Abstract
Background: Shigella remains the primary cause of diarrhoea in paediatric patients worldwide and accounts for up to 40,000 deaths per year. Current guidelines for the treatment of shigellosis are based on data which are over a decade old. In an era of increasing antimicrobial resistance, an updated review of the appropriate empirical therapy for shigellosis in children is necessary, taking into account susceptibility patterns, cost and the risk of adverse events.
Methods: A systematic review of the current published literature on the treatment of shigella dysentery was undertaken in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Results: The initial search produced 131 results, of which nine studies met the inclusion criteria. The quality of the studies was assessed as per the Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines. International guidelines were also reviewed. There is a lack of current research regarding the clinical treatment of shigellosis in paediatric and adult patients, despite rising antimicrobial resistance worldwide. In particular, there is a lack of studies assessing the non-susceptibility of community-acquired strains, with almost all published research pertaining to microbiological data from hospital-based settings.
Discussion: Current WHO guidelines support the use of fluoroquinolones (first-line), β-lactams (second-line) and cephalosporins (second-line) which accords with currently available evidence and other international guidelines, and there is no strong evidence for changing this guidance. Azithromycin is appropriate as a second-line therapy in regions where the rate of non-susceptibility of ciprofloxacin is known to be high, and research suggests that, from a cardiac point of view, azithromycin is safer than other macrolide antibiotics. Cefixime is also a reasonable alternative, although its use must be weighed against the risk of dissemination of extended-spectrum β-lactamase-producing organisms.
Keywords: Dysentery, shigellosis, shigella, antibiotics, antimicrobial resistance, treatment guidelines
Introduction
Shigella is a Gram-negative, non-motile bacillus belonging to the enterobacteriacae family of which four species exist: S. dysenteriae, S. flexneri, S. boydii and S. sonnei (designated as serogroups A, B, C and D, respectively) with multiple serotypes. Of the estimated 165 million shigella diarrhoeal episodes every year, 99% of cases occur in low- and middle-income countries (LMIC), mainly (69%) in children [1]. Shigella has recently been identified as the leading pathogen causing childhood diarrhoea worldwide, and has been estimated to be responsible for 1.1 million deaths per year, 61% of which are children <5 years of age [2,3].
Because of overcrowding and poor sanitation, shigellosis occurs predominantly in LMIC. Infants, non-breast fed or malnourished children and adults >50 years have a more severe illness and a greater risk of death [4]. Acquired immunity to shigella is serotype-specific. While S. boydii and S. sonnei usually cause a relatively mild illness (watery or bloody diarrhoea only), S. flexneri and S. dysenteriae are chiefly responsible for endemic and epidemic shigellosis, respectively, in developing countries, with high transmission rates and significant case fatality. S. dysenteriae (Type 1, also known as Shiga bacillus) is capable of causing a more severe and prolonged illness owing to the production of a potent cytotoxin (Shiga) which is associated with the development of haemolytic-uraemic syndrome [5]. Other complications of shigellosis include sepsis, rectal prolapse, arthralgia, intestinal perforation, toxic megacolon, electrolyte imbalance, seizures and leukaemoid reactions [1,2].
The species distribution of shigella infection varies globally. While S. sonnei is the predominant species worldwide, S. flexneri is more prominent in low-income settings in Africa and Asia [1,4] while the less virulent S. sonnei predominates in higher-income settings [5,6]. Transmission occurs via a number of mechanisms – the faecal/oral route, person-to-person contact, household flies, infected water, or inanimate objects following exposure to as few as 10–100 organisms [3,6]. Once infected, all shigella species multiply and cause acute bloody diarrhoea by invading the colonic epithelium where pro-inflammatory cytokines are released, and the subsequent inflammatory reaction (recruiting a number of polymorphonuclear cells) destroys the epithelial cells which line the gut mucosa, allowing for further direct invasion by shigella.
The resultant infectious diarrhoea causes a loss of water and electrolytes with a clinical picture of abdominal cramping, fever, and bloody/mucoid stools. Stool microscopy – a cheap, rapid and simple diagnostic test – demonstrates numerous polymorphonuclear cells on methylene blue stain; however, microbiological culture is required to differentiate between shigella and other causes of colitis [7]. Multiplex polymerase chain reaction (PCR) platforms for the detection of shigella are commercially available but in most health-care settings their availability is limited.
With effective antibiotic therapy, clinical improvement occurs within 48 h, resulting in a decreased risk of serious complications and death, shorter duration of symptoms, and the elimination of shigella from the stool. This results in diminished transmission of infection by decreasing the duration of faecal carriage from approximately 4 weeks to 3 days, conferring significant public health benefits [8,9]. Current guidelines for treating shigella were published by WHO in 2005 and they recommend ciprofloxacin as the first-line treatment (Table 1) [4]. The guidelines also noted that pivmecillinam (amdinocillin pivoxil) and ceftriaxone were ‘the only antimicrobials that are usually effective for the treatment of multi-resistant strains of Shigella in all age groups’, yet their usage is limited by their high cost and formulation (four times daily dosing for pivmecillinam, and parenteral administration for ceftriaxone). Pivmecillinam and ceftriaxone were therefore only listed for use when local strains of shigella are known to be resistant to ciprofloxacin. Azithromycin was included as a second-line therapy for adults. The 2005 guidelines also listed antimicrobials which should not be used to treat shigellosis owing to their poor mucosal penetration or increasing antimicrobial resistance (Table 2). The more recently published 2013 WHO Pocketbook of Hospital Care for Children included a chapter on the treatment of shigella dysentery, with recommendations which were the same as in the 2005 guidelines [10].
Table 1. 2005 WHO guidelines: antimicrobials for treatment of shigellosis (adapted) [4].
| Antimicrobial | Treatment schedule for children | Limitations |
|---|---|---|
| 1st-line: ciprofloxacin | 15 mg/kg orally twice daily for 3 days | Expensive |
| Resistance emerging | ||
| Drug interactions | ||
| 2nd-line: pivmecillinam | 20 mg/kg orally 4 times daily for 5 days | Cost |
| No paediatric formulation | ||
| Four times daily dosing | ||
| Resistance emerging | ||
| OR*: ceftriaxone | 50–100 mg/kg intramuscular injection for 2–5 days | Requires parenteral administration |
| Generates antimicrobial resistance | ||
| OR: (for adults) azithromycin | 6–20 mg/kg, orally once daily for 1–5 days | Cost |
| Drug interactions | ||
| Resistance emerges rapidly, spreads to other bacteria |
Ceftriaxone is listed as an alternative therapy ‘only for use when local strains of shigella are known to be resistant to ciprofloxacin’.
Table 2. Antimicrobials highlighted as inappropriate for shigellosis in the 2005 WHO guidelines [4].
| Antimicrobial | Rationale for not prescribing |
|---|---|
| Ampicillin | Antimicrobial resistance |
| Chloramphenicol | Antimicrobial resistance |
| Co-trimoxazole | Antimicrobial resistance |
| Tetracyclines | Antimicrobial resistance |
| Nalidixic acid | Antimicrobial resistance; cross-resistance to ciprofloxacin observed (MIC increased) |
| Nitrofurans (nitrofurantoin, furazolidone) | Penetrate the intestinal mucosa poorly |
| Oral aminoglycosides (gentamicin, kanamycin) | Penetrate the intestinal mucosa poorly |
| 1st- and 2nd-generation cephalosporins (cefazolin, cephalotin, cefaclor, cefoxitin) | Penetrate the intestinal mucosa poorly |
| Amoxicillin | Penetrates the intestinal mucosa poorly |
Current guidelines are therefore based on evidence which is increasingly outdated. In view of changing patterns of resistance to antimicrobials worldwide, this systematic review was undertaken to evaluate the current international literature on the treatment of shigellosis in children.
Methods
A systematic search for systematic reviews, meta-analyses, multi-centre studies and randomised controlled trials of antibiotic therapy was undertaken using the MeSH search terms ‘Shigella’, ‘dysentery’, ‘antibiotics’ and ‘antimicrobials’. The databases EMBASE, Cochrane database of systematic review and Pubmed were searched. To ensure accurate and up-to-date information on antimicrobial non-susceptibility patterns, the search was limited to trials in humans published since 2005. Inclusion and exclusion criteria are listed in Table 3.
Table 3. Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Initially, the search was restricted to studies in the paediatric population but published research in this age group was limited and the search was, therefore, expanded to include all ages. International clinical practice guidelines were also reviewed, including the Infectious Diseases Society of America (IDSA), BMJ Clinical Evidence, the American Academy of Pediatrics and Therapeutic Guidelines (Australia).
Results
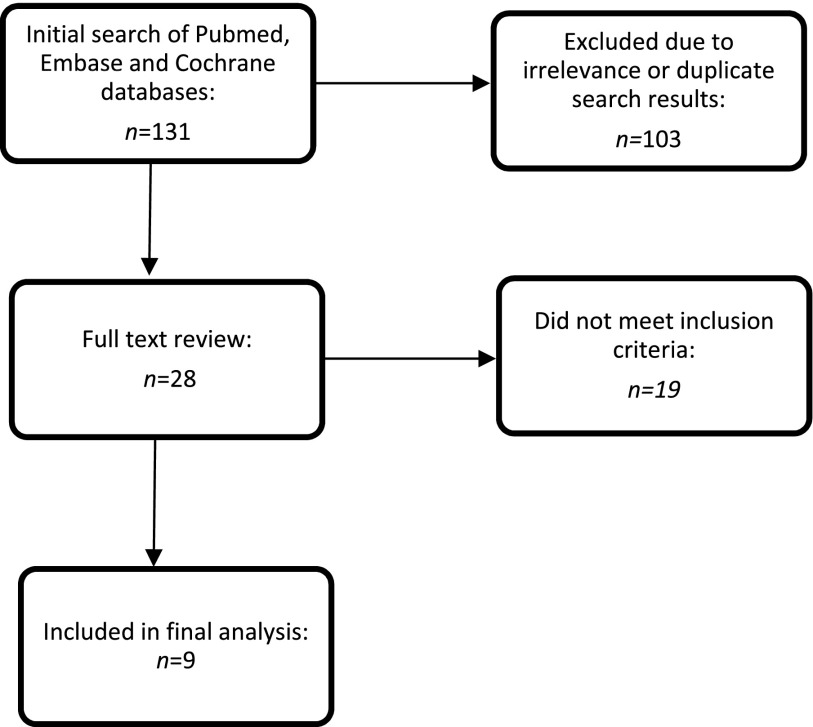
The initial search produced 131 results (Figure 1), 28 of which qualified for full-text review. Ultimately, nine studies met the inclusion criteria and were abstracted as detailed in Appendix 1. The quality of the studies was assessed as per the Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines (see Appendix 1 for description of methodologies) [11].
Figure 1.
Search strategy.
Characteristics of included studies
Of the papers eligible for inclusion, six studies were systematic reviews and meta-analyses (conducted across an international setting). One, a multi-centre study, evaluated 600,000 cases of shigella diarrhoea in six Asian countries. Two papers were based on data collated from a multi-centre randomised trial in Vietnam.
Four papers were classified as high-quality evidence, three as moderate-quality evidence and the two papers based on data investigating a multi-centre trial in Vietnam were classified as low-quality evidence.
Two systematic reviews exclusively assessed paediatric antimicrobial response, while the remainder included adults and children in their population. The papers based on a multi-centre trial in Vietnam investigated clinical outcomes in children < 16 years.
Evidence for current WHO recommendations: ciprofloxacin, pivmecillinam and ceftriaxone
A systematic review in 2013 [12] used CHERG standard rules [13] to analyse 48 high-quality randomised controlled trials in children aged <16 years mainly in low- and middle-income countries (LMIC), seven of which were ultimately eligible for inclusion. It demonstrated that current WHO guidelines for treatment with either ciprofloxacin, pivmecillinam or ceftriaxone reduced clinical failure rates (which the authors postulated were a proxy for shigella deaths) by 82% (95% CI 67–99%). It was reported that ciprofloxacin, pivmecillinam or ceftriaxone successfully cleared shigella pathogens in 96% of cases (95% CI 88–99%). The authors concluded that there is strong evidence that current antimicrobial guidelines are effective in preventing serious mortality and morbidity. Of note, 98% of the trials reviewed were undertaken in LMIC which increases the generalisability and applicability of the findings. However, all studies were hospital-based which limits the information available on resistance patterns for shigella infections typically treated within the community.
Another systematic review in 2010 [14] assessed ciprofloxacin, pivmecillinam or ceftriaxone for children in LMIC (limited to hospital-based settings) using CHERG rules to document clinical failure rates of 0.1% (95% CI-0.2–0.5%). It concluded that the antimicrobial antibiotics currently recommended by WHO are effective from both a clinical and bacteriological point of view.
A third systematic review [8] assessed changing patterns of resistance to ciprofloxacin (alone) and found resistance to be increasing in the Asia–Africa regions (analysed together), from 0.6% in 1998–2000 (95% CI 0.2–1.3%) to 29.1% in 2007–2009 (95% CI 0.9–74.8%) – a 49-fold increase over 12 years. This increase in resistance was significantly above the (minimal) increase documented in the Europe–America region which had reached only 0.6% (95% CI 0.2–1.2%) by 2007–2009. Of note, the review also found higher resistance patterns in children, with respective rates (globally) of 7.5% (95% CI 4.3–11.5%) in paediatric patients vs 3.6% in adults (95% CI 2.2–5.3%).
The same authors conducted a review which compared resistance to third-generation cephalosporins (ceftriaxone, cefotaxime and ceftazidime) between 1999 and 2012 [15]: it found markedly increased resistance in the Asia–Africa region [with ceftriaxone resistance reaching 14.2% (95% CI 3.9–29.4%) by 2012]. However, both studies lacked data pertaining to patient outcomes. The authors concluded that ceftriaxone and cefotaxime may not be appropriate for shigellosis in Asia–Africa.
Assessing the Asia region independently, a 2006 multi-centre study (of 2927 shigella isolates in children and adults) in Bangladesh, China, Pakistan, Indonesia, Thailand and Vietnam documented ciprofloxacin-resistant S. flexneri isolates in China (18/305, 6%), Pakistan (8/242, 3%) and Vietnam (5/282, 2%) [9].
Evidence for alternative antibiotic treatment options
In view of the research above documenting increasing resistance to ciprofloxacin and ceftriaxone, the international literature was reviewed for alternative antimicrobials that might be used for shigella dysentery. Previously effective agents (including nalidixic acid, amoxicillin and co-trimoxazole) have been removed from the WHO guidelines on dysentery because of extensive resistance, a decision which continues to be supported in view of the evidence above.
A 2010 Cochrane review [2] investigated antibiotic therapy for shigella dysentery but found no superior efficacy when comparing fluoroquinolones, β-lactams or macrolides. The authors noted that the current practice of presumptively treating shigella dysentery with antibiotics should continue because of the public health benefits conferred, but that no specific antibiotic, or antibiotic class, is universally effective for shigella. However, this study included several randomised controlled trials of low- to moderate-quality (many of which were conducted before 1990) and probably do not reflect current resistance patterns.
Aminoglycosides
A 2013 systematic review [16] which assessed worldwide patterns of aminoglycoside resistance in shigella (between 1999 and 2010) documented increasing levels of in vitro gentamicin resistance in the Asia–Africa region which reached 32.4% (95% CI 17.87–48.91%) in 2005–2007. Resistance to gentamicin, kanamycin and amikacin was higher in children than in adults. The 2010 Cochrane review outlined above further supported the ineffectiveness of aminoglycosides which tend to have poor absorption when administered orally, further limiting their usefulness.
Fluoroquinolones
Gatifloxacin, a fourth-generation fluoroquinolone, was investigated as an alternative therapy in a multi-centre randomised trial assessing the efficacy of gatifloxacin vs ciprofloxacin for shigellosis in Vietnamese children between 2006 and 2008 [17]. No superiority to gatifloxacin was found in terms of clinical failure rates which were similar in both groups (gatifloxacin 12% vs 11% for ciprofloxacin, p = 0.72), with gatifloxacin showing similar efficacy in the treatment of paediatric dysentery [2]. However, while gatifloxacin might be more convenient than ciprofloxacin owing to its longer half-life (allowing administration once daily rather than twice daily as required for ciprofloxacin), retrospective review of clinical outcomes in patients treated with gatifloxacin revealed significantly poorer clinical outcomes than in those treated with ciprofloxacin, regardless of isolate minimum inhibitory concentrations (MIC). Overall, no association between MIC and clinical outcome in paediatric shigellosis was found [17].
Macrolides
Azithromycin, a macrolide antibiotic, is listed as an alternative second-line therapy for adults in current WHO guidelines as well as in most international guidelines (for both paediatric and adult patients). To date, no trials comparing the efficacy of azithromycin vs ciprofloxacin in children have been published. In the late 1990s, azithromycin was found to be as effective as ciprofloxacin in adults in Kenya and Bangladesh [18]. In Tanzanian adults in 2004–2005, 90% of shigella strains isolated were S. flexneri and all were reported to be sensitive in vitro to ciprofloxacin, nalidixic acid and cefuroxime, while 98% were sensitive to azithromycin. By 2010/2011 in Dhaka, Bangladesh, in vitro susceptibilities to ciprofloxacin, pevmecillinam, azithromycin and ceftriaxone were 65, 50, 74 and 95%, respectively [19]. More recently, however, increasing reports of azithromycin-resistant strains of shigella spp. have been documented, including by the Centers for Disease Control and Prevention (CDC) [20–24].
Oral cephalosporins
Cefixime is an oral third-generation cephalosporin which inhibits bacterial cell wall synthesis by binding to one or more of the penicillin-binding proteins (PBPs), inhibiting cell wall synthesis. It is widely distributed throughout the body and reaches therapeutic concentration levels in most tissues and body fluids, with a time to peak serum concentration of 2–6 h and a half-life of 3–4 h [25]. Cefixime has been demonstrated to be effective (at 8 mg/kg/day in two divided doses) for shigellosis in adults and paediatric patients [26–29], although one study documented inferior efficacy to that of azithromycin [27]. Short (2-day) courses have been found to be as effective as 5-day courses [27,29]. Cefixime may be useful for paediatric patients when cephalosporin is necessary owing to high resistance to fluoroquinolones and β-lactams, and it can be taken orally. Cefixime is affordable and the suspension can be stored at room temperature [28]. Updated clinical trials to investigate this therapy as an alternative treatment option are urgently needed because previous randomised controlled trials investigating its efficacy are over a decade old.
No other antimicrobial agents were investigated in the research which met this review’s inclusion criteria.
Synopsis of international guidelines
Four evidence-based international guidelines were reviewed (listed in order of most recently updated): the Infectious Diseases Society of America (IDSA) [30], Therapeutic Guidelines (Australia) [31], the American Academy of Pediatrics (AAP) [7] and BMJ Clinical Evidence [32]. Their recommendations are summarised in Table 4. Apart from the IDSA guidelines which are currently under review (last published in 2001), in line with the 2005 WHO guidelines [4], most international guidelines currently recommend fluoroquinolones as first-line therapy.
Table 4. Current international guidelines for the treatment of shigellosis.
| Guideline | Last update | Recommendations |
|---|---|---|
| IDSA [30] | 2001; update in progress | Based on A-1 level of evidence |
| [Where: A = good evidence to support a recommendation for use; and I = Evidence from at least one properly randomised, controlled trial] | ||
| Selective therapy should be instituted for shigellosis | ||
| ||
| or | ||
| ||
| Therapeutic Guidelines (Australia) [31] | 2014 | Selective therapy for: |
| ||
| Empirical therapy (while awaiting local sensitivities): | ||
| ||
| or | ||
| ||
| or | ||
| ||
| Second-line therapy: | ||
| ||
| American Academy of Pediatrics [7] | 2015 |
|
| Empirical therapy (while awaiting culture/susceptibility results): any of (not hierarchical): | ||
| ||
| The guidelines also note that oral cephalosporins (cefixime) have been used successfully in treating shigellosis in adults. | ||
| BMJ Clinical Evidence [32] | 2016 | Selective therapy for: |
| ||
| Empirical therapy (while awaiting local sensitivities): | ||
| ||
| or | ||
| ||
| Second-line therapy: | ||
| ||
| or | ||
| ||
| All therapies state ‘consult with a specialist for guidance on duration of treatment’ | ||
| British National Formulary [33] | 2016 | Ciprofloxacin 20 mg/kg bd (higher dose than 15 mg/kg previously recommended) |
Of note, comparison of international guidelines reveals differing dosage ranges for ciprofloxacin, from 12.5 mg/kg [31] to 20 mg/kg (BNF) [33], and the WHO 2005 guidelines list 15 mg/kg as the currently recommended dosage [4]. Ciprofloxacin has high oral bio-availability (approximately 70%) which is not influenced by the concurrent administration of feeds, and no substantial loss by first-pass metabolism. Maximum serum concentrations are attained 1 or 2 h after oral dosing, and its half-life is approximately 4 h in patients with normal renal function [32]. References supporting the efficacy of a higher dose range (20 mg/kg, above the currently recommended 15 mg/kg) were not found in the literature, and, owing to the associated risk of adverse events when combined with other CYP3A4 inhibitors (discussed below), there is no current evidence base to suggest that a higher dose of ciprofloxacin than currently recommended is warranted in shigellosis. Furthermore, higher minimum inhibitory concentrations (MIC) of fluoroquinolones requiring increased ciprofloxacin concentrations have not been found to be significantly associated with poorer clinical outcomes [30].
Review of harms and toxicity – summary of evidence on safety
Adverse events
A 2010 systematic review of 1748 paediatric and adult patients [2] found no statistically significant differences in adverse events between patients taking fluoroquinolones, macrolides (including azithromycin) or β-lactams for shigellosis, concluding that all classes of currently available antibiotics for shigellosis are safe. The side effects of therapies currently recommended for shigellosis and those which may be considered in the future are highlighted in Table 5.
Table 5. Common adverse reactions to antibiotics currently indicated to treat shigellosis in children [7,33].
| Antibiotic | Life-threatening | Mild adverse effects which may result in discontinuation of treatment | Other | Relevant interactions |
|---|---|---|---|---|
| Fluoroquinolones:CiprofloxacinNorfloxacinOfloxacin | Hypersensitivity reactions; | Dyspepsia, headache, diarrhoea, vomiting, hypotension | Tendonitis and tendon rupture; peripheral neuropathy. | All fluoroquinolones should be used with caution in patients receiving drugs known to prolong the QT interval |
| Prolonged QT syndrome | The toxicity of fluoroquinolones is increased by the concurrent use of systemic steroidal medications | |||
| A 2010 systematic review of ciprofloxacin safety in paediatrics concluded that although musculoskeletal adverse effects occur owing to ciprofloxacin use, these events are reversible [14] | ||||
| Fluoroquinolones’ effects are reduced by the co-administration of iron- and zinc-containing products, of importance when zinc-containing products are used to treat diarrhoea in children. | ||||
| Fluoroquinolones cause additive toxicity with non-steroidal anti-inflammatory drugs (ibuprofen, meloxicam, naproxen) | ||||
| Azithromycin | Hypersensitivity reactions; | Dyspepsia, flatulence, headache, disturbance in taste, anorexia | Malaise, paraesthesia | Macrolides use not advised with other drugs which prolong the QT interval, (including anti-malarial medications such as artemether-lumefantrine) owing to the risk of ventricular arrhythmias. However, azithromycin has been identified as a safer macrolide (in terms of its ability to prolong the QT interval) in this class of antibiotics. |
| Prolonged QT syndrome | ||||
| Plasma concentrations of azithromycin are increased by ritonavir | ||||
| Azithromycin in combination with rifabutin results in increased side-effects of rifabutin, including neutropenia | ||||
| Ceftriaxone | Hypersensitivity reactions | Diarrhoea, headache, abdominal discomfort | Transient cholestatic jaundice owing to biliary sludge formation | Relevant interactions for all cephalosporins: |
| Increased risk of nephrotoxicity when co-administered with aminoglycosides | ||||
| Enhance anticoagulant effect of coumarins | ||||
| Cefixime | Hypersensitivity reactions; immune-mediated haemolytic anaemia | Flatulence, headache, abdominal pain, defaecation urgency, nausea, constipation, vomiting | Transient cholestatic jaundice owing to biliary sludge formation | As per ceftriaxone |
| Pivmecillinam | As with all penicillins: hypersensitivity reactions, serum-sickness-like reactions, anaphylaxis | Diarrhoea, joint pain, rashes, urticaria | Avoid use in acute porphyrias | Contra-indicated for concurrent use with sodium valproate |
Possible cardiac side effects
Previous case reports of fluoroquinolones and macrolides have been associated with prolongation of the QT interval [34,35]. Independently, mild delays in ventricular repolarisation are clinically unnoticeable, although these antimicrobials may serve to amplify the risk of torsades de pointes (TdP), a potentially fatal polymorphic ventricular tachyarrhythmia which may present as sudden death (owing to ventricular tachycardia), syncope, palpitations, seizures or asymptomatically if the duration is short and it terminates spontaneously [35]. Of note, the current literature identifies this risk as requiring the presence of other risk factors, as highlighted in Table 6. The main reported risk factor for TdP is co-administration of other medications which are substrates and/or inhibitors of cytochrome P450 (CYP) enzymes, and the possibility of metabolic instability resulting from synergistic interactions with this enzyme. This risk is enhanced by individual allelic variations in CYP3A4, the most important enzyme in human drug metabolism. CYP3A4 is responsible for the biotransformation of approximately 60% of all oxidised drugs and allelic variations can result in patients being poor metabolisers of CYP3A4-inducing medications, resulting in reduced clearance of drug substrates and increasing exposure to the effects of toxicity [36].
Table 6. Risk factors for the development of torsades de pointes [34].
| Risk factor | Examples |
|---|---|
| Genetic risk factors | Channelopathies |
| CYP3A4 poor metaboliser | |
| Underlying cardiac disease | Bradycardia |
| Congestive cardiac failure | |
| Myocardial ischaemia | |
| Atrial fibrillation | |
| Electrolyte derangements | Hypokalaemia |
| Hypomagnesaemia | |
| Hypocalcaemia | |
| Organ impairment, altering medication toxicity | Renal insufficiency |
| Severe hepatic disease | |
| Use of medication to increase QT liability | Concurrent CYP medications administered |
Existing evidence suggests that the individual risk of cardiac arrhythmias secondary to these antimicrobials is minimal; yet, when combined with a genetic propensity to poor metabolism of CYP3A4-inducing medications and co-administration with other CYP potentiators, the risk may be magnified. How this might affect clinical practice, however, remains unclear. Another important risk factor to consider is the possibility of acute renal failure in the setting of severe dehydration secondary to shigellosis which could result in decreased clearance and enhanced toxic effects, increasing prolongation of the QT interval in a clinical setting.
Prolonged QT syndrome and azithromycin
As discussed, the predominantly reported risk of macrolide-associated TdP is the co-administration of other CYP3A4 inhibitors, resulting in increased drug toxicity. However, azithromycin has been identified as distinguishable from other macrolides as a group in terms of its cardiac toxicity as it minimally inhibits CYP3A4, resulting in a lack of appreciable interaction with other CYP3A4 substrates, and is classified as one of the safer macrolide antibiotics from a cardiac perspective [35,37]. In recent years, however, increasing attention has been paid to azithromycin’s risks following a documented increased risk of cardiac death in a cohort of 347,795 patients aged 30–74 years taking azithromycin [38]. The study found that patients taking 5 days of azithromycin, in comparison with taking no antibiotics, had a statistically significantly increased risk of cardiac death (hazard ratio 2.88, 95% CI 1.25–2.75, p < 0.0001) as well as of death from any cause (HR 1.85, 95% CI 1.25–2.75, p = 0.002). However, the risk was found to be most pronounced in patients with a high baseline risk of cardiovascular disease, and there was evidence of confounding by factors associated with both azithromycin use and risk of cardiovascular disease – namely a history of smoking, high body mass index, poor diet and low physical activity. At present, published case reports of an increased risk of sudden cardiac deaths in patients taking azithromycin are limited to adults [39,40].
Prolonged QT syndrome and fluoroquinolones
As with macrolides, there is interclass variability in the QT prolongation effect of fluoroquinolones. Ciprofloxacin’s inhibition of CYP1A2 has been described as ‘relatively inconsequential’ [35] and the US Food and Drug Administration (FDA)’s Adverse Event Reporting System (AERS) supports the notion of several causes of fluoroquinolone-associated TdP, usually in the context of co-administration with another QT-prolonging drugs, underlying cardiac disease, renal impairment and electrolyte anomaly.
Fluoroquinolone use and polyneuropathy
In 2013, the FDA issued a communiqué to specifically address the risk of peripheral neuropathy (PN) for all oral fluoroquinolones [41], mainly in response to case reports of this adverse event [42], in the absence of large epidemiological studies. The neurotoxic mechanism is thought to be through the inhibition of GABA-receptors which occurs within days of use and may be permanent. Between 1997 and 2012, the FDA’s AERS recorded 539 reports (1% of all submitted events for fluoroquinolones) pertaining to peripheral neuropathy. A review of these reports found that the majority of affected patients were female with a median age of 48 years (range 9–100) [43]. This evidence was further investigated by a 2014 pharmaco-epidemiological study which quantified the risk and demonstrated a relative risk of developing peripheral neuropathy with fluoroquinolone use of 2.07 (95% CI 1.56–2.74) [44]. With regard to ciprofloxacin specifically, the increased risk was quantified as RR 1.93 (95% CI 1.32–2.82), a small but appreciable increase. However, the research was based on a cohort of men with a mean age of 68 years, and it is difficult to extrapolate these data to the paediatric population. Because of the risk of permanent peripheral neuropathy resulting from fluoroquinolone use, the FDA’s most recent advice warns against the use of fluoroquinolone except when no other treatment is available [41]; yet, in the setting of global shigella spp. non-susceptibility, the public health benefits of treatment outweigh the small yet statistically significant risk of this adverse event.
Co-administration of azithromycin with artemisinin-based antimalarial drugs
Co-administration of macrolides or quinolones with other QT-prolonging agents could present a clinical problem, yet nearly all data examining the risk are for adults (often with pre-existing cardiac risks), and genetic differences between populations may limit interpretation. Azithromycin is a weak antimalarial that has been used in combination with several other anti-malarials or co-administered to treat non-malarial infections. Current evidence evaluating the cardiac risk of azithromycin co-adminsitered with chloroquine [45–47], artesunate/arthmeter [48,49] or piperaquine [50,51] does not identify an increased risk of cardiac instability in paediatric patients.
Antimicrobial resistance patterns
Evidence suggests that antibiotic resistance is an increasing challenge in the therapeutic management of shigellosis, as recognised by the WHO prioritising ciprofloxacin-resistant shigella as a target of current international focus on antimicrobial resistance [52]. There are several mechanisms by which this may occur. In shigella spp., antimicrobial resistance is often owing to classes 1 and 2 integrons which contain resistance gene cassettes which are mobile and transferrable from one bacterium to another, providing a flexible way for bacteria to adapt to the environmental pressure caused by antibiotics. This may account for the dissemination of resistant genes and the emergence of multidrug-resistant strains, and explain why shigella resistance patterns vary worldwide – as the distribution of integrons varies according to the species and resistance phenotype (with. S. sonnei and S. boydii strains containing a single class 2 integron, while S. flexneri and S. dysenteriae carry a class 1 integron, often in combination with a class 2 integron which increases the propensity of dissemination of MDR strains of shigella) [53]. This underpins the importance of any antimicrobial resistance programme including surveillance to document changes in prevalent species in regions worldwide.
Resistance to fluoroquinolones
The primary target of fluoroquinolones is the DNA gyrase, a type II topoisomerase essential for DNA replication and transcription. Mutations in the gyrA gene have been shown to increase the MICs of fluoroquinolones for shigella spp. and other enterobacteriaceae, while plasmid-mediated quinolone resistance genes can also be acquired [54]. Of concern, complete ciprofloxacin resistance (MIC ≥ 4 mg/L) has recently been reported in domestic and imported S. sonnei isolates in the USA, Vietnam and elsewhere [55–57], and patients infected with fluoroquinolone-resistant shigella have a longer duration of diarrhoea than those with fluoroquinolone-susceptible strains [26]. Ciprofloxacin resistance must continue to be closely monitored and non-susceptibility should be reported when data are available.
Resistance to cephalosporins
Cephalosporin resistance is also of concern, arising from the production of plasmid-mediated β-lactamase [58]. Resistance to third-generation cephalosporins owing to the production of extended-spectrum β-lactamases (ESBLs) which confer resistance to all β-lactamases (except cephamycins and carbapenems) is increasingly prevalent and has been documented in recent laboratory analyses in Asia [59,60]. ESBL resistance in shigella spp. needs to be closely monitored in view of the necessity of treatment with expensive carbapenems, one of the last options for treating multi-resistant Gram-negative organisms, and there has been a considerable increase in the prevalence of ESBL resistance, including in shigella [53,59,60]. Thus, previous reports of the efficacy of ceftriaxone and cefixime might not reflect today’s susceptibility patterns; the use of these agents rapidly induces ESBLs and resistance to other antibiotic classes.
Discussion
Shigella, a Gram-negative enterobacteriaceae, is responsible for 165 million diarrhoeal episodes each year, 99% of which occur in LMIC, and 69% in the paediatric population. With effective antibiotic therapy, there is clinical improvement within 48 h, diminishing the risk of mortality and decreasing transmission by eliminating shigella from the stool. The WHO 2005 Guidelines for the Control of Shigellosis, Including Epidemics due to Shigella Dysenteriae Type 1 listed the fluoroquinolone ciprofloxacin (15 mg/kg orally twice daily for 3 days) as first-line treatment for shigellosis in children, and (more expensive and less available) pivmecillinam (amdinocillin pivoxil) and (parenteral) ceftriaxone were listed as second-line therapy when local strains were known to be resistant to ciprofloxacin. The macrolide azithromycin was listed as a second-line therapy for adults.
There is a lack of current research on the clinical treatment of shigellosis in paediatric or adult patients, despite rising antimicrobial non-susceptibility rates worldwide. In particular, there is a lack of research assessing the non-susceptibility of community-acquired strains; almost all published research pertains to microbiological data from hospital-based settings. Research investigating non-susceptibility of community-acquired shigellosis is urgently required. A large proportion of the current evidence is based on in vitro studies which do not necessarily correspond with clinical outcomes, and studies of clinical efficacy do not evaluate individual drugs.
A number of international guidelines currently list azithromycin as a first- and second-line therapy for shigellosis in children. While there are no published trials comparing the efficacy of azithromycin with that of ciprofloxacin for shigellosis in children, previous trials in adults have demonstrated similar efficacy and higher in vitro susceptibility; however, reports of azithromycin-resistant strains are increasing. In areas where ciprofloxacin-resistance is evident, azithromycin is an appropriate second-line alternative therapy owing to its oral administration and affordability. Safety concerns (the risk of polyneuropathy in ciprofloxacin use and prolonged QT syndrome secondary to azithromycin) are based mainly on retrospective studies in adults and results cannot necessarily be extrapolated to the paediatric population, although care should be taken when co-administering with other CYP450 inducing medications.
Prior research has investigated the antibiotics currently recommended by WHO together rather than assessing individual therapies. Research investigating non-susceptibility of ahigellosis – particularly community-acquired strains – is urgently required, and in vitro non-susceptibility studies need to be correlated with clinical outcomes. Further randomised controlled trials adhering to CONSORT guidelines are required to guide future treatment options for shigellosis, especially in populations at risk of high case fatality (such as malnourished or HIV-positive children). The efficacy of oral cephalosporins (cefixime) for shigellosis should be a priority for research as it is affordable and easily administered compared with (the currently recommended) parenteral ceftriaxone. Specifically, a large randomised controlled trial in children in the Asia–Africa region should compare the efficacy of ciprofloxacin, azithromycin and cefixime as the first-line treatment of shigellosis in children, including the assessment of MICs in relation to treatment outcomes and addressing the risks of exacerbating resistance in other intestinal bacteria, especially ESBL-producing enterobacteriaceae.
In conclusion, current WHO guidelines supporting the use of fluoroquinolones (first-line), β-lactams (second-line) and cephalosporins (second-line) accord with currently available evidence and other international guidelines, and there is no strong evidence to change this guidance. Azithromycin may be considered as an appropriate second-line therapy in regions with known high rates of ciprofloxacin non-susceptibility. Cefixime is also a reasonable alternative, although its use must be balanced against the risk of increasing antimicrobial resistance and the spread of ESBL.
Notes on contributors
Phoebe C. M. Williams, MBBS (Hons.), received her medical degree from The University of Sydney and a Masters in Global Health Science from The University of Oxford. She is a paediatric registrar and dual trainee in Infectious Diseases at Sydney Children’s Hospital, Australia. She is a DPhil candidate through The University of Oxford, with her research focusing on antimicrobial resistance in paediatric patients.
James A. Berkley FRCPCH, MD is a professor of Paediatric Infectious Diseases at The University of Oxford based at the KEMRI-Welcome Trust Research Programme in Kilifi, Kenya. He is the principal investigator of the CHAIN network with a research focus on serious infection and survival in highly vulnerable groups of infants and children.
Funding
This work was supported by The World Health Organization, The Nuffield Department of Medicine (University of Oxford), The General Sir John Monash Foundation, The Wellcome Trust and the Bill and Melinda Gates Foundation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Appendix 1
| Author | Year | Title | Setting | Population | Method | Findings | Level of evidence |
|---|---|---|---|---|---|---|---|
| Das et al. [12] | 2013 | Antibiotics for the treatment of cholera, ahigella and xryptosporidium in children | Systematic review and meta-analysis (international) | Children <16 years | The CHERG standard rules were applied to determine the final effect of treatment with antibiotics on diarrhoea morbidity and mortality | Using clinical failure rates as a proxy for shigella deaths (as there was no data on mortality), the authors propose that treating shigella dysentery with antibiotics results in an 82% (67–99%) reduction in diarrhoea mortality from shigella. | A |
| 48 papers relevant to Ahigella were included, 47 of which were from developing country settings | |||||||
| Studies were included if they reported the effect of antibiotics on morbidity and mortality associated with diarrhoea owing to cholera, shigella and cryptosporidiosis in children, as observed by clinical and bacteriological failure and mortality. No studies compared antibiotics with placebo or a control group, so studies with an antibiotic comparison group were also included. Only studies with a confirmed diagnosis of the respective infection and on immunocompetent patients were included | |||||||
| All studies were hospital-based | |||||||
| Bacteriological failure: Antibiotics (including ciprofloxacin, pivmecillinam and ceftriaxone) successfully clear shigella pathogens in 96% (88–99%) of cases | |||||||
| The overall conclusion is that there is strong evidence of effectiveness against serious mortality and morbidity | |||||||
| All antimicrobials were analysed together | |||||||
| Gu et al. [8] | 2012 | Comparison of the prevalence and changing resistance to nalidixic acid and ciprofloxacin of Ahigella between Europe–America and Asia–Africa from 1998 to 2009. | Systematic review comparing Africa-Asia region with Europe–America | 26,877 specimens from adults and children in LMIC and HIC | SR for articles published during 1998–2011 using search strategy ‘bacterial surveillance’ OR ‘antimicrobial resistance’ OR ‘bacterial resistance’ AND ‘shigella’ | The predominant species isolated was S. sonnei, representing 8900 (56.6%) of total isolates, followed by S. flexneri (5749 isolates, 36.5%), S. boydii (601 isolates, 3.8%) and S. dysenteriae (481 isolates, 3.1%) | A |
| 102 studies met inclusion criteria, LMIC and HIC all included | Quinolone resistance in Asia–Africa: | ||||||
| |||||||
| Quinolone resistance in Europe–America: | |||||||
| |||||||
| In all the studies collected, a total of 26,877 isolates were positive for shigella, 15,731 of which had information about prevalence of subtypes | |||||||
| Owing to widespread use of nalidixic acid as the first-line agent for empirical treatment of infectious diarrhoea, resistance to nalidixic acid in Asian–African countries increased to 64.5% (95% CI 13.8–99.3) in 2007–2009. Thus, this drug should no longer be considered appropriate empirical therapy | |||||||
| Progressively increasing resistance to ciprofloxacin is still a serious cause of concern and several studies have emphasised that most nalidixic acid-resistant strains exhibit some degree of cross-resistance to ciprofloxacin | |||||||
| Gu et al. [17] | 2013 | Prevalence and trends of aminoglycoside resistance in shigella worldwide, 1999–2010 | Systematic review and meta-analysis evaluating aminoglycoside resistance in shigella, Asia–Africa vs Europe–America | Adults and children | 3176 publications were retrieved from MEDLINE and EMBASE reported from 1999 to 2012, 68of which met the inclusion criteria |
|
A |
| Gu et al. [15] | 2015 | Comparison of resistance to 3rd–generation cephalosporins in shigella between Europe–America and Asia–Africa from 1999–2012 | Europe–America and Asia–Africa | Adults and children | A systematic review was conducted to compare resistance to 3rd-generation cephalosporins in shigella strains between Europe–America and Asia–Africa from 1998 to 2012 |
|
A |
| Only high quality studies were included for analysis (defined as prospective cohort or retrospective consecutive cohort studies where susceptibility tests were conducted according to CLSI guidelines with external quality control). 104 articles met these inclusion criteria | |||||||
| Traa et al. [14] | 2010 | Antibiotics for the treatment of dysentery in children. | Systematic review | Children aged <16 years | Systematic review investigating the effect of ciprofloxacin, ceftriaxone and pivmecillinam for dysentery in children in developing countries; CHERG Standard Rules were applied |
|
B |
| 8 papers were selected for abstraction; 4 reported on bacteriological failure and 5 on bacteriological relapse | |||||||
| All studies were RCTs judged to be of good quality | |||||||
| Developing countries | |||||||
| All studies were conducted in clinical or hospital settings | |||||||
| von Seidlein et al. [9] | 2006 | A multicentre study of shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology | Surveillance of 600,000 persons of all ages | Bangladesh, China, Pakistan, Indonesia, Vietnam and Thailand | Shigella was isolated from 2927 (5%) of 56,958 diarrhoea episodes detected between 2000 and 2004 |
|
B |
| Swabs were inoculated onto MacConkey agar and salmonella-shigella agar and incubated overnight then checked for non-lactose fermenting enteropathogens | |||||||
| Antimicrobial sensitivity testing against ampicillin, cotrimoxazole, nalidixic acid and ciprofloxacin was performed by disk diffusion following CLS methods, and subject to external laboratory validation | |||||||
| Vinh et al. [18] | 2011 | A multi-centre randomized trial to assess the efficacy of gatifloxacin vs ciprofloxacin for the treatment of shigellosis in Vietnamese children | Vietnam | 494 children <15 years admitted to a paediatric ward with a history of passing bloody or mucoid stools, with or without abdominal pain, tenesmus or fever for <72 hours prior to admission | Randomised, open-label, controlled trial with two parallel arms at two hospitals in southern Vietnam |
|
B |
| EXCLUSION CRITERIA = any prior treatment with a fluoroquinolone during the current bout of disease, or children with a trophozoite or Entamoeba histolytica in their stool on microscopic examination | |||||||
| The study was designed as a superiority trial and children with dysentery meeting the inclusion criteria were invited to participate | |||||||
| Participants received either gatifloxacin (10 mg/kg/day) in a single daily dose for 3 days or ciprofloxacin (30 mg/kg/day) in two divided doses for 3 days | |||||||
| The primary outcome measure was treatment failure; secondary outcome measures were time to the cessation of individual symptoms | |||||||
| 494 patients were randomised to receive either gatifloxacin (n = 249) or ciprofloxacin (n = 245), of 107 of whom had a positive shigella stool culture | |||||||
| Thompson et al. [21] | 2016 | Clinical implications of reduced susceptibility to fluoroquinolones in paediatric Shigella sonnei and Shigella flexneri infections | Vietnam | 490 paediatric patients <15 years admitted to tertiary units in Vietnam | Clinical information and bacterial isolates were derived from a randomised controlled trial comparing gatifloxacin with ciprofloxacin (above, Vinh et al.) for paediatric shigellosis. |
|
C |
| Time-kill experiments were performed to evaluate the impact of MIC on in-vitro growth of shigella, and Cox regression modelling was used to compare clinical outcome between treatments and shigella species | |||||||
| Patients were excluded if they had | |||||||
| received any fluoroquinolones within the time of this bacterial illness. | |||||||
| -Stool samples were collected on admission and standard microbiological techniques were employed to identify shigella and salmonella isolates-Antimicrobial susceptibility testing was performed by disc diffusion following methods prescribed by the CLSI-MICs were calculated by Etest as per the manufacturer’s instructions (AB Biodisk, Sweden). -Strains identified as resistant to ceftriaxone were subjected to further phenotypic tests to confirm ESBL production using discs containing only cefotaxime (30 mg) and both cefotaxime and ceftazidime combined with clavulanic acid (10 mg), according to current CLSI guidelines | |||||||
| Christopher et al. [2] | 2010 | Antibiotic therapy for shigella dysentery | Systematic review (international) | 16 RCTs met the inclusion criteria, which totalled 1748 children and adults based on clinical symptoms of dysentery prior to bacteriological confirmation | Of the 16 RCTs included, this was composed of 2 RCTs comparing antibiotics and placebo vs no drug; and 14 RCTs comparing effectiveness of different antibiotic regimens for treatment of shigella | There was insufficient evidence to consider any class of antibiotic superior in efficacy in treating shigella dysentery, but heterogeneity for some comparison limits confidence in the results | C |
| There were no statistically significant differences in adverse events between participants taking macrolides, β-lactams or fluoroquinolones, leading the authors to conclude that all antibiotics were safe | |||||||
| There was inadequate evidence regarding the role of antibiotics in prevention of complications] | |||||||
| Conclusion: Low- to moderate-quality evidence that antibiotic therapy significantly reduces the number of children with dysentery on follow-up compared with no antibiotic | |||||||
| All RCTs were low- to moderate-quality evidence: of the 16 trials, 7 were at risk of bias owing to inadequate allocation concealment, and 12 owing to incomplete reporting of outcome data | |||||||
| Limited data from one 3-armed trial of people with moderately severe illness suggest that antibiotics reduce the episodes of diarrhoea at follow-up | |||||||
| Many RCTs included in the review were conducted before 1990 and included Abx no longer used owing to high resistance (cotrimoxazole, ampicillin, nalidixic acid) | |||||||
| Reviewed both developed and developing countries, limiting generalisability of findings to developing country settings |
Acknowledgments
We are grateful to the WHO Department of Newborn, Child and Adolescent Health for valuable input to the conclusions of this review.
References
- [1]. Ashkenazi S. Shigella infections in children: new insights. Semin Pediatr Infect Dis. 2004;15:246–252. [DOI] [PubMed] [Google Scholar]
- [2]. Christopher PRH, David KV, John SM, et al. Antibiotic therapy for Shigella dysentery. Cochrane Database Sys Rev. 2010;8:CD006784 DOI: 10.1002/14651858.CD006784.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Liu J, Platts-Mills J, Juma J, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet. 2016;388:1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. World Health Organization Guidelines for the control of shigellosis, including epidemics due to Shigella dysenteriae type 1. 2005. Available from: http://www.who.int/cholera/publications/shigellosis/en/
- [5]. Taylor M. Enterohaemorrhagic Escherichia coli and Shigella dysenteriae type 1-induced haemolytic uraemic syndrome. Paediatr Nephrol. 2008;23:1425–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Kotloff K, Winickoff JP, Ivanoff B, et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull WHO. 1999;77:651–666. [PMC free article] [PubMed] [Google Scholar]
- [7]. American Academy of Pediatrics Shigellosis In: Kimberlin M, Jackson M, Long S, et al., editors. Red Book: 2015. report of the committee on infectious diseases, 30th ed. Elk Grove Village (IL): American Academy of Pediatrics, 2015; p. 706–709. [Google Scholar]
- [8]. Gu B, Pan S, Zhuang L, et al. Comparison of the prevalence and changing resistance to nalidixic acid and ciprofloxacin of Shigella between Europe–America and Asia–Africa from 1998 to 2009. Int J Antimicrob Agents. 2012;40:9–17. [DOI] [PubMed] [Google Scholar]
- [9]. von Seidlein KDR, Ali M, Lee H, et al. A multicentre study of Shigella Diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Med. 2006;3:e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. World Health Organization Pocket book of hospital care for children. 2nd ed Geneva: WHO; 2013. [PubMed] [Google Scholar]
- [11]. Balshem H, Helfand M, Schünemann H, et al. Grade guidelines 3: rating the quality of the evidence – introduction. J Clin Epidemiol. 2011;64:401–406. [DOI] [PubMed] [Google Scholar]
- [12]. Das A, Salam R, Bhutta Z, et al. Antibiotics for the treatment of cholera, shigella and cryptosporidium in children. BMC Public Health. 2013;Suppl. 3:S3–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Walker C, Bryce J, Bahl R, et al. Standards for CHERG reviews of intervention effects on child survival. Int J Epidemiol. 2010;39:i21–i31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Traa C, Walker C, Munos M, et al. Antibiotics for the treatment of dysentery in children. Int J Epidemiol. 2010;39(Supplement 1):i70–i74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Gu B, Zhou M, Ke X, et al. Comparison of resistance to third-generation cephalosporins in Shigella between Europe–America and Asia–Africa from 1998 to 2012. Epidemiol Infect. 2015;143:2687–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Gu B, Zhou M, Ke X, et al. Prevalence and trends of aminoglycoside resistance in Shigella worldwide, 1999–2010. J Biomed Res. 2013;27:103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Vinh V, Campbell J, Hoang N, et al. A multi-center randomized trial to assess the efficacy of gatifloxacin versus ciprofloxacin for the treatment of shigellosis in vietnamese children. PLoS Negl Trop Dis. 2011;5:e1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Shanks B, Aleman G, Oundo J, et al. Single dose of azithromycin or three‐day course of ciprofloxacin as therapy for epidemic dysentery in Kenya. Clin Infect Dis. 1999;29:942–943. [DOI] [PubMed] [Google Scholar]
- [19]. Das AS, Ferdous F, Farzana F, et al. Etiological diversity of diarrhoeal disease in Bangladesh. J Infect Dev Ctries. 2013;7:900–909. [DOI] [PubMed] [Google Scholar]
- [20]. Das SK, Klontz EH, Azmi IJ, et al. Characteristics of multidrug resistant Shigella and Vibrio cholerae 01 infections in patients treated at an urban and a rural hospital in Bangladesh. ISRN Microbiol. 2013;213915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Thompson N, Vinh P, Duc A, et al. Clinical implications of reduced susceptibility to fluoroquinolones in paediatric Shigella sonnei and Shigella flexneri infections. J Antimicrob Chemother. 2016;71:807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Heiman J, Sjolund-Karlsson M, Bowen A, et al. Shigellosis with decreased susceptibility to azithromycin. Paediatr Infect Dis J. 2016;33:1204–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Centers for Disease Control and Prevention Ciprofloxacin- and azithromycin-nonsusceptible Shigellosis in the United States. CDC Health Alert Network; 2015. Available from: https://emergency.cdc.gov/han/han00379.asp [Google Scholar]
- [24]. Howie RL, Bowen A, Barzilay EJ, et al. Reduced azithromycin susceptibility in Shigella sonnei, United States. Microbiol Drug Resist. 2010;16:245–248. [DOI] [PubMed] [Google Scholar]
- [25]. Guay R, Harding G, Meatherall D. Pharmacokinetics of cefixime in healthy subjects and patients with renal insufficiency. Antimicrob Agents Chemother. 1986;30:485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Ashkenazi S, Waisman Y, Rachmel A, et al. A randomised, double-blind study comparing cefixime and trimethoprim-sulfamethoxazole in the treatment of childhood Shigellosis. J Pediatr. 1993;123:817–821. [DOI] [PubMed] [Google Scholar]
- [27]. Basualdo W, Arbo A. Randomized comparison of azithromycin versus cefixime for treatment of shigellosis in children. Paediatr Infect Dis. 2003;22:374–373. [PubMed] [Google Scholar]
- [28]. Girgis N, Hammad O, Farid Z, et al. Comparison of the efficacy, safety and cost of cefixime, ceftriaxone and aztreonam in the treatment of multidrug-resistant Salmonella typhi septicemia in children. Pediatr Infect Dis J. 1995;14:603–605. [DOI] [PubMed] [Google Scholar]
- [29]. Martin R, Maffei F, Tritt J, et al. Treatment of shigellosis with cefixime: two days vs. five days. Paediatr. Infect. Dis. 2000;19:522–526. [DOI] [PubMed] [Google Scholar]
- [30]. Guerrant T, Steiner T, Thielman N, et al. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis. 2001;32:331–350. [DOI] [PubMed] [Google Scholar]
- [31]. Shigella [revised November 2014], eTG complete [Internet]. Melbourne: Therapeutic Guidelines Limited; 2015. [Google Scholar]
- [32]. Keshav A, Acheson D, Allerberger F, et al. BMJ best practice: Shigella infection. 2016. [Online]. Available from: http://bestpractice.bmj.com.acs.hcn.com.au/best-practice/monograph-pdf/1174.pdf
- [33]. British National Formulary for Children BMJ Publishing Group. 2016. Available from: https://www.medicinescomplete.com.acs.hcn.com.au/about/publications.htm
- [34]. Hancox M, Vieweg V, Crouse E, et al. Azithromycin, cardiovascular risks, QTc interval prolongation, torsade de pointes, and regulatory issues: a narrative review based on the study of case reports. Ther Adv Infec Dis. 2013;1:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Owens RC, Nolin T. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis. 2006;43:1603–1611. [DOI] [PubMed] [Google Scholar]
- [36]. Dresser GK, Bailey DG, Spence J. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet. 2000;38:41–57. [DOI] [PubMed] [Google Scholar]
- [37]. Ohara Y, Watanabe Y, Cao X, et al. Azithromycin can prolong QT interval and suppress ventricular contraction, but wi not induce Torsade de Pointes. Cardiovasc Toxicol. 2014;15:232–240. [DOI] [PubMed] [Google Scholar]
- [38]. Ray W, Murray K, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Samarendra P, Evans S, Sacchi TJ, et al. QT prolongation associated with azithromycin/amiodarone combination. Clin Electrophysiol. 2001;24:1572–1574. [DOI] [PubMed] [Google Scholar]
- [40]. Howard P. Azithromycin-induced proarrhythmia and cardiovascular death. Ann Pharmacol. 2013;47:1547–1551. [DOI] [PubMed] [Google Scholar]
- [41]. Food and Drug Safety Administration Drug Safety Communication. Azithromycin (Zithromax or Zmax) and the rik of potentially fatal heart rhythms. 2013. [Online]. Available from: https://www.fda.gov/Drugs/DrugSafety/ucm341822.htm
- [42]. Etminan J, Samii A, Brophy M. Oral fluoroquinolone use and risk of peripheral neuropathy. Neurology. 2014;83:1261–1271. [DOI] [PubMed] [Google Scholar]
- [43]. Food and Drug Administration FDA updates warnings for fluoroquinolone antibiotics. 2016. [Online]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm513183.htm.
- [44]. Ali A. Peripheral neuropathy and Guillain-Barré syndrome risks associated with exposure to systemic fluoroquinolones: a pharmacovigilance analysis. Ann Epidemiol. 2014;24:279–285. [DOI] [PubMed] [Google Scholar]
- [45]. Chandra P, Sagara I, Sie A, et al. Comparison of azithromycin plus chloroquine versus artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in children in Africa: a randomized, open-label study. Malaria J. 2015;14:108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Kimani K, Kamiza S, Duparc S, et al. Efficacy and safety of azithromycin-chloroquine versus sulfadoxine-pyrimethamine for intermittent preventive treatment of plasmodium falciparum malaria infection in pregnant women in Africa: an open-label, randomized trial. PLoS One. 2016;21:e0157045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Fossa T, Duncan J, Deng S, et al. Azithromycin/chloroquine combination does not increase cardiac instability despite an increase in monophasic action potential duration in the anesthetized guinea pig. Am J Trop Med Hyg. 2007;77:929–938. [PubMed] [Google Scholar]
- [48]. Egunsola K, Oshikoya O. Comparative safety of artemether-lumefantrine and other artemisinin-based combinations in children: a systematic review. Malaria J. 2013;12:385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Sykes I, Mtove G, Mandea V, et al. Azithromycin plus artesunate versus artemether‐lumefantrine for treatment of uncomplicated malaria in Tanzanian children: a randomized, controlled trial. Clin Infect Dis. 2009;49:1195–1201. [DOI] [PubMed] [Google Scholar]
- [50]. Baiden A, Oduro A, Halidou T, et al. Prospective observational study to evaluate the clinical safety of the fixed-dose artemisinin-based combination Eurartesim® (dihydroartemisinin/piperaquine), in public health facilities in Burkina Faso, Mozambique, Ghana, and Tanzania. Malaria J. 2015;14:160. doi: 10.1186/s12936-015-0664-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Manning P, Lon C, Spring M, et al. Randomized, double-blind, placebo-controlled clinical trial of a two-day regimen of dihydroartemisinin-piperaquine for malaria prevention halted for concern over prolonged corrected QT interval. Antimicrob Agents Chemother. 2014;58:6056–6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. World Health Organization Antimicrobial Resistance Global Report on Surveillance. 2014. [Online]. Available from: http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf.
- [53]. Zhang CL, Liu QZ, Wang J, et al. Epidemic and virulence characteristic of Shigella spp. with extended-spectrum cephalosporin resistance in Xiaoshan District, Hangzhou, China. BMC Infect. Dis. 2014;14:107 DOI: 10.1186/1471-2334-14-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Bowen A, Hoover J, Hurd C. Importation and domestic transmission of Shigella sonnei resistant to ciprofloxacin – United States, May 2014 – February 2015. Morbid Mortal Wkly Rep. 2015;64:318–320. [PMC free article] [PubMed] [Google Scholar]
- [55]. De Lappe N, Garvey P, O’Connor J. Ciprofloxacin-resistant Shigella sonnei associated with travel to India. Emerg Infect Dis. 2015;21:894–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Centers for Disease Control Nationwide dissemination of multiply resistant Shigella sonnei following a commonsource outbreak. Morbid Mortal Wkly Rep. 1987;36:633–634. [PubMed] [Google Scholar]
- [57]. Centers for Disease Control Shigella dysenteriae type 1 – Guatemala. Morbid Mortal Wkly Rep. 1991;40:421–428. [PubMed] [Google Scholar]
- [58]. Liu Y, Walsh T, Ling-Xian B, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16:161–168. [DOI] [PubMed] [Google Scholar]
- [59]. Li B, Ni Y, Sun J, et al. Molecular characterization of the extended-spectrum beta-lactamase (ESBL)-producing Shigella spp. in Shanghai. Eur J Clin Microbiol. Infect. Dis. 2015;34:447–451. [DOI] [PubMed] [Google Scholar]
- [60]. Sabra G, Kattar M, Abi-Rached R, et al. Molecular characterization of ESBL-producing Shigella sonnei isolates from patients with bacilliary dysentery in Lebanon. J Infect Dis. Dev Ctries. 2009;3:300–305. [DOI] [PubMed] [Google Scholar]