ABSTRACT
Recent reports highlighting the global significance of cryptosporidiosis among children have renewed efforts to develop control measures. We evaluated the efficacy of bumped kinase inhibitor (BKI) 1369 in the gnotobiotic piglet model of acute diarrhea caused by Cryptosporidium hominis, the species responsible for most human cases. Five-day treatment with BKI 1369 reduced signs of disease early during treatment compared to those of untreated animals. Piglets treated with BKI 1369 exhibited significant reductions of oocyst excretion, mucosal colonization by C. hominis, and mucosal lesions, which resulted in considerable symptomatic improvement. BKI 1369 reduced the parasite burden and disease severity in the gnotobiotic pig model. Together these data suggest that a BKI-mediated therapeutic may be an effective treatment against cryptosporidiosis.
KEYWORDS: BKI 1369, Cryptosporidium hominis, bumped kinase inhibitor, gnotobiotic pigs
INTRODUCTION
Cryptosporidium spp. are enteric protozoa that have a worldwide distribution and are found in all classes of vertebrates. Cryptosporidiosis is a major cause of diarrhea in young children under the age of 2 years in developing countries, resulting in an estimated 7.6 million cases and 202,000 deaths annually in sub-Saharan Africa and southern Asia (1–4). Cryptosporidium hominis and Cryptosporidium parvum are responsible for ∼75% and ∼20% of cases of illness, respectively (2, 5). While the disease in otherwise healthy individuals is self-limited, lasting 1 to 2 weeks, infection can be life-threatening in young children with malnutrition and in immunocompromised individuals (6).
Current therapeutic options for cryptosporidiosis are limited and only partially effective. Nitazoxanide (NTZ) is the only FDA-approved drug for treatment of Cryptosporidium in immunocompetent children and adults (7, 8). Although NTZ shortens the duration of diarrhea and parasite shedding in immunocompetent adults, its efficacy is partial (at best) in children, and it is ineffective in immunocompromised patients (9). Therefore, more effective parasite-specific drugs are urgently needed to treat cryptosporidiosis.
The calcium-dependent protein kinase 1 of C. parvum (CpCDPK1) has been considered a potential target for drug development against this infection because CDPK homologs have crucial roles in host cell invasion, microneme secretion, and gliding motility and differ from mammalian protein kinases. Selected bumped kinase inhibitors (BKIs) have a high specific affinity for CpCDPK1. In a previous study, BKI 1294 exhibited inhibitory activity against C. parvum in immunodeficient mice and calves (10, 11), and it reduced parasite and disease burdens of C. hominis in the gnotobiotic (GB) piglet model of acute diarrhea (unpublished data). However, it was reported to have cardiotoxicity (10), and consequently, other BKI derivatives were generated and evaluated.
In the present study, we used the GB piglet model to evaluate the efficacy of BKI 1369, a candidate with less potential cardiotoxicity than that of BKI 1294, using the well-characterized C. hominis strain TU502 (12). BKI 1369 has been well characterized for potency, stability, metabolism, toxicity, and pharmacokinetics and has been shown to be potent against C. parvum in infected mice and calves (13). Piglets treated with BKI 1369 exhibited a significant reduction of oocyst excretion in feces, with considerable symptomatic improvement. These findings validate BKI 1369 as a potential therapeutic agent to treat cryptosporidiosis.
RESULTS
Oocyst excretion.
GB piglets, delivered by caesarean section to ensure that they are germfree, are the only animal model that can reliably be used for C. hominis infections leading to clinical diarrhea and pathological damage typical of human C. hominis infection. Thus, this model is well suited for testing new drugs against C. hominis. After parasite challenge, we measured oocyst excretion, intensity of diarrhea, and body weight and compared the results between BKI 1369-treated and untreated groups, which included nine piglets each. Infectivity was measured by quantification of oocyst excretion and parasite DNA in feces. The oocyst counts in feces were high from days 4 to 11 postchallenge and then gradually declined in the infected and untreated group. In contrast, the BKI 1369-treated piglets shed significantly smaller numbers of oocysts throughout the study (Fig. 1A). Cryptosporidium DNA in feces was also measured by real-time PCR. Quantitative PCR (qPCR) analysis showed results similar to those for oocyst excretion, with the BKI 1369-treated piglets showing a log reduction of fecal DNA (Fig. 1B). The accumulated oocyst excretion level also indicated that BKI 1369 was significantly effective for oocyst reduction in feces, and the effect was prolonged to the end of the experiment (Fig. 1C and D).
FIG 1.
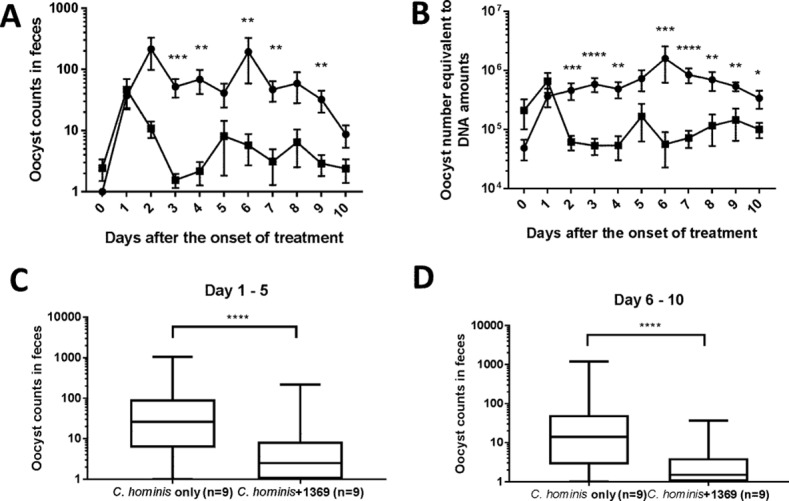
Fecal excretion of C. hominis oocysts after challenge. (A) Daily oocyst excretion. (B) Quantification of Cryptosporidium DNA in feces. (C and D) Accumulated counts of oocyst excretion from days 1 to 5 (C) and days 6 to 10 (D) after the start of BKI 1369 treatment. Piglets were inoculated orally with C. hominis oocysts 2 days after birth and were treated with BKI 1369 at 3 days postchallenge. Rectal swabs were processed for oocyst counts and DNA measurements. The plots in panels A and B show means ± standard errors of the means (SEM), and the plots in panels C and D are box-and-whisker (minimum to maximum) plots. The differences in the data on oocyst excretions and Cryptosporidium DNA between groups were compared by the Mann-Whitney test on each day, using GraphPad Prism 7.03. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. ●, C. hominis-infected group (n = 9); ■, C. hominis-infected and BKI 1369-treated group (n = 9).
Piglets challenged with heat-killed oocysts had no significant oocyst excretion in feces. Only one or two oocysts, which had most likely passed through the gastrointestinal (GI) tract, were observed in microscopic oocyst counts at 2 days postchallenge. However, no significant Cryptosporidium DNA was detected by quantitative real-time PCR in this experiment.
Clinical observations.
Diarrhea was monitored and scored daily. The infected control group developed moderate diarrhea from days 4 to 6 postchallenge. Treatment with BKI 1369 significantly reduced diarrhea in parasite-infected piglets (Fig. 2A and B). Two control piglets treated with BKI 1369 and two piglets treated with heat-killed oocysts and vehicle only had no to mild diarrhea during the study, as did the two untreated animals (data not shown).
FIG 2.
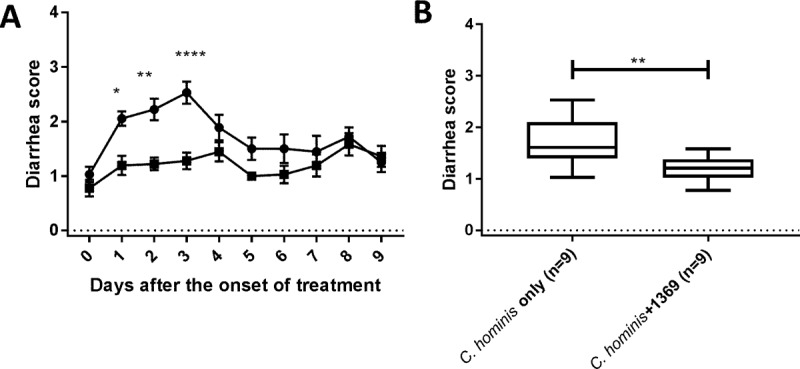
Diarrhea scores observed for piglets following oocyst challenge. (A) Daily diarrhea scores. (B) Accumulated diarrhea scores. Piglets were inoculated orally with C. hominis oocysts 2 days after birth. The clinical signs of cryptosporidiosis were monitored daily, and diarrheal symptoms were scored as described in Materials and Methods. The plot in panel A show means ± SEM, and the plot in panel B is a box-and-whisker (minimum to maximum) plot. Two-way analysis of variance (ANOVA) with Sidak's multiple-comparison test (A) and the Mann-Whitney test (B) were conducted using GraphPad Prism 7.03. *, P < 0.05; **, P < 0.01; ****, P < 0.0001. ●, C. hominis-infected group (n = 9); ■, C. hominis-infected and BKI 1369-treated group (n = 9).
Body weights, which were measured daily, showed that all piglets in both groups gained weight. No significance of differences was observed, although the BKI 1369-treated piglets had a slightly higher growth rate than that of the infected control piglets (Fig. 3).
FIG 3.
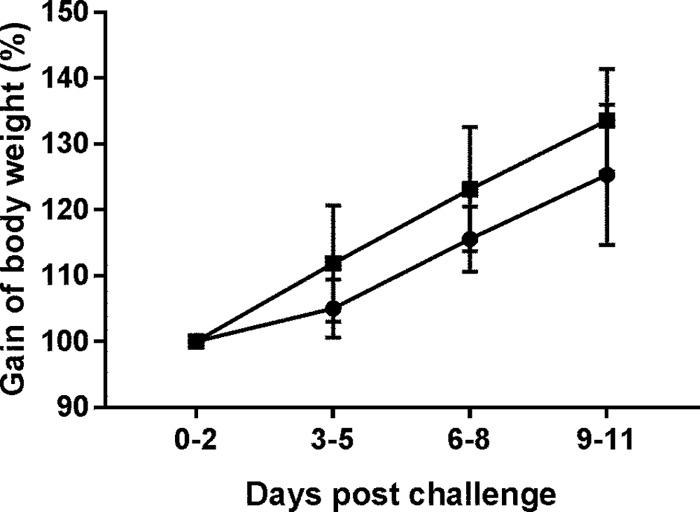
Body weight gain after drug treatment of C. hominis-infected piglets. Piglets were inoculated orally with C. hominis oocysts 2 days after birth and were treated with BKI 1369 3 days after challenge. Body weight was measured daily. The plot shows means ± standard deviations (SD). Two-way ANOVA with Tukey's multiple-comparison test was conducted using GraphPad Prism 7.03. No significance of differences between groups was observed. ●, C. hominis-infected group (n = 9); ■, C. hominis-infected and BKI 1369-treated group (n = 9).
Histopathology.
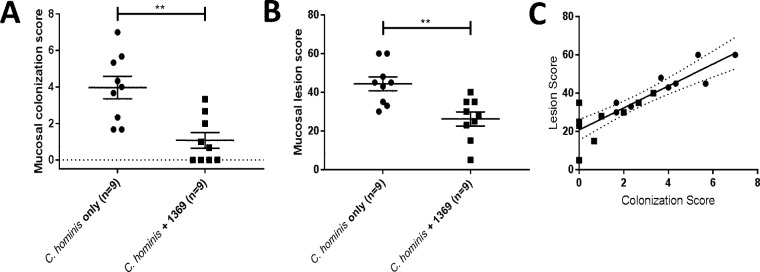
Piglets were euthanized 13 days after oral challenge, and gut sections were processed for microscopic examination. Compared to the histology of normal duodenal villi (Fig. 4A), infection with C. hominis caused marked villus blunting and fusion and moderate lymphocytic infiltrates in the lamina propria of duodenal villi (Fig. 4B). Compared to those in the uninfected spiral colon (Fig. 4C and E), glands of infected animals were sometimes heavily colonized with C. hominis and associated with epithelial cell loss, attenuation, and sloughing into the lumen (Fig. 4D), and sometimes epithelial surfaces were mildly colonized by C. hominis, with single-cell necrosis and sloughing (Fig. 4F). C. hominis infection was mostly observed in the cecum and spiral colon, and there was a significant reduction in the colonization score that was attributable to BKI 1369 (Fig. 5A), consistent with the results for oocyst excretion in feces. The mucosal lesion score was also significantly reduced by BKI 1369 treatment compared to that for piglets challenged with C. hominis alone (Fig. 5B). Note that the mucosal colonization and lesion scores are significantly and positively correlated (Spearman coefficient of 0.887) (Fig. 5C). These results indicate that treatment with BKI 1369 significantly decreases parasite colonization and ameliorates C. hominis-induced epithelial cell necrosis and loss and that the treatment may prevent villus blunting and fusion and minimize lymphocytic and neutrophilic inflammation.
FIG 4.
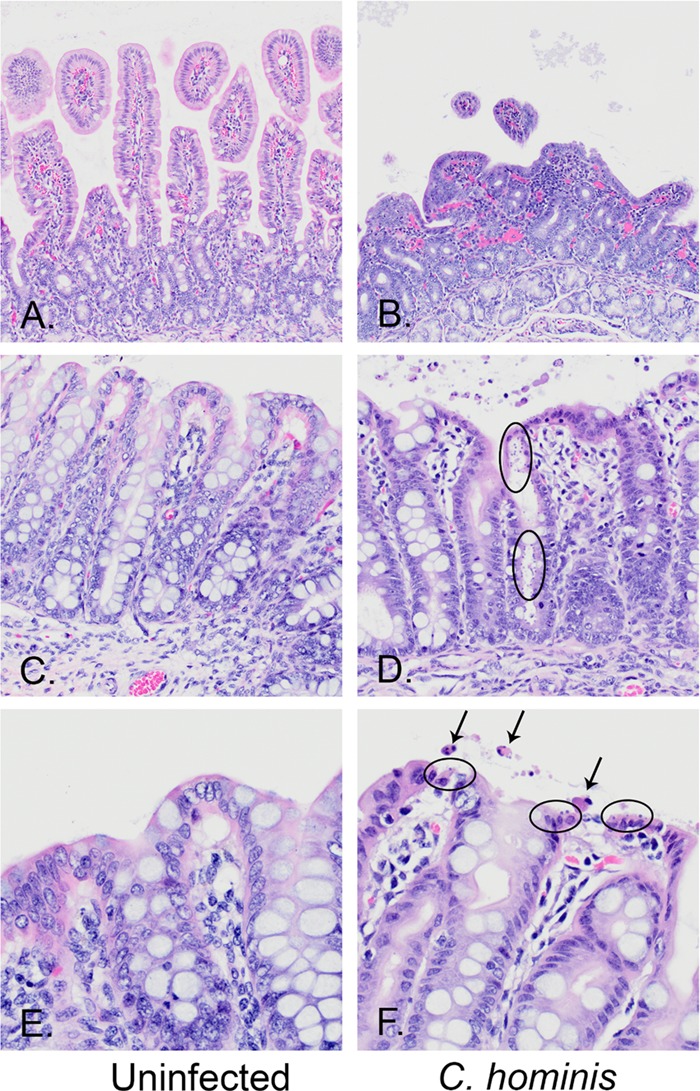
Hematoxylin- and eosin-stained sections from the spiral colons of uninfected (A, C, and E) and C. hominis-infected (B, D, and F) piglets, magnified ×20 (A to D) and ×40 (E and F) and showing normal structures (left panels) compared to representative lesions from C. hominis-infected pigs (right panels). (A and B) Normal duodenal villi within an uninfected pig (A) compared to marked villus blunting and fusion and moderate lymphocytic infiltrates in the lamina propria of a C. hominis-infected pig (B). (C and D) Normal spiral colon surface and glandular epithelia (C) compared to a gland heavily colonized with C. hominis, with epithelial cell loss, attenuation, and sloughing into the lumen (D). (E and F) Normal spiral colon surface (E) compared to a surface mildly colonized by C. hominis, with single-cell necrosis and sloughing (F). The arrows mark apoptotic epithelial cells sloughing into the lumen. The ovals encircle epithelial cells that are infected with C. hominis organisms at the very apical surface of the epithelial cells.
FIG 5.
Microscopic examination of C. hominis infection. (A) Mucosal colonization scores. (B) Mucosal lesion scores. (C) Correlation between colonization scores and lesion scores. The gastrointestinal tissues were fixed in formalin and processed for hematoxylin and eosin staining. Mucosal colonization was examined at a magnification of ×40 and given a score of 0 to 5 (0, no infection detected; 1, 1 to 20% of the epithelial surface infected; 2, 21 to 40% of the surface infected; 3, 41 to 60% of the surface infected; 4, 61 to 80% of the surface infected; and 5, 81 to 100% of the surface infected). Mucosal lesion scores were applied for villus changes, epithelial changes, and cellular inflammation in the lamina propria and within crypts/glands for all samples. The following scores were used: 0, normal; 2.5, equivocal; 5, mild; 10, moderate; and 15, marked. The final lesion score was the sum of scores for 5 anatomic sites (3 for the small intestine [duodenum, jejunum, and ileum] and 2 for the large intestine [cecum and colon]) for each piglet. The scatterplots (A and B) show means ± SEM. The Mann-Whitney test was conducted using GraphPad Prism 7.03. The plot in panel C shows the correlation between scores by linear regression. **, P < 0.01. ●, C. hominis-infected group (n = 9); ■, C. hominis-infected and BKI 1369-treated group (n = 9).
Pharmacokinetics.
BKI 1369 and its metabolites, BKI 1318 (metabolite 1 [13]) and BKI 1817 (metabolite 2 [13]), were measured in serum, urine, and gut contents after BKI 1369 was orally inoculated into piglets (Table 1). BKI 1369 concentrations of 2.8 μM and 3.4 μM were detected in plasma 2 h after the 1st dose of BKI 1369 at 10 mg/kg of body weight, while no BKI 1318 and BKI 1817 were detected. The plasma concentration of BKI 1369 increased to 10 μM after the 9th dose, indicating that BKI 1369 was eliminated slowly and accumulated with this dose regimen in piglets. The BKI 1369 plasma concentration decreased after dosing was stopped, and levels of 0.5 to 2.7 μM were detected 6 days after the last dose. Interestingly, BKI 1369 levels in urine and gut contents, also measured 6 days after the last dose, were 7.6 to 10.4 μM and 1.8 to 4.5 μM, respectively, demonstrating that urine levels during the drug excretion phase after dosing were higher than levels in plasma and that residual gut BKI 1369 may still have been yielding therapeutic effects. The metabolites (BKI 1318 and BKI 1817) were minimally present in plasma, and always under 1.1 μM. In contrast, the metabolite BKI 1318 was found at 9 μM in urine and 23.7 μM in gut contents 6 days after the last dose, while the metabolite BKI 1817 was found at 0.7 μM in urine and 1.8 μM in gut contents. Interestingly, the metabolite BKI 1817 was measured at 7 μM, on average, in daily rectal swabs after BKI 1369 treatment (data not shown). These data suggest that BKI 1369 is eliminated from the body in urine via the kidneys and through the GI tract as BKI 1369, but also as BKI 1318 and, to a lesser degree, BKI 1817. Prolonged gut excretion of BKI 1369 and the active metabolite BKI 1318 (13) may enhance the anti-Cryptosporidium therapeutic effect. We were able to measure the levels of BKI 1369 and the active metabolite, BKI 1318, in the intestinal tissue only after piglets were terminated, 6 days after dosing had ended. Yet the levels measured were still about the 50% effective concentrations (EC50s) of these compounds against Cryptosporidium, even after metabolism and excretion of compound had undoubtedly occurred. This suggests that the efficacy of BKI 1369 may be correlated with intestinal epithelial levels that exceed levels necessary for growth interruption in vitro.
TABLE 1.
Concentrations of BKI 1369 and its metabolites, BKI 1318 and BKI 1817, in piglets
| Sample type and time of collectiona | Drug concn (μM) |
|||
|---|---|---|---|---|
|
C. hominis-infected and BKI 1369-treated animals |
Uninfected animals treated with BKI 1369 |
|||
| BKI 1369 | BKI 1318 | BKI 1817 | BKI 1369 | |
| Plasma | ||||
| 3 dpi (0 h before 1st dose) | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 |
| 3 dpi (2 h after 1st dose) | 2.75 ± 0.50 | 0.0 ± 0.00 | 0.0 ± 0.00 | 3.44 ± 0.70 |
| 7 dpi (12 h after 8th dose) | 8.73 ± 0.79 | 0.92 ± 0.12 | 0.26 ± 0.03 | 8.20 ± 0.23 |
| 7 dpi (2 h after 9th dose) | 10.1 ± 0.99 | 0.85 ± 0.04 | 0.23 ± 0.02 | 7.12 ± 0.15 |
| 8 dpi (12 h after 10th dose) | 7.67 | 1.07 | 0.04 | |
| 10 dpi (3 days after 10th dose) | 2.21 ± 0.54 | 1.05 ± 0.17 | 0.09 ± 0.05 | |
| Termination (6 days after 10th dose) | 0.51 ± 0.19 | 0.21 ± 0.09 | 0.0 ± 0.00 | 2.66 ± 0.09 |
| Urine | ||||
| Termination (6 days after 10th dose) | 7.61 ± 1.78 | 9.01 | 0.69 | 10.4 ± 2.55 |
| Gut contents | ||||
| Termination (6 days after 10th dose) | 1.79 ± 0.43 | 23.7 ± 2.81 | 1.80 ± 0.25 | 4.47 ± 3.18 |
dpi, days postinfection.
Blood analysis.
The results of blood biochemical and hematological analyses after euthanasia are summarized in Table 2. While the gamma glutamyltransferase (GGT) level in BKI 1369-treated piglets was significantly lower than that in the infected control piglets, GGT levels in both groups were in the normal reference range (33 to 94 U/liter) for 6-week-old specific-pathogen-free Hampshire-Yorkshire crossbred pigs (14). Red blood cell (RBC), hemoglobin, and hematocrit levels in BKI 1369-treated piglets were also significantly lower than those in the infected control piglets. Interestingly, levels of RBC, hemoglobin, and hematocrit in both groups were lower than the normal reference ranges (5.52 to 9.11 million/μl, 8.8 to 12.7 g/dl, and 28.3 to 42.7%, respectively) (14). For all other parameters, no significant differences were observed between groups.
TABLE 2.
Serum biochemical and hematological data for blood from piglets
| Measurea | Units | Value |
|
|---|---|---|---|
| C. hominis-infected animals only (n = 9) | C. hominis-infected and BKI 1369-treated animals (n = 9) | ||
| Electrolytes | |||
| Sodium | meq/liter | 134.9 ± 0.89 | 134.0 ± 1.48 |
| Potassium | meq/liter | 4.0 ± 0.08 | 4.2 ± 0.18 |
| Chloride | meq/liter | 95.9 ± 0.42 | 95.9 ± 0.87 |
| Osmolarity | mmol/liter | 267.1 ± 1.88 | 265.0 ± 3.13 |
| Acid-base status | |||
| Bicarbonate | meq/liter | 22.8 ± 0.91 | 22.7 ± 1.72 |
| Anion gap | mmol/liter | 16.2 ± 0.36 | 15.4 ± 1.08 |
| Minerals | |||
| Calcium | mg/dl | 9.3 ± 0.11 | 9.1 ± 0.18 |
| Phosphorus (Pi) | mg/dl | 6.6 ± 0.18 | 6.5 ± 0.19 |
| Magnesium | mg/dl | 2.1 ± 0.06 | 2.1 ± 0.10 |
| Renal function parameters | |||
| Blood urea nitrogen | mg/dl | 1.6 ± 0.14 | 1.7 ± 0.21 |
| Creatinine | mg/dl | 0.5 ± 0.04 | 0.6 ± 0.02 |
| Liver function parameters | |||
| Bilirubin | mg/dl | 0.5 ± 0.03 | 0.5 ± 0.07 |
| Metabolites | |||
| Cholesterol | mg/dl | 96.3 ± 7.58 | 95.7 ± 3.61 |
| Glucose | mg/dl | 148.7 ± 7.75 | 133.6 ± 12.74 |
| Triglycerides | mg/dl | 64.2 ± 7.26 | 50.2 ± 8.10 |
| Enzymes | |||
| Alanine aminotransferase (ALT) | U/liter | 55.0 ± 1.39 | 53.8 ± 6.37 |
| Alkaline phosphatase (ALP) | U/liter | 2,039 ± 217.5 | 2,119 ± 298.3 |
| Amylase | U/liter | 3,544 ± 175.0 | 2,848 ± 292.1 |
| Aspartate aminotransferase (AST) | U/liter | 74.6 ± 25.72 | 29.6 ± 3.48 |
| Creatine kinase | U/liter | 523 ± 171.6 | 236 ± 33.3 |
| Gamma glutamyltransferase (GGT) | U/liter | 62.0 ± 5.16 | 43.1 ± 4.41 |
| Proteins | |||
| Protein (total) | g/dl | 2.0 ± 0.14 | 1.9 ± 0.09 |
| Albumin (A) | g/dl | 1.1 ± 0.08 | 1.1 ± 0.08 |
| Globulin (G) | g/dl | 0.9 ± 0.09 | 0.8 ± 0.06 |
| A/G ratio | 1.2 ± 0.11 | 1.5 ± 0.19 | |
| Hematology analysis | |||
| RBC | 106/μl | 4.21 ± 0.194 | 3.51 ± 0.151 |
| Hemoglobin | g/dl | 7.3 ± 0.34 | 6.1 ± 0.28 |
| Hematocrit | % | 24.4 ± 1.10 | 21.0 ± 0.76 |
| MCV | fl | 58.5 ± 1.23 | 60.4 ± 1.70 |
| MCH | pg | 17.4 ± 0.41 | 17.5 ± 0.54 |
| MCHC | g/dl | 29.7 ± 0.23 | 29.0 ± 0.54 |
| RDW | % | 19.6 ± 0.55 | 20.8 ± 0.54 |
| White blood cells (WBC) | 103/μl | 7.71 ± 0.745 | 6.47 ± 0.667 |
| Seg neutrophils | % | 58.6 ± 3.03 | 57.6 ± 4.27 |
| Lymphocytes | % | 39.9 ± 2.76 | 40.6 ± 4.29 |
| Monocytes | % | 1.4 ± 0.48 | 1.7 ± 0.55 |
| Seg Neutrophils | 103/μl | 4.61 ± 0.554 | 3.84 ± 0.562 |
| Lymphocytes | 103/μl | 3.05 ± 0.290 | 2.48 ± 0.236 |
| Monocytes | 103/μl | 0.10 ± 0.030 | 0.08 ± 0.035 |
The data represent means ± SEM. The Mann-Whitney test was conducted to assess differences between C. hominis-infected control piglets and BKI 1369-treated piglets. In BKI 1369-treated piglets, GGT was significantly lower than that in infected control piglets (P = 0.019). RBC, hemoglobin, and hematocrit levels in BKI 1369-treated piglets were also significantly lower than those in the infected control piglets (P = 0.013, P = 0.012, and P = 0.026, respectively). RBC, red blood cells; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red blood cell distribution width.
Tissue toxicity.
Tissue toxicity of BKI 1369 was investigated microscopically. Most piglets in this study showed diffuse, mild to marked hepatocellular cytoplasmic vacuolation of hepatocytes, an indicator of active metabolism and hepatocyte function in young animals, but there were no significant differences among experimental groups. One piglet from the C. hominis-infected and BKI 1369-treated group (n = 9) had an unusual microscopic lesion, namely, multifocal Purkinje cell necrosis in the cerebellum that corresponded to abnormal motor and balance clinical signs. Another piglet from the same group had lymphangiectasia characterized by submucosal, mural, and serosal neutrophilic, lymphocytic, and histiocytic enteritis surrounding dilated lymphatic vessels. Both piglets originated from the same litter. These pathological findings were not observed in the piglets treated with BKI 1369 only (n = 2) and vehicle only (n = 2), suggesting that these pathological signs are not associated with BKI 1369 or vehicle, to rule out rare toxic events. No other significant microscopic lesions were observed in any other extraintestinal tissues examined in the piglets.
DISCUSSION
Cryptosporidiosis, a previously neglected disease of childhood (15), remains near the top of the list among enteric diseases that contribute significantly to morbidity and mortality in children aged 6 to 18 months in many developing countries (1, 2, 16). It is second only to rotavirus, against which there are several effective vaccines, which further accentuates the significance of cryptosporidiosis. Furthermore, at this vulnerable age, cryptosporidiosis, more than other enteric diseases, often leads to chronic diarrhea, malnutrition, poor physical growth, and slow cognitive development (17). Hence, the search for effective treatment or control measures remains a priority.
Here we report experiments performed with the GB piglet model of acute diarrhea, using piglets challenged with C. hominis (12) and treated with BKI 1369, a known inhibitor of the CpCDPK-1 parasite enzymatic pathway (13). We previously established the GB piglet model for the purpose of evaluating prophylactic and therapeutic agents against cryptosporidiosis for children and showed limited efficacy of NTZ, the only drug approved by the FDA for treatment of cryptosporidiosis (12). The clinical efficacy of BKI 1369 for treatment of C. hominis infection in this piglet model appears to be superior to the efficacy of NTZ observed in our previous study (12). Five-day treatment with BKI 1369 significantly reduced the extent of parasite burden as judged by the reduction in oocyst excretion, leading to symptomatic improvement as manifested by the reduction of diarrhea induced by C. hominis.
BKIs targeting CpCDPK1 do not significantly inhibit mammalian protein kinases and are not known to be toxic to the host (10). Representative BKIs, including BKI 1294 and BKI 1369, were shown to considerably improve the clinical outcome of diarrhea as well as reduce oocyst fecal output in the calf model of C. parvum cryptosporidiosis (11, 13). While BKI 1294 was shown to be reasonably effective against cryptosporidiosis, it was considered unsuitable for human application because of its potent hERG-inhibitory activity (50% inhibitory concentration [IC50]= 0.3 μM), which is known to be associated with prolonged QTc intervals and potential cardiotoxicity in humans (10). BKI 1369 has a 5-fold increase in hERG-inhibitory activity (IC50 = 1.52 μM). This may not allow for its safe use in humans. The concentration of BKI 1369 was 11.4 μM in serum 2 h after 9 consecutive doses. The relevant estimate for hERG inhibition toxicity is the peak concentration of the free drug in plasma, and since BKI 1369 is 76% protein bound, about 2.4 μM free BKI 1369 would be expected with the therapeutic dosing used in this experiment. This exceeds the measured IC50 for BKI 1369 of 1.52 μM. It is very possible that efficacy can be maintained while reducing the dose of BKI 1369 to minimize accumulation of BKI 1369 and metabolites during the experiment and to increase the safety of BKI 1369. Alternatively, other BKIs with less hERG inhibition can be explored, such as BKI 1517 and BKI 1553, which have no observable hERG-inhibitory activity even at 30 μM (11, 18). Nevertheless, this report demonstrates proof of concept for BKI-mediated therapeutic and parasitologic improvement of C. hominis infection.
In summary, we observed a significant symptomatic improvement in piglets treated with BKI 1369, with considerably reduced oocyst excretion. Additional experiments are needed to further confirm the safety and efficacy of BKI 1369 as a candidate therapeutic agent for treatment of acute cryptosporidiosis.
MATERIALS AND METHODS
C. hominis oocysts and gnotobiotic piglets.
C. hominis strain TU502, which originated from a Ugandan diarrheic child, has been maintained for over a decade through numerous passages (every 8 to 10 weeks) in GB piglets (19). TU502 oocysts were purified from gut contents and feces and prepared for challenge experiments as described previously (20). Oocysts with an excystation rate of over 60% were used as inoculum.
Twenty-four GB piglets, derived from four litters delivered by caesarean section, were maintained inside sterile isolators for the duration of the experiments, as approved by the institutional animal care and use committee (IACUC) guidelines. Healthy piglets selected 2 days after birth were assigned to experimental groups.
C. hominis challenge and BKI 1369 treatment.
TU502 oocysts (1 × 106 to 5 × 106) were administered orally to 18 of the 24 animals 2 days after birth (1 × 106 oocysts for 13 piglets in three experiments and 5 × 106 oocysts for 5 piglets in one experiment). A group of 9 piglets were treated with BKI 1369 at the onset of diarrhea, 3 days after C. hominis challenge, while a second group of 9 piglets were left as untreated controls. Two piglets were orally administered heat-killed (70°C for 30 min) TU502 oocysts (1 × 106) 2 days after birth. Of the four uninfected control animals, two were treated with BKI 1369. Chemical and biological information relating to BKI 1369 and its synthesis was previously reported (21–24). The BKI 1369 compound for these experiments was prepared by Vas Bio, Hyderabad, India, and was >98% pure by high-pressure liquid chromatography (HPLC) and nuclear magnetic resonance (NMR) analyses. BKI 1369 at 10 mg/kg in 2 ml of vehicle (7% Tween 80-3% ethanol [EtOH]-90% saline) was given orally twice a day for 5 days. We found that a BKI 1369 dose of 5 mg/kg twice daily (BID) was effective in the calf model (13). We used a slightly higher dose in this study, based on the assumption that the smaller piglets might need a larger dose per kilogram than that for the larger calves. The piglets administered heat-killed oocysts were treated with 2 ml of vehicle (7% Tween 80-3% EtOH-90% saline) twice a day for 5 days.
Clinical observation and sample collection.
Clinical signs of illness were monitored twice daily, and symptoms of diarrhea were scored from 0 to 4 (0, no diarrhea; 1, brown or gray soft stool, mild diarrhea; 2, brown to yellow, mucoid, small-volume stool, mild to moderate diarrhea; 3, yellow, mucoid to watery, medium-volume stool, moderate diarrhea; and 4, yellow to white, watery, high-volume stool, severe diarrhea). Two lab personnel monitored and scored diarrhea individually and averaged the scores. Body weight was measured daily.
Rectal swabs were collected daily, while sera were collected on days 0, 4, 5, and 7 once drug treatment commenced. Daily rectal swabs were placed in 500 μl sterile H2O and incubated at room temperature for 10 min; agitated eluates were centrifuged at 13,000 × g for 10 min to separate the oocysts in the pellet from the residual drugs in supernatant. Three hundred microliters of supernatant was transferred to a new tube and stored at −80°C to be used for measuring the drug concentration. After the pellet was resuspended with 300 μl sterile H2O, 50-μl and 200-μl aliquots were transferred to new tubes for oocyst counting and DNA extraction, respectively. For oocyst counting, the 50-μl transfer aliquot was washed once, and 10 μl was mounted on welled glass slides (7-mm diameter) and stained by the modified Kinyoun acid-fast method (12, 25). The number of oocysts in 30 fields was counted under a magnification of ×1,000. The number of oocysts was counted independently by two lab individuals and averaged.
Quantitative real-time PCR.
For DNA extraction, the 200-μl transfer aliquot was subjected to 5 freeze-thaw cycles and applied to an EZNA Stool DNA kit (Omega Bio-Tek) following the manufacturer's instructions. The eluted DNA was stored at −20°C until it was used for PCR.
For quantitative real-time PCR, primers (HSP70 forward, 5′-TCTGAAGGAATGCGAACAACT-3′; and HSP70 reverse, 5′-GGGTTTGTGATTGCTTGTCTTT-3′) and a probe (5′-6-carboxyfluorescein [FAM]-TGGGCAGAG-ZEN-ATTGGTTGGTGAAGT-3IABkFQ-3′) targeting Cryptosporidium hsp70 were generated using the PrimerQuest design tool (Integrated DNA Technologies, Inc., Coralville, IA). A premixed solution of primers (5 μM [each]) and probe (2.5 μM) was prepared. For PCR, the following was added to give a 10-μl total volume in each well: 5 μl of PrimeTime Gene Expression master mix (Integrated DNA Technologies, Inc.), 1 μl of the premixed primers and probe, 2 μl of sample DNA, and 2 μl of H2O. Each sample was prepared in triplicate. PCR was performed in a StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA) under the following conditions: 45 cycles of polymerase activation at 95°C for 3 min and amplification at 95°C for 15 s and 60°C for 1 min. The standard DNA was prepared from TU502 oocysts counted by use of a hemocytometer. The DNA amounts equivalent to Cryptosporidium oocysts were calculated from the cycle threshold (CT) values for samples by using the equation for the standard curve. Sera and rectal swabs from representative animals from each group were extracted with acetonitrile and analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) to determine the concentrations of BKI 1369 and its metabolites, metabolite 1 (BKI 1318) and metabolite 2 (BKI 1817) (13).
Histopathology.
Piglets were euthanized 10 days after the onset of treatment. Blood was collected and sent to the Tufts Clinical Pathology Laboratory for hematology and serum biochemical data analysis. At necropsy, tissues were collected from the small and large intestines, stomach, liver, kidneys, lungs, spleen, and brain, embedded in wax, processed, sectioned, and stained using hematoxylin and eosin at the Tufts Cummings histology laboratory. Sections were examined and scored by a board-certified veterinary pathologist (G. Beamer) blinded to the identities of the individuals in any given group. The extent of C. hominis colonization in the small and large intestines was scored as described previously (12, 26). Mucosal lesion scores were applied for villus changes (blunting and fusion), epithelial changes (attenuation, necrosis, and loss), and cellular inflammation (neutrophils and lymphocytes) in the lamina propria and within crypts/glands for all samples. The following scores were used: 0, normal; 2.5, equivocal; 5, mild; 10, moderate; and 15, marked. The final lesion score was the sum of the scores for 5 anatomic sites (3 for the small intestine [duodenum, jejunum, and ileum] and 2 for the large intestine [cecum and colon]) for each piglet. The following organs were also fixed, sectioned, and examined for possible toxic effects: brain, kidneys, liver, lungs, and spleen.
Statistical analysis.
Statistical comparisons of the differences among experimental groups were conducted using GraphPad Prism 7.03 software (GraphPad Software, San Diego, CA, USA), and the results were graphed. Colonization and lesion scores were analyzed using the Mann-Whitney nonparametric t test, and correlations were analyzed using Spearman coefficients and linear regression modeling to assess curve fit and 95% confidence intervals.
ACKNOWLEDGMENTS
This work was supported by the Bill & Melinda Gates Foundation.
We thank Julia Dilo, John Feddo, and Brendon Davis for technical assistance, Lynn K. Barrett for managing the Van Voorhis laboratory, and Dustin J. Maly for valuable discussions.
We have no conflicts of interest to report except that W.C.V.V. is the president of and owns stock in ParaTheraTech Inc., a small animal health company that is developing BKIs as potential animal therapeutics.
W.C.V.V. helped to plan the experiments, wrote parts of the paper, and edited the paper. He did not carry out the experiments or do the initial interpretation.
REFERENCES
- 1.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 2.Sow SO, Muhsen K, Nasrin D, Blackwelder WC, Wu Y, Farag TH, Panchalingam S, Sur D, Zaidi AK, Faruque AS, Saha D, Adegbola R, Alonso PL, Breiman RF, Bassat Q, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ahmed S, Qureshi S, Quadri F, Hossain A, Das SK, Antonio M, Hossain MJ, Mandomando I, Nhampossa T, Acacio S, Omore R, Oundo JO, Ochieng JB, Mintz ED, O'Reilly CE, Berkeley LY, Livio S, Tennant SM, Sommerfelt H, Nataro JP, Ziv-Baran T, Robins-Browne RM, Mishcherkin V, Zhang J, Liu J, Houpt ER, Kotloff KL, Levine MM. 2016. The burden of Cryptosporidium diarrheal disease among children <24 months of age in moderate/high mortality regions of sub-Saharan Africa and South Asia, utilizing data from the Global Enteric Multicenter Study (GEMS). PLoS Negl Trop Dis 10:e0004729. doi: 10.1371/journal.pntd.0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molbak K, Andersen M, Aaby P, Hojlyng N, Jakobsen M, Sodemann M, da Silva AP. 1997. Cryptosporidium infection in infancy as a cause of malnutrition: a community study from Guinea-Bissau, west Africa. Am J Clin Nutr 65:149–152. doi: 10.1093/ajcn/65.1.149. [DOI] [PubMed] [Google Scholar]
- 4.Guerrant DI, Moore SR, Lima AA, Patrick PD, Schorling JB, Guerrant RL. 1999. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. Am J Trop Med Hyg 61:707–713. doi: 10.4269/ajtmh.1999.61.707. [DOI] [PubMed] [Google Scholar]
- 5.Tumwine JK, Kekitiinwa A, Nabukeera N, Akiyoshi DE, Rich SM, Widmer G, Feng X, Tzipori S. 2003. Cryptosporidium parvum in children with diarrhea in Mulago Hospital, Kampala, Uganda. Am J Trop Med Hyg 68:710–715. [PubMed] [Google Scholar]
- 6.Abubakar I, Aliyu SH, Arumugam C, Hunter PR, Usman NK. 2007. Prevention and treatment of cryptosporidiosis in immunocompromised patients. Cochrane Database Syst Rev 2007:CD004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong DK, Wong CJ, Gutierrez K. 2007. Severe cryptosporidiosis in a seven-year-old renal transplant recipient: case report and review of the literature. Pediatr Transplant 11:94–100. doi: 10.1111/j.1399-3046.2006.00593.x. [DOI] [PubMed] [Google Scholar]
- 8.Rossignol JF. 2010. Cryptosporidium and Giardia: treatment options and prospects for new drugs. Exp Parasitol 124:45–53. doi: 10.1016/j.exppara.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Amadi B, Mwiya M, Musuku J, Watuka A, Sianongo S, Ayoub A, Kelly P. 2002. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet 360:1375–1380. doi: 10.1016/S0140-6736(02)11401-2. [DOI] [PubMed] [Google Scholar]
- 10.Van Voorhis WC, Doggett JS, Parsons M, Hulverson MA, Choi R, Arnold S, Riggs MW, Hemphill A, Howe DK, Mealey RH, Lau AO, Merritt EA, Maly DJ, Fan E, Ojo KK. 2017. Extended-spectrum antiprotozoal bumped kinase inhibitors: a review. Exp Parasitol 180:71–83. doi: 10.1016/j.exppara.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaefer DA, Betzer DP, Smith KD, Millman ZG, Michalski HC, Menchaca SE, Zambriski JA, Ojo KK, Hulverson MA, Arnold SL, Rivas KL, Vidadala RS, Huang W, Barrett LK, Maly DJ, Fan E, Van Voorhis WC, Riggs MW. 2016. Novel bumped kinase inhibitors are safe and effective therapeutics in the calf clinical model for cryptosporidiosis. J Infect Dis 214:1856–1864. doi: 10.1093/infdis/jiw488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S, Harwood M, Girouard D, Meyers MJ, Campbell MA, Beamer G, Tzipori S. 2017. The therapeutic efficacy of azithromycin and nitazoxanide in the acute pig model of Cryptosporidium hominis. PLoS One 12:e0185906. doi: 10.1371/journal.pone.0185906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hulverson MA, Choi R, Arnold SLM, Schaefer DA, Hemphill A, McCloskey MC, Betzer DP, Muller J, Vidadala RSR, Whitman GR, Rivas KL, Barrett LK, Hackman RC, Love MS, McNamara CW, Shaughnessy TK, Kondratiuk A, Kurnick M, Banfor PN, Lynch JJ, Freiberg GM, Kempf DJ, Maly DJ, Riggs MW, Ojo KK, Van Voorhis WC. 2017. Advances in bumped kinase inhibitors for human and animal therapy for cryptosporidiosis. Int J Parasitol 47:753–763. doi: 10.1016/j.ijpara.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper CA, Moraes LE, Murray JD, Owens SD. 2014. Hematologic and biochemical reference intervals for specific pathogen free 6-week-old Hampshire-Yorkshire crossbred pigs. J Anim Sci Biotechnol 5:5. doi: 10.1186/2049-1891-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Striepen B. 2013. Time to tackle cryptosporidiosis. Nature 503:189–191. doi: 10.1038/503189a. [DOI] [PubMed] [Google Scholar]
- 16.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJ, McGrath M, Olortegui MP, Samie A, Shakoor S, Mondal D, Lima IF, Hariraju D, Rayamajhi BB, Qureshi S, Kabir F, Yori PP, Mufamadi B, Amour C, Carreon JD, Richard SA, Lang D, Bessong P, Mduma E, Ahmed T, Lima AA, Mason CJ, Zaidi AK, Bhutta ZA, Kosek M, Guerrant RL, Gottlieb M, Miller M, Kang G, Houpt ER, MAL-ED Network Investigators . 2015. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 3:e564–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Checkley W, White AC Jr, Jaganath D, Arrowood MJ, Chalmers RM, Chen XM, Fayer R, Griffiths JK, Guerrant RL, Hedstrom L, Huston CD, Kotloff KL, Kang G, Mead JR, Miller M, Petri WA Jr, Priest JW, Roos DS, Striepen B, Thompson RC, Ward HD, Van Voorhis WA, Xiao L, Zhu G, Houpt ER. 2015. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect Dis 15:85–94. doi: 10.1016/S1473-3099(14)70772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vidadala RS, Rivas KL, Ojo KK, Hulverson MA, Zambriski JA, Bruzual I, Schultz TL, Huang W, Zhang Z, Scheele S, DeRocher AE, Choi R, Barrett LK, Siddaramaiah LK, Hol WG, Fan E, Merritt EA, Parsons M, Freiberg G, Marsh K, Kempf DJ, Carruthers VB, Isoherranen N, Doggett JS, Van Voorhis WC, Maly DJ. 2016. Development of an orally available and central nervous system (CNS) penetrant Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) inhibitor with minimal human Ether-a-go-go-related gene (hERG) activity for the treatment of toxoplasmosis. J Med Chem 59:6531–6546. doi: 10.1021/acs.jmedchem.6b00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akiyoshi DE, Feng X, Buckholt MA, Widmer G, Tzipori S. 2002. Genetic analysis of a Cryptosporidium parvum human genotype 1 isolate passaged through different host species. Infect Immun 70:5670–5675. doi: 10.1128/IAI.70.10.5670-5675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chappell CL, Okhuysen PC, Langer-Curry R, Widmer G, Akiyoshi DE, Tanriverdi S, Tzipori S. 2006. Cryptosporidium hominis: experimental challenge of healthy adults. Am J Trop Med Hyg 75:851–857. [PubMed] [Google Scholar]
- 21.Ojo KK, Pfander C, Mueller NR, Burstroem C, Larson ET, Bryan CM, Fox AM, Reid MC, Johnson SM, Murphy RC, Kennedy M, Mann H, Leibly DJ, Hewitt SN, Verlinde CL, Kappe S, Merritt EA, Maly DJ, Billker O, Van Voorhis WC. 2012. Transmission of malaria to mosquitoes blocked by bumped kinase inhibitors. J Clin Invest 122:2301–2305. doi: 10.1172/JCI61822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy RC, Ojo KK, Larson ET, Castellanos-Gonzalez A, Perera BG, Keyloun KR, Kim JE, Bhandari JG, Muller NR, Verlinde CL, White AC Jr, Merritt EA, Van Voorhis WC, Maly DJ. 2010. Discovery of potent and selective inhibitors of calcium-dependent protein kinase 1 (CDPK1) from C. parvum and T. gondii. ACS Med Chem Lett 1:331–335. doi: 10.1021/ml100096t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z, Ojo KK, Vidadala R, Huang W, Geiger JA, Scheele S, Choi R, Reid MC, Keyloun KR, Rivas K, Siddaramaiah LK, Comess KM, Robinson KP, Merta PJ, Kifle L, Hol WG, Parsons M, Merritt EA, Maly DJ, Verlinde CL, Van Voorhis WC, Fan E. 2014. Potent and selective inhibitors of CDPK1 from T. gondii and C. parvum based on a 5-aminopyrazole-4-carboxamide scaffold. ACS Med Chem Lett 5:40–44. doi: 10.1021/ml400315s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson SM, Murphy RC, Geiger JA, DeRocher AE, Zhang Z, Ojo KK, Larson ET, Perera BG, Dale EJ, He P, Reid MC, Fox AM, Mueller NR, Merritt EA, Fan E, Parsons M, Van Voorhis WC, Maly DJ. 2012. Development of Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) inhibitors with potent anti-toxoplasma activity. J Med Chem 55:2416–2426. doi: 10.1021/jm201713h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morada M, Lee S, Gunther-Cummins L, Weiss LM, Widmer G, Tzipori S, Yarlett N. 2016. Continuous culture of Cryptosporidium parvum using hollow fiber technology. Int J Parasitol 46:21–29. doi: 10.1016/j.ijpara.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Tzipori S, Rand W, Griffiths J, Widmer G, Crabb J. 1994. Evaluation of an animal model system for cryptosporidiosis: therapeutic efficacy of paromomycin and hyperimmune bovine colostrum-immunoglobulin. Clin Diagn Lab Immunol 1:450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]