Abstract
Allyl isothiocyanate (AITC), a metabolite of the glucosinolate sinigrin, protects the liver of rats injured by carbon tetrachloride (CCl4). This study evaluated whether AITC reduces hepatic fibrosis in rats repetitively exposed to CCl4. Serum chemistry showed that AITC (doses of 5 and 50 mg) administered to rats exposed to CCl4 significantly reduced the levels of alanine aminotransferase and aspartate aminotransferase activity that were elevated in CCl4-intoxicated rats. The connective tissue in AITC-treated rats was significantly reduced based on Sirius staining. In addition, Kupffer cell activation was significantly reduced in the AITC and CCl4 co-treated groups. Collectively, this study suggests that AITC mitigates hepatic fibrosis in rats repetitively exposed to CCl4 with concurrent regulation of Kupffer cell and monocyte activation.
Keywords: allyl isothiocyanate, carbon tetrachloride, fibrosis, Kupffer cell
Allyl isothiocyanate (AITC) is a hydrolysis product of glucosinolates [6] which are constituents of cruciferous vegetables such as radish [12]. AITC possesses antioxidant [5], anti-inflammatory [13], and anti-lipogenic/adipogenic effects [9], as well as anticancer activities [14]. The metabolic sequence of AITC has been precisely studied in a rat model [9], which showed that AITC is metabolized in the liver and excreted in urine, suggesting that AITC may be more effective in the liver and urinary system.
Carbon tetrachloride (CCl4) is a potent hepatotoxicant that produces centrilobular hepatic necrosis in animals after a single injection [4], with concurrent activation of Kupffer cells [4] and subsequent fibrosis. It is used widely as a model to test antioxidative compounds and plant extracts containing antioxidants [1, 2].
AITC had a hepatoprotective role, via antioxidant mechanisms, in an acute CCl4-injured rat model [1]. However, little is known regarding the role of AITC in hepatic fibrosis, which is a major consequence of liver damage. Therefore, the aim of this study is to evaluate whether daily uptake of AITC affects the progression of hepatic fibrosis caused by chronic oxidative stress in a CCl4 injury model.
All chemicals, including AITC (Cat. 377430, Lot. MKBS7089V) and reagents, unless otherwise noted, were of analytical grade and were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). The commercial reagent kits for the determination of serum chemistry were obtained from Beckman Coulter Ireland Inc. (Clare, Ireland).
Sprague-Dawley rats (male, 6–8 weeks old) weighing 200–300 g were used for all experiments (Orient Bio, Gyeonggi-do, Korea). The animals were maintained at a controlled temperature of 22–26°C under a 12/12-hr light/dark cycle and fed a standard diet and water ad libitum. All experimental procedures were conducted in accordance with the guidelines for the Care and Use of Laboratory Animals at Jeju National University, Jeju City, Republic of Korea (permit number: 2016-0040). Rats were divided into five groups (n=5 animals per group): normal control; AITC (50 mg/kg) alone (AITC50 group); AITC (5 mg/kg) and CCl4 (AITC5 + CCl4 group); AITC (50 mg/kg) and CCl4 (AITC50 + CCl4 group); and vehicle and CCl4 (vehicle + CCl4 group). The AITC dose was that of our previous study [1]. AITC dissolved in phosphate-buffered saline (pH 7.4) was administered daily in rats for 14 days. Liver injury was induced every 72 hr via the intraperitoneal administration of a 1:1 (v/v) mixture of CCl4 (1 ml/kg) and sterile olive oil, a total of five times [2, 7]. CCl4 was injected 3 hr after administration of AITC and vehicle. Rats were fasted for 24 hr after final administration of AITC or vehicle and then sacrificed. Blood samples and liver tissues were collected at the time of sacrifice for serum chemistry and tissue examination, respectively. The laboratory examination described below followed the procedures used in our previous CCl4-induced liver injury studies [1, 2]. The liver tissues were fixed immediately in 10% neutral buffered formalin solution for 2 days and processed routinely for paraffin wax embedding. The liver tissues were cut into 5-µm-thick sections. After deparaffinization, sections were stained with hematoxylin and eosin solution (Sigma-Aldrich) and used for immunohistochemistry. In addition, Sirius Red (Direct Red 80, Cat. 365548; Sigma-Aldrich) staining was performed for fibrillar collagen. We subjected fixed frozen sections to Oil-Red-O staining to reveal fatty changes.
Blood samples (n=5 animals per group) were allowed to coagulate at room temperature and were centrifuged (3,000 rpm, 10 min, room temperature), and the serum fraction was collected. The serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were detected using a FUJI DRI-CHEM 4200 system (FUJIFILM, Tokyo, Japan).
To evaluate Kupffer cell and macrophage activation in hepatic tissue, immunohistochemistry using rabbit anti-ionized calcium binding protein-1 (Iba-1, 1 µg/ml; Cat. 019-19741, Lot. LKH4161; Wako Pure Chemical Industries, Ltd., Osaka, Japan) was performed in liver tissue using the VECTASTAIN ABC Elite Kit (Vector Labs, Burlingame, CA, U.S.A.), as described previously [2]. Quantitative analysis of Iba-1-immunostained areas (centrilobular regions) and Sirius Red-stained areas (n=3 animals per group) were performed with the aid of ImageJ software (NIH, Bethesda, MD, U.S.A.). At least five regions of each liver section were photographed. The Iba-1- and Sirius Red-positive area [(positive area/total area) ×100 (%)] were shown as the mean ± standard error of the mean (SEM). In addition, we counted the number of Iba-1-positive cells in 5 different centilobular regions of each liver section (n=3 animals per group). Data were subjected to one-way analysis of variance followed by the Student–Newman–Keuls post hoc test for multiple comparisons. In all cases, P values <0.05 were considered to indicate statistical significance.
To evaluate the effect of AITC on CCl4-induced liver injury, we performed a serum chemistry analysis, including of AST and ALT levels (Table 1). The AST and ALT levels in the AITC50 group were within the normal range (AST, 328.38 ± 17.93; ALT, 62.18 ± 7.45). Both AST (1,691.9 ± 190.27) and ALT (1,766.17 ± 157.81) levels in the vehicle + CCl4 group were significantly increased compared with the normal control group (315 ± 22.82, 54.98 ± 5.1, P<0.001). However, the ALT level (1,118.7 ± 157.67) in the AITC5 + CCl4 group was significantly reduced (P<0.05 vs. vehicle + CCl4 group). Furthermore, the AITC50 + CCl4 group showed a significant decrease in both AST (917.45 ± 237.05, P<0.05) and ALT (235.37 ± 55.45, P<0.001) levels compared with the vehicle + CCl4 group. These results suggest that AITC has an ameliorative effect on CCl4-induced liver injury.
Table 1. Effects of AITC on hepatotoxicity in CCl4-intoxicated rats.
| Group | Hepatotoxicity | |
|---|---|---|
| AST (IU/l) | ALT (IU/l) | |
| Normal control | 315.00 ± 22.82 | 54.98 ± 5.1 |
| AITC50 | 328.38 ± 17.93 | 62.18 ± 7.45 |
| Vehicle + CCl4 | 1,691.90 ± 190.27a,c) | 1,766.17 ± 157.81a,c) |
| AITC5 + CCl4 | 1,634.46 ± 261.92a,c) | 1,118.70 ± 157.67a,c,d) |
| AITC50 + CCl4 | 917.45 ± 237.05a,d) | 235.37 ± 55.45b,d) |
Values are means ± SEM. a) P<0.001 vs. normal control group. b) P<0.05 vs. AITC50 group. c) P<0.001 vs. AITC50 group. d) P<0.001 vs. Vehicle + CCl4 group. AITC, allyl isothiocyanate; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
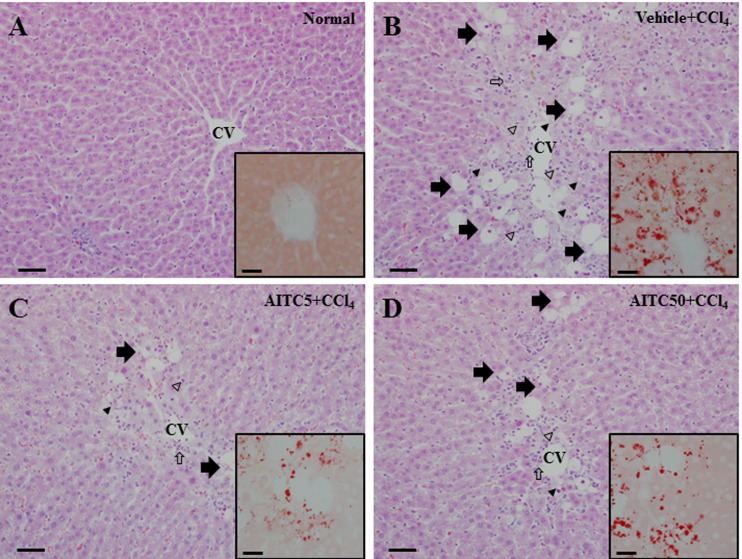
In the normal control group, the liver tissue exhibited a regular arrangement of hepatocytes and no cytopathogenic changes (Fig. 1A). Meanwhile, the livers of rats in the vehicle + CCl4 group showed extensive hydropic degeneration (arrows), vacuolar degeneration (arrowheads), necrosis (hollow arrowheads), and infiltration of inflammatory cells (hollow arrows) (Fig. 1B). Compared with the liver tissue of the vehicle + CCl4 group, the hepatic damage in the AITC5 and AITC50 + CCl4 groups was reduced (Fig. 1C and 1D). Oil-Red-O staining, which measures fatty changes, confirmed that AITC mitigated the histopathological changes in the liver caused by CCl4-induced injury.
Fig. 1.
Histopathological examination of the liver of normal control rats and carbon tetrachloride (CCl4)-treated rats pretreated or not with allyl isothiocyanate (AITC). Insets: Higher-magnification views of Oil-Red-O-stained areas. Arrows, hydropic degeneration; arrowheads, vacuolation; hollow arrows, inflammatory cells; hollow arrowheads, necrosis; CV, central vein. Scale bars=50 µm (A–D). Scale bars in insets=20 µm.
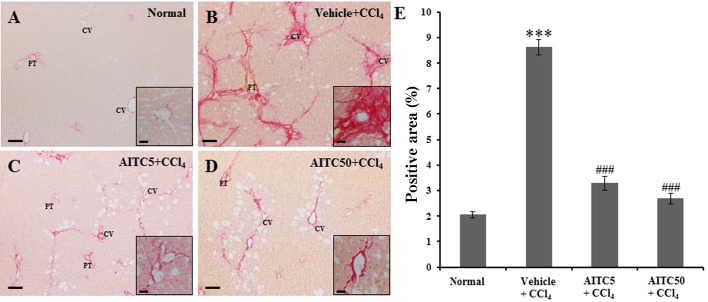
To evaluate the effect of AITC treatment on hepatic fibrosis in CCl4-induced liver injury, we performed Sirius Red staining. In the normal control group, Sirius Red-positive collagen fibers were detected around the portal triad regions (Fig. 2A). However, collagen tissue proliferation by fibrosis was observed in the livers of CCl4-injured rats (Fig. 2B). AITC treatment reduced the increase in collagen tissue proliferation (Fig. 2C and 2D), compared with the CCl4-injured rat liver tissue. The quantitative analysis of the Sirius Red-positive area using ImageJ confirmed the histopathological findings. In the vehicle + CCl4 group, the Sirius Red-positive area was significantly increased (8.62 ± 0.3%, P<0.001) compared with that of the normal control group. The AITC5 + CCl4 (3.28 ± 0.28%, P<0.001) and AITC50 + CCl4 (2.68 ± 0.19%, P<0.001) groups showed significantly decreased areas of Sirius Red-positive collagen tissue compared with the liver tissue in the vehicle + CCl4 group (Fig. 2E).
Fig. 2.
(A–D) Representative micrographs of Sirius Red-stained collagen fibers. (E) Quantitative analysis of the Sirius Red-positive regions in rat liver. Insets: Higher-magnification images. CV, central vein; PT, portal triad. ***P<0.001 vs. the control group. ###P<0.001 vs. the vehicle + CCl4 group. Scale bars=100 µm (A–D). Scale bars in insets=20 µm.
Kupffer cell and inflammatory cell activity in the liver tissue of CCl4-intoxicated rats is an important indicator of liver toxicity [1]. To evaluate macrophage activity, we performed immunostaining with Iba-1 antibody. In the hepatic tissues of the vehicle + CCl4 group, Iba-1-positive cells were markedly increased around the central vein (Fig. 3B) compared with the normal control group (Fig. 3A), and the intensity decreased with AITC treatment (Figs. 3C and 3D). The Iba-1-positive area in the vehicle + CCl4 group was significantly increased (17.80 ± 2.16%, P<0.001) compared with the normal control group. However, both the AITC5 + CCl4 (12.18 ± 0.49%, P<0.001) and AITC50 + CCl4 (11.39 ± 0.40%, P<0.001) groups showed significantly decreased areas compared to the vehicle + CCl4 group (Fig. 3E). Furthermore, the number of Iba-1-positive cells in the vehicle + CCl4 group was significantly greater (70.73 ± 3.46, P<0.01) than that in the normal control group (13.33 ± 0.37). However, these numbers were significantly reduced in the AITC5 + CCl4 (40.87 ± 5.16, P<0.05) and AITC50 + CCl4 (41.60 ± 3.49, P<0.05) groups (Fig. 3F).
Fig. 3.
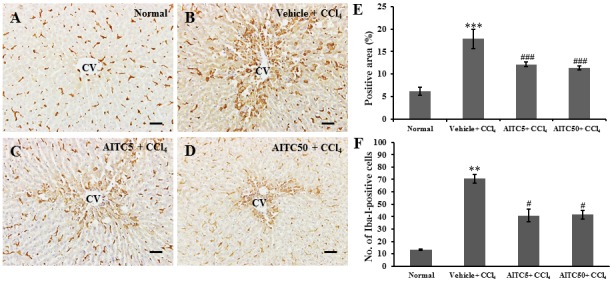
(A–D) Representative immunomicrographs of ionized calcium-binding adapter molecule 1 (Iba-1) and (E and F) Quantitative analysis of Iba-1-positive area and the number of Iba-1-positive cells in rat liver. **P<0.01, ***P<0.001 vs. the normal control group. #P<0.05, ###P<0.001 vs. the vehicle + CCl4 group. CV, central vein. Scale bars=50 µm (A–D).
In our previous study, we evaluated hepatoprotective effects, including the activation of antioxidant enzymes, radical scavenging, suppression of activated hepatic macrophages, and heme oxygenase-1 accumulation, in CCl4-induced acute liver injury [1]. In the present study, we confirmed the inhibitory effect of AITC, which is a metabolite of the glucosinolate sinigrin found in cruciferous vegetables (e.g., radish), against hepatic fibrosis, and its hepatoprotective effect (via antioxidant activity) against CCl4-induced liver injury. Serum AST and ALT levels, which are hepatotoxicity markers, decreased significantly in a dose-dependent manner in the AITC (5 and 50 mg/kg) + CCl4 groups compared to the vehicle + CCl4 group. Furthermore, the AITC + CCl4 groups showed a reduction in hepatic damage, such as hydropic degeneration, vacuolar degeneration, infiltration of inflammatory cells, and disappearance of hepatocyte arrangement (Fig. 2), based on the histopathological examination. These results suggested that hepatic tissue damage caused by CCl4-induced liver injury was alleviated by AITC.
Kupffer cell and macrophage activation accompany CCl4-induced liver injury [1, 2, 8] and are detected by Iba-1 immunostaining [11]. In a previous study of acute CCl4 liver injury, AITC pretreatment inhibited inflammatory responses, including by suppression of Kupffer cell and macrophage activation and reduction of pro-inflammatory cytokine expression [1]. In addition, AITC ameliorated mucosal inflammation in a dextran sulfate sodium-induced acute colitis model via a reduction in macrophage infiltration [3]. In the present study, we confirmed that AITC ameliorated Kupffer cell activation and the inflammatory response (e.g., macrophage infiltration) based on a decrease in Iba-1-postive area in the liver. This indicates that AITC also ameliorates the inflammatory response in CCl4-induced liver injury.
Hepatic fibrosis has been known to be associated with inflammation of liver [7], which is also caused by repeated administration of CCl4 in rats [2, 7]. Therefore, suppression of hepatic inflammation is pivotal to reduce the hepatic fibrosis. AITC has been known to ameliorate oxidative stress and fatty changes in a single CCl4-induced liver injury model [1], and also to reduce fibrosis in carboxymethyl-lysine-treated hepatic stellate cells via the suppression of α-smooth muscle actin mRNA [10]. Collectively, these findings suggest that AITC treatment reduces fibrosis in the liver with repeated exposure of CCl4 in rats.
Acknowledgments
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agri-Bio industry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (Grant number: 316006-05-1-HD040).
REFERENCES
- 1.Ahn M., Kim J., Bang H., Moon J., Kim G. O., Shin T.2016. Hepatoprotective effects of allyl isothiocyanate against carbon tetrachloride-induced hepatotoxicity in rat. Chem. Biol. Interact. 254: 102–108. doi: 10.1016/j.cbi.2016.05.037 [DOI] [PubMed] [Google Scholar]
- 2.Ahn M., Park J. S., Chae S., Kim S., Moon C., Hyun J. W., Shin T.2014. Hepatoprotective effects of Lycium chinense Miller fruit and its constituent betaine in CCl4-induced hepatic damage in rats. Acta Histochem. 116: 1104–1112. doi: 10.1016/j.acthis.2014.05.004 [DOI] [PubMed] [Google Scholar]
- 3.Davaatseren M., Hwang J. T., Park J. H., Kim M. S., Wang S., Sung M. J.2014. Allyl isothiocyanate ameliorates angiogenesis and inflammation in dextran sulfate sodium-induced acute colitis. PLoS One 9: e102975. doi: 10.1371/journal.pone.0102975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards M. J., Keller B. J., Kauffman F. C., Thurman R. G.1993. The involvement of Kupffer cells in carbon tetrachloride toxicity. Toxicol. Appl. Pharmacol. 119: 275–279. doi: 10.1006/taap.1993.1069 [DOI] [PubMed] [Google Scholar]
- 5.Ernst I. M., Wagner A. E., Schuemann C., Storm N., Höppner W., Döring F., Stocker A., Rimbach G.2011. Allyl-, butyl- and phenylethyl-isothiocyanate activate Nrf2 in cultured fibroblasts. Pharmacol. Res. 63: 233–240. doi: 10.1016/j.phrs.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 6.Hanschen F. S., Lamy E., Schreiner M., Rohn S.2014. Reactivity and stability of glucosinolates and their breakdown products in foods. Angew. Chem. Int. Ed. Engl. 53: 11430–11450. doi: 10.1002/anie.201402639 [DOI] [PubMed] [Google Scholar]
- 7.Islam M. A., Al Mamun M. A., Faruk M., Ul Islam M. T., Rahman M. M., Alam M. N., Rahman A. F. M. T., Reza H. M., Alam M. A.2017. Astaxanthin Ameliorates Hepatic Damage and Oxidative Stress in Carbon Tetrachloride-administered Rats. Pharmacognosy Res. 9Suppl 1: S84–S91. doi: 10.4103/pr.pr_26_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawashima R., Mochida S., Matsui A., YouLuTuZ Y., Ishikawa K., Toshima K., Yamanobe F., Inao M., Ikeda H., Ohno A., Nagoshi S., Uede T., Fujiwara K.1999. Expression of osteopontin in Kupffer cells and hepatic macrophages and Stellate cells in rat liver after carbon tetrachloride intoxication: a possible factor for macrophage migration into hepatic necrotic areas. Biochem. Biophys. Res. Commun. 256: 527–531. doi: 10.1006/bbrc.1999.0372 [DOI] [PubMed] [Google Scholar]
- 9.Kim Y. J., Lee D. H., Ahn J., Chung W. J., Jang Y. J., Seong K. S., Moon J. H., Ha T. Y., Jung C. H.2015. Pharmacokinetics, Tissue Distribution, and Anti-Lipogenic/Adipogenic Effects of Allyl-Isothiocyanate Metabolites. PLoS One 10: e0132151. doi: 10.1371/journal.pone.0132151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee B. H., Hsu W. H., Hsu Y. W., Pan T. M.2013. Suppression of dimerumic acid on hepatic fibrosis caused from carboxymethyl-lysine (CML) by attenuating oxidative stress depends on Nrf2 activation in hepatic stellate cells (HSCs). Food Chem. Toxicol. 62: 413–419. doi: 10.1016/j.fct.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 11.Pervin M., Golbar H. M., Bondoc A., Izawa T., Kuwamura M., Yamate J.2016. Immunophenotypical characterization and influence on liver homeostasis of depleting and repopulating hepatic macrophages in rats injected with clodronate. Exp. Toxicol. Pathol. 68: 113–124. doi: 10.1016/j.etp.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 12.Srivastava S. K., Xiao D., Lew K. L., Hershberger P., Kokkinakis D. M., Johnson C. S., Trump D. L., Singh S. V.2003. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits growth of PC-3 human prostate cancer xenografts in vivo. Carcinogenesis 24: 1665–1670. doi: 10.1093/carcin/bgg123 [DOI] [PubMed] [Google Scholar]
- 13.Wagner A. E., Boesch-Saadatmandi C., Dose J., Schultheiss G., Rimbach G.2012. Anti-inflammatory potential of allyl-isothiocyanate--role of Nrf2, NF-(κ) B and microRNA-155. J. Cell. Mol. Med. 16: 836–843. doi: 10.1111/j.1582-4934.2011.01367.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y.2010. Allyl isothiocyanate as a cancer chemopreventive phytochemical. Mol. Nutr. Food Res. 54: 127–135. doi: 10.1002/mnfr.200900323 [DOI] [PMC free article] [PubMed] [Google Scholar]