Abstract
The research was aimed to study the effect of the addition of Osmo‐air‐dried mulberry (TSS 29.33%) in yoghurt on syneresis and a bioactive component of yoghurt. Two types of yoghurts, with or without Osmo‐dried mulberry, were developed using standard culture (Streptococcus thermophilus and Lactobacillus bulgaricus), and changes at refrigerated temperature (<5°C) were studied. Fruit yoghurt showed high total soluble solids (TSSs) and low‐fat content (dry basis) (17.67% and 11.84%) compared with normal yoghurt (9.5% and 17.21%). The addition of fruits increased the ascorbic acid (0.77 to 5.96 mg/100 g yoghurt), anthocyanins content (0 to 7.9 mg/100 g yoghurt), total phenol content (TPC) (6.63 to 68.03 mg GAE/100 g yoghurt), and antioxidant activity (20.73% to 47.6% radical scavenging activity) in yoghurt. During 18 days of storage at refrigerated condition (<5°C), the acidity of all samples increased, while pH decreased. Syneresis increased with a storage period in control samples while fruit incorporated yoghurt showed decreased syneresis with time. The viability of lactic acid bacteria (LAB) count went on decreasing at similar rates for both with and without the Osmo‐dried mulberry incorporated yoghurt. There is an ample opportunity for utilization of Osmo‐air‐dried mulberry in yoghurt to prevent syneresis during storage with increased bioactive components.
Keywords: anthocyanin, antioxidant activity, ascorbic acid, lactic acid bacteria count, total phenol
1. INTRODUCTION
Yoghurt is a dairy‐based product, which popularity is increasing day by day due to its nutritional and therapeutic characteristics (Nazni & Komathi, 2014; Yousef, Nateghi, & Azadi, 2013). Yoghurt is a lactic acid fermented product from milk with an acidic taste (Aswal, Anubha, & Priyadarshi, 2012; Hassan & Amjad, 2010). Fruit and fruit preparation is used in yoghurt not only to improve the nutritional properties, but these fruit yoghurts also possess phytochemicals such as vitamin C, carotenoids, phenolic components, and antioxidant component (Ariaii, Mahmoudi, & Amoli, 2011; Bae & Suh, 2007; Nazni & Komathi, 2014; Shori & Baba, 2014). Mulberry belonging to family Moraceae and Genus Morus is grown in Nepal at an altitude of 500–2,000 m from tropical to subtropical climates (Shrestha, 2006). Mulberry is a seasonal fruit and is extremely perishable with limited market demand, so the postharvest loss is high (Doymaz, 2004). Its utilization is limited to silkworm and feed (Mehla, Patel, & Tripathi, 1987).
It is rich in anthocyanin and other bioactive components, which may provide beneficial health‐promoting properties (Kako, 2012; Kao, 2003). Berries possess a significant amount of anthocyanin, which possesses antioxidant properties (Brito, Areche, Sepúlveda, Kennelly, & Simirgiotis, 2014; Bunea et al., 2013). Osmo‐air drying not only results in better nutrient retention but also maintains the good integrity of the fruit and has good rehydration property (Djendoubi, Boudhrioua, Kechaou, Courtois, & Bonazzi, 2013; Chiralt et al., 2001; Giovanelli, Brambilla, Rizzolo, & Sinelli, 2012; Ojha et al., 2017; Tortoe, 2010). Most of the fruits are wasted in farm despite its high value. However, lack of proper processing techniques and unawareness of its health benefits, fruit like mulberry, rich in anthocyanins are not properly utilized. So, it is necessary to identify the proper technology which not only enhances the shelf‐life of mulberry fruit but also identify the proper usage.
One of the major problems of yoghurt industry is wheying‐off. Whey separation (syneresis) is the expulsion of whey from three‐dimensional networks, which become visible on the surface (Lucey, Munro, & Singh, 1997; Prothon et al., 2001). This results in short shelf‐life of yoghurt due to lack of body and texture. Whey separation is affected by various factors such as pH, acidity, total solid content, microbial culture, the addition of stabilizers, and hydrocolloid (Athar, Shah, & Khan, 2000; Celik & Bakirci, 2003; Koksoy & Kilic, 2004; Lee, Kim, Watkins, & Batt, 1994; Selvamuthukumaran & Farhath, 2014). Various literature cited that dried or partially dried fruit can improve the stability of yoghurt due to the low acidity and higher solid content compared with fruit juice (Athar et al., 2000; Celik & Bakirci, 2003; Sarmini, Sinniah, & Silva, 2014; Selvamuthukumaran & Farhath, 2014). It is not only necessary to prevent syneresis but also increase the health benefits of The research aimed to utilized the under‐rated mulberry fruit (in context to Nepal) as a potential source of bioactive components in yoghurt and also to minimize syneresis. So, this research was designed to study the stability and phytochemicals of Osmo‐dried mulberry incorporated yoghurt.
2. MATERIALS AND METHODS
2.1. Raw materials collection
Fresh mulberry fruits, grown in the field of Damauli, Nepal, which lies at an altitude of 650 m from sea level, were transported to the laboratory in the icebox and then refrigerated below 5°C. Standard direct vat set (DVS) freeze‐dried yoghurt culture (S. thermophilus and L. bulgaricus) was provided by Dairy Development Corporation (DDC), Nepal. Sugar was purchased from the local market of Kathmandu, Nepal, whereas standard milk (solid‐not‐fat: 10%, Fat: 3% and Protein: 3.6%) was obtained from DDC, Nepal.
2.2. Chemicals and reagents
2,2‐diphenyl‐1‐picrylhydrazyl (DPPH) was purchased from Sigma‐Aldrich Company, Germany. Phenol reagent was purchased from Finar Limited, India. Gallic acid was purchased from LOBA Chemie, India. Methanol was purchased from Fisher Scientific, India. Analytical grade chemicals were used for the analytical purpose. The spectrophotometer used was of model GENESYSTM 10S Vis Spectrophotometer from Thermo ScientificTM, Germany.
2.3. Preparation of Osmo‐air‐dried mulberry
Fruit (2.0 kg) was steeped in 0.1% potassium metabisulfite solution (1:1.5) for 10 min and then drained well with the help of muslin cloth followed by a quick rinse with potable water and treated with 2% Ca(OH)2 (1:1.5) for 10 min for texture improvement purpose as suggested by (Chavan & Amarowicz, 2012). Pretreated fruits were immersed in an osmotic solution of commercial sucrose (55° Bx) for about 5 hr at 40°C (Talens, Escriche, Martínez‐Navarrete, & Chiralt, 2003). The whole fruit was drained and was spread in monolayer thickness on a stainless steel sieve and dried in the convective dryer at 60 ± 5°C for 8 hr. The quality parameter and bioactive component of Osmo‐dried mulberry used were listed in Table 1.
Table 1.
Physiochemical characteristics of mulberry fruit (Osmo‐air‐dried)
| Analysis parameters | Osmo‐air‐dried fruit |
|---|---|
| Moisture content (%) | 9.59 ± 0.40 |
| Total dry weight (%) | 90.40 ± 0.04 |
| TSS (%) | 29.33 ± 0.57 |
| Vitamin C (mg%) | 9.6 ± 0.26 |
| Total phenols (mg/100 g db) | 722.33 ± 3.33 |
| Total anthocyanin (mg cyanidin‐3‐O‐glucoside equivalents (CGE)/100 g db) | 154.86 ± 3.32 |
| Antioxidant activity (% radical scavenging activity (RSA)) | 74.33 ± 1.53 |
The values in the table are the arithmetic mean of triplicate with standard deviation (±).
2.4. Preparation of yoghurt
Skim milk powder was added at 4% to preheated standardized milk (SNF: 10%, Fat: 3%, protein: 3.6%) at 45°C to increase total milk solid to 16.34%. The milk base was heated at 85 ± 2°C for 30 min in sterile stainless steel utensil and then cooled to 40°C. Standard DVS freeze‐dried yoghurt culture was inoculated according to the specification (78 g/2,000 L‐normal yoghurt). The cultured milk was filled to 100 ml in sterile plastic cups containing dried mulberries at the rate of 12% (from the trial) to develop set type fruit yoghurts, and control without fruit was also prepared. All these samples were then incubated at 42 ± 2°C (for about 3 hr) till the acidity (in terms of lactic acid) of yoghurt reached to about 0.9%. When yoghurt was well set they were cooled to 4°C. The samples were coded as NCY (normal control yoghurt‐without fruits) and NFY (normal fruit yoghurt).
2.5. Physiochemical analysis
Mulberries (Osmo‐air‐dried) were analyzed for various parameters. The moisture content, dry matter, total soluble solid (TSS), and pH were analyzed as per (AOAC, 2005). The yoghurt was analyzed for moisture content, total solids, acidity (as lactic acid), pH, and fat content, according to (AOAC, 2005). For TSS, mulberry and water (1:1) were squeezed to extract the juice and filter through cheesecloth for determination.
2.6. Analysis of bioactive properties (phytochemicals)
Ascorbic acid of fruits and yoghurt samples was determined according to (AOAC, 2005) using 2,6‐dichlorophenol indophenol visual titration method.
20 g of each sample was ground with 80% methanol (30 ml) and was agitated in a mechanical shaker for 20 min and then was filtered through Whatman No. 1 filter paper. Two more extraction cycle for 20 min was carried out, and volume was made 100 ml in a volumetric flask as suggested by (Kostic et al., 2013) with some modification.
The total phenol content of sample extracts was measured using the Folin–Ciocalteu method, as described by (Mahdavi, Nikniaz, Rafraf, & Jouyban, 2011). The absorbance was measured using an automated UV–VIS spectrophotometer at 750 nm. The results were expressed as mg of gallic acid equivalents (GAE) per 100 g of sample. The total anthocyanin content of the extracts was determined using the pH differential methods (Mónica Giusti & Wrolstad, 2005) using UV–VIS spectrophotometer. Results were expressed as mg of cyanidin‐3‐O‐glucoside equivalents (CGE) per 100 g. The antioxidant activity of the extract was determined by the free radical scavenging activity using DPPH assay (Mahmood, 2011; Stajcic et al., 2012). The antioxidant activity was calculated as the percent inhibition caused by the hydrogen donor activity of each sample according to the following Equation (1).
| (1) |
2.7. Storage stability
The control and fruit yoghurt were taken out of refrigerated storage (4°C) on every 3 days interval and were analyzed for pH, acidity, syneresis, and viable lactic acid bacteria (LAB) count. Syneresis was measured by the method described by Amatayakul, Sherkat, and Shah (2006) with slight modification. For syneresis, approximately 15 g of yoghurt gel was weighed and drained on muslin cloth for 30 min at room temperature (25°C). The syneresis was expressed as the percentage of the whey separated from gel over initial weight of the gel.
Viable lactic acid bacteria (LAB) count was determined by the method described by Ramakant (2006). For enumeration of LAB, De man Rogosa and Sharpe agar was used, and colonies were interpreted as colony‐forming units (CFU/g of yoghurt sample).
2.8. Statistical analysis
The data were analyzed with one‐way analysis of variance (ANOVA) (no blocking) using GenStat programming at the 5% level of significance (p < .5), and t‐test using Microsoft Excel 2007.
3. RESULTS AND DISCUSSION
3.1. Chemical composition of final yoghurts
The chemical composition of yoghurt with and without fruit is shown in Table 2. Control yoghurt has significantly lower total solid content (16.33%) while yoghurt receiving fruits had higher total solid content (23.16%) as fruit yoghurt was formulated with dried fruits. The fat content of control samples was high (17.21%) in comparison with fruit containing samples (11.84%), and this might be due to no fat contributing fruit which caused a decrease fat content which was similar to that of Temiz, Tarakci, Karaden, and Bak (2012). The addition of fruits significantly increased the total titratable acidity of yoghurts, along with reduced pH values, and the reduction was mainly due to low pH values of fruits. The increased total soluble solid content of fruit incorporated yoghurt might be due to the addition of Osmo‐air‐dried fruit. It was clear that the addition of fruits in yoghurt increased total dry weight (TDW) (%), TSS (%), and acidity, whereas pH and fat content decreased in the final product. Obtained results were in agreement with those reported by (Tarakçi & Küçüköner, 2003).
Table 2.
chemical composition of final yoghurt
| Parameters | NCY | NFY |
|---|---|---|
| Moisture (%) | 83.66 ± 0.57a | 76.83 ± 0.76b |
| Total dry weight (TDW) (%) | 16.33 ± 0.57a | 23.16 ± 0.76b |
| Fat (% db) | 17.21 ± 0.71a | 11.84 ± 0.36b |
| Acidity (% as lactic acid) | 0.84 ± 0.01a | 0.87 ± 0.01b |
| pH | 4.37 ± 0.01a | 4.31 ± 0.01b |
| TSS (%) | 9.5 ± 0.57a | 17.67 ± 0.57b |
The values in the table are the arithmetic mean of triplicate with standard deviation (±).
Values in the row bearing different superscript are significantly different at the 5% level of significance.
3.2. Phytochemical characteristics (bioactive components) of yoghurt
Phytochemical characteristics of yoghurt (with and without fruit) were analyzed, and the results were shown in Table 3. There was a significant increase in ascorbic acid, total phenols, anthocyanin, and antioxidant activity of yoghurt incorporated with fruit. Ascorbic acid was found to increase by more than 7.5 times (0.77 mg/100 g to 5.96 mg/100 g) in mulberry fortified yoghurt. Selvamuthukumaran and Farhath (2014) reported an ascorbic acid content of 20 mg/100 g yoghurt in yoghurt produce with the addition of sea buckthorn. The yoghurt formulated with mulberry fruits contained higher TPC (68.03 mg GAE/100 g) compared with control (6.63 mg GAE/100 g), which was more than 10 times. The detected TPC value of control yoghurt was in agreement with those who reported TPC 3–6 mg/100 g (Bakr, Mohamed, Tammam, & El‐gazzar, 2015) and 5.13–7.24 mg/100 g (Chouchouli et al., 2013). The TPC content in plain yoghurt might be due to break down of a phenolic side chain of milk proteins (Damin, Alcântara, Nunesb, & Oliveiraa, 2009; Shah, 2000). The clear increment of TPC in fruit yoghurt over the values of control yoghurt indicates the presence of mulberry polyphenols (phenolics, anthocyanins, and flavonoids) in the final products. A similar result was reported by Samh, Sherin, and Essam (2013). The mulberry fruit fortified yoghurt posses 7.9 mg CGE/100 g, whereas anthocyanin was not detected in normal yoghurt. Anthocyanin in fruit yoghurt was contributed by the fruit itself. Scibisz, Ziarno, Mitek, and Zare (2012) also found 12 mg/100 g of anthocyanin in yoghurt enriched with 20% blueberry preserve. Various researcher has illustrated anticancer activity, prevention of cardiovascular disease, antidiabetic role, anti‐obesity, and improved visual health from plant anthocyanins (Jayaprakasam, Vareed, Olson, & Nair, 2005; Miyake et al., 2012; Rechner & Kroner, 2005; Tsuda, Horio, Uchida, Aoki, & Osawa, 2003; Wang et al., 2009). 12–36 mg daily ingestion of anthocyanin extract has proven to improve night vision (Levy & Glovinsky, 1998). The result indicated that radical scavenging activity (RSA)% of yoghurt formulated with mulberry was highest (47.6 ± 2.51%) when compared to control yoghurt (20.73 ± 1.41%). The high antioxidant activity in fruit yoghurt than control yoghurt was most likely contributed by individual fruit phytochemical content (phenols, flavonoids, anthocyanins, and ascorbic acid) and as a result of microbial metabolic activities (Thompson, Lopetcharat, & Drake, 2007). The antioxidant activity of plain yoghurt was found to be in the range of 19% to 28.49% by different authors (Chouchouli et al., 2013; Shori & Baba, 2014). The high antioxidant activity of fruit yoghurt is a desirable characteristic that may enhance the therapeutic value of yoghurt and is reported to decrease the risk of some diseases such as cardiovascular and cancer (Kris‐etherton, Harris, & Appel, 2002).
Table 3.
Phytochemical characteristics of yoghurt (with and without fruits)
| Parameters | NCY | NFY |
|---|---|---|
| Ascorbic acid | 0.77 ± 0.01a | 5.96 ± 0.20b |
| Total phenols (mg GAE/100 g) | 6.63 ± 0.85a | 68.03 ± 2.86b |
| Anthocyanin (mg CGE/100 g) | – | 7.9 ± 0.86 |
| Antioxidant activity of (% RSA) | 20.73 ± 1.41a | 47.6 ± 2.51b |
The values in the table are the arithmetic mean of triplicate with standard deviation (±).
Values in the row bearing different superscript are significantly different at the 5% level of significance.
3.3. Study of storage of final products on various parameters
3.3.1. Change in pH and acidity
The change in acidity of NCY and NFY is shown in Figure 1. The initial mean pH value and mean acidity of the yoghurt (without mulberry) were lower than those of yoghurts with fruits as the fruits tend to show little higher acid and a lower pH value. The acidity of normal yoghurt increased from 0.84% to 1.3% during 18 days of storage at refrigerated temperature, whereas pH decreased from 4.37 to 3.87. Similarly, the acidity of normal yoghurt increased from 0.87% to 1.32% during 18 days of storage at refrigerated temperature, whereas pH decreased from 4.31 to 3.85. A similar trend of a rise in acidity and decreasing pattern of pH in yoghurt with storage period and the addition of fruits were revealed by various authors (Farahat & El‐batawy, 2013; Selvamuthukumaran & Farhath, 2014). The increase in acidity of fruit yoghurt is normal phenomenon due to low pH of fruits, and this low pH causes increase in acidity (Öztürk & Öner, 1999). According to Shah and Jelen (1990), postacidification of yoghurt during storage at 4°C occurs because lactic acid bacteria are active at this temperature and produce lactic acid resulting in noticeable decreased in pH and increased acidity.
Figure 1.
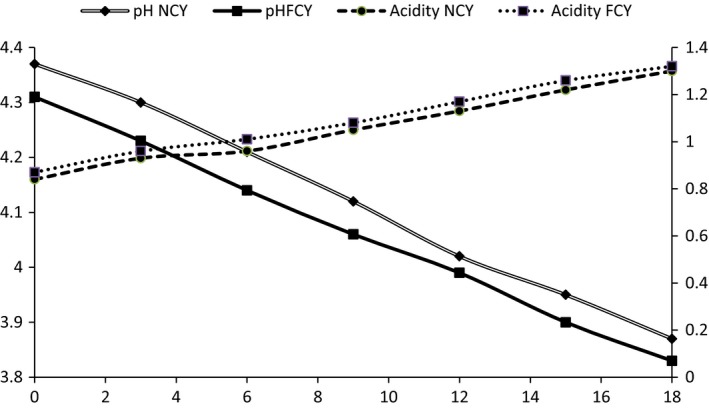
Change in acidity and pH of different yoghurt samples at refrigerated condition
3.3.2. Change in syneresis
The syneresis of control and fruit yoghurt is shown in Figure 2, and the trendline is shown in Table 4. The syneresis of plain yoghurt increased from 17.56% to 30.2% in 18 days of storage while the syneresis of fruit yoghurt decreased from 15.48% to 5.8% in 15 days of storage while again increased to 7.04% in further 3 days of storage. Syneresis of normal yoghurt follows linear trendline, whereas, for fruit yoghurt, syneresis follows logarithmic trendline. However, for normal yoghurt, syneresis increased with time, whereas in the case of fruit yoghurt, negative slope indicates a decrease in syneresis with time. Izadi, Nasirpour, Garoosi, and Tamjidi (2015) also reported a decrease in syneresis of phytosterol‐enriched yoghurt with storage period.
Figure 2.
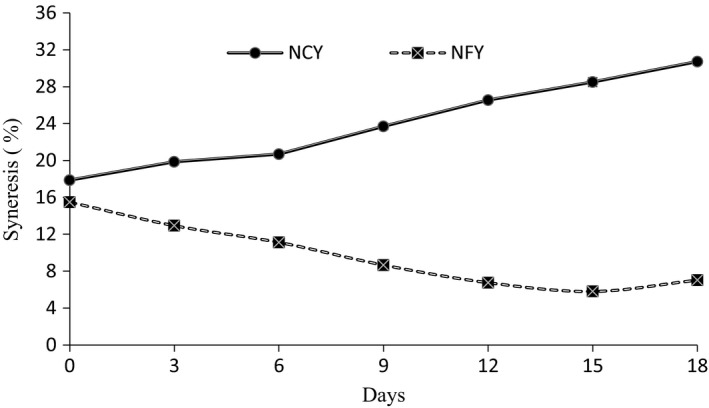
Change in syneresis of different yoghurts upon refrigerated storage
Table 4.
Trendline of syneresis of yoghurt with and without fruit
| Parameters | NCY | NFY |
|---|---|---|
| Equation | Y = 2.2046X + 15.16 | Y = −5.123ln(X) + 15.921 |
| Slope | 2.2046 | −5.123 |
| R 2 | 0.9874 | 0.9471 |
Athar et al. (2000) reported that decrease in pH value accelerates the syneresis in yoghurt. Increased syneresis in control yoghurt may be due to continued increases in acidity and decrease in pH of the product (Selvamuthukumaran & Farhath, 2014), while, yoghurts with dried fruits showed lower syneresis when compared to control yoghurt which can be correlated with absorption of unbound and free water by dried fruits. A similar result was reported by El‐Kholy, Osman, Gouda, and Ghareeb (2011) and Vahedi, Tehrani, and Shahidi (2008). Hence, this showed that dried fruits can serve as a potential source to control syneresis.
3.3.3. Change in viable count of lactic acid bacteria in yoghurt
The pattern of change in viability in for both yoghurt (with and without fruit) stored in refrigerated condition (4°C) is shown in Figure 3. For fruit yoghurt, LAB count decreased from 8.81 to 6.74 log cfu/ml, whereas for normal yoghurt, LAB count decreased from 8.81 to 6.57 log cfu/ml. The decrease in the LAB count for both yoghurts follows a similar trend. Various authors have reported decreased in the lactic count during storage of yoghurt (Mani‐López, Palou, & López‐Malo, 2014; Panesar & Shinde, 2012). The loss of cell viability may be due to a decrease in pH during storage and accumulation of organic acid as a result of growth and fermentation (Kailasapathy, Harmstorf, & Phillips, 2008).
Figure 3.
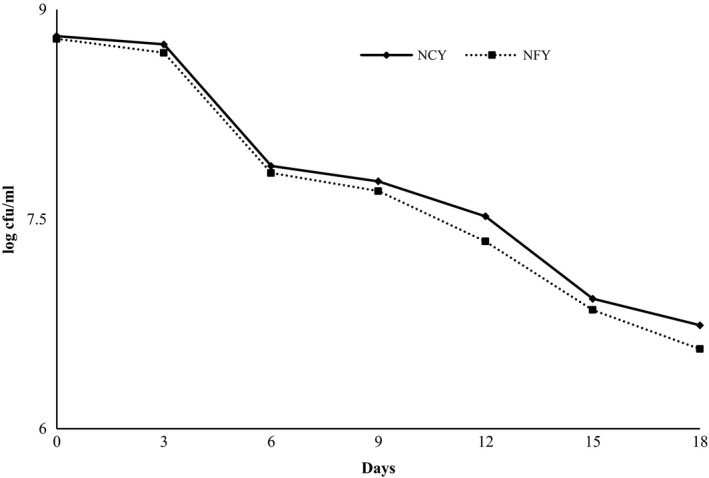
Change in viable count of lactic acid bacteria (LAB) at refrigerated storage
4. CONCLUSION
Osmo‐dried mulberry fruit incorporated yoghurt showed significant improvement in bioactive properties compared with normal yoghurts. These bioactive components may contribute to various health benefits. Antioxidant activity was also high, which was believed to reduce cardiovascular disease. Beside this, mulberry incorporated yoghurt also showed reduced syneresis with the time periods while in normal yoghurt, it increased with time
CONFLICT OF INTEREST
None declared.
Sigdel A, Ojha P, Karki TB. Phytochemicals and syneresis of osmo‐dried mulberry incorporated yoghurt. Food Sci Nutr. 2018;6:1045–1052. https://doi.org/10.1002/fsn3.645
REFERENCES
- Amatayakul, T. , Sherkat, F. , & Shah, N. P. (2006). Syneresis in set yogurt as affected by EPS starter cultures and levels of solids. International Journal of Dairy Technology, 59(3), 216–221. https://doi.org/10.1111/j.1471-0307.2006.00264.x [Google Scholar]
- AOAC (2005). Official method of analysis (18th ed.) (Horwitz W. & Latimer G.W., Eds.). Rockville, MD: AOAC International. [Google Scholar]
- Ariaii, P. , Mahmoudi, M. , & Amoli, R. I. (2011). The production of fruity yoghurt with banana flavor. Food Science and Technology Department of Islamic Azad University, 6, 368–370. [Google Scholar]
- Aswal, P. , Anubha, S. , & Priyadarshi, S. (2012). Yoghurt: Preparation, characteristics and recent advancements. Cibtech Journal of Bio‐Protocols, 1(2), 32–44. [Google Scholar]
- Athar, H. , Shah, M. A. , & Khan, U. (2000). Effect of various stabilizers on whey separation (syneresis) and quality of yoghurt. Pakistan Journal of Biological Sciences, 3(8), 1336–1339. [Google Scholar]
- Bae, S. H. , & Suh, H. J. (2007). Antioxidant activities of five different mulberry cultivars in Korea. LWT ‐ Food Science and Technology, 40(6), 955–962. https://doi.org/10.1016/j.lwt.2006.06.007 [Google Scholar]
- Bakr, I. , Mohamed, T. , Tammam, A. , & El‐gazzar, F. (2015). Characteristics of bioyoghurt fortified with fennel honey. International Journal of Current Microbiology and Applied Sciences, 4(3), 959–970. [Google Scholar]
- Brito, A. , Areche, C. , Sepúlveda, B. , Kennelly, E. J. , & Simirgiotis, M. J. (2014). Anthocyanin characterization, total phenolic quantification and antioxidant features of some chilean edible berry extracts. Molecules, 19(8), 10936–10955. https://doi.org/10.3390/molecules190810936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunea, A. , Ruginǎ, D. , Sconţa, Z. , Pop, R. M. , Pintea, A. , Socaciu, C. , … VanCamp, J. (2013). Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in B16‐F10 metastatic murine melanoma cells. Phytochemistry, 95, 436–444. https://doi.org/10.1016/j.phytochem.2013.06.018 [DOI] [PubMed] [Google Scholar]
- Celik, S. , & Bakirci, I. (2003). Some properties of yoghurt produced by adding mulberry pekmez (concentrated juice). International Journal of Dairy Technology, 56(1), 26–29. [Google Scholar]
- Chavan, U. D. , & Amarowicz, R. (2012). Osmotic dehydration process for preservation of fruits and vegetables. Journal of Food Research, 1(2), 202–209. https://doi.org/10.5539/jfr.v1n2p202 [Google Scholar]
- Chiralt, A. , Martínez‐Navarrete, N. , Martínez‐Monzó, J. , Talens, P. , Moraga, G. , Ayala, A. , & Fito, P. (2001). Changes in mechanical properties throughout osmotic processes. Journal of Food Engineering, 49(2–3), 129–135. https://doi.org/10.1016/s0260-8774(00)00203-x [Google Scholar]
- Chouchouli, V. , Kalogeropoulos, N. , Konteles, S. J. , Karvela, E. , Makris, D. P. , & Karathanos, V. T. (2013). Fortification of yoghurts with grape (Vitis vinifera) seed extracts. LWT – Food Science and Technology, 53(2), 522–529. https://doi.org/10.1016/j.lwt.2013.03.008 [Google Scholar]
- Damin, M. R. , Alcântara, M. R. , Nunesb, A. P. , & Oliveiraa, M. N. (2009). Effects of milk supplementation with skim milk powder, whey protein concentrate and sodium caseinate on acidification kinetics, rheological properties and structure of nonfat stirred yogurt. LWT ‐ Food Science and Technology, 42(10), 1744–1750. https://doi.org/10.1016/j.lwt.2009.03.019 [Google Scholar]
- Djendoubi, M. N. , Boudhrioua, M. N. , Kechaou, N. , Courtois, F. , & Bonazzi, C. (2013). Effect of osmo‐dehydration conditions on the quality attributes of pears. Journal of Food Processing & Technology, 4(8), 4–9. https://doi.org/10.4172/2157-7110.1000256 [Google Scholar]
- Doymaz, I. (2004). Pretreatment effect on sun drying of mulberry fruits (Morus alba L.). Journal of Food Engineering, 65(2), 205–209. https://doi.org/10.1016/j.jfoodeng.2004.01.016 [Google Scholar]
- El‐Kholy, A. M. , Osman, M. , Gouda, A. , & Ghareeb, W. A. (2011). Fortification of yoghurt with iron. Journal of Dairy & Food Sciences, 6(2), 159–165. [Google Scholar]
- Farahat, A. M. , & El‐batawy, O. (2013). Proteolytic activity and some properties of stirred fruit yoghurt made using some fruits containing proteolytic enzymes. World Journal of Dairy and Food Sciences, 8(1), 38–44. https://doi.org/10.5829/idosi.wjdfs.2013.8.1.23313 [Google Scholar]
- Giovanelli, G. , Brambilla, A. , Rizzolo, A. , & Sinelli, N. (2012). Effects of blanching pre‐treatment and sugar composition of the osmotic solution on physico‐chemical, morphological and antioxidant characteristics of osmodehydrated blueberries (Vaccinium corymbosum L.). Food Research International, 49(1), 263–271. https://doi.org/10.1016/j.foodres.2012.08.015 [Google Scholar]
- Hassan, A. , & Amjad, I. (2010). Nutritional evaluation of yoghurt prepared by different starter cultures and their physiochemical analysis during storage. African Journal of Microbiology Research, 4(4), 22–26. https://doi.org/10.5897/AJB09.098 [Google Scholar]
- Izadi, Z. , Nasirpour, A. , Garoosi, G. A. , & Tamjidi, F. (2015). Rheological and physical properties of yogurt enriched with phytosterol during storage. Journal of Food Science and Technology, 52(8), 5341–5346. https://doi.org/10.1007/s13197-014-1593-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaprakasam, B. , Vareed, S. K. , Olson, L. K. , & Nair, M. G. (2005). Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. Journal of Agricultural and Food Chemistry, 53(1), 28–31. [DOI] [PubMed] [Google Scholar]
- Kailasapathy, K. , Harmstorf, I. , & Phillips, M. (2008). Survival of Lactobacillus acidophilus and Bifidobacterium animalis ssp. lactis in stirred fruit yogurts. LWT ‐ Food Science and Technology, 41(7), 1317–1322. https://doi.org/10.1016/j.lwt.2007.08.009 [Google Scholar]
- Kako, S. M. (2012). The effect of auxin IBA and kinetin in budding success percentage of mulberry (Morus sp.). International Journal of Pure and Applied Science and Technology, 13(1), 50–56. [Google Scholar]
- Kao, M. S. (2003). A comparative study of antioxidant and physicochemical properties of blackberry and kiwifruit. Auburn University, Alabama, USA. Available from: https://etd.auburn.edu/bitstream/handle/10415/256/KAO_MING-WEI_32.pdf?sequence=1 [last accessed 2 April 2016].
- Koksoy, A. , & Kilic, M. (2004). Use of hydrocolloids in textural stabilization of a yoghurt drink, ayran. Food Hydrocolloids, 18(4), 593–600. https://doi.org/10.1016/j.foodhyd.2003.10.002 [Google Scholar]
- Kostic, D. A. , Dimitrijevic, D. S. , Mitic, S. S. , Mitic, M. N. , Stojanovic, G. S. , & Zivanovic, A. V. (2013). Phenolic content and antioxidant activities of fruit extracts of Morus nigra L. (Moraceae) from Southeast Serbia. Tropical Journal of Pharmaceutical Research, 12(1), 105–110. https://doi.org/10.4314/tjpr.v12i1.17 [Google Scholar]
- Kris‐etherton, P. M. , Harris, W. S. , & Appel, L. J. (2002). Fish consumption, fish oil, omega‐3 fatty acids, and cardiovascular disease epidemiological and observational studies. Circulation, 106, 2747–2776. [DOI] [PubMed] [Google Scholar]
- Lee, S. P. , Kim, D. S. , Watkins, S. , & Batt, C. A. (1994). Reducing whey syneresis in yogurt by the addition of a thermolabile variant of β‐lactoglobulin. Bioscience, Biotechnology and Biochemistry, 58(2), 309–313. https://doi.org/10.1080/bbb.58.309 [Google Scholar]
- Levy, Y. , & Glovinsky, Y. (1998). The effect of anthocyanosides on night vision. Eye, 12, 967–969. [DOI] [PubMed] [Google Scholar]
- Lucey, J. A. , Munro, P. A. , & Singh, H. (1997). Whey separation in acid skim milk gels made with glucono‐d‐lactone: Effects of heat treatment and gelation temperature. Journal of Texture Studies, 29(350), 413–426. [Google Scholar]
- Mahdavi, R. , Nikniaz, Z. , Rafraf, M. , & Jouyban, A. (2011). Determination and comparison of the total polyphenol contents of fresh and commercial fruit juices. British Food Journal, 113(10), 744–752. https://doi.org/10.1108/00070701111140089 [Google Scholar]
- Mahmood, T. (2011). Investigations of valuable phytochemicals in native berries and cherries fruits. University of Agriculture, Faisalabad, Pakistan. Available from: http://prr.hec.gov.pk/Thesis/1270S.pdf [last accessed 18 April 2016].
- Mani‐López, E. , Palou, E. , & López‐Malo, A. (2014). Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. Journal of Dairy Science, 97(5), 2578–2590. https://doi.org/10.3168/jds.2013-7551 [DOI] [PubMed] [Google Scholar]
- Mehla, R. K. , Patel, R. K. , & Tripathi, V. N. (1987). A model for sericulture and milk production. Agricultural Systems, 25(2), 125–133. https://doi.org/10.1016/0308-521X(87)90011-4 [Google Scholar]
- Miyake, S. , Takahashi, N. , Sasaki, M. , Kobayashi, S. , Tsubota, K. , & Ozawa, Y. (2012). Vision preservation during retinal inflammation by anthocyanin‐rich bilberry extract: Cellular and molecular mechanism. Laboratory Investigation, 92(1), 102–109. [DOI] [PubMed] [Google Scholar]
- Mónica Giusti, M. , & Wrolstad, R. E. (2005). Characterization and measurement of anthocyanins by UV‐visible spectroscopy. Handbook of Food Analytical Chemistry, 2–2, 19–31. https://doi.org/10.1002/0471709085.ch18 [Google Scholar]
- Nazni, P. , & Komathi, K. (2014). Quality evaluation of the fruit pulp added yoghurt. International Journal of Nutrition and Agriculture Research, 1(1), 48–54. Retrieved from http://www.ijnar.com/article/quality evaluation of the fruit pulp added yoghurt.pdf [Google Scholar]
- Ojha, P. , Sigdel, A. , Karki, R. , Mishra, A. , Subedi, U. , & Karki, T. B. (2017). Physiochemical and bioactive characteristics of osmo‐air dried mulberry fruit. Nepalese Horticulture, 12, 27–32. [Google Scholar]
- Öztürk, B. A. , & Öner, M. D. (1999). Production and evaluation of yogurt with concentrated grape juice. Journal of Food Science, 64(3), 530–532. [Google Scholar]
- Panesar, P. S. , & Shinde, C. (2012). Effect of storage on syneresis, pH, Lactobacillus acidophilus count, Bifidobacterium bifidum count of aloe vera fortified probiotic yoghurt. Current Research in Dairy Sciences, 4(1), 17–23. https://doi.org/10.3923/crds.2012.17.23 [Google Scholar]
- Prothon, F. , Ahrné, L. M. , Funebo, T. , Kidman, S. , Langton, M. , & Sjöholm, I. (2001). Effects of combined osmotic and microwave dehydration of apple on texture, microstructure and rehydration characteristics. LWT ‐ Food Science and Technology, 34(2), 95–101. https://doi.org/10.1006/fstl.2000.0745 [Google Scholar]
- Ramakant, S. (2006). Microbiological analysis of fermented milk products In Book I.D.C. (Ed.), Chemical and microbial analysis of milk and milk product (pp. 1–250, 1st ed.). Dehradun, India: International Book Distributing Company; Available from: https://www.amazon.com/Chemical-Microbiological-Analysis-Milk-Products/dp/8181891015 [last accessed 18 April 2016]. [Google Scholar]
- Rechner, A. R. , & Kroner, C. (2005). Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thrombosis Research, 116(4), 327–334. [DOI] [PubMed] [Google Scholar]
- Samh, M. M. , Sherin, A. A. , & Essam, H. H. (2013). Properties and antioxidant activity of probiotic yoghurt flavoured with black carrot, pumpkin and strawberry. International Journal of Dairy Science, 8(2), 48–57. https://doi.org/10.3923/ijds.2013.48.57 [Google Scholar]
- Sarmini, N. , Sinniah, J. , & Silva, K. F. S. T. (2014). Development of a ripened jack (Artocarpus heterophyllus Lain) fruit and soy (Glycine max) milk incorporated set yoghurt. International Journal of Dairy Science, 9(1), 15–23. https://doi.org/10.3923/ijds.2014.15.23 [Google Scholar]
- Scibisz, I. , Ziarno, M. , Mitek, M. , & Zare, D. (2012). Effect of probiotic cultures on the stability of anthocyanins in blueberry yoghurts. LWT – Food Science and Technology, 49(2), 208–212. https://doi.org/10.1016/j.lwt.2012.06.025 [Google Scholar]
- Selvamuthukumaran, M. , & Farhath, K. (2014). Evaluation of shelf stability of antioxidant rich seabuckthorn fruit yoghurt. International Food Research Journal, 21(2), 759–765. [Google Scholar]
- Shah, N. P. (2000). Effect of milk‐derived bioactives: An overview. British Journal of Nutrition, 84(1), 3–10. [DOI] [PubMed] [Google Scholar]
- Shah, N. , & Jelen, P. (1990). Survival of lactic acid bacteria and their lactase under acidic conditions. Journal of Food Science, 55(2), 506–509. https://doi.org/10.1002/jcc.540110605 [Google Scholar]
- Shori, A. B. , & Baba, A. S. (2014). Comparative antioxidant activity, proteolysis and in vitro α‐amylase and α‐glucosidase inhibition of Allium sativum‐yogurts made from cow and camel milk. Journal of Saudi Chemical Society, 18(5), 456–463. https://doi.org/10.1016/j.jscs.2011.09.014 [Google Scholar]
- Shrestha, J. B. (2006). Combating desertification with sericulture and apiculture. Agriculture and Environment: Gender Equity and Environment Division, 6–13. [Google Scholar]
- Stajcic, S. , Tepic, A. , Djilas, S. , Sumic, Z. , Canadanovic‐Brunet, J. , Cetkovic, G. , … Tumbas, V. (2012). Chemical composition and antioxidant activity of berry fruits. Acta Periodica Technologica, 342(43), 93–105. https://doi.org/10.2298/APT1243093S [Google Scholar]
- Talens, P. , Escriche, I. , Martínez‐Navarrete, N. , & Chiralt, A. (2003). Influence of osmotic dehydration and freezing on the volatile profile of kiwi fruit. Food Research International, 36(6), 635–642. https://doi.org/10.1016/S0963-9969(03)00016-4 [Google Scholar]
- Tarakçi, Z. , & Küçüköner, E. (2003). Physical, chemical, microbiological and sensory characteristics of some fruit‐flavored yoghurt. YYÜ Vet Fak Derg, 14(2), 10–14. [Google Scholar]
- Temiz, H. , Tarakci, Z. , Karaden, T. , & Bak, T. (2012). The effect of loquat fruit (Eriobotrya japonica) marmalade addition and storage time on phsico‐chemical and sensory properties of yoghurt. Journal of Agricultural Sciences, 18, 329–338. [Google Scholar]
- Thompson, J. L. , Lopetcharat, K. , & Drake, M. A. (2007). Preferences for commercial strawberry drinkable yogurts among African American, Caucasian, and Hispanic consumers in the United States. Journal of Dairy Science, 90(11), 4974–4987. https://doi.org/10.3168/jds.2007-0313 [DOI] [PubMed] [Google Scholar]
- Tortoe, C. (2010). A‐review‐of‐osmodehydration‐for‐food‐industry. African Journal of Food Science, 4(6), 303–324. [Google Scholar]
- Tsuda, T. , Horio, F. , Uchida, K. , Aoki, H. , & Osawa, T. (2003). Dietary cyanidin 3‐O‐β‐D‐glucoside‐rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. The Journal of Nutrition, 133(7), 2125–2130. [DOI] [PubMed] [Google Scholar]
- Vahedi, N. , Tehrani, M. , & Shahidi, F. (2008). Optimizing of fruit yoghurt formulation and evaluating its quality during storage. American‐Eurasian Journal of Agriculture and Environment Science, 3(6), 922–927. [Google Scholar]
- Wang, L. S. , Hecht, S. S. , Carmella, S. G. , Yu, N. , Larue, B. , Henry, C. , … Stoner, G. D. (2009). Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prevention Research, 2(1), 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef, M. , Nateghi, L. , & Azadi, E. (2013). Effect of different concentration of fruit additives on some physicochemical properties of yoghurt during storage. Annals of Biological Research, 4(4), 244–249. [Google Scholar]


