Abstract
The lung endothelium is vulnerable to both exogenous and endogenous insults, so a properly coordinated efficient repair system is essential for the timely recovery of the lung after injury. The agents that cause endothelial injury and dysfunction fall into a broad range from mechanical forces such as pathological cyclic stretch and shear stress to bacterial pathogens and their virulent components, vasoactive agonists including thrombin and histamine, metabolic causes including high glucose and oxidized low-density lipoprotein (OxLDL), circulating microparticles, and inflammatory cytokines. The repair mechanisms employed by endothelial cells (EC) can be broadly categorized into three groups: (1) intrinsic mechanism of recovery regulated by the cross-talk between small GTPases as exemplified by Rap1-mediated EC barrier recovery from Rho-mediated thrombin-induced EC hyperpermeability; (2) agonist-assisted recovery facilitated by the activation of Rac and Rap1 with subsequent inhibition of Rho signaling as observed with many barrier protective agonists including oxidized phospholipids, sphingosine 1-phosphate, prostacyclins, and hepatocyte growth factor; and (3) self-recovery of EC by the secretion of growth factors and other pro-survival bioactive compounds including anti-inflammatory molecules such as lipoxins during the resolution of inflammation. In this review, we will discuss the molecular and cellular mechanisms of pulmonary endothelium repair that is critical for the recovery from various forms of lung injuries.
Keywords: endothelial cells, lung injury, barrier recovery and repair, small GTPases, growth factors
Introduction
The pulmonary endothelium is composed of a continuous monolayer of endothelial cells (EC) that forms a semi-permeable barrier between the blood and interstitium. Being a major component of the alveolar-capillary unit, the endothelium is prone to injury from diverse insults including mechanical forces and various barrier disruptive agents such as bacterial pathogens, endotoxins, oxidized low-density lipoprotein (OxLDL), thrombin, histamine, and pro-inflammatory cytokines from the pulmonary circulation.1,2 The increased endothelial permeability caused by the disruption of the EC barrier leads to an influx of protein-rich edematous fluid into the airspaces and subsequent inflammatory responses play a key role in the pathogenesis of acute lung injury (ALI) and its most severe form acute respiratory distress syndrome (ARDS).3–6 Both ALI and ARDS are clinically characterized by acute respiratory failure with a high mortality rate of 20–50% and ALI alone has an incidence of 200,000/year in the US population.7,8 Since no effective therapeutics have been developed to date for both of these severe respiratory disorders, understanding the molecular and cellular mechanisms of endothelial barrier repair and recovery is key to the development of novel drugs targeting the prevention of vascular leak and resolution of inflammation.
When basal endothelial function is perturbed by EC barrier disruption or inflammation, EC can employ three broadly categorized mechanisms of repair and recovery from injury. First, EC can undergo self-repair which is facilitated by the activation of small GTPases Rac and Rap1. This intrinsic mechanism of auto-recovery of EC barrier disruption is best exemplified during thrombin-induced EC hyperpermeability where Rho-dependent barrier dysfunction is restored by the activation of Rap1.9 It appears that a cross-talk between these small GTPases mediates the recovery process. Second, various barrier protective agents also activate Rac and Rap1 to enhance basal endothelial barrier function as well as to induce the recovery from agonists-induced barrier disruption. The third described mechanism of EC barrier function restoration involves the secretion of various growth factors, numerous pro-survival bioactive metabolites, and anti-inflammatory molecules by the stimulated pulmonary endothelium. Mechanisms of auto- and agonist-assisted recovery will be discussed in the following sections.
In addition to the direct role of EC in the recovery of injured lung endothelium, its dynamic interaction and cross-talk with epithelial cells also contributes to the repair process. The precise mechanisms of epithelial–endothelial interactions and their role in EC barrier protection remains largely unknown, but the secretion of growth factors, cytokines, and other bioactive barrier protective molecules by one cell type may facilitate the protection and recovery of the other cell type against injurious stimuli, thereby enhancing the overall barrier function. For instance, soluble factors of endothelial origin have been shown to enhance epithelial barrier integrity.10,11 Conversely, a recent study showed that epithelial cell-derived prostaglandin E2 (PGE2) enhances endothelial barrier function via activation of EP4 and S1P1 receptors.12 Furthermore, the same study also showed that cyclooxygenase-2 (COX-2), an enzyme involved in the synthesis of PGE2, expression is increased in epithelial cells following lipopolysaccharide (LPS) challenge, suggesting a possible recovery response of epithelial–endothelial interactions against LPS. With regard to the role of cell–cell interactions in the repair of injured lung endothelium, it is noteworthy to mention that immune cells, especially resident or inflammation-induced circulating macrophages, play a critical role in the resolution of inflammation (reviewed in Herold et al.13). Macrophage-mediated resolution of inflammation is credited to their secretion of anti-inflammatory lipid mediators including lipoxins.14 In brief, it appears that organ remodeling during recovery from injury is possible by the combined interactive actions of epithelial, endothelial, immune cells, and fibroblasts.
Small GTPases in the regulation of endothelial permeability and barrier recovery
In response to barrier disruptive stimuli, EC have a self-repair mechanism in place which involves the activation of small GTPases and their cross-talk. By cycling between GTP-bound active and GDP-bound inactive states, small GTPases act as a molecular switch in numerous signaling pathways that ultimately regulate various cellular functions. The switch of GTPase between active and inactive states is mediated by different factors. Guanine nucleotide exchange factors (GEF) promote an active GTPase state by exchanging GDP to GTP. GTPase activating proteins (GAP) lead to inactive GTPase by promoting GTP hydrolysis. Finally, guanine nucleotide dissociation inhibitors (GDI) also suppress small GTPases functional activity by stabilizing them in GDP-bound state. The Rho family of small GTPases RhoA and Rac1 as well as the Ras family GTPase Rap1 play a central role in regulating EC barrier function by modulating cytoskeletal remodeling.15–17 Among these, RhoA is responsible for increasing endothelial permeability thereby decreasing EC barrier function. RhoA also mediates the barrier disruptive pathway induced by various agonists including thrombin and vascular endothelial growth factor (VEGF).18–21 The increased phosphorylation of myosin light chain (MLC) mediated by Rho-associated kinase (ROCK)-induced inactivation of MLC phosphatase is considered as the mechanism by which RhoA causes actomyosin contractility.22 On the other hand, Rac1 plays a major role in maintaining basal endothelial barrier function in resting cells and also mediates EC barrier enhancement induced by various barrier protective agents.5,23 In addition, recent studies, including from our group, have demonstrated the important role of Rap1 in positively modulating EC barrier function.9,24 These GTPases control EC barrier integrity by modifying cytoskeletal organization as evidenced by the formation of actin stress fibers, paracellular gaps with Rho activation and accumulation of peripheral actin, sealing of junctional gaps and enhanced junctional assembly with Rac or Rap1 activation.
Intrinsic mechanism of EC recovery
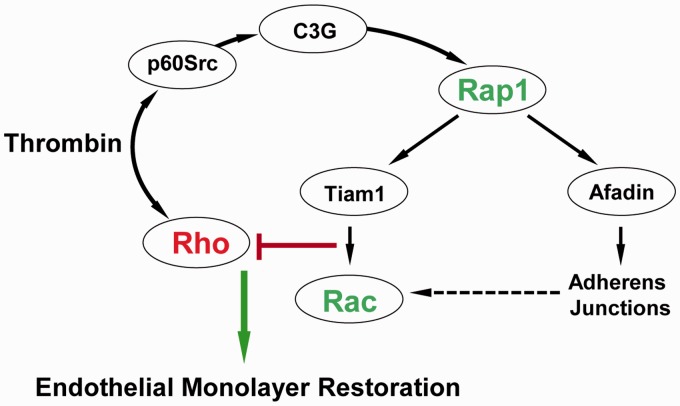
A dynamic cross-talk facilitated by the time-dependent activation of different small GTPases seems to regulate the auto-recovery of EC following the disruptive insults. This notion was established by our study where we showed that thrombin-induced EC permeability and recovery coincide with activation of Rho and Rap1, respectively9 (Fig. 1). The rapid activation of Rho mediates thrombin-induced EC permeability.19 In turn, barrier recovery is initiated by the activation of Src kinase-dependent phosphorylation of Rap1-specific GEF C3G leading to the activation of Rap1. Consistent with these functional roles of RhoA and Rap1, thrombin induced Rho activation and Rap1 inhibition at early time points (∼ 10 min), which was followed by activation of Rap1 and downregulation of Rho at later time points. Rap1-dependent recovery of EC barrier function was evident with the dissolution of actin stress fibers, resealing of intercellular gaps, and formation of lamelliopodia-like structures. The molecular inhibition of Rap1 with small interfering RNA abolished thrombin-induced EC barrier recovery, supporting that Rap1 controls the downregulation of Rho signaling during EC recovery during thrombin challenge. Similar time-dependent activation of Rho, Rac, and Cdc42 during thrombin-induced transient and reversible EC barrier disruption was postulated by Beckers et al. based on the findings from multiple studies (reviewed in Beckers et al.15).
Fig. 1.
Mechanism of EC barrier auto-recovery after thrombin challenge. In parallel with rapid activation of the Rho pathway leading to increased EC permeability, thrombin causes activation of Src kinase, which stimulates the Rap1-specific GEF C3G and, via Rap1-Tiam1, turns on the Rac1 signaling. Activation of the Rap1–Rac1 signaling axis downregulates the Rho pathway of barrier disruption and promotes reassembly of AJ complexes and endothelial monolayer barrier restoration.
In light of the pivotal role of cross-talk among small GTPases in governing self-repair of EC barrier dysfunction, it is essential to explore the role of various intermediate signaling molecules that facilitate the interaction between these GTPases. From our findings, Rap1-mediated association of afadin with p120-catenin appeared critical in downregulation of Rho.9 Moreover, Rap1 can also activate Rac1 via Tiam1, a Rac-specific GEF, and thus Rap1-mediated EC barrier recovery may also involve Rac, which is known to suppress Rho activation by multiple mechanisms including direct interaction with RhoGDI, PAK1-dependent inhibition of p115RhoGEF, and stimulation of p190RhoGAP.25–27 Based on the findings from a plethora of studies, it can be concluded that activation of a Rap1/Rac pathway might be a universal mechanism of EC barrier auto-recovery by reversing Rho-mediated disruption. This notion is further validated as various barrier protective agonists also upregulate Rap1/Rac to neutralize the detrimental effects of Rho activation during EC barrier protection which will be discussed in the next section.
EC recovery assisted by endogenous bioactive molecules and therapeutic agonists
In the past two decades, a significant amount of studies have focused on identifying potential EC barrier protective agents that can be developed into therapeutics against vascular leak and inflammation caused by endothelial barrier dysfunction. These studies have led to the discovery of a number of EC barrier protective agonists and their synthetic analogs with therapeutic potential to increase basal endothelial function and offer protection in clinical settings of lung injury. In addition, in response to noxious stimuli, the activated endothelium secretes various bioactive molecules that can trigger the recovery process. This type of EC recovery and resolution of lung inflammation is best exemplified by upregulated production of growth factors, and anti-inflammatory and barrier protective peptides.28–32 In addition, the change in repertoire of pro-inflammatory bioactive lipid mediators secreted during the acute phase of ALI to anti-inflammatory lipid mediators such as lipoxins, resolvins, and protectins synthesized during ALI resolution drives the recovery of inflammation.33,34 Barrier-promoting agonists and mechanisms of their action will be discussed in more detail below.
cAMP and cAMP derivatives
An elevation in cAMP levels in EC has been shown to enhance EC barrier function and also to provide protection via protein kinase A (PKA)-dependent mechanisms against EC barrier disruption evoked by disruptive agents such as thrombin.35,36 PKA-independent mechanism of cAMP-induced EC barrier protection against thrombin or VEGF occurs by the activation of Epac-Rap1 signaling which promotes enhancement of VE-cadherin-containing adherens junctions in endothelial cells.37 The role of Epac-Rap1 pathway in improving EC barrier function has been further substantiated by other studies.38–40 Several reports have also demonstrated the role of Rac in mediating cAMP-induced EC barrier function.41–43 Our study described the positive cross-talk between Rap1 and Rac, as a convergence mechanism of RhoA inhibition and EC barrier protection against thrombin caused by administration of atrial natriuretic peptide (ANP).44 Rac1 was determined as a central hub for both PKA-dependent and -independent EC barrier recovery pathways.45 The results from all of these studies strongly indicate that cAMP-elevating agents such as forskolin, rolipram, and other cell permeable synthetic cAMP analogs including 8-Bromoadenosine 3’, 5’-cyclic monophosphate and 8-(4-chlorophenylthio)adenosine 3’, 5’’-cyclic monophosphate could be potential therapeutics against lung injury and inflammation elicited by endothelial barrier dysfunction.
Prostaglandins
Prostaglandins (PGs) and some of their derivatives have been known to exert barrier protective and anti-inflammatory effects on the pulmonary endothelium.46–48 However, the cellular effects of PGs are determined by the engagement of different receptor types and often show detrimental effects in the lung.49 Nevertheless, a series of studies from our group have established the protective effects of various PGs in several models of ALI and inflammation in vitro and in vivo. Among these, PGE2 and PGI2 protected from thrombin-induced EC barrier disruption by activating both PKA-dependent and PKA-independent Epac-Rap1 pathways.50 In the same study, PGI2 analog beraprost protected mechanical ventilation-induced lung barrier dysfunction in mice. By employing pharmacological activators/inhibitors and knockout mice, our study further substantiated the role of beraprost-activated Rap1 pathway in protecting LPS-induced in vitro and in vivo lung injury.51 Multiple studies testing the role of another prostacyclin analog, iloprost, also showed its potent protective effects against mechanical ventilation and LPS-induced lung injury by activating Rap1 and inhibiting Rho.51–53 Given that both of the aforementioned prostacyclin analogs are clinically used to treat pulmonary hypertension, their marked protective effects in several models of lung injury and inflammation also bolsters their potential therapeutic use in these conditions.
Phospholipids
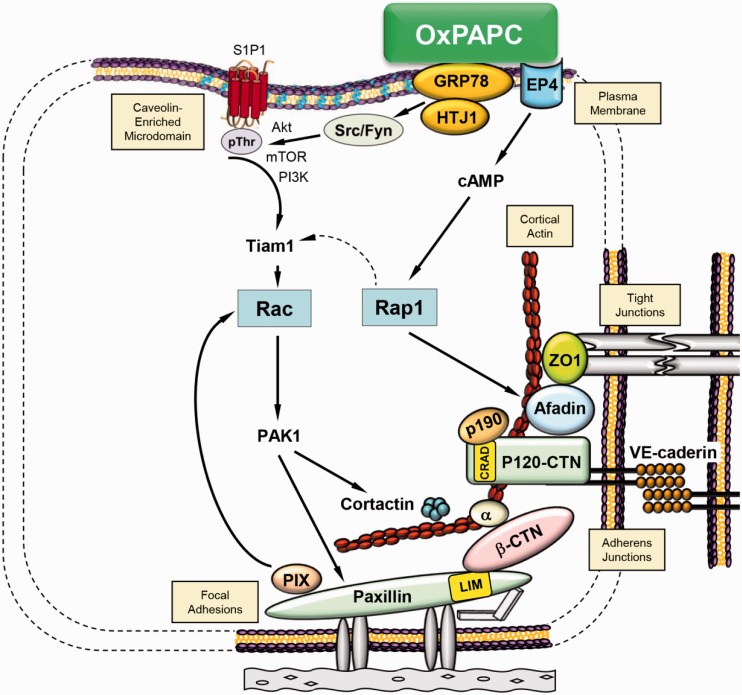
In recent years, a number of studies have highlighted EC barrier protective and anti-inflammatory properties of various phospholipids. In particular, treatment with oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC) has shown great promise in preventing lung injury and inflammation. Extensive studies by our and other groups have established that OxPAPC exerts protection against a diverse range of barrier disruptive and pro-inflammatory agents including mechanical forces (ventilator, cyclic stretch), thrombin, LPS, and heat-killed Staphylococcus aureus54–57 which was due to antagonistic effects of OxPAPC on agonist-induced activation of toll-like receptors TLR2, TLR4, and TLR9.58–61 Independently on TLR antagonism, OxPAPC exhibits potent barrier-enhancing effects on vascular endothelium which are initiated by interaction of OxPAPC with cell surface-associated molecular chaperone with signaling properties, GRP78.62 OxPAPC-induced barrier protection was facilitated by cytoskeletal remodeling with enhanced assembly of adherens junctions and tight junctions mediated by Rap1 and Rac activation.23,63,64 Additional activation of prostaglandin E receptor-4 (EP4) which triggers cAMP-PKA and cAMP-Epac1-Rap1 pathways of cytoskeleton remodeling and cell junction enhancement may also mediate the barrier protective effects of OxPAPC as evidenced in our latest study.65 More detailed analysis of phospholipid oxidation products contained in the OxPAPC preparation revealed that full-length PAPC oxidation products provide EC barrier protection while fragmented products evoke barrier disruption.66 A summary of OxPAPC-induced signaling pathways leading to EC barrier enhancement is presented in Fig. 2.
Fig. 2.
Summary of signaling mechanisms activated by barrier-protective oxidized phospholipids.
Our most recent study showed that preconditioning of both quiescent or inflamed pulmonary EC with OxPAPC or injection of OxPAPC to LPS-challenged mice induces the production of lipoxin A4 (LXA4), which contributes to anti-inflammatory effect of OxPAPC in vitro and in vivo; and the mechanism of LXA4 action involves activation of EC-expressed formyl-peptide receptor-2 (FPR2/ALX).67 EC-protective effects of LXA4 have also been confirmed by other groups.68–70
In addition to oxidized phospholipids, another lipid mediator, sphingosine-1-phosphate (S1P), also exhibits potent barrier protective effects on pulmonary EC via binding to its receptor S1PR1 and activation of Rac1 signaling.71–73 Interestingly, OxPAPC also transactivates S1PR1 and this event contributes to OxPAPC barrier-enhancing properties. FTY720, a potent agonist of S1PR1, also enhances EC barrier function, although involvement of S1PR1 receptor-mediated mechanism in FTY720 effects is controversial.74–76
Growth factors
Among growth factors, hepatocyte growth factor (HGF) is a prominent EC barrier protective agent. HGF increases basal endothelial function by cytoskeletal rearrangement mediated by the activation of various kinases including phosphatidylinositol 3’-kinase (PI3K), extracellular signal-related kinase (Erk), p38 mitogen-activated protein kinase, and PKC leading to the phosphorylation of glycogen synthase kinase-3β.77 HGF protects against thrombin-induced EC dysfunction by Tiam1-mediated activation of Rac and inhibition of Rho pathways.78 Likewise, HGF-induced protection against LPS-caused lung injury and inflammation was dependent on the activation of Asef, a Rac-specific GEF.79 Microtubules also appear to play a key role in mediating HGF-induced EC barrier enhancement.80 Contribution of other growth factors upregulated during lung injury in EC barrier function recovery still remains to be clarified. A recent study has shown that epidermal growth factor (EGF) protects blood–spinal cord barrier disruption during acute spinal cord injury by activating the PI3K/Akt/Rac pathway.81
Other barrier protective molecules
Statin, an inhibitor of the rate-limiting enzyme in cholesterol synthesis, has been shown to protect against thrombin-induced endothelial permeability in vitro by inhibiting membrane translocation of Rho and prevents vascular leaks in vivo.82 The EC barrier protective functions of simvastatin has been confirmed by several other studies.83–85 Hyaluronan is a major glycosaminoglycan component of extracellular matrix of many tissues. High molecular weight hyaluronan, a natural component of extracellular matrix also present in circulation in soluble form, enhances EC barrier function in part via transactivation of S1PR1 receptor86 and protects LPS-induced vascular leak.87 An endogenous peptide adrenomedullin is reported to protect vascular barrier integrity.88,89 Angiopoietin protects against VEGF-induced endothelial permeability by inhibiting Src activation and preventing the phosphorylation of VE-cadherin.90 The endothelial barrier protective and anti-inflammatory roles of angiopoietin have been observed in various settings.91,92
Conclusion
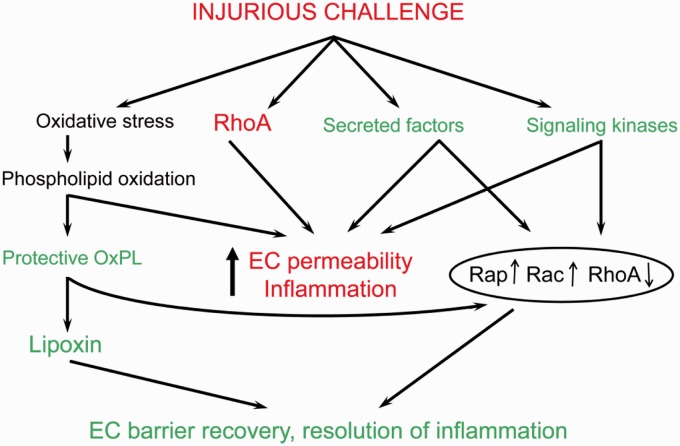
The timely and efficient recovery of the endothelium from various toxic insults and injuries is vital for the proper function of the lung. The repair process in EC is assisted by either intrinsic or agonist-induced small GTPases activation with cytoskeletal remodeling. Sequential activation and dynamic cross-talk between these GTPases regulate the overall endothelial function (Fig. 3). In response to toxic stimuli, the endothelium also generates various bioactive molecules which assist during the recovery. Among them, lipoxins appear to be of significant importance with their potent barrier protective and anti-inflammatory functions as well as their accumulation by EC barrier enhancing oxidized phospholipid species, OxPAPC. The comprehensive understanding of the molecular and cellular mechanisms of intrinsic or agonist-mediated endothelium repair and recovery will pave a way for the development of novel therapeutics against pulmonary disorders associated with endothelial dysfunction.
Fig. 3.
Balance of EC-disruptive and EC-protective mechanisms in the course of lung injury and resolution phase. Lung injurious factors (bacterial pathogens, excessive mechanical stretch, cytokines, disruptive bioactive molecules) trigger pathologic signaling (i.e. oxidative stress, RhoA pathway, signaling kinases, secreted factors) leading to endothelial hyperpermeability, inflammation, and lung dysfunction. However, pathologic stimuli also activate mechanisms of auto-recovery such as secretion of pro-survival growth factors and anti-inflammatory lipid mediators which suppress inflammation, downregulate pro-inflammatory and disruptive RhoA GTPase-mediated pathways, and stimulate Rap1 and Rac1 GTPase-mediated cytoskeletal remodeling leading to EC barrier recovery.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research has been supported in part by NHLBI grants HL076259 and HL087823, and NIGM grant GM122940.
2017 Grove Conference Series
This review article is part of the 2017 Grover Conference Series. The American Thoracic Society and the conference organizing committee gratefully acknowledge the education grants provided for the support of this conference by Actelion Pharmaceuticals US, Inc., Gilead Sciences, Inc., and United Therapeutics Corporation. Additionally, the American Thoracic Society is grateful for the support of the Grover Conference by the American Heart Association, the Cardiovascular Medical Research and Education Fund, and the National Institutes of Health.
References
- 1.Orfanos SE, Mavrommati I, Korovesi I, et al. Pulmonary endothelium in acute lung injury: from basic science to the critically ill. Intensive Care Med 2004; 30: 1702–1714. [DOI] [PubMed] [Google Scholar]
- 2.Birukov KG, Zebda N, Birukova AA. Barrier enhancing signals in pulmonary edema. Compr Physiol 2013; 3: 429–484. [DOI] [PubMed] [Google Scholar]
- 3.Minamino T, Komuro I. Regeneration of the endothelium as a novel therapeutic strategy for acute lung injury. J Clin Invest 2006; 116: 2316–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maniatis NA, Kotanidou A, Catravas JD, et al. Endothelial pathomechanisms in acute lung injury. Vascul Pharmacol 2008; 49: 119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 2006; 86: 279–367. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Y, Ridge K, Zhao J. Acute lung injury, repair, and remodeling: pulmonary endothelial and epithelial biology. Mediators Inflamm 2017; 2017: 9081521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv 2010; 23: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ragaller M, Richter T. Acute lung injury and acute respiratory distress syndrome. J Emerg Trauma Shock 2010; 3: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birukova AA, Tian X, Tian Y, et al. Rap-afadin axis in control of Rho signaling and endothelial barrier recovery. Mol Biol Cell 2013; 24: 2678–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuhaus W, Samwer F, Kunzmann S, et al. Lung endothelial cells strengthen, but brain endothelial cells weaken barrier properties of a human alveolar epithelium cell culture model. Differentiation 2012; 84: 294–304. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhury F, Howat WJ, Phillips GJ, et al. Interactions between endothelial cells and epithelial cells in a combined cell model of airway mucosa: effects on tight junction permeability. Exp Lung Res 2010; 36: 1–11. [DOI] [PubMed] [Google Scholar]
- 12.Barnthaler T, Maric J, Platzer W, et al. The role of PGE2 in alveolar epithelial and lung microvascular endothelial crosstalk. Sci Rep 2017; 7: 7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herold S, Mayer K, Lohmeyer J. Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front Immunol 2011; 2: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freire-de-Lima CG, Xiao YQ, Gardai SJ, et al. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem 2006; 281: 38376–38384. [DOI] [PubMed] [Google Scholar]
- 15.Beckers CM, van Hinsbergh VW, van Nieuw Amerongen GP. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost 2010; 103: 40–55. [DOI] [PubMed] [Google Scholar]
- 16.Wojciak-Stothard B, Potempa S, Eichholtz T, et al. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci 2001; 114: 1343–1355. [DOI] [PubMed] [Google Scholar]
- 17.Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovasc Res 2010; 87: 243–253. [DOI] [PubMed] [Google Scholar]
- 18.van Nieuw Amerongen GP, van Delft S, Vermeer MA, et al. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res 2000; 87: 335–340. [DOI] [PubMed] [Google Scholar]
- 19.Birukova AA, Smurova K, Birukov KG, et al. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 2004; 67: 64–77. [DOI] [PubMed] [Google Scholar]
- 20.Sun H, Breslin JW, Zhu J, et al. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation 2006; 13: 237–247. [DOI] [PubMed] [Google Scholar]
- 21.van Nieuw Amerongen GP, Koolwijk P, Versteilen A, et al. Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arterioscler Thromb Vasc Biol 2003; 23: 211–217. [DOI] [PubMed] [Google Scholar]
- 22.Essler M, Amano M, Kruse HJ, et al. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem 1998; 273: 21867–21874. [DOI] [PubMed] [Google Scholar]
- 23.Birukova AA, Chatchavalvanich S, Oskolkova O, et al. Signaling pathways involved in OxPAPC-induced pulmonary endothelial barrier protection. Microvasc Res 2007; 73: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pannekoek WJ, Post A, Bos JL. Rap1 signaling in endothelial barrier control. Cell Adh Migr 2014; 8: 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong KW, Mohammadi S, Isberg RR. Disruption of RhoGDI and RhoA regulation by a Rac1 specificity switch mutant. J Biol Chem 2006; 281: 40379–40388. [DOI] [PubMed] [Google Scholar]
- 26.Rosenfeldt H, Castellone MD, Randazzo PA, et al. Rac inhibits thrombin-induced Rho activation: evidence of a Pak-dependent GTPase crosstalk. J Mol Signal 2006; 1: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herbrand U, Ahmadian MR. p190-RhoGAP as an integral component of the Tiam1/Rac1-induced downregulation of Rho. Biol Chem 2006; 387: 311–317. [DOI] [PubMed] [Google Scholar]
- 28.Yanagita K, Matsumoto K, Sekiguchi K, et al. Hepatocyte growth factor may act as a pulmotrophic factor on lung regeneration after acute lung injury. J Biol Chem 1993; 268: 21212–21217. [PubMed] [Google Scholar]
- 29.Wang H, Zheng R, Chen Q, et al. Mesenchymal stem cells microvesicles stabilize endothelial barrier function partly mediated by hepatocyte growth factor (HGF). Stem Cell Res Ther 2017; 8: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu D, Perez RE, Ekekezie II, et al. Epidermal growth factor-like domain 7 protects endothelial cells from hyperoxia-induced cell death. Am J Physiol Lung Cell Mol Physiol 2008; 294: L17–23. [DOI] [PubMed] [Google Scholar]
- 31.Thebaud B, Ladha F, Michelakis ED, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation 2005; 112: 2477–2486. [DOI] [PubMed] [Google Scholar]
- 32.Itoh T, Obata H, Murakami S, et al. Adrenomedullin ameliorates lipopolysaccharide-induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol 2007; 293: L446–452. [DOI] [PubMed] [Google Scholar]
- 33.Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol 2010; 177: 1576–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy BD, Clish CB, Schmidt B, et al. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol 2001; 2: 612–619. [DOI] [PubMed] [Google Scholar]
- 35.Patterson CE, Lum H, Schaphorst KL, et al. Regulation of endothelial barrier function by the cAMP-dependent protein kinase. Endothelium 2000; 7: 287–308. [DOI] [PubMed] [Google Scholar]
- 36.Lum H, Jaffe HA, Schulz IT, et al. Expression of PKA inhibitor (PKI) gene abolishes cAMP-mediated protection to endothelial barrier dysfunction. Am J Physiol 1999; 277: C580–588. [DOI] [PubMed] [Google Scholar]
- 37.Fukuhara S, Sakurai A, Sano H, et al. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol 2005; 25: 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cullere X, Shaw SK, Andersson L, et al. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 2005; 105: 1950–1955. [DOI] [PubMed] [Google Scholar]
- 39.Kooistra MR, Corada M, Dejana E, et al. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett 2005; 579: 4966–4972. [DOI] [PubMed] [Google Scholar]
- 40.Wittchen ES, Worthylake RA, Kelly P, et al. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem 2005; 280: 11675–11682. [DOI] [PubMed] [Google Scholar]
- 41.Baumer Y, Spindler V, Werthmann RC, et al. Role of Rac 1 and cAMP in endothelial barrier stabilization and thrombin-induced barrier breakdown. J Cell Physiol 2009; 220: 716–726. [DOI] [PubMed] [Google Scholar]
- 42.Aslam M, Tanislav C, Troidl C, et al. cAMP controls the restoration of endothelial barrier function after thrombin-induced hyperpermeability via Rac1 activation. Physiol Rep 2014; 2: e12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waschke J, Drenckhahn D, Adamson RH, et al. cAMP protects endothelial barrier functions by preventing Rac-1 inhibition. Am J Physiol Heart Circ Physiol 2004; 287: H2427–2433. [DOI] [PubMed] [Google Scholar]
- 44.Birukova AA, Zagranichnaya T, Alekseeva E, et al. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J Cell Physiol 2008; 215: 715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birukova AA, Burdette D, Moldobaeva N, et al. Rac GTPase is a hub for protein kinase A and Epac signaling in endothelial barrier protection by cAMP. Microvasc Res 2010; 79: 128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farmer PJ, Bernier SG, Lepage A, et al. Permeability of endothelial monolayers to albumin is increased by bradykinin and inhibited by prostaglandins. Am J Physiol Lung Cell Mol Physiol 2001; 280: L732–738. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi K, Tsubosaka Y, Hori M, et al. Prostaglandin D2-DP signaling promotes endothelial barrier function via the cAMP/PKA/Tiam1/Rac1 pathway. Arterioscler Thromb Vasc Biol 2013; 33: 565–571. [DOI] [PubMed] [Google Scholar]
- 48.Ueno Y, Koike H, Annoh S, et al. Anti-inflammatory effects of beraprost sodium, a stable analogue of PGI2, and its mechanisms. Prostaglandins 1997; 53: 279–289. [DOI] [PubMed] [Google Scholar]
- 49.Park GY, Christman JW. Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am J Physiol Lung Cell Mol Physiol 2006; 290: L797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Birukova AA, Zagranichnaya T, Fu P, et al. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res 2007; 313: 2504–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Birukova AA, Meng F, Tian Y, et al. Prostacyclin post-treatment improves LPS-induced acute lung injury and endothelial barrier recovery via Rap1. Biochim Biophys Acta 2015; 1852: 778–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Birukova AA, Fu P, Xing J, et al. Lung endothelial barrier protection by iloprost in the 2-hit models of ventilator-induced lung injury (VILI) involves inhibition of Rho signaling. Transl Res 2010; 155: 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Birukova AA, Wu T, Tian Y, et al. Iloprost improves endothelial barrier function in lipopolysaccharide-induced lung injury. Eur Respir J 2013; 41: 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Birukov KG, Bochkov VN, Birukova AA, et al. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res 2004; 95: 892–901. [DOI] [PubMed] [Google Scholar]
- 55.Nonas S, Miller I, Kawkitinarong K, et al. Oxidized phospholipids reduce vascular leak and inflammation in rat model of acute lung injury. Am J Respir Crit Care Med 2006; 173: 1130–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nonas S, Birukova AA, Fu P, et al. Oxidized phospholipids reduce ventilator-induced vascular leak and inflammation in vivo. Crit Care 2008; 12: R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meliton AY, Meng F, Tian Y, et al. Oxidized phospholipids protect against lung injury and endothelial barrier dysfunction caused by heat-inactivated Staphylococcus aureus. Am J Physiol Lung Cell Mol Physiol 2015; 308: L550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oskolkova OV, Afonyushkin T, Preinerstorfer B, et al. Oxidized phospholipids are more potent antagonists of lipopolysaccharide than inducers of inflammation. J Immunol 2010; 185: 7706–7712. [DOI] [PubMed] [Google Scholar]
- 59.Erridge C, Kennedy S, Spickett CM, et al. Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition. J Biol Chem 2008; 283: 24748–24759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma Z, Li J, Yang L, et al. Inhibition of LPS- and CpG DNA-induced TNF-alpha response by oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol 2004; 286: L808–816. [DOI] [PubMed] [Google Scholar]
- 61.Bochkov VN, Kadl A, Huber J, et al. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature 2002; 419: 77–81. [DOI] [PubMed] [Google Scholar]
- 62.Birukova AA, Singleton PA, Gawlak G, et al. GRP78 is a novel receptor initiating a vascular barrier protective response to oxidized phospholipids. Mol Biol Cell 2014; 25: 2006–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Birukova AA, Malyukova I, Poroyko V, et al. Paxillin-beta-catenin interactions are involved in Rac/Cdc42-mediated endothelial barrier-protective response to oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol 2007; 293: L199–211. [DOI] [PubMed] [Google Scholar]
- 64.Birukova AA, Malyukova I, Mikaelyan A, et al. Tiam1 and betaPIX mediate Rac-dependent endothelial barrier protective response to oxidized phospholipids. J Cell Physiol 2007; 211: 608–617. [DOI] [PubMed] [Google Scholar]
- 65.Oskolkova O, Gawlak G, Tian Y, et al. Prostaglandin E receptor-4 receptor mediates endothelial barrier-enhancing and anti-inflammatory effects of oxidized phospholipids. FASEB J 2017; 31: 4187–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Birukova AA, Starosta V, Tian X, et al. Fragmented oxidation products define barrier disruptive endothelial cell response to OxPAPC. Transl Res 2013; 161: 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ke Y, Zebda N, Oskolkova O, et al. Anti-inflammatory effects of OxPAPC involve endothelial cell-mediated generation of LXA4. Circ Res 2017; 121: 244–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Souza MC, Padua TA, Torres ND, et al. Lipoxin A4 attenuates endothelial dysfunction during experimental cerebral malaria. Int Immunopharmacol 2015; 24: 400–407. [DOI] [PubMed] [Google Scholar]
- 69.Cezar-de-Mello PF, Nascimento-Silva V, Villela CG, et al. Aspirin-triggered Lipoxin A4 inhibition of VEGF-induced endothelial cell migration involves actin polymerization and focal adhesion assembly. Oncogene 2006; 25: 122–129. [DOI] [PubMed] [Google Scholar]
- 70.Vieira AM, Neto EH, Figueiredo CC, et al. ATL-1, a synthetic analog of lipoxin, modulates endothelial permeability and interaction with tumor cells through a VEGF-dependent mechanism. Biochem Pharmacol 2014; 90: 388–396. [DOI] [PubMed] [Google Scholar]
- 71.Li Q, Chen B, Zeng C, et al. Differential activation of receptors and signal pathways upon stimulation by different doses of sphingosine-1-phosphate in endothelial cells. Exp Physiol 2014. DOI: 10.1113/expphysiol.2014.082149. [DOI] [PubMed] [Google Scholar]
- 72.Peng X, Hassoun PM, Sammani S, et al. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 2004; 169: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 73.McVerry BJ, Peng X, Hassoun PM, et al. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med 2004; 170: 987–993. [DOI] [PubMed] [Google Scholar]
- 74.Natarajan V, Dudek SM, Jacobson JR, et al. Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury. Am J Respir Cell Mol Biol 2013; 49: 6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singleton PA, Chatchavalvanich S, Fu P, et al. Akt-mediated transactivation of the S1P1 receptor in caveolin-enriched microdomains regulates endothelial barrier enhancement by oxidized phospholipids. Circ Res 2009; 104: 978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dudek SM, Camp SM, Chiang ET, et al. Pulmonary endothelial cell barrier enhancement by FTY720 does not require the S1P1 receptor. Cell Signal 2007; 19: 1754–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu F, Schaphorst KL, Verin AD, et al. Hepatocyte growth factor enhances endothelial cell barrier function and cortical cytoskeletal rearrangement: potential role of glycogen synthase kinase-3beta. FASEB J 2002; 16: 950–962. [DOI] [PubMed] [Google Scholar]
- 78.Birukova AA, Alekseeva E, Mikaelyan A, et al. HGF attenuates thrombin-induced endothelial permeability by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway. FASEB J 2007; 21: 2776–2786. [DOI] [PubMed] [Google Scholar]
- 79.Meng F, Meliton A, Moldobaeva N, et al. Asef mediates HGF protective effects against LPS-induced lung injury and endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2015; 308: L452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tian X, Tian Y, Moldobaeva N, et al. Microtubule dynamics control HGF-induced lung endothelial barrier enhancement. PLoS One 2014; 9: e105912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng B, Ye L, Zhou Y, et al. Epidermal growth factor attenuates blood-spinal cord barrier disruption via PI3K/Akt/Rac1 pathway after acute spinal cord injury. J Cell Mol Med 2016; 20: 1062–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Nieuw Amerongen GP, Vermeer MA, Negre-Aminou P, et al. Simvastatin improves disturbed endothelial barrier function. Circulation 2000; 102: 2803–2809. [DOI] [PubMed] [Google Scholar]
- 83.Jacobson JR, Dudek SM, Birukov KG, et al. Cytoskeletal activation and altered gene expression in endothelial barrier regulation by simvastatin. Am J Respir Cell Mol Biol 2004; 30: 662–670. [DOI] [PubMed] [Google Scholar]
- 84.Chen W, Pendyala S, Natarajan V, et al. Endothelial cell barrier protection by simvastatin: GTPase regulation and NADPH oxidase inhibition. Am J Physiol Lung Cell Mol Physiol 2008; 295: L575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lampi MC, Faber CJ, Huynh J, et al. Simvastatin ameliorates matrix stiffness-mediated endothelial monolayer disruption. PLoS One 2016; 11: e0147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singleton PA, Dudek SM, Ma SF, et al. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J Biol Chem 2006; 281: 34381–34393. [DOI] [PubMed] [Google Scholar]
- 87.Singleton PA, Mirzapoiazova T, Guo Y, et al. High-molecular-weight hyaluronan is a novel inhibitor of pulmonary vascular leakiness. Am J Physiol Lung Cell Mol Physiol 2010; 299: L639–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hippenstiel S, Witzenrath M, Schmeck B, et al. Adrenomedullin reduces endothelial hyperpermeability. Circ Res 2002; 91: 618–625. [DOI] [PubMed] [Google Scholar]
- 89.Hocke AC, Temmesfeld-Wollbrueck B, Schmeck B, et al. Perturbation of endothelial junction proteins by Staphylococcus aureus alpha-toxin: inhibition of endothelial gap formation by adrenomedullin. Histochem Cell Biol 2006; 126: 305–316. [DOI] [PubMed] [Google Scholar]
- 90.Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell 2008; 14: 25–36. [DOI] [PubMed] [Google Scholar]
- 91.Pizurki L, Zhou Z, Glynos K, et al. Angiopoietin-1 inhibits endothelial permeability, neutrophil adherence and IL-8 production. Br J Pharmacol 2003; 139: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.David S, Ghosh CC, Mukherjee A, et al. Angiopoietin-1 requires IQ domain GTPase-activating protein 1 to activate Rac1 and promote endothelial barrier defense. Arterioscler Thromb Vasc Biol 2011; 31: 2643–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]