Abstract
Although several Edwardsiella tarda infections have been reported, its pathogenic role in marine mammals has not been investigated at the genome level. We investigated the genome of E. tarda strain KC-Pc-HB1, isolated from the false killer whale (Pseudorca crassidens) found bycaught in South Korea. The obtained genome was similar to that of human pathogenic E. tarda strains, but distinct from other Edwardsiella species. Although type III and VI secretion systems, which are essential for the virulence of other Edwardsiella species, were absent, several virulence-related genes involved in the pathogenesis of E. tarda were found in the genome. These results provide important insights into the E. tarda infecting marine mammals and give valuable information on potential virulence factors in this pathogen.
Keywords: Edwardsiella tarda, marine mammal, pathogen, virulence factor
The genus Edwardsiella, which is a member of the family Enterobacteriaceae (Proteobacteria: Gammaproteobacteria), comprises five valid species, namely, E. anguillarum, E. hoshinae, E. ictaluri, E. piscicida and E. tarda [26]. Among those species, E. tarda is considered a pathogenic inhabitant of animals including fish, reptiles, amphibians, and birds and is associated with opportunistic zoonotic infections in humans [1]. However, the classification of E. tarda has been a source of controversy, and therefore, several phenotypic and genetic analyses, including whole genome sequencing, have been conducted to understand the diversity and pathogenicity of this bacterium [5, 14, 23, 24, 26]. These studies demonstrated that isolates historically classified as E. tarda actually represent three genetically distinct taxa with various degrees of pathogenicity in different hosts, and almost all the pathogenic fish isolates were re-assigned as E. anguillarum and E. piscicida [5, 24]. Nevertheless, bacteria currently defined as E. tarda still contain several fish pathogenic isolates originating from disease outbreaks in catfish aquaculture in the 1970s and 1980s [24], as well as the human pathogenic strain ATCC 23685 [23], thus suggesting that bona fide E. tarda might have zoonotic potential.
Although several pathogens were recently recognized as causative agents of emerging infectious diseases in marine mammals [31, 32], little information is currently available on bacterial infections that might pose human health risks. Among those, E. tarda has been considered an opportunistic pathogen presumed to cause illness or death in the sperm whale (Physeter macrocephalus) [9], killer whale (Orcinus orca) [13], beluga whale (Delphinapterus leucas) [17], harbor porpoise (Phocena phocena) [8], and pinnipeds [21]. However, its pathogenic role in wild marine mammals remains unclear owing to some bacterial findings in free-ranging bottlenose dolphins (Tursiops truncatus) [4, 28] and the limitations of genetic (or genomic) information on bacteria isolated from marine mammals.
Since 2016, we have investigated the potential pathogens that can colonize and establish infection in endangered marine mammals present in coastal waters in the Republic of Korea [20]. In this study, we present the complete genome of E. tarda strain KC-Pc-HB1, which was isolated from a false killer whale (Pseudorca crassidens) found bycaught in 2017 along the South Sea (Republic of Korea). We aimed to provide genomic insights into the E. tarda infecting marine mammal species, and obtain useful information for the evaluation of its potential pathogenicity in those endangered species.
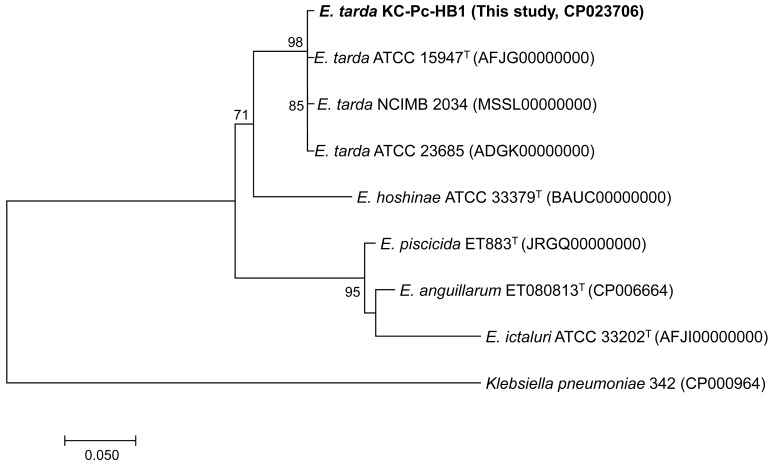
The general features and MIxS mandatory information for E. tarda strain KC-Pc-HB1 are summarized in Table 1. The bacterial strain was originally isolated from the blood collected from the heart of an adult female false killer whale (>480 cm in length, voucher no. CRI007391) found bycaught from troll fisheries in March 2017 along the South Sea (34°13ʹ12.0ʺN 128°21ʹ00.0ʺE, Republic of Korea). The motile, gram-negative, and flagellated straight-rod isolate (designated KC-Pc-HB1) was oxidase-negative and catalase-positive, and showed β-hemolysis on 5% sheep blood agar (Hanil Komed, Seongnam, Republic of Korea) after 24 hr of incubation at 37°C. The 16S rRNA of the isolate (MF973094) showed 99.9% similarity with the type strain of E. tarda ATCC 15947 (NR_024770) and E. hoshinae ATCC 33379 (AB682272) in the GenBank database, respectively. Sterile swabs from the blowhole and anus of the carcass were also collected and cultured under the same conditions mentioned above, and the same bacterium, which possessed 100% identical 16S rRNA sequence to that of the blood-isolate KC-Pc-HB1, was obtained from the both samples. Because the 16S rRNA was not able to discriminate the isolate as the species level, sodB sequence in KC-Pc-HB1 were obtained and used for the phylogenetic analysis, according to the previous report [24]. The resultant maximum-likelihood phylogeny indicated that the isolate KC-Pc-HB1 was well clustered with bona fide E. tarda strains (Fig. 1). Based on these results, KC-Pc-HB1 was classified as the species tarda and finally designated as E. tarda strain KC-Pc-HB1.
Table 1. General features of Edwardsiella tarda strain KC-Pc-HB1 and MIGS mandatory information.
| Items | Description | |
|---|---|---|
| Classification | Domain Bacteria | |
| Phylum Proteobacteria | ||
| Order Enterobacterales | ||
| Family Hafniaceae | ||
| Genus Edwardsiella | ||
| Species tarda | ||
| Strain: KC-Pc-HB1 | ||
| General features | ||
| Gram stain | Gram negative | |
| Cell shape | Nonsporeforming straight rod | |
| Motility | Motile | |
| Temperature | 4–42°C | |
| Pigmentation | Non-pigmented | |
| MIGS data | ||
| Investigation_type | Bacteria_archaea | |
| Project_name | Genome sequence of Edwardsiella tarda strain KC-Pc-HB1 | |
| Lat_lon | 34.22 N, 128.55 E | |
| Geo_loc_name | South Korea: South sea | |
| Collection_date | Mar-2017 | |
| Env_biome | Ocean [ENVO:00,000,015] | |
| Env_feature | Environmental material [ENVO:00,010,483] | |
| Env_material | Body fluid [ENVO:02,000,019] | |
| Num_replicons | 2 | |
| Extrachrom_elements | 1 | |
| Estimated_size | 3,720,168 | |
| Ref_biomaterial | None | |
| Source_mat_id | KCCM 90281 | |
| biotic_relationship | Infectious (or Commensal) | |
| host | False killer whale (Pseudorca crassidens) | |
| Rel_to_oxygen | Facultative anaerobic | |
| Isol_growth_condt | PMID: 26193880 | |
| Seq_meth | Illumina Hiseq 2000, PacBio RSII sequencing | |
| Annot_source | GenBank | |
| Finishing_strategy | Complete; 268 × coverage, 2 contigs | |
| Genome assembly data | ||
| Assembly method | HGAP | |
| Assembly name | HGAP algorithm ver. 3 | |
| Genome coverage | 268 × | |
| Sequencing technology | Illumina; PacBio | |
Fig. 1.
Maximum-likelihood phylogenetic tree based on the sodB genes from the available representative strains of Edwardsiella species. Klebsiella pneumoniae strain 342 (GenBank No. CP000964) was used as the outgroup. Numbers at the branches indicate bootstrapping values obtained with 1,000 replicates, and only bootstrap values >70% are indicated. The scale bar represents 0.05 nucleotide substitutions per site.
Genomic DNA of E. tarda KC-Pc-HB1 was obtained using a DNeasy blood and tissue kit (Qiagen Korea Ltd., Seoul, Republic of Korea), and it was sequenced at Macrogen Inc. (Seoul, Republic of Korea) according to the method reported by Lee et al. [20], using a hybrid approach with a PacBio RS II system (Pacific Biosciences, Menlo Park, CA, U.S.A.) and HiSeq 2000 instrument (Illumina, San Diego, CA, U.S.A.). The PacBio long read data (997,959,985 bp, 128,134 reads) were de novo assembled by the Hierarchical Genome Assembly Process program (ver. 3.0), and the Illumina pair end reads (3,716,099,262 bp, 36,793,062 reads) were mapped to the assembled contigs to improve the accuracy of the sequenced genome. Genome annotation was performed using the NCBI’s Prokaryotic Genome Annotation Pipeline (http://www.ncbi.nlm.nih.gov/books/NBK174280/), and functional categories of ORFs were analyzed by a PSI-BLAST search against the Clusters of Orthologous Groups (COG) database [30], with an E-value cutoff of 1E-4 and an identity cutoff of 20%. Bacterial tRNAs and rRNAs were respectively analyzed using tRNAscan-SE 1.21 [22] and RNAmmer 1.2 [18], and prophages were detected using PHASTER [2].
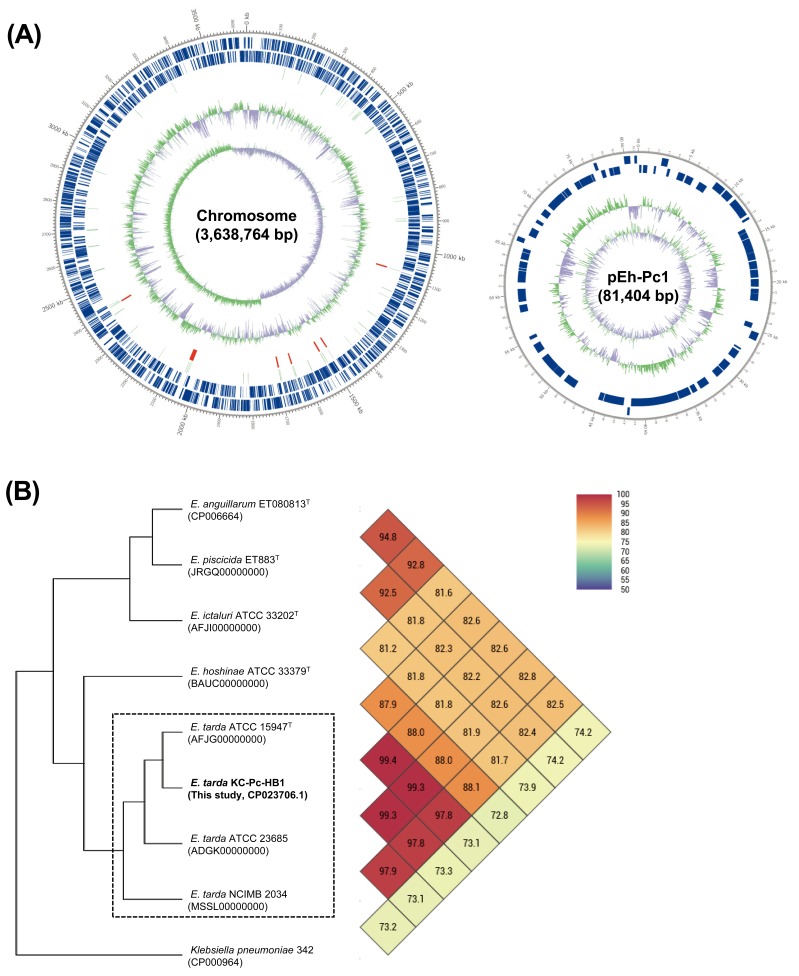
The sequenced E. tarda genome contained 3,720,168 bp consisting of one chromosome and one plasmid (designated pEh-Pc1) (Fig. 2A and Table 2). The final assembled circular chromosome of KC-Pc-HB1 was 3,638,764 bp (G+C content, 57.3%), and encoded 3,371 genes, 3,238 coding sequences (CDS), 28 rRNAs (5S, 16S and 23S), 101 tRNAs, and four noncoding RNAs. The result of the G+C content analysis of the isolate also supported those of a previous study [14], showing differences in G+C content between the groups of factual E. tarda and other Edwardsiella species. The plasmid pEh-Pc1 was 81,404 bp (G+C content, 52.0%), and encoded 90 CDS including several genes associated with plasmid conjugation (traC, traD, traL, traN and traX), thus revealing the genetic basis for its capability to transfer between bacteria. Additionally, six prophage regions (3 intact, 2 questionable, and 1 incomplete) and one additional incomplete prophage region were respectively detected in the chromosome and plasmid pEh-Pc1 (Supplementary Table S1).
Fig. 2.
(A) Circular maps of the E. tarda strain KC-Pc-HB1 genome. Marked characteristics are shown from the outside to the center: CDS on forward strand, CDS on reverse strand, tRNA, rRNA, GC content and GC skew. (B) Phylogenetic trees based on OrthoANI values calculated using available genomes of bona fide E. tarda strains (square box) and four other related Edwardsiella species. The results between two strains are given in the junction point of the diagonals departing from each strain, i.e., the OrthoANI value between E. tarda strain KC-Pc-HB1 (CP023706.1) and E. tarda ATCC 15947 (AFJG00000000) is 99.4% (two-column fitting image).
Table 2. General features of the Edwardsiella tarda strain KC-Pc-HB1 genome.
| Attribute | Value | ||
|---|---|---|---|
| Chromosome | Plasmid pEh-Pc1 | ||
| Size (bp) | 3,638,764 | 81,404 | |
| Coding regions (%) | 84.8 | 82.2 | |
| G+C content (%) | 57.3 | 52.0 | |
| Total genes | 3,371 | 90 | |
| tRNA genes | 101 | - | |
| rRNA genes | 28 | - | |
| ncRNA genes | 4 | - | |
| Protein-coding genes | 3,238 | 90 | |
| Pseudogenes | 50 | 7 | |
To assess overall genome similarity between KC-Pc-HB1 and other related Edwardsiella species, the average nucleotide identity (ANI) values were analyzed using the OrthoANI algorithm [19]. OrthoANI values were obtained, and a related phylogenetic tree was constructed based on OrthoANI analysis of the available representative bona fide E. tarda genomes (ATCC 15947T, ATCC 23685 and NCIMB 2034) in GenBank, and the four respective type strains of the other related Edwardsiella species (E. anguillarum ET080813T, E. hoshinae ATCC 33379T, E. ictaluri ATCC 33202T and E. piscicida ET883T) using the orthologous ANI tool. The resulting phylogenetic trees based on OrthoANI values for KC-Pc-HB1 and other related strains indicated that the genome of the isolate was most similar (>99%) to E. tarda ATCC 15947T, isolated from human fecal samples [12], and showed relatively low genome similarity (≤88%) to the other four Edwardsiella species (Fig. 2B).
The COG functional category analysis of E. tarda KC-Pc-HB1 revealed that the functional genes encoded on the bacterial chromosome were mainly involved in COG categories of J (translation, ribosomal structure, and biogenesis), K (transcription), M (cell wall/membrane/envelope biogenesis), C (energy production and conversion), G (carbohydrate transport and metabolism), and E (amino acid transport and metabolism), whereas 5.5 and 7.9% of the predicted genes were involved in S (function unknown in COG database) and failed to find a match in the database, respectively. In addition, several functional genes (>37%) encoded on plasmid pEh-Pc1 did not have matches in the COG database, and the remaining genes were mainly involved in L (replication, recombination, and repair) (Supplementary Fig. S1).
Although the pathogenesis of E. tarda is relatively not well understood at present, previous research has shown that several factors may contribute to the virulent mechanisms of this bacterium, for example, the two types of hemolysins (HlyA and EthAB) [6, 15]; fimbrial proteins related to adhesive properties (FimABC) and killing factor MukF [27]; superoxide dismutase B (SodB) [16]; chondroitinase, urease, and EacF (a putative Edwardsiella attenuation complex factor) [3, 10, 34]; and the twin arginine translocation (Tat) system consisting of tatABCDE [33]. Moreover, recent studies demonstrated that type III and type VI secretion systems (T3SS and T6SS), which contribute to the invasion and subversion of host cells, are essential for the virulence of Edwardsiella species [25, 29, 36]. Among the two reported hemolysin genes (hlyA and ethAB) in Edwardsiella species, ethAB was solely detected and two other putative hemolysin genes were also found to be encoded in KC-Pc-HB1. Moreover, several virulence-related genes homologous to sodB, fimABC, mukF, tatABCDE and chondroitinase were encoded on the genome. However, the T3SS and T6SS homologs were not found in KC-Pc-HB1, as was shown in the other bona fide E. tarda strains [35].
Additionally, the presence of other potential virulence genes was identified by searching the Virulence Factor DataBase (http://www.mgc.ac.cn/VFs/); and consequently, several virulence-related genes involved in other pathogenic bacterial species belonging to the family Enterobacteriaceae were detected in the KC-Pc-HB1 genome (Supplementary Table S2). Moreover, the antimicrobial-resistance genes in KC-Pc-HB1 were manually searched using the ARG-ANNOT database (http://en.mediterranee-infection.com/article.php?laref=283&titre=arg-annot-). The genome was found to possess a total of three genes involving β-lactam resistance, which can also be found in other bacterial genomes belonging to the family Enterobacteriaceae, including Edwardsiella strains in the GenBank database, whereas no antimicrobial-resistance gene was found in the plasmid pEh-Pc1 (Supplementary Table S3). The antimicrobial-resistance of KC-Pc-HB1 was quantitatively tested according to the guidelines of the Clinical and Laboratory Standards Institute [7]; however, no acceptable phenotypical resistance was observed in all the tested antibiotic classes (Data not shown).
According to Dunn et al. [11], E. tarda has been reported as one of the main causes of bacteremia and fatal septicemias in captive marine mammals; however, the evidence of its pathogenicity in wild marine mammals inevitably remains circumstantial owing to the limitations of postmortem analyses of stranded individuals and lack of genetic information of the bacterial isolates. Nevertheless, recent comparative studies indicate that Edwardsiella strains obtained from fish and humans are divergent [14], and most of the remaining bona fide E. tarda strains were potential human pathogenic ones [23]. Moreover, the genome of E. tarda KC-Pc-HB1 was almost identical to those of the bona fide E. tarda strains (Fig. 1) and possessed several virulence-related genes, making it a potentially virulent strain. Therefore, the chance for E. tarda transmission in humans is likely to happen during post-mortem examinations or inadvertent ingestion of infected (or contaminated) wild marine mammals and vice versa, because of its zoonotic potential. Consequently, more genetic information of E. tarda isolated from wild marine mammals is required to evaluate and clarify its potential pathogenesis in those animals. To the best of our knowledge, this is the first report of the isolation of E. tarda from the false killer whale and the first complete genome report of E. tarda found in marine mammals. The genomic data of KC-Pc-HB1 provide important insights into the biodiversity of E. tarda and give valuable information on potential virulence factors and antibiotic resistance for improving control strategies against this potential marine pathogen.
Edwardsiella tarda strain KC-Pc-HB1 was deposited in the Korean Culture Center of Microorganisms (KCCM) under KCCM 90281. The partial 16S rRNA and complete genome sequences of the strain KC-Pc-HB1 have been deposited in GenBank under accession numbers MF973094 (16S rRNA), CP023706 (chromosome), and CP023707 (plasmid pEh-Pc1).
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
Supplementary Material
Acknowledgments
This study was supported by grants from the KRIBB Initiative program [KGM4691612] and the Global Frontier Program [2015M3A6B2063544] funded by the Ministry of Science and ICT, and a grant from the National Institute of Fisheries Science [R2017028] funded by the Ministry of Oceans and Fisheries in Republic of Korea.
REFERENCES
- 1.Abbott S. L., Janda J. M.2006. The genus Edwardsiella. pp. 72–89. In: The Prokaryotes, Springer, New York. [Google Scholar]
- 2.Arndt D., Grant J. R., Marcu A., Sajed T., Pon A., Liang Y., Wishart D. S.2016. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 44W1: W16–21. doi: 10.1093/nar/gkw387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booth N. J., Beekman J. B., Thune R. L.2009. Edwardsiella ictaluri encodes an acid-activated urease that is required for intracellular replication in channel catfish (Ictalurus punctatus) macrophages. Appl. Environ. Microbiol. 75: 6712–6720. doi: 10.1128/AEM.01670-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buck J. D., Wells R. S., Rhinehart H. L., Hansen L. J.2006. Aerobic microorganisms associated with free-ranging bottlenose dolphins in coastal Gulf of Mexico and Atlantic Ocean waters. J. Wildl. Dis. 42: 536–544. doi: 10.7589/0090-3558-42.3.536 [DOI] [PubMed] [Google Scholar]
- 5.Buján N., Mohammed H., Balboa S., Romalde J. L., Toranzo A. E., Arias C. R., Magariños B.2017. Genetic studies to re-affiliate Edwardsiella tarda fish isolates to Edwardsiella piscicida and Edwardsiella anguillarum species. Syst. Appl. Microbiol. 41: 30–37. [DOI] [PubMed] [Google Scholar]
- 6.Chen J. D., Lai S. Y., Huang S. L.1996. Molecular cloning, characterization, and sequencing of the hemolysin gene from Edwardsiella tarda. Arch. Microbiol. 165: 9–17. doi: 10.1007/s002030050290 [DOI] [PubMed] [Google Scholar]
- 7.Clinical laboratory standards institute (CLSI). 2007. Performance standards for antimicrobial susceptibility testing: Seventeenth informational supplement. CLSI/NCCLS document M100-S17. Wayne.
- 8.Coles B. M., Stroud R. K., Sheggeby S.1978. Isolation of Edwardsiella tarda from three Oregon sea mammals. J. Wildl. Dis. 14: 339–341. doi: 10.7589/0090-3558-14.3.339 [DOI] [PubMed] [Google Scholar]
- 9.Cools P., Haelters J., Lopes dos Santos Santiago G., Claeys G., Boelens J., Leroux-Roels I., Vaneechoutte M., Deschaght P.2013. Edwardsiella tarda sepsis in a live-stranded sperm whale (Physeter macrocephalus). Vet. Microbiol. 166: 311–315. doi: 10.1016/j.vetmic.2013.05.020 [DOI] [PubMed] [Google Scholar]
- 10.Cooper R. K., Shotts E. B., Jr, Nolan L. K.1996. Use of a mini-transposon to study chondroitinase activity associated with Edwardsiella ictaluri. J. Aquat. Anim. Health 8: 319–324. doi: [DOI] [Google Scholar]
- 11.Dunn J. L., Buck J. D., Robeck T. R.2001. Bacterial diseases of cetaceans and pinnipeds. pp. 309–335. In: CRC Handbook of Marine Mammal Medicine, 2nd ed (Dierauf, L. A. and Gulland, M. D. eds.), CRC Press, Boca Raton. [Google Scholar]
- 12.Ewing W. H., McWhorter A. C., Escobar M. R., Lubin A. H.1965. Edwardsiella, a new genus of Enterobacteriaceae based on a new species, E. tarda. Int. J. Syst. Evol. Microbiol. 15: 33–38. [Google Scholar]
- 13.Gaydos J. K., Balcomb K. C., Osborne R. W., Dierauf L.2004. Evaluating potential infectious disease threats for southern resident kicker whales (Orcinus orca): a model for endangered species. Biol. Conserv. 117: 253–262. doi: 10.1016/j.biocon.2003.07.004 [DOI] [Google Scholar]
- 14.Griffin M. J., Quiniou S. M., Cody T., Tabuchi M., Ware C., Cipriano R. C., Mauel M. J., Soto E.2013. Comparative analysis of Edwardsiella isolates from fish in the eastern United States identifies two distinct genetic taxa amongst organisms phenotypically classified as E. tarda. Vet. Microbiol. 165: 358–372. doi: 10.1016/j.vetmic.2013.03.027 [DOI] [PubMed] [Google Scholar]
- 15.Hirono I., Tange N., Aoki T.1997. Iron-regulated haemolysin gene from Edwardsiella tarda. Mol. Microbiol. 24: 851–856. doi: 10.1046/j.1365-2958.1997.3971760.x [DOI] [PubMed] [Google Scholar]
- 16.Ishibe K., Osatomi K., Hara K., Kanai K., Yamaguchi K., Oda T.2008. Comparison of the responses of peritoneal macrophages from Japanese flounder (Paralichthys olivaceus) against high virulent and low virulent strains of Edwardsiella tarda. Fish Shellfish Immunol. 24: 243–251. doi: 10.1016/j.fsi.2007.11.001 [DOI] [PubMed] [Google Scholar]
- 17.Lair S., Measures L. N., Martineau D.2016. Pathologic findings and trends in mortality in the beluga (Delphinapterus leucas) population of the St Lawrence Estuary, Quebec, Canada, From 1983 to 2012. Vet. Pathol. 53: 22–36. doi: 10.1177/0300985815604726 [DOI] [PubMed] [Google Scholar]
- 18.Lagesen K., Hallin P., Rødland E. A., Staerfeldt H. H., Rognes T., Ussery D. W.2007. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 35: 3100–3108. doi: 10.1093/nar/gkm160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee I., Ouk Kim Y., Park S. C., Chun J.2016. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 66: 1100–1103. doi: 10.1099/ijsem.0.000760 [DOI] [PubMed] [Google Scholar]
- 20.Lee K., Kim H. K., Sohn H., Cho Y., Choi Y. M., Jeong D. G., Kim J. H.2018. Genomic insights into Photobacterium damselae subsp. damselae strain KC-Na-1, isolated from the finless porpoise (Neophocaena asiaeorientalis). Mar. Genomics 37: 26–30. [DOI] [PubMed] [Google Scholar]
- 21.Leotta G. A., Piñeyro P., Serena S., Vigo G. B.2009. Prevalence of Edwardsiella tarda in Antarctic wildlife. Polar Biol. 32: 809–812. doi: 10.1007/s00300-009-0610-9 [DOI] [Google Scholar]
- 22.Lowe T. M., Eddy S. R.1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25: 955–964. doi: 10.1093/nar/25.5.0955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura Y., Takano T., Yasuike M., Sakai T., Matsuyama T., Sano M.2013. Comparative genomics reveals that a fish pathogenic bacterium Edwardsiella tarda has acquired the locus of enterocyte effacement (LEE) through horizontal gene transfer. BMC Genomics 14: 642. doi: 10.1186/1471-2164-14-642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reichley S. R., Ware C., Steadman J., Gaunt P. S., García J. C., LaFrentz B. R., Thachil A., Waldbieser G. C., Stine C. B., Buján N., Arias C. R., Loch T., Welch T. J., Cipriano R. C., Greenway T. E., Khoo L. H., Wise D. J., Lawrence M. L., Griffin M. J.2017. Comparative phenotypic and genotypic analysis of Edwardsiella isolates from different hosts and geographic origins, with emphasis on isolates formerly classified as E. tarda, and evaluation of diagnostic methods. J. Clin. Microbiol. 55: 3466–3491. doi: 10.1128/JCM.00970-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogge M. L., Thune R. L.2011. Regulation of the Edwardsiella ictaluri type III secretion system by pH and phosphate concentration through EsrA, EsrB, and EsrC. Appl. Environ. Microbiol. 77: 4293–4302. doi: 10.1128/AEM.00195-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao S., Lai Q., Liu Q., Wu H., Xiao J., Shao Z., Wang Q., Zhang Y.2015. Phylogenomics characterization of a highly virulent Edwardsiella strain ET080813T encoding two distinct T3SS and three T6SS gene clusters: Propose a novel species as Edwardsiella anguillarum sp. nov. Syst. Appl. Microbiol. 38: 36–47. doi: 10.1016/j.syapm.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 27.Srinivasa Rao P. S., Lim T. M., Leung K. Y.2003. Functional genomics approach to the identification of virulence genes involved in Edwardsiella tarda pathogenesis. Infect. Immun. 71: 1343–1351. doi: 10.1128/IAI.71.3.1343-1351.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart J. R., Townsend F. I., Lane S. M., Dyar E., Hohn A. A., Rowles T. K., Staggs L. A., Wells R. S., Balmer B. C., Schwacke L. H.2014. Survey of antibiotic-resistant bacteria isolated from bottlenose dolphins Tursiops truncatus in the southeastern U.S.A.. Dis. Aquat. Organ. 108: 91–102. doi: 10.3354/dao02705 [DOI] [PubMed] [Google Scholar]
- 29.Tan Y. P., Zheng J., Tung S. L., Rosenshine I., Leung K. Y.2005. Role of type III secretion in Edwardsiella tarda virulence. Microbiology 151: 2301–2313. doi: 10.1099/mic.0.28005-0 [DOI] [PubMed] [Google Scholar]
- 30.Tatusov R. L., Natale D. A., Garkavtsev I. V., Tatusova T. A., Shankavaram U. T., Rao B. S., Kiryutin B., Galperin M. Y., Fedorova N. D., Koonin E. V.2001. The COG database: new developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res. 29: 22–28. doi: 10.1093/nar/29.1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Bressem M. F., Raga J. A., Di Guardo G., Jepson P. D., Duignan P. J., Siebert U., Barrett T., Santos M. C. D., Moreno I. B., Siciliano S., Aguilar A., Van Waerebeek K.2009. Emerging infectious diseases in cetaceans worldwide and the possible role of environmental stressors. Dis. Aquat. Organ. 86: 143–157. doi: 10.3354/dao02101 [DOI] [PubMed] [Google Scholar]
- 32.Waltzek T. B., Cortés-Hinojosa G., Wellehan J. F. X., Jr, Gray G. C.2012. Marine mammal zoonoses: a review of disease manifestations. Zoonoses Public Health 59: 521–535. doi: 10.1111/j.1863-2378.2012.01492.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Q., Yang M., Xiao J., Wu H., Wang X., Lv Y., Xu L., Zheng H., Wang S., Zhao G., Liu Q., Zhang Y.2009. Genome sequence of the versatile fish pathogen Edwardsiella tarda provides insights into its adaptation to broad host ranges and intracellular niches. PLoS One 4: e7646. doi: 10.1371/journal.pone.0007646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y. M., Wang Q. Y., Xiao J. F., Liu Q., Wu H. Z., Zhang Y. X.2011. Genetic relationships of Edwardsiella strains isolated in China aquaculture revealed by rep-PCR genomic fingerprinting and investigation of Edwardsiella virulence genes. J. Appl. Microbiol. 111: 1337–1348. doi: 10.1111/j.1365-2672.2011.05166.x [DOI] [PubMed] [Google Scholar]
- 35.Yang M., Lv Y., Xiao J., Wu H., Zheng H., Liu Q., Zhang Y., Wang Q.2012. Edwardsiella comparative phylogenomics reveal the new intra/inter-species taxonomic relationships, virulence evolution and niche adaptation mechanisms. PLoS One 7: e36987. doi: 10.1371/journal.pone.0036987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng J., Leung K. Y.2007. Dissection of a type VI secretion system in Edwardsiella tarda. Mol. Microbiol. 66: 1192–1206. doi: 10.1111/j.1365-2958.2007.05993.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.