Abstract
With the introduction of direct-acting antiviral agents (DAA), the rate of sustained virological response (SVR) in the treatment of hepatitis C virus (HCV) has radically improved to over 95%. Robust scientific evidence supports a beneficial role of SVR after interferon therapy in the progression of cirrhosis, resulting in a decreased incidence of hepatocellular carcinoma (HCC). However, a debate on the impact of DAAs on the development of HCC is ongoing. This review aimed to analyse the scientific literature regarding the risk of HCC in terms of its recurrence and occurrence after the use of DAAs to eradicate HCV infection. Among 11 studies examining HCC occurrence, the de novo incidence rate ranged from 0 to 7.4% (maximum follow-up: 18 mo). Among 18 studies regarding HCC recurrence, the rate ranged from 0 to 54.4% (maximum “not well-defined” follow-up: 32 mo). This review highlights the major difficulties in interpreting data and reconciling the results of the included studies. These difficulties include heterogeneous cohorts, potential misclassifications of HCC prior to DAA therapy, the absence of an adequate control group, short follow-up times and different kinds of follow-up. Moreover, no clinical feature-based scoring system accounts for the molecular characteristics and pathobiology of the tumours. Nonetheless, this review does not suggest that there is a higher rate of de novo HCC occurrence or recurrence after DAA therapy in patients with previous HCV infection.
Keywords: Hepatocellular carcinoma, Hepatitis C virus, Direct-acting antiviral agents, Occurrence, Recurrence
Core tip: A significant debate about the impact of direct-acting antiviral agents (DAAs) on the development of hepatocellular carcinoma (HCC) is currently ongoing. After a full review of the published literature, the evidence does not suggest that there is a higher rate of de novo HCC occurrence or recurrence after DAA therapy in patients with previous hepatitis C virus infection.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide. Cirrhosis is the strongest risk factor for HCC, and chronic hepatitis C (HCV) is the most common underlying aetiology in both the United States and Europe[1].
The annual risk of developing HCC in HCV-related cirrhotic patients is approximately 2%-8%[2].
Though curative treatments, such as liver resection (LR) and radiofrequency ablation (RFA), exist, once HCC develops, the rate of cumulative recurrence is known to be approximately 70% over 5 years[3].
Since 2013, the treatment of hepatitis C has dramatically changed. The generation of new direct-acting antiviral agents (DAAs) has been extraordinarily effective, safe and well-tolerated. These DAAs have spurred a revolution in the treatment of HCV patients, showing sustained virological response (SVR) rates exceeding 95% in real-life settings[4]. This tremendous advancement has provided therapeutic options for a larger number of patients, many of which have advanced liver disease and a higher risk of liver decompensation and HCC.
Over the past two decades, several meta-analyses have evaluated the role of SVR in preventing hepatic decompensation, reducing the necessity for liver transplantation, increasing both liver-related and overall survival, and reducing the risk of occurrence and recurrence of HCC in HCV cirrhotic patients. Studies from the interferon (IFN)-era taught us that HCV eradication reduces but does not eliminate the risk of HCC once cirrhosis has been established[5]. Accordingly, Nishiguchi et al[6] demonstrated that IFN reduced the incidence of HCC in patients with HCV infection and cirrhosis and showed that IFN might improve the prognosis of cirrhotic patients by improving liver function and by delaying the development or growth of hepatocellular carcinoma.
However, the impact that an SVR from a DAA regimen has on liver cancer occurrence and recurrence seems to be controversial. Due to the possibility of treating patients with more advanced liver disease, it is not surprising to observe the development of HCC after providing HCV therapy. In the spring of 2016, this topic became of particular interest because of the publication of two papers from Spain[7] and Italy[8] that suggested a potential increase in the occurrence and recurrence rates of HCC in patients who were treated with DAAs. Since these two original publications, more than 100 papers, letters and communications have also been published on this topic. Most of the controversies arise from the heterogeneity of populations, variations in the inclusion and exclusion criteria for each study, the follow-up time, the time points used to analyse the occurrence and recurrence rates, and so on.
The debate in the scientific community is still ongoing. Thus, the aim of this review was to analyse the published data concerning the occurrence and recurrence of HCC after DAA treatment to reconcile the conflicting results between studies.
LITERATURE SEARCH
Original published studies were identified by searching PubMed, Embase, and the Cochrane Library database (through April 2018). English language articles were selected using the following keywords: ‘hepatocellular carcinoma’, ‘HCC’, ‘chronic hepatitis C’, ‘HCV’, ‘DAA’, ‘direct-acting antivirals’, ‘occurrence’ and ‘recurrence.’ Two independent evaluators (MG and AS) identified all reports that may have pertained to the review issue. Case reports were excluded. A full-text evaluation was performed, and the references from relevant articles were reviewed to identify additional studies. Manual cross-referencing was performed, and relevant abstracts from the 2017 International Congress abstract books (AASLD and EASL) were included.
HCV-DAA AND HCC OCCURRENCE
Definition of HCC occurrence
HCC occurrence was defined as the de novo appearance of HCC in a subject (with or without an underlying liver disease) with no history or previous evidence or suspicion of a liver tumour.
The risk of HCC occurrence after DAA treatment
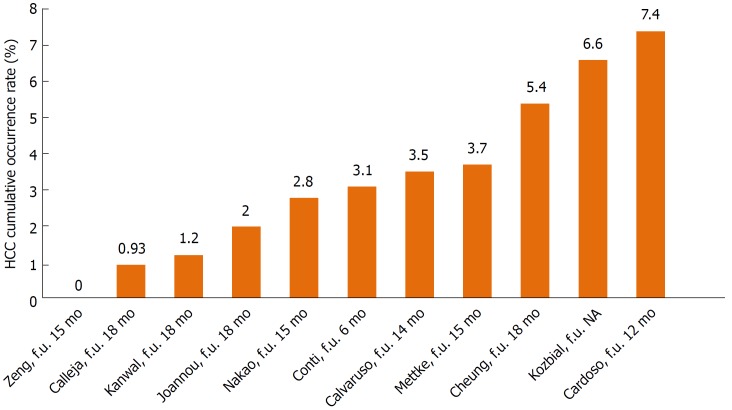
Recent studies have suggested that DAA may accelerate the occurrence of HCC in patients with liver cirrhosis. However, the annual risk of HCC in HCV-related cirrhotic patients is approximately 2%-8%[2]. Ultimately, the results of these studies did not show an increased risk of HCC in comparison to the expected annual risk for patients treated with DAAs. Table 1 summarises the results of the principal studies addressing de novo HCC occurrence after DAA treatment. Figure 1 shows the de novo HCC cumulative incidence rates reported by 11 studies.
Table 1.
Main characteristics of the studies on hepatocellular carcinoma occurrence
| Ref. | Journal, year | Country | Study design | Sample size | Median follow-up (mo) | HCC cumulative incidence (%) | Risk factors | Notes |
| Conti et al[8] | J Hepatol, 2016 | Italy | Retrospective | 207 | 6 | 3.1 | cirrhosis, child class B, low platelet count | No control group |
| Cheung et al[15] | J Hepatol, 2016 | United Kindom | Prospective | 406 | 18 | 5.4 in SVR group | NA | Control groups: Non-SVR with HCC incidence of 11.3% |
| Kozbial et al[12] | J Hepatol, 2016 | Austria | Retrospective | 16 | NA | 6.6 | NA | Letter to editor |
| Cardoso et al[10] | J Hepatol, 2016 | Portugal | Retrospective | 54 | 12 | 7.4 | NA | Letter to editor |
| Kanwal et al[13] | Gastroenterology, 2017 | United States | Retrospective | 22500 | NA | 1.2 | cirrhosis, alcohol use | HCC incidence: 0.9% in SVR, 2.9% in non-SVR |
| Zeng et al[18] | J Hepatol, 2016 | China | Retrospective | 21 | 15 | 0 | NA | Letter to editor |
| Calleja et al[14] | J Hepatol, 2017 | Spain | Retrospective | 3325 | 18 | 0.93 | NA | - |
| Ioannou et al[31] | J Hepatol, 2017 | United States | Retrospective | 21948 | 18 | 2 | NA | HCC incidence: 1.4% in SVR, 8.1% in non-SVR |
| Mettke et al[17] | Aliment Pharmacol Ther, 2017 | Germany | Prospective | 158 | 15 | 3.7 | MELD score, AFP level | HCC incidence in untreated: 7.6% |
| Nakao et al[9] | J Hepatol, 2017 | Japan | Retrospective | 242 | 15 | 2.8 | NA | Letter to editor |
| Calvaruso et al[19] | Gastroenterology, 2018 | Italy | PWrospective | 2249 | 14 | 3.5 | low albumin level, absence of SVR, low platelet count | The rate of HCC at 1 yr was 2.9%. |
AFP: Alpha-fetoprotein; DAAs: Direct-acting antiviral agents; HCC: Hepatocellular carcinoma; HCV: Hepatitis C virus; LR: Liver resection; LRT: Local-regional therapy; LT: Liver transplantation; SVR: Sustained virological response; RFA: Radiofrequency ablation; TACE: Trans-catheter arterial chemoembolization; NA: Not available.
Figure 1.
Hepatocellular carcinoma cumulative occurrence rates reported by 11 studies considered in the present review. HCC: Hepatocellular carcinoma; f.u.: Follow up; NA: Not available.
The first paper published was by Conti et al[8]. The authors reported an HCC occurrence rate of 3.2% at 6 mo of follow-up after beginning DAA therapy. Similarly, Nakao et al[9] observed cumulative HCC incidences after 1 year and 2 years of DAA therapy of 1.7% and 7%, respectively. Additionally, Cardoso et al[10] reported an HCC incidence of 7.4% in the first year after SVR from DAA treatment. This rate was higher than the previously reported incidence rate for IFN regimens (1.2%-1.4%)[10,11]. Median time for HCC development was 7.6 mo after HCV-RNA became non-detectable[10]. Accordingly, Kozbial et al[12] reported an overall cumulative incidence of de novo HCC after DAA treatment of 6.6%, while this rate was 5.2% in patients achieving SVR, with a follow-up time of 48 wk.
In contrast to these initial observations, several larger studies found different results (Table 1). Kanwal et al[13] reported that cirrhotic patients with SVR had a significantly reduced risk of HCC compared to patients without SVR (76% risk reduction). Moreover, they highlighted that the HCV-treated population has changed significantly in the DAA era and now includes many patients with other HCC risk factors (alcohol abuse, age older than 65 years and patients with advanced cirrhosis), which were previously criteria for exclusion from IFN therapy[13]. Because the risk of HCC in patients with HCV cirrhosis is 2%-8% per year[2] a 76% reduction represents a significant clinical advantage. In the largest real-world study, Calleja et al[14] enrolled almost 4000 patients who were treated with DAAs. The study reported an HCC incidence rate of 0.93% within 18 mo of starting DAA treatment, though measuring the incidence of HCC was not an objective of this study. They also observed that HCC was more common in patients with cirrhosis, but this was not related to the achievement of a SVR[14]. Similarly, a prospective study by Cheung et al[15] examined 406 patients with decompensated cirrhosis through the English Expanded Access Programme. The study found no evidence of an increased risk for liver cancer during DAA therapy or over the following 12 mo. Moreover, for HCC developing within 3 mo of starting DAA treatment, the authors suggested that there was a possibility that the patients already had an undiagnosed cancer before starting the treatment[15]. Additionally, Hasson et al[16] reported similar results showing that DAA-based therapy in HIV/HCV co-infected patients did not show an increasing cancer trend during 24 wk of follow-up, though, as expected, a high rate of SVR was confirmed (97%). During standard surveillance (liver ultrasound and AFP every six months) for a median follow-up of 87 wk after beginning DAA treatment, de novo HCC was diagnosed in 3/118 (2.5%) patients with advanced liver disease. The authors concluded that strict surveillance is mandatory, especially in patients with additional known risk factors for HCC (e.g., advanced cirrhosis, genotype 3, diabetes, metabolic syndrome, HIV co-infection)[16]. Finally, in a large prospective study, Mettke et al[17] showed that patients with liver cirrhosis who received DAA therapy had a similar HCC incidence over a short time compared to an untreated historical control cohort that was recruited from the same centre (158 DAA-treated and 184 control patients with HCV liver cirrhosis). HCC developed in 6 DAA patients and 14 untreated patients, yielding HCC incidence rates of 2.90 and 4.48 per 100 person-years, respectively. Moreover, they identified that higher MELD scores and AFP levels are independent HCC risk factors[17]. Similarly, Zeng et al[18] reported no cases of HCC occurrence in their 21 cirrhotic patients treated with 12 weeks of DAAs after a median follow-up time of 15 mo.
In the recent prospective observational study based on RESIST-HCV (Rete Sicilia Selezione Terapia - HCV), a web-based regional database, Calvaruso et al[19] confirmed the early benefit of viral eradication in HCV cirrhosis throughout all stages of cirrhosis. The authors analysed only patients with cirrhosis (2249 patients: 90.5% Child-Pugh class A, 9.5% Child-Pugh class B) treated with DAA from March 2015 until July 2016 in 22 academic and community liver centres in Sicily Region, Italy. Only 78 patients (3.4%) developed HCC during a mean observation of 14 mo from the start of DAA treatment; the overall cumulative rate of HCC at 1 year was 2.9%. Of interest, the occurrence of HCC is significantly reduced in patients with compensated cirrhosis without signs of portal hypertension and normal liver function. Moreover, the authors did not support the hypothesis that HCC that develops during DAA or after early follow-up is more aggressive and more difficult to treat with available therapies. In fact, none of the 78 patients had a multinodular or aggressive HCC pattern[19].
Several risk factors have been identified for HCC occurrence after DAA treatment (Table 1), such as HCV treatment failure, alcohol use, aged older than 65 years, male gender, presence of cirrhosis, HCV genotype 3 infection, diabetes, metabolic syndrome, HIV co-infection, higher MELD scores, lower albumin level, lower platelet count, and higher AFP levels[13,16,17,19,20].
In trying to summarize these data, we can conclude that de novo HCC occurrence risk does not appear to be reduced in the time immediately following DAA treatment compared to untreated patients. On the other hand, we do not have evidence to suggest that the occurrence rate increases either, as there was an absence of an adequate control group in most studies. Unfortunately, the risk of HCC does not vanish rapidly, and a longer observation period is probably necessary to observe any beneficial effects.
Comparing the risk of HCC occurrence after DAAs vs after IFN-based treatments
IFN-based regimens were approved for treating chronic hepatitis C more than 20 years ago. Many studies have demonstrated the long-term effects of this therapy in obtaining an SVR, which is considered a milestone in reducing the risk of liver-related complications, such as HCC[5,21-25].
However, though the benefits of this treatment in reducing HCC incidence are well documented, the risk is not completely removed in patients with severe fibrosis or cirrhosis. Such conditions occur with an annual incidence of 0.3%-1%[1]. These data were recently confirmed in an elegant study by D’Ambrosio et al[26], who investigated a cohort of 38 histological cirrhotic patients who had been prospectively followed for 10 years after achieving a SVR with IFN treatment. During a median follow-up of 86 mo after a liver biopsy, no patients developed clinical decompensation, while 5 patients (13%) developed HCC. The 8-year cumulative probability of HCC was thus 17% regardless of the cirrhosis regression, while the 8-year cumulative survival probability was 97% regardless of the cirrhosis regression (96% vs 100%, P = 1.0) or HCC development (100% vs 97%, P = 1.0)[26].
Compared to IFN regimens, less consistent data are available concerning the impact of DAA therapy on HCC occurrence. Moreover, the HCC risk after DAA treatment compared to IFN treatment faces recruitment bias since two different types of patients are involved in these treatments.
An interesting manuscript by Lu et al[27] showed data from a long-term follow-up for HCC detection in numerous HCV cohorts, including both patients who were treated with antiviral therapy and those who were not. The treated population was divided into patients who achieved an SVR and those who did not. The lack of SVR achievement emerged as a risk factor for HCC development. They also showed that an SVR reduced the risk of all-cause mortality in patients with advanced fibrosis[27]. Innes et al[28] showed similar results, confirming that there is a higher risk of HCC occurrence after DAA therapy compared to IFN therapy. This result reflects a change in the types of patients being treated (older with a higher Child-Pugh score, with both thrombocytopenic and treatment experienced). A major finding from this study was that upon adjusting for these differences, the risk of HCC that was associated with IFN-free regimens was similar to the risk related to INF regimens[28]. Furthermore, Bielen et al[29] did not find higher-than-expected early occurrence rates of HCC in patients treated with DAA regimens (1.1%; 4/355) compared to patients treated with IFN regimens (1.7%; 1/59). These rates were comparable to the estimated 1% per year frequency of HCC that is observed in patients with an SVR who were treated with IFN and ribavirin dual therapy. Additionally, in a retrospective study by Nagata et al[30], the risk rates of HCC occurrence after viral eradication were similar between IFN-based and IFN-free therapies. After viral eradication, 2.5% of patients developed HCC after IFN-based therapy during a median follow-up period of 6.8 years, while 1.1% of patients developed HCC after DAA therapy during a median follow-up period of 1.8 years (p-value: not significant). The Nagata study also showed that Wisteria floribunda agglutinin and Mac2-binding protein-positive (WFA + M2BP) were independently associated with HCC occurrence after viral eradication among patients without severe fibrosis[30]. Ioannou et al[31] recently published a study on 62,354 patients who received antiviral treatment in the Veterans Affairs National Healthcare system between 1/1/1999 and 12/31/2015. The study included 35871 (58%) IFN-based regimens, 4535 (7.2%) DAA+ IFN regimens and 21948 (35%) DAA-based regimens. They identified 3271 incident cases of HCC diagnosed at least 180 days after initiation of the antiviral treatment over a mean follow-up of 6.1 years. The incidence of HCC was highest in patients with cirrhosis and who were experiencing treatment failure. SVR was associated with a significantly decreased risk of HCC (a 71% risk reduction) in the DAA-treated cohort[31]. Kabayashi et al[32] demonstrated that the impact of DAA treatment was similar to that of IFN treatment with regard to HCC risk reduction in patients who achieved a SVR. The 3- and 5-year cumulative HCC development rates were 1.30% and 3.03%, respectively, in the DAA group, and 1.02% and 2.19% in the IFN group, respectively. Similarly, a study by Nagaoki et al[33] examined the cumulative incidence of HCC development after HCV eradication by IFN or DAA treatment and found that HCC development risk after HCV eradication was similar in patients with and without advanced liver fibrosis. A retrospective study by Li et al[34] used the Electronically Retrieved Cohort of HCV Infected Veterans (ERCHIVES) database (17836 persons). The study found that DAA treatment was not associated with a higher risk of HCC in cirrhotic patients, although a significantly higher rate of known risk factors for HCC (including cirrhosis, older age and higher baseline AFP levels) was found in DAA-treated patients compared to that in patients treated with IFN. Among people with cirrhosis who achieved an SVR, the HCC incidence rate was not significantly different in the DAA group compared to that in the IFN group. Furthermore, both groups had a significantly lower probability of HCC compared to patients who received no treatment. These results support the concept that treating HCV and achieving a SVR with any antiviral regimen is the most important determinant for lowering HCC risk[34].
Finally, in a recent meta-analyses (19 prospective, 5 retrospective, and 2 retrospective-prospective cohorts), Waziry et al[35] confirmed that there is no evidence to suggest that DAA therapy is associated with a higher risk of HCC development after curing HCV with DAA therapy in patients with cirrhosis. The treatments reduce individual risk by 63%, which is similar to previous results from IFN-based treatments. There is thus no reason to defer or withhold DAA therapy considering the advantages of this therapeutic strategy[35].
In conclusion, the studies comparing the HCC risk after DAA to the risk after IFN treatment showed similar results, with an SVR being a marker for obtaining a clinical advantage. On the other hand, it is important to be aware that the two populations are heterogeneous and incomparable for age, the presence of comorbidities and the stages of cirrhosis. Consequently, it is only possible to overcome this bias by statistical adjustment, which represents a good approach but is not a definitive demonstration. Even in presence of an SVR, patients with cirrhosis remain at risk for HCC and should continue to undergo HCC surveillance.
HCV-DAA AND HCC RECURRENCE
Definition of HCC recurrence
HCC recurrence was defined as the reappearance of HCC in a subject (with or without an underlying liver disease) who previously had HCC that was judged to be radically and successfully treated by one of the following techniques: surgical resection, liver transplantation, ablative techniques or TACE. The 6-mo recurrence rate of HCC successfully treated with resection or local ablation is estimated to be 20%[36].
Risk of HCC recurrence after treatment with DAAs
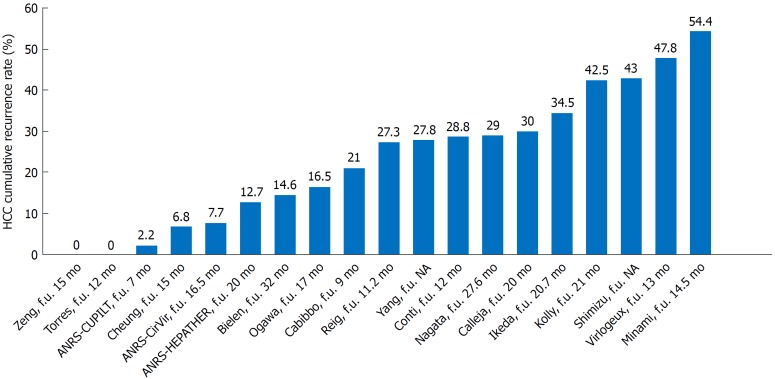
Several studies on the recurrence of HCC after DAA treatment produced intriguing data. Table 2 summarises the results of the key studies addressing this topic. Figure 2 shows the HCC cumulative recurrence rates reported by 18 studies.
Table 2.
Main characteristics of the studies on hepatocellular carcinoma recurrence
| Ref. | Journal, year | Country | Study design | Sample size | Previous HCC treatment | Median follow-up (mo) | HCC recurrence rate (%) | Risk factors | Notes |
| Reig et al[7,37,38] | J Hepatol, 2016 and 2017 - Semin Liv Dis, 2017 | Spain | Retrospective | 77 | Ablation, resection, TACE | 11.2 | 27.3 | NA | Updated cohort of the 58 patients reported in J Hepatol 2016 |
| Conti et al[8] | J Hepatol, 2016 | Italy | Retrospective | 59 | Ablation, resection, TACE | 12 | 28.8 | age, liver stiffness | - |
| ANRS - HEPATHER et al[43] | J Hepatol, 2016 | France | Prospective | 189 | NA | 20 | 12.7 | NA | - |
| ANRS - CirVir et al[43] | J Hepatol, 2016 | France | Prospective | 13 | Ablation, resection | 16.5 | 7.7 | NA | - |
| ANRS - CUPILT et al[43] | J Hepatol, 2016 | France | Prospective | 314 | Bridge therapy before LT (Ablation, resection, TACE, chemotherapy) | 7 | 2.2 | NA | - |
| Calleja et al[14] | J Hepatol, 2017 | Spain | Retrospective | 70 | NA | 20 | 30 | NA | - |
| Cabibbo et al[50] | APT, 2017 | Italy | Prospective | 143 | Ablation, resection, TACE | 9 | 21 | previous recurrence, tumour size | - |
| Minami et al[55,56] | J Hepatol 2016 - Abs AASLD 2017 | Japan | Retrospective | 163 | Ablation, resection, TACE, radiotherapy | 14.5 | 54.4 | AFP-L3, DCP, number of previous treatment for HCC, interval between last HCC treatment and DAA start | Abstract of AASLD 2017 |
| Virlogeux et al[46] | Liver Int 2017 | France | Retrospective | 23 | Ablation, resection, TACE | 13 | 47.8 | NA | - |
| Zeng et al[18] | J Hepatol, 2016 | China | Retrospective | 10 | Ablation | 15 | 0 | NA | Letter to Editor |
| Ogawa et al[48] | Aliment Pharmacol Ther, 2017 | Japan | Prospective | 152 | Ablation, resection, TACE, radiotherapy | 17 | 16.5 | cirrhosis, time from previous HCC treatment < 1 yr, non-curative HCC treatment (TACE, radiotherapy) | - |
| Cheung et al[15] | J Hepatol 2016 | United Kingdom | Prospective | 29 | NA | 15 | 6.8 | NA | - |
| Torres et al[44] | J Hepatol 2016 | United States | Prospective | 8 | Ablation, resection, proton therapy | 12 | 0 | NA | Letter to Editor |
| Yang et al[41] | J Hepatol 2016 | United States | Prospective | 18 | Bridge therapy before LT: Ablation, TACE | NA | 27.8 | NA | Letter to Editor; untreated patients: 9.5% |
| Bielen et al[29] | J Viral Hepat 2017 | Belgium | Retrospective | 41 | LT, ablation, resection, TACE | 32 | 14.6 | SVR | - |
| Ikeda et al[45] | Dig Dis Sci 2017 | Japan | Retrospective | 177 | Ablation, resection, TACE, radiotherapy | 20.7 | 34.5 | multiple HCC treatment, AFP level, Prothrombin time | - |
| Kolly et al[39] | J Hepatol 2017 | Germany, Belgium, Switzerland | Prospective | 56 | Ablation, resection, TACE | 21 | 42.5 | time from previous HCC treatment | Letter to Editor |
| Nagata et al[30] | J Hepatol 2017 | Japan | Retrospective | 83 | Ablation, resection | 27.6 | 29 | SVR, AFP, WFA-M2BP | - |
| Shimizu et al[52] | Indian J Gastroenterol 2017 | Indian | Retrospective | 23 | RFA, TACE, radiation | NA | 43 | HBcAb positivity, TACE | - |
| Mashiba et al[57] | Plos one 2018 | Japan | Retrospective | 368 | NA | 24 | NA | AFP level, SVR, clinical stage of HCC | - |
AFP: Alpha-fetoprotein; DAAs: Direct-acting antiviral agents; HCC: Hepatocellular carcinoma; HCV: Hepatitis C virus; LR: Liver resection; LRT: Local-regional therapy; LT: Liver transplantation; SVR: Sustained virological response; RFA: Radiofrequency ablation; TACE: Trans-catheter arterial chemoembolization; NA: Not available.
Figure 2.
Hepatocellular carcinoma cumulative recurrence rates reported by 17 studies considered in the present review. HCC: Hepatocellular carcinoma; f.u.: Follow up; NA: Not available.
There is ongoing uncertainty about the potential risks and benefits of DAA therapy in patients with a history of previous HCC. All published data should be analysed according to their methodological limitations and should consider the large differences between studies in terms of follow-up duration, clinical heterogeneity and patient selection. Analyses of the recurrence rates should also account for the time since previous HCC treatments due to the high HCC recurrence rate during the first 2 years after HCC treatment.
The first paper published on this topic came from a group in Barcelona[7]. The paper suggested that there is a potential increased risk of HCC recurrence after DAA treatment. In this multicentre retrospective study, Reig et al[7] observed that 16 of 58 (27.6%) patients who were treated with DAAs had HCC recurrence after a median follow-up of 5.7 mo. They observed that patients with a short amount of time between HCC treatment and the start of DAA therapy (under 4 mo) seemed to be at a greater risk, with a recurrence rate of 41.2%. Additionally, 6 patients had HCC recurrence within 2 wk of initiating their DAA treatment[7]. Moreover, after updating their cohort (with more patients who were followed for a longer time period), the updated results not only confirmed their initial findings but also exposed a more aggressive pattern of recurrence and a faster tumour progression[37,38].
At the same time, Conti et al[8] found that 17 out of 59 patients (29%) had HCC recurrence during the first 24 wk of follow-up after completing their DAA treatment. Recurrence was associated with being of a younger age and having more severe liver fibrosis[8]. Similarly, Calleja et al[14] reported an HCC recurrence rate of 30% within 12 mo of starting DAA treatment.
Three later retrospective studies reported a high rate of HCC recurrence after treatment with DAAs in cirrhotic patients[39,40] and in patients who underwent liver transplants[41]. The European multicentre study by Kolly et al[39] reported an HCC disease-free survival rate of 77% at six months after HCV therapy. This finding is in line with the findings reported by Reig et al[7] and Conti et al[8]. In this study, the time between the HCC treatment and the start of the DAA therapy was a significant, time-dependent predictor of recurrence. An Egyptian prospective study by El Kassas et al[40] showed that exposure to DAA treatment was associated with a significantly increased risk of HCC recurrence of 3.82 over a median follow-up period of 16 mo, compared to non-exposed patients. Yang et al[41] reported an increased risk of recurrence of HCC in a small group of transplant patients who received DAA therapy while they were waiting for their liver transplant. HCC recurrence was more frequently observed in DAA-treated recipients than untreated patients (27.8% vs 9.5%). Moreover, HCC recurrence occurred very early after the transplant (< 6 mo) in most recipients, with a higher frequency in the DAA-treated group compared to the untreated group (80% vs 33%). In most of cases, HCC recurred as an extrahepatic disease[41].
Despite the lower rate of occurrence after DAA treatment found in a cohort examined by Bielen et al[29], there was a high early recurrence rate of HCC (15%) within 6 mo after DAA treatment. In contrast, with a report by Yang et al[41], patients with HCC recurrence had a lower SVR rate. Interestingly, only patients who were treated with LR or RFA for the primary HCC experienced recurrences, whereas patients treated with liver transplantation (LT) did not. However, very recently Huang et al[42] performed a retrospective cohort study of 149 LT candidates with HCV and HCC to determine the impact of DAAs on HCC recurrence after local-regional therapy (LRT) and waitlist dropout. They showed advanced cirrhosis and lower rates of complete radiologic tumour response after LRT in patients who were not treated with DAAs (n = 87) compared to patients treated with DAA (n = 62). The cumulative incidence of HCC recurrence within 1-year of complete response after LRT was 47.0% in the DAA group and 49.8% in the untreated group (P = 0.93). In an adjusted competing risk analysis that used weighted propensity score modelling, the risk of HCC recurrence was similar between the DAA and the untreated groups (HR = 0.91, 95%CI: 0.58-1.42, P = 0.67). Patients treated with DAA had a lower risk of waitlist dropout due to tumour progression or death compared to the untreated group in the adjusted, weighted analysis (HR = 0.30, 95%CI: 0.13-0.69, P = 0.005)[42].
In contrast, the large ANRS study from France used three different prospective cohorts of HCC patients previously treated with curative therapies (including LT). The study reported no differences in the recurrence rates of patients who were treated with DAAs and those who were not[43]. Similarly, Zeng et al[18] and Torres et al[44] published their own prospective studies with median follow-up times of 12 and 15 mo, respectively. Both studies reported that there were no HCC recurrences among any of their patients.
Ikeda et al[45] then showed that DAA therapy significantly decreased recurrence rate when it is performed after an initial successfully HCC therapy, compared with the recurrence rate in untreated patients. They analysed a total of 270.81 patient-years, with 61 recurrence events (median follow-up of 20.7 mo). The 1- and 2-year cumulative recurrence rates in the DAA cohort were 30.1% and 38.9%, respectively. The HCC recurrence rate was significantly higher in patients with a history of previous recurrence or multiple treatments for HCC[45]. Additionally, Virlogeux et al[46] confirmed this trend by showing that the HCC recurrence rate was significantly lower among patients treated with DAAs compared with untreated patients (1.7/100 person-months vs 4.2/100 person-months, respectively). Similarly, Zavaglia et al[47] reported a recurrence rate of 3.1% over a median follow-up of 8 mo. They suggested that the longer time interval between complete eradication of the tumour and the start of the antiviral therapy (median 19.3 mo in this study and 11.2 mo in a study by Reig) may explain part of the observed difference. In fact, the longer this interval, the lower the risk that any residual tumour would be present at the start of DAA therapy[47]. A large multicentre cohort study conducted by Ogawa et al[48] showed no evidence of an increased risk of HCC recurrence in patients treated with DAA regimens. The study included 152 consecutive patients with a history of previous HCC who achieved an SVR after treatment with DAAs. During the follow-up period (median: 17 mo), 16.5% of patients developed HCC. The 1-year cumulative HCC recurrence rate was 23.1% when limited to cirrhotic patients[48]. Mazzarelli et al[49] commented that the risk of HCC recurrence may have been higher because there was a relatively short amount of time between the successful completion of the HCC treatment and the initiation of the DAAs. This suggests that patients may not have sufficient time to be deemed cancer-free.
Moreover, a large prospective study by Cabibbo et al[50] found that the 6-mo and 1-year probabilities of HCC recurrence after DAA therapy were 12% and 26.6%, respectively. Previous history of HCC recurrence and tumour size were the only two independent risk factors for early HCC recurrence. They concluded that the probability of early recurrence in patients who had previously been cured of HCC remained high, despite HCV eradication by DAA treatment. This risk was comparable, but not higher than, the risk reported in the literature concerning patients who did not receive DAA treatment[50].
Beste et al[51] reported that HCV can be cured in the majority of patients with HCC, in virtually all patients with a prior history of HCC and in patients who receive subsequent LT. The study also reported that these patients do not have an increased risk of recurrence. Compared to others, this study benefited from its use of a large cohort of HCC patients (624 HCC patients from 17487 HCV treatment recipients). HCC patients were divided into those who were treated with LT after HCC diagnosis (‘‘HCC/LT” group) and those who were treated with other modalities (‘‘HCC” group) prior to receiving DAA therapy. Overall, the SVR was 91.1% in the non-HCC patients, 74.4% in the HCC patients and 94.0% in the HCC/LT patients[51]. In addition, Shimizu et al[52] identified the anti-HBc positivity as a strong contributing factor for HCC recurrence after DAA therapy. In fact 8/10 patients with a positive value of anti-HBc developed HCC recurrence (80%), in the context of an overall recurrence rate of 43% in their 23 enrolled patients. So checking for anti-HBc seems to be mandatory before starting DAA therapy[52].
From a radiological point of view, a study of Renzulli et al[53] showed that in HCC, microvascular invasion (MVI) is a predictor of early HCC recurrence. In fact, in their retrospective study of patients who received DAA therapy, HCC was diagnosed in 29 of the 344 cirrhotic patients after DAA treatment (3.86% de novo HCC and 30.5% recurrent HCC). Both de novo and recurrent neoplastic nodules showed a significantly higher proportion of MVI (70.7%) than nodules that occurred before DAA therapy (33.3%). This finding supports the hypothesis that there is a similar oncogenic process after DAA therapy regardless of the patient’s history of HCC[53].
As a basis for comparison, recently Cabibbo et al[54] published a meta-analysis of 11 studies evaluating the HCC recurrences in HCV untreated patients. Patients in the studies included in the meta-analysis were characterized by an early HCC and a complete response after surgical resection or ablation. The meta-analysis reported that the actuarial probabilities of recurrence were 7.4%, 20% and 47% at six months, one year and two years, respectively. These data revealed a pooled 3-year survival probability of 79.8%. The authors highlighted that there was a high level of heterogeneity among studies on the recurrence and survival rates. They also reported that, currently, there is no clinical feature-based scoring system that accounts for the molecular characteristics and pathobiology of the tumours (invasiveness, doubling time, angiogenesis and microvascular invasion). These pooled, reported recurrence and survival probabilities provide a useful benchmark for making indirect comparisons of the benefits of HCV eradication using DAAs[54].
Several risk factors have been identified that may increase the risk of HCC recurrence after DAA treatment (Table 2), such as high liver stiffness, antiviral treatment failure, history of previous HCC recurrence, previous HCC shape and stage, AFP level values, des-γ-carbossi-prothrombin (DCP) > 40 mAU/mL, non-curative procedures (such as TACE), anti-HBc positivity, the time interval between HCC complete response and DAA initiation and the levels of WFA + M2BP[8,30,48,50,52,55-57].
In conclusion, DAA treatment does not reduce HCC recurrence risk, though we do not have sufficient data to assume that the recurrence rate is actually increased due to the lack of adequate control groups in many studies.
Comparing the risk of HCC recurrence after DAAs vs after IFN-based treatments
While there are few comparative studies examining patients receiving IFN treatment[28,33,54,55], the data suggest that DAA-treated patients have a similar risk of HCC recurrence as patients receiving IFN-treatments and untreated patients. However, these analyses are limited by several potential confounders such as the degree of liver function or the age of the patients.
A recent meta-analysis published by Waziry et al[35] reported that there was no evidence for different HCC recurrence risks following an SVR from DAA and IFN-based therapies. In this study, the baseline characteristics of the IFN- and DAA-cured populations were similar, including age and stage of cirrhosis, given that HCC is more likely to develop in older cirrhotic patients and that patients with advanced cirrhosis (Child- Pugh B/C) are not eligible for potentially curative HCC treatments.
Similarly, comparing 57 patients who were treated with IFN-based therapies to the 58 patients from the Reig et al[7] study whose HCC was successfully treated with DAAs, Petta et al[58] found recurrence rates of 3.7% vs 5.2% at six months, and 15.2% vs 26.3% at two years, respectively, with no statistically significant differences.
Vukotic el al[59] confirmed this trend and demonstrated the benefit of an SVR. In a retrospective study, they sought to compare the HCC recurrence-free survival of a historical cohort treated with IFN (SVR rate of 31.6%), an untreated cohort and a cohort treated with DAAs (SVR of 93.8%) with a median follow-up duration of 42 mo. In the two treated groups, the time to HCC recurrence measured from the start of the antiviral therapy was lower in the group that was treated with DAAs compared to the group treated with IFN, confirming the beneficial role of a SVR. However, in such retrospective comparisons, the majority of the DAA-treated cohorts had much shorter follow-up periods after antiviral therapy than the historical IFN-based cohorts. Interestingly, the median time to HCC recurrence since the last HCC treatment was significantly higher in both of the treated groups compared to the untreated cohort[59].
In a case-control study, Adhoute et al[60] suggested that DAA treatment should be started after a longer period of follow-up following the successful treatment of HCC. They suggested that a follow-up duration of least 12 mo be used to reduce the risk of early HCC recurrence[60]. Nagata et al[30] showed that there were no statistically significant differences between the DAA- and IFN-based therapies in terms of HCC recurrence. Specifically, 18 (53%) patients with a history of HCC had a recurrence after receiving IFN-based therapy over a median follow-up period of 6.2 years. For those treated with DAAs, 22 patients (29%) had an HCC recurrence over a median follow-up period of 2.3 years[30].
Finally Mashiba et al[57] analysed a Japanese cohort (556 patients) with 148 patients underwent IFN and achieving SVR in 52.7 % of cases, and 368 patients treated with DAA treatment achieving SVR in the 94.5%. The mean follow-up period after antiviral therapy was 25.5 mo for the IFN group and 7.7 mo for the DAA group. The DAA group was significantly older and included more patients with advanced fibrosis, a population at higher risk of HCC recurrence. Nonetheless, there were no differences in the recurrence rate between IFN and DAA therapy in SVR patients. Moreover, they identified AFP at the end of antiviral therapy, clinical stage of HCC, and antiviral treatment failure as independent factors associated with early recurrence of HCC[57].
The scientific debate on this topic has been particularly intense, which is likely a consequence of the limited number of studies and the high heterogeneity related to the study design, inclusion criteria, baseline patient and tumour characteristics, type of “curative” HCC treatment and assessment of response, time frame between tumour treatment and DAAs therapy and between the last assessment of tumour response and the DAA therapy and follow-up after DAA therapy. Nonetheless, we can conclude that the available evidence supports that DAA-treated patients have a similar risk for HCC recurrence as IFN-treated patients.
BIOMOLECULAR MECHANISMS OF HCC OCCURRENCE AND RECURRENCE AFTER DAA
Different pathogenetic hypotheses have been postulated to support the development of HCC after DAA therapy. These hypotheses are mainly based on the dysregulation of immune surveillance caused by the rapid decrease of HCV viral load induced by the DAAs[7], as also recently suggested by Kanda et al[61]. The rapid disappearance of HCV with DAA therapy leads to the reconstitution of innate immunity and the downregulation of type II and III IFNs, their receptors and IFN-stimulated genes. The lack of IFN activation may allow the growth of malignant cells. IFNs are known to exert anti-proliferative effects via intrinsic effects on the tumour regulation of angiogenesis and the activity of almost all immune cell types. This induces a strong immune response against malignancy. Although the exact mechanism behind the anti-tumour properties of IFN has not been fully elucidated, IFN has been widely used for the treatment of several cancers, including haematological malignancies and solid tumours[62]. Unlike IFN, DAAs have neither anti-angiogenic nor anti-proliferative properties and indeed have no effect on oncogenic buds.
Another speculated mechanism is the altered immunological balance that is secondary to a rapid decrease in HCV viral load that contributes to tumour development[63]. A rapid decrease in cancer immuno-surveillance due to an abrupt reduction of liver natural killer (NK) cells and of their cytotoxic activity has been suggested to be a possible factor enhancing a faster progression of HCC foci[64]. Debes et al[65] identified a group of 12 immune mediators showing significantly higher serum expression levels before DAA treatment in patients who developed subsequent HCC. The authors measured serum immune mediators in a cohort of 13 patients who developed HCC (10 occurrences and 3 recurrences) after DAA therapy and compared them to 10 matched controls who did not develop HCC during the follow-up period. In this analysis, the level of TNF α decreased 4 wk after DAA treatment in the controls, while it remained stable in patients who developed HCC. This observation suggests that the persistence of high levels of TNF-α may be involved in the development of HCC[65].
Ono et al[66] suggested that the persisting risk of hepatocellular carcinoma after curing HCV could be monitored by a liver transcriptome signatures. They underlined the necessity to identify a predictive molecular biomarker to stratify the risk of HCC after surgical resection. Finally, a recent study by Villani et al[67] supported this idea by demonstrating that during treatment with DAAs, an angiogenesis inducer (vascular endothelial growth factor) increased significantly and remained elevated for 3 mo after DAA treatment. Most of these theories remain to be confirmed and further studies are urgently needed to better understand the biological mechanisms underlying the hepatocarcinogenesis following viral eradication.
Faillaci et al[68] analysed RNA extracted from hepatic tissue of 242 patients (of whom 183 with cirrhosis) treated with DAAs and showed that Angiopoietin-2 levels were significantly higher in patients with recurrent (n = 14) and de novo HCC (n = 21) in comparison with those without HCC. Moreover, they demonstrated that DAAs are not per se able to determine the occurrence or recurrence of HCC, but that the DAA-mediated increase in VEGF acts as a trigger in predisposed patients, namely those with severe fibrosis and splanchnic collateralization, who already have abnormal activation in liver tissues of neo-angiogenetic pathways, as shown by increased Angiopoietin-2. Therefore, we can postulate that the combination of the clinical and biologic risk factors could give us the unique possibility of selecting the patients at real risk of developing HCC after DAAs[68].
For all these reasons, the use of serum biomarkers for HCC detection and follow-up, as currently recommended only by Asiatic guidelines, is becoming a necessity. Several microRNAs or others epigenetic molecules have been proposed for HCC detection, but the absence of a standardized method for their quantification is a major technical concern regarding the use of microRNAs as reliable markers[69].
In conclusion, patients with HCV cirrhosis who successfully underwent resection or ablation for HCC must undergo DAA therapy to prevent liver disease progression and decompensation. No accurate scientific evidence supports an increased risk of HCC recurrence.
CONCLUSION
In summary, in this review, we analysed the published data on the risk of developing HCC after DAA therapy, measured as the risks of occurrence and recurrence. The available data are conflicting, and the studies show different and relevant methodological limitations. Therefore, drawing definitive conclusions from these studies is difficult. On the other hand, the use of DAAs does not appear to increase the occurrence or recurrence of HCC. Ongoing multicentre prospective studies should provide some insight into this controversial issue. It has been pointed out that there is not an adequate control group for patients treated with DAAs because the population is very different from the historical population of untreated or IFN-treated patients. Therefore, a longer follow-up period and the selection of patients for making a control group is required to establish whether there is a real risk of HCC with IFN-free therapy or, inversely, if there is a real advantage of this new antiviral drug in terms of HCC occurrence and recurrence. Therefore, it is prudent to wait at least 12 mo after complete HCC response to initiate DAA therapy.
ACKNOWLEDGMENTS
Other members of Special Interest Group on “Hepatocellular carcinoma and new anti- hepatitis C virus therapies” of the Italian Association for the Study of the Liver (AISF)
Vigano’ Luca from Humanitas Clinical and Research Hospital, Humanitas University, Rozzano - Milan. Ponziani Francesca Romana, Pompili Maurizio from Fondazione Policlinico “A. Gemelli”, Catholic University of Rome. Umberto Cillo, Patrizia Burra, Claudia Mescoli, Martina Gambato, Russo Francesco Paolo, Vitale Alessandro from Padua University Hospital. Cabibbo Giuseppe from University of Palermo. Vigano’ Mauro from San Giuseppe Hospital, University of Milan. Galati Giovanni from Campus Bio Medico University of Rome. Villa Erica from Modena Hospital University of Modena and Reggio Emilia, Modena. Luigi G. Lupo, Rendina Maria from University of Bari. Francesco Losito, Fabio Fucilli from IRCCS from Castellana Grotte, Bari. Marcello Persico from University of Salerno, Baronissi, Salerno. Roberta D’Ambrosio, Angelo Sangiovanni, Iavarone Massimo from IRCCS Ca’ Granda Maggiore Hospital, University of Milan. Brancaccio Giuseppina from University of Campania Luigi Vanvitelli, Naples. Alessandro Cucchetti, Matteo Renzulli, Trevisani Franco from S. Orsola-Malpighi Hospital, University of Bologna. Luca Miele, Antonio Grieco, Gianlodovico Rapaccini, and Antonio Gasbarrini from Università Cattolica del S. Cuore. Roma. Giovanni Battisa Levi Sandri from San Camillo Forlanini Hospital, Roma. Fabio Melandro, Massimo Rossi, Lai Quirino from Sapienza University of Rome, Roma. Ilaria Lenci, Tommaso Maria Manzia from Policlinico Tor Vergata, Roma. Raffaella Tortora, Giovan Giuseppe Di Costanzo from Cardarelli Hospital, Napoli. Davide Ghinolfi, Erion Rreka, Paola Carrai, Natalia Simonetti, Sacco Rodolfo from Pisa University Hospital, Pisa. Carlo Sposito, Sherrie Bhoori from Fondazione IRCCS Istituto Nazionale Tumori, Milano. Stefano di Sandro from ASST Niguarda Hospital, Milan. Francesco Giuseppe Foschi from Faenza Hospital, Area Vasta Romagna, Ravenna. Andrea Casadei Gardini from IRCCS, Meldola. Daniele Nicolini, Susanna Mazzocato, Kostandini Alba from Polytechnic University of Marche, Ancona. Paola Violi from Verona University Hospital, Italy. Umberto Baccarani, Riccardo Pravisani from University of Udine, Italy. Valter Vincenzi from San Martino Hospital, Belluno.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: None of the authors had personal or financial conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 21, 2018
First decision: May 9, 2018
Article in press: June 12, 2018
P- Reviewer: Abenavoli L, Kanda T, Matsui K, Sazci A S- Editor: Gong ZM L- Editor: A E- Editor: Yin SY
Contributor Information
Maria Guarino, Filomena Morisco, Gastroenterology Unit, Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples 80131, Italy.
Anna Sessa, Filomena Morisco, Gastroenterology Unit, Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples 80131, Italy.
Valentina Cossiga, Filomena Morisco, Gastroenterology Unit, Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples 80131, Italy.
Federica Morando, Filomena Morisco, Gastroenterology Unit, Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples 80131, Italy.
Nicola Caporaso, Filomena Morisco, Gastroenterology Unit, Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples 80131, Italy. nicola.caporaso@unina.it.
References
- 1.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villanueva A, Minguez B, Forner A, Reig M, Llovet JM. Hepatocellular carcinoma: novel molecular approaches for diagnosis, prognosis, and therapy. Annu Rev Med. 2010;61:317–328. doi: 10.1146/annurev.med.080608.100623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Nguyen D, Hu KQ. Chronic Hepatitis C Virus Infection: A Review of Current Direct-Acting Antiviral Treatment Strategies. N Am J Med Sci (Boston) 2016;9:47–54. [PMC free article] [PubMed] [Google Scholar]
- 5.Bruno S, Di Marco V, Iavarone M, Roffi L, Boccaccio V, Crosignani A, Cabibbo G, Rossi S, Calvaruso V, Aghemo A, et al. Improved survival of patients with hepatocellular carcinoma and compensated hepatitis C virus-related cirrhosis who attained sustained virological response. Liver Int. 2017;37:1526–1534. doi: 10.1111/liv.13452. [DOI] [PubMed] [Google Scholar]
- 6.Nishiguchi S, Kuroki T, Nakatani S, Morimoto H, Takeda T, Nakajima S, Shiomi S, Seki S, Kobayashi K, Otani S. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051–1055. doi: 10.1016/s0140-6736(95)91739-x. [DOI] [PubMed] [Google Scholar]
- 7.Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719–726. doi: 10.1016/j.jhep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi G, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727–733. doi: 10.1016/j.jhep.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Nakao Y, Hashimoto S, Abiru S, Komori A, Yamasaki K, Nagaoka S, Saeki A, Bekki S, Kugiyama Y, Kuroki T, et al. Rapidly growing, moderately differentiated HCC: A clinicopathological characteristic of HCC occurrence after IFN-free DAA therapy? J Hepatol. 2017 doi: 10.1016/j.jhep.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso H, Vale AM, Rodrigues S, Gonçalves R, Albuquerque A, Pereira P, Lopes S, Silva M, Andrade P, Morais R, et al. High incidence of hepatocellular carcinoma following successful interferon-free antiviral therapy for hepatitis C associated cirrhosis. J Hepatol. 2016;65:1070–1071. doi: 10.1016/j.jhep.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Cardoso AC, Moucari R, Figueiredo-Mendes C, Ripault MP, Giuily N, Castelnau C, Boyer N, Asselah T, Martinot-Peignoux M, Maylin S, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol. 2010;52:652–657. doi: 10.1016/j.jhep.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 12.Kozbial K, Moser S, Schwarzer R, Laferl H, Al-Zoairy R, Stauber R, Stättermayer AF, Beinhardt S, Graziadei I, Freissmuth C, et al. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferon-free direct-acting antiviral treatment. J Hepatol. 2016;65:856–858. doi: 10.1016/j.jhep.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2017;153:996–1005.e1. doi: 10.1053/j.gastro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Calleja JL, Crespo J, Rincón D, Ruiz-Antorán B, Fernandez I, Perelló C, Gea F, Lens S, García-Samaniego J, Sacristán B, et al. Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: Results from a Spanish real-world cohort. J Hepatol. 2017;66:1138–1148. doi: 10.1016/j.jhep.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 15.Cheung MCM, Walker AJ, Hudson BE, Verma S, McLauchlan J, Mutimer DJ, Brown A, Gelson WTH, MacDonald DC, Agarwal K, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65:741–747. doi: 10.1016/j.jhep.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Hasson H, Merli M, Messina E, Bhoori S, Salpietro S, Morsica G, Regalia E, Bagaglio S, Lazzarin A, Uberti-Foppa C, et al. Occurrence of hepatocellular carcinoma in HIV/HCV co-infected patients treated with direct-acting antivirals. J Hepatol. 2017;67:415–417. doi: 10.1016/j.jhep.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 17.Mettke F, Schlevogt B, Deterding K, Wranke A, Smith A, Port K, Manns MP, Vogel A, Cornberg M, Wedemeyer H. Interferon-free therapy of chronic hepatitis C with direct-acting antivirals does not change the short-term risk for de novo hepatocellular carcinoma in patients with liver cirrhosis. Aliment Pharmacol Ther. 2018;47:516–525. doi: 10.1111/apt.14427. [DOI] [PubMed] [Google Scholar]
- 18.Zeng QL, Li ZQ, Liang HX, Xu GH, Li CX, Zhang DW, Li W, Sun CY, Wang FS, Yu ZJ. Unexpected high incidence of hepatocellular carcinoma in patients with hepatitis C in the era of DAAs: Too alarming? J Hepatol. 2016;65:1068–1069. doi: 10.1016/j.jhep.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 19.Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, Tinè F, Distefano M, Licata A, Giannitrapani L, Prestileo T, Mazzola G, Di Rosolini MA, Larocca L, Bertino G, Digiacomo A, Benanti F, Guarneri L, Averna A, Iacobello C, Magro A, Scalisi I, Cartabellotta F, Savalli F, Barbara M, Davì A, Russello M, Scifo G, Squadrito G, Cammà C, Raimondo G, Craxì A, Di Marco V; Rete Sicilia Selezione Terapia - HCV (RESIST-HCV) Incidence of Hepatocellular Carcinoma in Patients with HCV-associated Cirrhosis Treated with Direct-Acting Antiviral Agents. Gastroenterology. 2018 doi: 10.1053/j.gastro.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Alavi M, Janjua NZ, Chong M, Grebely J, Aspinall EJ, Innes H, Valerio H, Hajarizadeh B, Hayes PC, Krajden M, et al. Trends in hepatocellular carcinoma incidence and survival among people with hepatitis C: An international study. J Viral Hepat. 2018;25:473–481. doi: 10.1111/jvh.12837. [DOI] [PubMed] [Google Scholar]
- 21.Bruno S, Di Marco V, Iavarone M, Roffi L, Crosignani A, Calvaruso V, Aghemo A, Cabibbo G, Viganò M, Boccaccio V, et al. Survival of patients with HCV cirrhosis and sustained virologic response is similar to the general population. J Hepatol. 2016;64:1217–1223. doi: 10.1016/j.jhep.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 22.Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnù L, Mazzella G, Ascione A, Santantonio T, Piccinino F, Andreone P, Mangia A, Gaeta GB, Persico M, Fagiuoli S, Almasio PL; Italian Association of the Study of the Liver Disease (AISF) Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579–587. doi: 10.1002/hep.21492. [DOI] [PubMed] [Google Scholar]
- 23.Morisco F, Granata R, Stroffolini T, Guarino M, Donnarumma L, Gaeta L, Loperto I, Gentile I, Auriemma F, Caporaso N. Sustained virological response: a milestone in the treatment of chronic hepatitis C. World J Gastroenterol. 2013;19:2793–2798. doi: 10.3748/wjg.v19.i18.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Marco V, Calvaruso V, Ferraro D, Bavetta MG, Cabibbo G, Conte E, Cammà C, Grimaudo S, Pipitone RM, Simone F, et al. Effects of Eradicating Hepatitis C Virus Infection in Patients With Cirrhosis Differ With Stage of Portal Hypertension. Gastroenterology. 2016;151:130–139.e2. doi: 10.1053/j.gastro.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 25.van der Meer AJ, Feld JJ, Hofer H, Almasio PL, Calvaruso V, Fernández-Rodríguez CM, Aleman S, Ganne-Carrié N, D’Ambrosio R, Pol S, et al. Risk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradication. J Hepatol. 2017;66:485–493. doi: 10.1016/j.jhep.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 26.D’Ambrosio R, Aghemo A, Rumi MG, Degasperi E, Sangiovanni A, Maggioni M, Fraquelli M, Perbellini R, Rosenberg W, Bedossa P, et al. Persistence of hepatocellular carcinoma risk in hepatitis C patients with a response to IFN and cirrhosis regression. Liver Int. 2018 doi: 10.1111/liv.13707. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Lu M, Li J, Rupp LB, Holmberg SD, Moorman AC, Spradling PR, Teshale EH, Zhou Y, Boscarino JA, Schmidt MA, et al. Hepatitis C treatment failure is associated with increased risk of hepatocellular carcinoma. J Viral Hepat. 2016;23:718–729. doi: 10.1111/jvh.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Innes H, Barclay ST, Hayes PC, Fraser A, Dillon JF, Stanley A, Bathgate A, McDonald SA, Goldberg D, Valerio H, et al. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: role of the treatment regimen. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.10.033. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Bielen R, Moreno C, Van Vlierberghe H, Bourgeois S, Mulkay JP, Vanwolleghem T, Verlinden W, Brixco C, Decaestecker J, de Galocsy C, et al. The risk of early occurrence and recurrence of hepatocellular carcinoma in hepatitis C-infected patients treated with direct-acting antivirals with and without pegylated interferon: A Belgian experience. J Viral Hepat. 2017;24:976–981. doi: 10.1111/jvh.12726. [DOI] [PubMed] [Google Scholar]
- 30.Nagata H, Nakagawa M, Asahina Y, Sato A, Asano Y, Tsunoda T, Miyoshi M, Kaneko S, Otani S, Kawai-Kitahata F, et al. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J Hepatol. 2017;67:933–939. doi: 10.1016/j.jhep.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 31.Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.08.030. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi M, Suzuki F, Fujiyama S, Kawamura Y, Sezaki H, Hosaka T, Akuta N, Suzuki Y, Saitoh S, Arase Y, et al. Sustained virologic response by direct antiviral agents reduces the incidence of hepatocellular carcinoma in patients with HCV infection. J Med Virol. 2017;89:476–483. doi: 10.1002/jmv.24663. [DOI] [PubMed] [Google Scholar]
- 33.Nagaoki Y, Imamura M, Aikata H, Daijo K, Teraoka Y, Honda F, Nakamura Y, Hatooka M, Morio R, Morio K, et al. The risks of hepatocellular carcinoma development after HCV eradication are similar between patients treated with peg-interferon plus ribavirin and direct-acting antiviral therapy. PLoS One. 2017;12:e0182710. doi: 10.1371/journal.pone.0182710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li DK, Ren Y, Fierer DS, Rutledge S, Shaikh OS, Lo Re V 3rd, Simon T, Abou-Samra AB, Chung RT, Butt AA. The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: An ERCHIVES study. Hepatology. 2018;67:2244–2253. doi: 10.1002/hep.29707. [DOI] [PubMed] [Google Scholar]
- 35.Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, George J, Dore GJ. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67:1204–1212. doi: 10.1016/j.jhep.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 36.Llovet JM, Villanueva A. Liver cancer: Effect of HCV clearance with direct-acting antiviral agents on HCC. Nat Rev Gastroenterol Hepatol. 2016;13:561–562. doi: 10.1038/nrgastro.2016.140. [DOI] [PubMed] [Google Scholar]
- 37.Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Lens S, Díaz A, Vilana R, Darnell A, Varela M, Sangro B, et al. Tumour recurrence after interferon-free treatment for hepatitis C in patients with previously treated hepatocellular carcinoma discloses a more aggressive pattern and faster tumour growth. J Hepatol. 2017;66:S20. [Google Scholar]
- 38.Reig M, Boix L, Mariño Z, Torres F, Forns X, Bruix J. Liver Cancer Emergence Associated with Antiviral Treatment: An Immune Surveillance Failure? Semin Liver Dis. 2017;37:109–118. doi: 10.1055/s-0037-1601349. [DOI] [PubMed] [Google Scholar]
- 39.Kolly P, Waidmann O, Vermehren J, Moreno C, Vögeli I, Berg T, Semela D, Zeuzem S, Dufour JF. Hepatocellular carcinoma recurrence after direct antiviral agent treatment: A European multicentre study. J Hepatol. 2017;67:876–878. doi: 10.1016/j.jhep.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 40.El Kassas M, Funk AL, Salaheldin M, Shimakawa Y, Eltabbakh M, Jean K, El Tahan A, Sweedy AT, Afify S, Youssef NF, et al. Increased recurrence rates of hepatocellular carcinoma after DAA therapy in a hepatitis C-infected Egyptian cohort: A comparative analysis. J Viral Hepat. 2018;25:623–630. doi: 10.1111/jvh.12854. [DOI] [PubMed] [Google Scholar]
- 41.Yang JD, Aqel BA, Pungpapong S, Gores GJ, Roberts LR, Leise MD. Direct acting antiviral therapy and tumor recurrence after liver transplantation for hepatitis C-associated hepatocellular carcinoma. J Hepatol. 2016;65:859–860. doi: 10.1016/j.jhep.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 42.Huang AC, Mehta N, Dodge JL, Yao FY, Terrault NA. Direct-acting antivirals do not increase the risk of hepatocellular carcinoma recurrence after local-regional therapy or liver transplant waitlist dropout. Hepatology. 2018 doi: 10.1002/hep.29855. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts). Electronic address: stanislas.pol@aphp.fr. Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol. 2016;65:734–740. doi: 10.1016/j.jhep.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 44.Torres HA, Vauthey JN, Economides MP, Mahale P, Kaseb A. Hepatocellular carcinoma recurrence after treatment with direct-acting antivirals: First, do no harm by withdrawing treatment. J Hepatol. 2016;65:862–864. doi: 10.1016/j.jhep.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 45.Ikeda K, Kawamura Y, Kobayashi M, Kominami Y, Fujiyama S, Sezaki H, Hosaka T, Akuta N, Saitoh S, Suzuki F, et al. Direct-Acting Antivirals Decreased Tumor Recurrence After Initial Treatment of Hepatitis C Virus-Related Hepatocellular Carcinoma. Dig Dis Sci. 2017;62:2932–2942. doi: 10.1007/s10620-017-4739-z. [DOI] [PubMed] [Google Scholar]
- 46.Virlogeux V, Pradat P, Hartig-Lavie K, Bailly F, Maynard M, Ouziel G, Poinsot D, Lebossé F, Ecochard M, Radenne S, et al. Direct-acting antiviral therapy decreases hepatocellular carcinoma recurrence rate in cirrhotic patients with chronic hepatitis C. Liver Int. 2017;37:1122–1127. doi: 10.1111/liv.13456. [DOI] [PubMed] [Google Scholar]
- 47.Zavaglia C, Okolicsanyi S, Cesarini L, Mazzarelli C, Pontecorvi V, Ciaccio A, Strazzabosco M, Belli LS. Is the risk of neoplastic recurrence increased after prescribing direct-acting antivirals for HCV patients whose HCC was previously cured? J Hepatol. 2017;66:236–237. doi: 10.1016/j.jhep.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 48.Ogawa E, Furusyo N, Nomura H, Dohmen K, Higashi N, Takahashi K, Kawano A, Azuma K, Satoh T, Nakamuta M, Koyanagi T, Kato M, Shimoda S, Kajiwara E, Hayashi J; Kyushu University Liver Disease Study (KULDS) Group. Short-term risk of hepatocellular carcinoma after hepatitis C virus eradication following direct-acting anti-viral treatment. Aliment Pharmacol Ther. 2018;47:104–113. doi: 10.1111/apt.14380. [DOI] [PubMed] [Google Scholar]
- 49.Mazzarelli C, Cannon MD, Belli LS, Agarwal K. Direct-acting antiviral therapy in patients with hepatocellular cancer: The timing of treatment is everything. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.08.025. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 50.Cabibbo G, Petta S, Calvaruso V, Cacciola I, Cannavò MR, Madonia S, Distefano M, Larocca L, Prestileo T, Tinè F, Bertino G, Giannitrapani L, Benanti F, Licata A, Scalisi I, Mazzola G, Cartabellotta F, Alessi N, Barbàra M, Russello M, Scifo G, Squadrito G, Raimondo G, Craxì A, Di Marco V, Cammà C; Rete Sicilia Selezione Terapia - HCV (RESIST-HCV) Is early recurrence of hepatocellular carcinoma in HCV cirrhotic patients affected by treatment with direct-acting antivirals? A prospective multicentre study. Aliment Pharmacol Ther. 2017;46:688–695. doi: 10.1111/apt.14256. [DOI] [PubMed] [Google Scholar]
- 51.Beste LA, Green PK, Berry K, Kogut MJ, Allison SK, Ioannou GN. Effectiveness of hepatitis C antiviral treatment in a USA cohort of veteran patients with hepatocellular carcinoma. J Hepatol. 2017;67:32–39. doi: 10.1016/j.jhep.2017.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimizu H, Matsui K, Iwabuchi S, Fujikawa T, Nagata M, Takatsuka K, Tanemura H, Nakazaki H, Nakano M, Watanabe T. Relationship of hepatitis B virus infection to the recurrence of hepatocellular carcinoma after direct acting antivirals. Indian J Gastroenterol. 2017;36:235–238. doi: 10.1007/s12664-017-0755-3. [DOI] [PubMed] [Google Scholar]
- 53.Renzulli M, Buonfiglioli F, Conti F, Brocchi S, Serio I, Foschi FG, Caraceni P, Mazzella G, Verucchi G, Golfieri R, et al. Imaging features of microvascular invasion in hepatocellular carcinoma developed after direct-acting antiviral therapy in HCV-related cirrhosis. Eur Radiol. 2018;28:506–513. doi: 10.1007/s00330-017-5033-3. [DOI] [PubMed] [Google Scholar]
- 54.Cabibbo G, Petta S, Barbàra M, Missale G, Virdone R, Caturelli E, Piscaglia F, Morisco F, Colecchia A, Farinati F, et al. LI.CA study group. A meta-analysis of single HCV-untreated arm of studies evaluating outcomes after curative treatments of HCV-related hepatocellular carcinoma. Liver Int. 2017;37:1157–1166. doi: 10.1111/liv.13357. [DOI] [PubMed] [Google Scholar]
- 55.Minami T, Tateishi R, Wake T, Nishibatake M, Nakagomi R, Sato M, Uchino K, Enooku K, Nakagawa H, Asaoka Y, et al. Hepatocellular carcinoma recurrence after curative treatments in patients with chronic hepatitis C who underwent direct-acting. antiviral therapy. Hepatology. 2017;66:760A–761A. [Google Scholar]
- 56.Minami T, Tateishi R, Nakagomi R, Fujiwara N, Sato M, Enooku K, Nakagawa H, Asaoka Y, Kondo Y, Shiina S, et al. The impact of direct-acting antivirals on early tumor recurrence after radiofrequency ablation in hepatitis C-related hepatocellular carcinoma. J Hepatol. 2016;65:1272–1273. doi: 10.1016/j.jhep.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 57.Mashiba T, Joko K, Kurosaki M, Ochi H, Osaki Y, Kojima Y, Nakata R, Goto T, Takehiro A, Kimura H, et al. Does interferon-free direct-acting antiviral therapy for hepatitis C after curative treatment for hepatocellular carcinoma lead to unexpected recurrences of HCC? A multicenter study by the Japanese Red Cross Hospital Liver Study Group. PLoS One. 2018;13:e0194704. doi: 10.1371/journal.pone.0194704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petta S, Cabibbo G, Barbara M, Attardo S, Bucci L, Farinati F, Giannini EG, Tovoli F, Ciccarese F, Rapaccini GL, Di Marco M, Caturelli E, Zoli M, Borzio F, Sacco R, Virdone R, Marra F, Felder M, Morisco F, Benvegnù L, Gasbarrini A, Svegliati-Baroni G, Foschi FG, Olivani A, Masotto A, Nardone G, Colecchia A, Persico M, Boccaccio V, Craxì A, Bruno S, Trevisani F, Cammà C; Italian Liver Cancer (ITA. LI.CA) Group. Hepatocellular carcinoma recurrence in patients with curative resection or ablation: impact of HCV eradication does not depend on the use of interferon. Aliment Pharmacol Ther. 2017;45:160–168. doi: 10.1111/apt.13821. [DOI] [PubMed] [Google Scholar]
- 59.Vukotic R, Di Donato R, Conti F, Scuteri A, Serra C, Andreone P. Secondary prophylaxis of hepatocellular carcinoma: the comparison of direct-acting antivirals with pegylated interferon and untreated cohort. J Viral Hepat. 2017;24:13–16. doi: 10.1111/jvh.12651. [DOI] [PubMed] [Google Scholar]
- 60.Adhoute X, Penaranda G, Raoul JL, Sellier F, Castellani P, Oules V, Perrier H, Lefolgoc G, Pol B, Campanile M, et al. Hepatocellular carcinoma recurrence in hepatitis C virus-related cirrhosis treated with direct-acting antivirals: a case-control study. Eur J Gastroenterol Hepatol. 2018;30:368–375. doi: 10.1097/MEG.0000000000001082. [DOI] [PubMed] [Google Scholar]
- 61.Kanda T, Matsuoka S, Moriyama M. Early occurrence and recurrence of hepatocellular carcinoma in hepatitis C virus-infected patients after sustained virological response. Hepatol Int. 2018;12:90–93. doi: 10.1007/s12072-018-9862-1. [DOI] [PubMed] [Google Scholar]
- 62.Pasquali S, Mocellin S. The anticancer face of interferon alpha (IFN-alpha): from biology to clinical results, with a focus on melanoma. Curr Med Chem. 2010;17:3327–3336. doi: 10.2174/092986710793176393. [DOI] [PubMed] [Google Scholar]
- 63.Grandhe S, Frenette CT. Occurrence and Recurrence of Hepatocellular Carcinoma After Successful Direct-Acting Antiviral Therapy for Patients With Chronic Hepatitis C Virus Infection. Gastroenterol Hepatol (N Y) 2017;13:421–425. [PMC free article] [PubMed] [Google Scholar]
- 64.Chu PS, Nakamoto N, Taniki N, Ojiro K, Amiya T, Makita Y, Murata H, Yamaguchi A, Shiba S, Miyake R, et al. On-treatment decrease of NKG2D correlates to early emergence of clinically evident hepatocellular carcinoma after interferon-free therapy for chronic hepatitis C. PLoS One. 2017;12:e0179096. doi: 10.1371/journal.pone.0179096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Debes JD, van Tilborg M, Groothuismink ZMA, Hansen BE, Schulze Zur Wiesch J, von Felden J, de Knegt RJ, Boonstra A. Levels of Cytokines in Serum Associate With Development of Hepatocellular Carcinoma in Patients With HCV Infection Treated With Direct-Acting Antivirals. Gastroenterology. 2018;154:515–517.e3. doi: 10.1053/j.gastro.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 66.Ono A, Goossens N, Finn RS, Schmidt WN, Thung SN, Im GY, Hoshida Y; Precision Liver Cancer Prevention Consortium. Persisting risk of hepatocellular carcinoma after hepatitis C virus cure monitored by a liver transcriptome signature. Hepatology. 2017;66:1344–1346. doi: 10.1002/hep.29203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Villani R, Facciorusso A, Bellanti F, Tamborra R, Piscazzi A, Landriscina M, Vendemiale G, Serviddio G. DAAs Rapidly Reduce Inflammation but Increase Serum VEGF Level: A Rationale for Tumor Risk during Anti-HCV Treatment. PLoS One. 2016;11:e0167934. doi: 10.1371/journal.pone.0167934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faillaci F, Marzi L, Critelli R, Milosa F, Schepis F, Turola E, Andreani S, Vandelli G, Bernabucci V, Lei B, et al. Liver Angiopoietin-2 is a key predictor of de novo or recurrent hepatocellular cancer after HCV direct-acting antivirals. Hepatology. 2018 doi: 10.1002/hep.29911. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abenavoli L, Boccuto L. New serum markers for detection of early hepatocellular carcinoma. Panminerva Med. 2017;59:281–282. doi: 10.23736/S0031-0808.17.03356-0. [DOI] [PubMed] [Google Scholar]