Abstract
Alpha herpesviruses are common pathogens of mammals. They establish a productive infection in many cell types, but a life-long latent infection occurs in PNS neurons. A vast majority of the human population has latent HSV-1 infections. Currently, there is no cure to clear latent infections. Even though HSV-1 is among the best studied viral pathogens, regulation of latency and reactivation is not well understood due to several challenges including a lack of animal models that precisely recapitulate latency/reactivation episodes; a difficulty in modeling in vitro latency; and a limited understanding of neuronal biology. In this review, we discuss insights gained from in vitro latency models with a focus on the neuronal and viral factors that determine the mode of infection.
Keywords: : alpha herpesviruses, latency, reactivation, in vitro latency model
Alpha herpesviruses & the latency strategy
Herpesviruses (HV) are large, enveloped viruses with double stranded linear DNA genomes. The virions contain a characteristic tegument layer composed of several viral and host proteins as well as some mRNAs. All three subfamilies of human HV (α, β and γ) share a common replication strategy: they productively infect many cell types, but a specific tissue or cell type is targeted to establish a reactivatable, life-long silent infection called latency (see [1,2] for review). For β- and γ-HV, hematopoietic cells are the reservoir for latent infections, while α-HV target peripheral neurons. Productive infection proceeds in most susceptible and permissive cell types in a well characterized cascade fashion where viral immediate early proteins interact with host cell proteins and activate transcription of early (E) viral genes. These E proteins are required to prepare the optimum environment for viral DNA replication and subsequent structural viral late (L) protein synthesis [3,4]. This cascade of viral gene expression is perfectly orchestrated and results in the rapid production of large numbers of progeny virions ready to spread to other tissues and hosts.
α-HV infections (e.g., herpes simplex virus 1 and 2; HSV-1 and HSV-2, and varicella zoster virus; VZV) are among the most common virus infections in the world [5–7]. For HSV-1, the primary infection starts with a productive infection in the epithelial cells of mucosal surfaces (e.g., nasal-pharyngeal cavity, genitals), yielding hundreds to thousands of progeny particles per infected cell in less than a day. Some of these progeny virus particles move into the innervating axons of the PNS neurons. Because epithelial tissues are highly innervated by sensory neurons, the dorsal root ganglia (DRG) and trigeminal ganglia (TG) are the primary sites of latency [8,9]. However, neurons of other autonomic sympathetic ganglia that innervate peripheral tissues such as superior cervical ganglia (SCG) also harbor latent viral genomes [10–13].
The highly polarized and differentiated state of neurons affects the infection dynamics and contributes to the establishment of latency. As soon as viral particles enter PNS axons, viral capsids and inner tegument proteins (those bound to the capsid) separate from the envelope proteins and the outer tegument proteins [2,14–16]. The outer tegument contains the viral transcriptional activator, VP16 (i.e., UL48), which interacts with host cell factors to activate viral gene expression and to initiate productive infection [17–19]. Axonal transport of the genome-carrying capsids toward the neuronal nuclei is essential for the establishment of nervous system infection, but not much is known about how outer tegument proteins like VP16 are transported. If tegument proteins and viral genomes are transported separately in axons and arrive asynchronously at the cell bodies, this may bias the infection mode toward latency [20,21].
PNS axons can be centimeters to almost a meter long in humans (containing more than 90% of the neuron's cytoplasm). Axons are responsible for two-way transmission of information from the peripheral organs to the neuronal ganglia and then to the CNS. This long distance retrograde transport of viral particles to the cell body nucleus occurs on microtubules and relies on the interaction of viral inner tegument proteins with the cellular motor dynein [22]. This interaction represents a potential bottleneck in the establishment of neuronal infection because a finite number of dynein motors and adapters are found in axons. After the viral capsids reach the nucleus, the genomes are released and the specialized latency mode of infection initiates [23–25]. During this latent phase, herpesviral genomes are circularized and decorated with silencing nucleosomes with histone modifications that allow transcription of only a small segment of the viral genome (the latency associated transcript [LAT]) [26–28].
Infecting the nervous system seems an unlikely strategy for successful virus evolution because of the essential nature of the nervous system. If infection spreads to the CNS, virus replication and/or inflammation can be fatal [29,30]. Indeed, such infections often represent a ‘dead-end’ for the pathogen and the host. Well-known examples are the zoonotic infections of rabies viruses. However, the α-HV strategy is unique and obviously successful because infected peripheral neurons do not die and only occasionally produce measurable amounts of infectious virus particles. Indeed, asymptomatic shedding of HSV-1 viral DNA has been reported [31]. Unlike HSV latent infection, the VZV latent infection produces some viral proteins [9,32]. In general, because of the reduced expression of viral proteins, the adaptive immune system is stimulated but is not sufficient to clear the latently infected neurons. One contributing factor for such ineffective immune response may be that PNS neurons have limited responses to inflammatory cytokines such as interferon. Because neurons cannot be replaced and are not easily cleared by the immune cells, they represent an ideal reservoir for α-HV genomes.
For a latent infection to have any evolutionary advantage, the silenced viral genomes must retain the capacity to express and initiate the productive cycle (i.e., reactivate) so that transmission to other hosts can occur. Reactivation usually is triggered when the host experiences stress or damage (e.g., physical trauma, sunburn, fever, etc.) [4,33,34]. When well-known stress signaling and neuronal survival pathways are activated, transcription and replication of the viral genome occurs and new virus particles are produced that move back to the site of the original infection to ensure the spread of infection to other hosts [35]. Interestingly, despite the general global effect of stress on host cells, the threshold for reactivation of latently infected PNS neurons is high: reactivation does not occur in all the neurons that harbor silenced viral genomes. One idea has been that some neurons in the same ganglia contain more copies of the silenced viral genome and those might have a higher chance of reactivation [36]. Another observation is that within a single PNS nucleus, not all silenced genomes reactivate at the same time [25,37–39]. As currently appreciated, there are many host and viral factors affecting the establishment of latency and reactivation that are not well understood.
Usually, in a healthy latently infected host with a strong immune system, reactivations are well controlled, and symptoms are mild and are revealed as cold sores, genital lesions or shingles blisters. However, in rare cases, reactivation episodes may result in less well contained lesions leading to oral or genital ulcerations, keratitis, blindness and even encephalitis [40,41]. HSV-2 reactivations tend to be more frequent and do not always present as lesions [42,43]. A rare but serious complication of HSV-2 (or HSV-1) shedding in the genital tract is neonatal HSV, which is estimated to occur in 10 of 100,000 live births globally. This equates to approximately 14,000 cases per year (10,000 for HSV-2; 4000 for HSV-1) [44]. Neonatal HSV can lead to CNS or disseminated disease and is fatal in approximately 60% of untreated cases [45]. Asymptomatic reactivation and shedding of HSV-1 [46] and VZV [47,48] are also known. In some rare cases, reactivating virus particles are disseminated in the CNS causing seizures [48,49]. Control of latency, reactivation and subsequent spread of infection is affected by many cell intrinsic, tissue-specific and systemic factors that are challenging to dissect. Thus, latency has recently been referred to as a ‘Gordian knot’ for its many layers of complexity [50].
The apparent paradox of the latency-reactivation cycle
The switch between silenced and productive infection in a single neuron seems paradoxical at first and has been challenging to study. The latently infected neuron neither expresses detectable viral proteins nor replicates the viral genome, and therefore cannot be easily distinguished from its uninfected neighbors by inspection. The only ways researchers have been able to identify latently infected neurons are to isolate infected ganglia and look for LAT expression or to reactivate the silenced infection by various methods to trigger the productive cycle [4,51–54]. How can the genomes be silenced in neurons and still be capable of being productive and then silenced again? Because it is exceedingly difficult to study human α-HV infections in a controlled setting, veterinary α-HV infections have provided considerable insight (see Table 1 and references [4,8,12,33,55–58] for extensive reviews). Even with this excellent work, mechanistic details often remained elusive. Consequently, one solution to unravel such complicated biology is to use a reductionist approach and develop simplified, well-controlled model systems.
Table 1. . Selected human and veterinary pathogens of the alphaherpesvirinae subfamily studied in latency/reactivation models.
| Alpha herpesvirus | Genus | Native host | Host tropism | Tissue tropism | Primary sites of latency | Pathophysiology/disease |
|---|---|---|---|---|---|---|
| HSV-1 | Simplex virus | Human | Human | Mucosal epithelia PNS neurons |
Cranial sensory ganglia |
Orofacial lesions encephalitis |
| HSV-2 | Simplex virus | Human | Human | Mucosal epithelia PNS neurons |
Lumbar/sacral sensory ganglia |
Genital lesions Neonatal infections |
| VZV | Varicellovirus | Human | Human | Mucosal epithelia PNS neurons T cells |
Cranial, thoracic, lumbar/sacral sensory ganglia |
Chickenpox Shingles Postherpetic neuralgia |
| BHV-1 | Varicellovirus | Cattle | Cattle | Mucosal epithelia PNS neurons T cells |
Cranial, sacral sensory ganglia Tonsils |
Rhinotracheitis, Vulvovaginitis, Balanoposthitis, Abortion |
| PRV | Varicellovirus | Pig | Most mammals except higher primates |
Mucosal epithelia PNS neurons |
Cranial, sacral sensory ganglia Tonsils |
Aujesky's disease (mad itch) Abortion |
| SVV | Varicellovirus | Nonhuman Primates |
Nonhuman Primates |
Epithelia Alveolar myeloid cells PNS neurons T cells |
PNS neurons | Varicella (skin rash) |
BHV-1: Bovine herpesvirus 1; HSV: Herpes simplex virus; PRV: Pseudorabies virus; PNS: Peripheral nervous system; SVV: Simian varicella virus; VZV: Varicella zoster virus.
The in vivo rodent models
Several rodent model systems have been developed that recapitulate some aspects of latency and reactivation of the human and veterinary pathogens in their natural hosts (see reviews [4,12,23,54-56]). The mouse trigeminal explant model provided key insights into HSV-1 reactivation [52]. In this system, HSV-1 (or HSV-2) is used to infect peripheral epithelial tissues, and the infection spreads naturally into the PNS where latency is established. In this model, HSV-1 does not reactivate unless either the latently infected ganglia are excised and put into tissue culture systems or the animal is put through hyperthermia or injury-related stress. Approximately one to three neurons per TG shows signs of productive infection after in vivo hyperthermia-induced reactivation [52].
Rodent models exist for the study of stromal keratitis [61] and postherpetic neuralgia induced by HSV infection [62]. VZV has been more difficult to study in standard animal model systems, but successful models include human dorsal root ganglia tissue engrafted in SCID mice [63,64], Guinea pig [55,65] and the rhesus macaque–simian varicella virus [66,67]. Correlating the findings from these varied model systems has been complicated not only due to differences in virus strains and mutants used, but also due to differences in reactivation protocols and quantitation techniques [51,57,68].
Complementary in vitro systems are being developed to test the in vivo findings. However, these in vitro systems have their own issues for studying latency. A primary issue is that infection of dissociated, cultured peripheral neurons usually results in a productive, not a latent infection. Latency is often established by blocking viral replication with antiviral drugs or interferon. In the following section, we will summarize how researchers developed models to recapitulate latency and reactivation in rodent neuron cultures (also see reviews [33,69]).
Neuron culture models in retrospect
Initial attempts to model latency in culture were done in dissociated neurons where productive viral DNA replication was inhibited by acyclovir, an antiviral drug. Acyclovir has been the most effective antiherpetic drug used in humans since 1977 [70]. The drug is activated by a viral thymidine kinase in the infected cell, and only after activation will the drug interfere with DNA replication [71]. Acyclovir pretreatment of neurons, before infecting with HSV-1, blocks viral replication and forces the productive infection into a quiescent state similar to in vivo latency. By using this method, Wilcox and Johnson showed that NGF deprivation triggers herpesvirus reactivation in cultured primary neurons [72,73]. This paved the way toward a better molecular understanding of HSV reactivating stimuli.
More than 20 years after this pioneering work, Camarena et al., revisited and modified this experimental protocol to show that constant activation of PI3K signaling by NGF maintains the HSV genome in the silent state in SCG neurons [74]. Blocking NGF/PI3K signaling resulted in a productive infection. In this modified model, SCG ganglia isolated from embryonic rats are homogenized and seeded into 96-wells. Six days after seeding they are treated with acyclovir and subsequently infected, at a multiplicity of infection (MOI) of 1, with an HSV-1 recombinant expressing GFP) fusion to a late protein. After acyclovir treatment, no GFP signal is observed indicating establishment of a latent infection. Virus reactivation is assayed by appearance of the GFP signal. Spontaneous reactivation occurs in 10–20% of the wells, and this percentage rises to 70% in 1 week upon treatment with reactivating stimuli. Kobayashi et al., further elucidated the downstream signal transduction events required for reactivation and concluded that local mTOR signaling in axons maintains HSV-1 latency and controls reactivation [75].
While axons are the initial site of viral entry into PNS neurons, their cell bodies are quite distant. The idea of relaying information from axons to the distant cell bodies to control transcriptional events has attracted much attention particularly in neuronal biology [76,77]. However, experimental modeling of such long distance signaling is challenging especially when studying HSV infection. To prove that axonal signaling could lead to HSV-1 reactivation in the nucleus, Kobayashi et al. established HSV-1 latency by acyclovir treatment of SCG neurons seeded either onto Boyden chamber inserts or in microfluidic chambers [75]. Boyden chambers are established by cylindrical membrane inserts that separate a cell culture dish into upper and lower fluidic compartments. Cells are seeded onto the upper compartment, and only axons can penetrate through the membrane. Axons that are growing laterally on the bottom of the membrane are exposed to the media in the bottom compartment, while the cell bodies and axons growing on top of the membrane are not. While these inserts allow biochemical analysis of axons scraped from the bottom surface, it is challenging to perform axonal infections or microscopic analysis. Microfluidic chambers on the other hand, enable axonal infection and microscopic observations [78], but it is difficult to perform biochemical analyses and large scale screens in the microchannels.
To recapitulate the neuronal tissue architecture and the natural α-HV infection route, Hafezi et al. developed an organotypic model using chicken TG explants cultured in a double chamber where the two compartments are separated by a cloning cylinder ring [21]. In this model, ganglia are not dissociated, and their tightly packed structure is preserved in the culture dish. Axons growing out from the explant penetrated the cylinder ring barrier and formed the axon-only compartment. When the ganglionic compartment was infected with HSV-1, a productive infection was observed. When axons were infected with HSV-1 at a comparable MOI, a quiescent infection was established in a small number of neurons that expressed the LAT characteristic of a latent infection. The authors provided evidence that axonal delivery of viral particles favors a quiescent state of infection in the ganglia [20,21]. Remarkably, this quiescent state was established without the use of acyclovir, and viral genomes could be reactivated by drug treatment or co-infection with other α-HV. Although this model provided the principle of in vitro establishment of HSV-1 and HSV-2 latency via the axonal route, the explant setup of chicken TG explants consisting of different neuron populations tightly packed together and the presence of only one barrier between compartments challenged the isolation and quantitation of infected cell bodies and the reproducibility of the experiments.
Primary neuronal cultures in tri-chambers
A modified Campenot chamber consisting of three compartments, hence called the ‘tri-chamber’, has recently been used to establish in vitro latency in rodent neurons without the use of inhibitors [79,80]. Tri-chambers physically separate cell bodies from axons during the establishment of neuronal polarity and maturation. Cell bodies in the soma (S) compartment grow axons that first penetrate into the middle (M) and then into the neurite (N) compartments. SCG neurons from rat embryos yield a homogenous neuron culture that grows robust axons in the presence of NGF. These axons are capable of penetrating the two physical barriers in approximately 2 weeks. This system allows not only microscopic observations and biochemical analysis, but it also enables well controlled reproducible axonal infections. Use of this system facilitated an investigation of the viral and cellular factors regulating productive versus latent pseudorabies virus (PRV) infection.
PRV is a swine alpha herpesvirus that infects most mammals with the exception of primates (Table 1). Its value in part, stems from the fact that most of the PRV genes are related to those of the human α-HV. Therefore PRV shares most of the common functions and replication strategies with HSV-1. A PRV recombinant expressing a red fluorescent protein as a minor capsid protein fusion enables monitoring single virus particle motility in axons and imaging replication later in the neuronal nucleus. The red capsid fusion protein begins to accumulate in the nucleus only after viral DNA replication, which clearly demonstrates productive infection.
Local protein synthesis & pseudo-injury signaling: critical processes for establishing productive infection
How do viral particles reach the neuronal cell bodies to establish productive infection after axonal invasion? The answer involves events in the initial virion–axon interaction and in the way viral proteins engage cellular machineries. An important finding was that the number of virus particles that invade axons plays an essential role in the outcome of infection [80]. For example, infection of axons in N-compartments with a large number of PRV virions (sufficient to promote productive infection in all the cell bodies), activates translation of local neuronal mRNAs in axons, which leads to local synthesis of several axonal proteins. When protein synthesis is inhibited by cycloheximide or emetine, the efficiency of retrograde capsid transport is reduced. Some of these newly made neuronal proteins, including a dynein regulator, LIS1, are indeed crucial for efficient virus transport toward the cell bodies [81]. Induction of new protein synthesis in neuronal axons and dendrites has been a focus of research and debate in neurobiology since the early 2000s [82]. In PNS neurons, local axonal protein synthesis is now known to be essential for growth cone navigation, damage repair and communication with the distant cell body. Some of the proteins that are synthesized locally engage signaling molecules transported to the cell body where they act as messengers to alarm cell bodies in case of distal axonal injury [77].
Exposing axons to large numbers of virus particles triggers a mechanism similar to the axonal damage response. Both injury and infection activate local protein synthesis and fast transport of signaling molecules toward neuronal nuclei [81,83]. These observations led to the hypothesis that large number of viral particles invading axons stimulate translation of a subset of axonal messages to support efficient virus transport by repurposing the acute retrograde injury signaling machinery. Viral particles compete with injury signaling complexes that are directed toward the neuronal nucleus.
Establishment of PRV latency in compartmented neurons
The efficiency of retrograde transport and virus replication was correlated with the number of viral particles infecting axons in tri-chambers. When axons were exposed to large number of virions, all the cell bodies were infected by 24 h postinfection. However, when axons were infected with 10- to 100-fold fewer virions, not only was virus particle motility severely affected, but the cell body infection was delayed by 24 h. When the number of viral proteins attaching to or entering axons is reduced, particles cannot induce the ‘injury response’, local protein synthesis and efficient assembly of transport complexes [80].
Interestingly, when the concentration of virions added to axons was reduced further, no productive infection in the cell bodies was observed, even after weeks of incubation. However, the viral genomes did reach the neuronal nuclei, but were silenced. Such quiescent infections exhibited hallmarks of latency including increased LAT expression, no detectable infectious virus production, and no DNA replication. Importantly, these silenced genomes could be reactivated [79,80]. These studies showed that a simplified latency/reactivation system could be developed in the absence of non-neuronal support cells, immune control, or drug treatment simply by infecting isolated axons with a reduced number of viral particles.
Local antiviral responses in axons contribute to the establishment of latent infection
In a natural α-HV infection, axons innervating the peripheral epithelia are exposed to the cytokine milieu of infected epithelial cells even before neuronal invasion by the progeny initiates. How does this complicated environment affect axonal infection, particle transport and productive infection thresholds? Song et al., investigated this question by treating axons in N-compartments with type I (α/β) and II (γ) interferon before infecting them at high MOI with PRV [84]. These studies identified two different modes of local interferon responses in axons. When axons were treated with either IFN-β or IFN-γ, PRV and HSV-1 transport was reduced. However, only type II interferon exposure to axons induced transcription in the neuronal nuclei to further block viral replication. By contrast, treatment of axons with IFN-β had no effect on cell body transcription but instead activated STAT1 phosphorylation in axons. An important observation was that local antiviral responses in axons limited but did not block the number of particles reaching the neuronal nucleus. Since we know that productive infection requires a high number of capsids reaching the cell body, the cytokine response in axons probably contributes to the establishment of a latent infection in the ganglia by interfering with particle transport and reducing the number of capsids reaching the nuclei [84,85].
Both type I and type II IFNs limit replication and spread of α-HV in many cells by inducing the transcription of a multitude of interferon-stimulated genes [86,87]. In the sensory ganglia of latently infected mice, interferons and other cytokines secreted by infiltrating T cells contribute to the establishment and maintenance of latency [85,88–90]. Using porcine-dissociated TG neurons cultured in a two-compartment setup, De Regge et al., demonstrated that pretreatment of neurons with IFN-α suppresses axonally infecting α-HV replication leading to a stably silenced infection similar to latency [91]. Not only do IFN-β and IFN-γ promote establishment of latency, but they also block HSV-1 reactivation by interfering with an early step in the process [92]. Both types of interferon fail to block reactivation when they are introduced at a later phase.
Escape from silencing: modulating the switch
Roizman and Whitley, 2013 concluded in their review that “the path to latent state from the time viral DNA enters the nucleus is far from clear…” [4]. This path also involves the long distance transport of nucleocapsids in axons, and it is challenging to dissect due to the difficulty of monitoring early events in an animal or homogenized neuron culture model in which the fate of infection is hard to predict before the latent state is established.
In the tri-chamber neuron culture model, the natural route of infection is recapitulated by infecting axons with low numbers of virus particles leading to a silent infection in a small number of neuronal cell bodies without the use of drugs. The advantage of this approach is that it allows establishment of a ‘latent infection’ in a well-controlled and reproducible way; it facilitates treatment of isolated cell bodies or axons separately to activate or inhibit target pathways, and it enables study of not only the stimuli that promote reactivation, but also the factors that regulate the initial switch from productive to latent infection.
Stress- versus tegument-mediated escape from silencing
When axons are infected with very few viral particles, the viral genomes are destined to be silenced in the neuronal cell bodies but can be reactivated later. The challenge was to determine if the cell bodies could be manipulated to shift the mode of infection from silenced to productive (i.e., what would enable viral genomes to ‘escape from silencing’?).
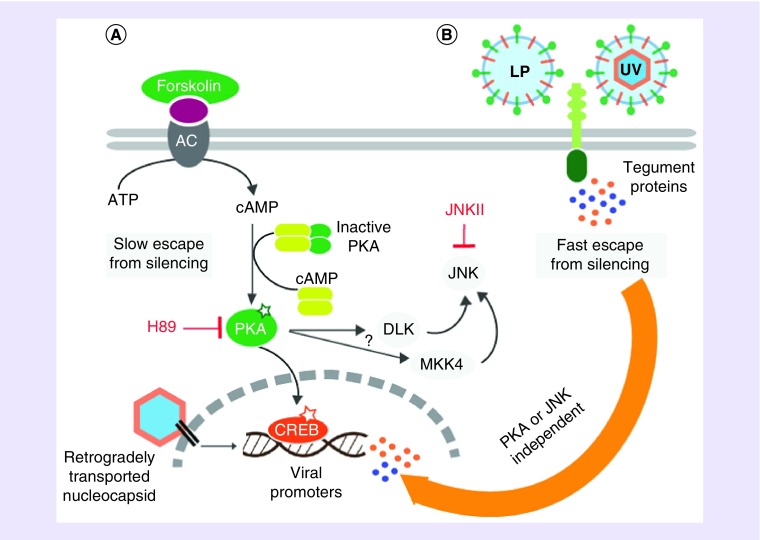
Two separate pathways were uncovered that can trigger a shift in the mode of infection from silenced to productive: the slow, stress-mediated pathway and the fast, viral tegument-mediated pathway. The stress-mediated pathway was triggered by the addition of cyclic adenosine monophosphate or forskolin to the cell body compartment, which subsequently activated PKA. If PKA was activated in the neuronal cell bodies at the same time that axons were infected with very few particles, genomes were not silenced. Spreading PRV infection was detected in S-compartments in approximately 7 days after the axonal infection. Importantly, this effect was dependent on the activity of PKA and the stress signaling kinase, JNK (Figure 1) [79]. These results align with previous findings suggesting that elevated cyclic adenosine monophosphate and PKA activation can reactivate quiescent HSV-1 infections [95,96]. Recently, it has been shown that various HSV-1 reactivating stimuli converge on the JNK pathway [97]. Induction of PKA apparently activates the JNK pathway, which is sufficient to promote PRV escape from silencing.
Figure 1. . Two distinct molecular mechanisms mediate escape from silencing.
(A) Forskolin mediates slow escape by activating adenylyl cyclase (AC) on the plasma membrane that converts ATP to cAMP, and cAMP activates PKA. Such escape takes almost 1 week and requires JNK activity. Nuclear translocation of PKA might activate CREB. Viral IE promoters carrying CRE might be activated by this way. Active PKA might directly activate DLK, or MKK4. DLK or MKK4 directly phosphorylates and activates JNK. (B) UVPRV and LP mediate fast escape. Several tegument proteins are released into the cytosol after receptor mediated fusion of viral envelope to the plasma membrane. Tegument proteins in the cell bodies activate the productive infection mode of axonally delivered viral genomes in 3 days. This pathway does not require active PKA or JNK, since inhibition of PKA by H89 or JNK by JNKII does not block escape.
LP: Light particles; UV: Ultraviolet.
Interestingly, when cell bodies in the S-compartment were exposed to a high concentration of UV-inactivated PRV virions at the same time that axons were infected with a small number of virions, the productive infection switch turned on in the cell bodies. Infection spread all over the S-compartment in 3 days, much faster than forskolin-mediated escape or the reactivation after a long latency period [79].
These experiments clearly showed that the latency/productive infection switch could be manipulated by treating cell bodies in the S-compartment. However, the UVPRV complementation did not allow distinguishing if it was the abundance of defective genomes or the tegument proteins delivered by UV-treated virions that enabled escape from silencing.
A well-known component of the α-HV productive replication cycle, capsid-less light particles (LP), provided an answer to that question [79]. LP preparations contained viral envelope and tegument proteins, but lacked capsids and viral genomes. Remarkably, exposure of cell bodies to LP was sufficient to bypass the silencing of axonally infecting PRV genomes as fast as UVPRV. This result demonstrates that excess genomes are not involved in escape from silencing, but viral tegument proteins are critical to override the latency program. It is important to note that neither UVPRV- nor LP-mediated productive infection switch was blocked by PKA or JNK inhibitors (Figure 1). This finding suggested that tegument-stimulated escape from silencing did not involve a stress activated escape pathway. The tegument protein or proteins that provide fast escape from silencing have not been identified yet. As, HSV-1 tegument component VP16 is thought to be a major player in the initiation of reactivation [98], PRV VP16 (UL48) is likely to contribute to the observed fast escape from silencing.
Upon infection of axons with large numbers of viral particles, thousands of copies of tegument proteins are released into the cytosol ready to interact with host proteins. However, the majority of these proteins are not carried by the capsid-transport complexes to the neuronal nucleus [15]. Apparently, with high MOI infections, sufficient tegument components reach the cell body to initiate the productive cycle. In the mouse model of HSV-1 latency, Thompson and Sawtell, 2000, suggested that productive replication of HSV-1 in a number of neurons in the ganglia occurs before latency is established [99]. This finding supports the idea that if tegument transport is efficient, productive replication can start in neuronal cell bodies. However, this productive phase is quickly shut off by the neurons that are supported by satellite cells and the immune system and latency is favored. In culture, it currently is difficult to switch the infection mode from productive to quiescent once the productive infection is initiated. Further studies are required to test the contribution of satellite cells and the effect of cytokine treatment during the establishment of latency in the compartmented culture model.
Escape from silencing versus reactivation
The capacity of tegument proteins to bypass viral genome silencing to enable the productive mode is conceptually different from reactivation. Usually, initiation of reactivation begins in the absence of tegument proteins, suggesting that the reactivation trigger must be intrinsic to the host neuron. This phenomenon has attracted much attention in the last decade, and the current research from many labs using different model systems has proven that reactivation is a multistep process consisting of at least two steps [34,57,100,101]. The first is activation of stress signaling kinases that lead to the modification of silencing histones [97,102]. This step is reversible and can be called initiation or preinitiation (i.e., Phase I) because it is a prerequisite for the relaxation of the viral heterochromatin [57]. As mentioned earlier, if neurons are exposed to type I or type II IFN at this phase, reactivation is blocked [92]. This nucleosome relaxation allows random transcription of viral genes, which has been called exit from latency. This transitional exit phase might yield sufficient tegument proteins to initiate the coordinated productive infection cycle (Phase II) resulting in infectious progeny production. These progeny are the measure of actual reactivation. From this point of view, tegument-mediated escape from latency bypasses the pre-initiation/initiation step of reactivation and resembles the exit from latency step. This conclusion might explain why escape from latency via tegument yields infectious virus much faster than the cellular stress mediated escape or reactivation. Similarly, the slow cellular stress mediated escape from silencing mechanistically resembles reactivation, albeit the silencing modifications on the viral episome most probably are not identical due to the shorter quiescence period.
Conclusion & future perspective
HV live up to the Greek origin of their name (herpein: to creep), not only illustrating their characteristic vesicular lesions, but also because latency/reactivation cycles ‘creep’ up on the infected hosts throughout their lives. Indeed, a vast majority of the human adult population is infected with at least one or multiple members of the Herpesviridae family. Reactivations likely occur more frequently than we know and although unseen, affect our health and quality of life and maintain the virus in the human population.
The evolutionary advantage of this remarkable host–virus interaction (silenced yet reactivatable infections in the PNS) must reflect, in part, the fact that neurons usually cannot be replaced, so cell death must be avoided. Most typical cell responses to viral infection often involve apoptosis or other forms of cell death. PNS neurons do not respond in this way. They deal with viral invasion as do many cell types, by silencing incoming foreign DNA. This decision must be the normal response when only a few viral genomes reach the nucleus. Accordingly, a silent infection is the ‘default’ pathway when tegument proteins are separated from genomes after a low MOI infection.
Reactivation of a silent infection in the PNS requires a signal from the host because no viral proteins are produced in latently infected neurons. One idea is that neurons respond to stress by producing gene products to protect themselves, and HV have evolved interactions to link the host stress signals to reactivation of silenced viral genomes. The reactivation threshold is high since only a small number of latent genomes will respond to stress. The logic for this tactic is twofold: the high threshold reflects the fact that the host must protect the integrity of the ganglion and ultimately the life of the host while only a few infectious virions are sufficient to spread to epithelial cells where productive infection will spread the infection to other hosts [103].
From the experiments with compartmented neuronal cultures, we learned that axonal biology, distinct from what happens in the cell body, plays an important role in the establishment of latent α-HV infections. It is clear that several bottlenecks or thresholds must be overcome to reach and establish infection in the neuronal nucleus. This in vitro model of α-HV latency, while highly reductionist yet inductive, has provided testable hypotheses and opens the way to investigate the decision making steps that result in a productive or silenced infection. This culture model can be further embellished to include satellite or epithelial cells or immune cells (NK cells, T cells, macrophages) that can be co-cultured in separate compartments.
It is evident that the combined use of simplified cell culture models along with in vivo animal models has exposed many mechanistic details of latency establishment, reactivation and the productive infection-latency switch. Yet, important questions remain regarding the consequences of latency/reactivation at both the cellular and organismal levels. Are latently infected neurons functionally different from their uninfected neighbors, and do they survive reactivation episodes? Does HV reach the CNS following reactivation, and can latency be established in the brain? Do recurrent reactivations play a role in the pathogenesis of neurodegenerative diseases? A detailed understanding of the complex latency/reactivation cycle will undoubtedly have vast implications for human health due to the high burden of latent α-HV infection.
Executive summary.
Alpha herpesviruses (α-HV) are among the most common human pathogens (e.g., HSV, VZV) causing cold sores, genital herpes, chickenpox and shingles.
Less frequently, HSV infections can lead to blindness, encephalitis and hard-to-treat neonatal infections.
Veterinary α-HV pathogens (e.g., PRV, BHV) cause devastating diseases in swine and cattle and lead to agricultural losses.
α-HV have co-evolved with their native hosts; they are highly species specific, and viral products interact with several cellular machineries and pathways.
α-HV infections are productive in various non-neuronal cells but can be latent in the neurons of the PNS ganglia.
α-HV latency is life-long, and there is no cure that eliminates latent viral genomes from the host neurons.
PNS neurons extend long axons that innervate the skin and other peripheral tissues, and α-HV particles invade these axons to travel to the neuron nucleus and establish latency.
During entry and subsequent long distance travel in axons, virus particles dissociate from their envelope and the outer tegument proteins.
In the neuronal nucleus, viral genomes are covered by histones and silenced by histone modifications.
Viral genomes persist in host cell nuclei as facultative heterochromatic nucleosomes.
Latent HSV, PRV and BHV genomes do not express measurable amounts of viral proteins but do express high levels of a long noncoding RNA (latency associated transcript).
Not all neurons in a ganglion harbor latent viral genomes, and only a few of the latently infected neurons show reactivating infection at a later time.
Various stress signaling mechanisms (e.g., hormonal changes, hyperthermia, nerve injury, DNA damage) reactivate latent viral genomes.
Reactivation results in the production of infectious progeny virions that can spread the infection to other tissues and hosts.
The latency-reactivation cycle is difficult to study because it involves many cell-intrinsic, tissue-specific and systemic factors.
Rodent models do not recapitulate all the aspects of human α-HV latency and reactivation.
α-HV infections of dissociated neuron cultures usually lead to a productive infection unless DNA replication is artificially blocked.
Compartmented neuronal cultures enabled physical separation of axons from cell bodies and showed that axonal infection by HSV and PRV leads to quiescent infections resembling in vivo latency, while cell body infection initiates the productive phase.
Tri-chamber neuron culture model of in vitro α-HV infection showed that:
Axons autonomously sense viral invaders and respond by making new proteins and activating injury signaling pathways.
Virus particles repurpose axonal machineries to ensure efficient long-distance transport in axons.
The number of viral particles infecting axons as well as innate immune responses affects the choice of latency or productive infection in the neuronal nucleus.
Reproducible and reactivateable in vitro latency can be established by infecting axons with a low concentration of PRV virions without the use of DNA replication inhibitors or cytokines.
Since the outcome of infection is predicted in such axonal infections, how viral genomes can escape from silencing can be studied.
PRV genome silencing can be overcome by activating neuronal PKA and JNK. Such stress-mediated escape takes almost a week to promote productive infection.
PRV genome silencing is rapidly overcome by the presence of tegument proteins independent of cellular PKA and JNK pathways. The hypothesis is that axonal infection by low concentrations of virions results in separate long-distance transport of viral particles and tegument that leads to the establishment of latency in the neuronal nucleus.
Compartmented neuronal cultures provide mechanistic understanding of the decision-making step of α-HV infection before viral genomes reach the nucleus.
Identification of viral proteins and their cellular interaction partners promoting escape from silencing as well as reactivation will lead to novel therapeutics blocking α-HV shedding and spread.
Acknowledgements
We thank the members of the Enquist lab, especially N Shree Tanneti for critical reading of the manuscript.
Footnotes
Financial & competing interests disclosure
LW Enquist acknowledges support from US NIH grants NS033506 and NS060699 and F30 fellowship – NINDS F30 NS090640 (MAM). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Cohrs RJ, Gilden DH. Human herpesvirus latency. Brain Pathol. 2001;11(4):465–474. doi: 10.1111/j.1750-3639.2001.tb00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penkert RR, Kalejta RF. Tegument protein control of latent herpesvirus establishment and animation. Herpesviridae. 2011;2(1):3. doi: 10.1186/2042-4280-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Honess RW, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 1974;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roizman B, Whitley RJ. An inquiry into the molecular basis of HSV latency and reactivation. Ann. Rev. Microbiol. 2013;67:355–374. doi: 10.1146/annurev-micro-092412-155654. [DOI] [PubMed] [Google Scholar]; •• A comprehensive review on the mechanism of HSV replication, latency and reactivation.
- 5.Looker KJ, Magaret AS, May MT, et al. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE. 2015;10(10):e0140765. doi: 10.1371/journal.pone.0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS ONE. 2015;10(1):e114989. doi: 10.1371/journal.pone.0114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moffat J, Ku CC, Zerboni L, Sommer M, Arvin A. VZV: pathogenesis and the disease consequences of primary infection. In: Arvin A, Campadelli-Fiume G, Mocarski E, et al., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press; Cambridge, UK: 2007. [PubMed] [Google Scholar]
- 8.Mitchell BM, Bloom DC, Cohrs RJ, Gilden DH, Kennedy PG. Herpes simplex virus-1 and varicella-zoster virus latency in ganglia. J. Neurovirol. 2003;9(2):194–204. doi: 10.1080/13550280390194000. [DOI] [PubMed] [Google Scholar]
- 9.Croen KD, Ostrove JM, Dragovic LJ, Straus SE. Patterns of gene expression and sites of latency in human nerve ganglia are different for varicella-zoster and herpes simplex viruses. Proc. Natl Acad. Sci. USA. 1988;85(24):9773–9777. doi: 10.1073/pnas.85.24.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung AK. Detection of pseudorabies virus transcripts in trigeminal ganglia of latently infected swine. J. Virol. 1989;63(7):2908–2913. doi: 10.1128/jvi.63.7.2908-2913.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enquist LW, Tomishima MJ, Gross S, Smith GA. Directional spread of an alpha-herpesvirus in the nervous system. Vet. Microbiol. 2002;86(1–2):5–16. doi: 10.1016/s0378-1135(01)00486-2. [DOI] [PubMed] [Google Scholar]
- 12.Jones C. Herpes simplex virus type 1 and bovine herpesvirus 1 latency. Clin. Microbiol. Rev. 2003;16(1):79–95. doi: 10.1128/CMR.16.1.79-95.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richter ER, Dias JK, Gilbert JE, 2nd, Atherton SS. Distribution of herpes simplex virus type 1 and varicella zoster virus in ganglia of the human head and neck. J. Infect. Dis. 2009;200(12):1901–1906. doi: 10.1086/648474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antinone SE, Shubeita GT, Coller KE, et al. The Herpesvirus capsid surface protein, VP26, and the majority of the tegument proteins are dispensable for capsid transport toward the nucleus. J. Virol. 2006;80(11):5494–5498. doi: 10.1128/JVI.00026-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohannon KP, Jun Y, Gross SP, Smith GA. Differential protein partitioning within the herpesvirus tegument and envelope underlies a complex and variable virion architecture. Proc. Natl Acad. Sci. USA. 2013;110(17):E1613–E1620. doi: 10.1073/pnas.1221896110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfstein A, Nagel CH, Radtke K, Dohner K, Allan VJ, Sodeik B. The inner tegument promotes herpes simplex virus capsid motility along microtubules in vitro . Traffic. 2006;7(2):227–237. doi: 10.1111/j.1600-0854.2005.00379.x. [DOI] [PubMed] [Google Scholar]
- 17.Campbell ME, Palfreyman JW, Preston CM. Identification of herpes simplex virus DNA sequences which encode a trans-acting polypeptide responsible for stimulation of immediate early transcription. J. Mol. Biol. 1984;180(1):1–19. doi: 10.1016/0022-2836(84)90427-3. [DOI] [PubMed] [Google Scholar]
- 18.Wilson AC, Cleary MA, Lai JS, LaMarco K, Peterson MG, Herr W. Combinatorial control of transcription: the herpes simplex virus VP16-induced complex. Cold Spring Harb. Symp. Quant. Biol. 1993;58:167–178. doi: 10.1101/sqb.1993.058.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Wysocka J, Herr W. The herpes simplex virus VP16-induced complex: the makings of a regulatory switch. Trends Biochem. Sci. 2003;28(6):294–304. doi: 10.1016/S0968-0004(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 20.Roizman B, Sears AE. An inquiry into the mechanisms of herpes simplex virus latency. Annu. Rev. Microbiol. 1987;41:543–571. doi: 10.1146/annurev.mi.41.100187.002551. [DOI] [PubMed] [Google Scholar]
- 21.Hafezi W, Lorentzen EU, Eing BR, et al. Entry of herpes simplex virus type 1 (HSV-1) into the distal axons of trigeminal neurons favors the onset of nonproductive, silent infection. PLoS Pathog. 2012;8(5):e1002679. doi: 10.1371/journal.ppat.1002679. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An article showing that the infection route affects the outcome of alpha herpesvirus infections.
- 22.Zaichick SV, Bohannon KP, Hughes A, Sollars PJ, Pickard GE, Smith GA. The herpesvirus VP1/2 protein is an effector of dynein-mediated capsid transport and neuroinvasion. Cell Host Microbe. 2013;13(2):193–203. doi: 10.1016/j.chom.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preston CM, Efstathiou S. Molecular basis of HSV latency and reactivation. In: Arvin A, Campadelli-Fiume G, Mocarski E, et al., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press; Cambridge, UK: 2007. [PubMed] [Google Scholar]
- 24.Lomonte P. The interaction between herpes simplex virus 1 genome and promyelocytic leukemia nuclear bodies (PML-NBs) as a hallmark of the entry in latency. Microb. Cell. 2016;3(11):569–572. doi: 10.15698/mic2016.11.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maroui MA, Calle A, Cohen C, et al. Latency entry of herpes simplex virus 1 is determined by the interaction of its genome with the nuclear environment. PLoS Pathog. 2016;12(9):e1005834. doi: 10.1371/journal.ppat.1005834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deshmane SL, Fraser NW. During latency herpes simplex virus type 1 DNA is associated with nucleosomes in a chromatin structure. J. Virol. 1989;63(2):943–947. doi: 10.1128/jvi.63.2.943-947.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubat NJ, Tran RK, McAnany P, Bloom DC. Specific histone tail modification and not DNA methylation is a determinant of herpes simplex virus type 1 latent gene expression. J. Virol. 2004;78(3):1139–1149. doi: 10.1128/JVI.78.3.1139-1149.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Efstathiou S, Minson AC, Field HJ, Anderson JR, Wildy P. Detection of herpes simplex virus-specific DNA sequences in latently infected mice and in humans. J. Virol. 1986;57(2):446–455. doi: 10.1128/jvi.57.2.446-455.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tirabassi RS, Townley RA, Eldridge MG, Enquist LW. Molecular mechanisms of neurotropic herpesvirus invasion and spread in the CNS. Neurosci. Biobehav. Rev. 1998;22(6):709–720. doi: 10.1016/s0149-7634(98)00009-8. [DOI] [PubMed] [Google Scholar]
- 30.Koyuncu OO, Hogue IB, Enquist LW. Virus infections in the nervous system. Cell Host Microbe. 2013;13(4):379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufman HE, Azcuy AM, Varnell ED, Sloop GD, Thompson HW, Hill JM. HSV-1 DNA in tears and saliva of normal adults. Invest. Ophthalmol. Vis. Sci. 2005;46(1):241–247. doi: 10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohrs RJ, Gilden DH, Kinchington PR, Grinfeld E, Kennedy PG. Varicella-zoster virus gene 66 transcription and translation in latently infected human Ganglia. J. Virol. 2003;77(12):6660–6665. doi: 10.1128/JVI.77.12.6660-6665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson AC, Mohr I. A cultured affair: HSV latency and reactivation in neurons. Trends Microbiol. 2012;20(12):604–611. doi: 10.1016/j.tim.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cliffe AR, Wilson AC. Restarting lytic gene transcription at the onset of herpes simplex virus reactivation. J. Virol. 2016;91(2):e01419-16. doi: 10.1128/JVI.01419-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith G. Herpesvirus transport to the nervous system and back again. Annu. Rev. Microbiol. 2012;66:153–176. doi: 10.1146/annurev-micro-092611-150051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawtell NM, Poon DK, Tansky CS, Thompson RL. The latent herpes simplex virus type 1 genome copy number in individual neurons is virus strain specific and correlates with reactivation. J. Virol. 1998;72(7):5343–5350. doi: 10.1128/jvi.72.7.5343-5350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Catez F, Picard C, Held K, et al. HSV-1 genome subnuclear positioning and associations with host-cell PML-NBs and centromeres regulate LAT locus transcription during latency in neurons. PLoS Pathog. 2012;8(8):e1002852. doi: 10.1371/journal.ppat.1002852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lomonte P. Herpesvirus latency: on the importance of positioning oneself. Adv. Anat. Embryol. Cell Biol. 2017;223:95–117. doi: 10.1007/978-3-319-53168-7_5. [DOI] [PubMed] [Google Scholar]
- 39.Sawtell NM. The probability of in vivo reactivation of herpes simplex virus type 1 increases with the number of latently infected neurons in the ganglia. J. Virol. 1998;72(8):6888–6892. doi: 10.1128/jvi.72.8.6888-6892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsatsos M, MacGregor C, Athanasiadis I, Moschos MM, Hossain P, Anderson D. Herpes simplex virus keratitis: an update of the pathogenesis and current treatment with oral and topical antiviral agents. Clin. Exp. Ophthalmol. 2016;44(9):824–837. doi: 10.1111/ceo.12785. [DOI] [PubMed] [Google Scholar]
- 41.Gnann JW, Jr, Whitley RJ. Herpes simplex encephalitis: an update. Curr. Infect. Dis. Rep. 2017;19(3):13. doi: 10.1007/s11908-017-0568-7. [DOI] [PubMed] [Google Scholar]
- 42.Groves MJ. Genital herpes: a review. Am. Fam. Physician. 2016;93(11):928–934. [PubMed] [Google Scholar]
- 43.Bertke AS, Ma A, Margolis MS, Margolis TP. Different mechanisms regulate productive herpes simplex virus 1 (HSV-1) and HSV-2 infections in adult trigeminal neurons. J. Virol. 2013;87(11):6512–6516. doi: 10.1128/JVI.00383-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Looker KJ, Magaret AS, May MT, et al. First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob. Health. 2017;5(3):e300–e309. doi: 10.1016/S2214-109X(16)30362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N. Engl. J. Med. 2009;361(14):1376–1385. doi: 10.1056/NEJMra0807633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller CS, Danaher RJ. Asymptomatic shedding of herpes simplex virus (HSV) in the oral cavity. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008;105(1):43–50. doi: 10.1016/j.tripleo.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Amlie-Lefond C, Gilden D. Varicella zoster virus: a common cause of stroke in children and adults. J. Stroke Cerebrovasc. Dis. 2016;25(7):1561–1569. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilden D, Grose C, White T, et al. Successful antiviral treatment after 6 years of chronic progressive neurological disease attributed to VZV brain infection. J. Neurol. Sci. 2016;368:240–242. doi: 10.1016/j.jns.2016.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitley RJ. Herpes simplex virus infections of the central nervous system. Continuum (Minneap Minn) 2015;21(6 Neuroinfectious Disease):1704–1713. doi: 10.1212/CON.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 50.Goodrum F. Human cytomegalovirus latency: approaching the gordian knot. Annu. Rev. Virol. 2016;3(1):333–357. doi: 10.1146/annurev-virology-110615-042422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doll JR, Sawtell NM. Analysis of herpes simplex virus reactivation in explant reveals a method-dependent difference in measured timing of reactivation. J. Virol. 2017;91(16) doi: 10.1128/JVI.00848-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sawtell NM, Thompson RL. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J. Virol. 1992;66(4):2150–2156. doi: 10.1128/jvi.66.4.2150-2156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A paper showing that stress due to elevated body temperatures reactivates latent HSV-1 infection in the murine model.
- 53.Thompson RL, Sawtell NM. The herpes simplex virus type 1 latency associated transcript locus is required for the maintenance of reactivation competent latent infections. J. Neurovirol. 2011;17(6):552–558. doi: 10.1007/s13365-011-0071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagner EK, Bloom DC. Experimental investigation of herpes simplex virus latency. Clin. Microbiol. Rev. 1997;10(3):419–443. doi: 10.1128/cmr.10.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen JJ, Gershon AA, Li ZS, Lungu O, Gershon MD. Latent and lytic infection of isolated guinea pig enteric ganglia by varicella zoster virus. J. Med. Virol. 2003;70(Suppl. 1):S71–S78. doi: 10.1002/jmv.10325. [DOI] [PubMed] [Google Scholar]
- 56.Kennedy PG, Rovnak J, Badani H, Cohrs RJ. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J. Gen. Virol. 2015;96(Pt 7):1581–1602. doi: 10.1099/vir.0.000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sawtell NM, Thompson RL. Herpes simplex virus and the lexicon of latency and reactivation: a call for defining terms and building an integrated collective framework. F1000Res. 2016;5:2038. doi: 10.12688/f1000research.8886.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pomeranz LE, Reynolds AE, Hengartner CJ. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 2005;69(3):462–500. doi: 10.1128/MMBR.69.3.462-500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; • An extensive review of pseudorabies virus biology.
- 59.Inman M, Zhou J, Webb H, Jones C. Identification of a novel bovine herpesvirus 1 transcript containing a small open reading frame that is expressed in trigeminal ganglia of latently infected cattle. J. Virol. 2004;78(10):5438–5447. doi: 10.1128/JVI.78.10.5438-5447.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang Y, Hossain A, Winkler MT, Holt T, Doster A, Jones C. A protein encoded by the latency-related gene of bovine herpesvirus 1 is expressed in trigeminal ganglionic neurons of latently infected cattle and interacts with cyclin-dependent kinase 2 during productive infection. J. Virol. 1998;72(10):8133–8142. doi: 10.1128/jvi.72.10.8133-8142.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yun H, Rowe AM, Lathrop KL, Harvey SA, Hendricks RL. Reversible nerve damage and corneal pathology in murine herpes simplex stromal keratitis. J. Virol. 2014;88(14):7870–7880. doi: 10.1128/JVI.01146-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kinchington PR, Goins WF. Varicella zoster virus-induced pain and post-herpetic neuralgia in the human host and in rodent animal models. J. Neurovirol. 2011;17(6):590–599. doi: 10.1007/s13365-011-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arvin AM, Moffat JF, Sommer M, et al. Varicella-zoster virus T cell tropism and the pathogenesis of skin infection. Curr. Top. Microbiol. Immunol. 2010;342:189–209. doi: 10.1007/82_2010_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ku CC, Zerboni L, Ito H, Graham BS, Wallace M, Arvin AM. Varicella-zoster virus transfer to skin by T cells and modulation of viral replication by epidermal cell interferon-alpha. J. Exp. Med. 2004;200(7):917–925. doi: 10.1084/jem.20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gan L, Wang M, Chen JJ, Gershon MD, Gershon AA. Infected peripheral blood mononuclear cells transmit latent varicella zoster virus infection to the guinea pig enteric nervous system. J. Neurovirol. 2014;20(5):442–456. doi: 10.1007/s13365-014-0259-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arnold N, Girke T, Sureshchandra S, Messaoudi I. Acute simian varicella virus infection causes robust and sustained changes in gene expression in the sensory ganglia. J. Virol. 2016;90(23):10823–10843. doi: 10.1128/JVI.01272-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Messaoudi I, Barron A, Wellish M, et al. Simian varicella virus infection of rhesus macaques recapitulates essential features of varicella zoster virus infection in humans. PLoS Pathog. 2009;5(11):e1000657. doi: 10.1371/journal.ppat.1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sawtell NM, Thompson RL. Comparison of herpes simplex virus reactivation in ganglia in vivo and in explants demonstrates quantitative and qualitative differences. J. Virol. 2004;78(14):7784–7794. doi: 10.1128/JVI.78.14.7784-7794.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thellman NM, Triezenberg SJ. Herpes simplex virus establishment, maintenance, and reactivation: in vitro modeling of latency. Pathogens. 2017;6(3):28. doi: 10.3390/pathogens6030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elion GB, Furman PA, Fyfe JA, de Miranda P, Beauchamp L, Schaeffer HJ. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc. Natl Acad. Sci. USA. 1977;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reardon JE, Spector T. Herpes simplex virus type 1 DNA polymerase. Mechanism of inhibition by acyclovir triphosphate. J. Biol. Chem. 1989;264(13):7405–7411. [PubMed] [Google Scholar]
- 72.Wilcox CL, Johnson EM., Jr Nerve growth factor deprivation results in the reactivation of latent herpes simplex virus in vitro . J. Virol. 1987;61(7):2311–2315. doi: 10.1128/jvi.61.7.2311-2315.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The discovery that growth factor signaling in neurons maintains alpha herpesvirus latency.
- 73.Wilcox CL, Smith RL, Freed CR, Johnson EM., Jr Nerve growth factor-dependence of herpes simplex virus latency in peripheral sympathetic and sensory neurons in vitro . J. Neurosci. 1990;10(4):1268–1275. doi: 10.1523/JNEUROSCI.10-04-01268.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Camarena V, Kobayashi M, Kim JY, et al. Nature and duration of growth factor signaling through receptor tyrosine kinases regulates HSV-1 latency in neurons. Cell Host Microbe. 2010;8(4):320–330. doi: 10.1016/j.chom.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A paper describing the molecular mechanism of growth factor signaling [72].
- 75.Kobayashi M, Wilson AC, Chao MV, Mohr I. Control of viral latency in neurons by axonal mTOR signaling and the 4E-BP translation repressor. Genes Dev. 2012;26(14):1527–1532. doi: 10.1101/gad.190157.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Michaelevski I, Segal-Ruder Y, Rozenbaum M, et al. Signaling to transcription networks in the neuronal retrograde injury response. Sci. Signal. 2010;3(130):ra53. doi: 10.1126/scisignal.2000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perry RB, Doron-Mandel E, Iavnilovitch E, et al. Subcellular knockout of importin beta1 perturbs axonal retrograde signaling. Neuron. 2012;75(2):294–305. doi: 10.1016/j.neuron.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu WW, Goodhouse J, Jeon NL, Enquist LW. A microfluidic chamber for analysis of neuron-to-cell spread and axonal transport of an alpha-herpesvirus. PLoS ONE. 2008;3(6):e2382. doi: 10.1371/journal.pone.0002382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koyuncu OO, MacGibeny MA, Hogue IB, Enquist LW. Compartmented neuronal cultures reveal two distinct mechanisms for alpha herpesvirus escape from genome silencing. PLoS Pathog. 2017;13(10):e1006608. doi: 10.1371/journal.ppat.1006608. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A paper investigating escape from silencing in compartmented neurons culture model of pseudorabies virus infection of primary rodent neurons.
- 80.Koyuncu OO, Song R, Greco TM, Cristea IM, Enquist LW. The number of alphaherpesvirus particles infecting axons and the axonal protein repertoire determines the outcome of neuronal infection. mBio. 2015;6(2):e00276-15. doi: 10.1128/mBio.00276-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koyuncu OO, Perlman DH, Enquist LW. Efficient retrograde transport of pseudorabies virus within neurons requires local protein synthesis in axons. Cell Host Microbe. 2013;13(1):54–66. doi: 10.1016/j.chom.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jung H, Yoon BC, Holt CE. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat. Rev. Neurosci. 2012;13(5):308–324. doi: 10.1038/nrn3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Michaelevski I, Medzihradszky KF, Lynn A, Burlingame AL, Fainzilber M. Axonal transport proteomics reveals mobilization of translation machinery to the lesion site in injured sciatic nerve. Mol. Cell Proteomics. 2010;9(5):976–987. doi: 10.1074/mcp.M900369-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Song R, Koyuncu OO, Greco TM, Diner BA, Cristea IM, Enquist LW. Two modes of the axonal interferon response limit alphaherpesvirus neuroinvasion. mBio. 2016;7(1):e02145–e02115. doi: 10.1128/mBio.02145-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Enquist LW, Leib DA. Intrinsic and innate defenses of neurons: detente with the herpesviruses. J. Virol. 2017;91(1):e01200-16. doi: 10.1128/JVI.01200-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schoggins JW. Interferon-stimulated genes: roles in viral pathogenesis. Curr. Opin. Virol. 2014;6:40–46. doi: 10.1016/j.coviro.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cunningham AL, Mikloska Z. The Holy Grail: immune control of human herpes simplex virus infection and disease. Herpes. 2001;8(Suppl. 1):6A–10A. [PubMed] [Google Scholar]
- 88.Leib DA, Harrison TE, Laslo KM, Machalek MA, Moorman NJ, Virgin HW. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo . J. Exp. Med. 1999;189(4):663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.St Leger AJ, Hendricks RL. CD8+ T cells patrol HSV-1-infected trigeminal ganglia and prevent viral reactivation. J. Neurovirol. 2011;17(6):528–534. doi: 10.1007/s13365-011-0062-1. [DOI] [PubMed] [Google Scholar]
- 90.Mikloska Z, Cunningham AL. Alpha and gamma interferons inhibit herpes simplex virus type 1 infection and spread in epidermal cells after axonal transmission. J. Virol. 2001;75(23):11821–11826. doi: 10.1128/JVI.75.23.11821-11826.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Regge N, Van Opdenbosch N, Nauwynck HJ, Efstathiou S, Favoreel HW. Interferon alpha induces establishment of alphaherpesvirus latency in sensory neurons in vitro . PLoS ONE. 2010;5(9) doi: 10.1371/journal.pone.0013076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Linderman JA, Kobayashi M, Rayannavar V, et al. Immune escape via a transient gene expression program enables productive replication of a latent pathogen. Cell Rep. 2017;18(5):1312–1323. doi: 10.1016/j.celrep.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cai W, Schaffer PA. Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J. Virol. 1992;66(5):2904–2915. doi: 10.1128/jvi.66.5.2904-2915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moriuchi H, Moriuchi M, Dean H, Cheung AK, Cohen JI. Pseudorabies virus EPO is functionally homologous to varicella-zoster virus ORF61 protein and herpes simplex virus type 1 ICPO. Virology. 1995;209(1):281–283. doi: 10.1006/viro.1995.1256. [DOI] [PubMed] [Google Scholar]
- 95.Danaher RJ, Savells-Arb AD, Black SA, Jr, Jacob RJ, Miller CS. Herpesvirus quiescence in neuronal cells IV: virus activation induced by pituitary adenylate cyclase-activating polypeptide (PACAP) involves the protein kinase A pathway. J. Neurovirol. 2001;7(2):163–168. doi: 10.1080/13550280152058825. [DOI] [PubMed] [Google Scholar]
- 96.Rodriguez A, Sainz De La Maza M, Missry J, Foster CS. The role of cyclic nucleotide mediators in latency and reactivation of HSV-1 infected neuroblastoma cells. Eye. 1991;5(Pt 5):627–635. doi: 10.1038/eye.1991.109. [DOI] [PubMed] [Google Scholar]
- 97.Cliffe AR, Arbuckle JH, Vogel JL, et al. Neuronal stress pathway mediating a histone methyl/phospho switch is required for herpes simplex virus reactivation. Cell Host Microbe. 2015;18(6):649–658. doi: 10.1016/j.chom.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; • An article describing the initial molecular steps of HSV reactivation in an in vitro rodent model of HSV-1 latency.
- 98.Thompson RL, Sawtell NM. Therapeutic implications of new insights into the critical role of VP16 in initiating the earliest stages of HSV reactivation from latency. Future Med. Chem. 2010;2(7):1099–1105. doi: 10.4155/fmc.10.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thompson RL, Sawtell NM. Replication of herpes simplex virus type 1 within trigeminal ganglia is required for high frequency but not high viral genome copy number latency. J. Virol. 2000;74(2):965–974. doi: 10.1128/jvi.74.2.965-974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sawtell NM, Thompson RL. De novo herpes simplex virus VP16 expression gates a dynamic programmatic transition and sets the latent/lytic balance during acute infection in trigeminal ganglia. PLoS Pathog. 2016;12(9):e1005877. doi: 10.1371/journal.ppat.1005877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim JY, Mandarino A, Chao MV, Mohr I, Wilson AC. Transient reversal of episome silencing precedes VP16-dependent transcription during reactivation of latent HSV-1 in neurons. PLoS Pathog. 2012;8(2):e1002540. doi: 10.1371/journal.ppat.1002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cliffe AR, Garber DA, Knipe DM. Transcription of the herpes simplex virus latency-associated transcript promotes the formation of facultative heterochromatin on lytic promoters. J. Virol. 2009;83(16):8182–8190. doi: 10.1128/JVI.00712-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Taylor MP, Kobiler O, Enquist LW. Alphaherpesvirus axon-to-cell spread involves limited virion transmission. Proc. Natl Acad. Sci. USA. 2012;109(42):17046–17051. doi: 10.1073/pnas.1212926109. [DOI] [PMC free article] [PubMed] [Google Scholar]