Abstract
A 2-year-old, exotic shorthair cat presented with baldness and mild scaling on trunk that was confirmed as Microsporum canis (M. canis) infection by the following methods. Wood’s lamp and trichogram were used to demonstrate fungal elements suggestive of dermatophytosis consistent with M. canis. Dermatophyte test medium (DTM) and polymerase chain reaction (PCR) were used for identification. E-test and broth microdilution test were then utilized to estimate antifungal minimal inhibitory concentrations (MICs) towards ITZ and TRF respectively. The strain was isolated from the patient and revealed TRF MIC >32 µg/ml and ITZ MIC 0.023 µg/ml. Patient was cured of dermatophytosis with systemic ITZ.
Keywords: Microsporum canis, resistance, terbinafine
Microsporum canis is the most common cause of dermatophytosis in cats worldwide, and its diagnosis of fungi can depend upon various methods [6]. While the trend of low susceptibility towards common antifungal agents have been documented in numerous fungi such as Aspergilus spp. and Candida spp., low susceptibility of M. canis towards antifungals has yet to be reported. We wish to report the first case of M. canis with low susceptibility towards terbinafin (TRF) in a feline patient.
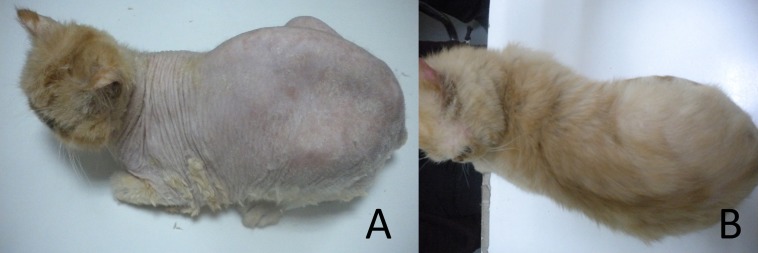
An indoor, female 2-year-old exotic shorthair, from a single cat household, at Beijing, China, was presented with chronic dermatophytosis and alopecia. The initial lesion was noticing at age of one and half year-old, exhibited on the trunk as multifocal circular patches of alopecia, mild scaling with minimal pruritus. The cat was then prescribed with a topical TRF spray (JinDun, terbinafine hydrochloride solution 1%, Nanjing, China) once a day for 3 months. Neither systemic medication nor medicated shampoo was prescribed. However, no clinical efficacy was reported despite the topical treatment (Fig. 1A).
Fig. 1.
Cl inical picture of the cat. (A) The cat exhibited totally baldness on trunk and spares to the head as generalized dermatophytosis. (B) The normal hair coat was presented after 12 weeks ITZ treatment.
Under Wood’s light, hair shafts fluoresced in typical apple-green color. Trichogram revealed numerous small, translucent spores (2.5 × 3.5 µm) around the shafts also suggestive of an ectothrix infection. The affected hairs were cultured on DTM (BD BBLTM, San Jose, CA, U.S.A.) and Sabouraud’s glucose agar (BD BBLTM, Heidelberg, Germany) at 37°C for 10–14 days. The fungus was identified as M. canis, based on colony morphology and macroconidia. Also, the molecular characteristics were identified by sequence analysis of the internal transcribed spacer (ITS) region. Results of comparative sequence analyses (by nucleotide BLAST analysis on the National Center for Biotechnology Information [NCBI] website) showed that the sequences amplified were 99% conserved among strains, including the reference strain of Arthroderma otae (teleomorph of M. canis), and matched the A. otae-defining ITS sequence deposited in the database (GenBank accession nos. JX122196.1 and JX122195.1).
The isolates’ MIC to ITZ was estimated with E-test gradient strips and performed based on the manufacturers’ recommendation [1]. For quality control, the strain Candida parapsilosis ATCC22019 was used in each experiment to check the accuracy of drug dilution [9]. Each isolate was tested in duplicate on separate occasions. The MIC of ITZ was 0.023 µg/ml, which was considered as a susceptible to ITZ.
On the other hand, broth microdilution of TRB was performed based on the CLSI M38-A2 with modifications [4, 7, 9]. The MIC of TRB was >32 µg/ml, which was considered as a resistance to TRF. Based on the results, 10 mg/kg ITZ PO once a day (SPORANOX®, Itraconazole, Janssen, Gyeonggi, Korea) was administered with food continuing for 3 months. Fungal re-cultures were scheduled every three weeks with first negative culture obtained at six. After that, the subsequent reculture were all negative (Fig. 2). At present, the cat remains asymptomatic after a year since the declaration of cure (Fig. 1B).
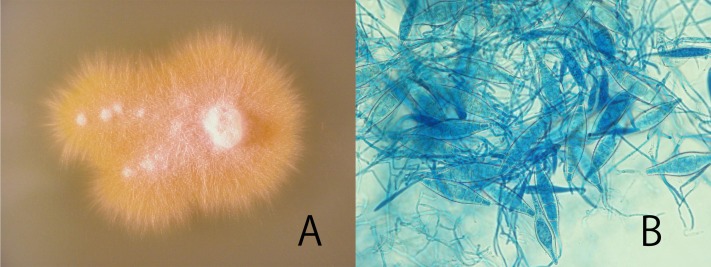
Fig. 2.
The reexaminations for dermatophytosis, based on Wood’s light, trichgram and DTM culture at different time points. Colony of the isolate on Sabouraud’s glucose agar (A) and macroconidia (B). The colony was flat, spreading, yellow-coloured. Macroconidia were spindle-shaped with 1–7 cells, verrucose, thick-walled and terminal knob.
We described the first isolation of a TRF-resistant M. canis strain from a feline dermatophytosis in China. This strain exhibited a TRF minimum inhibitory concentration (MIC) of >32 µg/ml but remained susceptible to itraconazole (ITZ) (MIC 0.023 µg/ml). Therefore, the patient cat improved following ITZ treatment, indicating that the results of the in vitro MIC test were consistent with in vivo susceptibility.
Feline dermatophytosis is generally treated with azoles and terbinafine (TRF), compounds that demonstrate efficacy against both human and animal dermatophytoses [3, 8]. There is a report of TRF resistance in Trichophyton rubrum isolated from a human dermatophytosis patient [2]. Furthermore, Martins et al. reported that the resistance of dermatophytes to antifungal drugs frequently results from overexpression of genes encoding ATP-binding cassette (ABC) transporter proteins, including the pleiotropic drug resistance (PDR1) and multidrug resistance (MDR2 and MDR4) genes [5]. We speculated that the TRF-resistant strain of M. canis characterized here was also isolated from a patient that had been receiving TRF therapy and exhibited overexpression of genes encoding members of the ABC transporter family.
In recent work, Yamada et al. reported that a TRF-resistant T. rubrum strain harbored missense mutations in the squalene epoxidase (SQLE) –encoding gene [10]. TRF is a member of the allylamine class of antifungal agents, which are known to target the SQLE enzyme in dermatophytes. However, TRF-resistant T. rubrum isolates were from patients with tinea pedis and/or onychomycosis who did not respond to terbinafine treatment [10]. Therefore, inducing to TRF resistance mechanism has not been clarified.
Further studies are required to determine the TRF resistance mechanism of this and other M. canis isolates. Anti-fungal susceptibility of clinical isolates could be a major determinant of treatment outcome.
Acknowledgments
The authors would like to thank Gau Jing for providing the case and clinical picture. This study was supported by a grant (“International joint research and training of young researchers for zoonosis control in the globalized world”) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (S1491007).
REFERENCES
- 1.Biodisk A.2008. Etest Technical Manual. AB Biodisk, Solna. [Google Scholar]
- 2.Digby S. S., Hald M., Arendrup M. C., Hjort S. V., Kofoed K.2017. Darier Disease Complicated by Terbinafine-resistant Trichophyton rubrum: A Case Report. Acta Derm. Venereol. 97: 139–140. doi: 10.2340/00015555-2455 [DOI] [PubMed] [Google Scholar]
- 3.Greene C. E.2013. Infectious Diseases of the Dog and Cat, 4th ed., Elsevier Health Sciences, Philadelphia. [Google Scholar]
- 4.Itoi S., Kano R., Hasegawa A., Kamata H.2012. In vitro activities of antifungal agents against clinical isolates of dermatophytes from animals. J. Vet. Med. Sci. 74: 1067–1069. doi: 10.1292/jvms.12-0057 [DOI] [PubMed] [Google Scholar]
- 5.Martins M. P., Franceschini A. C., Jacob T. R., Rossi A., Martinez-Rossi N. M.2016. Compensatory expression of multidrug-resistance genes encoding ABC transporters in dermatophytes. J. Med. Microbiol. 65: 605–610. doi: 10.1099/jmm.0.000268 [DOI] [PubMed] [Google Scholar]
- 6.Moriello K. A., Coyner K., Paterson S., Mignon B.2017. Diagnosis and treatment of dermatophytosis in dogs and cats.: Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 28: 266–e68. doi: 10.1111/vde.12440 [DOI] [PubMed] [Google Scholar]
- 7.NCCLS2002. National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disc Susceptibility Testing of Yeasts. Approved Standard M38-A2. National Committee for Clinical laboratory Standards. Philadelphia, Wayne. [Google Scholar]
- 8.Reiss E., Shadomy H. J., Lyon G. M.2011. Fundamental Medical Mycology. John Wiley & Sons. Hoboken. [Google Scholar]
- 9.Tan D., Seyyal A.2008. Antifungal Susceptibility Testing to Different Antifungal Agents to Isolats of M. canis from Dogs. J. Anim. Vet. Adv. 7: 226–230. [Google Scholar]
- 10.Yamada T., Maeda M., Alshahni M. M., Tanaka R., Yaguchi T., Bontems O., Salamin K., Fratti M., Monod M.2017. Terbinafine Resistance of Trichophyton Clinical Isolates Caused by Specific Point Mutations in the Squalene Epoxidase Gene. Antimicrob. Agents Chemother. 61: e00115–e00117. doi: 10.1128/AAC.00115-17 [DOI] [PMC free article] [PubMed] [Google Scholar]