Abstract
Objectives
Spinal stabilization surgery is an integral part of the treatment of spinal metastatic disease. Bony fusion is the hallmark of spinal stabilization in non-oncology patients. Spinal oncology patients are unlikely to achieve bony fusion due to their overall prognosis and concurrent therapies. Stabilization surgery without fusion may be a reasonable approach for these patients. Literature evaluating the effectiveness of this approach is limited. The object of this study was to investigate the rate of instrumentation failure in patients undergoing posterior spinal instrumented stabilization without fusion for spinal metastatic disease.
Methods
Data from consecutive cases of spinal surgery at our institution during an 81-month period were reviewed. Demographics, clinical notes, and computed tomography (CT) findings were recorded and used to evaluate instrumentation failures. Patients who underwent separation surgery that included laminectomy and posterior spinal instrumentation without fusion for spinal metastatic disease and had follow-up CT scans >3 months postoperatively were selected for the study.
Results
Twenty-seven patients were included in the study. Mean age was 64.85±6.53 years. Nine patients were women. A mean of 1.61±0.96 laminectomy levels was performed. A mean of 8.26±1.48 screws was inserted. The mean postoperative discharge date was 5.07±1.47 days. Mean follow-up duration was 12.17±11.73 months. None of the patients had a change in instrumentation position, pedicle screw pullout, change in spinal alignment, or progressive deformity. No patient required reoperation or instrumentation revision or replacement.
Conclusions
Our experience suggests that instrumented spinal stabilization without fusion is an acceptable approach for patients with spinal metastatic disease.
Keywords: fusion, instrumentation, metastases, spine, spinal metastatic disease, spinal stabilization
INTRODUCTION
The spinal column is the most common site of metastatic bone disease.1 It is also the third most common site for solid tumor metastatic disease, following the lung and the liver.1 Metastatic lesions account for more than 90% of spinal tumors, and the most common origins of metastasis are lung, breast, prostate, and kidney.2–6 The most frequent locations for metastases within the spinal neural axis are the thoracic and thoracolumbar spine (70%), followed by the lumbar spine and sacrum (20%), whereas the least frequent location is the cervical spine.6–8 Spinal metastases may cause neural compression and spinal fracture and can lead to debilitating pain and neurological deterioration. Surgical decompression and spinal stabilization are integral components of the treatment of spinal metastatic disease.6, 7, 9
The hallmark of spinal stabilization surgery in non-oncology patients is the achievement of solid bony fusion.10 Studies have shown that patients with degenerative spinal disease, spinal stenosis, adult scoliosis, spondylolysis, or spondylolisthesis who underwent spinal decompression and fusion and achieved fusion had a better clinical outcome than those who did not achieve spinal fusion.11–26 This improved outcome is due to the lasting spinal stability provided by bony fusion.10 Spinal fusion indications and success rates have evolved in the past 2 decades as an increasing variety of titanium instrumentation and fusion substrates has become commercially available.24, 27–29
The goals of care for spinal oncology patients can differ from those for non-oncology patients due to their overall prognosis and concurrent therapies.30–32 Patients treated for spinal metastatic disease may not live long enough to achieve bony fusion or develop hardware failure.33–35 The healing capacity of their bone is often reduced as a result of continuous chemotherapy, radiation therapy, and poor nutritional status.10, 36, 37 Furthermore, the decortication procedure that is required to stimulate fusion may disturb the body’s natural anatomic barriers that prevent the tumor from additional spread.38 For these reasons, the goals of spinal stabilization in oncology patients include pain relief, preservation of neurological function, prevention of progressive spinal deformity, and improvement of overall survival and quality of life.1 In patients with spinal metastatic disease, fusion might not be essential to achieving these goals. However, instrumented internal bracing of the spine is effective.
The use of instrumented stabilization without fusion has been described for other spinal diseases or disorders. Studies in the spinal trauma literature describe the satisfactory outcome of spinal stabilization without fusion in the management of vertebral fractures.39–45 These studies included the treatment of fractures specifically by posterior instrumentation. Further, a 10-year follow-up study of patients who underwent posterior instrumentation without fusion for traumatic thoracic and lumbar spine fractures supported the effectiveness of non-fusion stabilization.46
Nevertheless, several authors have reported failure of instrumentation in non-oncology patients who underwent stabilization without fusion.47–49 These findings suggest that instrumentation can only stabilize the spine and maintain its alignment while the spine is being fused. The findings further assert that bony elements of the spine cannot be replaced by implanted hardware.48, 50 In addition, other authors suggest that the amount of stress at the bone-screw interface can increase in the absence of a fusion construct, which can lead to instrument loosening or fracture.51
Literature evaluating the effectiveness of spinal stabilization without fusion in patients with spinal metastatic disease is limited. Studies evaluating outcomes of separation surgery likely include many patients that did not achieve bony fusion; however, to the best of the authors’ knowledge, this patient population has yet to be specifically evaluated. The aim of the present study was to evaluate the rate of instrumentation failure in spinal oncology patients undergoing posterior spinal instrumented stabilization without fusion.
MATERIALS AND METHODS
Population, Setting, and Study Design
Consecutive spinal operations performed by a single neurosurgeon over an 81-month period (January 1, 2010 – September 30, 2016) at our institute were reviewed to reduce the possibility of selection bias in this study. Data from patients undergoing surgery before November 11, 2015 were retrospectively collected. After that date, data were prospectively collected. The Research Electronic Data Capture (REDCap) system (Vanderbilt University, Nashville, TN) was used to manage the data. This chart review was conducted after obtaining institutional review board approval. The approval included a Health Insurance Portability and Accountability Act waiver of patient authorization owing to the retrospective nature of and use of de-identified data in this study.
Patients undergoing separation surgery consisting of laminectomy and posterior spinal instrumentation for spinal metastatic disease who had a follow-up computed tomographic (CT) scan >3 months after the surgery were included in the study. Patients with non-metastatic cancer, anterior instrumentation, use of any fusion substrate at surgery, or previous radiation therapy or surgery on the same spinal level were excluded. Partial corpectomy was performed on some study participants. Less than 50% of the vertebral body was removed during corpectomy and no anterior construct was placed. All included patients underwent posterior pedicle screw stabilization with bilateral screw-rod constructs.
Demographic data such as sex, age at the time of surgery, origin of primary carcinoma, and comorbidities were obtained from each patient’s electronic medical record. The number of pedicle screws placed and number of laminectomy levels were also documented. In addition, duration of the surgical procedure, estimated blood loss, type of guidance used for pedicle screw placement (2D fluoroscopy or 3D fluoroscopy with spinal neuronavigation), postoperative date of discharge, and follow-up data and duration were recorded. Any case of postoperative radiation at the same spinal level, postoperative infection, or reoperation for instrumentation revision was documented.
Evaluation of Instrumentation Failure
Preoperative, immediate postoperative, and follow-up CT scans were obtained and reviewed by a neuroradiologist and a neurosurgeon. Patients typically underwent postoperative CT scans at 6 weeks, 3 months, 6 months, and 1 year and were then followed with a CT scan biannually. Criteria for instrumentation failure were identified on CT imaging. The criteria included any change in instrumentation position, pedicle screw pullout, change in spinal alignment, or progressive deformity. Patients were further evaluated during follow-up office visits to assess for any instrumentation failure and need for reoperation and instrumentation revision. Postoperative CT scans were evaluated for any evidence of new bone growth or fusion between the stabilized levels.
Statistical Evaluation
Differences between patient groups who experienced instrumentation failure and those without instrumentation failure were compared using a Student’s t-test.
RESULTS
One hundred and thirty-nine consecutive patients were evaluated. One hundred and twelve of the evaluated patients were excluded from the study. The patients excluded did not require instrumentation (n=63), had non-metastatic disease (n=17), had anterior instrumentation (n=3), did not have a 3-month follow-up examination (n=15), or received fusion material (n=11). One patient underwent a previous surgery on the same spinal level. Finally, 2 additional patients were excluded because they had multiple separate laminectomies on different spinal levels.
The study group was comprised of the remaining 27 patients, 9 of whom were women. The mean age of the patients at the time of surgery was 64.85±6.53 years (Table 1). All included patients had an expected prognosis of 6 months or more as determined by their primary medical oncologist. The primary disease sites and their prevalence in the study group patients were as follows: lung (n=10; 37.0%), kidney (n=10; 37.0%), prostate (n=3; 11.1%), breast (n=1; 3.7%), colon (n=1; 3.7%), thyroid (n=1; 3.7%), and adrenal glands (n=1; 3.7%). The types of comorbidities with their prevalence were diabetes (n=2; 7.4%), hypertension (n=20; 74.1%), renal insufficiency (n=1; 3.7%), heart failure (n=1; 3.7%), and coronary artery disease (n=2; 7.4%). The indication for surgery was the presence of a neurologic deficit, myelopathy, or spinal instability secondary to spinal metastatic disease in a patient with an overall prognosis of >6 months as determined by the patient’s medical oncologist. Operations were performed with 2D fluoroscopy in 5 cases (18.5%) and 3D fluoroscopy with spinal neuronavigation in 22 cases (81.5%). Constructs included 1 (3.7%) cervicothoracic (C7 pedicle fixation), 18 (66.7%) thoracic, 1 (3.7%) thoracolumbar, and 7 (25.9%) lumbar. The mean number of laminectomy levels was 1.61±0.96; the mean number of screws inserted was 8.26±1.48; the mean duration of the surgical procedure was 198.74±43.15 minutes; and the mean estimated blood loss was 836.11±1239mL. Images from a representative case are shown in Figure 1. Partial corpectomy at one vertebral level was performed on 4 patients (14.8%). None of the study patients had a major perioperative complication. Patients were discharged a mean of 5.07±1.47 days after surgery, and there were no postoperative wound infections. The mean follow-up duration was 12.17±11.73 months postoperation. Twenty-four patients (88.9%) underwent postoperative radiotherapy.
Table 1.
Summary of Results
| Summary of Results | |
|---|---|
| Mean Age | 64.85±6.53 years |
| Primary Disease Site | Lung (10), Kidney (10), Prostate (3), Breast (1), Colon (1), Thyroid (1), Adrenal Glands (1) |
| Mean Laminectomy Levels | 1.61±0.96 |
| Mean Pedicle Screws Placed | 8.26±1.48 |
| Spinal Segment Instrumented | Cervicothoracic (1), Thoracic (18), Thoracolumbar (1), Lumbar (7) |
| Average Postoperative Discharge Day | 5.07±1.47 |
| Average Follow-up | 12.17±11.73 months |
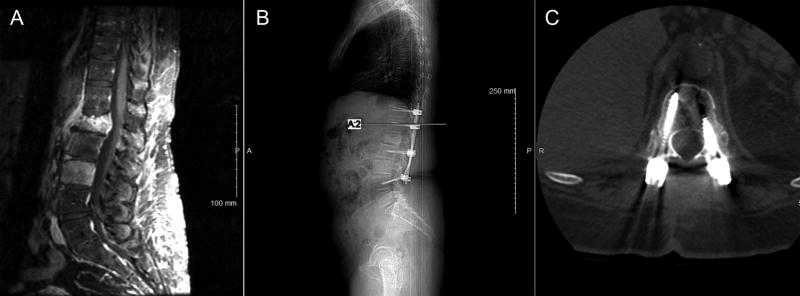
Figure 1.
Representative images from a 61-year-old woman with renal cell carcinoma who presented with intractable axial loading back pain. The patient had an L1 pathologic compression fracture and L3 vertebral body tumor involvement (A, sagittal T1- weighted gadolinium-enhanced magnetic resonance image). She underwent a separation surgery that included an L1 laminectomy and T11–L4 posterior spinal stabilization without fusion. The patient underwent postoperative fractionated radiotherapy. Follow-up spinal CT scan 16 months after the surgical procedure shows stable spinal alignment without evidence of instrumentation failure (B, sagittal and C, axial).
None of the 27 patients experienced any instances of instrumentation failure such as pedicle screw pullout, rod bending, or rod breakage. No changes in spinal alignment, development of kyphosis, or progressive deformity were observed on the follow-up CT scans (Figure 1). As a result, none of these patients required reoperation for instrumentation revision, removal, or replacement. There was no evidence of new bone growth or bony fusion for any of the postoperative CT scans analyzed. No instances of pain or neurologic deficit attributed to spinal instrumentation were noted during clinical evaluations.
DISCUSSION
This study describes a cohort of patients with spinal metastatic disease who underwent instrumented surgical stabilization without fusion. The patients were assessed clinically and with postoperative CT imaging. Instrumentation failure was not identified in any of the patients. The average follow-up time was 12.17 months.
Several characteristics of patients with spinal metastatic disease make them unlikely to achieve bony fusion following surgery. Fehlings et al. reported a median survival time of 7.7 months for patients undergoing surgery for spinal metastatic disease in a prospective, multicenter trial.52 Similar median survival data has been observed in other studies.53–57 In our study, the mean follow-up time was 12.2 months. This contrasts with the process of bony fusion that may occur over 1 year or longer.58, 59 Patients with spinal metastatic disease typically receive postoperative radiation and chemotherapy that may impair new bone formation.36, 37, 60–62 Corticosteroid administration can decrease bone mineral density and compromise osseous healing capacity.63–65 The nutritional compromise observed in oncology patients may further preclude fusion occurrence.38, 61
A potential disadvantage of the lack of fusion in oncology patients is instrumentation failure. Instrumentation failure has been reported in the degenerative and trauma spinal literature; however, it often occurs in a delayed fashion and is unlikely to impact patients with a limited survival expectation.66–69 Screw loosening also typically occurs in a delayed fashion and may not be clinically significant in patients with spinal metastatic disease.51 Rod breakage is a rare event.
There are some potential advantages to eliminating fusion. Operative time, as well as total blood loss, may decrease.44 The risk of further destabilization of the spinal column caused by posterolateral decortication as part of the fusion procedure is eliminated. The possibility that the fusion substrate could stimulate the growth of cancer cells is removed.70–73 Finally, fusion materials are expensive. Cellular allograft and demineralized bone matrix are fusion substrate options in cancer patients; however; they carry a substantial financial cost. List prices for commonly used fusion substrates may be as much as $5,000 (USD).74–76 Excluding these materials represents a substantial cost saving, particularly in high-volume oncology settings.
Surgical outcome studies can be challenging when examining a disease with a relatively short survival rate. Hardware failure is more likely to occur with increasing time from surgery.66 Therefore, we selected patients with >3 months follow-up because they were more likely to develop instrumentation issues. Our median follow-up of 7 months and mean follow-up of 12.17 months is comparable to the results achieved by Fehlings et al.52 in a study with similar group of patients. This represents an adequate follow-up time for the condition studied. Clinical and radiographic data were utilized to assess patients for instrumentation failure. CT imaging accurately detects hardware issues;51, 77–80 and, therefore, it was used to assess instrumentation integrity and spinal alignment for all of the patients in our study.
In most of the patients, the spine was stabilized with long-segment fixation. Pedicle screws were typically placed from 2 levels above to 2 levels below the affected vertebral segment. An average of 8.3 pedicle screws was placed per patient. Following this classic approach to spinal stabilization was likely a contributing factor to the lack of hardware failures. Although an increasing trend exists toward minimally-invasive and short-segment constructs,81, 82 such surgical techniques can result in hardware failure.81, 83 Further, bilateral screw rod constructs were placed in all cases, and the authors would discourage unilateral fixation in the setting of spinal metastatic disease.
The patients included in this study underwent a posterior separation surgery involving posterior and posterolateral decompression of the neural elements and spinal stabilization.9 Many of these patients had significant anterior vertebral body disease, and 4 patients had a partial corpectomy performed from a posterolateral approach without cage placement. Presumably, patients with anterior disease, a posterior and posterolateral decompression, and a long posterior construct would be at risk for hardware failure. However, hardware failure in this setting was not observed. Patients undergoing surgery for spinal metastatic disease are unlikely to achieve bony fusion or hardware failure regardless of the technique/substrate employed in large part due to their life expectancy.
The heterogeneity of patients with spinal metastatic disease makes them a difficult population to study because characteristics such as pathology, chemotherapeutic regimens, sites of distant metastases, and patient treatment goals vary. Studies of diverse populations can be limited by selection bias. We restricted the population to those patients with adequate follow-up who met our selection criteria; however, this inclusion process also limited our study population. There were no instances of instrumentation failure in our study group so a true failure rate could not be determined.
Recent advances in immune therapy and targeted therapy for systemic cancers has improved the expected survival for certain cancer types.84–86 If significant survival improvements occur for patients with spinal metastatic disease, long-term bony fusion may become achievable and also necessary. The spinal surgeon should work closely with a medical oncologist to accurately assess a patient’s prognosis given the available therapies and determine the spinal treatment goals.
CONCLUSIONS
Instrumented spinal stabilization without fusion is an acceptable surgical approach for the treatment of spinal metastatic disease. The risk of hardware failure or progressive deformity following posterior spinal instrumentation without fusion for spinal metastatic disease is low. Spinal surgeons, particularly those accustomed to routinely performing fusion procedures in non-oncology patients, should be aware of these findings.
Highlights.
To the best of our knowledge, this is the first reported analysis of spinal instrumentation without fusion for spinal metastatic disease.
Our report provides a useful outcomes analysis for a cohort of patients with spinal metastatic disease undergoing surgical stabilization.
Acknowledgments
The authors thank Paul H. Dressel BFA for preparation of the illustrations and Elaine C. Mosher MLS and Debra J. Zimmer for editorial assistance.
Funding Source:
This work was supported by Roswell Park Cancer Institute and National Cancer Institute (NCI) grant P30CA016056.
Dr. Fabiano receives research funding from Arbor Pharmaceuticals.
Abbreviations
- CT
computed tomographic
- REDCap
Research Electronic Data Capture
Footnotes
Ethics Approval: Roswell Park Cancer Institute IRB: I 275815
Declaration of Interests:
Ms. Drakhshandeh and Mr. Miller report no disclosures.
Previous Presentation: None
Author Contributions:
Conception and design: AJF; Data acquisition: DD, JAM; Data analysis and interpretation: AJF, DD, JAM; Drafting the manuscript AJF, DD, JAM, Critically revising the manuscript: AJF, DD, JAM; Final approval of the manuscript: AJF, DD, JAM
References
- 1.Lee CS, Jung CH. Metastatic spinal tumor. Asian Spine J. 2012;6:71–87. doi: 10.4184/asj.2012.6.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi D, Crockard A, Bunger C, Harms J, Kawahara N, Mazel C, Melcher R, Tomita K. Review of metastatic spine tumour classification and indications for surgery: the consensus statement of the Global Spine Tumour Study Group. Eur Spine J. 2010;19:215–22. doi: 10.1007/s00586-009-1252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciftdemir M, Kaya M, Selcuk E, Yalniz E. Tumors of the spine. World J Orthop. 2016;7:109–16. doi: 10.5312/wjo.v7.i2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ecker RD, Endo T, Wetjen NM, Krauss WE. Diagnosis and treatment of vertebral column metastases. Mayo Clin Proc. 2005;80:1177–86. doi: 10.4065/80.9.1177. [DOI] [PubMed] [Google Scholar]
- 5.Ha K-Y, Kim YH, Ahn J-H, Park H-Y. Factors Affecting Survival in Patients Undergoing Palliative Spine Surgery for Metastatic Lung and Hepatocellular Cancer: Dose the Type of Surgery Influence the Surgical Results for Metastatic Spine Disease? Clin Orthop Surg. 2015;7:344–50. doi: 10.4055/cios.2015.7.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klimo P, Schmidt MH. Surgical Management of Spinal Metastases. Oncologist. 2004;9:188–96. doi: 10.1634/theoncologist.9-2-188. [DOI] [PubMed] [Google Scholar]
- 7.Fanous AA, Fabiano AJ. Surgical management of spinal metastatic disease. J Neurosurg Sci. 2016;61:316–24. doi: 10.23736/S0390-5616.16.03914-X. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1978;3:40–51. doi: 10.1002/ana.410030107. [DOI] [PubMed] [Google Scholar]
- 9.Laufer I, Rubin DG, Lis E, Cox BW, Stubblefield MD, Yamada Y, Bilsky MH. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist. 2013;18:744–51. doi: 10.1634/theoncologist.2012-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vrionis FD. Surgical management of metastatic spinal neoplasms. Neurosurg Focus. 2003;15:1–8. doi: 10.3171/foc.2003.15.5.12. [DOI] [PubMed] [Google Scholar]
- 11.Andersen T, Videbaek TS, Hansen ES, Bunger C, Christensen FB. The positive effect of posterolateral lumbar spinal fusion is preserved at long-term follow-up: a RCT with 11-13 year follow-up. Eur Spine J. 2008;17:272–80. doi: 10.1007/s00586-007-0494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daffner SD, Vaccaro AR. Adult degenerative lumbar scoliosis. Am J Orthop (Belle Mead NJ) 2003;32:77–82. discussion. [PubMed] [Google Scholar]
- 13.Ekman P, Moller H, Hedlund R. The long-term effect of posterolateral fusion in adult isthmic spondylolisthesis: a randomized controlled study. Spine J. 2005;5:36–44. doi: 10.1016/j.spinee.2004.05.249. [DOI] [PubMed] [Google Scholar]
- 14.Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am. 1991;73:802–8. [PubMed] [Google Scholar]
- 15.Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine (Phila Pa 1976) 2004;29:726–33. doi: 10.1097/01.brs.0000119398.22620.92. discussion 33-4. [DOI] [PubMed] [Google Scholar]
- 16.Kuroki H, Tajima N, Kubo S. Clinical results of posterolateral fusion for degenerative lumbar spinal diseases: a follow-up study of more than 10 years. J Orthop Sci. 2002;7:317–24. doi: 10.1007/s007760200054. [DOI] [PubMed] [Google Scholar]
- 17.Mardjetko SM, Connolly PJ, Shott S. Degenerative lumbar spondylolisthesis. A meta-analysis of literature 1970-1993. Spine (Phila Pa 1976) 1994;19:2256s–65s. [PubMed] [Google Scholar]
- 18.Martin CR, Gruszczynski AT, Braunsfurth HA, Fallatah SM, O'Neil J, Wai EK. The surgical management of degenerative lumbar spondylolisthesis: a systematic review. Spine (Phila Pa 1976) 2007;32:1791–8. doi: 10.1097/BRS.0b013e3180bc219e. [DOI] [PubMed] [Google Scholar]
- 19.McPhee IB, Swanson CE. The surgical management of degenerative lumbar scoliosis. Posterior instrumentation alone versus two stage surgery. Bull Hosp Jt Dis. 1998;57:16–22. [PubMed] [Google Scholar]
- 20.Nasca RJ. Rationale for spinal fusion in lumbar spinal stenosis. Spine (Phila Pa 1976) 1989;14:451–4. doi: 10.1097/00007632-198904000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Resnick DK, Watters WC, 3rd, Sharan A, Mummaneni PV, Dailey AT, Wang JC, Choudhri TF, Eck J, Ghogawala Z, Groff MW, Dhall SS, Kaiser MG. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: lumbar fusion for stenosis with spondylolisthesis. J Neurosurg Spine. 2014;21:54–61. doi: 10.3171/2014.4.SPINE14274. [DOI] [PubMed] [Google Scholar]
- 22.Topalidou A, Tzagarakis G, Balalis K, Papaioannou A. Posterior decompression and fusion: whole-spine functional and clinical outcomes. PLoS One. 2016;11:e0160213. doi: 10.1371/journal.pone.0160213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turunen V, Nyyssonen T, Miettinen H, Airaksinen O, Aalto T, Hakumaki J, Kroger H. Lumbar instrumented posterolateral fusion in spondylolisthetic and failed back patients: a long-term follow-up study spanning 11-13 years. Eur Spine J. 2012;21:2140–8. doi: 10.1007/s00586-012-2320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang JC, Mummaneni PV, Haid RW. Current treatment strategies for the painful lumbar motion segment: posterolateral fusion versus interbody fusion. Spine (Phila Pa 1976) 2005;30:S33–43. doi: 10.1097/01.brs.0000174559.13749.83. [DOI] [PubMed] [Google Scholar]
- 25.Wu CH, Wong CB, Chen LH, Niu CC, Tsai TT, Chen WJ. Instrumented posterior lumbar interbody fusion for patients with degenerative lumbar scoliosis. J Spinal Disord Tech. 2008;21:310–5. doi: 10.1097/BSD.0b013e318148b256. [DOI] [PubMed] [Google Scholar]
- 26.Zagra A, Giudici F, Minoia L, Corriero AS, Zagra L. Long-term results of pediculo-body fixation and posterolateral fusion for lumbar spondylolisthesis. Eur Spine J. 2009;18:151–5. doi: 10.1007/s00586-009-0997-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersen T, Christensen FB, Niedermann B, Helmig P, Hoy K, Hansen ES, Bunger C. Impact of instrumentation in lumbar spinal fusion in elderly patients: 71 patients followed for 2-7 years. Acta Orthop. 2009;80:445–50. doi: 10.3109/17453670903170505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu SS, Pashman RS. Spinal instrumentation. Evolution and state of the art. Invest Radiol. 1992;27:632–47. doi: 10.1097/00004424-199208000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976) 2012;37:67–76. doi: 10.1097/BRS.0b013e31820cccfb. [DOI] [PubMed] [Google Scholar]
- 30.Ghogawala Z, Mansfield FL, Borges LF. Spinal radiation before surgical decompression adversely affects outcomes of surgery for symptomatic metastatic spinal cord compression. Spine (Phila Pa 1976) 2001;26:818–24. doi: 10.1097/00007632-200104010-00025. [DOI] [PubMed] [Google Scholar]
- 31.McPhee IB, Williams RP, Swanson CE. Factors influencing wound healing after surgery for metastatic disease of the spine. Spine (Phila Pa 1976) 1998;23:726–32. doi: 10.1097/00007632-199803150-00015. discussion 32–3. [DOI] [PubMed] [Google Scholar]
- 32.Sundaresan N, Rothman A, Manhart K, Kelliher K. Surgery for solitary metastases of the spine: rationale and results of treatment. Spine (Phila Pa 1976) 2002;27:1802–6. doi: 10.1097/00007632-200208150-00021. [DOI] [PubMed] [Google Scholar]
- 33.Finkelstein JA, Zaveri G, Wai E, Vidmar M, Kreder H, Chow E. A population-based study of surgery for spinal metastases. Survival rates and complications. J Bone Joint Surg Br. 2003;85:1045–50. doi: 10.1302/0301-620x.85b7.14201. [DOI] [PubMed] [Google Scholar]
- 34.Onimus M, Papin P, Gangloff S. Results of surgical treatment of spinal thoracic and lumbar metastases. Eur Spine J. 1996;5:407–11. doi: 10.1007/BF00301969. [DOI] [PubMed] [Google Scholar]
- 35.Wibmer C, Leithner A, Hofmann G, Clar H, Kapitan M, Berghold A, Windhager R. Survival analysis of 254 patients after manifestation of spinal metastases: evaluation of seven preoperative scoring systems. Spine (Phila Pa 1976) 2011;36:1977–86. doi: 10.1097/BRS.0b013e3182011f84. [DOI] [PubMed] [Google Scholar]
- 36.Georgiou KR, Scherer MA, Fan CM, Cool JC, King TJ, Foster BK, Xian CJ. Methotrexate chemotherapy reduces osteogenesis but increases adipogenic potential in the bone marrow. J Cell Physiol. 2012;227:909–18. doi: 10.1002/jcp.22807. [DOI] [PubMed] [Google Scholar]
- 37.Sugimoto M, Takahashi S, Toguchida J, Kotoura Y, Shibamoto Y, Yamamuro T. Changes in bone after high-dose irradiation. Biomechanics and histomorphology. J Bone Joint Surg Br. 1991;73:492–7. doi: 10.1302/0301-620X.73B3.1670456. [DOI] [PubMed] [Google Scholar]
- 38.Rose PS, Clarke MJ, Dekutoski MB. Minimally invasive treatment of spinal metastases: techniques. Int J Surg Oncol. 2011;2011:6. doi: 10.1155/2011/494381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chou PH, Ma HL, Wang ST, Liu CL, Chang MC, Yu WK. Fusion may not be a necessary procedure for surgically treated burst fractures of the thoracolumbar and lumbar spines: a follow-up of at least ten years. J Bone Joint Surg Am. 2014;96:1724–31. doi: 10.2106/JBJS.M.01486. [DOI] [PubMed] [Google Scholar]
- 40.Dai LY, Jiang LS, Jiang SD. Posterior short-segment fixation with or without fusion for thoracolumbar burst fractures. a five to seven-year prospective randomized study. J Bone Joint Surg Am. 2009;91:1033–41. doi: 10.2106/JBJS.H.00510. [DOI] [PubMed] [Google Scholar]
- 41.Fu MC, Nemani VM, Albert TJ. Operative Treatment of Thoracolumbar Burst Fractures: Is Fusion Necessary? HSS J. 2015;11:187–9. doi: 10.1007/s11420-015-9439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jindal N, Sankhala SS, Bachhal V. The role of fusion in the management of burst fractures of the thoracolumbar spine treated by short segment pedicle screw fixation: a prospective randomised trial. J Bone Joint Surg Br. 2012;94:1101–6. doi: 10.1302/0301-620X.94B8.28311. [DOI] [PubMed] [Google Scholar]
- 43.Sanderson PL, Fraser RD, Hall DJ, Cain CM, Osti OL, Potter GR. Short segment fixation of thoracolumbar burst fractures without fusion. Eur Spine J. 1999;8:495–500. doi: 10.1007/s005860050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian NF, Wu YS, Zhang XL, Wu XL, Chi YL, Mao FM. Fusion versus nonfusion for surgically treated thoracolumbar burst fractures: a meta-analysis. PLoS One. 2013;8:e63995. doi: 10.1371/journal.pone.0063995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang ST, Ma HL, Liu CL, Yu WK, Chang MC, Chen TH. Is fusion necessary for surgically treated burst fractures of the thoracolumbar and lumbar spine?: a prospective, randomized study. Spine (Phila Pa 1976) 2006;31:2646–52. doi: 10.1097/01.brs.0000244555.28310.40. discussion 53. [DOI] [PubMed] [Google Scholar]
- 46.Kocanli O, Komur B, Duymus TM, Guclu B, Yilmaz B, Sesli E. Ten-year follow-up results of posterior instrumentation without fusion for traumatic thoracic and lumbar spine fractures. J Orthop. 2016;13:301–5. doi: 10.1016/j.jor.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burkus JK. Surgical treatment of the painful motion segment: matching technology with indications. Spine (Phila Pa 1976) 2005;30:S7–15. doi: 10.1097/01.brs.0000175093.48994.4d. [DOI] [PubMed] [Google Scholar]
- 48.Slone RM, McEnery KW, Bridwell KH, Montgomery WJ. Fixation techniques and instrumentation used in the thoracic, lumbar, and lumbosacral spine. Radiol Clin North Am. 1995;33:233–65. [PubMed] [Google Scholar]
- 49.Young PM, Berquist TH, Bancroft LW, Peterson JJ. Complications of spinal instrumentation. Radiographics. 2007;27:775–89. doi: 10.1148/rg.273065055. [DOI] [PubMed] [Google Scholar]
- 50.Nouh MR. Spinal fusion-hardware construct: Basic concepts and imaging review. World J Radiol. 2012;4:193–207. doi: 10.4329/wjr.v4.i5.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galbusera F, Volkheimer D, Reitmaier S, Berger-Roscher N, Kienle A, Wilke HJ. Pedicle screw loosening: a clinically relevant complication? Eur Spine J. 2015;24:1005–16. doi: 10.1007/s00586-015-3768-6. [DOI] [PubMed] [Google Scholar]
- 52.Fehlings MG, Nater A, Tetreault L, Kopjar B, Arnold P, Dekutoski M, Finkelstein J, Fisher C, France J, Gokaslan Z, Massicotte E, Rhines L, Rose P, Sahgal A, Schuster J, Vaccaro A. Survival and Clinical Outcomes in Surgically Treated Patients With Metastatic Epidural Spinal Cord Compression: Results of the Prospective Multicenter AOSpine Study. J Clin Oncol. 2016;34:268–76. doi: 10.1200/JCO.2015.61.9338. [DOI] [PubMed] [Google Scholar]
- 53.Bauer HC, Wedin R. Survival after surgery for spinal and extremity metastases. Prognostication in 241 patients. Acta Orthop Scand. 1995;66:143–6. doi: 10.3109/17453679508995508. [DOI] [PubMed] [Google Scholar]
- 54.Cho DC, Sung JK. Palliative surgery for metastatic thoracic and lumbar tumors using posterolateral transpedicular approach with posterior instrumentation. Surg Neurol. 2009;71:424–33. doi: 10.1016/j.surneu.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 55.Hirabayashi H, Ebara S, Kinoshita T, Yuzawa Y, Nakamura I, Takahashi J, Kamimura M, Ohtsuka K, Takaoka K. Clinical outcome and survival after palliative surgery for spinal metastases: palliative surgery in spinal metastases. Cancer. 2003;97:476–84. doi: 10.1002/cncr.11039. [DOI] [PubMed] [Google Scholar]
- 56.Sioutos PJ, Arbit E, Meshulam CF, Galicich JH. Spinal metastases from solid tumors. Analysis of factors affecting survival. Cancer. 1995;76:1453–9. doi: 10.1002/1097-0142(19951015)76:8<1453::aid-cncr2820760824>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 57.van der Linden YM, Dijkstra SP, Vonk EJ, Marijnen CA, Leer JW. Prediction of survival in patients with metastases in the spinal column: results based on a randomized trial of radiotherapy. Cancer. 2005;103:320–8. doi: 10.1002/cncr.20756. [DOI] [PubMed] [Google Scholar]
- 58.Boriani S, Biagini R, Bandiera S, Gasbarrini A, De Iure F. Reconstruction of the anterior column of the thoracic and lumbar spine with a carbon fiber stackable cage system. Orthopedics. 2002;25:37–42. doi: 10.3928/0147-7447-20020101-14. [DOI] [PubMed] [Google Scholar]
- 59.Wilden JA, Moran SL, Dekutoski MB, Bishop AT, Shin AY. Results of vascularized rib grafts in complex spinal reconstruction. J Bone Joint Surg Am. 2006;88:832–9. doi: 10.2106/JBJS.E.00409. [DOI] [PubMed] [Google Scholar]
- 60.Emery SE, Brazinski MS, Koka A, Bensusan JS, Stevenson S. The biological and biomechanical effects of irradiation on anterior spinal bone grafts in a canine model. J Bone Joint Surg Am. 1994;76:540–8. doi: 10.2106/00004623-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 61.Itshayek E, Cohen JE, Yamada Y, Gokaslan Z, Polly DW, Rhines LD, Schmidt MH, Varga PP, Mahgarefteh S, Fraifeld S, Gerszten PC, Fisher CG. Timing of stereotactic radiosurgery and surgery and wound healing in patients with spinal tumors: a systematic review and expert opinions. Neurol Res. 2014;36:510–23. doi: 10.1179/1743132814Y.0000000380. [DOI] [PubMed] [Google Scholar]
- 62.Kim TK, Cho W, Youn SM, Chang UK. The Effect of Perioperative Radiation Therapy on Spinal Bone Fusion Following Spine Tumor Surgery. J Korean Neurosurg Soc. 2016;59:597–603. doi: 10.3340/jkns.2016.59.6.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Narayan P, Haid RW, Subach BR, Comey CH, Rodts GE. Effect of spinal disease on successful arthrodesis in lumbar pedicle screw fixation. J Neurosurg. 2002;97:277–80. doi: 10.3171/spi.2002.97.3.0277. [DOI] [PubMed] [Google Scholar]
- 64.Sawin PD, Dickman CA, Crawford NR, Melton MS, Bichard WD, Sonntag VK. The effects of dexamethasone on bone fusion in an experimental model of posterolateral lumbar spinal arthrodesis. J Neurosurg. 2001;94:76–81. doi: 10.3171/spi.2001.94.1.0076. [DOI] [PubMed] [Google Scholar]
- 65.Soares-Schanoski A, Gomez-Pina V, del Fresno C, Rodriguez-Rojas A, Garcia F, Glaria A, Sanchez M, Vallejo-Cremades MT, Baos R, Fuentes-Prior P, Arnalich F, Lopez-Collazo E. 6-Methylprednisolone down-regulates IRAK-M in human and murine osteoclasts and boosts bone-resorbing activity: a putative mechanism for corticoid-induced osteoporosis. J Leukoc Biol. 2007;82:700–9. doi: 10.1189/jlb.1106673. [DOI] [PubMed] [Google Scholar]
- 66.Amankulor NM, Xu R, Iorgulescu JB, Chapman T, Reiner AS, Riedel E, Lis E, Yamada Y, Bilsky M, Laufer I. The incidence and patterns of hardware failure after separation surgery in patients with spinal metastatic tumors. Spine J. 2014;14:1850–9. doi: 10.1016/j.spinee.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 67.Bellato RT, Teixeira WG, Torelli AG, Cristante AF, de Barros TE, de Camargo OP. Late failure of posterior fixation without bone fusion for vertebral metastases. Acta Ortop Bras. 2015;23:303–6. doi: 10.1590/1413-785220152306151402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elder BD, Ishida W, Goodwin CR, Bydon A, Gokaslan ZL, Sciubba DM, Wolinsky JP, Witham TF. Bone graft options for spinal fusion following resection of spinal column tumors: systematic review and meta-analysis. Neurosurg Focus. 2017;42:E16. doi: 10.3171/2016.8.FOCUS16112. [DOI] [PubMed] [Google Scholar]
- 69.Renshaw TS. The role of Harrington instrumentation and posterior spine fusion in the management of adolescent idiopathic scoliosis. Orthop Clin North Am. 1988;19:257–67. [PubMed] [Google Scholar]
- 70.Sayama C, Willsey M, Chintagumpala M, Brayton A, Briceno V, Ryan SL, Luerssen TG, Hwang SW, Jea A. Routine use of recombinant human bone morphogenetic protein-2 in posterior fusions of the pediatric spine and incidence of cancer. J Neurosurg Pediatr. 2015;16:4–13. doi: 10.3171/2014.10.PEDS14199. [DOI] [PubMed] [Google Scholar]
- 71.Skovrlj B, Koehler SM, Anderson PA, Qureshi SA, Hecht AC, Iatridis JC, Cho SK. Association Between BMP-2 and Carcinogenicity. Spine (Phila Pa 1976) 2015;40:1862–71. doi: 10.1097/BRS.0000000000001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sonn KA, Kannan AS, Bellary SS, Yun C, Hashmi SZ, Nelson JT, Ghodasra JH, Nickoli MS, Parimi V, Ghosh A, Shawen N, Ashtekar A, Stock SR, Hsu EL, Hsu WK. Effect of recombinant human bone morphogenetic protein-2 on a novel lung cancer spine metastasis model in rodents. J Orthop Res. 2016;34:1274–81. doi: 10.1002/jor.23139. [DOI] [PubMed] [Google Scholar]
- 73.Vavken J, Mameghani A, Vavken P, Schaeren S. Complications and cancer rates in spine fusion with recombinant human bone morphogenetic protein-2 (rhBMP-2) Eur Spine J. 2016;25:3979–89. doi: 10.1007/s00586-015-3870-9. [DOI] [PubMed] [Google Scholar]
- 74.Bostrom MP, Seigerman DA. The clinical use of allografts, demineralized bone matrices, synthetic bone graft substitutes and osteoinductive growth factors: a survey study. HSS J. 2005;1:9–18. doi: 10.1007/s11420-005-0111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Epstein NE. Pros, cons, and costs of INFUSE in spinal surgery. Surg Neurol Int. 2011;2:10. doi: 10.4103/2152-7806.76147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Glassman SD, Carreon LY, Campbell MJ, Johnson JR, Puno RM, Djurasovic M, Dimar JR. The perioperative cost of Infuse bone graft in posterolateral lumbar spine fusion. Spine J. 2008;8:443–8. doi: 10.1016/j.spinee.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 77.Abul-Kasim K, Ohlin A. Evaluation of implant loosening following segmental pedicle screw fixation in adolescent idiopathic scoliosis: a 2 year follow-up with low-dose CT. Scoliosis. 2014;9:13. doi: 10.1186/1748-7161-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Castro WH, Halm H, Jerosch J, Malms J, Steinbeck J, Blasius S. Accuracy of pedicle screw placement in lumbar vertebrae. Spine (Phila Pa 1976) 1996;21:1320–4. doi: 10.1097/00007632-199606010-00008. [DOI] [PubMed] [Google Scholar]
- 79.Farber GL, Place HM, Mazur RA, Jones DE, Damiano TR. Accuracy of pedicle screw placement in lumbar fusions by plain radiographs and computed tomography. Spine (Phila Pa 1976) 1995;20:1494–9. doi: 10.1097/00007632-199507000-00010. [DOI] [PubMed] [Google Scholar]
- 80.Ohtori S, Inoue G, Orita S, Yamauchi K, Eguchi Y, Ochiai N, Kishida S, Kuniyoshi K, Aoki Y, Nakamura J, Ishikawa T, Miyagi M, Kamoda H, Suzuki M, Kubota G, Sakuma Y, Oikawa Y, Inage K, Sainoh T, Takaso M, Toyone T, Takahashi K. Comparison of teriparatide and bisphosphonate treatment to reduce pedicle screw loosening after lumbar spinal fusion surgery in postmenopausal women with osteoporosis from a bone quality perspective. Spine (Phila Pa 1976) 2013;38:E487–92. doi: 10.1097/BRS.0b013e31828826dd. [DOI] [PubMed] [Google Scholar]
- 81.Logroscino CA, Proietti L, Tamburrelli FC. Minimally invasive spine stabilisation with long implants. Eur Spine J. 2009;18(Suppl 1):75–81. doi: 10.1007/s00586-009-0995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mobbs RJ, Sivabalan P, Li J. Technique, challenges and indications for percutaneous pedicle screw fixation. J Clin Neurosci. 2011;18:741–9. doi: 10.1016/j.jocn.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 83.Lin F, Yamaguchi U, Matsunobu T, Kobayashi E, Nakatani F, Kawai A, Chuman H. Minimally invasive solid long segmental fixation combined with direct decompression in patients with spinal metastatic disease. Int J Surg. 2013;11:173–7. doi: 10.1016/j.ijsu.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 84.Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res. 2015;4:36–54. doi: 10.3978/j.issn.2218-6751.2014.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thurlimann B, Senn HJ, Panel M. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26:1533–46. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman DM, Sequist LV, Smith DC, Leming P, Carbone DP, Pinder-Schenck MC, Topalian SL, Hodi FS, Sosman JA, Sznol M, McDermott DF, Pardoll DM, Sankar V, Ahlers CM, Salvati M, Wigginton JM, Hellmann MD, Kollia GD, Gupta AK, Brahmer JR. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2015;33:2004–12. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]