ABSTRACT
Acute kidney injury (AKI) increases during empirical antimicrobial therapy with the combination of piperacillin-tazobactam (TZP) and vancomycin (VAN) compared to the number of incidences with monotherapy or the combination of cefepime and VAN. Limited data regarding the impact of meropenem (MEM) combined with VAN exist. This study examined the AKI incidence among patients treated with MEM plus VAN (MEM+VAN) or TZP+VAN. Data were collected from the University of Kentucky Center for Clinical and Translational Science Enterprise Data Trust from September 2007 through October 2015. Adults without previous renal disease who received MEM+VAN or TZP+VAN for at least 2 days were included. AKI was assessed using risk, injury, failure, loss, and end-stage (RIFLE) criteria. Inverse probability of treatment weighting was utilized to control for differences between groups. In total, 10,236 patients met inclusion criteria, with 9,898 receiving TZP+VAN and 338 receiving MEM+VAN. AKI occurred in 15.4% of MEM+VAN patients and in 27.4% of TZP+VAN patients (P < 0.001). TZP+VAN was associated with increased AKI compared to the level with MEM+VAN (odds ratio [OR], 2.53; 95% confidence interval [CI], 1.82 to 3.52), after controlling for confounders. Use of MEM+VAN should be considered an appropriate alternative therapy to TZP+VAN if nephrotoxicity is a major concern. The results of this study demonstrate that judicial use of TZP+VAN for empirical coverage of infection is needed.
KEYWORDS: acute kidney injury, combination therapy, vancomycin, beta-lactams
INTRODUCTION
Empirical combination antimicrobial therapy is critical for the treatment of infections and sepsis (1, 2). Piperacillin-tazobactam (TZP) is a beta-lactam/beta-lactamase inhibitor combination that is frequently used concomitantly with vancomycin (VAN; TZP+VAN) for empirical coverage of infections. This combination provides coverage of clinically important Gram-negative and Gram-positive organisms. While generally considered safe, recent literature suggests a significant increase in the incidence of nephrotoxicity with the TZP+VAN combination compared to the level with either agent as monotherapy or to other empirical combinations (3–8). However, this phenomenon has not been noted in all studies (9, 10).
While studies have demonstrated that TZP+VAN has significantly increased acute kidney injury (AKI) incidence compared to that with cefepime and VAN (4, 8, 11), only one study to date has attempted to compare the use of TZP+VAN to that of meropenem (MEM) plus VAN (12). In that study, Al Yami was unable to find a significant difference in AKI incidence among patients receiving TZP+VAN compared to that among patients using MEM+VAN. However, this result is limited by small sample size and lower than anticipated overall AKI incidence.
The current study was designed to determine if a difference in AKI incidence exists between TZP or MEM in combination with VAN, with the hypothesis that TZP+VAN would exhibit increased AKI incidence compared to that with MEM+VAN.
RESULTS
In total, 10,236 patients met all inclusion criteria, with 338 receiving MEM+VAN and 9,898 receiving TZP+VAN. Mean age was 53.7 (±16.4) years of age, and 58.8% of patients were male (Table 1). At baseline the MEM+VAN cohort tended to be more ill than patients in the TZP+VAN cohort (Charlson comorbidity index [CCI] of 4 [interquartile range, 2 to 6] versus 3 [interquartile range, 1 to 7], respectively; P = 0.014) and more MEM+VAN patients had baseline creatinine clearance (CrCl) of ≥90 ml/min (58.5% versus 50.6% for TZP+VAN; P = 0.011). Significantly more patients in the MEM+VAN group had diabetes mellitus (34.0% versus 28.0% for the TZP+VAN group, P = 0.018) and hypotension (60.9% versus 51.8% for TZP+VAN, P = 0.001), with a trend toward significance in heart failure. The MEM+VAN cohort was more likely to be exposed to concomitant aminoglycosides (3.5% versus 1.5% for the TZP+VAN cohort; P = 0.007), calcineurin inhibitors (6.5% versus 3.7%; P = 0.011), and vasopressors (16.3% versus 11.3%; P = 0.006).
TABLE 1.
Patient characteristics in treatment groups
| Variablea | Value for the group |
P value | |
|---|---|---|---|
| MEM+VAN (n = 338) | TZP+VAN (n = 9,898) | ||
| Mean age (yr [SD]) | 52.3 (16.7) | 53.8 (16.4) | 0.122 |
| Gender (no. of patients [%]) | 0.003 | ||
| Male | 172 (50.9) | 5,847 (59.1) | |
| Female | 166 (49.1) | 4,051 (40.9) | |
| No. (%) of Caucasian patients | 317 (93.8) | 8,884 (89.8) | 0.020 |
| Mean weight (kg [SD]) | 84.1 (24.7) | 83.3 (24.5) | 0.591 |
| BMI (mean [SD]) | 29 (8.3) | 28.6 (17.0) | 0.431 |
| Charlson comorbidity index (median [IQR]) | 4 (2–6) | 3 (1–7) | 0.014 |
| Median baseline CrCl (ml/min [IQR]) | 98.3 (69.7–132.5) | 90.6 (65.5–120.4) | 0.002 |
| Baseline CrCl by group (no. of patients [%]) | 0.011 | ||
| 30–59 ml/min | 61 (18.0) | 1,954 (19.7) | |
| 60–89 ml/min | 79 (23.4) | 2,939 (29.7) | |
| ≥90 ml/min | 198 (58.5) | 5,005 (50.6) | |
| No. of days of therapy (median [IQR]) | 5 (3–8.75) | 5 (3–8) | 0.891 |
| Comorbidities (no. of patients [%]) | |||
| Diabetes mellitus | 115 (34.0) | 2,771 (28.0) | 0.018 |
| Heart failure | 64 (18.9) | 1,485 (15.0) | 0.057 |
| Hypertension | 192 (56.8) | 5,338 (53.9) | 0.324 |
| Hypotension | 206 (60.9) | 5,131 (51.8) | 0.001 |
| Concomitant nephrotoxin treatment (no. of patients [%]) | |||
| Aminoglycoside | 60 (17.7) | 1,625 (16.4) | 0.565 |
| Amphotericin B | 12 (3.5) | 151 (1.5) | 0.007 |
| ACE inhibitor | 63 (18.6) | 1,966 (19.9) | 0.627 |
| ARB | 9 (2.7) | 294 (3.0) | 0.869 |
| Contrast dye | 38 (11.2) | 484 (4.9) | <0.001 |
| Loop diuretic | 105 (31.1) | 3,603 (36.4) | 0.051 |
| NSAID | 43 (12.7) | 1,515 (15.3) | 0.221 |
| Calcineurin inhibitor | 22 (6.5) | 364 (3.7) | 0.011 |
| Vasopressor | 55 (16.3) | 1,117 (11.3) | 0.006 |
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor antagonist; CrCl, creatinine clearance; IQR, interquartile range; MEM, meropenem; NSAID, nonsteroidal anti-inflammatory drugs; SD, standard deviation; TZP, piperacillin-tazobactam; VAN, vancomycin.
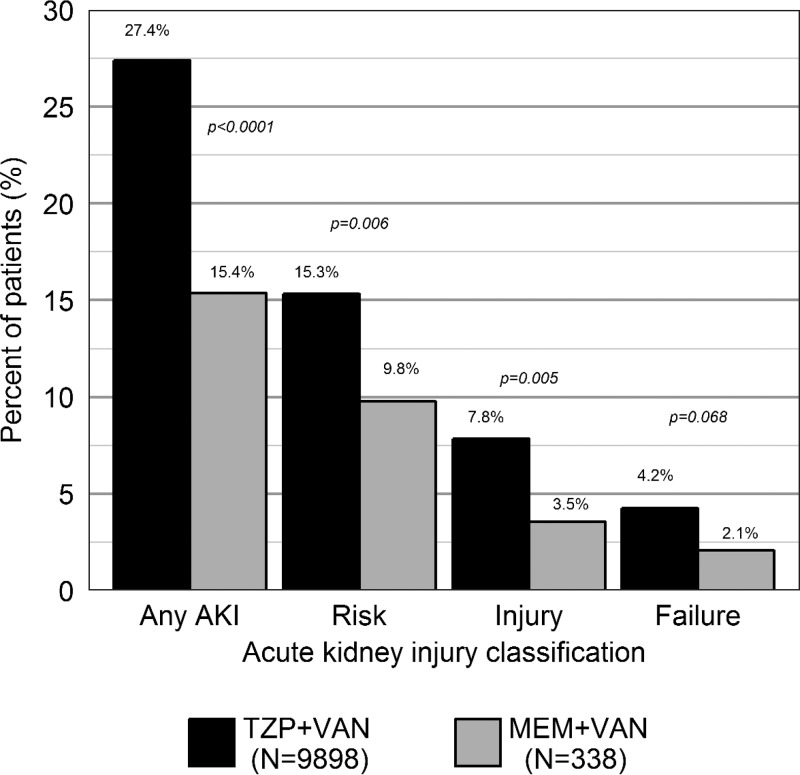
RIFLE-defined AKI occurred in 2,765 (27.0%) patients overall, with AKI being more common in the TZP+VAN group (27.4% versus 15.4%, P < 0.0001) (Table 2). Risk and injury stratifications were significantly more common among the TZP+VAN cohort (15.3% versus 9.8% for MEM+VAN [P = 0.006], and 7.8% for versus 3.5% for MEM+VAN [P = 0.005], respectively) (Fig. 1). There was no significant difference in failure stratification (4.2% for TZP+VAN versus 2.1% for MEM+VAN, P = 0.068). A sensitivity analysis was conducted with AKI being defined as an absolute change in serum creatinine of 0.3 mg/dl from baseline level. AKI was significantly more common in TZP+VAN patients in this analysis (25.9% versus 11.8% for MEM+VAN patients, P < 0.0001). These findings suggest that our results are robust to changes in AKI definition.
TABLE 2.
Primary and secondary outcomes
| Outcome by type | Value for the groupc |
P value | |
|---|---|---|---|
| MEM+VAN (n = 338) | TZP+VAN (n = 9,898) | ||
| Primary outcomes (no. of patients [%]) | |||
| AKI | 52 (15.4) | 2,713 (27.4) | <0.001 |
| Risk | 33 (9.8) | 1,516 (15.3) | 0.006 |
| Injury | 12 (3.5) | 777 (7.8) | 0.005 |
| Failure | 7 (2.1) | 420 (4.2) | 0.068 |
| Secondary outcomes | |||
| Inpatient mortality (no. of patients [%]) | 35 (10.3) | 1,144 (11.6) | 0.552 |
| No. of days of hospitalization (median [IQR])a | 9 (6–15) | 10 (5–18) | 0.482 |
| AKI recovery (no. of patients [%])b | 31 (59.6) | 1276 (47.0) | 0.097 |
IQR, interquartile range.
Percentages represent patients with AKI in the treatment group.
MEM, meropenem; TZP, piperacillin-tazobactam; VAN, vancomycin.
FIG 1.
Acute kidney injury incidence stratified by RIFLE criteria. N, number; MEM, meropenem; TZP, piperacillin-tazobactam; VAN, vancomycin.
In inverse probability-weighted logistic regression, TZP+VAN treatment was associated with a significant increase in AKI odds compared with treatment with MEM+VAN (odds ratio, 2.53; 95% confidence interval, 1.82 to 3.52).
Secondary endpoints did not differ between treatment groups, with 10.3% and 11.6% of patients in the MEM+VAN and TZP+VAN groups experiencing mortality (P = 0.552), respectively. Median length of stay was similar between cohorts (MEM+VAN cohort, 9 days [range, 6 to 15 days]; TZP+VAN cohort, 10 days [range, 5 to 18 days] days; P = 0.482).
DISCUSSION
In this large retrospective study of AKI among patients receiving meropenem or piperacillin-tazobactam in combination with vancomycin, we found that combination therapy with piperacillin-tazobactam is associated with significant increases in AKI incidence compared to levels with meropenem combination therapy. To our knowledge, this is the largest study to examine this comparison.
Previous investigations of AKI related to TZP+VAN therapy have shown incidence ranges from 9.5% to 34.8% (4, 13). Our findings are consistent with this estimate, with 27.4% of TZP+VAN patients experiencing an AKI during therapy. The rate of MEM+VAN treatment-related AKI in the present study differs significantly from that in the study by Al Yami (15.4% in the present study versus 5.33% in the Al Yami study) (12). This may be due to differences in patient populations or AKI definitions. In the sensitivity analysis utilizing a definition of AKI similar to that of Al Yami, 11.8% of patients treated with MEM+VAN experienced AKI. It is important to note that our study included a heterogeneous population of critically ill and general medicine patients, similar to the population in the Al Yami study. Additionally, patients in the TZP+VAN cohort in the Al Yami study had lower AKI incidence than reported in previous literature (7.41%). Overall differences in AKI between the two studies are likely due to differences in severity of illness. While Al Yami does not provide a severity-of-illness measure, severe comorbidities were more prevalent in our study. For example, only 6% of patients in the Al Yami study had underlying heart failure while the rate was 15.1% in the current evaluation. Our study differs by having a larger patient sample (10,236 versus 183 patients) than the previous study on this topic, ensuring statistical power to detect a difference in AKI incidence and to control for confounding.
Antimicrobial resistance is a global health concern, and the overuse of carbapenem agents can lead to increased carbapenem-resistant organisms being isolated from patients (14). While significant increases in AKI were noted in TZP+VAN-treated patients, the risk of AKI might be outweighed by resistance development. Cefepime has been compared to TZP and has consistently been shown to be less nephrotoxic when used in combination with VAN (4, 8, 11, 15). Head-to-head comparisons of AKI rates with meropenem and cefepime have not been conducted. In choosing combination regimens, other clinical factors should be weighed when a patient's risk for nephrotoxicity is minimal.
To our knowledge, no human or animal studies have been conducted investigating the mechanism for this observed increase in AKI with TZP+VAN. One hypothesis is that the addition of a beta-lactamase inhibitor to the beta-lactam/vancomycin combination may result in increased AKI. However, in a study comparing TZP to ampicillin-sulbactam, no difference in the incidence of AKI was noted between monotherapy agents (6). The addition of VAN to TZP or ampicillin-sulbactam resulted in increased AKI incidence compared to results with either monotherapy, but the magnitude of the increase was dissimilar between agents. This suggests that the addition of a beta-lactamase inhibitor is not the factor associated with increased AKI incidence. Gomes and colleagues suggest that subclinical interstitial nephritis caused by TZP in combination with oxidative stress of VAN may lead to increased renal injury (4). Burgess and Drew posited that TZP may reduce VAN clearance, resulting in increased exposure in the kidney and thus in further injury (5). Neither of these mechanisms has been investigated further.
The current study has several limitations. This was a retrospective study, which limits the causal relationship between drug exposure and outcome; however, we established a temporal link between the incidence of AKI and administration of the medications being studied. Additionally, to mimic randomization and limit the impact of confounders, we performed inverse probability weighting to create a balanced pseudopopulation on which the regression analysis was performed. Sensitivity analyses suggest that the inverse probability scores were adequate. Nephrotoxin exposures were assessed as binary variables, a method which does not account for any dosing frequency or intensity. This may change the estimate of the confounding variables; however, sensitivity analyses using the number of days of nephrotoxin therapy suggests that the binary treatment is acceptable. Further work to identify optimal handling of concomitant nephrotoxins is needed. Duration of beta-lactam infusion was not assessed in this study; however, previous studies demonstrate that AKI incidence is not associated with duration of infusion (13, 16, 17). Despite the rigorous study design, there is a possibility of uncontrolled confounding as unknown confounders may remain. In conclusion, we found a significant increase in the incidence of AKI with TZP+VAN treatment compared to that with MEM+VAN treatment. This finding further underscores the need for judicial use of TZP+VAN as an empirical antimicrobial therapy. Meropenem may be an acceptable alternative to piperacillin-tazobactam when nephrotoxicity is a major concern. Further studies of alternative combination therapies are needed to determine what alternatives have the best safety profile.
MATERIALS AND METHODS
This was an institutional review board (IRB)-approved, retrospective cohort study of adult patients admitted to the University of Kentucky Medical Center (UKMC) between September 2007 and October 2015. Patients were included if they received MEM or TZP in combination with VAN for at least 2 days. Patients were excluded for ICD-9 (where ICD is International Classification of Diseases) codes, indicating a past medical history of chronic kidney disease or cystic fibrosis, baseline creatinine clearance less than 30 ml/min, current procedural codes (CPT) indicating hemodialysis, and pregnancy or breastfeeding.
Data were obtained from the University of Kentucky Center for Clinical and Translational Science Enterprise Data Trust (EDT). The EDT is an electronic repository of clinical data collected at UKMC and contains a copy of the digital health record. The EDT is updated nightly and contains demographics, financial classification, provider-level detail, medical diagnosis, medical procedures, lab tests and results, medication administration details, visit details, and vital signs. Data collected included patient demographics and visit information, antimicrobial drug administration data, concomitant nephrotoxin administration, laboratory results, and baseline comorbidity information.
The primary outcome of this study was the incidence of AKI as defined by the glomerular filtration rate (GFR) criteria of risk, injury, failure, loss, and end-stage (RIFLE) (18). Only the risk, injury, and failure stratifications of RIFLE were assessed due to limited follow-up data. GFR was estimated with the adjusted Cockcroft-Gault equation (19) at baseline and throughout each patient's hospitalization. AKI that occurred before treatment, within 48 h treatment initiation, or after 7 days after treatment discontinuation were excluded. Secondary outcomes included length of hospitalization and inpatient mortality, defined as mortality on date of discharge or transfer to hospice. Additionally, recovery of baseline renal function was assessed in patients who experienced AKI. Recovery was defined as a return to baseline renal function or improvement in renal function from baseline at the last collected serum creatinine level.
Severity of baseline comorbidity was assessed with the Charlson comorbidity index (CCI) (20). Hypotension was defined as mean arterial pressure of <65 mmHg or exposure to vasopressors. Concomitant nephrotoxin exposure was assessed as receiving at least one dose of the agent within 24 h of initiation of TZP or MEM through the duration of therapy. Concomitant nephrotoxins analyzed included aminoglycosides, amphotericin B, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, intravenous radiocontrast dye, loop diuretics, nonsteroidal anti-inflammatory drugs, calcineurin inhibitors, and vasopressors.
Basic descriptive statistics were performed. Continuous variables were assessed with a Student t test or Wilcoxon test as appropriate. Categorical variables were assessed with a chi-square or Fisher's exact test. Following bivariable analysis, variables that significantly differed between groups and known confounders regardless of statistical differences were included in an inverse probability of treatment model to generate weights for the final logistic regression model of AKI odds (21). Statistical significance was defined as an alpha of 0.05. All data analysis and statistical procedures were conducted with R, version 3.3.2 (Vienna, Austria), and RStudio, version 0.99.903 (Boston, MA) (22, 23).
ACKNOWLEDGMENTS
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1TR001998. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The sponsors did not have any role in the design and conduct of the study or collection, management, analysis, and interpretation of the data.
We have no potential conflicts of interest to declare.
REFERENCES
- 1.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche J-D, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, et al. . 2017. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 2.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratalà J, El Solh AA, Ewig S, Fey PD, File TM, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. 2016. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meaney CJ, Hynicka LM, Tsoukleris MG. 2014. Vancomycin-associated nephrotoxicity in adult medicine patients: incidence, outcomes, and risk factors. Pharmacotherapy 34:653–661. doi: 10.1002/phar.1423. [DOI] [PubMed] [Google Scholar]
- 4.Gomes DM, Smotherman C, Birch A, Dupree L, Della Vecchia BJ, Kraemer DF, Jankowski CA. 2014. Comparison of acute kidney injury during treatment with vancomycin in combination with piperacillin-tazobactam or cefepime. Pharmacotherapy 34:662–669. doi: 10.1002/phar.1428. [DOI] [PubMed] [Google Scholar]
- 5.Burgess LD, Drew RH. 2014. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy 34:670–676. doi: 10.1002/phar.1442. [DOI] [PubMed] [Google Scholar]
- 6.Rutter WC, Burgess DS. 2017. Acute kidney injury in patients treated with IV beta-lactam/beta-lactamase inhibitor combinations. Pharmacotherapy 37:593–598. doi: 10.1002/phar.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rutter WC, Burgess DR, Talbert JC, Burgess DS. 2017. Acute kidney injury in patients treated with vancomycin and piperacillin-tazobactam: a retrospective cohort analysis. J Hosp Med 12:77–82. doi: 10.12788/jhm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutter WC, Cox JN, Martin CA, Burgess DR, Burgess DS. 2017. Nephrotoxicity during vancomycin therapy in combination with piperacillin-tazobactam or cefepime. Antimicrob Agents Chemother 61:e02089-. doi: 10.1128/AAC.00314-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammond DA, Smith MN, Painter JT, Meena NK, Lusardi K. 2016. Comparative incidence of acute kidney injury in critically ill patients receiving vancomycin with concomitant piperacillin-tazobactam or cefepime: a retrospective cohort study. Pharmacotherapy 36:463–471. doi: 10.1002/phar.1738. [DOI] [PubMed] [Google Scholar]
- 10.Moenster RP, Linneman TW, Finnegan PM, Hand S, Thomas Z, McDonald JR. 2014. Acute renal failure associated with vancomycin and β-lactams for the treatment of osteomyelitis in diabetics: piperacillin-tazobactam as compared with cefepime. Clin Microbiol Infect 20:O384–O389. doi: 10.1111/1469-0691.12410. [DOI] [PubMed] [Google Scholar]
- 11.Navalkele B, Pogue JM, Karino S, Nishan B, Salim M, Solanki S, Pervaiz A, Tashtoush N, Shaikh H, Koppula S, Koons J, Hussain T, Perry W, Evans R, Martin ET, Mynatt RP, Murray KP, Rybak MJ, Kaye KS. 2017. Risk of acute kidney injury in patients on concomitant vancomycin and piperacillin-tazobactam compared to those on vancomycin and cefepime. Clin Infect Dis 64:116–123. doi: 10.1093/cid/ciw709. [DOI] [PubMed] [Google Scholar]
- 12.Al Yami MS. 2017. Comparison of the incidence of acute kidney injury during treatment with vancomycin in combination with piperacillin–tazobactam or with meropenem. J Infect Public Health 10:770–773. doi: 10.1016/j.jiph.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 13.McCormick H, Tomaka N, Baggett S, Heierman T, LaFosse J, Gilbert S, Imhof K. 2015. Comparison of acute renal injury associated with intermittent and extended infusion piperacillin/tazobactam. Am J Health Syst Pharm 72:S25–S30. doi: 10.2146/sp150007. [DOI] [PubMed] [Google Scholar]
- 14.McDougall DAJ, Morton AP, Playford EG. 2013. Association of ertapenem and antipseudomonal carbapenem usage and carbapenem resistance in Pseudomonas aeruginosa among 12 hospitals in Queensland, Australia. J Antimicrob Chemother 68:457–460. doi: 10.1093/jac/dks385. [DOI] [PubMed] [Google Scholar]
- 15.Jeon N, Staley B, Klinker KP, Hincapie Castillo J, Winterstein AG. 2017. Acute kidney injury risk associated with piperacillin/tazobactam compared with cefepime during vancomycin therapy in hospitalised patients: a cohort study stratified by baseline kidney function. Int J Antimicrob Agents 50:63–67. doi: 10.1016/j.ijantimicag.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Cotner SE, Rutter WC, Burgess DR, Wallace KL, Martin CA, Burgess DS. 2017. Influence of beta-lactam infusion strategy on acute kidney injury. Antimicrob Agents Chemother 61:e00871-17. doi: 10.1128/AAC.00871-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karino S, Kaye KS, Navalkele B, Nishan B, Salim M, Solanki S, Pervaiz A, Tashtoush N, Shaikh H, Koppula S, Martin ET, Mynatt RP, Murray KP, Rybak MJ, Pogue JM. 2016. Epidemiology of acute kidney injury among patients receiving concomitant vancomycin and piperacillin-tazobactam: opportunities for antimicrobial stewardship. Antimicrob Agents Chemother 60:3743–3750. doi: 10.1128/AAC.03011-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup . 2004. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilhelm SM, Kale-Pradhan PB. 2011. Estimating creatinine clearance: a meta-analysis. Pharmacotherapy 31:658–664. doi: 10.1592/phco.31.7.658. [DOI] [PubMed] [Google Scholar]
- 20.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel J-M, Sundararajan V. 2011. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 21.van der Wal WM, Geskus RB, others . 2011. ipw: an R package for inverse probability weighting. J Stat Softw 43:1–23. [Google Scholar]
- 22.R Foundation for Statistical Computing. 2014. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 23.RStudio Team. 2015. RStudio: integrated Development for R. RStudio, Inc., Boston, MA. [Google Scholar]