Abstract
Silver nanoparticles (AgNPs) have been incorporated into several consumer products. While these advances in technology are promising and exciting, the effects of these nanoparticles have not equally been studied. Due to the size, AgNPs can penetrate the body through oral exposure and reach the gastrointestinal tract. The present study was designed as a comparative proteomic analysis of Caco-2 cells, used as an in vitro model of the small intestine, exposed to 30 nm citrate stabilized-silver nanoparticles (AgNPs) for 24 or 72 h. Using two complementary proteomic approaches, 2D gel-based and label-free mass spectrometry, we present insight into the effects of AgNPs at proteins level. Exposure of 1 or 10 μg/mL AgNPs to Caco-2 cells resulted in 56 and 88 altered proteins at 24 h and 72 h respectively, by 2D gel-based technique. Ten of these proteins were found to be common between the two time-points. Using label-free mass spectrometry technique, 291 and 179 altered proteins were found at 24 h and 72 h, of which 24 were in common. Analysis of the proteomes showed several major biological processes altered, from which, cell cycle, cell morphology, cellular function and maintenance were the most affected.
Keywords: Nanomaterial safety assessment, Systems biology analysis, 2D-gel based proteomic approach, Label-free MS-based proteomic approach, Qualitative and quantitative proteomics
Highlights
-
•
Comparison between 2D gel-based vs label-free MS based proteomics study
-
•
Significant changes in the protein profiles of Caco-2 cells exposed to AgNPs.
-
•
Contribute to understand the mechanisms of action of AgNPs
1. Introduction
Silver nanoparticles (AgNPs) are the most commercialised nanotechnological products on the market according to the Consumer Products Inventory (2016), with over 400 registered applications to date (Calderón-Jiménez et al., 2017). Due to their unique antibacterial properties against both Gram-positive and negative bacteria (Panáček et al., 2006), AgNPs have been incorporated into a large number of consumer products. AgNPs are found in clothing, kitchenware, toys, cosmetics, medicinal products, medical devices, food packaging materials, plant protection and biocidal products.
Considering the broad application of AgNPs, a wide public is likely exposed to them on a regular basis. The increased rate of introduction of NPs-based consumer products to the market prompts the need for a better understanding of the fate and potential impacts on the biological systems.
Silver is considered to be more toxic than other metals when in nanoscale form (Bar-Ilan et al., 2009) and AgNPs have a different toxicity mechanism compared to dissolved silver ions (Li et al., 2013). The mechanism employed for the uptake of NPs and their effects on cellular function appear to be critically dependent on the particle characteristics, such as the size (of the primary particle and potential aggregates/agglomerates), hydrophobicity, surface modification, and shape (Win and Feng, 2005). However, less research has been done to evaluate these interactions and their impact on human health. Previous works have shown that AgNPs can induce potential harmful effects, including the generation of dangerous radicals (Li et al., 2013).
Due to their size, NPs can readily penetrate the body and cells through various routes. It has been reported that inhaled NPs cleared by mucociliary escalator, can be ingested and reach the gastrointestinal tract (Teow et al., 2011). It is estimated that the average person in a developed country is exposed orally to 1012 to 1014 man-made fin (0.1–1 μm) to ultrafine (<100 nm) particles every day (Lomer et al., 2002, Kim et al., 2010, Hartemann et al., 2015). As the Caco-2 human epithelial cell line is one of the most relevant in vitro models to study intestinal functions (Lefebvre et al., 2015), it was selected to investigate AgNPs toxicity.
The present study was designed to elucidate the effects of AgNPs when interacting with Caco-2 cells and to address the limited literature available. We had previously shown the advantages of 2D gel-based proteomics as a potent tool to accurately quantify and identify proteins involved in cellular events, underlying nano-bio interactions and understanding the potential mechanism of actions (Gioria et al., 2016). Here, we have applied the technique to unravel the cellular networks regulated by AgNPs.
We report on the two complementary proteomic approaches for the investigation of the interactions of AgNPs with Caco-2 cells: (i) the 2D gel-based proteomics, which included two-dimensional gel electrophoresis (2DE), coupled with ultra-high-performance liquid chromatography high-resolution mass spectrometry (UHPLC-HRMS/MS); and (ii) the label-free MS-based proteomics, based on UHPLC-HRMS/MS, followed by extensive bioinformatics and data mining procedures. Also, the two proteomic approaches were complemented with additional analytical techniques for a complete analysis of cellular response to NPs.
Overall this research advances the mechanistic understanding of AgNP toxicity and contribute to a more effective assessment of the growing number of new nanomaterials, which is difficult to achieve by traditional, single end-point approaches (Costa and Fadeel, 2016) (Matysiak et al., 2016). In addition, data sharing in mass-spectrometry (MS)-based proteomics opens a plethora of opportunities (Martens and Vizcaíno, 2017) for scientific progress.
2. Materials and methods
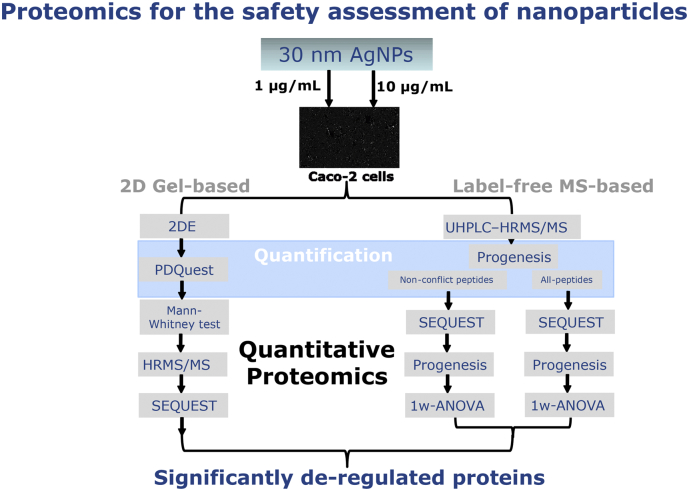
A schematic diagram of the experimental design of the 2D-gel based and label-free MS-based approaches and bioinformatics tools employed is provided in Fig. 1.
Fig. 1.
Schematic diagram of the experimental design of the 2D-gel based and label-free MS-based.
2.1. AgNPs synthesis
In controlling the colloidal stability, citrate was used as a capping agent. The synthesis of AgNPs was carried out by the reduction of AgNO3 with citrate and tannic acid based on the procedure described in (Dadosh, 2009) with some modifications. More specifically, 120 μL of tannic acid (2 mM) were added to 6 mL of citrate 28 mM, the solution was and stirred at 60 °C for 15 min. Then, 6 mL of this solution were added to 94 mL of AgNO3 0.55 mM in boiling condition under vigorous stirring. The mixture was kept at 97 °C for further 40 min. The solution was heated up using a microwave synthesis reactor (Discover S by CEM corporation) to ensure a highly reproducible rapid heating. Afterwards, the solution was rapidly cooled down at 40 °C, and then to room temperature. The nanoparticles were directly characterized after synthesis.
2.2. AgNPs characterisation
The size and size distribution of the synthesized AgNPs in dispersion solution were assessed by Centrifugal Liquid Sedimentation (CLS), and Dynamic Light Scattering (DLS) while the shape and size were verified by Scanning Electron Microscopy (SEM) imaging. The CLS measurements (instrument model DC24000UHR, CPS Instruments Inc., USA) were performed in an 8 wt%–24 wt% sucrose density gradient with a disc speed of 22,000 rpm. Each sample injection of 100 μL was preceded by a calibration step using certified polyvinyl chloride (PVC) particle size standards with a weight mean size of 280 nm. Measurements of particle size distribution by DLS were done using a Zetasizer Nano-ZS by Malvern Ltd., UK. Each sample was measured in triplicate at 25 °C after an equilibration step of 120 s using an acquisition time of 80 s. The hydrodynamic diameter was calculated using the DLS internal software.
The size distribution and shape of the particles were also verified using TEM image. The software ImageJ was used for image analysis with a minimum of 100 particles being counted for size and size distribution calculations.
The behaviour of citrate-stabilized AgNPs in complete culture medium was monitored by DLS analysis for up to 72 h at 37 °C.
The particle stock suspension was analysed for potential endotoxin contamination using a commercially available endotoxin quantitation kit (Thermo Fisher Scientific, 88,282) according to the manufacturer's instructions and no endotoxin contamination was detected.
2.3. Quantification of dissolved ionic silver and internalised AgNPs by ICP-MS
The amount of dissolved ionic Ag was evaluated by ultrafiltration in 1 and 10 μg/mL AgNPs suspensions in Caco-2 complete culture media. Two mL of the stock was filtered through Amicon Ultra Centrifugal Filter of regenerated cellulose (cut off 3KDa). To 500 μL of the resulting filtrate, 200 μL of concentrated nitric acid was added (Carlo Erba SpA, Italy) and the solution was made up to 3 mL with Milli-Q water (Millipore, USA) before analysis by ICP-MS (Agilent ICP-MS 7700x, Agilent Technologies, Santa Clara, USA). The instrument was operated using collision cell technology (CCT) with He gas (4.3 mL/min) and monitoring isotope Ag107. Rhodium (50 μg/L) was added on-line as an internal standard.
Total concentration of Ag internalised in cells incubated for 24 h, or 72 h was evaluated after 10 min microwave digestion (200 °C, 300 W, 400 psi) with 2 mL of HNO3 and 0.4 mL H2O2 using a microwave acid digestion system (Discover SP-D, CEM Co., USA). Samples were diluted with milli-Q water before ICP-MS analysis, as described above. ICP-MS was also performed on initial solution of 1 and 10 μg/mL (t = 0) in order to calculate the percentage of dissolved ionic Ag.
2.4. Cell culture conditions and AgNP exposure
Human colon adenocarcinoma Caco-2 cells were obtained from the American Type Culture Collection (LGC standards, Milano, Italy). Caco-2 cells (passage 43–49) were cultured in complete culture medium, composed of Dulbecco's Modified Eagle Medium (DMEM) high glucose (4500 g/L) supplemented with 10% (v/v) Fetal Bovine Serum (FBS, North America Origin), 0.5% (v/v) penicillin/streptomycin, 4 mM l-glutamine and 1% (v/v) not essential amino acids. All cell culture reagents were purchased from Life Technologies, Italy. For routine culture, cells were maintained in a sub-confluent state under standard cell culture conditions in a humidified incubator (37 °C, 5% CO2, 95% humidity) (Heraeus Thermo Fisher®, Belgium).
2.5. Sample preparation
For proteomic experiments, 1 × 106 Caco-2 cells between passage 43 and 49 were seeded in 5 mL complete culture medium in 100 × 20 mm Petri dish (Corning, Italy). For treatment, the medium was replaced with 30 nm AgNPs at final concentrations of 1 or 10 μg/mL. In each experiment, untreated cells were used as control. Six biological replicates were performed for each experimental condition. Proteins extraction from the cytoplasmatic compartment was performed at 24 and 72 h of exposure time as described in our previous work (Gioria et al., 2014).
For 2D gel-based experiments, protein pellets were re-suspended in the buffer for two-dimensional polyacrylamide gel electrophoresis. Experiments were run using an equal protein amount of 100 μg per sample (control or treated). For each experimental condition (Control and treated), six replicate gels were run.
For MS-based proteomic experiments, protein pellets (100 μg protein each) were re-suspended in 100 μL 0.2% (w/v) RapiGest solution and vortexed. 5 μL of 50 mM DTT solution was added, and the sample was heated at 60 °C for 30 min at 300 rpm using a Thermomixer. The sample was cooled to room temperature, and 10 μL of 100 mM iodoacetamide (IoAc) solution was added. The sample was placed in the dark at room temperature for 30 min and 40 μL 0.1 μg/μL of trypsin solution was added to the protein tube (1:50, protease: protein ratio). Samples were incubated at 37 °C for 12 h (Thermomixer at 300 rpm) for optimum enzymatic digestion. 5 μL of 500 mM HCl solution was then added to neutralise the RapiGest. The sample was transferred into molecular-mass cut-off filtration units (3000 MWCO) and the units were centrifuged at 14,000 ×g for 10 min before LC-MS/MS analysis.
2.6. 2D gel-based quantitative proteomic experiments
2.6.1. 2D gel electrophoresis and 2D map differential analysis
In order to better estimate the difference between untreated (control) and AgNPs-treated cells, a randomised block design on 6 biological replicates for each experimental condition (control, 1 or 10 μg/mL AgNPs) was performed to reduce the bias and variance in the 2D-gel protein patterns.
The 2D gel electrophoresis technique was described in our previous work (Gioria et al., 2014). Briefly, protein samples were separated by isoelectric focusing using immobilised non-linear pH range 3.0–10.0 strips (GE Healthcare) followed by SDS-PAGE in a 16 × 14 cm 8–14% linear gradient. After 2D electrophoresis, gels stained with fluorescent dye Sypro Ruby (Molecular Probe Inc., Lifetechnologies, Italy) were scanned with a GS-800 imaging densitometer (BioRad) under the same scanning conditions. For each 2D map protein pattern analysis, background subtraction, spot detection, gel alignment and spot matching were performed using PDQuest v. 7.3.0 software package (BioRad) as already reported (Gioria et al., 2016). Using Mann-Whitney test along with ± two folds change in expression level, differentially regulated proteins were selected. Apparent molecular weight (MW) and isoelectric points (pI) were established by comparison with known proteins used as internal standards.
2.6.2. Preparative 2D gels and protein spot picking
A preparative experiment was run using 200 μg of protein from control and treated samples. Experimental conditions for electrophoresis were the same as the ones described for the analytical gel. The gels were Sypro Ruby stained and digitized for image analysis. Preparative gels were matched with analytical gels for protein selection in the 2D map using PDQuest software. Corresponding spots were listed and numbered accordingly for further MS/MS identification. Selected protein spots were excised and transferred to a 96-well plate using a ProteomeWorks Plus Spot Cutter System (BioRad).
2.6.3. In-gel protein hydrolysis and peptide extraction
Sample preparation was carried out under a laminar flow cabinet using powder-free gloves and sterile equipment. Protein spots were washed three times with Milli-Q water and dried three times with acetonitrile (CH3CN), reduced (using 10 mM DTT in 50 mM ammonium bicarbonate for 30 min at 56 °C) and alkylated (using 50 mM iodoacetamide in 50 mM ammonium bicarbonate for 30 min in the dark). The enzymatic digestion (using 1 ng/μl sequencing grade trypsin in 50 mM ammonium bicarbonate) was performed at 37 °C overnight. The resulting hydrolysates were extracted three times with a total volume of 40 μl solution (CH3CN 100%) and transferred into Eppendorf tubes. Extracts were combined (120 μl) and samples were evaporated to dryness using a rotary evaporator equipped with a vacuum system and re-suspended in 20 μL solution of 0.1% formic acid (HCOOH) in milli-Q water: methanol, 95:5.
2.7. Label-free MS-based quantitative proteomic experiments
2.7.1. Capillary-UHPLC and LTQ Orbitrap mass spectrometry
For label-free MS-based quantitative proteomics, the UHPLC-HRMS/MS configuration and experimental conditions were similar as described for the 2D-gel based approach (Protein spot identification by UHPLC-HRMS/MS, Supplementary Methods). 6 biological replicates were analysed to increase the impact of this study. Peptides extracted from the digested gel were transferred to the Ultimate 3000 autosampler. A 5 μL aliquot of the extract was injected and loaded onto the pre-column. Experiment design involved the analysis of quality control (QC) samples (Waters Mass PREP Digestion standard bovine serum albumin in establishing the repeatability of the method), analytical blanks (for possible contamination) and the study samples (control and treated). Control and treated samples were run in randomised order with the analytical blanks and QCs during the sequence. Peptides extracted from the digested gel were transferred to the Ultimate 3000 autosampler. A 5 μL aliquot of the extract was injected and loaded onto the pre-column. An experimental design table was created for each of the 6 batches of analysis (not shown). To each batch was associated a *.csv file containing the following information on the analysis sequence: sample name (QC, blank, sample), sample code, file name and treatment, nanoparticle size.
2.7.2. HRMS/MS data processing
The raw data obtained from the label-free MS-based proteomic analysis (*.raw) were imported and processed using Progenesis QI for Proteomics software (NonLinear Dynamics, UK). The software processed the raw data in two steps. Firstly, each sample run was subjected to peak extraction and alignment. The sample run that yielded most features (i.e. peptide ions) was used as the reference run to which retention time of all of the other runs was aligned, and peak intensities were normalised. The Progenesis peptide quantification algorithm calculates peptide abundance as the sum of the peak areas. Each abundance value is then transformed to a normalised abundance value by applying a global scaling factor. Protein abundance was calculated as the sum of the abundances of all peptide ions identified as coming from the same protein. For the purpose of this experiment, the quantification based on i) all peptides and ii) non-conflict peptides was performed and compared. A number of criteria were used to filter the data before exporting the MS/MS output files for protein identification; (1) peptide features with analysis of variance (ANOVA) p-value ≤0.05 between experimental groups, (2) mass peaks with charge states from +2 to +4, and (3) maximum number of MS/MS spectra per mass set to 5. All MS/MS spectra were exported from Progenesis software as a MASCOT generic file (*.mgf) and used for peptide identification with Proteome Discoverer 1.4 (Thermo Fisher Scientific) using the SEQUEST algorithm, (licence Thermo Scientific, registered trademark University of Washington, USA) against the UniProtKB database (taxonomy: Homo sapiens). The search parameters used were as follows: (1) peptide mass tolerance set to 20 ppm, (2) MS/MS mass tolerance set to 0.6 Da, (3) up to two missed cleavages were allowed, (4) carbamidomethylation set as a fixed modification and (5) methionine oxidation set as a variable modification. A number of criteria were applied to assign a protein as identified; proteins with ≥2 peptides matched, a ≥ 1.5 fold difference in abundance. For re-importation back into Progenesis LC–MS software for further analysis, only peptides with XCorr scores >1.9 for singly charged ions, >2.2 for doubly charged ions and > 3.75 for triply charged ions or more (from SEQUEST) were selected. A number of criteria were applied to ensure proper identification of proteins, including an ANOVA score between experimental groups of ≤0.05 and proteins with ≥2 peptides matched. The quantitative protein data (normalised abundances) for each sample of biological replicated analysis were exported into Excel file.
2.7.3. Identification of differentially abundant proteins
In detecting statistically significant alterations in protein abundances between control and treated samples, one-way ANOVA was used to compare the different treatments (control, 1 μg/mL AgNPs, 10 μg/mL AgNPs).
2.7.4. Systems biology analysis
The relation between the identified proteins was evaluated using the software Ingenuity Pathways Analysis (IPA) (Ingenuity Systems®, Redwood City, CA, USA). A pair-wise analysis of deregulated protein was performed throughout the experiment. This pair-wise comparison of proteins-features is a representation of data where the individual values contained in the table were represented as colours. The range was set from −0.58 to 0.58 (Base 2 logarithm = 0.58), where < −0.58 was set to green, 0 to black and > 0.58 to red. The values in between are shown as colour gradients. The significantly different features are the ones that are lower than −0.58 and higher than 0.58. Identified proteins were analysed using Ingenuity Pathways Analysis (IPA) (Ingenuity Systems®, Redwood City, CA, USA). Identified proteins were mapped onto Ingenuity's Knowledge Database to generate networks on the base of their algorithmic connectivity. Canonical pathway analysis identified the most significant ones from the IPA library, based on the number of molecules from the data set that map the pathway. Functional analysis of networks revealed the biological functions most significant to the molecules in the network (p < 0.05, right-tailed Fisher's exact test).
2.8. Other techniques used
For complementary techniques (immunocytochemistry analysis, cytokines and apoptosis array membrane) refer to Supplementary methods.
3. Results
3.1. Physico-chemical characterisation of AgNPs
In controlling the colloidal stability of the AgNPs, citrate was used as a capping agent. The main physicochemical properties of the NPs used in this work are summarised in Table 1Sa. The behaviour of citrate-stabilized AgNPs in complete culture medium was monitored by DLS analysis for up to 72 h of incubation at 37 °C. The NPs remained well dispersed with no aggregation.
Initial preliminary experiments were performed to confirm the suitability of regenerated cellulose filters for quantifying ionic silver release. To this end, different aqueous Ag+ solutions in the range 5–1000 μg/L were submitted to ultrafiltration and recoveries were calculated. The values ranged from 92.4–98.4%, thus confirming negligible Ag+ adsorption to the ultrafiltration units and therefore the suitability of the chosen regenerated cellulose material for ionic release quantification (data not shown).
The amount of dissolved ionic silver was measured through ICP-MS in complete cell culture medium, and it was found <0.01% and 0.075% for AgNPs 1 and 10 μg/mL respectively at the highest time point of 72 h (Table 1Sb).
3.2. Cell viability
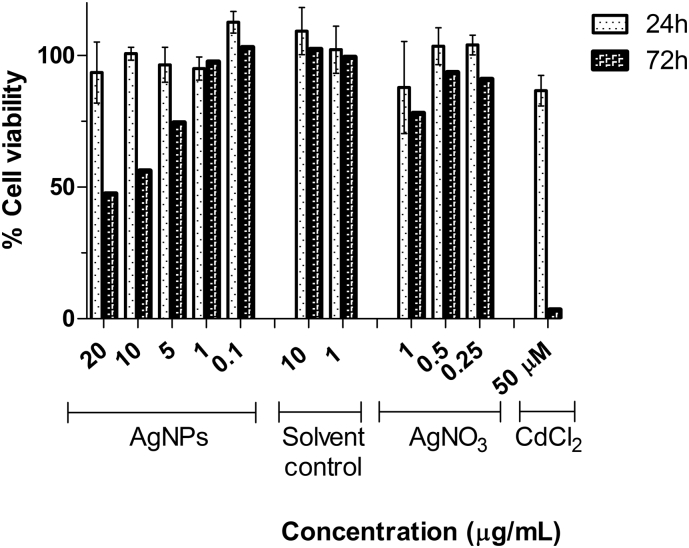
The cytotoxic effect of AgNPs and AgNO3 on Caco-2 cells was quantified with the analysis of DAPI-stained nuclei using the IN Cell Analyzer. Cell viability was calculated by determining the number of nuclei in the exposure conditions compared to the number of nuclei in negative control wells. The analysis showed that at 24 h exposure, AgNPs for all concentrations that tested up to 20 μg/mL did not induce a significant reduction in cell number compared to the negative control (Fig. 2). However, after 72 h exposure, the cell numbers were significantly reduced for concentrations above 1 μg/mL with a calculated IC50 of 15.4 μg/mL. At lower concentrations of AgNPs, no significant differences in cell number were detected (p > 0.05).
Fig. 2.
Cell viability of CaCo-2, assessed by PI/Hoechst staining using IN Cell Analyzer 2200 (GE Healthcare®). Cells were exposed to AgNPs (0.1, 1, 5, 10 and 20 μg/mL) for 24 and 72 H. medium control, solvent controls, AgNO3 (1, 0.5 and 0.25 μg/mL) and a positive control for toxicity (50 μM CdCl2) were used. Data are expressed as the mean ± standard deviation, and three independent experiments were performed in triplicates.
Based on the dose-response toxicity results for Caco-2 cells exposed to 30 nm AgNPs, two concentrations were selected for the proteomic analysis: 1 and 10 μg/mL, corresponding to a low and high toxic concentration at 72 h exposure.
Citrate used to stabilise the NPs was also tested at the same concentrations selected for the study (1 or 10 μg/mL) as solvent control, and no effects on cell viability were observed. AgNO3 was used as a control for silver ions release. The data shows no effects due to Ag ions for concentrations of AgNO3 below 0.5 μg/mL. These controls indicate that the toxicity observed for AgNPs is caused by the NPs and not by impurities derived from the synthesis process or by silver ion leaching.
3.3. AgNPs internalization
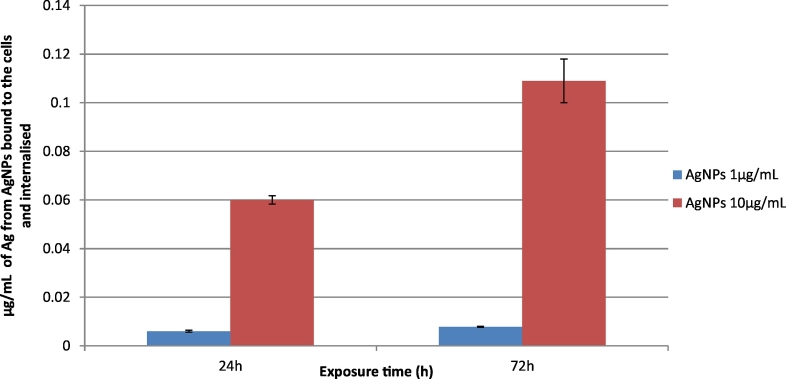
The amount of AgNPs bound to the cells and internalised have been quantified through ICP-MS. Data shows that at the highest concentration and exposure time, only approximately 1% of the initial amount of AgNPs exposed to the cells is internalised or bound to the external cell membrane (Fig. 1S).
Fig. 1S.
Quantification by ICP-MS of the amount of AgNPs internalised or bound to the cells.
3.4. Investigation of the differentially expressed proteins using the 2D gel-based method
We assessed the differences between the cytoplasmic proteome of Caco-2 cells exposed to 30 nm AgNPs (1 or 10 μg/mL) at two-time points (24 or 72 h), on the untreated cells.
As preliminary work, we assessed if there were any differences in the proteome profile of the untreated cells compared to cells exposed to the solvent of the NPs at the same doses intended to use in the study (1 or 10 μg/mL). The results showed that there were very few significant differences: two proteins in the case of Control vs. solvent of 1 μg/mL AgNPs and three proteins in the case of control vs. solvent of 10 μg/mL AgNPs-treated cells. Therefore, untreated cells were considered as the control in our study.
The analysis of imaged gels returned a total of 960 (range 672–1112), 954 (range 674–1238) and 1015 (range 734–1258) protein spots for the control, 1 and 10 μg/mL AgNPs treated samples at 24 h. A total of 1103 (range 721–1303), 1013 (range 776–1264) and 1092 (range 818–1332) protein spots were detected for the control, 1 and 10 μg/mL AgNPs treated samples at 72 h, respectively. The average percentage of matched spots across gels was 91% for 24 h and 87% for 72 h.
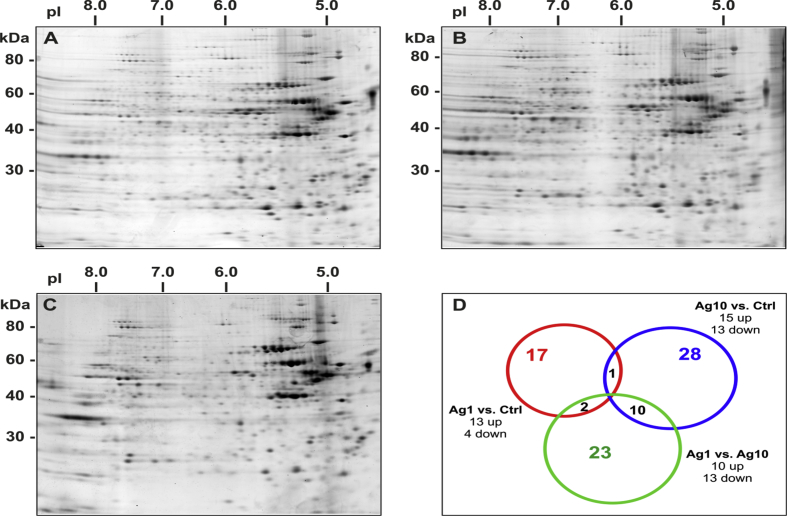
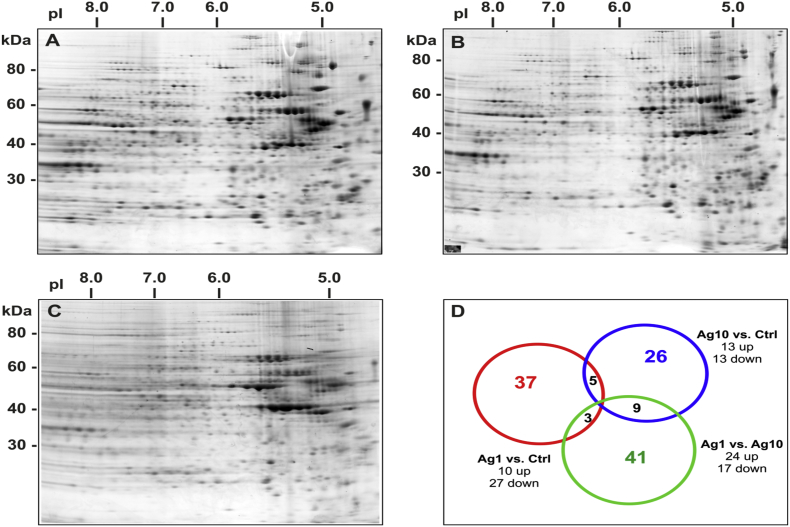
The Mann-Whitney test was used to compare the overall protein expression profile. Among the protein spots detected, 4 were found to be down-regulated by at least 2-folds, and 13 were up-regulated by at least 2-folds in cells treated with 1 μg/mL AgNPs and 13 down-regulated and 15 up-regulated by 10 μg/mL AgNPs exposure for 24 h (Fig. 2Sa). In the case of 72 h exposure, 27 spots were down-regulated, and 10 spots were up-regulated with at least a two-fold change by the lower concentration tested, whereas 13 proteins were found down-regulated and 13 up-regulated by the highest dose used of 10 μg/μL (Fig. 2Sb). Differences were also found between the two doses of AgNPs-treated cells. Some spots were differentially expressed in cells treated with 1 μg/mL AgNPs with respect to 10 μg/mL AgNPs (10 up-regulated and 13 down-regulated at 24 h and 24 up-regulated and 17 down-regulated at 72 h of exposure) (Fig. 2Sa, 2Sb).
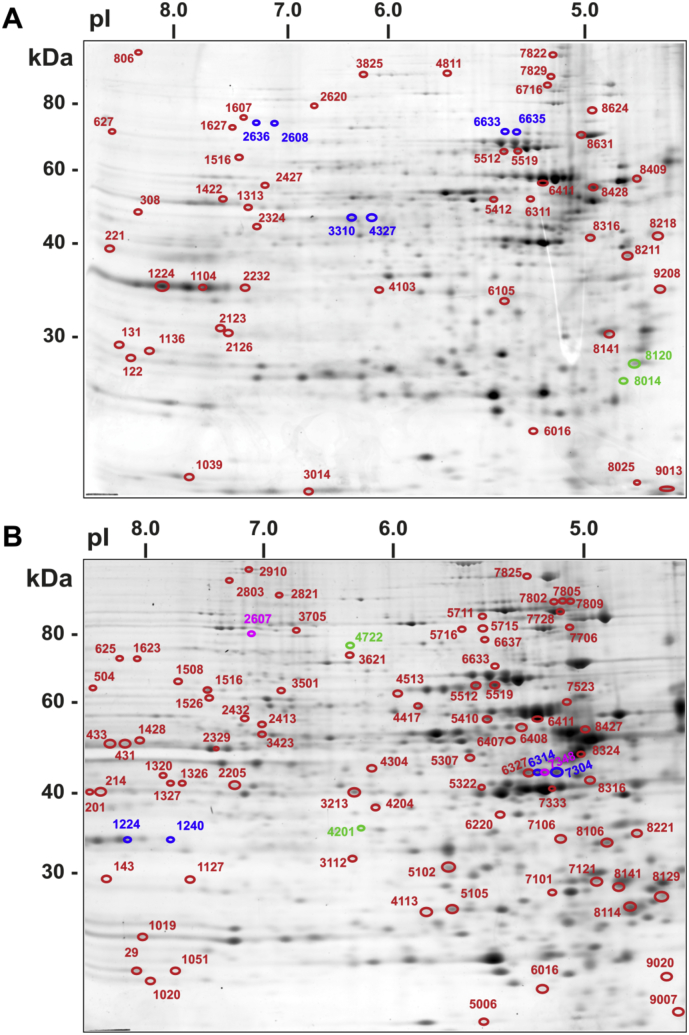
Proteins of interest (up- or down-regulated) were defined from the images of the control and treated samples, and their corresponding spots in the image of the preparative gel were matched. The 132 spots were selected from the preparative gel for spot picking (Fig. 3S). The aforementioned deregulated protein targets were identified using LC-MS/MS. Proteome Discoverer (Thermo Scientific®) with the Sequest workflow and UniProtKB/Swiss-Prot database was used for protein identification of the selected deregulated protein spots.
Fig. 3S.
Selection of differentially expressed proteins for HRMS identification. Sypro Ruby-stained gel of cytoplasmic proteins extracted from untreated Caco-2 cells. The 61 selected protein spots are highlighted in circles and marked with code numbers, as specified in Table 1S. a) 24 h and b) 72 h experiment. Isoforms and proteolitic products of de-regulated proteins are also highlighted (see Table 5S).
In total, 123 unique differentially regulated spots were identified. Table 2S reports the UniProt Accession Number, the protein symbol, the protein coverage, the number of identified peptides and amino acids, the molecular mass, a probability score, a description and a biological function, symbols and the level of de-regulation of the identified proteins (p value and fold change). UniProtKB/Swiss-Prot and Gene Ontology (GO) were used to gain insight into the cellular processes, molecular functions of the proteins differentially regulated by the AgNPs. In Table 1, deregulated proteins by 1 or 10 μg/mL AgNPs exposure at 24 or 72 h are visualised grouped for the main function.
Table 2.
List of deregulated proteins identified by the label-free nano UHPLC-Orbitrap MS/MS analysis of Caco-2 cytoplasmic extracts. Proteins have been classified according to their main function, based on UniProtKB/Swiss-Prot and Gene Ontology (GO). a) 24 h and b) 72 h experiment.
 |
 |
 |
 |
 |
 |
 |
 |
Table 1.
List of deregulated proteins identified in individual 2D gel spots of Caco-2 cytoplasmic extracts. Proteins have been classified according to their main function, based on UniProtKB/Swiss-Prot and Gene Ontology (GO). 24 h and 72 h data are presented.
 |
 |
 |
3.5. Relative quantification of identified proteins using the label-free MS-based method
A label-free MS-based proteomics approach was also used to investigate the effects of 1 or 10 μg/mL AgNPs on Caco-2 cells. The analysis of imaged peptide maps returned a total of 9177 and 11,333 co-detected peaks after matching the control, 1 and 10 μg/mL AgNPs treated sample groups at 24 h and 72 h exposure, respectively. The relative quantification was based on all peptides. Data were filtered (MS/MS spectra: rank <5; peptide charge: <5) before exporting the MS/MS output files for protein identification. 105,729 and 94,213 MS/MS spectra were exported into Proteome Discoverer for the 24 h and 72 h experiment, respectively. 75,162 and 57,457 search hits were re-imported into Progenesis QI for Proteomics and assigned to peptide ions. After signal normalisation, the quantification was made for all peptides using the relative quantification method (use of all peptide identified as part of the protein). The total number of quantifiable proteins was 7232 and 7295 for the 24 h and 72 h experiment, respectively. One-way ANOVA was used to identify statistically significant differentially abundant proteins between non-treated Caco-2 cells (control) and cells treated with 1 or 10 μg/mL AgNPs for the two-time points considered in this study. Only deregulated proteins, by at least 2-folds in cells, were validated. This results in 39 and 71 deregulated proteins for 1 and 10 μg/mL regarding, with respect to the control at 24 h exposure. For the 72 h experiment, 12 and 44 proteins were deregulated for 1 and 10 μg/mL, with respect to the control. Differences were also found between the two doses of AgNPs treated cells. 181 and 123 proteins were differentially expressed in cells treated with 1 μg/mL AgNPs with respect to 10 μg/mL AgNPs at 24 h and 72 h exposure, respectively. Table 3S reports the UniProt Accession Number, the protein symbol, the protein coverage, the number of identified peptides and amino acids, the molecular mass, a probability score, a description and a biological function, symbols and the level of deregulation of the identified proteins (p value and fold change).
Table 3.
Overlap of protein identifications between the two proteomics approaches at two experimental times. a) 24 h and b) 72 h experiment.
| UniProt accession number | Name | Protein description |
|---|---|---|
| a) | ||
| P0DMV8 | HSPA1A | Heat shock 70 kDa protein 1A OS=Homo sapiens GN=HSPA1A PE = 1 SV = 1 - [HS71A_HUMAN] |
| P25705 | ATP5A1 | ATP synthase subunit alpha, mitochondrial OS=Homo sapiens GN = ATP5A1 PE = 1 SV = 1 - [ATPA_HUMAN] |
| P07355 | ANXA2 | Annexin A2 OS=Homo sapiens GN = ANXA2 PE = 1 SV = 2 - [ANXA2_HUMAN] |
| P04406 | GAPDH | Glyceraldehyde-3-phosphate dehydrogenase OS=Homo sapiens GN = GAPDH PE = 1 SV = 3 - [G3P_HUMAN] |
| b) | ||
| P49411 | TUFM | Elongation factor Tu, mitochondrial OS=Homo sapiens GN = TUFM PE = 1 SV = 2 - [EFTU_HUMAN] |
| P0DMV8 | HSPA1A | Heat shock 70 kDa protein 1A OS=Homo sapiens GN=HSPA1A PE = 1 SV = 1 - [HS71A_HUMAN] |
In Table 2, deregulated proteins after 1 or 10 μg/mL AgNPs exposure at 24 or 72 h are visualised and grouped for the main function.
3.6. Comparison of the two experimental approaches
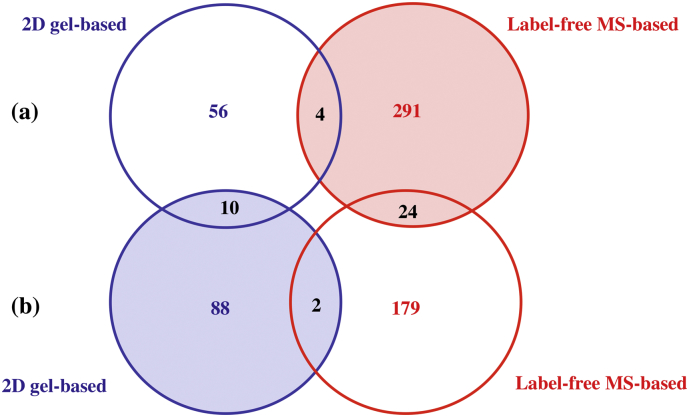
Sample requirement is an important parameter when dealing with in vitro experiments. There is a difference in the total amount of proteins required to obtain an adequate dataset with each of the techniques. 200 μg of loaded proteins are needed in the 2D-gel based method to generate enough peptides from protein spots for their MS characterisation. Only 3 μg loaded in the UHPLC are sufficient in the MS-based for a qualitative and quantitative analysis of the peptide mixture. Furthermore, the 2D-gel based experiment requires a total instrumental time of 130 h (based on the number of selected spots for MS characterisation) whereas the label-free MS-based experiment is performed for 54 h (see Table 4S). The average data analysis time is similar to both approaches. The average number of peptides identified per confident protein assignment for the 2D gel-based method is 10, compared to an average of 7 for the label-free MS-based method, which is slightly lower. Fig. 4S shows the number of deregulated proteins identified by the two experimental approaches, label-free MS-based and 2D-gel based at 24 (a) and 72 h (b). It shows that 291 proteins were identified independently by the label-free MS-based and 56 proteins by the 2D-gel based methods at 24 h exposure. At 72 h exposure, 179 and 88 proteins were identified independently by the label-free MS-based and 2D-gel based methods, respectively. Four and two proteins were found in common irrespective of the applied approach in the case of the 24 h and 72 h experiments, respectively (Table 3). For the 24 h experiment, the four proteins found in common were P0DMV8, P25705, P07355 and P04406; whereas for the 72 h experiment, the two proteins in common were P49411 and P0DMV8. The low overlap observed cannot only be interpreted as a poor performance, but also demonstrates the advantage of the multimodal approach to the characterisation of the proteome (Yeung et al., 2008).
Table 4.
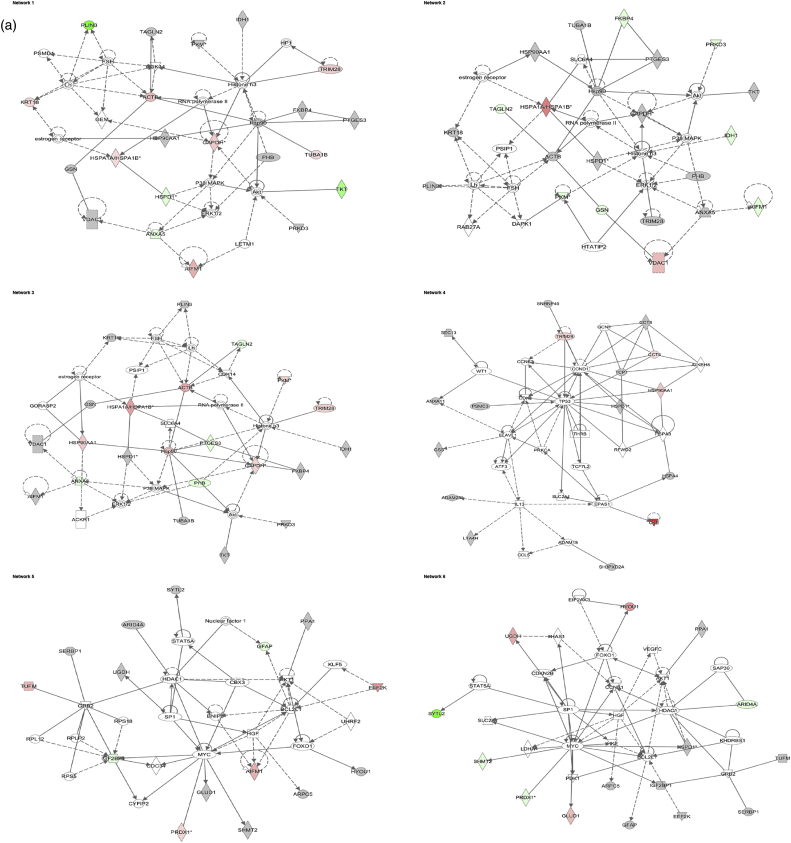
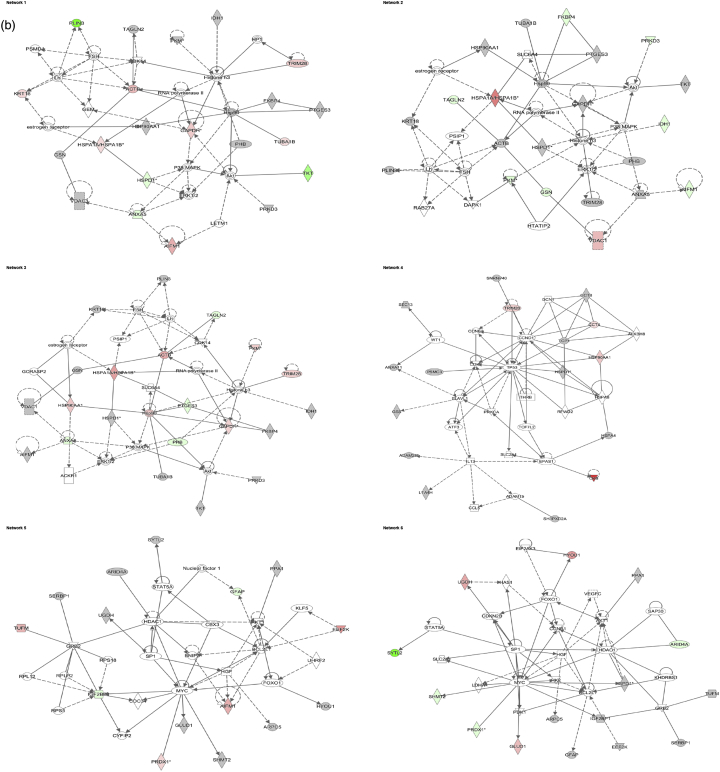
Data analysis from the 2D gel-based approach - Identified molecular networks using Ingenuity IPA. a) 24 h and b) 72 h experment. The table reports the most significant molecular networks in response to 1 and 10 μg/mL AgNP treatments, by analysing the differentially expressed proteins from the cells. The network number, the list of all proteins and metabolites involved in the network, the number of molecules overlapping between our data set and the network and the top functions related to the network are shown. Focus molecules are indicated in bold and deregulation is indicated with a coloured arrow (red: up-regulated, green: down-regulated).
 |
 |
Fig. 4S.
Overlap of protein identifications between the two proteomics approaches and the two experimental times. A number of proteins identified by the two experimental approaches, label-free MS-based and 2D-gel based based on a) 24 h and (b) 72 h experiment. Identification based on a minimum of two peptides.
It is important to note that the analysis by differential 2D gel can provide additional information on the analysis only by MS. This is quite a novelty as this work can provide a perfect integration between two proteomic techniques that are normally used independently. The results obtained show that the integration of both techniques is advantageous for two main reasons. On the one hand, the label-free MS-based proteomics analysis, which is less costly in terms of time, allows acquiring more information on the differentially expressed proteins. This technique does not have the limitations of the analysis 2D by which it is possible to investigate only a defined range of pI and molecular weights. In our work, we covered from about pI 4.5 to 8.5 and from about 20 to 200 kDa. Also, label-free MS-based proteomics analysis does not have any limitations, either in the case of proteins with a poor solubility (mainly hydrophobic proteins of the membrane) or proteins which tend to form protein aggregates due to bridges disulfide formation (the case of cytoskeleton proteins). Here, the latter problem was minimised by the sample preparation method applied. On the other hand, the 2D gel analysis provides the advantage of identifying the different isoforms of a protein e.g. due to post-translational modification (PTMs) and/or by alternative splicing, as well as altered by the action of proteases. This is a relevant factor considering that isoforms of the same protein might have different biological effects, ranging from a complete loss of function to its acquisition.
Specifically, referring to Fig. 3S, isoforms or proteolytic products of a particular protein found to be differently regulated by AgNPs exposure are highlighted. The data are presented in Table 5S.
Table 5.
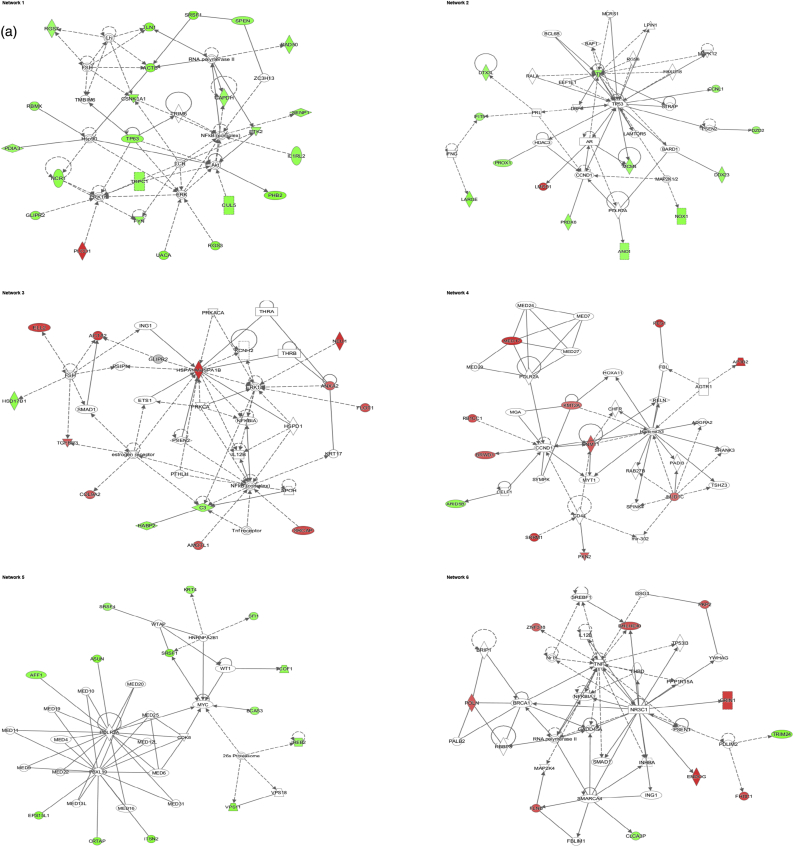
Data analysis from the label-free MS-based approach - Identified molecular networks using Ingenuity IPA. a) 24 h and b) 72 h experment. The table reports the most significant molecular networks in response to 1 and 10 μg/mL AgNP treatments, by analysing the differentially expressed proteins from the cells.
 |
 |
3.7. De-regulated networks
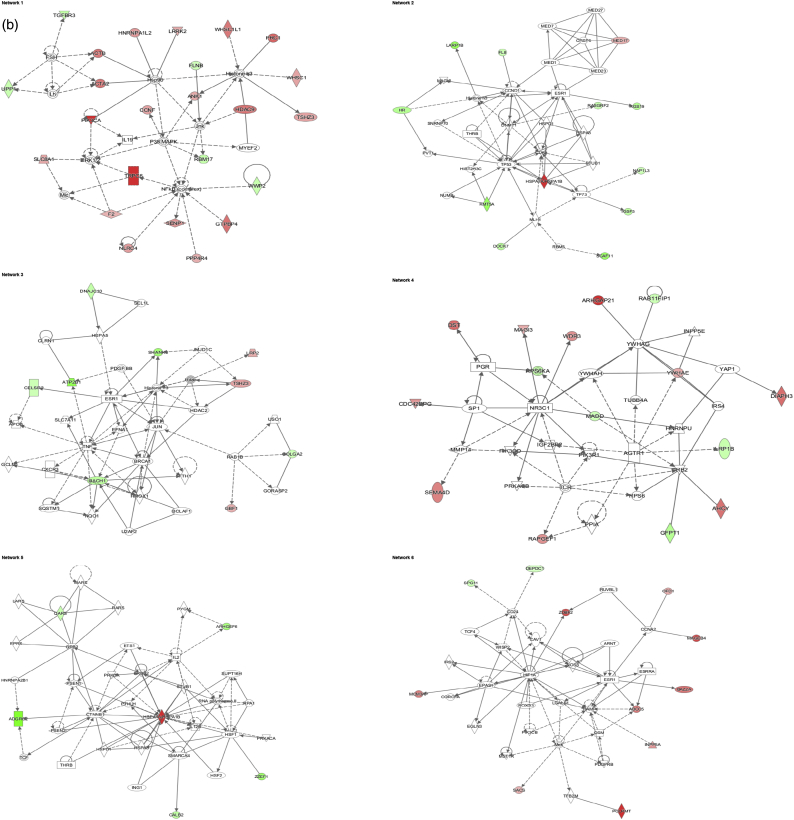
The IPA software was used for the combination and interpretation of complex data for both proteomic platforms. The data were obtained by analysing the differentially expressed proteins. Several biological activities were found to be altered in Caco-2 cells exposed to AgNPs. Figs. 3 (a, b) and 4 (a, b) report the most significant molecular networks affected in response to 1 or 10 μg/mL AgNPs treatment for 24 h (a) and 72 h (b), for the 2D-gel based and label-free MS-based approach respectively. The focus molecules and de-regulation in response to 1 or 10 μg/mL AgNPs treatment for 24 h and 72 h are highlighted in Tables 4 (a, b) and 5 (a, b) for the 2D-gel based and label-free MS-based approach respectively.
Fig. 3.
Data analysis from the 2D gel-based approach - De-regulated molecular networks in response to 1 or 10 μg/mL AgNPs exposure in Caco-2 cells. a) 24 h and b) 72 h experiment. The networks are obtained by analysing the differentially expressed proteins using Ingenuity IPA. Identified deregulated proteins and metabolites involved in the network are highlighted in bold. The colour indicates the deregulation (red: up-regulated, green: down regulated). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
Data analysis from the label-free MS-based approach - Deregulated molecular networks in response to 1 or 10 μg/mL AgNPs exposure in Caco-2 cells. a) 24 h and b) 72 h experiment. The networks are obtained by analysing the differentially expressed proteins using Ingenuity IPA. Identified deregulated proteins and metabolites involved in the network are highlighted in bold. The colour indicates the deregulation (red: up-regulated, green: down regulated). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Interestingly, the major proteins altered in response to AgNPs were associated with cell cycle, cell morphology, cellular function and maintenance.
3.8. Additional studies
To support our findings, we cross-linked omics data with a broad set of complementary techniques.
We investigated the effects of 72 h AgNPs exposure on the nuclei and cytoskeleton organisation of Caco-2 cells by immunocytochemistry. In the Supplementary information, Fig. 5S shows Hoechst and Alexa Fluor® 488 phalloidin staining, used to visualise nuclei and F-actin filaments respectively (63× magnification). Upon AgNPs incubation, some nuclei are fragmented; this phenomenon is not observed in untreated cells or cells exposed to the solvent control. The cytoskeleton organisation appears disrupted and damaged. The F-actin distribution shows to be altered in cells exposed to AgNPs, with a higher effect seen at 10 μg/mL concentration.
Fig. 5S.
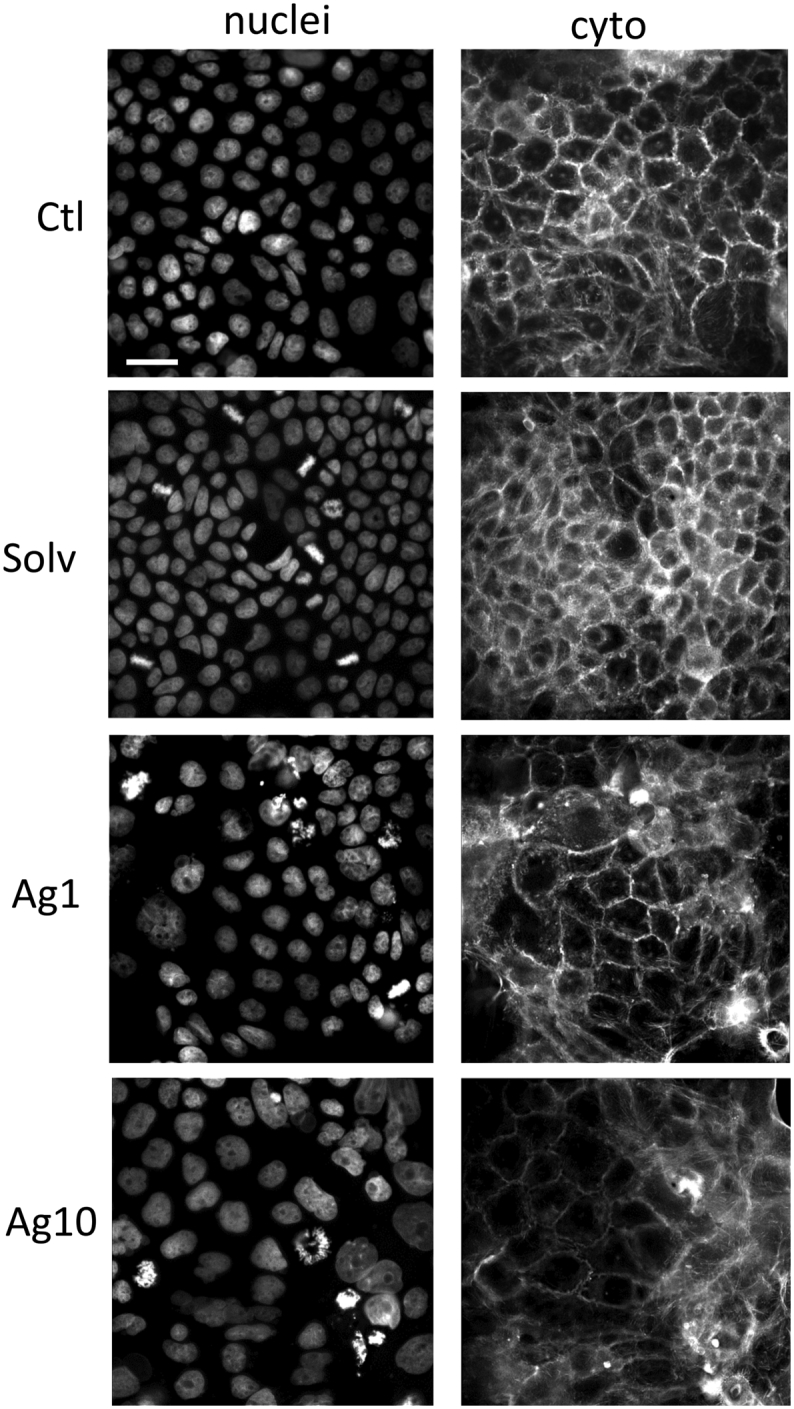
Images of the nuclei (DAPI) and cytoskeleton staining (Phalloidin) of Caco-2 cells untreated (Ctl), and exposed to 1 or 10 μg/mLAgNPs for 72 h were acquired using INCell Analyzer. Images of solvent control (Solv) are also reported. Scale bar: 30 μm.
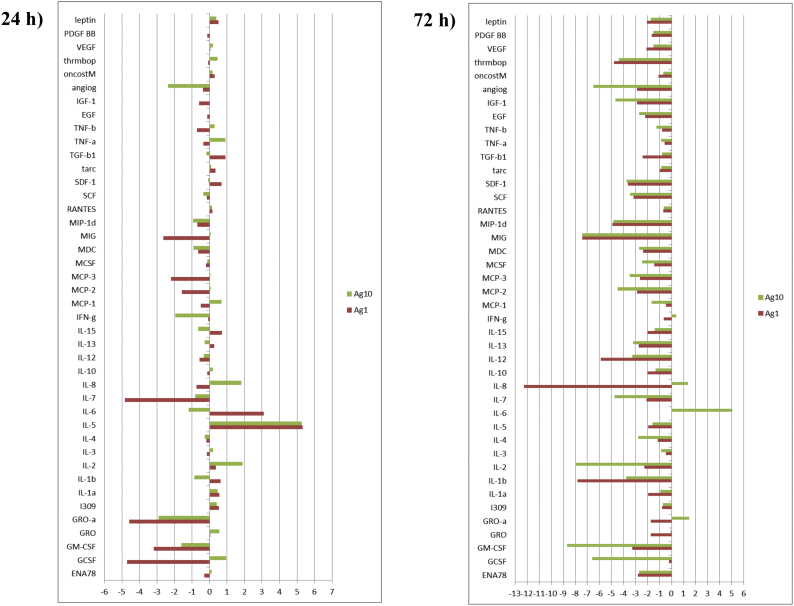
Also, to overcome the limited sensitivity of proteomics to investigate inflammatory cell response induced by AgNPs, we used antibody arrays, which can detect cytokines in the ranges of pg/mL. We performed a simultaneous screening of 42 human markers using a human cytokine specific antibody array.
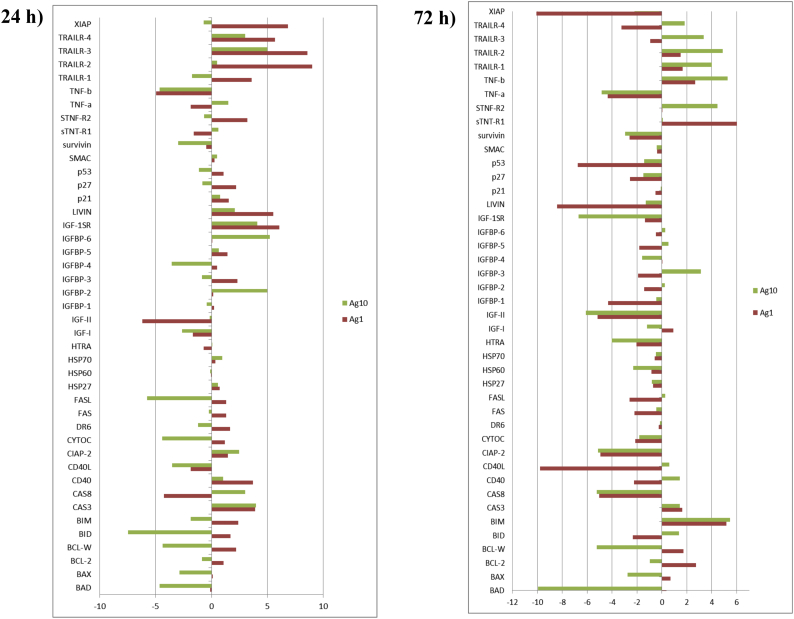
For the completeness of the study, apoptosis-specific antibody array was performed. Arrays data are reported in Fig. 6S, Fig. 7S and highlight significant changes in the expression of several proteins involved in cytokines production, as well as in the apoptosis process, respectively.
Fig. 6S.
Cytokine antibody array. Cell lysates (300 μg) were incubated overnight with Abcam's human cytokine antibody array-membrane. The antibody array membranes were then washed, and a cocktail of biotinylated antibody mix was used to detect cytokines and cytokines related proteins. After incubation with HRP-conjugated streptavidin, the signal was visualised after 1-min exposure by chemiluminescence. Comparison of signal intensity was used to determine relative differences in expression levels of the proteins. Positive control spots were used for normalisation. Ag 1 is for 1 μg/mL AgNPs, Ag 10 is for 10 μg/mL.
Fig. 7S.
Apoptosis antibody array. Cell lysates (300 μg) were incubated overnight with Abcam's human apoptosis antibody array-membrane. The antibody array membranes were then washed, and a cocktail of biotinylated antibody mix was used to detect apoptosis-related proteins. After incubation with HRP-conjugated streptavidin, the signal was visualised after 1- min exposure by chemiluminescence. Comparison of signal intensity was used to determine relative differences in expression levels of the proteins. Positive control spots were used for normalisation. Ag 1 is for 1 μg/mL AgNPs, Ag 10 is for 10 μg/mL.
4. Discussion
4.1. Biological significance of proteomic results obtained
The increased use of AgNPs in consumer production as dietary supplements or food packaging materials creates concerns for humans, which could directly or indirectly be exposed to the NPs. Oral exposure to AgNPs and its subsequent systemic absorption have been observed in rats (Kim et al., 2008), indicating that the gastrointestinal tract is a potential target organ. For this reason, the Caco-2 cells, extensively used over the last twenty years as an in vitro model of the intestinal barrier for toxicity testing of classical toxicants (Brandon et al., 2006) and more recently for nanomaterials (Sahu et al., 2016) (Gerloff et al., 2009) was selected for this study.
Effective assessment of the growing number of new nanomaterials benefits from a more comprehensive understanding of their toxicological mechanisms, which is difficult to achieve by traditional, single end-point approaches (Costa and Fadeel, 2016) (Matysiak et al., 2016). In this regard, system biology approaches have started to be applied to the nanotoxicological sciences to overcome the limitations of end-point assays.
Several key questions remain to be answered on the toxicity mechanism of AgNPs, in particular, the identification of the key signalling pathways involved (McShan et al., 2014). With this work, we aim to provide a valid contribution by applying a multimodal approach, which allows analysing Caco-2 cellular interactions with 30 nm citrate coated AgNPs.
Firstly, we deeply characterized the NPs used as particle size, surface area, aggregation/agglomeration state, parameters that are all likely to influence the biological availability and effects of the NPs. We demonstrated that the AgNPs selected were homogeneous, well dispersed and stable at the experimental conditions considered, including in complete cell culture medium. As next step, we performed in vitro cytotoxicity testing showing a concentration-dependent toxic effect of AgNPs. Based on these data, we selected two doses for the subsequent proteomic analysis: a low no toxic concentration of 1 μg/mL and a high concentration of 10 μg/mL. Acute exposure as the common exposure model in nanotoxicology was applied, which provide useful indication in terms of quantitative ranking of NPs hazards. Two exposure durations were considered: 24 and 72 h. Doses and time points selected were in line with available studies (Zhang et al., 2016) (Verano-Braga et al., 2014). At his regards, no specific exposure limits have been calculated for nanosilver in the EU, whereas for the general population the World Health Organisation (WHO) set a “No Observable Adverse Effect Level”-(NOAEL) related to the sum of all exposure routes of 5 μg/kg bw(body weight) AgNPs/day. Recently, for AgNP a NOAEL (for rats) was observed, based on a 90 day oral exposure of 30 mg/kg bw/day; this assessment was based on signs of liver toxicity (Hartemann et al., 2015, Kim et al., 2010).
Since silver ions might be slowly released from NPs due to surface oxidation, surface reactions, and dissolution of nanosilver in a biological medium, the toxicity contribution from the ionic form versus the nano-form of silver has been taken into account. We quantified the ions released from AgNPs, and investigated the cellular interaction and uptake of AgNPs using ICP-MS. Our results showed that approximately only 1% of the initial amount of AgNPs exposed to the cells is internalised or strictly bound to the membrane of cells at the highest concentration (10 μg/mL) and exposure time (72 h) analysed. Based on literature data, AgNPs stability, in terms of ions release is of considerable variability. (Beer et al., 2012), detected between 39% and 71% of Ag+ ion fraction from commercial available AgNPs powder, reporting that the high concentration of Ag ionic fraction is linked to the dispersion protocol. AgPure (listed as NM-300 reference material of the Joint Research Centre (JRC, Italy) used by (Oberemm et al., 2016) resulted in 15.1% as ionic silver after 24 h incubation in cell culture medium, whereas (Verano-Braga et al., 2014) reported a 6 to 17% of the silver content of the AgNPs suspensions to be in the ionic form (Ag+) in LoVo cells treated with 20 and 100 nm AgNPs. In our experimental conditions, the amount of free ionic silver was found to be <0.1% after incubation in cell culture media at the highest dose and time point considered. As we observed a very limited release of Ag+, we account that the toxicity of AgNPs is mainly related to AgNPs per se.
This is in agreement with several other previous studies that had reported that AgNPs-induced toxicity is primarily the result of oxidative damage and is independent of the toxicity of Ag+ ions (Kim et al., 2009). Also, (Verano-Braga et al., 2014) and (Xu et al., 2015) have shown that more proteins were deregulated by AgNPs than by the free silver ions released into the solution by the NPs. More recently, (Oberemm et al., 2016) concluded that the high number of deregulated proteins in the AgNP-treated cells points towards particle-specific effects not exerted by silver ions. (Sahu et al., 2016) reported that 20 nm AgNPs nanoparticle are genotoxic in Caco-2 cell line, however, it is not dependent on the contribution of ionic silver. (Beer et al., 2012) concluded that for an AgNPs suspension containing 5.5% of Ag+, they could not detect any difference in the toxicity between AgNPs suspension and its supernatant.
As a preliminary step, we assessed if there were any differences in the proteome profile of the untreated cells compared to cells exposed to the solvent of the NPs at the same doses intended to use in the study (1 or 10 μg/mL). The results showed that very few significant differences were found: two proteins in the case of control vs. solvent of 1 μg/mL AgNPs and three proteins in the case of control vs. solvent of 10 μg/mL AgNPs-treated cells (Data not shown). This indicates no specific effects of the coating matrix on cells. Solvent control did not also show any decrease in cell viability on the control. Based on these observations, we focused on the analysis of the differentially regulate proteins by only the NPs and we compared the profiling among controls and AgNPs-treated Caco-2 cells at the two different doses and at two different exposure time (24 and 72 h).
In analysing the molecular effects of AgNPs in the most comprehensive manner, the combination of two proteomic approaches, complemented with other techniques were applied. In contrast with classical end-points methods, the strength of omics methods is to provide the opportunity for an unbiased assessment and may result in identifying novel and/or unanticipated end-points, which could also yield to novel biomarkers (Costa and Fadeel, 2016). Also, when coupled with bioinformatics, it may lead to indicate novel and/or low dose effects, not detected by conventional cellular assays. Although only a small amount of AgNPs was taken up by the cells and as ICP-MS data have shown and in accordance with literature (Bouwmeester et al., 2011), image analysis and biostatistics of the 2-DE gel image spot data revealed noticeable differences in protein spot expression in a concentration and timely manner. De-regulated proteins were classified through biological functions. In particular, a considerable fraction of the proteins identified as altered by AgNPs was related to energy and metabolism, protein synthesis or stability/transcription, cell morphology and transport, as well as stress response.
For example, citrate coated-AgNPs 30 nm triggered a deregulation of several cytoskeleton proteins. Among the most affected proteins are: Tubulin beta-4B (TUBB4), one of the major constituents of microtubules; Actin-related protein 2/3 complex subunit 5 (ARPC5), which functions as a component of the Arp2/3 complex and it is involved in the regulation of actin polymerization and, together with an activating nucleation-promoting factor (NPF), mediates the formation of branched actin networks.
Cofilin expression (CFL1) was sharply decreased at 24 h in 10 μg/mL treated cells; this protein belongs to the actin-binding proteins which disassemble actin filaments. T-complex protein (TCP1 and CCT8) involved in complex folds, including actin and tubulin, were also found deregulated by AgNPs. Isoform 4 of Perilipin-3 (PLIN3), a cadherin binding protein involved in cell-cell adhesion was found to be strongly down-regulated at 24 h at both tested concentrations. Of interest is the deregulation of KRT8 and KRT18, which are essential proteins for the integrity of the epithelial cells, and playing an important role under stress. As (Wang et al., 2007) and (Georgantzopoulou et al., 2016) have reported, KRT8 and KRT18 are involved in IL-6 mediated cell protection. Accordingly, our cytokines array data shows a significant increase in IL-6 expression in cells exposed to 10 μg/mL of AgNPs for 72 h.
Also, 10 μg/mL AgNPs treatment for 72 h led to a significant deregulation of glutathione synthetase (GSS), a protein involved in inflammatory response and oxidative stress neutralisation. As a defence mechanism against oxidative injury, cells expressed altered levels of glutaredoxin-3 (GLRX3) and peroxiredoxin-1 (PRDX1) when exposed to 10 μg/mL of AgNPs for 72 h. Moreover, several heat shock proteins among which mitochondrial heat shock 60KDa (HSPD1), Serpin H1 (SERPINH1), Heath shock 70 KDa (HSPA1A) were found deregulated to counteract the oxidative damage. Altered expression of several heat shock proteins was in line with published observations (Oberemm et al., 2016). Protein disulphide-isomerase (P4HB), which catalyses the formation, breakage and rearrangement of disulfide bonds and 3-mercaptopyruvate sulfurtransferase (MPST) that acts as an antioxidant by transfering sulfur ion to cyanide or to other thiol compounds, were found deregulated at both time points tested (24 and 72 h) only at the lower concentration examined (1 μg/mL).
(Miethling-Graff et al., 2014) reported that AgNPs increased ROS level in LoVo cells at 24 h of exposure and correlated this finding with changes in the proteomic response of proteins involved in oxidative stress.
Both concentrations tested led to altered levels of Annexin A5 (ANXA5), a key apoptosis regulator, suggested to be an early marker of apoptosis (Herzog et al., 2004). (van der Zande et al., 2016) also reported that at gene levels, among the most dominant functional pathways affected by AgNPs exposure in CaCo-2 cells were the ones connected to oxidative stress and apoptosis.
In addition, by 2D technique, different isoforms of proteins have been identified. Glyceraldehyde-3- Phosphate dehydrogenase (GADPH), which is involved in several biological processes such as apoptosis, glycolysis, translational, presents three different isoforms whose expression levels were altered by AgNPs cell exposure. These results are in a good agreement with literature data on the influence of post-translational modification and oxidation of GADPH (Zhang et al., 2015, Kosova et al., 2017).
Oxidative stress can promote the formation of high molecular weight disulfide-linked GAPDH aggregates through a process called nucleocytoplasmic coagulation. Oxidation at Met-46 may play a pivotal role in the formation of these insoluble structures. This modification has been proposed to destabilise nearby residues, increasing the likelihood of secondary oxidative damages (Samson et al., 2014).
Also of interest was elongation factor 2 (EEF2), whose proteolytic product was found to be over expressed in cells treated with 10 μg/mL AgNPs for 72 h, also reported as among the top deregulated proteins by (Oberemm et al., 2016). Phosphorylation by EEF-2 kinase completely inactivates EEF-2, resulting in a drastic inhibition of polyphenylalanine synthesis in poly(U)-directed translation, therefore, completely inactive in translation (Ryazanov et al., 1988).
When results of the nanoparticle-cell interaction mechanisms induced by nanogold (AuNPs) obtained from previous studies (Gioria et al., 2014, Gioria et al., 2016) were compared with the data obtained by AgNPs in the present study, both the similarities and differences underlying biological processes and proteins regulation were found. To highlight is the expression of ENO1, IDH1 and P4HB, proteins involved in glycolysis, isocitrate metabolic process and cell redox homeostasis respectively, resulted altered in Caco-2 when exposed to AgNPs, as well as in Caco-2 or Balb-3 T3 cells exposed to AuNPs. Their modified expression could be suggested as a general response of cells exposed to NMs, whereas ETFA, HSPD1, PP1r7/PPP1R12A, Anx2, NUDC deregulation could potentially be considered as a specific Caco-2 response to NPs. It should be noted that no similar pathways were found to be activated in the two studies (Caco-2 cells exposed to AgNPs or AuNPs). This revealed that even if common deregulated proteins are found, they may link and coordinate different molecular pathways in the same Caco-2 cells model when exposed to different metal NPs.
Due to the extensively documented difficulties to quantitate in 2D gels proteins with high net charges, high pI and low Mr values, (Rabilloud et al., 2009), for a complete study, we employed in parallel label-free MS-based proteomics. Gel-free approaches were initially pitched as replacements for 2DE-MS; however, due as well to their limitations, they turned out to be complementary. It is evident that both approaches, with their respective advantages and disadvantages, should be used in parallel to get a complete comprehension of protein expression and interactions in a certain physiological condition (Abdallah et al., 2012).
De-regulated proteins identified using the label-free technique was also classified by biological functions. After 24 and 72 h of treatment with the selected AgNPs particles, a large number of proteins were found to be deregulated, particularly related to energy and metabolism, protein synthesis or stability/transcription, cell structure and transport, signal transduction.
As expected, the higher AgNPs concentration caused more protein deregulation. Interestingly, by label-free approach, more deregulated spots were observed for cells treated for 24 h, as compared to 72 h. Furthermore, 24 h exposure resulted in a predominant up-regulation of proteins, in particular, for the high dose tested, whereas, at 72 h exposure, altered proteins were mainly down regulated. Several zinc finger proteins involved in transcription regulation were found to be altered by AgNPs at the dose of 10 μg/mL at both time points analysed, in line with what was observed by (Oberemm et al., 2016) and co-workers who identified six different zinc finger proteins specifically deregulated by AgNPs treatment.
The low overlap observed between the two techniques that we found demonstrates the advantage of the multimodal approach in the characterisation of the proteome (Yeung et al., 2008).
Omics techniques provide more holistic approaches than offered by conventional techniques, in particular, here we addressed the advantage of a multimodal proteomics approach in the characterisation of the proteome (Yeung et al., 2008), definitely of great value for mechanistic (Adverse Outcome Pathway) studies and further integration of knowledge obtained from in vitro data for the safety assessment of NMs.
It is accepted that due to the complexity of NPs- cell interactions, comprehensive computational modelling approaches are needed to understand the cellular mechanisms, evolution, and dynamics of cellular proteins. Since omics approaches, as proteomics allows for computational modelling, we applied Ingenuity pathway analysis for interpreting the data of both proteomic platforms and gained insights into the main networks affected by the deregulated proteins.
The most significant molecular networks affected in response to 1 or 10 μg/mL AgNPs treatment for 24 h and 72 h were presented. Interestingly, the major proteins altered in response to AgNPs were associated with cell morphology, cellular assembly and organisation, cell cycle, cell death and survival. The findings are in line with what was reported by (Ma et al., 2011) at the level of global gene expression profiles, analysed by the integration of clustering, gene ontology (GO) and biological pathway analysis. By investigating the molecular mechanisms of interaction between AgNPs and human dermal fibroblasts-fetal (HDF-f), the results of Ma and co-workers suggest that AgNPs may cause the disruption of the cytoskeleton and cellular membrane, disturbance of energy metabolism and gene expression associated pathways, DNA damage, accompanied by cell cycle arrest.
At first glance, this work might not see as of any progress beyond the current status of knowledge. However, by conducting the experiments flawlessly and by interpreting the proteomics findings as much as possible, this work contributes to the goal of cooperation and openness in the pursuit of scientific progress. Indeed, data sharing in mass-spectrometry (MS)-based proteomics opens a plethora of opportunities for data scientists (Martens and Vizcaíno, 2017). Standardization efforts have ensured that a large proportion of the public data can be read and processed by any interested researcher with the great opportunity for (orthogonal) reuse of public data or integration with other public omics data sets. Unfortunately, the lack of experimental and technical metadata has been highlighted many times as the main issue for the reuse of biological data, and particularly in proteomics (Griss et al., 2015).
4.2. Additional studies
At proteomics level, we reported alterations of molecular networks involved in cell morphology, cellular assembly and organisation, cell death and survival. We supported our findings, by cross-linking omics data with a broad set of complementary techniques. Cell morphology investigation revealed apparent dose-dependent changes in cell shape and a less define cytoskeleton structure in AgNPs treated cells compared to the controls.
The inflammatory response was also examined. Treatment with AgNPs resulted in an increase of IL-8, both at 24 and 72 h only at the highest dose tested of 10 μg/mL, whereas IL-6 was found overexpressed at the highest dose tested only at 72 h. AgNPs are reported to have both stimulatory and suppressive effects on the production of cytokines. Furthermore, the differential effects are dependent on dose and cell type (Nguyen et al., 2016). Treatment with low doses of AgNPs resulted in inhibitor effects, and higher doses led to increased pro-inflammatory cytokine levels. The dose and time-dependent effects in cytokine production however, need to be further investigated.
At proteome level, we identified several proteins involved in oxidative stress and apoptosis, in line with previous studies that reported apoptosis induced by various AgNPs (Gopinath et al., 2010). Based on the data achieved by using a human apoptosis antibody array membrane, we suggest that following the exposure of Caco-2 cells to AgNPs, TRAIL proteins, which are members of the tumor necrosis factor (TNF) family of ligands, are capable of initiating apoptosis through engagement of its death receptors (Wiley et al., 1995).
Some members of the Bcl-2 family inhibit apoptosis, while others facilitate this physiological process of cell death (O'Connor et al., 1998). In particular, AgNPs exposed to Caco-2 cells for 72 h increase BIM expression, a protein that binds to Bcl-2 provoking apoptosis.
Normally, DNA damage and cellular stress signalling activate p53, which, through DNA-specific transcription activation, transcriptional repression, and protein-protein interactions, triggers cell cycle arrest and apoptosis. One of the genes induced by p53 was identified as that encoding the insulin-like growth factor binding protein IGFBP-3, which is reported as the primary regulator of the amount of free IGF-I available to interact with the IGF-1 receptor. By sequestering IGFs from stimulating the IGF-1R, IGFBP-3 inhibits the IGF-survival signalling, thus functioning as a pro-apoptotic agent (Grimberg, 2000). There are accumulating evidences that IGFBP-3 can also cause apoptosis in an IGF-independent manner. Thus, IGFBP-3 induction by p53 constitutes a means of cross talk between the p53 and IGF axes. Also, by down regulating IGF-II, as we reported, P53 reduces the IGF/IGF-1R survival and mitogenic stimulation.
5. Conclusions
In this work, we have focused specifically on how systematic proteomic and structural studies could be used to define the critical protein interaction networks affecting Caco-2 human cells when exposed to AgNPs. We applied two different proteomic platforms for the assessment of the potential human health risks of AgNPs present e.g. in consumer products or medical applications. We have shown how this integration of techniques is crucial to obtaining biological insight for a correct hazard assessment. With these two proteomic platforms, we were able to detect significant changes in the protein profiles of Caco-2 cells that were treated with 30 nm AgNPs at the concentrations of 1 and 10 μg/mL at 24 or 72 h exposure.
We believe that these techniques could support an informed decision-making platform to assess the potential health effects of existing nanomaterials. This work is intended to contribute to an in-depth understanding of the mechanisms of action of AgNPs and should help in the development of safe NMs for nanotechnology-based consumer products without harmful side effects. As additional value, it contributes to the accumulation of data in the public domain, with the potential to generate new knowledge.
Funding
The research described in this work was supported by the European Commission‘s Joint Research Centre (JRC) within the Consumer Products Safety of the Directorate of Health, Consumers and Reference Materials through the JRC Multiannual Work Programme.
The following are the supplementary data related to this article.
Fig. 2S.
Venn diagram for the selection of deregulated proteins from the cytoplasmic extract of Caco-2 cells exposed to AgNPs. Selection is made using the Mann-Whitney test.
Physico-chemical characterisation of 30 nm AgNP citrate coated.
List of deregulated proteins identified in individual 2D gel spots of Caco-2 cytoplasmic extracts. Each protein spot has been assigned a UniProt Accession Number, the protein symbol, the protein coverage, the number of identified peptides and amino acids, the molecular mass, the calculated pI and a probability score. Quantitative changes after AgNPs treatment (1 μg/mL AgNPs vs Ctrl and 10 μg/mL AgNPs vs Ctrl) are reported (p value and fold change) for individual proteins. a) 24 h and b) 72 h experiment.
List of deregulated proteins identified by the label-free nano UHPLC-Orbitrap MS/MS analysis of Caco-2 cytoplasmic extracts when “non-conflict” quantification approach was used. The table shows the results of one-way ANOVA, followed by sequential Bonferroni correction (p < .05). a) 24 h and b) 72 h experiment.
Comparison of the experimental requirements for each of the two approaches (2D-gel based and label-free MS-based) and information obtained from the data generated.
List of isoforms or proteolytic products of proteins found to be differently regulated by AgNPs exposure. Spot Id, UniProt accession number, Gene, protein identification are also provided.
Supplementary material
Acknowledgments
Acknowledgements
We are very thankful to Angela Kaempfer, François Rossi and Agnieszka Kinser-Ovaskainen for their helpful discussions. We acknowledge Emmanuel Duh for proofreading.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Abdallah C., Dumas-Gaudot E., Renaut J., Sergeant K. Gel-based and gel-free quantitative proteomics approaches at a glance. International Journal of Plant Genomics. 2012:2012. doi: 10.1155/2012/494572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Ilan O., Albrecht R.M., Fako V.E., Furgeson D.Y. Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small. 2009;5:1897–1910. doi: 10.1002/smll.200801716. [DOI] [PubMed] [Google Scholar]
- Beer C., Foldbjerg R., Hayashi Y., Sutherland D.S., Autrup H. Toxicity of silver nanoparticles—nanoparticle or silver ion? Toxicol. Lett. 2012;208:286–292. doi: 10.1016/j.toxlet.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H., Poortman J., Peters R.J., Wijma E., Kramer E., Makama S., Puspitaninganindita K., Marvin H.J., Peijnenburg A.A., Hendriksen P.J. Characterization of translocation of silver nanoparticles and effects on whole-genome gene expression using an in vitro intestinal epithelium coculture model. ACS Nano. 2011;5:4091–4103. doi: 10.1021/nn2007145. [DOI] [PubMed] [Google Scholar]
- Brandon E.F., Bosch T.M., Deenen M.J., Levink R., van DER Wal E., van Meerveld J.B., Bijl M., Beijnen J.H., Schellens J.H., Meijerman I. Validation of in vitro cell models used in drug metabolism and transport studies; genotyping of cytochrome P450, phase II enzymes and drug transporter polymorphisms in the human hepatoma (HepG2), ovarian carcinoma (IGROV-1) and colon carcinoma (CaCo-2, LS180) cell lines. Toxicol. Appl. Pharmacol. 2006;211:1–10. doi: 10.1016/j.taap.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Calderón-Jiménez B., Johnson M.E., Bustos A.R.M., Murphy K.E., Winchester M.R., Baudrit J.R.V. Silver nanoparticles: technological advances, societal impacts, and metrological challenges. Front. Chem. 2017;5 doi: 10.3389/fchem.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa P.M., Fadeel B. Emerging systems biology approaches in nanotoxicology: towards a mechanism-based understanding of nanomaterial hazard and risk. Toxicol. Appl. Pharmacol. 2016;299:101–111. doi: 10.1016/j.taap.2015.12.014. [DOI] [PubMed] [Google Scholar]
- Dadosh T. Synthesis of uniform silver nanoparticles with a controllable size. Mater. Lett. 2009;63:2236–2238. [Google Scholar]
- Georgantzopoulou A., Serchi T., Cambier S., Leclercq C.C., Renaut J., Shao J., Kruszewski M., Lentzen E., Grysan P., Eswara S. Effects of silver nanoparticles and ions on a co-culture model for the gastrointestinal epithelium. Particle fibre Toxicol. 2016;13:9. doi: 10.1186/s12989-016-0117-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff K., Albrecht C., Boots A.W., Förster I., Schins R.P. Cytotoxicity and oxidative DNA damage by nanoparticles in human intestinal Caco-2 cells. Nanotoxicology. 2009;3:355–364. [Google Scholar]
- Gioria S., Chassaigne H., Carpi D., Parracino A., Meschini S., Barboro P., Rossi F. A proteomic approach to investigate AuNPs effects in Balb/3T3 cells. Toxicol. Lett. 2014;228:111–126. doi: 10.1016/j.toxlet.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Gioria S., Lobo Vicente J., Barboro P., La Spina R., Tomasi G., Urbán P., Kinsner-Ovaskainen A., François R., Chassaigne H. A combined proteomics and metabolomics approach to assess the effects of gold nanoparticles in vitro. Nanotoxicology. 2016;10:736–748. doi: 10.3109/17435390.2015.1121412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath P., Gogoi S.K., Sanpui P., Paul A., Chattopadhyay A., Ghosh S.S. Signaling gene cascade in silver nanoparticle induced apoptosis. Colloids Surf. B: Biointerfaces. 2010;77:240–245. doi: 10.1016/j.colsurfb.2010.01.033. [DOI] [PubMed] [Google Scholar]
- Grimberg A. P53 and IGFBP-3: apoptosis and cancer protection. Mol. Genet. Metab. 2000;70:85–98. doi: 10.1006/mgme.2000.3008. [DOI] [PubMed] [Google Scholar]
- Griss J., Perez-Riverol Y., Hermjakob H., Vizcaíno J.A. Identifying novel biomarkers through data mining—A realistic scenario? PROTEOMICS-Clin. Appl. 2015;9:437–443. doi: 10.1002/prca.201400107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartemann P., Hoet P., Proykova A., Fernandes T., Baun A., DE Jong W., Filser J., Hensten A., Kneuer C., Maillard J.-Y. Nanosilver: safety, health and environmental effects and role in antimicrobial resistance. Mater. Today. 2015;18:122–123. [Google Scholar]
- Herzog A., Kuntz S., Daniel H., Wenzel U. Identification of biomarkers for the initiation of apoptosis in human preneoplastic colonocytes by proteome analysis. Int. J. Cancer. 2004;109:220–229. doi: 10.1002/ijc.11692. [DOI] [PubMed] [Google Scholar]
- Kim Y.S., Kim J.S., Cho H.S., Rha D.S., Kim J.M., Park J.D., Choi B.S., Lim R., Chang H.K., Chung Y.H. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2008;20:575–583. doi: 10.1080/08958370701874663. [DOI] [PubMed] [Google Scholar]
- Kim S., Choi J.E., Choi J., Chung K.-H., Park K., Yi J., Ryu D.-Y. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol. in Vitro. 2009;23:1076–1084. doi: 10.1016/j.tiv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Kim Y.S., Song M.Y., Park J.D., Song K.S., Ryu H.R., Chung Y.H., Chang H.K., Lee J.H., Oh K.H., Kelman B.J. Subchronic oral toxicity of silver nanoparticles. Particle Fibre Toxicol. 2010;7:20. doi: 10.1186/1743-8977-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosova A., Khodyreva S., Lavrik O. Role of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in DNA repair. Biochem. Mosc. 2017;82:643–654. doi: 10.1134/S0006297917060013. [DOI] [PubMed] [Google Scholar]
- Lefebvre D.E., Venema K., Gombau L., Valerio Jr L.G., Raju J., Bondy G.S., Bouwmeester H., Singh R.P., Clippinger A.J., Collnot E.-M. Utility of models of the gastrointestinal tract for assessment of the digestion and absorption of engineered nanomaterials released from food matrices. Nanotoxicology. 2015;9:523–542. doi: 10.3109/17435390.2014.948091. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhang W., Niu J., Chen Y. Surface-coating-dependent dissolution, aggregation, and reactive oxygen species (ROS) generation of silver nanoparticles under different irradiation conditions. Environ. Sci. & Technol. 2013;47:10293–10301. doi: 10.1021/es400945v. [DOI] [PubMed] [Google Scholar]
- Lomer M.C., Thompson R.P., Powell J.J. Proceedings of the Nutrition Society. Vol. 61. 2002. Fine and ultrafine particles of the diet: influence on the mucosal immune response and association with Crohn's disease; pp. 123–130. [DOI] [PubMed] [Google Scholar]
- Ma J., Lü X., Huang Y. Genomic analysis of cytotoxicity response to nanosilver in human dermal fibroblasts. J. Biomed. Nanotechnol. 2011;7:263–275. doi: 10.1166/jbn.2011.1286. [DOI] [PubMed] [Google Scholar]
- Martens L., Vizcaíno J.A. A golden age for working with public proteomics data. Trends Biochem. Sci. 2017;42(5):333–341. doi: 10.1016/j.tibs.2017.01.001. (Epub 2017 Jan 22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matysiak M., Kapka-Skrzypczak L., Brzóska K., Gutleb A.C., Kruszewski M. Proteomic approach to nanotoxicity. J. Proteome. 2016;137:35–44. doi: 10.1016/j.jprot.2015.10.025. [DOI] [PubMed] [Google Scholar]
- Mcshan D., Ray P.C., Yu H. Molecular toxicity mechanism of nanosilver. J. Food Drug Anal. 2014;22:116–127. doi: 10.1016/j.jfda.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miethling-Graff R., Rumpker R., Richter M., Verano-Braga T., Kjeldsen F., Brewer J., Hoyland J., Rubahn H.-G., Erdmann H. Exposure to silver nanoparticles induces size-and dose-dependent oxidative stress and cytotoxicity in human colon carcinoma cells. Toxicol. in Vitro. 2014;28:1280–1289. doi: 10.1016/j.tiv.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Nguyen K.C., Richards L., Massarsky A., Moon T.W., Tayabali A.F. Toxicological evaluation of representative silver nanoparticles in macrophages and epithelial cells. Toxicol. in Vitro. 2016;33:163–173. doi: 10.1016/j.tiv.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Oberemm A., Hansen U., Böhmert L., Meckert C., Braeuning A., Thünemann A.F., Lampen A. Proteomic responses of human intestinal Caco-2 cells exposed to silver nanoparticles and ionic silver. J. Appl. Toxicol. 2016;36:404–413. doi: 10.1002/jat.3231. [DOI] [PubMed] [Google Scholar]
- O'connor L., Strasser A., O'reilly L.A., Hausmann G., Adams J.M., Cory S., Huang D.C. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panáček A., Kvítek L., Prucek R., Kolář M., Večeřová R., Pizúrová N., Sharma V.K., Nevěčná T.J., Zbořil R. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B. 2006;110:16248–16253. doi: 10.1021/jp063826h. [DOI] [PubMed] [Google Scholar]
- Rabilloud T., Vaezzadeh A.R., Potier N., Lelong C., Leize-Wagner E., Chevallet M. Power and limitations of electrophoretic separations in proteomics strategies. Mass Spectrom. Rev. 2009;28:816–843. doi: 10.1002/mas.20204. [DOI] [PubMed] [Google Scholar]
- Ryazanov A.G., Shestakova E.A., Natapov P.G. 1988. Phosphorylation of Elongation Factor 2 by EF-2 Kinase Affects Rate of Translation. [DOI] [PubMed] [Google Scholar]
- Sahu S.C., Njoroge J., Bryce S.M., Zheng J., Ihrie J. Flow cytometric evaluation of the contribution of ionic silver to genotoxic potential of nanosilver in human liver HepG2 and colon Caco2 cells. J. Appl. Toxicol. 2016;36(4):521–531. doi: 10.1002/jat.3276. (Epub 2016 Jan 6) [DOI] [PubMed] [Google Scholar]
- Samson A.L., Knaupp A.S., Kass I., Kleifeld O., Marijanovic E.M., Hughes V.A., Lupton C.J., Buckle A.M., Bottomley S.P., Medcalf R.L. Oxidation of an exposed methionine instigates the aggregation of glyceraldehyde-3-phosphate dehydrogenase. J. Biol. Chem. 2014;289:26922–26936. doi: 10.1074/jbc.M114.570275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teow Y., Asharani P., Hande M.P., Valiyaveettil S. Health impact and safety of engineered nanomaterials. Chem. Commun. 2011;47:7025–7038. doi: 10.1039/c0cc05271j. [DOI] [PubMed] [Google Scholar]
- Van Der Zande M., Undas A.K., Kramer E., Monopoli M.P., Peters R.J., Garry D., Antunes Fernandes E.C., Hendriksen P.J., Marvin H.J., Peijnenburg A.A. Different responses of Caco-2 and MCF-7 cells to silver nanoparticles are based on highly similar mechanisms of action. Nanotoxicology. 2016;10:1431–1441. doi: 10.1080/17435390.2016.1225132. [DOI] [PubMed] [Google Scholar]
- Verano-Braga T., Miethling-Graff R., Wojdyla K., Rogowska-Wrzesinska A., Brewer J.R., Erdmann H., Kjeldsen F. Insights into the cellular response triggered by silver nanoparticles using quantitative proteomics. ACS Nano. 2014;8:2161–2175. doi: 10.1021/nn4050744. [DOI] [PubMed] [Google Scholar]
- Wang L., Srinivasan S., Theiss A.L., Merlin D., Sitaraman S.V. Interleukin-6 induces Keratin expression in intestinal epithelial cells potential role of keratin-8 in interleukin-6-induced barrier function alterations. J. Biol. Chem. 2007;282:8219–8227. doi: 10.1074/jbc.M604068200. [DOI] [PubMed] [Google Scholar]
- Wiley S.R., Schooley K., Smolak P.J., Din W.S., Huang C.-P., Nicholl J.K., Sutherland G.R., Smith T.D., Rauch C., Smith C.A. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Win K.Y., Feng S.-S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26:2713–2722. doi: 10.1016/j.biomaterials.2004.07.050. [DOI] [PubMed] [Google Scholar]
- Xu L., Shi C., Shao A., Li X., Cheng X., Ding R., Wu G., Chou L.L. Toxic responses in rat embryonic cells to silver nanoparticles and released silver ions as analyzed via gene expression profiles and transmission electron microscopy. Nanotoxicology. 2015;9:513–522. doi: 10.3109/17435390.2014.948942. [DOI] [PubMed] [Google Scholar]
- Yeung A.T., Patel B.B., Li X.-M., Seeholzer S.H., Coudry R.A., Cooper H.S., Bellacosa A., Boman B.M., Zhang T., Litwin S. One-hit effects in cancer: altered proteome of morphologically normal colon crypts in familial adenomatous polyposis. Cancer Res. 2008;68:7579–7586. doi: 10.1158/0008-5472.CAN-08-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.-Y., Zhang F., Hong C.-Q., Giuliano A.E., Cui X.-J., Zhou G.-J., Zhang G.-J., Cui Y.-K. Critical protein GAPDH and its regulatory mechanisms in cancer cells. Cancer Biol. & Med. 2015;12:10. doi: 10.7497/j.issn.2095-3941.2014.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.-F., Shen W., Gurunathan S. Silver nanoparticle-mediated cellular responses in various cell lines: an in vitro model. Int. J. Mol. Sci. 2016;17:1603. doi: 10.3390/ijms17101603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Physico-chemical characterisation of 30 nm AgNP citrate coated.
List of deregulated proteins identified in individual 2D gel spots of Caco-2 cytoplasmic extracts. Each protein spot has been assigned a UniProt Accession Number, the protein symbol, the protein coverage, the number of identified peptides and amino acids, the molecular mass, the calculated pI and a probability score. Quantitative changes after AgNPs treatment (1 μg/mL AgNPs vs Ctrl and 10 μg/mL AgNPs vs Ctrl) are reported (p value and fold change) for individual proteins. a) 24 h and b) 72 h experiment.
List of deregulated proteins identified by the label-free nano UHPLC-Orbitrap MS/MS analysis of Caco-2 cytoplasmic extracts when “non-conflict” quantification approach was used. The table shows the results of one-way ANOVA, followed by sequential Bonferroni correction (p < .05). a) 24 h and b) 72 h experiment.
Comparison of the experimental requirements for each of the two approaches (2D-gel based and label-free MS-based) and information obtained from the data generated.
List of isoforms or proteolytic products of proteins found to be differently regulated by AgNPs exposure. Spot Id, UniProt accession number, Gene, protein identification are also provided.
Supplementary material