceftazidime-avibactam MIC values for six ceftazidime-nonsusceptible P. aeruginosa isolates ranged from 8 to 32 mg/liter (Table 4). There was a trend for a left shift in the MIC distribution for ceftazidime-avibactam versus ceftazidime alone in these ceftazidime-nonsusceptible isolates, with two (33.3%) of the six ceftazidime-nonsusceptible isolates being brought into the susceptible range when ceftazidime was combined with avibactam. For these ceftazidime-nonsusceptible P. aeruginosa isolates, the MICs of meropenem and imipenem ranged from 2 to >8 mg/liter and 1 to 16 mg/liter, respectively.
Overall, 85 Enterobacteriaceae isolates (61 E. coli and 24 K. pneumoniae) were phenotypically positive for an ESBL (Table 5). Ceftazidime-avibactam MIC90 values against ESBL-positive E. coli and K. pneumoniae isolates were 0.25 mg/liter and 1 mg/liter, respectively. The respective MIC90 values against ESBL-negative E. coli and K. pneumoniae isolates were 0.12 mg/liter.
TABLE 5.
In vitro activities of ceftazidime-avibactam against Escherichia coli and Klebsiella pneumoniae baseline isolates by the presence or absence of extended-spectrum β-lactamases for all randomized patientsa
| ESBL status by baseline pathogen (no. of pathogens tested) | MIC (mg/liter) |
||
|---|---|---|---|
| Range | MIC50 | MIC90 | |
| Escherichia coli | |||
| ESBL positive (61) | ≤0.008 to 0.5 | 0.12 | 0.25 |
| ESBL negative (487) | ≤0.008 to 4 | 0.06 | 0.12 |
| Klebsiella pneumoniae | |||
| ESBL positive (24) | 0.12 to 2 | 0.25 | 1 |
| ESBL negative (76) | ≤0.008 to >256 | 0.12 | 0.12 |
Total of 1,066 randomized patients. ESBL, extended-spectrum β-lactamase; NA, not applicable. ESBL status was determined by phenotype based on CLSI confirmatory tests. Some patients had more than one pathogen isolated. Multiple isolates of the same species from the same patient were counted only once using the isolate with the highest MIC to the study drug received. For bacteremic patients, multiple isolates of the same species from the same patient were counted only once using the isolate with the highest MIC to the study drug received across culture source (intra-abdominal site or blood).
DISCUSSION
The in vitro activities of ceftazidime-avibactam and comparators against 1,440 clinical isolates obtained from intra-abdominal and blood cultures from all randomized patients (n = 1,066) with cIAI enrolled in a phase 3 clinical trial (ClinicalTrials.gov identifier NCT01499290) (10) were evaluated in this study. Overall, based on the modified-intention-to-treat (MITT) population (which may reflect fewer patients and isolates than the all-randomized-patient set and comprised 1,043 patients who met the disease definition of cIAI and received any amount of study drug), 414 patients (39.7%) in this study had monomicrobial infections, and 417 patients (40.0%) had polymicrobial infections, with the remainder having no study-qualifying pathogen identified (10). In addition, bacteremia was identified in 36 patients (3.5%) (10). These findings are similar to those of another phase 3 ceftazidime-avibactam trial in adult patients with cIAI enrolled in Asian countries, where 42.9%, 25.5%, and 3.5% of patients were found to have monomicrobial infections, polymicrobial infections, and bacteremia, respectively (12).
Gram-negative species were found to predominate in this study, with 56% of the isolates being members of the Enterobacteriaceae family, 5% being P. aeruginosa, 21% being Gram-positive aerobes, and 18% being anaerobic species. These findings are similar to those of recent surveillance studies (13), other recent phase 3 studies in adult patients with cIAIs (12, 14), and the Complicated Intra-Abdominal Infections Worldwide Observational (CIAOW) study (15). The CIAOW study included 1,898 patients from 68 medical centers worldwide between October 2012 and March 2013 and identified Enterobacteriaceae (most commonly E. coli and K. pneumoniae) as the major pathogens involved in cIAI (15). Thus, the pathogens isolated in the ceftazidime-avibactam phase 3 cIAI study described here are representative of those seen in clinical practice (8, 13, 15, 16, 17, 18), and the current study provides further confirmation of the association between Enterobacteriaceae and cIAIs (10).
In this study, ceftazidime-avibactam was found to be highly active in vitro against baseline Enterobacteriaceae isolates, with an overall MIC90 of 0.25 mg/liter (128-fold lower than that of ceftazidime alone) and an MIC90 of ≤2 mg/liter against each of the individual members of the Enterobacteriaceae family. These results are in agreement with the clinical results of the phase 3 study, which showed that ceftazidime-avibactam plus metronidazole is effective in patients with cIAI, with a clinical cure rate similar to that for meropenem in patients with Gram-negative infection (10). In addition, no significant trends in clinical outcomes were observed between groups of patients subdivided according to patient or disease baseline characteristics, including mono- versus polymicrobial infection and the presence of bacteremia (10).
The in vitro activity of ceftazidime-avibactam against clinical Enterobacteriaceae isolates has also been investigated in another phase 3 study (ClinicalTrials.gov identifiers NCT01599806 and NCT01595438) (19), which evaluated the efficacy and safety of ceftazidime-avibactam versus doripenem in patients with complicated urinary tract infections (cUTIs). Although the in vitro results of the phase 3 cIAI study presented here and the phase 3 cUTI study cannot be directly compared because of differences in the patient populations, study centers, and countries included, the MIC90 values of 0.25 mg/liter and 1 mg/liter against E. coli and K. pneumoniae, respectively, in the cUTI study were not that dissimilar from those observed in the current cIAI study (0.12 mg/liter and 0.5 mg/liter, respectively) (19).
A similar trend was seen in the in vitro results from the phase 3 cUTI study against P. aeruginosa isolates; the MIC90 for ceftazidime-avibactam in the cUTI study was 8 mg/liter and was 32 mg/liter for ceftazidime alone (the MIC90 for ceftazidime-avibactam in the current cIAI study was 4 mg/liter and was 8 mg/liter for ceftazidime alone) (19). The results of recent surveillance studies performed in the United States (13) also confirmed the increased susceptibility of P. aeruginosa isolates to ceftazidime-avibactam compared with ceftazidime alone. In this recent surveillance study, which included Gram-negative isolates collected from abdominal infection sites between 2012 and 2014 in U.S. hospitals, the MIC90 for ceftazidime-avibactam against P. aeruginosa was also found to be 4 mg/liter (13), and the MIC90 for ceftazidime alone was 32 mg/liter (13).
The majority of the 109 ceftazidime-nonsusceptible Gram-negative isolates in the current study tested with a ceftazidime-avibactam MIC of ≤2 mg/liter, with only four isolates not susceptible to ceftazidime-avibactam (Fig. 1). These findings are in line with the in vitro data from the phase 3 cUTI study and also an open-label study (ClinicalTrials.gov identifier NCT01644643) evaluating the efficacy and safety of ceftazidime-avibactam versus the best available therapy in patients with ceftazidime-resistant cIAI and cUTI; both of these studies indicated that the addition of avibactam to ceftazidime restores the in vitro activity of ceftazidime against ceftazidime-nonsusceptible Enterobacteriaceae (MIC90, 1 mg/liter in both studies) (19, 20).
The CIAOW study data identified ESBL-producing bacteria as the most common drug-resistant pathogens associated with cIAI, comprising 13.7% of all intraoperatively obtained E. coli isolates and 18.6% of K. pneumoniae isolates (15). Similar to these results, 10.6% of the Enterobacteriaceae isolates identified in the current study were confirmed as being phenotypically positive for an ESBL, including 11.1% of the E. coli isolates and 24.0% of the K. pneumoniae isolates. Also confirming the presence of ESBL-producing pathogens in patients with cIAI, 7.2% of Enterobacteriaceae isolates from cIAI patients in the phase 3 ceftolozane-tazobactam clinical trial tested positive for an ESBL-producing pathogen (14). Of note, the CIAOW study highlighted a difference in the proportion of ESBL-producing pathogens in patients with health care-associated and community-acquired cIAIs, with 20.6% of E. coli and 42.8% of K. pneumoniae isolates from patients with health care-associated infection confirmed to be ESBL positive. Any variations in the overall proportions of ESBL-producing pathogens identified between these studies could be due to differences in the geographical area included, patient population, hospital epidemiology, and study timing (14, 15).
Previous molecular characterization of Gram-negative isolates in the current cIAI study that met MIC screening criteria for potential ESBLs identified CTX-M variants alone (29.7% [41/138]) or in combination with OXA-1/30 (35.5% [49/138]) as the most commonly carried β-lactamases (11). The prevalence and type of ESBLs among isolates of Enterobacteriaceae from this study are representative of the global distribution of ESBLs (21).
Molecular characterization determined that the four ceftazidime-nonsusceptible Enterobacteriaceae isolates (two E. cloacae and two K. pneumoniae) identified in the cIAI study were New Delhi metallo-β-lactamase-producing isolates with a ceftazidime-avibactam MIC of >256 mg/liter (11). Avibactam does not inhibit Ambler class B metallo-β-lactamases, and this is likely to be the cause of the observed nonsusceptibility of these isolates (2, 3, 22). The molecular analysis also identified that the six ceftazidime-nonsusceptible isolates of P. aeruginosa in the current cIAI study demonstrated overexpression of chromosomal AmpC alone or in combination with blaOXA-10 or blaPER-1 (11). It is possible that these isolates may also exhibit other unidentified resistance mechanisms, such as decreased permeability to antimicrobial agents, but all affected patients (two patients in the ceftazidime-avibactam group and four patients in the meropenem group) reached a clinical cure in the study (10). Reassuringly, the clinical cure rates in the ceftazidime-avibactam group as a whole were shown to be similar irrespective of ESBL status (clinical cure rate of 82.2% in patients in whom pathogens did not meet the screening criteria for ESBLs versus 87.5% in patients meeting the MIC screening criteria) (11).
Similar to the CIAOW study (15), the most common Gram-positive and anaerobic pathogens identified in the current cIAI study were in the Streptococcus and Bacteroides species categories, respectively. Vancomycin, linezolid, and daptomycin were permitted per protocol in the study to treat suspected or confirmed Enterococcus or methicillin-resistant Staphylococcus aureus (MRSA) infection. The MIC90 values for these drugs were within the range of susceptibility to provide coverage against the Gram-positive pathogens isolated. Furthermore, the metronidazole and meropenem MIC90 values were also within the range of susceptibility for the anaerobic pathogens isolated.
In conclusion, ceftazidime-avibactam was highly active in vitro against isolates of Enterobacteriaceae and P. aeruginosa obtained from clinical specimens from patients in the phase 3 cIAI clinical trial. These included ESBL-producing Enterobacteriaceae isolates and those that were nonsusceptible to ceftazidime.
MATERIALS AND METHODS
The clinical isolates for this study were obtained from two double-dummy double-blind randomized controlled trials that were subsequently combined and analyzed as one study (ClinicalTrials.gov identifier NCT01499290) to assess the efficacy, safety, and tolerability of ceftazidime-avibactam plus metronidazole versus meropenem in adult patients hospitalized with cIAI (10). In addition to the assigned study therapy, in the case of suspected or confirmed concomitant infection with Enterococcus or methicillin-resistant Staphylococcus aureus (MRSA), patients in either group received open-label vancomycin, linezolid, or daptomycin at the discretion of the investigator (10). Overall, 1,066 patients from 136 study sites in 30 countries in Asia, Europe, North and South America, and South Africa were included between March 2012 and April 2014. Detailed descriptions of the methods for the clinical study and patient demographics have been published previously (10).
Abdominal and blood culture specimens isolated from all randomized patients (n = 1,066) were processed at the study sites' (or regional) laboratories according to local practices and culture methods. Bacterial isolates from the patient specimens were submitted to a central laboratory (Covance CLS, Indianapolis, IN, USA) for identification and susceptibility testing by broth microdilution, according to Clinical and Laboratory Standards Institute (CLSI) methods (23, 24).
In vitro activity was assessed for ceftazidime-avibactam and various reference antibiotics, including the comparator in the phase 3 trial, meropenem, and representative agents in relevant classes. All agents were tested by reference broth microdilution methods using frozen panels according to the manufacturer's recommendations (Trek Diagnostics, Westlake, OH, USA). For susceptibility testing of ceftazidime-avibactam, avibactam was tested at a constant concentration of 4 mg/liter in doubling dilutions of ceftazidime. CLSI interpretive criteria were used for all isolates, except tigecycline, for which FDA interpretative criteria were used (25). At the time of the cIAI trials, breakpoints for ceftazidime-avibactam had not been approved, but the presumptive interpretive criteria used in the analyses have since been confirmed (9). Ceftazidime-nonsusceptible Enterobacteriaceae and P. aeruginosa isolates were defined as those testing with ceftazidime MICs of ≥8 mg/liter and ≥16 mg/liter, respectively.
If there was more than one isolate of a given species in an individual patient at baseline, the strain that tested with the highest MIC to the received study drug was used for the analysis. Phenotypic detection of ESBL production is limited to a few species and was performed according to CLSI guidelines using MIC screening and confirmatory tests (24).
ACKNOWLEDGMENTS
We thank the patients, their families, and all investigators involved in the study.
Medical writing support was provided by Jennifer Shepherd and Valerie Moss of Prime, Knutsford, Cheshire, funded by Pfizer.
The ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
This phase 3 cIAI study was originally sponsored by AstraZeneca and is now sponsored by Pfizer. AstraZeneca's rights to ceftazidime-avibactam were acquired by Pfizer in December 2016.
G.G.S., P.A.B., and P.N. were employees of and shareholders in AstraZeneca at the time of the study. G.G.S. is currently an employee of Pfizer.
REFERENCES
- 1.Bush K. 2013. Proliferation and significance of clinically relevant beta-lactamases. Ann N Y Acad Sci 1277:84–90. doi: 10.1111/nyas.12023. [DOI] [PubMed] [Google Scholar]
- 2.Stachyra T, Levasseur P, Pechereau MC, Girard AM, Claudon M, Miossec C, Black MT. 2009. In vitro activity of the β-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J Antimicrob Chemother 64:326–329. doi: 10.1093/jac/dkp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehmann DE, Jahic H, Ross PL, Gu RF, Hu J, Kern G, Walkup GK, Fisher SL. 2012. Avibactam is a covalent, reversible, non-beta-lactam beta-lactamase inhibitor. Proc Natl Acad Sci U S A 109:11663–11668. doi: 10.1073/pnas.1205073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lahiri SD, Mangani S, Jahic H, Benvenuti M, Durand-Reville TF, De Luca F, Ehmann DE, Rossolini GM, Alm RA, Docquier JD. 2015. Molecular basis of selective inhibition and slow reversibility of avibactam against class D carbapenemases: a structure-guided study of OXA-24 and OXA-48. ACS Chem Biol 10:591–600. doi: 10.1021/cb500703p. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Estabrook M, Jacoby GA, Nichols WW, Testa RT, Bush K. 2015. In vitro susceptibility of characterized beta-lactamase-producing strains tested with avibactam combinations. Antimicrob Agents Chemother 59:1789–1793. doi: 10.1128/AAC.04191-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flamm RK, Stone GG, Sader HS, Jones RN, Nichols WW. 2014. Avibactam reverts the ceftazidime MIC90 of European Gram-negative bacterial clinical isolates to the epidemiological cut-off value. J Chemother 26:333–338. doi: 10.1179/1973947813Y.0000000145. [DOI] [PubMed] [Google Scholar]
- 7.de Jonge BL, Karlowsky JA, Kazmierczak KM, Biedenbach DJ, Sahm DF, Nichols WW. 2016. In vitro susceptibility to ceftazidime-avibactam of carbapenem-nonsusceptible Enterobacteriaceae isolates collected during the INFORM Global Surveillance Study (2012 to 2014). Antimicrob Agents Chemother 60:3163–3169. doi: 10.1128/AAC.03042-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfizer. 2016. Summary of product characteristics. Zavicefta (ceftazidime-avibactam). European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004027/WC500210234.pdf. [Google Scholar]
- 9.Allergan. 2017. AVYCAZ prescribing information. Allergan, Irvine, CA: https://www.allergan.com/assets/pdf/avycaz_pi. [Google Scholar]
- 10.Mazuski JE, Gasink LB, Armstrong J, Broadhurst H, Stone GG, Rank D, Llorens L, Newell P, Pachl J. 2016. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis 62:1380–1389. doi: 10.1093/cid/ciw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendes RE, Castanheira M, Woosley LN, Stone GG, Bradford PA, Flamm RK. 2017. Molecular beta-lactamase characterization of aerobic Gram-negative pathogens recovered from patients enrolled in the ceftazidime-avibactam phase 3 trials for complicated intra-abdominal infections, with efficacies analyzed against susceptible and resistant subsets. Antimicrob Agents Chemother 61:e02447-16. doi: 10.1128/AAC.02447-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin X, Tran BG, Kim MJ, Wang L, Nguyen DA, Chen Q, Song J, Laud PJ, Stone GG, Chow JW. 2017. A randomised, double-blind, phase 3 study comparing the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem for complicated intra-abdominal infections in hospitalised adults in Asia. Int J Antimicrob Agents 49:579–588. doi: 10.1016/j.ijantimicag.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Sader HS, Castanheira M, Flamm RK, Huband MD, Jones RN. 2016. Ceftazidime-avibactam activity against aerobic Gram-negative organisms isolated from intra-abdominal infections in United States hospitals, 2012–2014. Surg Infect (Larchmt) 17:473–478. doi: 10.1089/sur.2016.036. [DOI] [PubMed] [Google Scholar]
- 14.Solomkin J, Hershberger E, Miller B, Popejoy M, Friedland I, Steenbergen J, Yoon M, Collins S, Yuan G, Barie PS, Eckmann C. 2015. Ceftolozane/tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: results from a randomized, double-blind, phase 3 trial (ASPECT-cIAI). Clin Infect Dis 60:1462–1471. doi: 10.1093/cid/civ097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sartelli M, Catena F, Ansaloni L, Coccolini F, Corbella D, Moore EE, Malangoni M, Velmahos G, Coimbra R, Koike K, Leppaniemi A, Biffl W, Balogh Z, Bendinelli C, Gupta S, Kluger Y, Agresta F, Di Saverio S, Tugnoli G, Jovine E, Ordonez CA, Whelan JF, Fraga GP, Gomes CA, Pereira GA, Yuan KC, Bala M, Peev MP, Ben-Ishay O, Cui Y, Marwah S, Zachariah S, Wani I, Rangarajan M, Sakakushev B, Kong V, Ahmed A, Abbas A, Gonsaga RA, Guercioni G, Vettoretto N, Poiasina E, Diaz-Nieto R, Massalou D, Skrovina M, Gerych I, Augustin G, Kenig J, Khokha V, Trana C, et al. 2014. Complicated intra-abdominal infections worldwide: the definitive data of the CIAOW study. World J Emerg Surg 9:37. doi: 10.1186/1749-7922-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucasti C, Hershberger E, Miller B, Yankelev S, Steenbergen J, Friedland I, Solomkin J. 2014. Multicenter, double-blind, randomized, phase II trial to assess the safety and efficacy of ceftolozane-tazobactam plus metronidazole compared with meropenem in adult patients with complicated intra-abdominal infections. Antimicrob Agents Chemother 58:5350–5357. doi: 10.1128/AAC.00049-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babinchak T, Badal R, Hoban D, Hackel M, Hawser S, Lob S, Bouchillon S. 2013. Trends in susceptibility of selected Gram-negative bacilli isolated from intra-abdominal infections in North America: SMART 2005–2010. Diagn Microbiol Infect Dis 76:379–381. doi: 10.1016/j.diagmicrobio.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 18.Sartelli M, Chichom-Mefire A, Labricciosa FM, Hardcastle T, Abu-Zidan FM, Adesunkanmi AK, Ansaloni L, Bala M, Balogh ZJ, Beltran MA, Ben-Ishay O, Biffl WL, Birindelli A, Cainzos MA, Catalini G, Ceresoli M, Che Jusoh A, Chiara O, Coccolini F, Coimbra R, Cortese F, Demetrashvili Z, Di Saverio S, Diaz JJ, Egiev VN, Ferrada P, Fraga GP, Ghnnam WM, Lee JG, Gomes CA, Hecker A, Herzog T, Kim JI, Inaba K, Isik A, Karamarkovic A, Kashuk J, Khokha V, Kirkpatrick AW, Kluger Y, Koike K, Kong VY, Leppaniemi A, Machain GM, Maier RV, Marwah S, McFarlane ME, Montori G, Moore EE, Negoi I, et al. 2017. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg 12:29. doi: 10.1186/s13017-017-0141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stone GG, Bradford PA, Yates K, Newell P. 2017. In vitro activity of ceftazidime/avibactam against urinary isolates from patients in a phase 3 clinical trial programme for the treatment of complicated urinary tract infections. J Antimicrob Chemother 72:1396–1399. doi: 10.1093/jac/dkw561. [DOI] [PubMed] [Google Scholar]
- 20.Stone GG, Bradford PA, Newell P, Wardman A. 2017. In vitro activity of ceftazidime-avibactam against isolates in a phase 3 open-label clinical trial for complicated intra-abdominal and urinary tract infections caused by ceftazidime-nonsusceptible Gram-negative pathogens. Antimicrob Agents Chemother 61:e01820-16. doi: 10.1128/AAC.01820-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livermore DM, Canton R, Gniadkowski M, Nordmann P, Rossolini GM, Arlet G, Ayala J, Coque TM, Kern-Zdanowicz I, Luzzaro F, Poirel L, Woodford N. 2007. CTX-M: changing the face of ESBLs in Europe. J Antimicrob Chemother 59:165–174. doi: 10.1093/jac/dkl483. [DOI] [PubMed] [Google Scholar]
- 22.Zasowski EJ, Rybak JM, Rybak MJ. 2015. The beta-lactams strike back: ceftazidime-avibactam. Pharmacotherapy 35:755–770. doi: 10.1002/phar.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 8th ed CLSI document M07-A8. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 24.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing; 22nd informational supplement. CLSI M100-S22 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 25.Wyeth Pharmaceuticals, Inc. 2013. Highlights of prescribing information. TYGACIL (tigecycline) for injection for intravenous use. Wyeth Pharmaceuticals, Inc, Philadelphia, PA: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021821s026s031lbl.pdf. [Google Scholar]