ABSTRACT
The purpose of this study was to investigate the population pharmacokinetics of vancomycin in patients undergoing open heart surgery. In this observational pharmacokinetic study, multiple blood samples were drawn over a 48-h period of intravenous vancomycin in patients who were undergoing open heart surgery. Blood samples were analyzed using an Architect i4000SR immunoassay analyzer. Population pharmacokinetic models were developed using Monolix 4.4 software. Pharmacokinetic-pharmacodynamic (PK-PD) simulations were performed to explore the ability of different dosage regimens to achieve the pharmacodynamic targets. A total of 168 blood samples were analyzed from 28 patients. The pharmacokinetics of vancomycin are best described by a two-compartment model with between-subject variability in clearance (CL), the volume of distribution of the central compartment (V1), and volume of distribution of the peripheral compartment (V2). The CL and the V1 of vancomycin were related to creatinine CL (CLCR), body weight, and albumin concentration. Dosing simulations showed that standard dosing regimens of 1 and 1.5 g failed to achieve the PK-PD target of AUC0–24/MIC > 400 for an MIC of 1 mg/liter, while high weight-based dosing regimens were able to achieve the PK-PD target. In summary, the administration of standard doses of 1 and 1.5 g of vancomycin two times daily provided inadequate antibiotic prophylaxis in patients undergoing open heart surgery. The same findings were obtained when 15- and 20-mg/kg doses of vancomycin were administered. Achieving the PK-PD target required higher doses (25 and 30 mg/kg) of vancomycin.
KEYWORDS: vancomycin, open heart surgery, prophylaxis, population pharmacokinetics, Monte Carlo simulation, PK-PD
INTRODUCTION
There are several complications that can occur after open heart surgery, including wound infections. Patients who have had this major surgery are at risk for certain severe postoperative infections. It has been reported that the incidence of sternal surgical site infections after open heart surgery ranges from 1.3 to 8.6% (1, 2), and the incidence of deep sternal infections, such as mediastinitis, is 4.6% (2). Furthermore, the 1-year mortality rate increases for patients with deep sternal infections to reach ∼22% (3). Staphylococcus aureus (32%) and coagulase-negative staphylococci (12%), as well as Gram-negative enteric organisms (40%), are considered the most common pathogens responsible for postoperative infections (1).
One of the most common complications of cardiac surgical procedures is infection due to S. aureus. Surgical-site infections (SSIs) due to S. aureus following open heart surgery have been associated with significant increases in morbidity and mortality and substantial burden on both patients and health care systems, particularly from serious infections such as deep sternal infections. It has been reported that the total length of hospital stay after coronary artery bypass graft surgery for patients with SSIs is significantly longer than that for patients without SSIs (4). In addition, several investigators have reported that S. aureus is the most common cause of sternal wound infections and the leading cause of infective endocarditis in developed countries (5–7). Moreover, according to recent published study, 7.9% of the reported infections are due to methicillin-resistant S. aureus (MRSA) strains (1), which result in more mortality and morbidity than infections from methicillin-sensitive S. aureus strains (6). In Saudi Arabia, the prevalence of MRSA was estimated to be 35.6% from a pooled estimation of 22,793 S. aureus strains from 2002 to 2012 (8).
A number of effective infection control strategies can be applied to reduce the risk of SSIs, including appropriate antibiotic prophylaxis, effective patient skin preparation, and surveillance systems to monitor and report SSIs after specific procedures with feedback to surgeons and hospitals (9). There is strong evidence that using antibiotic prophylaxis significantly lowers postoperative infections. According to the clinical practice guidelines for antimicrobial prophylaxis, a β-lactam antibiotic is indicated as the single antibiotic of choice for standard cardiac surgical prophylaxis in populations that do not have a high incidence of MRSA, and the duration of routine postoperative administration of prophylactic antibiotics can be no longer than 48 h (10–12). However, the prevalence and emergence of MRSA strains within hospitals and the community have become increasingly significant, with the possibility of causing more serious infections in the cardiac surgery patient (12, 13). Thus, more aggressive use of prophylactic antibiotics, such as vancomycin, is recommended even for patients with no history of penicillin or cephalosporin allergy (12, 13).
Vancomycin is a glycopeptide antibacterial developed many years ago as an alternative to penicillin to treat MRSA strains and is one of the most widely used antibacterials in the treatment of serious Gram-positive infections involving MRSA (14). Vancomycin is administered intravenously for various indications. Vancomycin has the ability to distribute to body liquids and penetrate into various body spaces (15, 16). Its level of protein binding (mainly to albumin in the plasma) is 55% (15, 16). Its volume of distribution (V) is 0.4 to 1 liter/kg (15, 16). Vancomycin is excreted mainly unchanged in the urine (∼80%), and only a small amount is eliminated by nonrenal mechanisms (15, 16). In addition, its elimination half-life ranges from 3 to 9 h in individuals with normal renal function (15, 16).
After vancomycin administration, the dosing is commonly titrated to achieve trough serum concentrations of 10 to 20 mg/liter (16). Low concentrations are associated with treatment failure and emergence of resistance, while high concentrations are commonly associated with nephrotoxicity and ototoxicity (17). However, previous studies have indicated that the vancomycin trough level may not be the optimal pharmacokinetic measure for predicting treatment outcomes in invasive MRSA infections (16, 18). It has been reported that a vancomycin area under the concentration-time curve from 0 to 24 h (AUC0–24)/MIC index is the best measure for clinical outcomes, with an AUC0–24/MIC > 400 (as the pharmacokinetic-pharmacodynamic [PK-PD] target) associated with optimal outcomes for invasive MRSA infections (16, 18–22). However, the aforementioned PK-PD target is for bactericidal activity against invasive infections, and the vancomycin PK-PD target in a prophylaxis setting has yet to be established. The importance and effectiveness of vancomycin in the reduction of severe infections caused by MRSA should be considered, as supported by two recent published studies (1, 2).
The pharmacokinetics of vancomycin during open heart surgery, including cardiopulmonary bypass (CPB), has been widely investigated (23–28). However, vancomycin use as a prophylactic agent in open heart surgery has not been evaluated by coupling population pharmacokinetic modeling and Monte Carlo simulation to determine the probability of target attainment (PTA) at various MICs. In addition, the PK-PD target of vancomycin in the prophylaxis setting needs to be determined. Therefore, the aim of this study was to develop a population pharmacokinetic model of vancomycin in patients who underwent open heart surgery and to investigate whether PK-PD targets are achieved with current dosing strategies; the potential of alternative dosing regimens and strategies was also investigated.
RESULTS
Sample collection and patient demographics.
In total, 168 blood samples were analyzed from 28 patients enrolled in this study, and 17 patients (61%) were males. The baseline demographic and general clinical characteristics of the patients used for model building are shown in Table 1. The average (± the standard deviation [SD]) age and weight in the population were 51.7 ± 15.9 years and 79.6 ± 17 kg. The average CLCR estimated by the Cockcroft-Gault formula was 78.5 ± 24 ml/min. The mean total and plasma vancomycin concentrations at different sampling intervals are shown in Table 2.
TABLE 1.
Summary of patient characteristics
| Characteristics | Mean (SD) | Range |
|---|---|---|
| Age (yr) | 51.7 (15.9) | 18–78 |
| Wt (kg) | 79.6 (17) | 52–111.8 |
| Body mass index | 29.8 (5.6) | 20.2–41.9 |
| Serum creatinine (μmol/liter) | 77.2 (22.2) | 41–134 |
| Albumin (g/liter) | 35.5 (4.5) | 25–44 |
| CLCR (ml/min)a | 83.5 (29.3) | 33.4–125 |
| Sex (% male/% female) | 61/39 |
As determined by the Cockcroft-Gault formula.
TABLE 2.
Plasma concentrations of vancomycin for all patients at different sampling intervals
| Sample intervala | Avg concn (mg/liter) | SD |
|---|---|---|
| 1 | 11.6 | 2.8 |
| 2 | 8.6 | 2.4 |
| 3 | 7 | 2.2 |
| 4 | 7.6 | 5.5 |
| 5 | 16.1 | 4.4 |
| 6 | 13 | 7.1 |
Intervals: 1, immediately before skin incision; 2, at the start of CPB; 3, 1 h after starting CPB; 4, immediately before skin closure; 5, 24 h after administration of the first dose; 6, 48 h after administration of the first dose.
Population pharmacokinetics.
Visual inspection of the observed vancomycin data supported a two-compartment model, which was confirmed by the goodness-of-fit (GOF) plots and a statistically significant drop in the objective function value with respect to the one-compartment model. The pharmacokinetic model was parameterized in terms of the CL, the volume of the central compartment (V1), the volume of the peripheral compartment (V2), and the intercompartmental clearance (Q). A combined-error model was the most accurate for residual and interpatient variability. After covariate testing, CLCR and serum albumin concentration were the only covariates showing correlation with vancomycin CL, while body weight was the only significant covariate for V1. We used a power formula to describe the effects of these covariates on CL and V1. All covariates were scaled to the mean, and these covariates decreased the interpatient variability for CL and V1 by approximately 55%. Therefore, the CLCR, serum albumin, and body weight were included in the model, while other covariates that displayed no correlation with the pharmacokinetic parameters were not investigated further. The values of the parameters for the final models are summarized in Table 3.
TABLE 3.
Population pharmacokinetic model estimates for vancomycin following intravenous infusiona
| Parameter | Population estimate | RSE (%) |
|---|---|---|
| CL (liters/h) | 6.13 | 19 |
| V1 (liters) | 40 | 15 |
| Q (liters/h) | 0.22 | 10 |
| V2 (liters) | 3.88 | 16 |
| IIV (%) | ||
| IIV for CL | 22.1 | 16 |
| IIV for V1 | 6.34 | 17 |
| IIV for Q | 57.8 | 12 |
| IIV for V2 | 61.2 | 19 |
| Residual errors | ||
| a (mg/liters) | 0.055 | 11 |
| b (%) | 15.2 | 7 |
Interindividual variability (IIV) is expressed as a coefficient of variation; RSE represents the relative standard error. In residual errors, a is the additive constant, and b is the proportional error. CL = 6.13 · (CLCR/83.5)0.514 · (albumin/35.5)0.854; V1 = 40 · (weight/79.6)0.466.
Model evaluation.
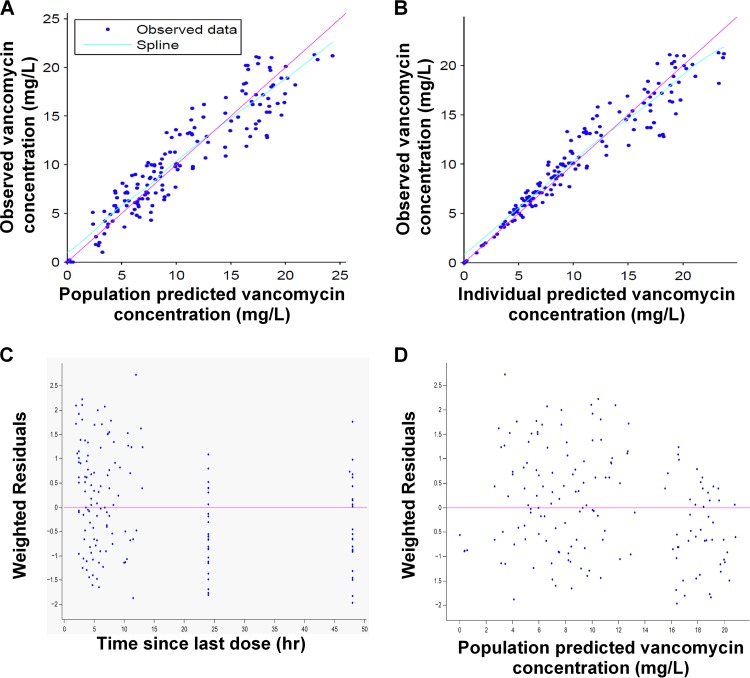
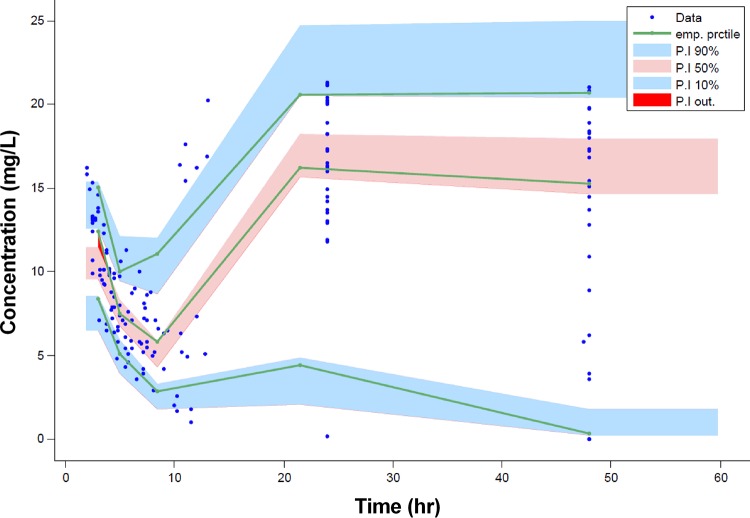
Diagnostic GOF plots for the vancomycin final covariate model are shown in Fig. 1. The relative standard errors (RSEs), expressed as a percentage, shown in Table 3 reveal that all parameters were precisely estimated. In addition, inspection of the prediction-corrected visual predictive check (pcVPC) (Fig. 2) revealed a good correlation between the percentile intervals obtained by simulation in the final model with those of the observed data. Both figures show that the final pharmacokinetic model describes the measured concentrations adequately. This model was used to simulate all subsequent dosing regimens.
FIG 1.
GOF plots obtained from the final model for vancomycin. (A) Individual predictions of vancomycin versus observed concentrations. (B) Population predictions of vancomycin versus observed concentrations. (C) Weighted residuals versus time since last dose. (D) Weighted residuals versus population predicted concentrations.
FIG 2.
Predicted-corrected visual predictive check (pcVPC) for vancomycin concentration versus time based on 1,000 Monte Carlo simulations. The solid green line represents the 10th, 50th, and 90th percentiles of the observed data. The shaded regions represent the 90% confidence intervals around the 10th, 50th, and 90th percentiles of the simulated data. The observed plasma concentrations are represented by blue circles.
Monte Carlo simulations.
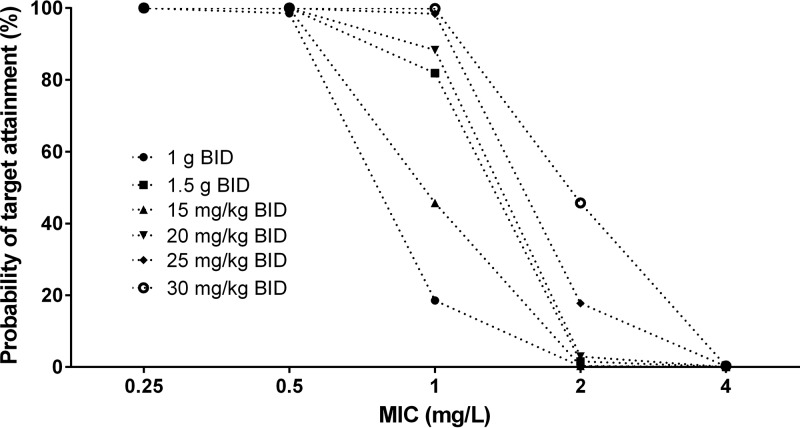
We used Monte Carlo simulations to evaluate different dosing regimens of vancomycin. The PTA values for different dosing regimens of vancomycin are presented in Fig. 3. All simulated dosing regimens produced an adequate target attainment for patients up to an MIC of 0.5 mg/liter. These dosing regimens lead to >90% PTA for an MIC of 0.5 mg/liter, even at 15-mg/kg administered every 12 h (q12h). At an MIC of 1 mg/liter, target attainment was very low for 1 g q12h, 1.5 g q12h, 15 mg/kg q12h, and 20 mg/kg q12h and approached 98 and 99% at the 25- and 30-mg/kg q12h doses, respectively. For an MIC of 2 mg/liter, all dosing regimens showed poor target attainment (e.g., 45.7% for 30 mg/kg q12h).
FIG 3.
Target attainment analysis for vancomycin (AUC0–24/MIC > 400) for all of the various dosing regimens at different MICs.
DISCUSSION
During open heart surgery, contamination of the surgical site can occur at any time during the operation period. The risk is the highest during the postoperative period, when there is exposure of the tissue and entry of microorganisms into the surgical site is possible. There are several factors that can increase risk of infection, which may be endogenous, such as advanced age, obesity, and diabetes, or exogenous, such as breaking the aseptic barrier, inadequate hand hygiene, wound classification, duration of the procedures, and the surgical technique (29–31). Open heart surgical procedures often are invasive, involving the prolonged use of supporting devices and indwelling intravenous lines, and the introduction of foreign materials and tissues, such as prosthetic valves, into the body. All of these factors increase the chance for postoperative S. aureus infections, which have been associated with poor outcomes, including significant morbidity, prolonged hospitalization, higher medical costs, increased likelihood of readmission within 3 months of the procedure, and death (7, 32, 33). Antibiotic prophylaxis is regularly administered to patients undergoing open heart surgery because the benefits of preoperative antibiotic administration in these patients have been clearly supported in placebo-controlled studies (34).
According to the Society of Thoracic Surgeons Practice Guidelines, a β-lactam antibiotic is indicated as the single antibiotic of choice for standard cardiac surgical prophylaxis in populations that do not have a high incidence of MRSA, and the duration of routine postoperative administration of prophylactic antibiotics should be no longer than 48 h (10, 11). However, in settings with known staphylococcal colonization, at institutions with a high incidence of MRSA infections, with patients who are susceptible to MRSA colonization, or with patients who have prosthetic valves or vascular graft insertions, it is recommended to combine the β-lactam with vancomycin (10, 11). The same practice guidelines recommend either a fixed dose of 1 to 1.5 g of vancomycin or a weight-adjusted dose of 15 mg/kg within 60 min of surgical incision (10, 11).
The population pharmacokinetics of vancomycin as a prophylactic antibiotic in open heart surgery has not been evaluated yet. In the present study, we evaluated the kinetic profile of vancomycin in patients undergoing open heart surgery using population modeling and applied the proposed models to Monte Carlo simulations to understand how patient characteristics influence the adequacy of the prophylaxis. The plasma concentration-time curves for vancomycin were fitted to a two-compartment model, as shown in other studies (35–40). Vancomycin is eliminated mainly by renal excretion, and we found that vancomycin CL was related to CLCR and serum albumin concentration, but with notable interpatient variability, which was consistent with other studies (35–40). Our study also showed that the vancomycin central compartment volume of distribution (V1) was related to body weight. Therefore, all three covariates were included in the final covariate model. The estimated pharmacokinetic parameters of vancomycin were 6.13 liters/h, 40 liters, 0.22 liter/h, and 3.88 liters for CL, V1, Q, and V2, respectively, which were consistent with the estimated values reported in other studies (35–40).
Previous studies have reported that the use of CPB during open heart may have a significant impact on the pharmacokinetics of administered drugs, including vancomycin. This is due to several reasons, including hemodilution, hypotension, hypothermia, and alteration of protein binding, and can lead to suboptimal antibiotic prophylaxis (23, 24, 26–28). Cotogni et al. (25) evaluated the effect of CPB on vancomycin plasma concentrations during open heart surgery. These researchers found that the median Cmax, AUC, and CL of vancomycin were similar in patients undergoing cardiac surgery with or without CPB, whereas the V of vancomycin was only ∼10% larger in patients with CPB (25). These authors concluded that vancomycin pharmacokinetics are not significantly altered by CPB and that dose modifications are not required (25). In our study, we tested the use of CPB as one of the covariates that may affect the CL and V of vancomycin and found that there was no significant effect on these parameters, an observation that was in total agreement with the previous studies.
When simulations were performed for different dosing regimens and the PTA was investigated, we demonstrated that all dosing regimens were able to achieve a PTA of >90% at MICs of 0.25 and 0.5 mg/liter. However, only 25 and 30 mg/kg q12h were able to achieve the target at an MIC of 1 mg/liter. The doses would also depend on the susceptibility pattern of MRSA at each hospital. Institutions with elevated MIC levels might need higher starting doses. For MRSA isolates with an MIC > 2 mg/liter, target attainment was poor even at the 30-mg/kg q12h dose. Increased vancomycin doses resulted in a greater likelihood of patients achieving an AUC0–24/MIC > 400. Our findings are in agreement with those of previously published studies. Salem et al. (41) investigated the PTA for vancomycin at two dosing regimens (1 and 1.5 g q12h). Our findings were similar to their results at MICs of 0.25, 0.5, and 1 mg/liter for a 1-g q12h dosing regimen and at MICs of 0.25 and 0.5 mg/liter for 1.5 g q12h (41). However, in their study, a 1.5-g q12h dosing regimen was able to achieve the PTA at an MIC of 1 mg/liter, which was not consistent with our findings (41). Furthermore, Hafermann et al. reported that weight-based vancomycin dosing before open heart surgery or valve replacement results in vancomycin concentrations greater than those seen with standard 1-g dosing (42). Although an MIC of 2 mg/liter is considered within the susceptible range, our findings showed that all tested dosing regimens did not achieve an AUC0–24/MIC > 400 at this MIC (Fig. 3). Our predicted poor pharmacodynamic response at an MIC of 2 mg/liter is in agreement with findings from clinical studies in which patients with an increased MIC (>1 mg/liter) had poor treatment responses (43–45).
Regarding the PK-PD analysis, the PK-PD target that best predicted the activity of vancomycin was the AUC0–24/MIC index (16, 18, 19). It has been reported that a vancomycin AUC0–24/MIC index, and not the trough concentrations, is the best measure for clinical outcomes, with an AUC0–24/MIC > 400 associated with optimal outcomes (16, 18, 19). The use of vancomycin in prophylaxis settings supposes a completely new concept where the risk of infection and life-threatening consequences from MRSA infections is higher. Therefore, the goal of antibiotic prophylaxis is to achieve unbound plasma and tissue concentrations of vancomycin that exceed the MICs for the MRSA organisms likely to be encountered during the surgery until the wound has been closed. Consequently, in this study, we used the highest MIC with a PTA of at least 90% as the PK-PD MIC breakpoint.
To the best of our knowledge, this is the first study to evaluate the population pharmacokinetics of vancomycin in patients undergoing open heart surgery. Nevertheless, it is important to note the limitations of the present study. First, we have not measured free vancomycin concentrations or concentrations at the site of infection. It is essential to identify the range of protein binding observed clinically for vancomycin, so that the free drug AUC0–24/MIC target can be properly estimated, and the true therapeutic window for vancomycin is determined robustly. Second, the small cohort of 28 patients examined here could be considered a limitation. This small number of patients may have prevented other covariates from being revealed as significant and predictive of the variability of the pharmacokinetic parameters. Third, the PK-PD target we used in the present study is the target for documented invasive infections, and we assumed the same target in order to prevent severe MRSA infection during cardiac surgery. Further investigations about the optimal PK-PD target for vancomycin and optimal outcomes are required. However, this study will provide the first step toward more investigation and studies.
In conclusion, a population pharmacokinetic model has been developed for vancomycin used as a prophylactic agent in patients undergoing open heart surgery. The pharmacokinetics of vancomycin is best reflected by a two-compartment model. A relationship between the vancomycin CL and the CLCR, the vancomycin CL and serum albumin levels, and body weight and the V1 was detected. This model was useful for establishing dosing recommendations to reduce the incidence of SSI, depending on patient characteristics. The results of the dosing simulations showed that dosing regimens of 1 and 1.5 g of vancomycin administered twice daily as a bolus infusion failed to achieve the PK-PD target, whereas weight-based dosing regimens of 25 and 30 mg/kg of vancomycin administered twice daily were more likely to achieve the PK-PD targets.
MATERIALS AND METHODS
Study design and settings.
A prospective, open-label study was conducted in patients who underwent cardiac surgical procedures at King Fahad Cardia Centre, King Saud University Medical City (Riyadh, Saudi Arabia). All study procedures were approved by the Institutional Review Board at the hospital and were conducted in accordance with Good Clinical Practice. Written informed consent was obtained from all patients. Patients aged ≥18 years scheduled to undergo surgical procedures and vancomycin was used for prophylaxis were included in the study. The creatinine clearance (CLCR) was estimated for each patient using the Cockcroft-Gault equation (46). Patients were excluded if they had been administered vancomycin therapy in the last 72 h before the surgery.
Drug administration and sampling procedure.
According to the surgical protocol of the hospital, patients received 1 g of vancomycin as an intravenous infusion over 30 min if they were MRSA positive, if they had a long preparative hospital stay, or if they were undergoing valve or aortic surgery. The first dose of vancomycin was administered 2 h before skin incision and then q12h for 48 h. An extra dose (redosing) was administered if the surgery lasted for more than 4 h (the procedure takes from 3 to 6 h, and about 12 patients have received an extra dosing). Blood samples were collected from the radial artery catheter. Six blood samples were collected as follows: (i) immediately before skin incision, (ii) at the start of CPB, (iii) 1 h after starting CPB, (iv) immediately before skin closure, (v) 24 h from after the first dose, and (vi) 48 h after the first dose. Blood samples were collected in EDTA blood collection tubes, stored on ice, mixed, and centrifuged at 5,000 rpm for 10 min directly after the procedure; the plasma was stored at −80°C until analysis.
Analytical method.
The vancomycin drug concentrations were analyzed using Architect iVancomycin (Abbott, Wiesbaden, Germany) with the Architect i4000SR immunoassay analyzer (Abbott Laboratories, North Chicago, IL). Architect iVancomycin is an in vitro chemiluminescent microparticle immunoassay for the quantitative measurement of vancomycin in human serum or plasma. The analytical measurement range of the Architect iVancomycin assay is 0.24 to 100 μg/ml, and the clinical reportable range of the assay is 0.5 to 83 μg/ml. The standard curve ranged from 3 to 100 μg/ml.
Population pharmacokinetics.
The population pharmacokinetic model for vancomycin was developed using Monolix 4.4 software. Monolix estimates pharmacokinetic parameters using the stochastic approximation expectation maximization algorithm (47). Initially, we developed the base structural model for vancomycin. One- and two-compartment systems were evaluated with linear or nonlinear elimination. Pharmacokinetic parameters were assumed to follow a lognormal distribution. For the residual variability, the following types of error models were tested: proportional, constant, exponential, and combined. Selection between models was based on the following: (i) the decrease in the minimum objective function value (log-likelihood value); (ii) the precision of the parameter estimation expressed as the RSE (%) and calculated as the ratio between the standard error and the final parameter estimate; (iii) physiological plausibility; and (iv) GOF plots, including the observed versus predicted concentration, residuals plot, and the visual predictive check (VPC).
After the appropriate base model was established, nine covariates (age, weight, height, serum creatinine, gender, CLCR, plasma albumin concentration, body mass index, and CPB) were tested. For covariate testing, we started by plotting the empirical Bayesian estimates versus covariates to screen for potentially significant correlations. Then, we performed a stepwise regression analysis to test the significant covariates identified above using the log-likelihood ratio test (see Tables S1 and S2 in the supplemental material). Inclusion was based on a significance level of 0.05; backward elimination was tested at a significance level of 0.01.
Model evaluation.
GOF plots were used as the first indicator of suitability, including the plotting of model-based individual predictions (IPRED) and population predictions (PRED) versus the observed concentrations. A pcVPC was constructed to study the performance of the final model. A pcVPC was constructed with the 10th, 50th, and 90th percentiles of the observed data. Then, 1,000 data sets were simulated from the final model parameter estimates, and the 95% confidence intervals for the 5th, 50th, and 95th percentiles based on the simulated data sets were calculated and represented together.
Monte Carlo simulations.
The final population model was utilized to simulate different dosing regimens for vancomycin using Monte Carlo simulations. The simulated dosage regimens included: 1 g of vancomycin q12h, 1.5 g of vancomycin q12h, and weight-based doses ranging from 15 to 30 mg/kg q12h in increments of 5 mg/kg. We assessed the PTA for each dosing regimen by counting the subjects who achieved an AUC0–24/MIC > 400 (19, 48). Target attainment was evaluated at different MICs (0.5, 1, 2, and 4 mg/liter).
In this analysis, the PTA was calculated as follows. First, we simulated 10,000 random vancomycin CL values based on the final pharmacokinetic model. Then, the AUC0–24/MIC value was calculated for each individual by using the following equation: AUC/MIC = (D/CL)/MIC. For each dosing regimen and MIC combination, we calculated the proportion of virtual patients that achieved the therapeutic target of an AUC/MIC > 400. The PK-PD susceptibility breakpoint was defined as the highest MIC at which the PTA was ≥90% (49). All simulations and graphical representations were performed using R statistical software.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge financial support from the College of Pharmacy Research Center and the Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00088-18.
REFERENCES
- 1.Branch-Elliman W, Ripollone JE, O'Brien WJ, Itani KMF, Schweizer ML, Perencevich E, Strymish J, Gupta K. 2017. Risk of surgical site infection, acute kidney injury, and Clostridium difficile infection following antibiotic prophylaxis with vancomycin plus a beta-lactam versus either drug alone: a national propensity-score-adjusted retrospective cohort study. PLoS Med 14:e1002340. doi: 10.1371/journal.pmed.1002340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reineke S, Carrel TP, Eigenmann V, Gahl B, Fuehrer U, Seidl C, Reineke D, Roost E, Bachli M, Marschall J, Englberger L. 2017. Adding vancomycin to perioperative prophylaxis decreases deep sternal wound infections in high-risk cardiac surgery patients. Eur J Cardiothorac Surg doi: 10.1093/ejcts/ezx328. [DOI] [PubMed] [Google Scholar]
- 3.Hollenbeak CS, Murphy DM, Koenig S, Woodward RS, Dunagan WC, Fraser VJ. 2000. The clinical and economic impact of deep chest surgical site infections following coronary artery bypass graft surgery. Chest 118:397–402. doi: 10.1378/chest.118.2.397. [DOI] [PubMed] [Google Scholar]
- 4.Cristofolini M, Worlitzsch D, Wienke A, Silber RE, Borneff-Lipp M. 2012. Surgical site infections after coronary artery bypass graft surgery: incidence, perioperative hospital stay, readmissions, and revision surgeries. Infection 40:397–404. doi: 10.1007/s15010-012-0275-0. [DOI] [PubMed] [Google Scholar]
- 5.Cosgrove SE, Fowler VG Jr. 2008. Management of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 46(Suppl 5):S386–S393. doi: 10.1086/533595. [DOI] [PubMed] [Google Scholar]
- 6.Lee BY, Wiringa AE, Bailey RR, Goyal V, Lewis GJ, Tsui BY, Smith KJ, Muder RR. 2010. Screening cardiac surgery patients for MRSA: an economic computer model. Am J Manag Care 16:e163–e173. [PMC free article] [PubMed] [Google Scholar]
- 7.Soderquist B. 2007. Surgical site infections in cardiac surgery: microbiology. APMIS 115:1008–1011. doi: 10.1111/j.1600-0463.2007.00833.x. [DOI] [PubMed] [Google Scholar]
- 8.Al-Yousef SA MS, Taha EM. 2013. Prevalence of methicillin-resistant Staphylococcus aureus in Saudi Arabia: systemic review and meta-analysis. Afr J Clin Exp Microbiol 14:146–154. [Google Scholar]
- 9.Si D, Rajmokan M, Lakhan P, Marquess J, Coulter C, Paterson D. 2014. Surgical site infections following coronary artery bypass graft procedures: 10 years of surveillance data. BMC Infect Dis 14:318. doi: 10.1186/1471-2334-14-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards FH, Engelman RM, Houck P, Shahian DM, Bridges CR, Society of Thoracic Surgeons . 2006. The Society of Thoracic Surgeons practice guideline series: antibiotic prophylaxis in cardiac surgery. I. Duration. Ann Thorac Surg 81:397–404. doi: 10.1016/j.athoracsur.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 11.Engelman R, Shahian D, Shemin R, Guy TS, Bratzler D, Edwards F, Jacobs M, Fernando H, Bridges C, Society of Thoracic Surgeons Workforce on Evidence-Based Medicine . 2007. The Society of Thoracic Surgeons practice guideline series: antibiotic prophylaxis in cardiac surgery. II. Antibiotic choice. Ann Thorac Surg 83:1569–1576. doi: 10.1016/j.athoracsur.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 12.Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, Fish DN, Napolitano LM, Sawyer RG, Slain D, Steinberg JP, Weinstein RA, American Society of Health-System Pharmacists, Infectious Diseases Society of America, Surgical Infection Society, Society for Healthcare Epidemiology of America . 2013. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect 14:73–156. doi: 10.1089/sur.2013.9999. [DOI] [PubMed] [Google Scholar]
- 13.Chenoweth CE, DePestel DD, Prager RL. 2005. Are cephalosporins adequate for antimicrobial prophylaxis for cardiac surgery involving implants? Clin Infect Dis 41:122–124. doi: 10.1086/430452. [DOI] [PubMed] [Google Scholar]
- 14.Levine DP. 2006. Vancomycin: a history. Clin Infect Dis 42(Suppl 1):S5–S12. doi: 10.1086/491709. [DOI] [PubMed] [Google Scholar]
- 15.Matzke GR, Zhanel GG, Guay DR. 1986. Clinical pharmacokinetics of vancomycin. Clin Pharmacokinet 11:257–282. doi: 10.2165/00003088-198611040-00001. [DOI] [PubMed] [Google Scholar]
- 16.Vandecasteele SJ, De Vriese AS, Tacconelli E. 2013. The pharmacokinetics and pharmacodynamics of vancomycin in clinical practice: evidence and uncertainties. J Antimicrob Chemother 68:743–748. doi: 10.1093/jac/dks495. [DOI] [PubMed] [Google Scholar]
- 17.de Hoog M, Mouton JW, van den Anker JN. 2005. New dosing strategies for antibacterial agents in the neonate. Semin Fetal Neonatal Med 10:185–194. doi: 10.1016/j.siny.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Rybak MJ. 2006. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis 42(Suppl 1):S35–S39. doi: 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- 19.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 43:925–942. doi: 10.2165/00003088-200443130-00005. [DOI] [PubMed] [Google Scholar]
- 20.Casapao AM, Lodise TP, Davis SL, Claeys KC, Kullar R, Levine DP, Rybak MJ. 2015. Association between vancomycin day 1 exposure profile and outcomes among patients with methicillin-resistant Staphylococcus aureus infective endocarditis. Antimicrob Agents Chemother 59:2978–2985. doi: 10.1128/AAC.03970-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finch NA, Zasowski EJ, Murray KP, Mynatt RP, Zhao JJ, Yost R, Pogue JM, Rybak MJ. 2017. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob Agents Chemother 61:e01293-17. doi: 10.1128/AAC.01293-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pai MP, Neely M, Rodvold KA, Lodise TP. 2014. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev 77:50–57. doi: 10.1016/j.addr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Klamerus KJ, Rodvold KA, Silverman NA, Levitsky S. 1988. Effect of cardiopulmonary bypass on vancomycin and netilmicin disposition. Antimicrob Agents Chemother 32:631–635. doi: 10.1128/AAC.32.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vuorisalo S, Pokela R, Syrjala H. 1997. Is single-dose antibiotic prophylaxis sufficient for coronary artery bypass surgery? An analysis of peri- and postoperative serum cefuroxime and vancomycin levels. J Hosp Infect 37:237–247. [DOI] [PubMed] [Google Scholar]
- 25.Cotogni P, Passera R, Barbero C, Gariboldi A, Moscato D, Izzo G, Rinaldi M. 2013. Intraoperative vancomycin pharmacokinetics in cardiac surgery with or without cardiopulmonary bypass. Ann Pharmacother 47:455–463. doi: 10.1345/aph.1R669. [DOI] [PubMed] [Google Scholar]
- 26.Kitzes-Cohen R, Farin D, Piva G, Ivry S, Sharony R, Amar R, Uretzky G. 2000. Pharmacokinetics of vancomycin administered as prophylaxis before cardiac surgery. Ther Drug Monit 22:661–667. doi: 10.1097/00007691-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Miglioli PA, Merlo F, Grabocka E, Padrini R. 1998. Effects of cardiopulmonary bypass on vancomycin plasma concentration decay. Pharmacol Res 38:275–278. doi: 10.1006/phrs.1998.0370. [DOI] [PubMed] [Google Scholar]
- 28.Ortega GM, Marti-Bonmati E, Guevara SJ, Gomez IG. 2003. Alteration of vancomycin pharmacokinetics during cardiopulmonary bypass in patients undergoing cardiac surgery. Am J Health Syst Pharm 60:260–265. [DOI] [PubMed] [Google Scholar]
- 29.Haley VB, Van Antwerpen C, Tsivitis M, Doughty D, Gase KA, Hazamy P, Tserenpuntsag B, Racz M, Yucel MR, McNutt LA, Stricof RL. 2012. Risk factors for coronary artery bypass graft chest surgical site infections in New York State, 2008. Am J Infect Control 40:22–28. doi: 10.1016/j.ajic.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Harrington G, Russo P, Spelman D, Borrell S, Watson K, Barr W, Martin R, Edmonds D, Cocks J, Greenbough J, Lowe J, Randle L, Castell J, Browne E, Bellis K, Aberline M. 2004. Surgical-site infection rates and risk factor analysis in coronary artery bypass graft surgery. Infect Control Hosp Epidemiol 25:472–476. doi: 10.1086/502424. [DOI] [PubMed] [Google Scholar]
- 31.Mannien J, Wille JC, Kloek JJ, van Benthem BH. 2011. Surveillance and epidemiology of surgical site infections after cardiothoracic surgery in The Netherlands, 2002-2007. J Thorac Cardiovasc Surg 141:899–904. doi: 10.1016/j.jtcvs.2010.09.047. [DOI] [PubMed] [Google Scholar]
- 32.Anderson DJ, Kaye KS, Chen LF, Schmader KE, Choi Y, Sloane R, Sexton DJ. 2009. Clinical and financial outcomes due to methicillin resistant Staphylococcus aureus surgical site infection: a multi-center matched outcomes study. PLoS One 4:e8305. doi: 10.1371/journal.pone.0008305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tom TS, Kruse MW, Reichman RT. 2009. Update: methicillin-resistant Staphylococcus aureus screening and decolonization in cardiac surgery. Ann Thorac Surg 88:695–702. doi: 10.1016/j.athoracsur.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Kreter B, Woods M. 1992. Antibiotic prophylaxis for cardiothoracic operations: meta-analysis of thirty years of clinical trials. J Thorac Cardiovasc Surg 104:590–599. [PubMed] [Google Scholar]
- 35.Dolton M, Xu H, Cheong E, Maitz P, Kennedy P, Gottlieb T, Buono E, McLachlan AJ. 2010. Vancomycin pharmacokinetics in patients with severe burn injuries. Burns 36:469–476. doi: 10.1016/j.burns.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Llopis-Salvia P, Jimenez-Torres NV. 2006. Population pharmacokinetic parameters of vancomycin in critically ill patients. J Clin Pharm Ther 31:447–454. doi: 10.1111/j.1365-2710.2006.00762.x. [DOI] [PubMed] [Google Scholar]
- 37.Mulla H, Pooboni S. 2005. Population pharmacokinetics of vancomycin in patients receiving extracorporeal membrane oxygenation. Br J Clin Pharmacol 60:265–275. doi: 10.1111/j.1365-2125.2005.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez JL, Dominguez AR, Lane JR, Anderson PO, Capparelli EV, Cornejo-Bravo JM. 2010. Population pharmacokinetics of vancomycin in adult and geriatric patients: comparison of eleven approaches. Int J Clin Pharmacol Ther 48:525–533. doi: 10.5414/CPP48525. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto M, Kuzuya T, Baba H, Yamada K, Nabeshima T. 2009. Population pharmacokinetic analysis of vancomycin in patients with gram-positive infections and the influence of infectious disease type. J Clin Pharm Ther 34:473–483. doi: 10.1111/j.1365-2710.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- 40.Yasuhara M, Iga T, Zenda H, Okumura K, Oguma T, Yano Y, Hori R. 1998. Population pharmacokinetics of vancomycin in Japanese adult patients. Ther Drug Monit 20:139–148. doi: 10.1097/00007691-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Salem AH, Zhanel GG, Ibrahim SA, Noreddin AM. 2014. Monte Carlo simulation analysis of ceftobiprole, dalbavancin, daptomycin, tigecycline, linezolid, and vancomycin pharmacodynamics against intensive care unit-isolated methicillin-resistant Staphylococcus aureus. Clin Exp Pharmacol Physiol 41:437–443. doi: 10.1111/1440-1681.12195. [DOI] [PubMed] [Google Scholar]
- 42.Hafermann MJ, Kiser TH, Lyda C, Fish DN, Barber GR, Wempe MF, Cleveland JC Jr. 2014. Weight-based versus set dosing of vancomycin for coronary artery bypass grafting or aortic valve surgery. J Thorac Cardiovasc Surg 147:1925–1930. doi: 10.1016/j.jtcvs.2013.12.037. [DOI] [PubMed] [Google Scholar]
- 43.Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. 2006. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med 166:2138–2144. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]
- 44.Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC Jr, Eliopoulos GM. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 42:2398–2402. doi: 10.1128/JCM.42.6.2398-2402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soriano A, Marco F, Martinez JA, Pisos E, Almela M, Dimova VP, Alamo D, Ortega M, Lopez J, Mensa J. 2008. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 46:193–200. doi: 10.1086/524667. [DOI] [PubMed] [Google Scholar]
- 46.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 47.Lavielle M, Mentre F. 2007. Estimation of population pharmacokinetic parameters of saquinavir in HIV patients with the MONOLIX software. J Pharmacokinet Pharmacodyn 34:229–249. doi: 10.1007/s10928-006-9043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lodise TP, Drusano GL, Zasowski E, Dihmess A, Lazariu V, Cosler L, McNutt LA. 2014. Vancomycin exposure in patients with methicillin-resistant Staphylococcus aureus bloodstream infections: how much is enough? Clin Infect Dis 59:666–675. doi: 10.1093/cid/ciu398. [DOI] [PubMed] [Google Scholar]
- 49.Dudley MN, Ambrose PG. 2000. Pharmacodynamics in the study of drug resistance and establishing in vitro susceptibility breakpoints: ready for prime time. Curr Opin Microbiol 3:515–521. doi: 10.1016/S1369-5274(00)00132-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.