Abstract
The broad-spectrum lytic capability of Salmonella bacteriophages against various Salmonella species was evaluated to determine their potential as an alternative for antibiotics, and the safety and preventive effects of the bacteriophages were assessed on mice and pigs. Four bacteriophage cocktails were prepared using 13 bacteriophages, and the lytic capability of the four bacteriophage cocktails was tested using Salmonella reference strains and field isolates. Bacteriophage cocktail C (SEP-1, SGP-1, STP-1, SS3eP-1, STP-2, SChP-1, SAP-1, SAP-2; ≥109 pfu/ml) showed the best lytic activity against the Salmonella reference strains (100% of 34) and field isolates (92.5% of 107). Fifty mice were then orally inoculated with bacteriophage cocktail C to determine the distribution of bacteriophages in various organs, blood and feces. The effects of bacteriophages on Salmonella infection in weaned pigs (n=15) were also evaluated through an experimental challenge with Salmonella Typhimurium after treatment with bacteriophage cocktail C. All mice exhibited distribution of the bacteriophages in all organs, blood and feces until 15 days post infection (dpi). After 35 dpi, bacteriophages were not detected in any of these specimens. As demonstrated in a pig challenge study, treatment with bacteriophage cocktail C reduced the level of Salmonella shedding in feces. The metagenomic analyses of these pig feces also revealed that bacteriophage treatment decreased the number of species of the Enterobacteriaceae family without significant disturbance to the normal fecal flora. This study showed that bacteriophages effectively controlled Salmonella in a pig challenge model and could be a good alternative for antibiotics to control Salmonella infection.
Keywords: bacteriophage, Salmonella, distribution of bacteriophage, pig intestinal bacteria flora, next-generation sequencing
Salmonella is a common food-borne pathogen worldwide [3, 33, 35], and its major route of transmission to humans is the consumption of Salmonella-contaminated food products, such as pork and poultry products. Salmonella causes acute enteric infections, and the intestinal contents of pigs infected with Salmonella can contaminate their carcasses [9, 27]. Subclinically infected pigs with Salmonella can also act as super-shedders of Salmonella in lairage and slaughterhouses because the shedding can be increased by stress, such as changes in environment, transport, and other factors [39]. Effective public health measures for the control of Salmonella contamination in farms are needed to prevent transmission to the carcasses and thus to humans. A recent study demonstrated that the prevalence of Salmonella in 1,194 samples from slaughterhouses and swine farms (977 fecal contents and 67 organ samples) was 5.3% [29]. In another study, Salmonella was isolated from 15.7 to 21.3% of animals over 30 days of age in Korean swine farms [31].
Antibiotics have been widely used in pig and poultry farms, but multidrug-resistant (MDR) bacteria are a serious emerging concern in the fields of food safety and animal disease control [6]. Antibiotic resistance that originated from pigs and poultry threatens human health due to the transferability of antibiotic-resistance genes to zoonotic pathogens [47], and the frequency of antibiotic resistance among food-borne pathogens has increased [37, 38]. Therefore, the use of antibiotics as growth promoters in animal feed was banned in most developed countries [12, 17]. The difficulties in controlling the disease caused by various pathogenic bacteria without antibiotics, particularly during the weaning period, have reduced product quality and livestock yield [45, 46]. Therefore, an available and inexpensive method for the prevention and treatment of Salmonella is urgently needed.
Bacteriophages are viruses that can specifically infect and lyse bacteria. Bacteriophages are natural, nontoxic, and inexpensive and replicate only in specific bacteria, allowing avoidance of the gut flora imbalance caused by broad-spectrum antibiotics. Bacteriophages have also been used successfully to treat various bacterial infections in animals [5, 18, 22, 36, 42]. Furthermore, bacteriophages can also be effective against MDR pathogens [1, 16, 32, 44]. Notably, several studies have reported that some bacteriophages can reduce the enteric Salmonella levels in pigs [10, 48]. Additionally, a cocktail of bacteriophages is more effective than the use of one bacteriophage to control bacterial disease in pigs [44], and Yoichi et al. [51] reported that the use of a bacteriophage mixture delayed bacteriophage-resistant bacterial growth.
In the present study, we evaluated the efficiency of four bacteriophage cocktails against Salmonella isolates from pig farms and slaughterhouses in Korea. Additionally, we evaluated the distribution of bacteriophages in mouse organs and the changes in the pig intestinal bacterial flora induced by bacteriophage cocktail treatment.
MATERIALS AND METHODS
Bacterial strains
One hundred seven Salmonella isolates from fecal samples or carcasses collected from slaughterhouses or farms during 2009 to 2011 were used assessing the lytic spectra of bacteriophages and antimicrobial susceptibility. Additionally, 34 reference strains representing 34 serotypes were used for assessment of the bacteriophage lytic spectrum. All the isolates were streaked on xylose lactose deoxycholate agar to assess their purity and were genetically confirmed by polymerase chain reaction (PCR) [11]. All the isolates were serotyped by a slide agglutination test according to the Kauffmann-White scheme at the Salmonella reference center RIVM (National Institute of Public Health and the Environment) at Bilthoven, The Netherlands.
Antibiotic susceptibility tests
One hundred seven Salmonella isolates were subjected to an antibiotic susceptibility test. The antibiotic susceptibility test was performed using the agar disk diffusion test on Mueller Hinton agar plate [23]. The concentrations of antibiotics on BBL Sensi-Disk (BD, Franklin Lakes, NJ, U.S.A.) were as follows; ampicillin (AM, 10 µg), streptomycin (S, 10 µg), gentamicin (GM, 10 µg), neomycin (N, 30 µg), tetracycline (Te, 30 µg), nalidixic acid (NA, 30 µg), enrofloxacin (ENR, 5 µg), trimethoprim/sulfamethoxazole (SXT, 1.25/23.75 µg), and chloramphenicol (C, 30 µg). MDR isolates were defined as isolates resistant to three or more antibiotics belonging to different antibiotic classes [in the following order, one penicillin (Pe), three aminoglycosides (Ag), one tetracycline (Te), two quinolones (Qu), one sulfonamide (Su) and one phenicol (Ph)]. Each isolate was plated in Mueller Hinton agar, and each antibiotic disk was placed over the bacteria layer. The plates were incubated at 37°C for 18 hr according to the method described by CLSI guidelines [15]. The antibiotic susceptibilities of the isolates were then classified as susceptible, intermediate, and resistant according to the zone diameter on each antibiotic disk.
Isolation of bacteriophages and preparation of bacteriophage cocktails
Isolation of bacteriophages was carried out as previously described [43]. Briefly, six Salmonella isolates (Salmonella enterica Enteritidis SE30, Salmonella enterica Gallinarum SG40, Salmonella enterica Typhimurium ST11, Salmonella enterica Typhimurium ST2, Salmonella enterica Enteritidis SE5, and Salmonella enterica Choleraesuis SC1) were used as the host strains for bacteriophage isolation. To isolate bacteriophages that had lytic activity against the Salmonella isolates, various samples were collected from the sewage and feces in five swine farms at Paju, Asan and Yesan in Korea (Table S1). All the isolates were cultured at 37°C in TSB (tryptic soy broth, BD) and then co-cultivated with each farm specimen overnight at 37°C. Following overnight growth, the culture was centrifuged for 20 min at 10,000 × g. The resultant supernatant was filtered with 0.45-µm syringe filter. This procedure, from co-cultivation to filtration, was repeated twice to enhance the bacteriophage titer. A conventional double-layered agar method was used to examine whether the filtrates contained lytic bacteriophages for the Salmonella strains, respectively. A series of purification steps (plaque isolation, co-cultivation, centrifugation and filtration) were performed at least three times to obtain pure bacteriophages.
Thirteen lytic bacteriophages (SEP-1, SGP-1, STP-1, SS3eP-1, EK99P-1, SalTP-2, SChP-1, SAP-1, SAP-2, E41P-1, EK88P-1, CPP-3 and CPP-5) were isolated using the procedures described above. The lytic activity of 13 bacteriophages against Salmonella reference strains was summarized in Table S1. Based on the lytic spectrum and final titer of each bacteriophage, 4 bacteriophage cocktails were prepared with a mixture selected from the 13 individual bacteriophages: cocktail A (SEP-1, SGP-1, STP-1, SS3eP-1 and EK99P-1; ≥109 plaque forming units (pfu)/ml), cocktail B (SEP-1, SGP-1, STP-1, SS3eP-1 and EK99P-1; ≥1011 pfu/ml), cocktail C (SEP-1, SGP-1, STP-1, SS3eP-1, SalTP-2, SChP-1, SAP-1 and SAP-2; ≥109 pfu/ml), and cocktail D (SalTP-2, SChP-1, SAP-1, SAP-2, E41P-1, EK88P-1, EK99P-1, CPP-3 and CPP-5; ≥109 pfu/ml). Each cocktail was prepared with equal numbers of the selected bacteriophages.
Assessment of bacteriophage host spectrum
Bacteriolytic activity of 13 bacteriophages was preferentially examined for 141 Salmonella isolates, respectively. The bacterial susceptibilities of all the Salmonella isolates to bacteriophage cocktails were assayed. One hundred microliters of bacterial culture were spread on a TSA (tryptic soy agar, BD) plate with five squares and allowed to dry. Ten microliters of each bacteriophage cocktail suspension were dropped into the center of each square and allowed to dry. A drop of saline, as the negative control, was used on one square area per plate. Following overnight incubation at 37°C for 18 hr, the plates were examined for the following: a clear zone of complete lysis, incomplete lysis (not all the infected cell underwent lysis), and no lysis in the bacterial lawn.
Distribution of bacteriophages in mice after oral administration
To determine the residual period of bacteriophages after oral administration, 50 four-week-old female BALB/c mice (DBL, Eumseong, Korea) were purchased and randomly divided into 10 groups: nine groups of five mice were orally administered 200 µl of bacteriophage cocktail C at 1 × 108 PFU/ml in sodium chloride-magnesium sulfate (SM) buffer with gelatin (100 mM NaCl, 10 mM MgSO4 [heptahydrate], 50 mM Tris-HCl [pH 7.5], and 0.01% gelatin), and one group was the non-treatment group and administered 200 µl of SM buffer. The mice were housed in a temperature-controlled animal room with a 12-hr light-dark cycle. The reuptake of bacteriophages through the feces, respiratory system, body fluids, feed, and water was minimized by providing fresh water and feed and changing the bedding every day until the end of the experiment. The mice in the nine bacteriophage-treated groups were humanly euthanized 0, 3, 6, 9, 12, 15, 21, 28 or 35 days after bacteriophage treatment. Samples of blood, feces, and eight organs (liver, lung, spleen, kidney, small intestine, large intestine, brain, and heart) were collected at each time point.
The bacteriophage titer was measured by calculating the weight of each organ and feces in the mice. The bacteriophage titer was measured through a double agar overlay plaque assay, which was performed as follows: each organ, blood and feces sample from mice was suspended in PBS, homogenized and centrifuged at 10,000 × g for 5 min. The bacteriophage was then purified using a 0.45-µm syringe filter. The filtered bacteriophage was serially diluted 10-fold with PBS, and the diluted solution was mixed with the Salmonella Typhimurium culture broth at 1 × 108 colony-forming units (cfu)/ml. The mixture of bacteriophage and Salmonella was suspended in Top agar (TSB with 0.6% agar and 0.2% magnesium chloride), poured onto Bottom agar (TSB with 1.5% agar) and incubated at 37°C for 24 hr, and the bacteriophage titer was then calculated by assessing the clear zone of complete lysis. The animal experimental protocol was approved by the Chonbuk National University Institutional Animal Care and Use Committee (Approval Number: CBNU 2016-0041).
Evaluation of bacteriophage treatment against Salmonella infection in weaned pigs
To evaluate the preventive effects of bacteriophage treatment against Salmonella infection, 15 four-week-old pigs were purchased and divided into three groups. The bacteriophage control (PC) group and bacteriophage treatment (PT) group were fed 5 ml of the Salmonella-specific bacteriophage cocktail C at 109 pfu/ml until the end of the study. One week after initiation of the bacteriophage treatment in the PT group, the PT and challenge control (CC) groups were simultaneously challenged with 10 ml of Salmonella Typhimurium (ATCC 14028) culture at 108 cfu/ml. Fecal swabs were collected daily from all the groups at 2 and 8 days post infection (dpi) and tested for bacterial shedding. The weight of the pigs was also measured on a weekly basis. All the pigs were sacrificed for pathological evaluation two weeks after infection. The animal experimental protocol was approved by the Chonbuk National University Institutional Animal Care and Use Committee (Approval Number: CBNU 2016-0041).
Salmonella PCR
Nucleic acids were extracted from fecal samples using a MagMax™ Total Nucleic Acid Isolation Kit (Applied Biosystems, Foster city, CA, U.S.A.) according to the manufacturer’s instructions. Briefly, 0.01 M PBS, pH 7.4, was added to each sample to generate 30% fecal homogenates. After centrifugation for 1 min at 100 × g to pellet the larger-size particles, 175 µl of the supernatant of each sample was added to a bead tube containing zirconia beads and 235 µl of the lysis/binding solution. The bead tube was beaten at maximum speed for 5 min with a Bullet Blender® (Next Advance, Inc., Troy, NY, U.S.A.). After the beating process, the bead tubes were centrifuged at 16,000 × g for 3 min, and the supernatant was carefully transferred to clean microcentrifuge tubes. After another centrifugation at 16,000 × g for 6 min, 115 µl of the supernatant, as well as washing and elution buffers, was transferred to a 96-well microplate, and the plate was placed in a MagMax™ Express magnetic particle processor (Applied Biosystems) for automated extraction, which consisted of a 5-min lysis/binding step, two 90-sec wash steps, two 150-min wash steps, a 1-min drying step, and elution for 3 min. The extracted total nucleic acids in the elution plate were stored at −80°C until used for PCR.
The sequence information of the primers and probes used for real-time PCR is as follows: forward primer, 5ʹ-GCCATGCTGTTCGATGAT-3ʹ; reverse primer, 5ʹ-GTTACCGATAGCGGGAAAGG-3ʹ; and probe primer, 5ʹ-Cy5-TTTTGCACCACMGCCAGCCC-BHQ-3ʹ [14]. The PCR was optimized with an AgPath-IDTM Multiplex RT-PCR Kit (Applied Biosystems) following the manufacturer’s recommended protocol in a 25-µl reaction volume using 8 µl of extracted template. The final concentration of each primer or probe was 0.2 µM. The PCR amplification was performed on an ABI 7500 Fast Real-Time PCR System (Applied Biosystems) with the following cycling conditions: a) reverse transcription for 10 min at 45°C (this step was omitted for bacterial/protozoan PCR), b) a 10-min activation step at 95°C, and c) 35 cycles of 15 sec at 95°C and 60 sec at 60°C. Samples with a threshold cycle (Ct) of 35 cycles or less were considered positive.
Fecal metagenomic analysis
To evaluate the negative effects of bacteriophage treatment on the normal intestinal flora, fecal swabs collected from the above-described pig challenge study was subjected to a fecal metagenomic analysis. The DNA in each fecal sample was extracted using a stool DNA extraction kit (PowerFecal® DNA Isolation Kit, Qiagen, Hilden, Germany), and each extracted fecal DNA was pooled to obtain each corresponding group for library construction and sequenced using a NGS system. The microflora analysis was performed as follows: Bacterial 16S rRNA genes were amplified by PCR from DNA samples using the V1-V12 variable regions (Ion 16STM Metagenomics Kit, Life Technologies, Carlsbad, CA, U.S.A.). The library preparation and 16S metagenomic sequencing were analyzed using the Ion Torrent Personal Genome Machine (PGM, Life Technologies) according to the manufacturers’ specifications and as described previously [40, 49]. All sequencing reactions were performed using 316 chips and the Ion Torrent 400-bp sequencing kit (Life Technologies) with an approximate runtime of 4.5 hr per chip. Primary base calling was performed using Torrent Suite v5.0 software (Life Technologies), and sequences were exported in FastQ format. FastQ files were used for all subsequent analyses.
Data analysis
Graphs were constructed using GraphPad Prism 5.0.2 (GraphPad, San Diego, CA, U.S.A.), and the statistical analyses were performed using SPSS Advanced Statistics 17.0 software (SPSS, Inc., Chicago, IL, U.S.A.). The levels of Salmonella shedding were analyzed by ANOVA with repeated measurements. A contrast was constructed when direct comparisons between the groups were necessary.
RESULTS
Host spectra of bacteriophage cocktails
The abilities of four bacteriophage cocktails to lyse 34 reference strains of 34 serotypes and 107 field isolates of 12 serotypes isolated from swine farms and slaughterhouses in Korea were evaluated. As summarized in Table 1, all the reference strains were clearly lysed by cocktails B and C, whereas eight (23.5%) serotypes of reference strains showed complete lysis by cocktails A and D. Among 107 Salmonella isolates, 99 (92.5%) isolates were lysed by cocktails B and C, but 63 (58.9%) and 57 (55.3%) of the isolates were lysed by cocktails A and D, respectively. All the isolates of Salmonella Typhimurium (n=64), the most prevalent serotype in Korean swine farms, were completely lysed with cocktails B and C. Serotypes such as Schwarzengrund (n=7), Derby (n=3), London (n=2) and Hadar (n=2) were also clearly lysed by cocktails B and C. However, the testing of 23 isolates belonging to serotype Rissen with cocktails A and D revealed no lysis, but 16 (69.6%) of the isolates were completely lysed with cocktails B and C. Essen (n=1), one of the least frequently isolated serotypes in Korea, was not sensitive to any of the tested bacteriophage cocktails (Table 2).
Table 1. Lytic spectra of four bacteriophage cocktails determined on 34 serotypes of Salmonella reference strains.
| Groups | Serotypes | No. of completely lysed isolates with the indicated phage solution |
|||
|---|---|---|---|---|---|
| A | B | C | D | ||
| B | Derby, Eko, Haifa, Paratyphi B, Typhimurium (n=5) | 5 | 5 | 5 | 5 |
| C1 | Bareilly, Braenderup, Choleraesuis, Colindale, Oranienburg, Tennessee, Virchow (n=7) | 0 | 7 | 7 | 0 |
| C2–C3 | Glostrup, Kentucky, Litchfield, Muenchen, Tallahassee, Wippra (n=6) | 0 | 6 | 6 | 0 |
| D1 | Berta, Dublin, Enteritidis (n=3) | 3 | 3 | 3 | 3 |
| D2 | Hillingdon (n=1) | 0 | 1 | 1 | 0 |
| E1 | Amsterdam, Give, Illinois, Muenster, Onireke, Westhampton (n=6) | 0 | 6 | 6 | 0 |
| E4 | Liverpool, Senftenberg (n=2) | 0 | 2 | 2 | 0 |
| F | Abaetetuba, Aberdeen (n=2) | 0 | 2 | 2 | 2 |
| G | Kedougou (n=1) | 0 | 1 | 1 | 0 |
| H | Sundsvall (n=1) | 0 | 1 | 1 | 0 |
Table 2. Lytic spectra of four bacteriophage cocktails determined on 107 Salmonella field isolates.
| Serotypes | No. of completely lysed isolates with the indicated phage solution (%) |
|||
|---|---|---|---|---|
| A | B | C | D | |
| Typhimurium (n=64) | 57 | 64 | 64 | 54 |
| Teddington (n=1) | 0 | 1 | 1 | 0 |
| Derby (n=3) | 3 | 3 | 3 | 0 |
| Othmarshen (n=1) | 0 | 1 | 1 | 0 |
| Rissen (n=23) | 0 | 16 | 16 | 0 |
| Essen (n=1) | 0 | 0 | 0 | 0 |
| London (n=2) | 0 | 2 | 2 | 0 |
| Hadar (n=2) | 2 | 2 | 2 | 2 |
| Schwarzengrund (n=7) | 0 | 7 | 7 | 0 |
| Virchow (n=1) | 0 | 1 | 1 | 0 |
| Newport (n=1) | 0 | 1 | 1 | 0 |
| Mendoxa (n=1) | 1 | 1 | 1 | 1 |
| Total (n=107) | 63 (58.9) | 99 (92.5) | 99 (92.5) | 57 (53.3) |
Antibacterial properties of bacteriophage cocktails against antibiotic-resistant Salmonella isolates
One hundred seven Salmonella isolates were tested for antibiotic susceptibility (Table 3). Among 107 Salmonella isolates, all isolates were resistant to one or more of the antibiotics tested in this study. The antibiotic susceptibility of the isolates varied as follows: N (80.4%), SXT (76.7%), ENR (61.7%), C (60.7%), GM (60.7%), AM (48.6%), S (34.6%), Te (18.7%) and NA (0%). Of the 107 resistant isolates, 80 (74.8%) were MDR isolates showing resistance to at least three antibiotics belonging to different antibiotic classes. Most of the MDR isolates (65%, 52/80) were Salmonella Typhimurium, and only Salmonella Typhimurium isolates were resistant to all eight antibiotics (Table 3). Among the 80 MDR isolates, 74 (92.5%) isolates were clearly lysed with cocktails B and C (Table 4). As described above, five isolates of serotype Rissen and one isolate of serotype Essen (n=1) were not sensitive to any of the tested bacteriophage cocktails (Table 2).
Table 3. Rate of host lysis in cocktail C observed among antibiotic-resistant Salmonella serotypes.
| Serotypes | No. of isolates to complete lysis in
cocktail C/ No. of isolates resistant to indicated number of antibiotics classes |
||||||
|---|---|---|---|---|---|---|---|
| 1a) | 2a) | 3a) | 4a) | 5a) | 6a) | MDRb) (≥3) |
|
| Rissen (n=23) | 1/1 | 5/7 | 2/5 | 6/7 | 2/3 | 10/15 | |
| Schwarzengrund (n=7) | 1/1 | 6/6 | 6/6 | ||||
| Typhimurium (n=64) | 5/5 | 7/7 | 5/5 | 23/23 | 14/14 | 10/10 | 52/52 |
| Other serotypes (n=13) | 4/4 | 2/2 | 6/6 | 0/1 c) | 6/7 | ||
| Total (n=107) | 11/11 | 14/16 | 19/22 | 29/30 | 16/18 | 10/10 | 74/80 |
a) Number of resistant to indicated number of antibiotics classes, b) Multi antibiotics-resistance to more than three antibiotics classes, c) Isolates of Salmonella Essen.
Table 4. Percentage of multidrug-resistant Salmonella isolates lysed by the bacteriophage cocktails.
| No. of classes of resistant antibioticsa) |
Patterns of resistant antibiotic classes |
No. of isolates completely lysed by the indicated phage solution (%) |
|||
|---|---|---|---|---|---|
| A | B | C | D | ||
| 3 (n=22) | PeAgTeb) (n=1) | 10 | 19 | 19 | 3 |
| PeTeQu (n=1) | |||||
| AgTeQu (n=16) | |||||
| AgQuPh (n=2) | |||||
| TeQuPh (n=2) | |||||
| 4 (n=30) | PeAgQuPh (n=1) | 20 | 29 | 29 | 20 |
| PeTeQuSu (n=3) | |||||
| PeAgTeQu (n=18) | |||||
| PeTeQuPh (n=2) | |||||
| AgTeQuPh (n=5) | |||||
| AgTeQuSu (n=1) | |||||
| 5 (n=18) | PeAgTeQuPh (n=8) | 11 | 16 | 16 | 12 |
| PeAgTeSuPh (n=2) | |||||
| PeAgTeQuSu (n=5) | |||||
| AgTeQuSuPh (n=3) | |||||
| 6 (n=10) | PeAgTeQuSuPh (n=10) | 10 | 10 | 10 | 10 |
| Total (n=80) | 51 (63.8) | 74 (92.5) | 74 (92.5) | 45 (56.3) | |
a) Number of isolates resistant to the indicated number of antimicrobials classes; b) penicillins (Pe), aminoglycosides (Ag), tetracyclines (Te), quinolones (Qu), sulfonamides (Su) and phenicols (Ph).
Evaluation of the distribution and stability of bacteriophages in mice
Three days post-bacteriophage inoculation (dpi), bacteriophages were detected in all the organs examined in the current study at the range of 104.544(liver) to 108.301(lung) pfu/ml. At 3 dpi, a high bacteriophage titer (108.041 pfu/ml) was also detected in feces, whereas a relatively low bacteriophage titer was observed in blood (103.041 pfu/ml). At 15 dpi, higher titers of bacteriophages (103.342–104.462 pfu/ml) were detected in the digestive system (small and large intestine, respectively) and feces than in any other specimen, and low titers of bacteriophages (100.602 and 100.699 pfu/ml) were continuously detected in the digestive system until 28 dpi, whereas no bacteriophage was detected elsewhere. Although high titers of bacteriophages (102.362–103.322 pfu/ml) were maintained in the lung, spleen, brain and heart until 15 dpi, a low bacteriophage titer was detected in blood and other organs only up to 21 dpi. No bacteriophages were detected in any specimen at 35 dpi (Table 5).
Table 5. Distribution of bacteriophages in the organs, blood and feces of mice treated with the bacteriophage cocktail C.
| DPI | Blood | Liver | Lung | Spleen | Kidney | Small intestine | Large intestine | Brain | Heart | Feces |
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 3.041a) ± 2.7 | 4.544 ± 4.4 | 8.301 ± 8.5 | 7.230 ± 6.8 | 8.041 ± 7.1 | 7.380 ± 7.2 | 6.792 ± 5.5 | 6.000 ± 6.0 | 6.447 ± 6.1 | 8.041 ± 7.7 |
| 6 | 2.176 ± 1.2 | 5.230 ± 4.4 | 5.398 ± 4.4 | 6.863 ± 6.6 | 7.279 ± 6.0 | 6.114 ± 5.6 | 5.740 ± 5.1 | 6.519 ± 4.3 | 6.301 ± 6.4 | 7.580 ± 5.1 |
| 9 | 1.204 ± 0.7 | 5.146 ± 5.1 | 4.908 ± 5.1 | 6.799 ± 6.6 | 6.000 ± 7.8 | 6.230 ± 6.0 | 5.477 ± 6.7 | 5.613 ± 6.7 | 6.079 ± 6.9 | 7.591 ± 7.0 |
| 12 | 0.322 ± 0.6 | 0.934 ± 0.4 | 4.903 ± 4.7 | 4.934 ± 4.6 | 1.643 ± 1.0 | 3.643 ± 3.1 | 5.491 ± 5.4 | 4.699 ± 5.3 | 5.633 ± 5.0 | 6.987 ± 7.5 |
| 15 | 0.342 ± 0.1 | 0.672 ± 0.1 | 3.079 ± 2.9 | 3.322 ± 3.0 | 1.663 ± 1.1 | 3.342 ± 3.0 | 4.462 ± 4.2 | 2.690 ± 2.0 | 2.362 ± 1.9 | 3.633 ± 3.1 |
| 21 | 0.079 ± 0.1 | 0.255 ± 0.0 | 1.041 ± 0.8 | ND | 1.000 ± 0.9 | 2.301 ± 1.9 | 3.000 ± 3.3 | ND | 1.771 ± 1.5 | 2.301 ± 2.6 |
| 28 | ND | ND | ND | ND | ND | 0.602 ± 0.8 | 0.699 ± 1.4 | ND | ND | ND |
| 35 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
a) (PFU/ml), Log10 ± standard deviation. ND: no detection.
Effects of Salmonella-specific bacteriophages on weaned pigs infected with Salmonella Typhimurium
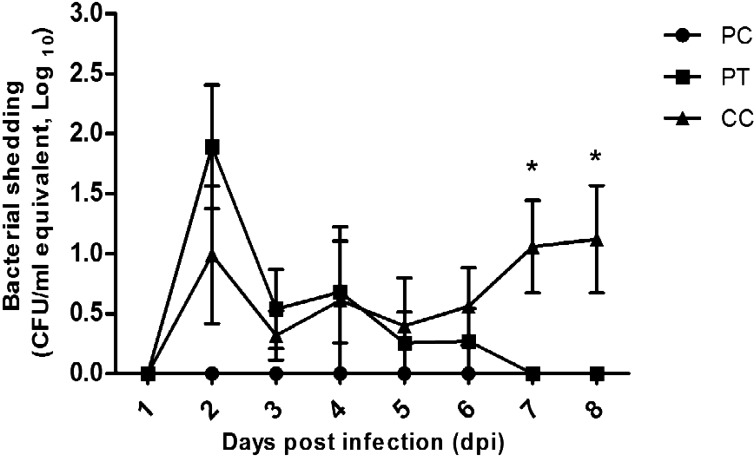
To evaluate the effects of Salmonella-specific bacteriophage cocktail C, we measured Salmonella shedding in pig feces after experimental challenge with Salmonella Typhimurium in the PT and CC groups until 8 dpi. The PT group showed the highest level of Salmonella shedding at 2 dpi (101.8 cfu/ml), and thereafter, a continuous decrease in Salmonella shedding was observed until 7 dpi. The CC group showed similar levels of Salmonella shedding compared with the PT group until 6 dpi and a continuous increase in Salmonella shedding until at the end of the experiment (8 dpi, Fig. 1).
Fig. 1.
Levels of Salmonella shedding in feces (means ± SEMs) collected from pigs treated or not treated with Salmonella-specific bacteriophages after challenge with Salmonella Typhimurium. PC, Phage control; PT, Phage treatment; CC, Challenge control.
Evaluation of changes in the pig intestinal bacterial flora after Salmonella bacteriophage cocktail C treatment
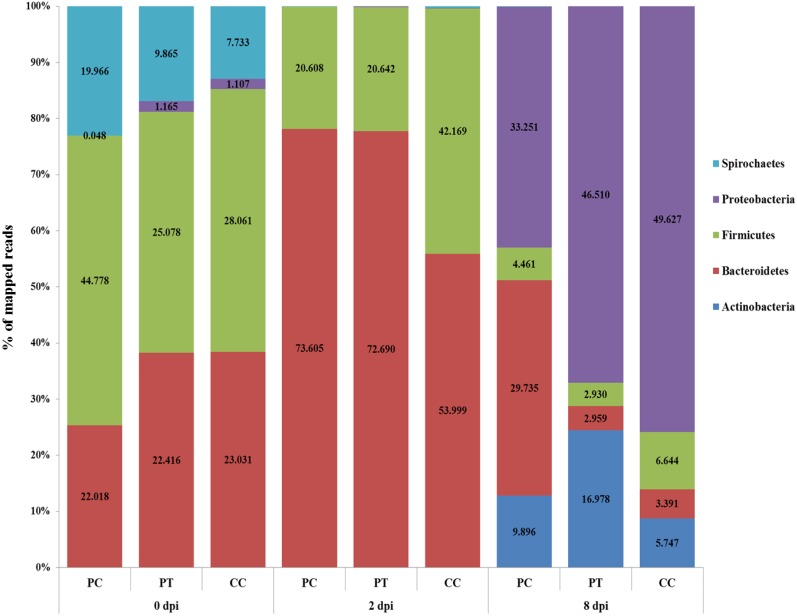
To compare the fecal bacterial flora in the pigs belonging to the three experimental groups (PC, PT and CC) after bacteriophage treatment and/or Salmonella challenge, we analyzed the relative ratio of the phylum- and family-level assignments through metagenomics. As shown in Table 6 and Fig. 2, the unique phylum- and family-level compositions of fecal bacterial flora in each pig group challenged with Salmonella or treated with bacteriophage cocktail C were observed.
Table 6. Composition of family level of fecal microbes in pigs with or without Salmonella-specific bacteriophage cocktail C.
| Phylum | Family | 0 dpi | 2 dpi | 8 dpi | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PC a) | PT | CC | PC | PT | CC | PC | PT | CC | ||
| Actinobacteria | Nocardiaceae | 0.000 b) | 0.000 | 0.000 | 0.056 | 0.000 | 0.018 | 9.896 | 16.978 | 5.747 |
| Bacteroidetes | Bacteroidaceae | 17.069 | 17.487 | 12.656 | 1.568 | 2.899 | 2.922 | 9.416 | 0.187 | 0.152 |
| S24-7 | 0.000 | 0.000 | 0.000 | 2.819 | 0.590 | 7.358 | 0.201 | 0.101 | 0.350 | |
| Porphyromonadaceae | 0.085 | 0.021 | 0.074 | 3.211 | 22.413 | 4.792 | 18.182 | 0.244 | 1.095 | |
| Prevotellaceae | 4.320 | 4.394 | 9.807 | 64.495 | 46.736 | 38.838 | 0.635 | 2.269 | 1.657 | |
| Rikenellaceae | 0.544 | 0.514 | 0.494 | 1.512 | 0.052 | 0.089 | 1.301 | 0.158 | 0.137 | |
| Firmicutes | Acidaminococcaceae | 9.959 | 4.555 | 6.870 | 0.075 | 0.052 | 0.214 | 0.046 | 0.000 | 0.000 |
| Erysipelotrichaceae | 10.128 | 5.112 | 7.579 | 1.027 | 0.174 | 2.815 | 0.093 | 0.029 | 0.258 | |
| Clostridiaceae | 1.511 | 2.333 | 2.100 | 1.941 | 1.406 | 17.584 | 1.580 | 2.011 | 3.056 | |
| Lachnospiraceae | 5.717 | 2.551 | 0.829 | 9.334 | 8.472 | 2.298 | 1.301 | 0.172 | 0.958 | |
| Lactobacillaceae | 0.350 | 0.813 | 0.087 | 1.157 | 1.875 | 0.338 | 0.248 | 0.316 | 0.076 | |
| Christensenellaceae | 8.907 | 4.214 | 6.679 | 0.187 | 0.017 | 0.499 | 0.170 | 0.000 | 0.000 | |
| Ruminococcaceae | 6.013 | 5.458 | 3.846 | 6.384 | 6.667 | 4.810 | 0.883 | 0.158 | 1.140 | |
| Peptostreptococcaceae | 0.193 | 0.042 | 0.071 | 0.504 | 1.979 | 13.611 | 0.139 | 0.244 | 1.156 | |
| Proteobacteria | Comamonadaceae | 0.000 | 0.000 | 0.000 | 0.000 | 0.017 | 0.018 | 11.817 | 15.010 | 6.599 |
| Enterobacteriaceae | 0.048 | 1.165 | 1.107 | 0.000 | 0.000 | 0.018 | 2.292 | 4.955 | 26.653 | |
| Pseudomonadaceae | 0.000 | 0.000 | 0.000 | 0.000 | 0.017 | 0.053 | 6.257 | 8.475 | 8.925 | |
| Xanthomonadaceae | 0.000 | 0.000 | 0.000 | 0.000 | 0.069 | 0.000 | 12.885 | 18.070 | 7.450 | |
| Spirochaetes | Spirochaetaceae | 19.966 | 9.865 | 7.733 | 0.019 | 0.000 | 0.285 | 0.015 | 0.000 | 0.000 |
a) Phage control (PC), Phage treatment (PT), Challenge control (CC), b) % of mapped reads.
Fig. 2.
Phylum-level metagenomic analyses of fecal bacteria in pigs treated or not treated with Salmonella-specific bacteriophages after challenge with Salmonella Typhimurium. Five fecal samples were pooled for each group. PC, Phage control; PT, Phage treatment; CC, Challenge control.
To investigate the changes in fecal bacterial flora during the experimental period, the pig fecal bacterial flora before and after Salmonella and bacteriophage administration was examined. At 0 dpi, Firmicutes was the predominant phylum in the fecal bacterial flora (42.78, 25.08 and 28.06% in the PC, P and CC groups, respectively), and Bacteroidetes was the next most abundant phylum (22.02, 22.42 and 23.03% in the PC, PT and CC groups, respectively). At 2 dpi, Bacteroidetes was the predominant phylum in the fecal bacterial flora of all the groups (71.76, 76.01 and 51.16% for the PC, PT, and CC groups, respectively), and Firmicutes was the next most abundant phylum (26.72, 22.56 and 41.31% for the PC, PT, and CC groups, respectively; Fig. 2). At 8 dpi, however, Proteobacteria became the predominant phylum (44.37, 67.78 and 67.69%,for the PC, PT and CC groups, respectively), and the next most common phylum differed between the groups: Bacteroidetes, Actinobacteria and Firmicutes were the next most common phyla in the PC (30.09%), PT (24.94%) and CC (18.90%) groups (Fig. 2). Based on the results of the phylum levels, the fecal bacterial flora were identified at the family level (Table 6). Prevotellaceae, which belongs to the phylum Bacteroidetes, was the predominant family among the fecal bacterial flora found in all the groups at 2 dpi (64.495, 46.736 and 38.838% in the PC, PT, and CC groups, respectively; Table 6). At 8 dpi, Porphyromonadaceae (18.182%), which belongs to the phylum Bacteroidetes, and Xanthomonadaceae (12.885%) and Comamonadaceae (11.817%), which belong to the phylum Proteobacteria, were the predominant families in the fecal bacterial flora found in the PC group, whereas Xanthomonadaceae (18.070%) and Nocardiaceae (16.978%), which belong to the Actinobacteria phylum, and Comamonadaceae (15.010%) were the predominant families in the fecal bacterial flora found in the PT group (Table 6). However, Enterobacteriaceae (26.653%), which belongs to the phylum Proteobacteria and includes Salmonella species, was the predominant family in the fecal bacterial flora found in the CC group, and markedly lower percentages of Enterobacteriaceae were observed in the PC (2.292%) and PT (4.955%) groups at 8 dpi (Table 6).
DISCUSSION
The Salmonella serotypes London, Newport, Rissen, Schwanzengrund and Typhimurium have been isolated from human clinical samples in Korea. Among these, Typhimurium is the most common serotype that causes human salmonellosis [13]. In this study, Salmonella Typhimurium isolates exhibited markedly higher antibiotic resistance than other serotypes. Additionally, among 107 resistant isolates, 80 (74.8%) were MDR, and most of these were Salmonella Typhimurium (Table 4). These results are consistent with the results of Salmonella prevalence surveys conducted at swine farms in Korea [34]. Salmonella Typhimurium is an important public health concern because it is a zoonotic bacterium that is frequently isolated from swine production systems and exhibits high resistance to antibiotics [19, 20, 26]. Thus, a standard management protocol is required to avoid the introduction and transmission of Salmonella in swine farms and during processing. Previous studies have suggested that the direct feeding of bacteriophages to supplemented feeds or water, which results in decreased bacterial shedding and colonization, the spraying of bacteriophages in the environment to reduce bacterial contamination, or the rinsing of carcasses are practical and effective means for reducing transmission to humans [21, 25, 48]. Although Salmonella can be controlled by vaccines, bacteriophages are cost effective and as efficient as vaccines. Thus, bacteriophage treatments could be a good candidate for animal disease control [41].
In the present study, Salmonella-specific bacteriophage cocktails demonstrated broad-spectrum lytic capability against Salmonella reference strains and field isolates (Tables 1 and 2). Atterbury et al. [2] reported that among 232 Salmonella bacteriophages, three bacteriophages were effective against the broadest host range of the serotypes Enteritidis, Hadar and Typhimurium. Zhang et al. [52] reported that 10 bacteriophages were effective against Salmonella Typhimurium isolated from diseased pigs. Unexpectedly, among the 23 isolates of Salmonella Rissen, only 16 isolates (69.6%) were completely lysed with cocktails B and C, and Salmonella Essen (n=1), one of least frequently isolated serotypes in Korea, was not sensitive to any of the tested bacteriophage cocktails (Table 2). S. Rissen and S. Essen are generally detected in samples from pigs at a slaughterhouse rather than in clinical samples, as observed in previous studies. Therefore, the risk of clinical disease outbreaks caused by these particular isolates was considered very low [7, 8, 30]. In general, the bacteriophage cocktails B and C showed more efficient lytic capability against various Salmonella species than the other two cocktails. The bacteriophage cocktail B (≥1011 pfu/ml), which had the same bacteriophage components as cocktail A (≥109 pfu/ml) except for the bacteriophage titer, was more efficient against Salmonella than cocktail A. Bacteriophage cocktail C, which had a different bacteriophage titer and components compared with cocktail B, showed similar levels of lytic performance against Salmonella. Thus, these results demonstrated that the bacteriophage titer and bacteriophage components are important factors determining the lytic capability of bacteriophages. This result is similar to the in vivo results obtained in previous studies, in which the use of a high bacteriophage titer significantly reduced Salmonella colonization in chicken [2, 4, 24].
The distribution and duration of bacteriophages cocktail C in various organs, blood and feces of mice after oral inoculation with bacteriophages were investigated until 35 dpi. As a result, bacteriophages were detected in all the organs, blood and feces until 21 dpi and in the digestive organs (small and large intestine) of the mice until 28 dpi (Table 5). The detection of bacteriophages in all the tested organs might be due to bacteriophage delivery through the blood circulation. Therefore, further research is needed to evaluate the possibility of the induction of antibodies against bacteriophages during long-term bacteriophage treatment. In addition, even though the bacteriophages are quite specific for Salmonella spp., they might be able to replicate in other bacteria of the Enterobacteriaceae family at low levels because the orally injected bacteriophages were detected in intestines for a relatively long duration.
To evaluate the protective effects of bacteriophage cocktail C against Salmonella infection, we measured the levels of Salmonella shedding in feces until 8 dpi. The highest levels of Salmonella shedding were observed in the PT group at 2 dpi, Salmonella shedding gradually decreased from 3 dpi, and no Salmonella shedding was detected at 7 dpi. However, the levels of Salmonella shedding continued to increase from 6 until 8 dpi, which indicated that Salmonella had been successfully colonized and started replicating in the pig intestines (Fig. 1). Thus, this result demonstrated that bacteriophage cocktail C can efficiently resolve Salmonella infection in pigs. Callaway et al. [10] reported that the groups treated with a bacteriophage cocktail for 48 hr presented a decreased Salmonella population in the cecal and rectal intestinal contents of pigs compared with the controls. Jamalludeen et al. [28] reported that six bacteriophages were effective in enterotoxigenic Escherichia coli (ETEC)-challenged pigs, and six days after the inoculation, the bacteriophage-treated group showed a decreased average number of E. coli.
One of the most important changes in the pig intestinal microbial flora due to the administration of Salmonella-specific bacteriophages is the decline in the abundance of phyla and families containing Salmonella in the PT groups until 8 dpi. The PC and PT groups showed increases in the phylum-level percentages of mapped reads of Proteobacteria due to increases in Comamonadaceae and Xanthomonadaceae until 8 dpi. Enterobacteriaceae, which include Salmonella spp., was observed at a low percentage of mapped reads in the PT group, probably due to the effect of bacteriophage cocktail C. However, the CC group showed an increase in the phylum-level percentage of mapped reads of Proteobacteria due to an increase in Enterobacteriaceae until 8 dpi. As previously reported, dietary treatment of pigs with bacteriophages resulted in decreases in the Salmonella and E. coli concentrations in the fecal microflora compared with the concentrations obtained with a normal diet [50]. Thus, these metagenomic analyses revealed that treatment with Salmonella bacteriophage cocktails could reduce Salmonella colonization in pig intestines without causing any significant negative impacts on the normal intestinal flora (Table 6 and Fig. 2).
In conclusion, Salmonella-specific bacteriophage cocktails might be a safe and efficient tool for controlling Salmonella infection in pigs.
Supplementary Material
Acknowledgments
This study was supported by a grant from the Korea Institute of Planning and Evaluation for Technology of Food, Agriculture, Forestry and Fisheries (No. 1121314).
REFERENCES
- 1.Alisky J., Iczkowski K., Rapoport A., Troitsky N.1998. Bacteriophages show promise as antimicrobial agents. J. Infect. 36: 5–15. doi: 10.1016/S0163-4453(98)92874-2 [DOI] [PubMed] [Google Scholar]
- 2.Atterbury R. J., Van Bergen M. A., Ortiz F., Lovell M. A., Harris J. A., De Boer A., Wagenaar J. A., Allen V. M., Barrow P. A.2007. Bacteriophage therapy to reduce salmonella colonization of broiler chickens. Appl. Environ. Microbiol. 73: 4543–4549. doi: 10.1128/AEM.00049-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Authority E.2011. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2009. EFSA J. 9: 2090. doi: 10.2903/j.efsa.2011.2090 [DOI] [Google Scholar]
- 4.Bardina C., Spricigo D. A., Cortés P., Llagostera M.2012. Significance of the bacteriophage treatment schedule in reducing Salmonella colonization of poultry. Appl. Environ. Microbiol. 78: 6600–6607. doi: 10.1128/AEM.01257-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrow P., Lovell M., Berchieri A., Jr.1998. Use of lytic bacteriophage for control of experimental Escherichia coli septicemia and meningitis in chickens and calves. Clin. Diagn. Lab. Immunol. 5: 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berends B. R., van den Bogaard A. E., Van Knapen F., Snijders J. M.2001. Human health hazards associated with the administration of antimicrobials to slaughter animals. Part II. An assessment of the risks of resistant bacteria in pigs and pork. Vet. Q. 23: 10–21. doi: 10.1080/01652176.2001.9695069 [DOI] [PubMed] [Google Scholar]
- 7.Bonardi S.2017. Salmonella in the pork production chain and its impact on human health in the European Union. Epidemiol. Infect. 145: 1513–1526. doi: 10.1017/S095026881700036X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonardi S., Alpigiani I., Bruini I., Barilli E., Brindani F., Morganti M., Cavallini P., Bolzoni L., Pongolini S.2016. Detection of Salmonella enterica in pigs at slaughter and comparison with human isolates in Italy. Int. J. Food Microbiol. 218: 44–50. doi: 10.1016/j.ijfoodmicro.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 9.Botteldoorn N., Herman L., Rijpens N., Heyndrickx M.2004. Phenotypic and molecular typing of Salmonella strains reveals different contamination sources in two commercial pig slaughterhouses. Appl. Environ. Microbiol. 70: 5305–5314. doi: 10.1128/AEM.70.9.5305-5314.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callaway T. R., Edrington T. S., Brabban A., Kutter B., Karriker L., Stahl C., Wagstrom E., Anderson R., Poole T. L., Genovese K., Krueger N., Harvey R., Nisbet D. J.2011. Evaluation of phage treatment as a strategy to reduce Salmonella populations in growing swine. Foodborne Pathog. Dis. 8: 261–266. doi: 10.1089/fpd.2010.0671 [DOI] [PubMed] [Google Scholar]
- 11.Cardona-Castro N., Sánchez-Jiménez M., Lavalett L., Múñoz N., Moreno J.2009. Development and evaluation of a multiplex polymerase chain reaction assay to identify Salmonella serogroups and serotypes. Diagn. Microbiol. Infect. Dis. 65: 327–330. doi: 10.1016/j.diagmicrobio.2009.07.003 [DOI] [PubMed] [Google Scholar]
- 12.Castanon J. I.2007. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 86: 2466–2471. doi: 10.3382/ps.2007-00249 [DOI] [PubMed] [Google Scholar]
- 13.Cheong H. J., Lee Y. J., Hwang I. S., Kee S. Y., Cheong H. W., Song J. Y., Kim J. M., Park Y. H., Jung J. H., Kim W. J.2007. Characteristics of non-typhoidal Salmonella isolates from human and broiler-chickens in southwestern Seoul, Korea. J. Korean Med. Sci. 22: 773–778. doi: 10.3346/jkms.2007.22.5.773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho Y. I., Kim W. I., Liu S., Kinyon J. M., Yoon K. J.2010. Development of a panel of multiplex real-time polymerase chain reaction assays for simultaneous detection of major agents causing calf diarrhea in feces. J. Vet. Diagn. Invest. 22: 509–517. doi: 10.1177/104063871002200403 [DOI] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. 2013. Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Third Informational (Supplement): M100–S23CLSI, Wayne. [Google Scholar]
- 16.Connerton P., Connerton I., Mead G.2005. Microbial treatments to reduce pathogens in poultry meat. pp. 414–427. In: Food Safety Control in the Poultry Industry, Woodhead Publishing Ltd., Cambridge. [Google Scholar]
- 17.Dibner J. J., Richards J. D.2005. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 84: 634–643. doi: 10.1093/ps/84.4.634 [DOI] [PubMed] [Google Scholar]
- 18.Fiorentin L., Vieira N. D., Barioni W., Jr.2005. Oral treatment with bacteriophages reduces the concentration of Salmonella Enteritidis PT4 in caecal contents of broilers. Avian Pathol. 34: 258–263. doi: 10.1080/01445340500112157 [DOI] [PubMed] [Google Scholar]
- 19.Futagawa-Saito K., Hiratsuka S., Kamibeppu M., Hirosawa T., Oyabu K., Fukuyasu T.2008. Salmonella in healthy pigs: prevalence, serotype diversity and antimicrobial resistance observed during 1998−1999 and 2004−2005 in Japan. Epidemiol. Infect. 136: 1118–1123. doi: 10.1017/S0950268807009570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García-Feliz C., Collazos J. A., Carvajal A., Herrera S., Echeita M. A., Rubio P.2008. Antimicrobial resistance of Salmonella enterica isolates from apparently healthy and clinically ill finishing pigs in Spain. Zoonoses Public Health 55: 195–205. doi: 10.1111/j.1863-2378.2008.01110.x [DOI] [PubMed] [Google Scholar]
- 21.Gebru E., Lee J. S., Son J. C., Yang S. Y., Shin S. A., Kim B., Kim M. K., Park S. C.2010. Effect of probiotic-, bacteriophage-, or organic acid-supplemented feeds or fermented soybean meal on the growth performance, acute-phase response, and bacterial shedding of grower pigs challenged with Salmonella enterica serotype Typhimurium. J. Anim. Sci. 88: 3880–3886. doi: 10.2527/jas.2010-2939 [DOI] [PubMed] [Google Scholar]
- 22.Greer G. G.2005. Bacteriophage control of foodborne bacteriat. J. Food Prot. 68: 1102–1111. doi: 10.4315/0362-028X-68.5.1102 [DOI] [PubMed] [Google Scholar]
- 23.Harada K., Usui M., Asai T.2014. Application of enrofloxacin and orbifloxacin disks approved in Japan for susceptibility testing of representative veterinary respiratory pathogens. J. Vet. Med. Sci. 76: 1427–1430. doi: 10.1292/jvms.14-0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins J. P., Higgins S. E., Guenther K. L., Huff W., Donoghue A. M., Donoghue D. J., Hargis B. M.2005. Use of a specific bacteriophage treatment to reduce Salmonella in poultry products. Poult. Sci. 84: 1141–1145. doi: 10.1093/ps/84.7.1141 [DOI] [PubMed] [Google Scholar]
- 25.Hooton S. P., Atterbury R. J., Connerton I. F.2011. Application of a bacteriophage cocktail to reduce Salmonella Typhimurium U288 contamination on pig skin. Int. J. Food Microbiol. 151: 157–163. doi: 10.1016/j.ijfoodmicro.2011.08.015 [DOI] [PubMed] [Google Scholar]
- 26.Huang T. M., Lin T. L., Wu C. C.2009. Serovar distribution and antimicrobial susceptibility of swine Salmonella isolates from clinically ill pigs in diagnostic submissions from Indiana in the United States. Lett. Appl. Microbiol. 48: 331–336. doi: 10.1111/j.1472-765X.2008.02530.x [DOI] [PubMed] [Google Scholar]
- 27.Hurd H. S., Enøe C., Sørensen L., Wachmann H., Corns S. M., Bryden K. M., Grenier M.2008. Risk-based analysis of the Danish pork Salmonella program: past and future. Risk Anal. 28: 341–351. doi: 10.1111/j.1539-6924.2008.01034.x [DOI] [PubMed] [Google Scholar]
- 28.Jamalludeen N., Johnson R. P., Shewen P. E., Gyles C. L.2009. Evaluation of bacteriophages for prevention and treatment of diarrhea due to experimental enterotoxigenic Escherichia coli O149 infection of pigs. Vet. Microbiol. 136: 135–141. doi: 10.1016/j.vetmic.2008.10.021 [DOI] [PubMed] [Google Scholar]
- 29.Jung H. K., Lee S. S., Kim C. Y., Sunwoo S. Y., Lyoo Y. S.2011. Serovars distribution and antimicrobial resistance patterns of Salmonella spp. isolated from the swine farms and slaughter houses. Korean J. Vet. Res. 51: 123–128. [Google Scholar]
- 30.Keelara S., Scott H. M., Morrow W. M., Gebreyes W. A., Correa M., Nayak R., Stefanova R., Thakur S.2013. Longitudinal study of distributions of similar antimicrobial-resistant Salmonella serovars in pigs and their environment in two distinct swine production systems. Appl. Environ. Microbiol. 79: 5167–5178. doi: 10.1128/AEM.01419-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim E. M., Kim H. K., Park S. J., Lee C. S., Luo Y., Moon H. J., Yang J. S., Park B. K.2007. Prevalence and antimicrobial resistance patterns of Salmonella spp. isolated from different aged pigs in Korea. Korean J. Vet. Res. 47: 395–398. [Google Scholar]
- 32.Kutter E.1997. Phage Therapy: Bacteriophages As Antibiotics. Evergreen State College, Olympia. [Google Scholar]
- 33.Lee W. C., Lee M. J., Kim J. S., Park S. Y.2001. Foodborne illness outbreaks in Korea and Japan studied retrospectively. J. Food Prot. 64: 899–902. doi: 10.4315/0362-028X-64.6.899 [DOI] [PubMed] [Google Scholar]
- 34.Lim S. K., Lee H. S., Nam H. M., Jung S. C., Koh H. B., Roh I. S.2009. Antimicrobial resistance and phage types of Salmonella isolates from healthy and diarrheic pigs in Korea. Foodborne Pathog. Dis. 6: 981–987. doi: 10.1089/fpd.2009.0293 [DOI] [PubMed] [Google Scholar]
- 35.Lynch M., Painter J., Woodruff R., Braden C.2006. Surveillance for Foodborne: Disease Outbreaks: United States, 1998–2002. United States Department of Health and Human Services, Washington, D.C. [PubMed] [Google Scholar]
- 36.Marčuk L. M., Nikiforov V. N., Ščerbak J. F., Levitov T. A., Kotljarova R. I., Naumšina M. S., Davydov S. U., Monsur K. A., Rahman M. A., Latif M. A., Northrup R. S., Cash R. A., Hug I., Dey C. R., Phillips R. A.1971. Clinical studies of the use of bacteriophage in the treatment of cholera. Bull. World Health Organ. 45: 77–83. [PMC free article] [PubMed] [Google Scholar]
- 37.McDowell S. W., Porter R., Madden R., Cooper B., Neill S. D.2007. Salmonella in slaughter pigs in Northern Ireland: prevalence and use of statistical modelling to investigate sample and abattoir effects. Int. J. Food Microbiol. 118: 116–125. doi: 10.1016/j.ijfoodmicro.2007.05.010 [DOI] [PubMed] [Google Scholar]
- 38.Mejía W., Casal J., Zapata D., Sánchez G. J., Martín M., Mateu E.2006. Epidemiology of salmonella infections in pig units and antimicrobial susceptibility profiles of the strains of Salmonella species isolated. Vet. Rec. 159: 271–276. doi: 10.1136/vr.159.9.271 [DOI] [PubMed] [Google Scholar]
- 39.Morgan I. R., Krautil F. L., Craven J. A.1987. Effect of time in lairage on caecal and carcass salmonella contamination of slaughter pigs. Epidemiol. Infect. 98: 323–330. doi: 10.1017/S0950268800062075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salipante S. J., Sengupta D. J., Rosenthal C., Costa G., Spangler J., Sims E. H., Jacobs M. A., Miller S. I., Hoogestraat D. R., Cookson B. T., McCoy C., Matsen F. A., Shendure J., Lee C. C., Harkins T. T., Hoffman N. G.2013. Rapid 16S rRNA next-generation sequencing of polymicrobial clinical samples for diagnosis of complex bacterial infections. PLoS One 8: e65226. doi: 10.1371/journal.pone.0065226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shryock T. R.2004. The future of anti-infective products in animal health. Nat. Rev. Microbiol. 2: 425–430. doi: 10.1038/nrmicro887 [DOI] [PubMed] [Google Scholar]
- 42.Smith H. W., Huggins M. B.1983. Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets and lambs. J. Gen. Microbiol. 129: 2659–2675. [DOI] [PubMed] [Google Scholar]
- 43.Son J. S., Jun S. Y., Kim E. B., Park J. E., Paik H. R., Yoon S. J., Kang S. H., Choi Y. J.2010. Complete genome sequence of a newly isolated lytic bacteriophage, EFAP-1 of Enterococcus faecalis, and antibacterial activity of its endolysin EFAL-1. J. Appl. Microbiol. 108: 1769–1779. doi: 10.1111/j.1365-2672.2009.04576.x [DOI] [PubMed] [Google Scholar]
- 44.Sulakvelidze A., Alavidze Z., Morris J. G., Jr.2001. Bacteriophage therapy. Antimicrob. Agents Chemother. 45: 649–659. doi: 10.1128/AAC.45.3.649-659.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sulakvelidze A., Barrow P.2005. Phage therapy in animals and agribusiness. pp. 335–380. In: Bacteriophages: Biology and Applications (Kutter, E. and Sulakvelidze, A. eds.), CRC Press, Boca Raton. [Google Scholar]
- 46.Summers W. C.2001. Bacteriophage therapy. Annu. Rev. Microbiol. 55: 437–451. doi: 10.1146/annurev.micro.55.1.437 [DOI] [PubMed] [Google Scholar]
- 47.Tollefson L., Flynn W. T.2002. Impact of antimicrobial resistance on regulatory policies in veterinary medicine: status report. AAPS PharmSci 4: E37. doi: 10.1208/ps040437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wall S. K., Zhang J., Rostagno M. H., Ebner P. D.2010. Phage therapy to reduce preprocessing Salmonella infections in market-weight swine. Appl. Environ. Microbiol. 76: 48–53. doi: 10.1128/AEM.00785-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whiteley A. S., Jenkins S., Waite I., Kresoje N., Payne H., Mullan B., Allcock R., O’Donnell A.2012. Microbial 16S rRNA Ion Tag and community metagenome sequencing using the Ion Torrent (PGM) Platform. J. Microbiol. Methods 91: 80–88. doi: 10.1016/j.mimet.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 50.Yan L., Hong S. M., Kim I. H.2012. Effect of bacteriophage supplementation on the growth performance, nutrient digestibility, blood characteristics, and fecal microbial shedding in growing pigs. Asian-Australas. J. Anim. Sci. 25: 1451–1456. doi: 10.5713/ajas.2012.12253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoichi M., Morita M., Mizoguchi K., Fischer C. R., Unno H., Tanji Y.2004. The criterion for selecting effective phage for Escherichia coli O157: H7 control. Biochem. Eng. J. 19: 221–227. doi: 10.1016/j.bej.2004.02.001 [DOI] [Google Scholar]
- 52.Zhang J., Kraft B. L., Pan Y., Wall S. K., Saez A. C., Ebner P. D.2010. Development of an anti-Salmonella phage cocktail with increased host range. Foodborne Pathog. Dis. 7: 1415–1419. doi: 10.1089/fpd.2010.0621 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.