Abstract
Background
The changes in body composition that occur in response to therapy for localized PDAC and during the early survivorship period, as well as their clinical significance, are poorly understood.
Methods
127 consecutive patients with PDAC who received preoperative therapy followed by pancreatoduodenectomy at a single institution between 2009–2012 were longitudinally evaluated. Changes in skeletal muscle (SKM), visceral adipose tissue (VAT), and subcutaneous adipose tissue (SAT) were measured on serial computed tomography images obtained upon presentation, prior to pancreatectomy, and approximately 3 and 12 months after surgery.
Results
Prior to therapy, patients’ mean baseline BMI was 26.5±4.7 Kg/m2 and 63.0% met radiographic criteria for sarcopenia. During a mean 5.4±2.3 months of preoperative therapy, minimal changes in SKM (−0.5±7.8%, p>0.05), VAT (−1.8±62.6%, p<0.001), and SAT (−4.8±27.7%, p<0.001) were observed. In contrast, clinically significant changes were observed on post-operative CT compared to baseline anthropometry: SKM −4.1±10.7%, VAT −38.7±30.2%, and SAT −24.1±22.6% (all p<0.001) and these changes persisted at one year following PD. While anthropometric changes during preoperative therapy were not independently associated with survival, SKM gain between the postoperative period and one year follow-up was associated with improved overall survival (OR 0.50, 95% CI 0.29–0.87).
Conclusions
In contrast to the minor changes that occur during preoperative therapy for PDAC, significant losses in key anthropometric parameters tend to occur over the first year following PD. Ongoing SKM loss in the postoperative period may represent an early marker for worse outcomes.
Keywords: pancreatic ductal adenocarcinoma, body composition, neoadjuvant therapy, whipple, pancreatoduodenectomy, pancreatectomy
Introduction
Patients with pancreatic ductal adenocarcinoma (PDAC) commonly experience anthropometric changes in association with their cancer [1]. The involuntary loss of skeletal muscle is a poor prognostic factor among patients with lung, gastrointestinal, and hepatopancreatobiliary cancers[2,3], including advanced PDAC[4]. Among patients with newly diagnosed PDAC, the depletion of skeletal muscle has been associated with shorter survival following pancreatectomy [3,5,6]. Although the etiology of this observation is probably multifactorial, it may in part reflect relative differences in the physiologic reserve between patients who present with early and advanced disease or differences in patients’ ability to tolerate therapy [7]. On the other hand, treatments for PDAC may themselves contribute to nutritional and physiologic depletion [4].
We have previously shown that depletion of skeletal muscle, visceral adipose tissue, and subcutaneous adipose tissue occurs concurrent with preoperative therapy but that these changes do not preclude subsequent pancreatectomy [8]. Other studies have reported that clinically significant anthropometric changes occur during neoadjuvant therapy for various malignancies [9–11]. However, the anthropometric and nutritional changes that occur following curative therapy for localized PDAC and throughout the survivorship period have not previously been investigated. Such changes might have prognostic value and/or reflect physiologic disturbances that might be targeted to optimize treatment outcomes.
The purpose of this study was to quantify and characterize the anthropometric and nutritional changes that occur in patients with localized PDAC over the course of therapy and the first postoperative year and to document their associations, if any, with survival. We hypothesized that clinically significant derangements of key anthropometric indices would occur during therapy.
Materials & Methods
The University of Texas MD Anderson Cancer Center’s (MDACC) institutional review board approved this retrospective study. Patients were identified from a prospectively maintained institutional pancreatic tumor database [12]. Consecutive patients with PDAC who completed preoperative chemotherapy and/or chemoradiation and underwent pancreatoduodenectomy (PD) between January 2009 and December 2012 were included.
Staging
Prior to the initiation of preoperative therapy, patients underwent comprehensive clinical and radiographic staging that included cross-sectional computed tomography (CT) imaging of the abdomen and pelvis, a chest x-ray or CT scan, a physical examination, and full laboratory studies, including serum CA 19-9 level. Tumors were anatomically staged as potentially resectable, borderline resectable, or locally advanced based on previously published MD Anderson criteria [13].
Preoperative Therapy
Preoperative therapy was administered, either on or off protocol, according to the recommendations of each patient’s multidisciplinary care team, and it was delivered either at MD Anderson or at the referring facility in close collaboration with MD Anderson physicians. Several chemotherapy regimens were utilized during the study period [14]. External-beam radiation therapy was generally delivered to a total of 50.4 Gy over 6 weeks (standard fractionated: 1.8 Gy, 28 fractions) or to a total of 30 Gy over 2 weeks (hypofractionated: 3 Gy, 10 fractions) with concurrent 5-fluorouracil (FU), capecitabine, or gemcitabine [15].
Pancreatoduodenectomy
Within 4–8 weeks following completion of all intended preoperative therapy, patients underwent a comprehensive restaging evaluation. Patients with a performance status sufficient for major abdominal surgery and who had no radiographic or intraoperative findings of disease progression were selected for pancreatectomy [16]. PD was performed at MD Anderson using a standardized technique [17].
Postoperative Therapy and Follow-up
Following pancreatectomy, postoperative therapy was administered selectively based on individual patient and pathology characteristics and physician preference. Patients were typically evaluated initially every 3–4 months, later extended to every 6 months, with cross-sectional imaging, physical examination, and CA 19-9 analysis according to a standardized surveillance protocol [18].
Anthropometric Analysis
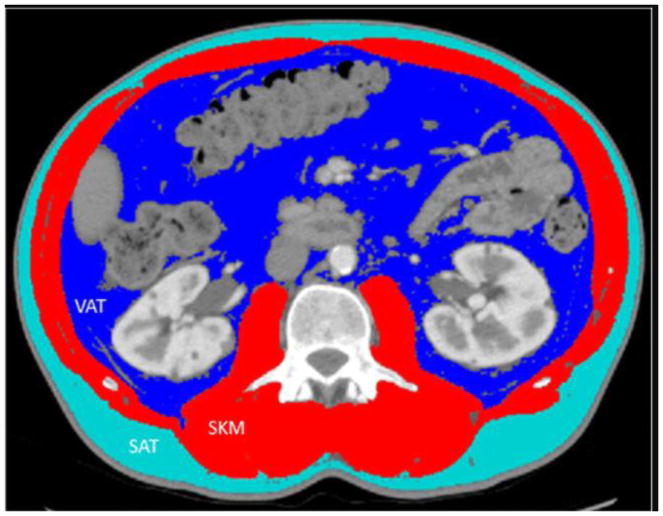
The cross-sectional areas of skeletal muscle (SKM), visceral adipose tissue (VAT), and subcutaneous adipose tissue (SAT) were assessed at the L3 vertebral body midpoint on serial CT images (Figure 1) using sliceOmatic v5.0 software (TomoVision, Magog, Canada). Cross-sectional areas were standardized to the square of the patient’s height in meters. Gender-specific thresholds defining radiographic evidence of sarcopenia were established as ≤38.9 cm2/m2 for women and ≤55.4 cm2/m2 for men [19]. Body mass index (BMI), calculated by dividing the patient’s weight in kilograms by height in meters squared, and serum albumin were also measured at corresponding time points.
Figure 1.
Representative computed tomography image with anthropometric measurements. SKM, skeletal muscle; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue
Statistical Analysis
Anthropometric and nutritional parameters were measured upon presentation, prior to PD, and approximately 3 and 12 months after surgery. Differences compared to baseline were first assessed using paired t-tests. These comparisons only test for differences in the setting of complete data, which were not available for all patients. In order to control for missing data, changes over time for each anthropometric and nutritional parameter were also analyzed utilizing a multilevel mixed-effects linear regression model, which takes into account correlations between measurements and fixed effects. Beta coefficients (B) and 95% confidence intervals (CIs) were calculated.
Next, univariate Cox proportional hazards regression models were created to evaluate the association between clinicopathologic and anthropometric factors and overall survival, which was calculated from the date of tissue diagnosis to the date of death. Baseline values as well as changes in anthropometrics over time, per 10 units cm2/m2, were analyzed for their potential association with survival. Hazard ratios (HR) and 95% CIs were calculated. Statistical significance was set at a two-tailed p-value <0.05. All statistical analyses were performed using Stata/SE version 14.1 statistical software (StataCorp LLC, College Station, TX).
Results
The clinicopathologic profile of 127 consecutive patients with PDAC who received chemotherapy and/or chemoradiation prior to PD is reported in Table 1. Upon presentation, patients’ mean age was 64.6 ± 8.9 years, their mean BMI was 26.5 ± 4.7 kg/m2, and 80 (63.0%) met established radiographic criteria for evidence of sarcopenia. Prior to PD, 12 (9.4%) patients received chemotherapy alone, 44 (34.6%) received chemoradiation alone, and 71 (55.9%) received both, over a mean duration of 5.4 ± 2.3 months. The surgical specimens of 122 (96.1%) patients had negative (R0) margins; 62 (48.8%) had negative lymph nodes. Following surgery, 64 (50.4%) patients received postoperative systemic chemotherapy.
Table 1.
Clinicopathologic profile of 127 patients with pancreatic ductal adenocarcinoma who received chemotherapy and/or chemoradiation prior to pancreatoduodenectomy.
| Parameter | Value |
|---|---|
| Profile | |
| Patient characteristics | |
| Mean age, years (SD) | 64.6 (8.9) |
| Sex, n (%) | |
| Male | 68 (53.5) |
| Female | 59 (46.5) |
| Mean BMI, kg/m2 (SD) | 26.5 (4.7) |
| Radiographic Sarcopenia, n (%) | 80 (63.0) |
| Tumor characteristics | |
| Radiographic stage, n (%) | |
| Potentially resectable | 91 (71.7) |
| Borderline resectable | 23 (18.1) |
| Locally advanced | 13 (10.2) |
| Median pretreatment CA 19-9, U/mL (1st, 3rd quartile) | 136 (45, 395) |
| Nonoperative therapy | |
| Preoperative systemic chemotherapy, n (%) | 83 (65.4) |
| Gemcitabine | 21 (25.3) |
| Gemcitabine+platinum | 48 (57.8) |
| FOLFIRINOX | 11 (13.3) |
| Other | 3 (3.6) |
| Preoperative radiation, n (%) | 115 (90.6) |
| Hypofractionated (30 Gy) | 30 (26.1) |
| Standard fractionated (45–50.4 Gy) | 85 (73.9) |
| Mean preoperative treatment duration, months (SD) | 5.4 (2.3) |
| Postoperative systemic chemotherapy, n (%) | 64 (50.4) |
| Surgery | |
| Vascular resection, n (%) | 58 (45.7) |
| Venous | 44 (75.9) |
| Arterial | 3 (5.2) |
| Both | 14 (24.1) |
| Pathology | |
| Mean tumor size, cm (SD) | 2.3 (1.3) |
| Differentiation, n (%) | |
| Well/moderate | 78 (61.4) |
| Poor | 49 (38.6) |
| Margin status, n (%) | |
| R0 | 122 (96.1) |
| R1 | 5 (3.9) |
| Positive lymph nodes, n (%) | 65 (51.2) |
| % Viable cells 1 | |
| 0–5% | 13 (10.5) |
| >5% | 111 (89.5) |
| Mean lymph node ratio (SD) | 0.10 (0.1) |
| Lymphovascular invasion, n (%) | 61 (48.0) |
| Perineural invasion, n (%) | 94 (74.0) |
| Survival | |
| Vital status at last follow-up, n (%) | |
| No evidence of disease | 35 (27.6) |
| Alive with disease | 12 (9.4) |
| Died of disease | 80 (63.0) |
| Median overall survival, months (95% confidence interval) | 32.8 (27.7–37.9) |
FOLFIRINOX, fluorouracil, leucovorin, irinotecan, and oxaliplatin; SD, standard deviation; BMI, body mass index.
Data available for 124 patients.
Table 2 reports changes in anthropometric and key laboratory parameters as measured prior to the administration of preoperative therapy, prior to PD, and approximately 3 and 12 months following surgery. Among all 127 patients, CT scans were available for 126 (99.2%) at baseline, 124 (97.6%) preoperative, 121 (95.3%) postoperative, and 90 (74.4%) at one year. During a mean 5.4±2.3 months of preoperative therapy, minimal changes in SKM (−0.5±7.8%, p>0.05), VAT (−1.8±62.6%, p<0.001), and SAT (−4.8±27.7%, p<0.001) were observed. In contrast, clinically significant changes were observed on post-operative CT compared to baseline anthropometry: SKM −4.1±10.7%, VAT −38.7±30.2%, and SAT −24.1±22.6% (all p<0.001) and these changes persisted at one year following PD. Similar results were found when we accounted for all data while using mixed models (Table 3).
Table 2.
Anthropometric and nutritional changes occurring during treatment of pancreatic ductal adenocarcinoma
| Pretreatment (n=126) | Preoperative (n=124) | Postoperative (n=121) | 12 Month Follow-up (n=90) | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Mean months since baseline (SD): 5.1 (2.5) | Mean months since PD (SD): 3.3 (1.6) | Mean months since PD (SD): 11.9 (2.1) | ||||
| Parameter | Absolute | Absolute | % Change1 | Absolute | % Change1 | Absolute | % Change1 |
| SKM, cm2/m2 | 46.6 (8.9) | 46.2 (8.3) | −0.5 (7.8) | 44.0 (7.7) | −5.1 (10.7) | 42.3 (8.0) | −8.5 (12.4) |
| VAT, cm2/m2 | 47.9 (32.2) | 40.7 (29.1) | −1.8 (62.6) | 26.4 (23.4) | −41.4 (41.7) | 22.9 (21.5) | −48.9 (46.3) |
| SAT, cm2/m2 | 67.5 (37.1) | 62.0 (36.8) | −4.8 (27.7) | 46.6 (30.9) | −28.1 (28.0) | 40.6 (30.4) | −34.3 (41.5) |
| Albumin, g/dL | 4.2 (0.5) | 4.0 (0.4) | −3.6 (14.5) | 3.7 (0.5) | −10.4 (15.0) | 3.9 (0.5) | −5.8 (15.7) |
| BMI, kg/m2 | 26.5 (4.7) | 26.2 | −0.9 (6.9) | 24.1 (4.2) | −9.1 (7.6) | 23.5 (4.4) | −10.9 (10.2) |
Results are mean (SD).
PD, pancreatoduodenectomy; SD, standard deviation; SKM, skeletal muscle; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; BMI, body mass index.
Compared to pretreatment scan.
Bold p<0.001; Non-bold p>0.05.
Table 3.
Results of multilevel mixed-effects linear regression for anthropometric and nutritional parameters
| Parameter | Changes in Relation to Baseline (Pretreatment) Values | |||||
|---|---|---|---|---|---|---|
| Preoperative | Postoperative | 12 month Follow-up | ||||
| B (95% Confidence interval) | p Value | B (95% Confidence interval) | p Value | B (95% Confidence interval) | p Value | |
| SKM, cm2/m2 | −0.45 (−1.43, 0.53) | 0.368 | −2.66 (−3.65, −1.68) | <0.001 | −4.29 (−5.38, −3.19) | <0.001 |
| VAT, cm2/m2 | −7.13 (−10.23, −4.04) | <0.001 | −21.58 (−24.70, −18.46) | <0.001 | −25.06 (−28.53, −21.60) | <0.001 |
| SAT, cm2/m2 | −5.44 (−8.70, −2.18) | <0.001 | −19.74 (−23.03, −16.45) | <0.001 | −25.28 (−28.93, −21.63) | <0.001 |
| Albumin, g/dL | −0.18 (−0.29, −0.06) | 0.003 | −0.47 (−0.58, −0.35) | <0.001 | −0.29 (−0.40, −0.17) | <0.001 |
| BMI, kg/m2 | −0.29 (−0.67, 0.09) | 0.133 | −2.48 (−2.87, −2.10) | <0.001 | −3.02 (−3.44, −2.60) | <0.001 |
SKM, skeletal muscle; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; BMI, body mass index.
The median overall survival duration of patients was 32.8 months (95% CI 27.7–37.9 months). On univariate Cox proportional hazards regression, tumor size, differentiation, lymph node status, histopathologic treatment effect[20], lymph node ratio, lymphovascular invasion, and perineural invasion were associated with overall survival (Table 4). However, baseline body composition characteristics as well as anthropometric changes that occurred during preoperative therapy were not associated with overall survival. On the other hand, relative increases in SKM (HR 0.50, 95% CI 0.29–0.87) and albumin (HR 0.57, 95% CI 0.36–0.89) between the postoperative period and 12-month follow-up were associated with improved overall survival.
Table 4.
Factors associated with overall survival in univariate analysis
| Parameter | Hazard ratio (95% confidence interval) | p-value |
|---|---|---|
| Clinical | ||
| Age, years | ||
| <50 | Ref | |
| 50–70 | 2.00 (0.62–6.41) | 0.24 |
| >70 | 2.84 (0.86–9.39) | 0.09 |
| Male sex | 1.40 (0.90–2.19) | 0.14 |
| Radiographic staging | ||
| Potentially resectable | Ref | |
| Borderline resectable | 0.95 (0.54–1.68) | 0.87 |
| Locally advanced | 0.54 (0.23–1.23) | 0.15 |
| Pretreatment CA 19-9>200 U/mL | 1.20 (0.77–1.86) | 0.42 |
| Preoperative chemotherapy | 0.82 (0.52–1.29) | 0.40 |
| Preoperative radiation | 0.73 (0.37–1.47) | 0.38 |
| Adjuvant chemotherapy | 0.92 (0.60–1.43) | 0.72 |
| Surgical | ||
| EBL | ||
| ≤500 mL | Ref | |
| >500–1000 mL | 0.95 (0.58–1.56) | 0.84 |
| >1000 mL | 1.58 (0.57–2.96) | 0.15 |
| Vascular resection | 1.34 (0.86–2.07) | 0.19 |
| Lymph nodes excised | ||
| <15 | Ref | |
| 15–30 | 2.34 (0.84–6.48) | 0.10 |
| >30 | 2.24 (0.78–6.45) | 0.13 |
| Pathology | ||
| Tumor size | 1.35 (1.13–1.60) | 0.001 |
| Differentiation | ||
| Well/moderate | Ref | |
| Poor | 1.82 (0.16–2.83) | 0.009 |
| R1 margin status | 1.57 (0.57–4.30) | 0.38 |
| Positive lymph nodes | 1.98 (1.26–3.10) | 0.003 |
| >5% viable cells | 7.95 (1.95–32.45) | 0.004 |
| Lymph node ratio | ||
| 0 | Ref | |
| 0–0.2 | 1.55 (0.95–2.54) | 0.08 |
| ≥0.2 | 4.34 (2.36–7.96) | <0.001 |
| Lymphovascular invasion | 2.04 (1.31–3.19) | 0.002 |
| Perineural invasion | 2.66 (1.49–4.75) | 0.001 |
| Anthropometrics | ||
| Baseline | ||
| SKM (per10 cm2/m2) | 0.96 (0.73–1.25) | 0.76 |
| VAT (per10 cm2/m2) | 1.00 (0.93–1.07) | 0.99 |
| SAT (per10 cm2/m2) | 0.97 (0.90–1.03) | 0.39 |
| Albumin (per 1 g/dL) | 1.34 (0.80–2.25) | 0.27 |
| BMI (per 1 kg/m2) | 0.99 (0.95–1.04) | 0.69 |
| Change between pretreatment and preoperative | ||
| SKM (per10 cm2/m2) | 0.70 (0.39–1.29) | 0.25 |
| VAT (per10 cm2/m2) | 0.96 (0.84–1.10) | 0.56 |
| SAT (per10 cm2/m2) | 1.00 (0.86–1.16) | 0.98 |
| Albumin (per 1 g/dL) | 0.81 (0.51–1.29) | 0.38 |
| BMI (per 1 kg/m2) | 0.94 (0.83–1.06) | 0.29 |
| Change between preoperative and 3 months following surgery | ||
| SKM (per10 cm2/m2) | 1.16 (0.74–1.82) | 0.51 |
| VAT (per10 cm2/m2) | 1.07 (0.90–1.27) | 0.42 |
| SAT (per10 cm2/m2) | 0.97 (0.85–1.12) | 0.72 |
| Albumin (per 1 g/dL) | 1.19 (0.70–2.03) | 0.52 |
| BMI (per 1 kg/m2) | 1.01 (0.88–1.16) | 0.89 |
| Change between postoperative and 12 months following surgery | ||
| SKM (per10 cm2/m2) | 0.50 (0.29–0.87) | 0.01 |
| VAT (per10 cm2/m2) | 0.92 (0.77–1.10) | 0.35 |
| SAT (per10 cm2/m2) | 0.95 (0.82–1.10) | 0.50 |
| Albumin (per 1 g/dL) | 0.57 (0.36–0.89) | 0.01 |
| BMI (per 1 kg/m2) | 0.95 (0.84–1.08) | 0.48 |
EBL, estimated blood loss; SKM, skeletal muscle; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; BMI, body mass index.
Discussion
We conducted this study to investigate the hypothesis that clinically significant changes in body composition may occur in patients during and immediately following treatment for localized PDAC. By analyzing standard CT images that were serially and routinely performed throughout the course of therapy and the first postoperative year, we found that the skeletal muscle and body mass of patients selected for pancreatectomy were maintained during the administration of preoperative chemotherapy and/or chemoradiation. However, progressive depletion of body mass, fat, and muscle occurred over the first postoperative year. Relative increases in skeletal muscle and serum albumin between the perioperative period and 12-month follow-up were associated with improved overall survival.
Although minor decreases in visceral and subcutaneous adiposity were observed during preoperative therapy, on average patients were able to maintain their skeletal muscle and body mass indices. This is important as preoperative sarcopenia has been shown to be an important risk factor for postoperative complications following pancreatectomy [21–28] and that some studies have demonstrated either a decrease in SKM [8] or muscle attenuation [11] during preoperative therapy for PDAC. In fact, we previously showed that minor changes in SKM, VAT and SAT occur during preoperative therapy for PDAC but that these changes did not preclude subsequent pancreatectomy nor were they associated with survival [8]. However, the current study may be somewhat more generalizable in its patient population (as the previous study was of a small cohort of clinical trial patients with, in general, excellent performance status) and also differed in that only patients who underwent surgery were included. Nevertheless, a greater understanding of the etiology and magnitude of changes in body composition that occur in association with preoperative therapy, and the extent to which they are reversible and/or preventable, is clearly needed.
Although few prior studies have characterized changes in either nutritional indices or body composition following pancreatectomy [29–32], the results of those studies, together with the data described herein, clearly document that adverse physiologic changes do occur and, more importantly, that they persist long after surgery. In this study, patients experienced a significant loss of weight and serum albumin, and a progressive depletion of both muscle and fat, in the year following PD. In a study of physiologic changes that occurred in 27 patients during the first 6 months following PD, Aslani et al. found that both fat mass and total body protein declined in the early postoperative period but by 6 months measures of protein (but not fat mass) returned to preoperative levels [29]. In a more recent study, Hashimoto et al found that 93 patients who underwent pancreatectomy had lost 8.4% of body weight at 2 months after surgery and 9% of body weight by 4 months [33]. Given that patients who lose significant weight are at risk for receiving lower doses of chemotherapy, a shorter duration of chemotherapy, and greater dose-limiting toxicities [34], heightened attention to postoperative nutrition and optimizing patient performance status and function seems warranted.
Although changes during preoperative therapy and immediately following surgery were not associated with the overall survival of patients in this study, the inability to restore skeletal muscle mass during the follow-up period was. Other studies have found significant decreases in body weight following gastrointestinal cancer surgery and that greater losses were predictive of early recurrence and poor survival [35–37]. Whether loss of skeletal muscle is a direct cause of increased mortality (and therefore potentially modifiable) or, more likely, an early indicator of disease recurrence is unknown. Regardless, this anthropometric parameter is easily measured and may confer clinically important information to patients and providers. In the future, combining clinically relevant anthropometric information with other validated assessment tools may permit accurate prediction of short term outcomes [24,25].
That adverse anthropometric changes occur during the course of therapy for PDAC suggests that opportunities to enhance nutritional and physiologic support exist throughout the treatment and survivorship periods. In the preoperative setting, we and others have implemented exercise and nutrition programs designed to improve patients’ physiologic status prior to surgery [38] or counter the cytotoxic effects of chemotherapy and radiation on fat and muscle mass [39,40]. Despite this interest, there is a scarcity of published literature on the topic of prehabilitation among patients with localized PDAC treated with preoperative therapy [41]. Given the findings of the current study, efforts at maintaining muscle mass in the ongoing survivorship period are also clearly justified. Such efforts might include exercise programs, extended rehabilitation, nutritional supplementation, regular follow-up with trained nutritional professionals, or drugs targeting inflammation and cachexia [42–44].
A major strength of the current study is the longitudinal assessment of serial anthropometric measurements in a relatively large cohort of patients with PDAC throughout their course of therapy over a full postoperative year. Nevertheless, several limitations should be acknowledged. First, although inexpensive, comprehensive, and reproducible, the methods we utilized in this study for anthropometric measurement are only one of several possible approaches; previous studies have used other measures that include total psoas volume [23], total psoas area [27], psoas muscle mass index [45], intramuscular adipose tissue content [45], Hounsfield unit average calculation [6], total psoas index [6], psoas thickness [46], and the psoas-vertebral index [47]. And automated image processing software and more sophisticated techniques, such as analytic morphomics, now allows investigators to take advantage of whole-body anthropometric data instead of data generated at a single vertebral level [24,48]. In addition, we used validated Hounsfield unit thresholds for tissue labeling; to the best of our knowledge, this methodology has not been validated in the postoperative setting. However, measurements were made at the level of the L3 vertebra, a level at which few anatomic alterations occur secondary to PD, and none of the 3 month postoperative scans had evidence of significant complications (e.g. fluid collections). Second, our study only included patients who completed all intended aspects of therapy—namely, preoperative chemotherapy and/or radiation and surgical resection. Third, most of the patients in this study received gemcitabine- or 5-FU-based preoperative regimens. Whether the results here are generalizable to patients with PDAC who received different sequencing strategies or regimens (e.g., FOLFIRINOX) is unclear. It is possible that more aggressive regimens could result in even more pronounced anthropometric changes than were seen in the current study. Finally, we acknowledge that the loss of skeletal muscle mass and function are both important components of sarcopenia [49–51]; however, the primary purpose of our study was to characterize longitudinal changes in body composition as they occur within the first year of pancreatectomy, and only characterized patients on the basis of pre-specified, radiographic norms of sarcopenia to provide additional context.
Conclusion
In summary, we performed a longitudinal assessment of anthropometric changes occurring throughout the course of therapy and follow-up for patients with PDAC. We found that in contrast to the relatively minor changes in body composition that occur during preoperative therapy, significant losses in key anthropometric parameters tend to occur over the first year following PD, and ongoing skeletal muscle loss following surgery may represent an early indicator of prognosis. Heightened attention to physiologic metrics both prior to and following completion of therapy is warranted.
Acknowledgments
Financial Support: Supported in part by the National Institutes of Health/National Cancer Institute under award number P30CA016672 (used the Clinical Trials Support Resource and the Biostatistics Resource Group).
Footnotes
Presented at 2017 Society for Surgery of the Alimentary Tract meeting in Chicago, IL.
- conception or design of the work; or the acquisition, analysis, or interpretation of data for the work
- drafting the work or revising it critically for important intellectual content
- final approval of the version to be published
- agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. The Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 2.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 3.Levolger S, van Vugt JLA, de Bruin RWF, IJzermans JNM. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br J Surg. 2015;102:1448–1458. doi: 10.1002/bjs.9893. [DOI] [PubMed] [Google Scholar]
- 4.Choi Y, Oh DY, Kim TY, Lee KH, Han SW, Im SA, Kim TY, Bang YJ. Skeletal Muscle Depletion Predicts the Prognosis of Patients with Advanced Pancreatic Cancer Undergoing Palliative Chemotherapy, Independent of Body Mass Index. PloS One. 2015;10:e0139749. doi: 10.1371/journal.pone.0139749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mei KL, Batsis JA, Mills JB, Holubar SD. Sarcopenia and sarcopenic obesity: do they predict inferior oncologic outcomes after gastrointestinal cancer surgery? Perioper Med Lond Engl. 2016;5:30. doi: 10.1186/s13741-016-0052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joglekar S, Asghar A, Mott SL, Johnson BE, Button AM, Clark E, Mezhir JJ. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol. 2015;111:771–775. doi: 10.1002/jso.23862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 8.Cooper AB, Slack R, Fogelman D, Holmes HM, Petzel M, Parker N, Balachandran A, Garg N, Ngo-Huang A, Varadhachary G, Evans DB, Lee JE, Aloia T, Conrad C, Vauthey JN, Fleming JB, Katz MHG. Characterization of Anthropometric Changes that Occur During Neoadjuvant Therapy for Potentially Resectable Pancreatic Cancer. Ann Surg Oncol. 2015;22:2416–2423. doi: 10.1245/s10434-014-4285-2. [DOI] [PubMed] [Google Scholar]
- 9.Yip C, Goh V, Davies A, Gossage J, Mitchell-Hay R, Hynes O, Maisey N, Ross P, Gaya A, Landau DB, Cook GJ, Griffin N, Mason R. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol. 2014;24:998–1005. doi: 10.1007/s00330-014-3110-4. [DOI] [PubMed] [Google Scholar]
- 10.Rutten IJG, van Dijk DPJ, Kruitwagen RFPM, Beets-Tan RGH, Olde Damink SWM, van Gorp T. Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle. 2016;7:458–466. doi: 10.1002/jcsm.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akahori T, Sho M, Kinoshita S, Nagai M, Nishiwada S, Tanaka T, Tamamoto T, Ohbayashi C, Hasegawa M, Kichikawa K, Nakajima Y. Prognostic Significance of Muscle Attenuation in Pancreatic Cancer Patients Treated with Neoadjuvant Chemoradiotherapy. World J Surg. 2015;39:2975–2982. doi: 10.1007/s00268-015-3205-3. [DOI] [PubMed] [Google Scholar]
- 12.Hwang RF, Wang H, Lara A, Gomez H, Chang T, Sieffert N, Moon Y, Ram S, Zimmerman S, Lee JH, Pisters PWT, Tamm EP, Fleming JB, Abbruzzese JL, Evans DB. Development of an integrated biospecimen bank and multidisciplinary clinical database for pancreatic cancer. Ann Surg Oncol. 2008;15:1356–1366. doi: 10.1245/s10434-008-9833-1. [DOI] [PubMed] [Google Scholar]
- 13.Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, Lee JE, Pisters PWT, Evans DB, Wolff RA. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Cloyd JM, Katz MHG, Prakash L, Varadhachary GR, Wolff RA, Shroff RT, Javle M, Fogelman D, Overman M, Crane CH, Koay EJ, Das P, Krishnan S, Minsky BD, Lee JH, Bhutani MS, Weston B, Ross W, Bhosale P, Tamm EP, Wang H, Maitra A, Kim MP, Aloia TA, Vauthey JN, Fleming JB, Abbruzzese JL, Pisters PWT, Evans DB, Lee JE. Preoperative Therapy and Pancreatoduodenectomy for Pancreatic Ductal Adenocarcinoma: a 25-Year Single-Institution Experience. J Gastrointest Surg Off J Soc Surg Aliment Tract. 2017;21:164–174. doi: 10.1007/s11605-016-3265-1. [DOI] [PubMed] [Google Scholar]
- 15.Cloyd JM, Crane CH, Koay EJ, Das P, Krishnan S, Prakash L, Snyder RA, Varadhachary GR, Wolff RA, Javle M, Shroff RT, Fogelman D, Overman M, Wang H, Maitra A, Lee JE, Fleming JB, Katz MHG. Impact of hypofractionated and standard fractionated chemoradiation before pancreatoduodenectomy for pancreatic ductal adenocarcinoma. Cancer. 2016;122:2671–2679. doi: 10.1002/cncr.30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tzeng CWD, Fleming JB, Lee JE, Xiao L, Pisters PWT, Vauthey JN, Abdalla EK, Wolff RA, Varadhachary GR, Fogelman DR, Crane CH, Balachandran A, Katz MHG. Defined clinical classifications are associated with outcome of patients with anatomically resectable pancreatic adenocarcinoma treated with neoadjuvant therapy. Ann Surg Oncol. 2012;19:2045–2053. doi: 10.1245/s10434-011-2211-4. [DOI] [PubMed] [Google Scholar]
- 17.Katz MHG, Lee JE, Pisters PWT, Skoracki R, Tamm E, Fleming JB. Retroperitoneal Dissection in Patients with Borderline Resectable Pancreatic Cancer: Operative Principles and Techniques. J Am Coll Surg. 2012;215:e11–e18. doi: 10.1016/j.jamcollsurg.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzeng CWD, Fleming JB, Lee JE, Wang X, Pisters PWT, Vauthey JN, Varadhachary G, Wolff RA, Katz MHG. Yield of clinical and radiographic surveillance in patients with resected pancreatic adenocarcinoma following multimodal therapy. HPB. 2012;14:365–372. doi: 10.1111/j.1477-2574.2012.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab Physiol Appl Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 20.Cloyd JM, Wang H, Egger ME, Tzeng C-WD, Prakash LR, Maitra A, Varadhachary GR, Shroff R, Javle M, Fogelman D, Wolff RA, Overman MJ, Koay EJ, Das P, Herman JM, Kim MP, Vauthey J-N, Aloia TA, Fleming JB, Lee JE, Katz MHG. Association of Clinical Factors With a Major Pathologic Response Following Preoperative Therapy for Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2017 doi: 10.1001/jamasurg.2017.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onesti JK, Wright GP, Kenning SE, Tierney MT, Davis AT, Doherty MG, Chung MH. Sarcopenia and survival in patients undergoing pancreatic resection. Pancreatol Off J Int Assoc Pancreatol IAP Al. 2016;16:284–289. doi: 10.1016/j.pan.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Pecorelli N, Carrara G, De Cobelli F, Cristel G, Damascelli A, Balzano G, Beretta L, Braga M. Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br J Surg. 2016;103:434–442. doi: 10.1002/bjs.10063. [DOI] [PubMed] [Google Scholar]
- 23.Amini N, Spolverato G, Gupta R, Margonis GA, Kim Y, Wagner D, Rezaee N, Weiss MJ, Wolfgang CL, Makary MM, Kamel IR, Pawlik TM. Impact Total Psoas Volume on Short- and Long-Term Outcomes in Patients Undergoing Curative Resection for Pancreatic Adenocarcinoma: a New Tool to Assess Sarcopenia. J Gastrointest Surg Off J Soc Surg Aliment Tract. 2015;19:1593–1602. doi: 10.1007/s11605-015-2835-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamin AJ, Buschmann MM, Schneider A, Derstine BA, Friedman JF, Wang SC, Dale W, Roggin KK. Can Comprehensive Imaging Analysis with Analytic Morphomics and Geriatric Assessment Predict Serious Complications in Patients Undergoing Pancreatic Surgery? J Gastrointest Surg Off J Soc Surg Aliment Tract. 2017;21:1009–1016. doi: 10.1007/s11605-017-3392-3. [DOI] [PubMed] [Google Scholar]
- 25.Sur MD, Namm JP, Hemmerich JA, Buschmann MM, Roggin KK, Dale W. Radiographic Sarcopenia and Self-reported Exhaustion Independently Predict NSQIP Serious Complications After Pancreaticoduodenectomy in Older Adults. Ann Surg Oncol. 2015;22:3897–3904. doi: 10.1245/s10434-015-4763-1. [DOI] [PubMed] [Google Scholar]
- 26.Buettner S, Wagner D, Kim Y, Margonis GA, Makary MA, Wilson A, Sasaki K, Amini N, Gani F, Pawlik TM. Inclusion of Sarcopenia Outperforms the Modified Frailty Index in Predicting 1-Year Mortality among 1,326 Patients Undergoing Gastrointestinal Surgery for a Malignant Indication. J Am Coll Surg. 2016;222:397–407.e2. doi: 10.1016/j.jamcollsurg.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Peng P, Hyder O, Firoozmand A, Kneuertz P, Schulick RD, Huang D, Makary M, Hirose K, Edil B, Choti MA, Herman J, Cameron JL, Wolfgang CL, Pawlik TM. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg Off J Soc Surg Aliment Tract. 2012;16:1478–1486. doi: 10.1007/s11605-012-1923-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaap K, Hunsinger M, Dove J, McGinty K, Stefanowicz E, Fera J, Wild J, Shabahang M, Blansfield J. Morphometric Predictors of Morbidity after Pancreatectomy. Am Surg. 2016;82:1221–1226. [PubMed] [Google Scholar]
- 29.Aslani A, Roach PJ, Smith RC. Long-term changes in body composition after pancreaticoduodenectomy. ANZ J Surg. 2012;82:173–178. doi: 10.1111/j.1445-2197.2011.05970.x. [DOI] [PubMed] [Google Scholar]
- 30.Curran FT, Stokes MA, Hill GL. Long-term changes in body composition after pancreatoduodenectomy. J R Coll Surg Edinb. 1991;36:32–34. [PubMed] [Google Scholar]
- 31.Royall D, Jeejeebhoy KN, O’Connor B, Taylor BR, Langer B, McLeod RS. Nutritional status and function in patients following Whipple procedure compared with controls. J Am Coll Nutr. 1996;15:73–78. doi: 10.1080/07315724.1996.10718567. [DOI] [PubMed] [Google Scholar]
- 32.Jang J-Y, Kim S-W, Park S-J, Park Y-H. Comparison of the functional outcome after pylorus-preserving pancreatoduodenectomy: pancreatogastrostomy and pancreatojejunostomy. World J Surg. 2002;26:366–371. doi: 10.1007/s00268-001-0234-x. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto D, Chikamoto A, Ohmuraya M, Abe S, Nakagawa S, Beppu T, Takamori H, Hirota M, Baba H. Impact of Postoperative Weight Loss on Survival After Resection for Pancreatic Cancer. JPEN J Parenter Enteral Nutr. 2015;39:598–603. doi: 10.1177/0148607114520992. [DOI] [PubMed] [Google Scholar]
- 34.Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer Oxf Engl 1990. 1998;34:503–509. doi: 10.1016/s0959-8049(97)10090-9. [DOI] [PubMed] [Google Scholar]
- 35.Lee SE, Lee JH, Ryu KW, Nam B, Kim CG, Park SR, Kook M-C, Kim Y-W. Changing pattern of postoperative body weight and its association with recurrence and survival after curative resection for gastric cancer. Hepatogastroenterology. 2012;59:430–435. doi: 10.5754/hge09218. [DOI] [PubMed] [Google Scholar]
- 36.Davis JL, Selby LV, Chou JF, Schattner M, Ilson DH, Capanu M, Brennan MF, Coit DG, Strong VE. Patterns and Predictors of Weight Loss After Gastrectomy for Cancer. Ann Surg Oncol. 2016;23:1639–1645. doi: 10.1245/s10434-015-5065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Journo XB, Ouattara M, Loundou A, Trousse D, Dahan L, Nathalie T, Doddoli C, Seitz JF, Thomas P-A. Prognostic impact of weight loss in 1-year survivors after transthoracic esophagectomy for cancer. Dis Esophagus Off J Int Soc Dis Esophagus. 2012;25:527–534. doi: 10.1111/j.1442-2050.2011.01282.x. [DOI] [PubMed] [Google Scholar]
- 38.Moran J, Guinan E, McCormick P, Larkin J, Mockler D, Hussey J, Moriarty J, Wilson F. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: A systematic review and meta-analysis. Surgery. 2016 doi: 10.1016/j.surg.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Fogelman DR, Holmes H, Mohammed K, Katz MHG, Prado CM, Lieffers J, Garg N, Varadhachary GR, Shroff R, Overman MJ, Garrett C, Wolff RA, Javle M. Does IGFR1 inhibition result in increased muscle mass loss in patients undergoing treatment for pancreatic cancer? J Cachexia Sarcopenia Muscle. 2014;5:307–313. doi: 10.1007/s13539-014-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barret M, Antoun S, Dalban C, Malka D, Mansourbakht T, Zaanan A, Latko E, Taieb J. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer. 2014;66:583–589. doi: 10.1080/01635581.2014.894103. [DOI] [PubMed] [Google Scholar]
- 41.Ngo-Huang A, Parker N, Martinez VA, Petzel MQ, Fogelman D, Holmes HM, Dhah SS, Katz M. Poster 68 Feasibility of a Prehabilitation Program for Patients with Potentially Resectable Pancreatic Cancer: Pilot Study. PM R. 2016;8:S183. doi: 10.1016/j.pmrj.2016.07.111. [DOI] [PubMed] [Google Scholar]
- 42.Afaneh C, Gerszberg D, Slattery E, Seres DS, Chabot JA, Kluger MD. Pancreatic cancer surgery and nutrition management: a review of the current literature. Hepatobiliary Surg Nutr. 2015;4:59–71. doi: 10.3978/j.issn.2304-3881.2014.08.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyamoto Y, Hanna DL, Zhang W, Baba H, Lenz H-J. Molecular Pathways: Cachexia Signaling-A Targeted Approach to Cancer Treatment. Clin Cancer Res Off J Am Assoc Cancer Res. 2016;22:3999–4004. doi: 10.1158/1078-0432.CCR-16-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeo TP, Burrell SA, Sauter PK, Kennedy EP, Lavu H, Leiby BE, Yeo CJ. A progressive postresection walking program significantly improves fatigue and health-related quality of life in pancreas and periampullary cancer patients. J Am Coll Surg. 2012;214:463–475. doi: 10.1016/j.jamcollsurg.2011.12.017. discussion 475–477. [DOI] [PubMed] [Google Scholar]
- 45.Okumura S, Kaido T, Hamaguchi Y, Fujimoto Y, Masui T, Mizumoto M, Hammad A, Mori A, Takaori K, Uemoto S. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery. 2015;157:1088–1098. doi: 10.1016/j.surg.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Taguchi S, Akamatsu N, Nakagawa T, Gonoi W, Kanatani A, Miyazaki H, Fujimura T, Fukuhara H, Kume H, Homma Y. Sarcopenia Evaluated Using the Skeletal Muscle Index Is a Significant Prognostic Factor for Metastatic Urothelial Carcinoma. Clin Genitourin Cancer. 2016;14:237–243. doi: 10.1016/j.clgc.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 47.Ebbeling L, Grabo DJ, Shashaty M, Dua R, Sonnad SS, Sims CA, Pascual JL, Schwab CW, Holena DN. Psoas:lumbar vertebra index: central sarcopenia independently predicts morbidity in elderly trauma patients. Eur J Trauma Emerg Surg Off Publ Eur Trauma Soc. 2014;40:57–65. doi: 10.1007/s00068-013-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Englesbe MJ, Lee JS, He K, Fan L, Schaubel DE, Sheetz KH, Harbaugh CM, Holcombe SA, Campbell DA, Sonnenday CJ, Wang SC. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg. 2012;256:255–261. doi: 10.1097/SLA.0b013e31826028b1. [DOI] [PubMed] [Google Scholar]
- 49.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel J-P, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M. European Working Group on Sarcopenia in Older People, Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, Fearon KC, Laviano A, Maggio M, Rossi Fanelli F, Schneider SM, Schols A, Sieber CC. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr Edinb Scotl. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]