ABSTRACT
Although guidelines recommend amikacin (AMK) inhalation therapy for difficult-to-treat nontuberculous mycobacterial lung disease (NTM-LD), data are limited regarding the safety and clinical efficacy of this salvage therapy. We retrospectively evaluated the treatment outcomes of 77 patients with refractory NTM-LD caused by Mycobacterium abscessus complex (MABC) or M. avium complex (MAC) who initiated AMK inhalation therapy between February 2015 and June 2016. MABC was the most common etiology (n = 48, 62%), followed by MAC (n = 20, 26%) and mixed infections (n = 9, 12%). Isolates with macrolide resistance and baseline AMK resistance were identified in 63 (82%) patients and 5 (6%) patients, respectively. At 12 months after AMK inhalation therapy, 49% of patients had symptomatic improvement, whereas 42% had radiological improvement. Conversion to a negative sputum culture occurred in 14 (18%) patients, and the culture conversion rate was higher in patients infected with macrolide-susceptible isolates (7/14, 50%) than in those infected with macrolide-resistant isolates (7/63, 11%) (P = 0.003). Significant decreases in sputum semiquantitative culture positivity occurred after AMK inhalation therapy (P < 0.001). On multivariate analysis, conversion to a negative sputum culture was associated with mixed infections (P = 0.009), a forced expiratory volume in 1 s of greater than 60% (P = 0.008), and the absence of macrolide resistance (P = 0.003). Thirty-eight percent of patients experienced adverse effects, with ototoxicity (n = 15) being the most common. AMK inhalation salvage therapy may improve the treatment responses in some patients with refractory NTM-LD. However, considering the common adverse effects, further evaluation of the optimal dosage and intervals for AMK inhalation therapy is needed.
KEYWORDS: nontuberculous mycobacteria, Mycobacterium avium complex, Mycobacterium abscessus, amikacin, inhalation
INTRODUCTION
Nontuberculous mycobacterial (NTM) lung disease (NTM-LD) is a chronic progressive disease caused by various NTM species. The prevalence and burden of NTM-LD are increasing worldwide (1–3). The current guidelines for the treatment of NTM-LD recommend long-term, multidrug therapy (4–6); however, the treatment success rates are unsatisfactory (7–9). In pulmonary disease caused by Mycobacterium avium complex (MAC), the most common etiologic organism of NTM-LD, the overall sputum culture conversion rate is approximately 50 to 60% (7, 8). Moreover, M. abscessus complex (MABC) lung disease (MABC-LD) has sometimes been considered chronic and incurable, given the lack of effective drugs, based on in vitro antibiotic susceptibilities (9).
In patients with cavitary or extensive MAC-LD and those with MABC-LD, guidelines recommend the use of injectable aminoglycoside antibiotics, such as amikacin (AMK), in addition to the standard oral regimens (4–6). However, long-term systemic administration of AMK is often limited by serious adverse effects, such as auditory or renal toxicity (10, 11). Therefore, aerosolized antibiotic inhalation therapy has recently been applied in efforts to maximize drug delivery to the lungs and limit the potential systemic adverse effects. Such drug delivery systems are considered to improve the tolerability of long-term antibiotic therapy and clinical outcomes (12).
Recent case series reported the potential role of AMK inhalation therapy for NTM-LD (13–16), and current guidelines recommend its use in patients with refractory MAC- and MABC-LD (5, 6). However, data regarding the clinical efficacy and adverse effects of AMK inhalation in the treatment of NTM-LD are still very limited. Therefore, the purpose of this study was to evaluate the treatment outcomes and safety of salvage therapy with AMK inhalation in patients with refractory NTM-LD caused by MAC or MABC, the two major NTM pathogens.
RESULTS
Patient characteristics.
The baseline characteristics of the 77 patients at the time of initiation of AMK inhalation therapy are shown in Table 1. The median age was 60 years (interquartile range [IQR], 53 to 69 years), and 69% of patients were female. Fifty-seven (74%) patients had undergone previous treatment for pulmonary tuberculosis. Seventeen (22%) patients had chronic cavitary pulmonary aspergillosis, and most of these patients (16/17) had received itraconazole therapy. Nine (12%) patients had chronic obstructive pulmonary disease.
TABLE 1.
Baseline characteristics at time of initiation of AMK inhalation therapya
| Clinical characteristics | Value(s) |
|---|---|
| Median (IQR) age (yr) | 60 (53–69) |
| No. (%) of female patients | 53 (69) |
| Median (IQR) body mass index (kg/m2) | 19.8 (18.1–21.8) |
| No. (%) of nonsmokers | 60 (78) |
| No. (%) of patients with the following underlying lung disease: | |
| Previous pulmonary tuberculosis | 57 (74) |
| Chronic cavitary pulmonary aspergillosis | 17 (22) |
| Chronic obstructive pulmonary disease | 9 (12) |
| No. (%) of patients in whom the etiologic organism was: | |
| M. abscessus complex | 48 (62) |
| M. abscessus | 35 |
| M. massiliense | 12 |
| M. bolletii | 1 |
| M. avium complex | 20 (26) |
| M. intracellulare | 14 |
| M. avium | 6 |
| Mixture of bacteria | 9 (12) |
| No. (%) of patients with the following radiological findings: | |
| Nodular bronchiectatic form | 47 (61) |
| With cavity | 25 |
| Without cavity | 22 |
| Fibrocavitary form | 30 (39) |
| Laboratory findings | |
| No. (%) of patients with acid-fast bacillus smear-positive sputum | 51 (66) |
| Median (IQR) albumin concn (g/dl) | 3.8 (3.5–4.1) |
| Median (IQR) C-reactive protein concn (mg/dl) | 1.4 (0.4–3.6) |
| Median (IQR) erythrocyte sedimentation rate (mm/h) | 49 (34–90) |
| Median (IQR) forced expiratory vol in 1 s (%) | 63 (46–75) |
| No. (%) of patients whose isolates had: | |
| Macrolide resistance | 63 (82) |
| Amikacin resistance | 5 (7) |
Data are for a total of 77 patients. Abbreviations: AMK, amikacin; IQR, interquartile range.
MABC was the most common etiologic organism (n = 48 [62%]; 35 cases of M. abscessus infection, 12 cases of M. massiliense infection, and 1 case of M. bolletii infection), followed by MAC (n = 20 [26%]; 14 cases of M. intracellulare infection and 6 cases of M. avium infection) and a mixture of bacteria (n = 9 [12%]; 3 cases of M. abscessus with M. intracellulare, 3 cases of M. abscessus with M. avium, 2 cases of M. massiliense with M. avium, and 1 case of M. abscessus with M. massiliense). High-resolution computed tomography (HRCT) scans of the chest revealed that 47 (61%) patients had the nodular bronchiectatic form of NTM-LD, whereas 30 (39%) had the fibrocavitary form. Cavitary lesions were identified in 55 (71%) patients. Sputum specimens with positive acid-fast bacillus (AFB) smears were identified in 66% of patients. On pulmonary function testing, the median percentage of predicted forced expiratory volume in 1 s (FEV1) was 63% (IQR, 46 to 75%).
Macrolide resistance was identified in 63 (82%) patients at the time of initiation of AMK inhalation therapy. Among the 48 MABC patients, 29 M. abscessus isolates and 1 M. bolletii isolate had inducible resistance to macrolides, whereas 6 M. abscessus isolates and 6 M. massiliense isolates were resistant to macrolides. Among the 20 MAC patients, 8 M. intracellulare isolates and 6 M. avium isolates had macrolide resistance. Among the patients with mixed infections, the isolates from seven of nine patients had macrolide resistance: two patients infected with M. intracellulare and inducibly resistant M. abscessus, three patients infected with M. avium and inducibly resistant M. abscessus, one patient infected with M. avium and resistant M. massiliense, and one patient infected with M. massiliense and resistant M. abscessus. AMK resistance was identified in five (6%) patients at the time of initiation of AMK inhalation therapy: two patients infected with M. abscessus, one patient infected with M. massiliense, one patient infected with M. avium, and one patient with a mixed infection with M. abscessus and AMK-resistant M. intracellulare.
Antibiotic treatment regimens.
The antibiotic treatment regimens before and after AMK inhalation therapy are shown in Table 2. All patients who received salvage treatment with AMK inhalation had persistent positive cultures after previous antibiotic therapy for a median of 37.8 months (IQR, 15.7 to 61.4 months). Sixty-eight (88%) patients had previously received injectable aminoglycoside therapy, in the form of intravenous injection of AMK for MABC-infected patients and intramuscular injection of streptomycin or AMK for MAC-infected patients, for a median of 2.0 months (IQR, 1.0 to 4.7 months) before AMK inhalation therapy.
TABLE 2.
Antibiotic treatment regimensa
| Antibiotic | Value(s) for patients with the following infection: |
||
|---|---|---|---|
| MABC (n = 48) | MAC (n = 20) | Mixed (n = 9) | |
| No. (%) of patients receiving the following before amikacin inhalation therapy: | |||
| Macrolide | 48 (100) | 20 (100) | 9 (100) |
| Ethambutol | 0 | 20 (100) | 8 (89) |
| Rifampin | 0 | 20 (100) | 8 (89) |
| Clofazimine | 33 (69) | 5 (25) | 7 (78) |
| Moxifloxacin | 19 (40) | 12 (60) | 3 (33) |
| Imipenem | 34 (71) | 0 (0) | 8 (89) |
| Cefoxitin | 14 (29) | 0 (0) | 1 (11) |
| Amikacin or streptomycin injection | 48 (100) | 15 (75) | 5 (56) |
| Median (IQR) total treatment duration (mo) | 39.5 (15.8–84.2) | 38.5 (14.8–50.4) | 25.9 (17.5–47.1) |
| No. (%) of patients receiving the following after amikacin inhalation therapy: | |||
| Macrolide | 48 (100) | 20 (100) | 9 (100) |
| Ethambutol | 0 | 18 (90) | 8 (89) |
| Rifampin | 0 | 13 (65) | 4 (44) |
| Clofazimine | 43 (90) | 16 (80) | 8 (89) |
| Moxifloxacin | 0 | 1 (8) | 1 (11) |
| Amikacin inhalation | 48 (100) | 20 (100) | 9 (100) |
| Median (IQR) duration of amikacin inhalation therapy (mo) | 12.0 (6.8–12.0) | 12.0 (5.3–12.0) | 12.0 (12.0–12.0) |
Abbreviations: MABC, M. abscessus complex; MAC, M. avium complex; IQR, interquartile range.
After starting AMK inhalation therapy, all MABC-infected patients continued therapy with an oral macrolide, and 43 (90%) patients also received clofazimine. In patients with MAC-LD, all continued an oral macrolide, and most also continued companion drugs, such as ethambutol (n = 18, 90%), rifampin (n = 13, 65%), or clofazimine (n = 16, 80%). In the mixed infection group, all patients continued an oral macrolide as well as other drugs, such as ethambutol (n = 8, 89%), rifampin (n = 4, 44%), and clofazimine (n = 8, 89%). Overall, the median duration of AMK inhalation therapy during the study period was 12.0 months (IQR, 6.8 to 12.0 months).
Treatment response evaluation 12 months after initiating AMK inhalation therapy.
The treatment responses were evaluated 12 months after initiating AMK inhalation therapy (Table 3). There was overall symptomatic improvement in 49% of patients (MABC-infected patients, 44%; MAC-infected patients, 55%; patients with mixed infection, 67%; P = 0.242). The radiological response was evaluated using HRCT in 65 patients and chest X ray in the remaining 12 patients. There was overall radiological improvement in 42% of patients (MABC-infected patients, 33%; MAC-infected patients, 50%; patients with mixed infection, 67%; P = 0.154).
TABLE 3.
Treatment response 12 months after initiating amikacin inhalation therapya
| Response | Value(s) for patients with the following infection: |
P value | |||
|---|---|---|---|---|---|
| Total (n = 77) | MABC (n = 48) | MAC (n = 20) | Mixed (n = 9) | ||
| No. (%) of patients with the following symptomatic responses: | 0.242 | ||||
| Improved | 38 (49) | 21 (44) | 11 (55) | 6 (67) | |
| Unchanged | 14 (18) | 11 (23) | 1 (5) | 2 (22) | |
| Worsened | 25 (33) | 16 (33) | 8 (40) | 1 (11) | |
| No. (%) of patients with the following radiological responses: | 0.154 | ||||
| Improved | 32 (42) | 16 (33) | 10 (50) | 6 (67) | |
| Unchanged | 12 (15) | 11 (23) | 1 (5) | 0 (0) | |
| Worsened | 33 (43) | 21 (44) | 9 (45) | 3 (33) | |
| No. (%) of patients with at least one negative culture | 26 (34) | 14 (29) | 5 (25) | 7 (78) | 0.015 |
| No. (%) of patients with sputum culture conversion | 14 (18) | 6 (13) | 3 (15) | 5 (56) | 0.019 |
| Median (IQR) time to culture conversion (mo) | 3.1 (1.3–7.3) | 5.4 (2.1–9.9) | 3.4 (1.4–5.1) | 2.3 (1.0–4.5) | 0.365 |
| No. (%) of patients lost to follow-up | 3 (4) | 3 (6) | 0 | 0 | NA |
| No. (%) of patients who died | 10 (13) | 7 (15) | 3 (15) | 0 | 0.624 |
| Median (IQR) follow-up period (mo) | 12.0 (12.0–12.0) | 12.0 (12.0–12.0) | 12.0 (12.0–12.1) | 12.0 (12.0–12.0) | 0.396 |
Abbreviations: MABC, M. abscessus complex; MAC, M. avium complex; NA, not available; IQR, interquartile range.
Twenty-six (34%) patients had at least one negative culture, and conversion to a negative sputum culture occurred in 14 (18%) patients during the study period. The rates for at least one negative culture (P = 0.015) and conversion to a negative culture (P = 0.019) were different among the three groups (Table 3). With regard to macrolide resistance, the culture conversion rate was significantly higher in patients infected with macrolide-susceptible isolates (7/14, 50%) than it was in patients infected with macrolide-resistant isolates (7/63, 11%, P = 0.003). The culture conversion rate was also higher in patients infected with AMK-susceptible isolates (14/72, 19%) than it was in patients infected with AMK-resistant isolates (0/5, 0%). However, this finding was not statistically significant (P = 0.578). The median time to conversion to a negative sputum culture was 3.1 months (IQR, 1.3 to 7.3 months) (Fig. 1). During the study period, 64 patients had follow-up data on the results of testing for susceptibility to AMK within 12 months after the initiation of AMK inhalation therapy, and an AMK-resistant strain was identified in only 1 of these patients. This patient had a mixed infection with M. massiliense and M. avium, and AMK resistance was detected in the M. avium strain after 6 months of AMK inhalation therapy. This patient subsequently failed culture conversion.
FIG 1.
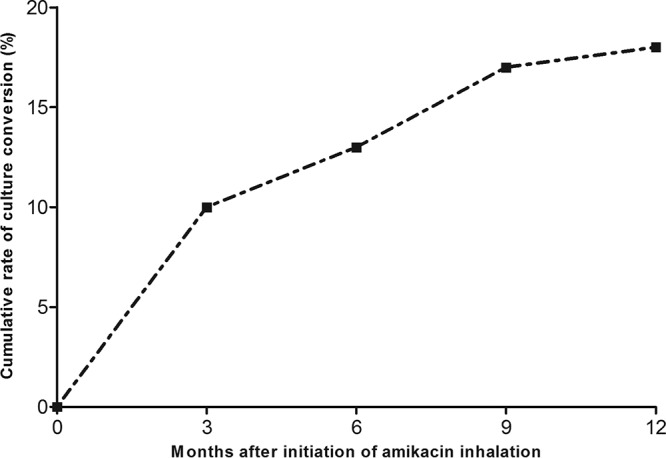
Cumulative sputum culture conversion rates after initiation of amikacin inhalation therapy.
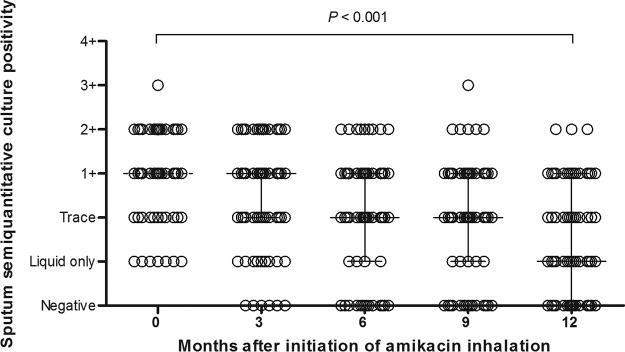
According to the culture results on solid medium, 59 (77%) patients had ≥1+ AFB culture positivity at the time of initiation of AMK inhalation therapy. Twenty (26%) patients had ≥1+ AFB culture positivity after 12 months of AMK inhalation therapy. There were significant decreases in sputum semiquantitative culture positivity after AMK inhalation therapy (P < 0.001) (Fig. 2).
FIG 2.
Trends in serial changes in semiquantitative sputum culture positivity for acid-fast bacilli after amikacin inhalation therapy during 12 months determined using generalized estimating equations. Vertical bars indicate interquartile ranges.
During the study period, three (4%) patients were lost to follow-up after a median of 3.6 months of AMK inhalation therapy. Ten (13%) patients died due to the progression of NTM-LD (n = 7) or pneumonia (n = 3), and nine of these patients died within 12 months after the initiation of AMK inhalation therapy, whereas the remaining patient died after 19.0 months of AMK inhalation therapy. There were no differences in the death rates according to the etiologic organisms (P = 0.624).
Adverse effects associated with amikacin inhalation therapy.
Of the 77 patients in this study, 76 patients initially received 500-mg daily AMK inhalation therapy. Of these 76 patients, 27 (35%) patients maintained daily AMK inhalation therapy for 12 months without adverse effects, whereas 29 (38%) patients experienced adverse effects associated with AMK inhalation therapy (Table 4). Additionally, 21 (27%) of these patients discontinued AMK inhalation therapy after 6.4 months (IQR, 3.4 to 9.9 months), and 8 (10%) patients had a change in the AMK dosage from 500 mg once daily to 500 mg three times weekly after 6.9 months (IQR, 1.7 to 8.0 months) of AMK inhalation therapy and maintained this dosage without adverse effects.
TABLE 4.
Adverse effects associated with amikacin inhalation-containing regimens
| Adverse effect | No. (%) of patients with: |
||
|---|---|---|---|
| Discontinuation | Regimen changea | Total | |
| Total | 21 (27) | 8 (10) | 29 (38) |
| Ototoxicity | 11 (14) | 4 (6) | 15 (19) |
| Fatigue | 6 (8) | 1 (1) | 7 (9) |
| Tinnitus | 3 (4) | 1 (1) | 4 (5) |
| Cough | 1 (1) | 1 (1) | 2 (3) |
| Hoarseness | 0 | 1 (1) | 1 (1) |
| Nephrotoxicity | 0 | 0 | 0 |
Change of amikacin inhalation from 500 mg once daily to 500 mg three times weekly.
Starting in September 2016, our AMK inhalation treatment protocol was changed from 500 mg once daily to 500 mg three times weekly. On the basis of this protocol change, 20 (26%) of the 76 patients changed from daily AMK inhalation to three-times-weekly AMK inhalation after 8.9 months (IQR, 4.8 to 9.8 months) without adverse effects. Out of the total number of 77 study patients, only 1 (1%) patient was treated with 500-mg three-times-weekly AMK inhalation at the outset of therapy as a consequence of the protocol change. The most common reason for therapy discontinuation or a regimen change was ototoxicity (n = 15 patients), followed by fatigue (n = 7), tinnitus (n = 4), and cough (n = 2) (Table 4). All 29 patients who had adverse effects were initially treated with 500-mg once-daily AMK inhalation. However, after the protocol change at the Samsung Medical Center, no additional patients experienced adverse effects with AMK inhalation therapy.
Factors associated with conversion to a negative culture.
We also evaluated the factors that were associated with conversion to a negative sputum culture. On univariate analysis, a mixed organism infection, an FEV1 of >60%, and the absence of macrolide resistance were associated with conversion to a negative sputum culture. On multivariate analysis, a mixed organism infection (P = 0.015), an FEV1 of >60% (P = 0.014), and no macrolide resistance (P = 0.002) retained statistical significance (Table 5).
TABLE 5.
Univariate and multivariable analyses of factors associated with culture-negative conversiona
| Variable | No. (%) of patients with: |
Univariate analysis |
Multivariable analysis |
|||
|---|---|---|---|---|---|---|
| Culture conversion (n = 14) | No culture conversion (n = 63) | OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
| Age | 8 (57) | 31 (49) | 1.38 (0.43–4.43) | 0.592 | ||
| Female | 10 (71) | 43 (68) | 1.16 (0.32–4.16) | 0.817 | ||
| No previous pulmonary tuberculosis | 6 (43) | 14 (22) | 2.63 (0.78–8.84) | 0.119 | ||
| Etiologic organism | ||||||
| M. abscessus complex | 6 (43) | 42 (67) | Reference | |||
| M. avium complex | 3 (21) | 17 (27) | 1.24 (0.28–5.51) | 0.782 | ||
| Mixed infection | 5 (36) | 4 (6) | 8.75 (1.82–42.00) | 0.007 | 17.79 (2.06–153.86) | 0.009 |
| Albumin concn > 3.5 g/dl | 12 (86) | 42 (67) | 3.00 (0.61–14.65) | 0.175 | ||
| FEV1 > 60% | 12 (86) | 29 (46) | 7.03 (1.45–34.04) | 0.015 | 28.67 (2.42–339.16) | 0.008 |
| Sputum smear negative for AFB | 7 (50) | 19 (30) | 2.32 (0.71–7.52) | 0.162 | ||
| No cavity | 5 (36) | 17 (27) | 1.50 (0.44–5.13) | 0.515 | ||
| No macrolide resistance | 7 (50) | 7 (11) | 8.00 (2.16–29.64) | 0.002 | 20.51 (2.75–52.86) | 0.003 |
| AMK inhalation dosage | ||||||
| 500 mg daily → stopped (adverse effects) | 2 (14) | 19 (30) | Reference | |||
| 500 mg daily → TIW or initial TIW | 9 (64) | 20 (32) | 4.28 (0.82–22.39) | 0.086 | ||
| 500-mg daily maintenance | 3 (21) | 24 (38) | 1.19 (0.18–7.84) | 0.858 | ||
Abbreviations: OR, odds ratio; CI, confidence interval; FEV1, forced expiratory volume in 1 s; AFB, acid-fast bacilli; TIW, three times weekly.
DISCUSSION
In this study, we evaluated the efficacy and safety of salvage therapy with AMK inhalation in 77 refractory NTM-LD patients. To the best of our knowledge, this is the largest case series of treatment-refractory NTM-infected patients treated with AMK inhalation. One of the most notable findings in this study was that approximately half of all patients exhibited clinicoradiological improvement, while approximately 18% (14/77) achieved conversion to a negative sputum culture. We also found a significant decrease in sputum AFB semiquantitative culture positivity in patients with refractory NTM-LD after AMK inhalation therapy. These findings suggest that adjunctive AMK inhalation therapy may help to achieve symptomatic and radiographic improvement and decrease the mycobacterial burden. Considering that only limited data regarding the benefits of AMK inhalation therapy in NTM-LD patients are available (13–16), our results are important in the determination of appropriate therapeutic strategies for refractory NTM-LD.
Four prior case series described AMK inhalation therapy for refractory NTM-LD (Table 6) (13–16). The sputum culture conversion rate in our study (18%) seemed to be lower than that observed in two of those previous studies (13, 16). Davis et al. found that four of six (67%) patients with refractory MAC-LD became culture negative after 6 months of AMK inhalation therapy (13), and Yagi et al. recently found that 10 of 23 patients (43%) with refractory NTM-LD achieved sputum culture conversion after AMK inhalation therapy (16). However, our sputum culture conversion rate was comparable to that in the report by Olivier et al., who showed a culture conversion rate of 25% in 20 patients with refractory NTM-LD (15). Interestingly, a recently published prospective study evaluating the clinical efficacy of liposomal AMK for inhalation (a novel AMK formulation) reported a slightly higher culture conversion rate (32%) in 44 patients with refractory NTM-LD (17).
TABLE 6.
Amikacin inhalation therapy for refractory nontuberculous mycobacterial lung disease in previous case seriesa
| Treatment and author(s), year (reference) | No. of patients | Organism (no. of patients) | Median (IQR) treatment duration before AMK inhalation (mo) | % of patients with macrolide- resistant isolates (no. of patients with macrolide-resistant isolates/total no. of patients) | Median (IQR) duration of AMK inhalation (mo) | % of patients intolerant of AMK inhalation (no. of patients with intolerance/total no. of patients) | % of patients with sputum conversion (no. of patients with sputum conversion/total no. of patients) |
|---|---|---|---|---|---|---|---|
| Amikacin inhalation | |||||||
| Davis et al., 2007 (13) | 6 | MAC (6) | 24.5 (9.1–60.0) | 0 (0/6) | 11.0 (7.0–25.0) | 33 (2/6) | 67 (4/6) |
| Safdar, 2012 (14) | 9 | MAC (2) | ≥3.0 | NA | 2.5 (1.5–5.9) | 11 (1/9) | NAb |
| MABC (3) | |||||||
| M. kansasii (3) | |||||||
| MAC + MABC (1) | |||||||
| Olivier et al., 2014 (15) | 20 | MAC (5) | 60.0 | 75 (15/20) | 19.0 | 35 (7/20) | 25 (5/20) |
| MABC (15) | 6.0–190.0c | 1.0–50.0c | |||||
| Yagi et al., 2017 (16) | 26 | MAC (23) | 61.0 (28.0–107.0) | 39 (9/23) | 7.0 (6.0–12.3) | 8 (2/26) | 43 (10/23)d |
| MABC (3) | |||||||
| This study | 77 | MAC (20) | 37.8 (15.7–61.4) | 82 (63/77) | 12.0 (6.8–12.0) | 38 (29/77) | 18 (14/77) |
| MABC (48) | |||||||
| MAC + MABC (9) | |||||||
| Liposomal amikacin inhalation, Olivier et al., 2017 (17) | 44 | MAC (29) | ≥12.0 | 18 (8/44) | ≥6.0 | 18 (8/44) | 32 (14/44) |
| MABC (15) |
Abbreviations: AMK, amikacin; MAC, M. avium complex; MABC, M. abscessus complex; IQR, interquartile range; NA, not available.
All patients demonstrated complete (n = 8) or partial (n = 1) clinicoradiographic responses.
Values are ranges.
Sputum conversion could not be evaluated in three patients.
These discrepancies may be explained by the fact that relatively small numbers of patients were analyzed in previous studies, and the characteristics of the cohorts varied. In our study, 55 (71%) patients had MABC infection or mixed infection caused by MABC and MAC. This high proportion of patients with MABC infection was similar to that in the study by Olivier et al. (15). MABC-LD is the most difficult-to-treat NTM-LD because of the intrinsic resistance of MABC to many antibiotics (18–21). In addition, two-thirds of our patients (52/77, 68%) had been treated for longer than 24 months prior to initiation of AMK inhalation therapy, and nine patients died within 12 months after initiation of AMK inhalation therapy. These findings suggest that a significant proportion of our patients already had extensive disease, which may have contributed to the relatively low sputum culture conversion rate. The rate of macrolide resistance was also much higher (82%) in our study than in the studies by Davis et al. (13) and Yagi et al. (16), although our resistance rate was comparable to that reported by Olivier et al. (15). In our study population, a relatively higher culture conversion rate was observed in the mixed infection group. Five of nine (56%) patients with mixed infection achieved conversion after adding AMK inhalation therapy (three cases of infection with M. abscessus with M. avium, one case of infection with M. massiliense with M. avium, and one case of infection with M. abscessus with M. intracellulare). None of these five patients had cavitary lesions, and three patients with mixed infections had negative AFB smears at the time of initiation of AMK inhalation therapy. These characteristics could have contributed to the high sputum culture conversion rate in patients with mixed infections in our study.
The culture conversion rate in this study for patients infected with macrolide-resistant isolates was only 11%, a rate significantly lower than that for patients infected with macrolide-susceptible isolates (47%). Additionally, macrolide resistance was associated with persistent positive cultures after AMK inhalation therapy. Macrolides are the cornerstone drugs in the treatment of NTM-LD (4–6). Therefore, patients with macrolide-resistant MAC-LD (22–25) or macrolide-resistant MABC-LD, including patients with lung disease caused by both isolates with intrinsic resistance and isolates with acquired resistance (26–30), have a very poor prognosis. In our study, salvage therapy with AMK inhalation was added to the existing (failing) regimens in all patients. The majority of patients had already received recently repurposed medications, such as clofazimine (31, 32). Moreover, no patients infected with AMK-resistant isolates achieved culture conversion after AMK inhalation therapy in the present study, which is consistent with the findings of a recent study using liposomal AMK inhalation therapy in patients with refractory NTM-LD (17). Therefore, the clinical efficacy of AMK inhalation therapy in patients with AMK-resistant NTM-LD could be considered limited on the basis of these results. However, the clinical efficacy of AMK inhalation therapy combined with other drugs for the initial treatment of macrolide-resistant NTM-LD must be evaluated further.
Aerosolized antibiotic delivery was initially shown to be beneficial for cystic fibrosis patients with chronic Pseudomonas infection (33–35). Subsequently, inhalation antibiotic therapy was used to treat various respiratory diseases, including bronchiectasis (12) and nosocomial bacterial pneumonia (36). A major advantage to aerosolized antibiotic delivery is that drug delivery is maximized at the target site, allowing higher drug concentrations within the macrophage, the site of mycobacterial replication, without introducing systemic adverse effects. However, it is unknown whether aerosolized antibiotics are effectively delivered or absorbed at the target sites of patients with underlying structural lung damage due to long-standing refractory NTM-LD. A previous study found a rapid decline in lung function in patients with NTM-LD who did not respond to antibiotic therapy (37). In this context, our finding that a low FEV1 was significantly associated with a failure of culture conversion may have clinical implications. AMK inhalation therapy might be effective in patients with less advanced NTM-LD and more preserved lung function. This finding may also help to predict the treatment outcomes of adjunctive AMK inhalation therapy in patients with NTM-LD. Regardless, further studies are needed to substantiate these findings.
In our study, more than one-third of patients discontinued the AMK inhalation therapy or had their dosage reduced due to adverse effects. These findings are comparable to those of Olivier et al., who found that 7 out of 20 (35%) patients stopped AMK inhalation therapy because of various adverse effects, including ototoxicity (15). However, other studies that used similar AMK doses (15 mg/kg of body weight/day) reported more tolerable adverse effect profiles. Yagi et al. found that only 2 of 26 patients discontinued AMK inhalation therapy within 1 month of starting the inhalation therapy (16). Similarly, Davis et al. reported that only one of six patients with MAC-LD was unable to tolerate prolonged AMK inhalation therapy (13). In a recent study evaluating the efficacy of liposomal AMK inhalation therapy in patients with refractory NTM-LD, serious adverse events, including exacerbation of bronchiectasis or pneumonia, were reported in up to 18% of patients (17). These findings suggest that the development of AMK-associated adverse effects may be affected not only by drug pharmacokinetics/dynamics but also by intrinsic host factors. However, to date, there are no guidelines regarding the optimum dose of AMK in AMK inhalation therapy for the treatment of NTM-LD. Therefore, further studies are needed to clarify optimal dosing regimens.
This study has several limitations. For example, its retrospective observational nature introduces potential biases. In addition, given the retrospective design, the assessment of symptomatic and radiographic improvement was not evaluated using objective measures. A third limitation is that, because sputum specimens were not collected monthly, it was not possible to report the accurate time to sputum culture conversion. Finally, the long-term clinical responses and safety of three-times-weekly AMK inhalation therapy could not be fully evaluated because the follow-up duration was relatively short.
In conclusion, salvage therapy with AMK inhalation may help to achieve symptomatic and radiographic improvement and to decrease the mycobacterial disease burden in patients with refractory NTM-LD. Considering its relatively common adverse effects, however, further evaluation regarding the optimal dosage and timing of AMK inhalation therapy is needed.
MATERIALS AND METHODS
Study population.
AMK inhalation therapy was introduced for the treatment of NTM-LD at the Samsung Medical Center in February 2015. We identified consecutive patients who initiated salvage therapy with AMK inhalation for refractory MAC- or MABC-LD between February 2015 and June 2016 using the database of the NTM Registry of the Samsung Medical Center (a 1,979-bed referral hospital in Seoul, South Korea). All study patients met the diagnostic criteria for NTM-LD (4). Refractory NTM-LD was defined as persistent positive sputum cultures after at least 6 months of multidrug treatment. All patients received standardized multidrug treatment according to the etiologic organisms, as reported previously (26, 38–42). None of the included patients had previously received AMK inhalation therapy. AMK inhalation therapy was added to each patient's respective, existing treatment regimen.
This retrospective study was approved by the Institutional Review Board (IRB) of the Samsung Medical Center (IRB no. 2017-06-018). Patient information was anonymized and deidentified prior to analysis. Therefore, informed consent was waived.
Amikacin inhalation protocol.
Commercially available intravenous AMK sulfate solution (250 mg/ml) was diluted with 2 ml of saline and applied using a compressor nebulizer (model NE-C28; Omron Colin Co., Ltd., Tokyo, Japan). Patients were initially treated with 250-mg AMK inhalation therapy once daily in February 2015. In the absence of adverse effects, the dose was uptitrated to 500 mg once daily 2 to 4 weeks later. However, after several patients in our study developed adverse effects (such as ototoxicity), our protocol was changed from 500 mg once daily to 500 mg three times weekly in September 2016.
Patients were followed up at 1, 3, and 6 months after the initiation of AMK inhalation therapy. They were then seen at 3-month intervals at an outpatient clinic with laboratory and audiometry examinations. Nephrotoxicity was defined as an increase in serum creatinine levels of ≥0.5 mg/dl from those at the baseline (11). Ototoxicity was defined by any of the following criteria: hearing loss of at least 10 dB at two or more consecutive frequencies, hearing loss of at least 20 dB at one isolated frequency, or loss of a response at any of three consecutive frequencies on a posttreatment audiogram compared with that on the baseline audiogram in either ear at any frequency (250, 500, 1,000, 2,000, 4,000, or 8,000 Hz), as previously described (11).
Radiological and microbiological evaluation.
Either a chest X ray or a high-resolution computed tomography (HRCT) image was available at the time of initial AMK inhalation therapy and during follow-up. The fibrocavitary form of NTM-LD was defined by the presence of cavitary opacities predominantly in the upper lobes. The nodular bronchiectatic form was defined by the presence of multifocal bronchiectasis and clusters of small nodules, regardless of the presence of small cavities in the lungs (26, 40, 42).
Sputum acid-fast bacillus (AFB) smears and cultures were performed using standard methods (43). All specimens were cultured both on 3% Ogawa solid medium (Shinyang, Seoul, South Korea) and in liquid broth medium in mycobacterial growth indicator tubes (MGIT; Becton, Dickinson and Co., Sparks, MD, USA). For semiquantitative culture analysis, each culture was scored using a slight modification of methods described in previous reports (31, 44) as follows: (i) negative, no growth in liquid or solid medium; (ii) liquid only, growth in liquid medium only; (iii) trace, growth of <50 colonies on solid medium; (iv) 1+, growth of 50 to 100 colonies on solid medium; (v) 2+, growth of 100 to 200 colonies on solid medium; (vi) 3+, growth of 200 to 500 colonies on solid medium; and (vii) 4+, growth of >500 colonies on solid medium.
NTM species were identified using a reverse blot hybridization assay of the rpoB gene (26, 40, 42). Drug susceptibility testing was performed using the broth microdilution method (45). MAC isolates with a MIC of ≥32 μg/ml for clarithromycin were considered resistant to macrolides. In MABC isolates, the MIC of clarithromycin was determined on days 3 and 14 after incubation. The isolates were considered clarithromycin susceptible if the MIC was ≤2 μg/ml at days 3 and 14, clarithromycin resistant if the MIC was ≥8 μg/ml at day 3, or inducibly clarithromycin resistant if they were susceptible at day 3 but resistant at day 14 (45). Isolates were considered macrolide resistant if they were resistant or inducibly resistant to clarithromycin in this study. Isolates of both MAC and MABC with an MIC of AMK of ≤16 μg/ml were considered susceptible, and those with an MIC of ≥64 μg/ml were considered resistant to AMK (45, 46). A mixed infection was considered resistant to a macrolide or AMK when at least one of the strains was resistant to the drug.
Evaluation of treatment response.
The treatment response was assessed at 12 months after starting AMK inhalation therapy, and for patients who were treated with AMK inhalation therapy for less than 12 months, the data obtained at the last follow-up date were included in the analysis. The symptomatic responses during the study period were determined by the attending physician without the use of objective standardized methods. The radiological response was evaluated by comparing the initial and follow-up chest X rays or HRCT images. The images were reviewed by two of the authors (B.Y., W.-J.K.), between whom consensus was obtained for classifying the findings as “improved,” “unchanged,” or “worse.” Conversion to a negative sputum culture was defined as three consecutive negative cultures. The time of conversion was defined as the date of the first negative culture (26, 40, 42).
Statistical analysis.
All data are presented as medians and interquartile ranges (IQRs) for continuous variables and as numbers (percentages) for categorical variables. Data were compared using the Mann-Whitney U test for continuous variables and the Pearson χ2 test or Fisher's exact test for categorical variables. Trends in serial changes in AFB culture positivity were analyzed with generalized estimating equations. In order to assess the associated factors for culture conversion, univariate and multivariate analyses with a logistic regression were performed by including variables that had a P value of <0.20 on univariate analysis in the regression. All analyses were performed using SAS (version 9.4) software (SAS Institute, Cary, NC, USA). Two-sided P values of <0.05 were considered statistically significant for all analyses.
ACKNOWLEDGMENTS
Charles L. Daley has received grants from Insmed, Inc. However, this relationship is not associated with the submitted work. Hye Yun Park has received lecture fees from AstraZeneca, Novartis, and Boehringer-Ingelheim; however, these are not associated with the submitted work. We have no other conflicts of interest to declare.
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, and Future Planning (NRF-2015R1A2A1A01003959). Additional support was provided by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI15C2778).
The sponsors had no role in the study design, data collection and analysis, or manuscript preparation.
REFERENCES
- 1.Stout JE, Koh WJ, Yew WW. 2016. Update on pulmonary disease due to non-tuberculous mycobacteria. Int J Infect Dis 45:123–134. doi: 10.1016/j.ijid.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Morimoto K, Hasegawa N, Izumi K, Namkoong H, Uchimura K, Yoshiyama T, Hoshino Y, Kurashima A, Sokunaga J, Shibuya S, Shimojima M, Ato M, Mitarai S. 2017. A laboratory-based analysis of nontuberculous mycobacterial lung disease in Japan from 2012 to 2013. Ann Am Thorac Soc 14:49–56. doi: 10.1513/AnnalsATS.201607-573OC. [DOI] [PubMed] [Google Scholar]
- 3.Diel R, Jacob J, Lampenius N, Loebinger M, Nienhaus A, Rabe KF, Ringshausen FC. 2017. Burden of non-tuberculous mycobacterial pulmonary disease in Germany. Eur Respir J 49:1602109. doi: 10.1183/13993003.02109-2016. [DOI] [PubMed] [Google Scholar]
- 4.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 5.Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, Leitch A, Loebinger MR, Milburn HJ, Nightingale M, Ormerod P, Shingadia D, Smith D, Whitehead N, Wilson R, Floto RA. 2017. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 72(Suppl 2):ii1–ii64. doi: 10.1136/thoraxjnl-2017-210927. [DOI] [PubMed] [Google Scholar]
- 6.Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann JL, Nick JA, Noone PG, Bilton D, Corris P, Gibson RL, Hempstead SE, Koetz K, Sabadosa KA, Sermet-Gaudelus I, Smyth AR, van Ingen J, Wallace RJ, Winthrop KL, Marshall BC, Haworth CS. 2016. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 71(Suppl 1):i1–i22. doi: 10.1136/thoraxjnl-2015-207360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasipanodya JG, Ogbonna D, Deshpande D, Srivastava S, Gumbo T. 2017. Meta-analyses and the evidence base for microbial outcomes in the treatment of pulmonary Mycobacterium avium-intracellulare complex disease. J Antimicrob Chemother 72(Suppl 2):i3–i19. doi: 10.1093/jac/dkx311. [DOI] [PubMed] [Google Scholar]
- 8.Kwak N, Park J, Kim E, Lee CH, Han SK, Yim JJ. 2017. Treatment outcomes of Mycobacterium avium complex lung disease: a systematic review and meta-analysis. Clin Infect Dis 65:1077–1084. doi: 10.1093/cid/cix517. [DOI] [PubMed] [Google Scholar]
- 9.Diel R, Ringshausen F, Richter E, Welker L, Schmitz J, Nienhaus A. 2017. Microbiological and clinical outcomes of treating non-Mycobacterium avium complex nontuberculous mycobacterial pulmonary disease: a systematic review and meta-analysis. Chest 152:120–142. doi: 10.1016/j.chest.2017.04.166. [DOI] [PubMed] [Google Scholar]
- 10.Ellender CM, Law DB, Thomson RM, Eather GW. 2016. Safety of IV amikacin in the treatment of pulmonary non-tuberculous mycobacterial disease. Respirology 21:357–362. doi: 10.1111/resp.12676. [DOI] [PubMed] [Google Scholar]
- 11.Lee H, Sohn YM, Ko JY, Lee SY, Jhun BW, Park HY, Jeon K, Kim DH, Kim SY, Choi JE, Moon IJ, Shin SJ, Park HJ, Koh WJ. 2017. Once-daily dosing of amikacin for treatment of Mycobacterium abscessus lung disease. Int J Tuberc Lung Dis 21:818–824. doi: 10.5588/ijtld.16.0791. [DOI] [PubMed] [Google Scholar]
- 12.Brodt AM, Stovold E, Zhang L. 2014. Inhaled antibiotics for stable non-cystic fibrosis bronchiectasis: a systematic review. Eur Respir J 44:382–393. doi: 10.1183/09031936.00018414. [DOI] [PubMed] [Google Scholar]
- 13.Davis KK, Kao PN, Jacobs SS, Ruoss SJ. 2007. Aerosolized amikacin for treatment of pulmonary Mycobacterium avium infections: an observational case series. BMC Pulm Med 7:2. doi: 10.1186/1471-2466-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safdar A. 2012. Aerosolized amikacin in patients with difficult-to-treat pulmonary nontuberculous mycobacteriosis. Eur J Clin Microbiol Infect Dis 31:1883–1887. doi: 10.1007/s10096-011-1516-3. [DOI] [PubMed] [Google Scholar]
- 15.Olivier KN, Shaw PA, Glaser TS, Bhattacharyya D, Fleshner M, Brewer CC, Zalewski CK, Folio LR, Siegelman JR, Shallom S, Park IK, Sampaio EP, Zelazny AM, Holland SM, Prevots DR. 2014. Inhaled amikacin for treatment of refractory pulmonary nontuberculous mycobacterial disease. Ann Am Thorac Soc 11:30–35. doi: 10.1513/AnnalsATS.201307-231OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yagi K, Ishii M, Namkoong H, Asami T, Iketani O, Asakura T, Suzuki S, Sugiura H, Yamada Y, Nishimura T, Fujiwara H, Funatsu Y, Uwamino Y, Kamo T, Tasaka S, Betsuyaku T, Hasegawa N. 2017. The efficacy, safety, and feasibility of inhaled amikacin for the treatment of difficult-to-treat non-tuberculous mycobacterial lung diseases. BMC Infect Dis 17:558. doi: 10.1186/s12879-017-2665-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olivier KN, Griffith DE, Eagle G, McGinnis JP II, Micioni L, Liu K, Daley CL, Winthrop KL, Ruoss S, Addrizzo-Harris DJ, Flume PA, Dorgan D, Salathe M, Brown-Elliott BA, Gupta R, Wallace RJ Jr. 2017. Randomized trial of liposomal amikacin for inhalation in nontuberculous mycobacterial lung disease. Am J Respir Crit Care Med 195:814–823. doi: 10.1164/rccm.201604-0700OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. 2012. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 67:810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 19.Koh WJ, Stout JE, Yew WW. 2014. Advances in the management of pulmonary disease due to Mycobacterium abscessus complex. Int J Tuberc Lung Dis 18:1141–1148. doi: 10.5588/ijtld.14.0134. [DOI] [PubMed] [Google Scholar]
- 20.Mougari F, Guglielmetti L, Raskine L, Sermet-Gaudelus I, Veziris N, Cambau E. 2016. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther 14:1139–1154. doi: 10.1080/14787210.2016.1238304. [DOI] [PubMed] [Google Scholar]
- 21.Ryu YJ, Koh WJ, Daley CL. 2016. Diagnosis and treatment of nontuberculous mycobacterial lung disease: clinicians' perspectives. Tuberc Respir Dis (Seoul) 79:74–84. doi: 10.4046/trd.2016.79.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffith DE, Brown-Elliott BA, Langsjoen B, Zhang Y, Pan X, Girard W, Nelson K, Caccitolo J, Alvarez J, Shepherd S, Wilson R, Graviss EA, Wallace RJ Jr. 2006. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 174:928–934. doi: 10.1164/rccm.200603-450OC. [DOI] [PubMed] [Google Scholar]
- 23.Moon SM, Park HY, Kim SY, Jhun BW, Lee H, Jeon K, Kim DH, Huh HJ, Ki CS, Lee NY, Kim HK, Choi YS, Kim J, Lee SH, Kim CK, Shin SJ, Daley CL, Koh WJ. 2016. Clinical characteristics, treatment outcomes, and resistance mutations associated with macrolide-resistant Mycobacterium avium complex lung disease. Antimicrob Agents Chemother 60:6758–6765. doi: 10.1128/AAC.01240-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadota T, Matsui H, Hirose T, Suzuki J, Saito M, Akaba T, Kobayashi K, Akashi S, Kawashima M, Tamura A, Nagai H, Akagawa S, Kobayashi N, Ohta K. 2016. Analysis of drug treatment outcome in clarithromycin-resistant Mycobacterium avium complex lung disease. BMC Infect Dis 16:31. doi: 10.1186/s12879-016-1877-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morimoto K, Namkoong H, Hasegawa N, Nakagawa T, Morino E, Shiraishi Y, Ogawa K, Izumi K, Takasaki J, Yoshiyama T, Hoshino Y, Matsuda S, Hayashi Y, Sasaki Y, Ishii M, Kurashima A, Nishimura T, Betsuyaku T, Goto H. 2016. Macrolide-resistant Mycobacterium avium complex lung disease: analysis of 102 consecutive cases. Ann Am Thorac Soc 13:1904–1911. doi: 10.1513/AnnalsATS.201604-246OC. [DOI] [PubMed] [Google Scholar]
- 26.Koh WJ, Jeong BH, Kim SY, Jeon K, Park KU, Jhun BW, Lee H, Park HY, Kim DH, Huh HJ, Ki CS, Lee NY, Kim HK, Choi YS, Kim J, Lee SH, Kim CK, Shin SJ, Daley CL, Kim H, Kwon OJ. 2017. Mycobacterial characteristics and treatment outcomes in Mycobacterium abscessus lung disease. Clin Infect Dis 64:309–316. doi: 10.1093/cid/ciw724. [DOI] [PubMed] [Google Scholar]
- 27.Jeon K, Kwon OJ, Lee NY, Kim BJ, Kook YH, Lee SH, Park YK, Kim CK, Koh WJ. 2009. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med 180:896–902. doi: 10.1164/rccm.200905-0704OC. [DOI] [PubMed] [Google Scholar]
- 28.Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. 2011. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis 52:565–571. doi: 10.1093/cid/ciq237. [DOI] [PubMed] [Google Scholar]
- 29.Choi H, Kim SY, Kim DH, Huh HJ, Ki CS, Lee NY, Lee SH, Shin S, Shin SJ, Daley CL, Koh WJ. 2017. Clinical characteristics and treatment outcomes of patients with acquired macrolide-resistant Mycobacterium abscessus lung disease. Antimicrob Agents Chemother 61:e01146-17. doi: 10.1128/AAC.01146-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi H, Kim SY, Lee H, Jhun BW, Park HY, Jeon K, Kim DH, Huh HJ, Ki CS, Lee NY, Lee SH, Shin SJ, Daley CL, Koh WJ. 2017. Clinical characteristics and treatment outcomes of patients with macrolide-resistant Mycobacterium massiliense lung disease. Antimicrob Agents Chemother 61:e02189-16. doi: 10.1128/AAC.02189-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang B, Jhun BW, Moon SM, Lee H, Park HY, Jeon K, Kim DH, Kim SY, Shin SJ, Daley CL, Koh WJ. 2017. A clofazimine-containing regimen for the treatment of Mycobacterium abscessus lung disease. Antimicrob Agents Chemother 61:e02052-16. doi: 10.1128/AAC.02052-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martiniano SL, Wagner BD, Levin A, Nick JA, Sagel SD, Daley CL. 2017. Safety and effectiveness of clofazimine for primary and refractory nontuberculous mycobacterial infection. Chest 152:800–809. doi: 10.1016/j.chest.2017.04.175. [DOI] [PubMed] [Google Scholar]
- 33.Kuhn RJ. 2001. Formulation of aerosolized therapeutics. Chest 120:94S–98S. doi: 10.1378/chest.120.3_suppl.94S. [DOI] [PubMed] [Google Scholar]
- 34.Ramsey BW, Dorkin HL, Eisenberg JD, Gibson RL, Harwood IR, Kravitz RM, Schidlow DV, Wilmott RW, Astley SJ, McBurnie MA, Wentz K, Smith AL. 1993. Efficacy of aerosolized tobramycin in patients with cystic fibrosis. N Engl J Med 328:1740–1746. doi: 10.1056/NEJM199306173282403. [DOI] [PubMed] [Google Scholar]
- 35.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, Vasiljev KM, Borowitz D, Bowman CM, Marshall BC, Marshall S, Smith AL. 1999. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med 340:23–30. [DOI] [PubMed] [Google Scholar]
- 36.Ioannidou E, Siempos I, Falagas ME. 2007. Administration of antimicrobials via the respiratory tract for the treatment of patients with nosocomial pneumonia: a meta-analysis. J Antimicrob Chemother 60:1216–1226. doi: 10.1093/jac/dkm385. [DOI] [PubMed] [Google Scholar]
- 37.Park HY, Jeong BH, Chon HR, Jeon K, Daley CL, Koh WJ. 2016. Lung function decline according to clinical course in nontuberculous mycobacterial lung disease. Chest 150:1222–1232. doi: 10.1016/j.chest.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Koh WJ, Jeong BH, Jeon K, Lee NY, Lee KS, Woo SY, Shin SJ, Kwon OJ. 2012. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M avium complex lung disease. Chest 142:1482–1488. doi: 10.1378/chest.12-0494. [DOI] [PubMed] [Google Scholar]
- 39.Jeong BH, Jeon K, Park HY, Kim SY, Lee KS, Huh HJ, Ki CS, Lee NY, Shin SJ, Daley CL, Koh WJ. 2015. Intermittent antibiotic therapy for nodular bronchiectatic Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 191:96–103. doi: 10.1164/rccm.201408-1545OC. [DOI] [PubMed] [Google Scholar]
- 40.Koh WJ, Moon SM, Kim SY, Woo MA, Kim S, Jhun BW, Park HY, Jeon K, Huh HJ, Ki CS, Lee NY, Chung MJ, Lee KS, Shin SJ, Daley CL, Kim H, Kwon OJ. 2017. Outcomes of Mycobacterium avium complex lung disease based on clinical phenotype. Eur Respir J 50:1602503. doi: 10.1183/13993003.02503-2016. [DOI] [PubMed] [Google Scholar]
- 41.Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, Park YK, Kim CK, Shin SJ, Huitt GA, Daley CL, Kwon OJ. 2011. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med 183:405–410. doi: 10.1164/rccm.201003-0395OC. [DOI] [PubMed] [Google Scholar]
- 42.Koh WJ, Jeong BH, Jeon K, Kim SY, Park KU, Park HY, Huh HJ, Ki CS, Lee NY, Lee SH, Kim CK, Daley CL, Shin SJ, Kim H, Kwon OJ. 2016. Oral macrolide therapy following short-term combination antibiotic treatment for Mycobacterium massiliense lung disease. Chest 150:1211–1221. doi: 10.1016/j.chest.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 43.American Thoracic Society. 2000. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 161:1376–1395. [DOI] [PubMed] [Google Scholar]
- 44.Griffith DE, Adjemian J, Brown-Elliott BA, Philley JV, Prevots DR, Gaston C, Olivier KN, Wallace RJ Jr. 2015. Semiquantitative culture analysis during therapy for Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 192:754–760. doi: 10.1164/rccm.201503-0444OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clinical and Laboratory Standards Institute. 2011. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard, 2nd ed CLSI document no. M24-A2, Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]
- 46.Brown-Elliott BA, Iakhiaeva E, Griffith DE, Woods GL, Stout JE, Wolfe CR, Turenne CY, Wallace RJ Jr. 2013. In vitro activity of amikacin against isolates of Mycobacterium avium complex with proposed MIC breakpoints and finding of a 16S rRNA gene mutation in treated isolates. J Clin Microbiol 51:3389–3394. doi: 10.1128/JCM.01612-13. [DOI] [PMC free article] [PubMed] [Google Scholar]