Abstract
Mesenchymal stem cells (MSCs) are multipotent stem cells characterized by self-renewal, production of clonal cell populations, and multilineage differentiation. They exist in nearly all tissues and play a significant role in tissue repair and regeneration. Additionally, MSCs possess wide immunoregulatory properties via interaction with immune cells in both innate and adaptive immune systems, leading to immunosuppression of various effector functions. Numerous bioactive molecules secreted by MSCs, particularly cytokines, growth factors, and chemokines, exert autocrine/paracrine effects that modulate the physiological processes of MSCs. These invaluable virtues of MSCs provide new insight into potential treatments for tissue damage and inflammation. In particular, their extensive immunosuppressive properties are being explored for promising therapeutic application in immune disorders. Recently, clinical trials for MSC-mediated therapies have rapidly developed for immune-related diseases following reports from preclinical studies declaring their therapeutic safety and efficacy. Though immunotherapy of MSCs remains controversial, these clinical trials pave the way for their widespread therapeutic application in immune-based diseases. In this review, we will summarize and update the latest research findings and clinical trials on MSC-based immunomodulation.
1. Background
Mesenchymal stem cells (MSCs) are nonhematopoietic stem cells with multipotent properties and self-renewal capability. In addition to bone marrow, MSCs can also be derived from various tissues, including adipose, muscle, umbilical cord blood, peripheral blood, liver, placenta, skin, amniotic fluid, breast milk, synovial membrane, and tooth root [1, 2].
MSCs can act on immune and inflammatory responses following bone marrow-derived MSC-induced T-cell suppression [3]. In addition, MSCs stimulate metabolism, not only through secreting a vast array of chemokines, growth factors, and cytokines but also through production of many secretomes and proteomes. These factors play an important role in immunomodulatory activities, mediating hematopoietic stem cell (HSC) engraftment, and MSC differentiation, as well as regulating angiogenesis and apoptosis. [4]
Because of their remarkable properties for multipotential differentiation and immune mediation, there is potential for using MSCs as a novel therapy for many diseases [5]. Furthermore, MSC-based clinical trials in multiple sclerosis, myocardial infarction, and type 1 diabetes mellitus have been reported [6]. It has also been shown that using soluble factors derived from MSCs improves treatment efficacy for autoimmune disease, which has gained much attention [7]. New insights into the immune-regulatory capacities of MSCs have focused on inflammatory status [8]. The interaction between MSCs and the inflammatory niche furnish vast potential for using MSCs in the treatment of all sorts of diseases, particularly disorders of the immune system [9].
In this review, we will summarize MSC-modulated immunoregulation through description of their constitutive functions, secretion factors, basic functions in regulating immune responses, and clinical value with respect to immunomodulatory treatments.
2. Characterization of MSCs
Mesenchymal stem cells have mesodermal lineage differentiation potential and the potential to regulate tissue regeneration [10]. Major characteristics of MSCs include the advantage of multilineage differentiation potential that can generate adipocytes, chondrocytes, and osteocytes due to expression of several pluripotency genes [11–13], thus mediating tissue and organ repair, as well as replacing damaged cells [14].
Currently, MSCs are regarded as a potential new therapy for a variety of human diseases. Recently, studies have focused on regulation of MSC fate with respect to their pluripotency and differentiation to promote regenerative therapeutic development [15, 16]. Increasing numbers of clinical trials are reporting the success of MSC-based immunomodulation based on the measurement of soluble secretors and their interaction with immune cells [17]. Treatment with MSC transplants has attracted much attention based on MSC engraftment studies over the past few years. More importantly, increasing studies have attempted to apply MSCs for the treatment of several autoimmune disorders, such as multiple sclerosis, Crohn's disease, graft versus host disease (GVHD), and systemic lupus erythematosus (SLE) [18].
3. MSCs and Immune Modulation
In 2002, it was first shown that MSCs had the ability to modulate immunosuppression by Bartholomew and colleagues, who demonstrated suppression of a mixed lymphocyte response in vitro and prevention of rejection in a baboon skin allograft model in vivo [19]. Since the immune response properties of MSCs were first reported, subsequent studies have shown that MSCs mediate immunosuppression in animal models and human.
Considering the promising preliminary clinical outcomes, the mechanisms of MSC interactions with the immune response as we currently understand them are worth outlining. MSCs have the ability to interact with many kinds of immune cells, including B cells, T cells, dendritic cells (DCs), natural killer (NK) cells, neutrophil, and macrophages [20]. Mechanisms of interaction were shown to rely on cell–cell contact working in collaboration with secretion of soluble immune factors to induce MSC-regulated immunosuppression [21]. These specific modulators, including a multitude of immune-modulatory factors, cytokines, and growth factors, modulate inflammatory responses and balance immune profiles [22]. Namely, soluble immune secretomes, such as prostaglandin E2 (PGE-2), indoleamine 2,3-dioxygenase (IDO), or nitric oxide (NO), respond to immune cells to activate immunoregulation by MSCs [23].
Adhesion molecules, intracellular secretomes, and the main histocompatibility complex (MHC) antigens are all required to induce immune suppression. Particularly T cells, as well as the Fas ligand/Fas receptor interaction (FasL/FasR), play a vital role in T-cell reaction function [24]. Extracellular vesicles produced by MSCs accelerate generation of M2 macrophages and regulatory T cells, while at the same time suppressing maturation of monocytes and proliferation of T cells and B cells [17, 25].
MSCs also have the ability to regulate inflammatory progress and repair damaged cells and tissues by adhering to inflammatory sites [26]. MSC integration with inflammatory actions can both fortify and restrain the immune response and is dependent on the function of immune suppressants, the kinds of inflammatory secretomes, and the general condition of the immune system [27]. Interestingly, MSCs only modulate immunosuppression when they are first stimulated by inflammatory cytokines, such as tumor necrosis factor (TNF) and interleukin- (IL-) 1 [28]. MSCs not only respond to inflammatory cytokines but also produce immune-regulatory secretors that mediate the process of inflammation. For example, a large number of indoleamine 2,3-dioxygenase (IDO) in humans, nitric oxide (NO) in mice, and chemokines produced by MSCs play a key part in MSC-mediated immunomodulation [29]. Furthermore, MSC secretomes, including growth factors hepatocyte growth factor (HGF) or tumor-specific glycoprotein (TSG6), have been effectively utilized to treat immune diseases [30]. MSCs themselves have also been used to successfully treat patients with severe immune disorders, including Crohn's disease and SLE [31].
3.1. Immune Cells Interact with MSCs in Immunomodulation
Both in vivo and in vitro studies have shown that MSCs present their multipotency as a mediator of immunomodulation. MSCs exert significant effects on immunosuppression by refraining immune cells in both the innate and adaptive immune systems (Table 1).
Table 1.
The function of MSCs in mediating immune cells both in innate and adaptive immune systems.
| Immune cell type | MSC functions |
|---|---|
| Innate immune systems | |
| DCs | Inhibiting DC migration, activation, differentiation, maturation, and endocytosis |
| NK cell | Inhibiting NK cell migration, proliferation, differentiation, maturation, and activation |
| Macrophage | Activating M2 macrophage polarization in general; activating M1 macrophage polarization in specific microenvironment |
|
| |
| Adaptive immune systems | |
| T cell | Inhibiting T-cell survival proliferation, differentiation, maturation, and activation, while accelerating T-cell recruitment |
| B cell | Inhibiting B-cell proliferation, differentiation, maturation, chemotaxis, and activation |
3.1.1. Innate Immunity
The innate immune system plays a pivotal role not only in the adaptive immune reaction but also in the elimination of pathogens targeted by an adaptive immune response [32]. DCs, NK cells, and macrophages constitute the innate immune system, and their interaction with MSCs promotes regenerative processes and inhibits inflammatory responses [33].
(1) Myeloid Dendritic Cells (DCs). Myeloid dendritic cells (DCs) maintain and modulate immune responses through acceleration of antigen-specific T-cell processes, as well as activation of cells in the innate immune reaction following DC maturation [34, 35]. Recent studies demonstrated that MSCs have immunosuppressive functions on DCs in the form of restraining DC differentiation from monocytes and decreasing the cell-surface expression of CD1-α, CD40, CD80, CD83, CD86, and MHCII [36]. After incubation with MSCs, DCs would lose their capability to motivate lymphocytes by downregulating interferon-γ (IFN-γ) and TNF-α expression as well as accelerating IL-10 release [37]. The MSC-DC interaction is mediated by the Notch pathway relied on IFN-γ-secretase [38]. The molecular mechanisms of MSCs restraining DC maturation seem to be regulated by PGE-2 [39]. In addition, MSCs have the ability to damage the migration of DCs by suppressing molecules tied to DCs and presenting antigens for activating T cells [40, 41]. MSCs can also depress the proinflammatory capacity of DCs by inhibiting the formation of TNF [4]. Importantly, the inhibitory effects of MSCs play a significant role in relieving some immune disorders, such as allograft rejection [38], type 1 diabetes, and acute GVHD [42–44].
(2) Natural Killer (NK) Cells. Natural killer (NK) cells produce proinflammatory cytokines and have cytolytic activity [45].
MSCs inhibit the effects of NK cells with immunosuppressive secretors, such as PGE2, TGF-β, and sHLA-G, leading to induction of cytotoxic effects against virus-infected cells and reduction of IFN-γ secretion [46]. This inhibitory action is completed by suppressing the activating NK-cell receptor expression, which is mediated by IDO and PGE-2 [47]. In addition to these findings, direct cell–cell contact also plays a distinct role in suppressing NK cells, which is related to expression of Toll-like receptor- (TLR-) 4 on MSCs [48]. MSCs promote cytotoxic movement by suppressing the secretion of NKp30 and NKG2D, which are the surface receptors related to NK-cell activation [49]. However, the potent suppressive actions of MSCs were only apparent at high MSC-to-NK ratios [46]. Furthermore, it has been demonstrated that activated NK cells have the ability to dissolve MSCs when there are enough activating receptors on NK cells [50]. Together, these discoveries indicate that interaction between MSCs and NK cells relies on the ratios of both cells, as well as their microenvironment [3].
(3) Macrophages. It is well known that macrophages are important cells in the innate immune system with significant plasticity [51]. Based on the specific microenvironment of MSCs, macrophages may be polarized into classically activated M1 macrophages or alternatively activated M2 macrophages [52]. Generally, M1 macrophages possess prominent antimicrobial properties by releasing a variety of chemokines and inflammatory cytokines, whereas M2 macrophages are able to alleviate inflammation and expedite tissue repair via secretion of IL-10 and trophic factors [53]. In addition, the coculture of macrophages with MSCs induces production of M2 macrophages, which upregulates the phagocytic activity and secretion of IL-10, and downregulates levels of inflammatory cytokines, such as IFNγ, TNF-α, IL-1β, and IL-12 [54, 55]. Recent studies reported that MSCs facilitated monocyte chemotactic protein-1 (MCP1) secretion by responding to TLR4 ligation, then inducing monocyte emigration [56]. In a model of zymosan-induced peritonitis injury, human MSCs activate peritoneal macrophages by secreting TNF-stimulated gene 6 (TSG6), which regulates TLR2 nuclear factor-κB (NF-κB) signaling [57]. Additionally, MSCs have been shown to ameliorate immune disorders and accelerate tissue regeneration by increasing the concentration of macrophages at locations of injury [58, 59].
3.1.2. Adaptive Immunity
The adaptive immune system has its own distinct properties, specifically antigen-specific immune response and immunological memory. The system consists of CD4+ T helper and CD8+ cytotoxic T lymphocytes that transmit a suitable antigen-specific immune response after antigen-presenting cells (APCs) undergo antigen processing and presentation [32].
(1) T Cells. T cells are widely distributed in both animal and human tissues and, once activated, can differentiate into T helper (Th) 1, regulatory T cell (Treg) subpopulation, Th2, Th9, or Th17, according to the intensity of stimulation and the cytokine microenvironment [60, 61]. It has been demonstrated that MSCs interact tightly with T cells [62]. More importantly, as a key mediator of the adaptive immune system, T cells modulate various autoimmune diseases and protect organisms from infections and malignancies [63].
On the other hand, MSCs secrete a great quantity of immunosuppressive factors, chemokines, and adhesion molecules, which are responsible for effective T-cell suppression, involved in T-cell proliferation and apoptosis, as well as differentiation [26, 64]. For example, MSCs are capable of repressing T-cell proliferation through cellular or nonspecific mitogenic stimuli [65] and promoting apoptosis of activated T cells via the Fas/Fas ligand pathway [66]. MSCs constitutively secrete coinhibitory molecule B7-H4 and HLA-G, which present an immunosuppressive action on T cell and influence their proliferation as well as T cell-mediated cytotoxicity [67]. However, the immunosuppressive capacity of MSCs is not activated at all times and relies on the strength and type of the inflammatory stimulation [68]. MSCs no longer restrain T-cell proliferation in the presence of pathogen-associated molecules and TLRs such as TLR3 and TLR4 which damage Notch signaling, thereby recovering effective T cell to respond to pathogens [69]. In addition, regulatory T cells, as a specialized subset of T cells, restrain effects of the immune system, leading to relieving their own antigens and sustaining homeostasis [70].
(2) B Cells. B cells are the second major cell genre related to adaptive immune responses. These cells resist and hunt down outside pathogens through the production of specific antibodies [71, 72]. Both murine and human MSCs have the ability to inhibit B-cell proliferation and activation in vitro [73]. Additionally, MSCs also suppress differentiation of B cells, as well as expression of chemokine receptors owing to cell–cell contact and secretion of soluble molecules [74]. Metalloproteinase-processed CC-chemokine ligand 2(CCL2) released by MSCs suppress signal transducer and activator of transcription 3 (STAT3) activity, resulting in downregulating Paired box 5 (PAX5), thereby inhibiting immunoglobulin synthesis [75]. Several other signaling pathways, such as p38, extracellular response kinase 1/2, B lymphocyte-induced maturation protein 1 (Blimp1), and Akt signaling also modulate B-cell activation [76]. However, inadequate inflammatory signal-activated MSCs from patients with SLE may support proliferation and differentiation of antibody-releasing B cells [77]. Taken together, MSCs suppress antibody production by B cells, and this effect is dependent upon the strength of the inflammatory stimulation, as well as the ratio of MSCs to B cells [78, 79].
3.2. Soluble Factors Secreted by MSCs in Immunomodulation
MSCs could interact with immune cells in both the innate and adaptive immune systems by secreting multiple soluble immune factors to induce MSC-regulated immunosuppression [80]. During an immune response, a number of soluble factors are released by MSCs, such as cytokines, growth factors, hormones, and chemokines, which act on immune cells and exert their functions by repairing damaged cells or suppressing immunology activity [81, 82] (Table 2). The inflammatory response is pivotal for MSCs to exert effects on immunomodulation, owing to an inflammatory cytokine-licensing process by MSCs. Consequently, the immunoregulatory activities of MSCs require inflammatory cytokines secreted by antigen-presenting cells and T cells, which include interferon- (IFN-) γ, IL-1α, IL-1β, and TNF-α [83]. These inflammatory cytokines can activate MSCs to secrete immunosuppressive factors consisting of IDO, TSG6, NO, IL-10, CCL2, galectins, PGE2, and TGF-β and then modulating tissue homeostasis [13, 25].
Table 2.
Biological function of soluble factor secreted by MSCs.
| Soluble factors | Biological function |
|---|---|
| IDO | Suppressing proliferation and effect of immune cells |
| TSG6 | Anti-inflammatory effect |
| NO | Suppressing proliferation and modulation of T cell, promoting apoptosis of immune cells |
| IL-10 | Suppressing apoptosis of immune cells |
| CCL2 | Suppressing activation and migration of TH17 cells, promoting migration of monocyte |
| PGE2 | Suppressing generation and migration of TNF, proliferation of T cell, and cytolytic activity of NK cell |
3.2.1. Indoleamine 2,3-Dioxygenase (IDO)
Recently, it has been reported that indoleamine 2,3-dioxygenase (IDO) mediates immunomodulation by suppressing various immune cells, including T cells and NK cells [78, 84]. IDO can restrain the proliferation and effect of immune cells by transforming tryptophan into its metabolite kynurenine [85]. Furthermore, IDO secreted by MSCs has the ability to suppress allogeneic T-cell reactivity and promotes kidney allograft tolerance [86]. In addition, IDO has been proposed to be one of the representative immunosuppressive molecules for human MSCs [78, 87].
3.2.2. TNF-Stimulated Gene 6 (TSG6)
TNF-stimulated gene 6 (TSG6) is a multifunctional protein with anti-inflammatory effects [88]. Proinflammatory mediators, such as TNF-a and IL-1, may stimulate the secretion of TSG6 [89]. It has been reported that microembolization induces TSG6 to interact with damaged lung in a mouse model of myocardial infarction. Thus, TSG6 plays a significant role in reducing inflammation and infarct size, as well as enhancing cardiac function [7].
3.2.3. NO
In the presence of proinflammatory cytokines, MSCs facilitate high expression of inducible NO synthase (iNOS), which stimulates the secretion of NO, giving rise to inhibition of T-cell proliferation [90]. Both in vivo and in vitro studies showed that murine MSCs lacking iNOS exhibited diminished inhibition capability [26]. Intriguingly, high concentrations of NO may suppress immune modulation and lead to immune cell apoptosis via inhibition of signal transducer and activator of transcription 5 (STAT5) phosphorylation and signal transducer in T cells [90, 91]. However, NO is an extremely unstable oxidative molecule, and both adhesion molecules and chemokines can assist it in exerting immunosuppressive action [77, 92].
3.2.4. IL-10
IL-10 was reported to play a crucial part in MSC-regulated immunosuppression [4]. Antigen-presenting cells, including monocytes and dendritic cells, could work with MSCs to induce secretion of IL-10 [93]. In addition, Macrophages can deliver large quantities of IL-10 by stimulation of E prostanoid receptors, thereby protecting tissues against migration of neutrophils [94].
3.2.5. CC-Chemokine Ligand 2 (CCL2)
CC-chemokine ligand 2 (CCL2), as a metalloproteinase-processed chemokine, antagonizes the function of CC-chemokine receptor 2 (CCR2), which is the cognate receptor of CCL2 [95]. Binding of CCL2 to CCR2 has been shown to mediate immunosuppression of MSCs by inhibiting activation and migration effects on TH17 cells in experimental autoimmune encephalomyelitis (EAE) [96]. Furthermore, CCL2 secreted by mouse MSCs accelerates monocyte migration from the bone marrow into the blood stream, verifying the notion that MSC interaction with innate immune responses affects the immune system [96].
3.2.6. Prostaglandin E2 (PGE2)
Prostaglandin E2 (PGE2) is another immunosuppressive factor secreted by inflammatory stimulus-induced MSCs. PGE2 regulates immunosuppression of MSCs in T cells, DCs, NK cells, and macrophages [83, 84]. In vitro, PGE2 produced by mouse MSCs restrain several cell functions, such as TNF generation and migration [97]. Additionally, in an experimental mouse model of sepsis, IL-10-dependent PGE2 has been described to play a significant role in treating effectively mice with MSCs [83]. More significantly, PGE2 collaboration with IDO exerts immunosuppressive actions in human MSCs, such as inhibiting T-cell proliferation, as well as NK cell cytolytic activity [84].
It seems that all these molecules exert their functions in reliance on the inflammatory microenvironment. Therefore, future research should focus on mediator mechanisms that regulate the immunosuppressive characteristics of MSCs, as well as their local microenvironments, which will provide a broad perspective for therapeutic application of MSCs [13].
4. MSC Clinical Applications in Immune-Mediated Disease
Since MSCs derived from bone marrow were first suggested for use in regenerative medicine owing to their stem cell-like qualities, there have been many major studies on applying the multipotential capacity of MSCs in promoting transplanted HSC engraftment and facilitating damaged tissue repair [98]. The immunosuppressive capacities of MSCs have provided new insight for the treatment of immune-mediated diseases. In addition to restraining immunocompetent cells by suppressing their response to antigen and sustaining them in a silent state, MSCs also promote peripheral tolerance [3]. In addition, MSCs could induce T-cell tolerance and damage pathogenic T- and B-cell response.
MSCs bring new vitality to the study of various immune disorder-related diseases and tissue regeneration through their immune-regulatory properties. Some studies have verified that MSCs induce tissue regeneration in the liver [99], kidney [100], and heart [101]. These damaged tissues may be directly replaced by MSCs with multipotent differentiation abilities. Additionally, MSCs can exert immune-regulatory capabilities to treat immunological disorders by decreasing inflammation and promoting tissue repair. For instance, the inhibitory function of MSCs contributes to relieving several immune disorders, including peritonitis, endotoxemia, type 1 diabetes, type 2 diabetes, acute GVHD, ischemia–reperfusion injury, acute liver injury, arthritis, allograft rejection, and atherosclerosis [102].
In fact, a variety of immune disorder diseases, including multiple sclerosis, Crohn's disease, GVHD, SLEs, and type 1 diabetes, have entered clinical trials for treatment with MSCs [80]. Moreover, prochymal and cupistem products based on the immunomodulatory capabilities of MSCs have been widely applied for disease therapy in a number of disorders [103].
More importantly, recent preclinical and human studies support the hypothesis that MSCs derived from allogeneic donors could be utilized in clinical therapy [104]. Furthermore, in many subacute conditions, as in autoimmune diseases, there is enough time to obtain and culture autologous MSCs in vitro, whereas allogeneic MSCs may be the only option for major acute conditions [105].
There have been over 700 MSC-based clinical trials registered on the National Institutes of Health (NIH) Clinical Trial Databank (https://clinicaltrials.gov/) around the world as of the end of October 2017. Surprisingly, although the immunomodulatory capacities of MSCs have been only recently confirmed, MSC-based therapies have quickly risen in prominence among immunology disease treatments in the past few years. There are 105 clinical trials related to the immunomodulatory effects of MSC and 44 clinical trials linked to graft enhancement, utilizing the immunosuppressive functions of MSCs (Table 3).
Table 3.
Clinical trials using mesenchymal stem cells (registered as of October 26, 2017).
| Indication | Number of studies |
|---|---|
| Immunomodulation | 105 |
| Multiple sclerosis/atherosclerosis | 31 |
| Type 1 diabetes | 18 |
| Crohn's disease | 22 |
| Systemic lupus (erythematosus/colitis) | 11 |
| Rheumatoid arthritis/Sjögren's syndrome | 7 |
| Buerger's disease/sickle cell disease | 3 |
| HIV | 2 |
| Limbus corneae insufficiency syndrome | 1 |
| Periodontitis | 5 |
| Progressive hemifacial atrophy | 2 |
| Retinitis pigmentosa | 3 |
| Graft enhancement | 44 |
| GvHD | 41 |
| Hematopoietic malignancies | 3 |
All values have been extracted from https://clinicaltrials.gov/.
To date, most of the MSC-based clinical trials related to immunomodulation have been administered in China and Europe, as well as the United States (Figure 1). The major clinical indications within the clinical trial database consist of multiple sclerosis/atherosclerosis (n = 31), Type 1 diabetes (n = 18), Crohn's disease (n = 22), systemic lupus erythematosus (n = 11), and GvHD (n = 41) (Table 1).
Figure 1.
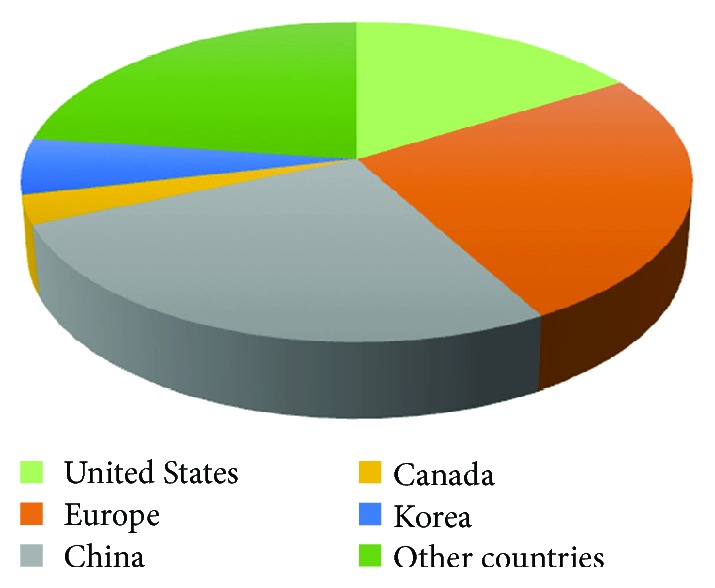
The clinical trial distribution of MSC-based immunomodulation in the world. Up to now, most of the clinical trials using MSCs for treating inflammatory or autoimmune diseases have been conducted in the China, US, and Europe. All values have been extracted from https://clinicaltrials.gov/.
4.1. Graft versus Host Disease (GVHD)
The first successful MSC therapy case comprised infusion of MSCs obtained from bone marrow into an IV for an acute GVHD-grade patient with cyclosporine- and steroid-resistant features [106]. HSC remains the most successful treatment among all stem cell therapies [102]. However, in spite of the immunosuppressive effects of allogeneic HSC transplantation, immune rejection still causes 30–40% morbidity and mortality in GVHD therapeutic applications [107]. GVHD pathogenesis includes an alloresponse to donor lymphocytes, which leads to damage in multiple organs, especially liver, skin, and gastrointestinal tract [108]. Importantly, the treatment for GVHD using MSCs developed more quickly than for other immune-based diseases [106]. Published reports from completed clinical trials included the conclusion that MSCs to treat GVHD decreased the 2-year mortality rate [109]. These hopeful results provide valuable resources for larger-scale clinical trials. Recently, the mesoblast has received fast-track designation for MSC-100-IV for treatment of steroid-refractory acute GVHD in children, so far this allogeneic mesenchymal stem cell (MSC) product has completed enrollment and topline results are expected in 2018. MSCs have their own advantages in the treatment of GVHD; however, both strict standard MSC processing and precise tailoring for patients are necessary to reach coherent and repeatable results.
4.2. Crohn's Disease
In the early 1990s, Crohn's disease patients were reported to experience relief from their inflammatory bowel disease following infusion of HSCs [110]. Subsequently, the development of HSC transplantation has been widely applied in Crohn's disease therapy [111]. However, serious adverse effects also accompanied HSC transplantation treatment. More importantly, MSCs have the privilege of exerting immune-mediated actions without a requirement for host-recipient matching, attributed to low expression of HLA class I antigen and HLA class II antigen on MSC surfaces [57, 112]. Early phase studies have demonstrated that allogeneic MSCs exert effects on luminal disease, while either allogeneic or autologous MSCs exhibit efficacy on fistula disease [113]. A phase 3 study is now evaluating the efficacy of allogenic adipose-derived MSC in 212 patients to treat refractory, perianal fistulizing Crohn's disease. The primary end point showed that remarkably more patients treated with MSC had achieved complete closure of fistula at 24 weeks [114]. In addition, TiGenix following phase 3 has successfully achieved European Medicines Agency (EMA) approval for their allogeneic MSCs being developed to treat complex patients with Crohn's disease. MSCs have extensive application prospects for Crohn's disease treatment, but future work should focus on defining accurate phenotypic and functional features of conducted cells.
4.3. Multiple Sclerosis (MS)
Multiple sclerosis (MS) is a CD4 T cell-modulated autoimmune disease dependent on myelin-based protein (MBP), a protein discovered uniquely in myelin sheaths [115]. Currently, MS is one of the most prevalent autoimmune diseases mediated by CD4 T cells in the central nervous system (CNS). Astonishingly, there are 2.3 million people influenced by this disease that has no cure [116]. It has been demonstrated that MSCs exert productive therapeutic effects in an EAE mouse model, one of the best MS models [117]. Furthermore, both demyelinating regions and lymphoid organs could be used to test the presence of either human or mouse MSCs by intravenous infusion in the EAE mouse model [117, 118]. Adipose-derived MSCs decrease the symptoms of EAE and inflammation of spinal cord and brain according to MSCs targeting in CNS and lymphoid organs [119]. It has also been demonstrated that intravenous administration of human MSCs could prolong EAE mice life and ameliorate the disease by the mechanisms of immunomodulation [120]. Various preclinical investigations have reported MSC therapeutic efficacy in animal models for MS. Up to now, there have been 31 clinical trials of MS registered on the NIH Clinical Trial Database (Table 1), which are researching the MSC effectiveness and adverse effects for treatment MS patients all over the world, and a clinical trial has reported the validity of intravenous administration of MSCs from autologous bone marrow for patients with MS [121]. However, there are no significant positive achievements reached until now, though conducting many phase I/II researches and further investigations are needed before they can be widely applied to clinical treatment.
4.4. Type 1 Diabetes
Diabetes mellitus is a common metabolic disease, with nearly 171 million adult patients around the world [122]. Type 1A diabetes mellitus is characterized by T cell-mediated autoimmune disorder, giving rise to the destruction of pancreatic β cells, as well as decreasing insulin secretion owing to existing anti-islet cell antibodies [123]. Currently, insulin injections and blood glucose control is still the standard therapeutic methods for type 1 diabetes [124]. However, the urgent need for a new option to treat type 1 diabetes is necessary due to the adverse effects accompanied by insulin treatment and the difficulties in maintaining metabolic balance.
In fact, compared with other stem cells, MSCs have more advantages for therapeutic application in type 1 diabetes owing to their immunosuppressive ability and multilineage differentiation [125]. The preliminary results indicated that MSCs derived from the umbilical cord blood reverse the autoimmune that could regenerate islet beta cells and reinforce glycemic control of type 1 diabetes [126]. Human bone marrow-derived MSCs took effects on immune tolerance in a mouse model transplanted with human islets [127]. Furthermore, Urban's study has suggested that MSCs may be a new way to treat insulin-dependent diabetes, and these encouraging results in vivo and in vitro provide enthusiasm for MSC-based theoretical use in clinical trials [128]. However, some cautions should be focused on MSC transformation and tumorigenicity with passages due to the gene mutation.
4.5. Systemic Lupus Erythematosus (SLE)
Systemic lupus erythematosus (SLE) is also a chronic autoimmune disease characterized by a multiorgan inflammatory response [129]. Both the proinflammatory cytokine stimulation and autoantibody complex production in SLE bring about activating immune cells in both innate and adaptive immune systems. Over the past 50 years, there is only one drug permitted by the USA Food and Drug Administration (FDA) for the treatment of SLE disease that remains controversial [130]. Consequently, new treatments targeting immune intervention would represent significant advantages for SLE patients. Recently reports have suggested that extracellular vesicle (EV) secreted from MSCs could be used as a cell-free therapy. The preclinical results showed MSC-derived EVs inhibit inflammatory responses and suppress autoimmune disease pathogenesis [131]. Most promising animal studies' data and clinical trials about SLE treatment promoted MSC application in the therapy of SLE patients [131]. The Nanjing Drum Tower Hospital in China has used MSCs to treat over 300 refractory SLE patients. Their results showed 32.5% patients reached a significant clinical efficacy with a well-tolerated safety and a dramatic decline in disease activity scores. And the group published that SLE patients transplanted with MSCs achieved remarkable effect and long-term safety at a 4-year review [113]. However, some clinical trials reported that MSCs convey tumorigenic potential, and the immunosuppressive efficacy could be influenced by donor alteration as well as ex vivo amplification. [102]
5. Controversy over Mesenchymal Stem Cell Therapy
Currently, MSC treatment has already entered clinical trials for the treatment of organ transplantations, tissue regeneration, and autoimmune diseases [132]. Even though the FDA appointed that MSC transplantation was safe, recent researches stated that potential long-term risks involved in MSC treatment may have not appeared in the short term [133]. Many possible complications of MSC therapy are associated with immunosuppressive properties of MSCs on account of reduction of immunosurveillance to host and foreign pathogens or virus [134]. Furthermore, autologous MSCs may, in theory, induce tumors by changing the action of cancer cells and accelerating tumor cell growth. In addition, allogeneic MSCs derived from donors may accelerate infectious risk [135]. Notable questions need to be concentrated about MSCs on the durability of response and long-term safety. For example, less is known about the route administration of MSC home to the site of inflammation and survival in tissues. Furthermore, delivery dose, safety control, as well as optimized standards for MSC derivation and amplification are also needed to be explored for the better development of MSC-based clinical trials [136].
6. Conclusion
MSCs are multipotent progenitor cells with multilineage differentiation potential and immune-modulatory properties. MSCs have the ability to interact with immune cells both in innate and adaptive immune systems. In addition, MSC-mediated immunosuppression is dependent on the combined reaction of chemokines, inflammatory cytokines, and effector factors secreted by MSCs, as well as the microenvironment and strength of the inflammatory stimulus [137]. In the past few years, emerging data have demonstrated that MSCs exert immunoregulatory effects that provide a promising tool for the therapy of tissue repair, inflammatory diseases, and immune disorders [3]. MSCs showed unique advantages and achieved significant improvements in the therapy of immune diseases. Nevertheless, MSC-based treatment does not always provide advantages according to tightly interacting with the microenvironmental milieu. The risks of MSC therapy conclude potential complications of immunosuppression, ectopical differentiation, and promotion of tumor growth [138]. In consequence, many issues need to be settled before clinical application of MSCs [23]. Even though these immunomodulatory characteristics are not entirely elucidated, the immunosuppressive potential of MSCs makes them a promising therapy for various autoimmune diseases [20]. We believe further investigation of MSC-based molecular mechanisms as well as additional clinical trials will enhance our understanding of MSC immunomodulation and aid in the development of clinical applications utilizing MSC treatment [136].
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81321002 and 81600842).
Abbreviations
- MSCs:
Mesenchymal stem cells
- HSC:
Hematopoietic stem cell
- GVHD:
Graft versus host disease
- SLE:
Systemic lupus erythematosus
- TNF:
Tumor necrosis factor
- IL:
Interleukin
- IDO:
Indoleamine2,3-dioxigenase
- NO:
Nitric oxide
- HGF:
Hepatocyte growth factor
- TSG6:
TNF-stimulated gene 6
- DCs:
Dendritic cells
- NK cells:
Natural killer cells
- PGE-2:
Prostaglandin E2
- MHC:
Main histocompatibility complex
- FasL/FasR:
Fas ligand/Fas receptor interaction
- IFN-γ:
Interferon-γ
- EGF:
Epidermal growth factor
- MCP1:
Monocyte chemotactic protein-1
- NF-κB:
Nuclear factor-κB
- PAX5:
Paired box 5
- Blimp1:
B lymphocyte-induced maturation protein 1
- FGF:
Fibroblast growth factor
- PDGF:
Platelet-derived growth factor
- VEGF:
Vascular endothelial growth factor
- IGF-1:
Insulin-like growth Factor-1
- SDF-1:
Stromal cell-derived factor 1
- ICAM:
Intercellular cell adhesion molecule
- HLA:
Human leukocyte antigen
- VCAM:
Vascular cell adhesion molecule
- CCL2:
CC-chemokine ligand 2
- iNOS:
Inducible NO synthase
- STAT5:
Signal transducer and activator of transcription 5
- CCR2:
CC-chemokine receptor 2
- EAE:
Experimental autoimmune encephalomyelitis
- TLR:
Toll-like receptor
- APCs:
Antigen-presenting cells
- Th:
T helper
- Treg:
Regulatory T cell
- MS:
Multiple sclerosis
- MBP:
Myelin-based protein
- CNS:
Central nervous system
- FDA:
Food and Drug Administration
- NIH:
National Institutes of Health
- EV:
Extracellular vesicle.
Contributor Information
Quan Yuan, Email: yuanquan@scu.edu.cn.
Liang Xie, Email: lxie@scu.edu.cn.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
All authors have made substantial, direct, and intellectual contributions to the work. At the same time, all authors participated in designing the study, drafting and writing the manuscript, and approving it for submission.
References
- 1.Hass R., Kasper C., Böhm S., Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Communication and Signaling: CCS. 2011;9(1):p. 12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie L., Zeng X., Hu J., Chen Q. Characterization of nestin, a selective marker for bone marrow derived mesenchymal stem cells. Stem Cells International. 2015;2015:9. doi: 10.1155/2015/762098.762098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uccelli A., Moretta L., Pistoia V. Mesenchymal stem cells in health and disease. Nature Reviews Immunology. 2008;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal S., Pittenger M. F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 5.Qu M., Yuan X., Liu D., et al. Bone marrow-derived mesenchymal stem cells attenuate immune-mediated liver injury and compromise virus control during acute hepatitis B virus infection in mice. Stem Cells and Development. 2017;26(11):818–827. doi: 10.1089/scd.2016.0348. [DOI] [PubMed] [Google Scholar]
- 6.Ankrum J., Karp J. M. Mesenchymal stem cell therapy: two steps forward, one step back. Trends in Molecular Medicine. 2010;16(5):203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee R. H., Pulin A. A., Seo M. J., et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M. O., Flavell R. A. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28(4):468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Chen X., Gan Y., Li W., et al. The interaction between mesenchymal stem cells and steroids during inflammation. Cell Death & Disease. 2014;5(1, article e1009) doi: 10.1038/cddis.2013.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caplan A. I. Mesenchymal stem cells. Journal of Orthopaedic Research. 1991;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 11.Greco S. J., Corcoran K. E., Cho K. J., Rameshwar P. Tachykinins in the emerging immune system: relevance to bone marrow homeostasis and maintenance of hematopoietic stem cells. Frontiers in Bioscience. 2004;9(1-3):1782–1793. doi: 10.2741/1373. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y., Zhou C., Yuan Q. Role of DNA and RNA N6-adenine methylation in regulating stem cell fate. Current Stem Cell Research & Therapy. 2017;13(1) doi: 10.2174/1574888X12666170621125457. [DOI] [PubMed] [Google Scholar]
- 13.Shi Y., Su J., Roberts A. I., Shou P., Rabson A. B., Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends in Immunology. 2012;33(3):136–143. doi: 10.1016/j.it.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park M., Kim Y. H., Ryu J. H., Woo S. Y., Ryu K. H. Immune suppressive effects of tonsil-derived mesenchymal stem cells on mouse bone-marrow-derived dendritic cells. Stem Cells International. 2015;2015:12. doi: 10.1155/2015/106540.106540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y., Zhang S., Yuan Q. N 6-Methyladenosine methyltransferases and demethylases: new regulators of stem cell pluripotency and differentiation. Stem Cells and Development. 2016;25(14):1050–1059. doi: 10.1089/scd.2016.0062. [DOI] [PubMed] [Google Scholar]
- 16.Zhou C. C., Xiong Q. C., Zhu X. X., et al. AFF1 and AFF4 differentially regulate the osteogenic differentiation of human MSCs. Bone Res. 2017;5, article 17044 doi: 10.1038/boneres.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prockop D. J. Concise review: two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells. 2013;31(10):2042–2046. doi: 10.1002/stem.1400. [DOI] [PubMed] [Google Scholar]
- 18.Djouad F., Plence P., Bony C., et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102(10):3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 19.Bartholomew A., Sturgeon C., Siatskas M., et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental Hematology. 2002;30(1):42–48. doi: 10.1016/S0301-472X(01)00769-X. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Chen X., Cao W., Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nature Immunology. 2014;15(11):1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 21.Kean T. J., Lin P., Caplan A. I., Dennis J. E. MSCs: delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells International. 2013;2013:13. doi: 10.1155/2013/732742.732742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aboalola D., Han V. K. M. Different effects of insulin-like growth Factor-1 and insulin-like growth Factor-2 on myogenic differentiation of human mesenchymal stem cells. Stem Cells International. 2017;2017:15. doi: 10.1155/2017/8286248.8286248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gebler A., Zabel O., Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends in Molecular Medicine. 2012;18(2):128–134. doi: 10.1016/j.molmed.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Mielcarek M., Storb R., Georges G. E., et al. Mesenchymal stromal cells fail to prevent acute graft-versus-host disease and graft rejection after dog leukocyte antigen-haploidentical bone marrow transplantation. Biology of Blood and Marrow Transplantation. 2011;17(2):214–225. doi: 10.1016/j.bbmt.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernardo M. E., Fibbe W. E. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13(4):392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Ren G., Zhang L., Zhao X., et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Anzalone R., Iacono M. L., Corrao S., et al. New emerging potentials for human Wharton’s jelly mesenchymal stem cells: immunological features and hepatocyte-like differentiative capacity. Stem Cells and Development. 2010;19(4):423–438. doi: 10.1089/scd.2009.0299. [DOI] [PubMed] [Google Scholar]
- 28.Abomaray F. M., al Jumah M. A., Alsaad K. O., et al. Phenotypic and functional characterization of mesenchymal stem/multipotent stromal cells fromdecidua basalisof human term placenta. Stem Cells International. 2016;2016:18. doi: 10.1155/2016/5184601.5184601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su J., Chen X., Huang Y., et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death and Differentiation. 2014;21(3):388–396. doi: 10.1038/cdd.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bai L., Lennon D. P., Caplan A. I., et al. Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nature Neuroscience. 2012;15(6):862–870. doi: 10.1038/nn.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalal J., Gandy K., Domen J. Role of mesenchymal stem cell therapy in Crohn’s disease. Pediatric Research. 2012;71(4-2):445–451. doi: 10.1038/pr.2011.56. [DOI] [PubMed] [Google Scholar]
- 32.Yamane H., Paul W. E. Early signaling events that underlie fate decisions of naive CD4+ T cells toward distinct T-helper cell subsets. Immunological Reviews. 2013;252(1):12–23. doi: 10.1111/imr.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Blanc K., Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nature Reviews. Immunology. 2012;12(5):383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 34.Bassi E. J., Aita C. A., Camara N. O. Immune regulatory properties of multipotent mesenchymal stromal cells: where do we stand? World J Stem Cells. 2011;3(1):1–8. doi: 10.4252/wjsc.v3.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuettenberg A., Becker C., Correll A., Steinbrink K., Jonuleit H. Immune regulation by dendritic cells and T cells—basic science, diagnostic, and clinical application. Clinical Laboratory. 2011;57(1-2):1–12. [PubMed] [Google Scholar]
- 36.Jiang X. X., Zhang Y., Liu B., et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 37.Gao W. X., Sun Y. Q., Shi J., et al. Effects of mesenchymal stem cells from human induced pluripotent stem cells on differentiation, maturation, and function of dendritic cells. Stem Cell Research & Therapy. 2017;8(1):p. 48. doi: 10.1186/s13287-017-0499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu L. L., Fu H. X., Zhang J. M., et al. Impaired function of bone marrow mesenchymal stem cells from immune thrombocytopenia patients in inducing regulatory dendritic cell differentiation through the Notch-1/Jagged-1 signaling pathway. Stem Cells and Development. 2017;26(22):1648–1661. doi: 10.1089/scd.2017.0078. [DOI] [PubMed] [Google Scholar]
- 39.Yañez R., Oviedo A., Aldea M., Bueren J. A., Lamana M. L. Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Experimental Cell Research. 2010;316(19):3109–3123. doi: 10.1016/j.yexcr.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Consentius C., Akyüz L., Schmidt-Lucke J. A., et al. Mesenchymal stromal cells prevent allostimulation in vivo and control checkpoints of Th1 priming: migration of human DC to lymph nodes and NK cell activation. Stem Cells. 2015;33(10):3087–3099. doi: 10.1002/stem.2104. [DOI] [PubMed] [Google Scholar]
- 41.Chiesa S., Morbelli S., Morando S., et al. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(42):17384–17389. doi: 10.1073/pnas.1103650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Favaro E., Carpanetto A., Caorsi C., et al. Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia. 2016;59(2):325–333. doi: 10.1007/s00125-015-3808-0. [DOI] [PubMed] [Google Scholar]
- 43.Li H., Guo Z. K., Jiang X. X., Zhu H., Li X. S., Mao N. Mesenchymal stem cells alter migratory property of T and dendritic cells to delay the development of murine lethal acute graft-versus-host disease. Stem Cells. 2008;26(10):2531–2541. doi: 10.1634/stemcells.2008-0146. [DOI] [PubMed] [Google Scholar]
- 44.Huang Y., Chen P., Zhang C. B., et al. Kidney-derived mesenchymal stromal cells modulate dendritic cell function to suppress alloimmune responses and delay allograft rejection. Transplantation. 2010;90(12):1307–1311. doi: 10.1097/TP.0b013e3181fdd9eb. [DOI] [PubMed] [Google Scholar]
- 45.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nature Reviews Immunology. 2002;2(12):957–965. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 46.Sotiropoulou P. A., Perez S. A., Gritzapis A. D., Baxevanis C. N., Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 47.Cui R., Rekasi H., Hepner-Schefczyk M., et al. Human mesenchymal stromal/stem cells acquire immunostimulatory capacity upon cross-talk with natural killer cells and might improve the NK cell function of immunocompromised patients. Stem Cell Research & Therapy. 2016;7(1):p. 88. doi: 10.1186/s13287-016-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michelo C. M., Fasse E., van Cranenbroek B., et al. Added effects of dexamethasone and mesenchymal stem cells on early natural killer cell activation. Transplant Immunology. 2016;37:1–9. doi: 10.1016/j.trim.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Najar M., Fayyad-Kazan M., Meuleman N., Bron D., Fayyad-Kazan H., Lagneaux L. Immunomodulatory effects of foreskin mesenchymal stromal cells on natural killer cells. Journal of Cellular Physiology. 2018;233(7):5243–5254. doi: 10.1002/jcp.26305. [DOI] [PubMed] [Google Scholar]
- 50.Götherström C., Lundqvist A., Duprez I. R., Childs R., Berg L., le Blanc K. Fetal and adult multipotent mesenchymal stromal cells are killed by different pathways. Cytotherapy. 2011;13(3):269–278. doi: 10.3109/14653249.2010.523077. [DOI] [PubMed] [Google Scholar]
- 51.Wynn T. A., Vannella K. M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glass C. K., Natoli G. Molecular control of activation and priming in macrophages. Nature Immunology. 2016;17(1):26–33. doi: 10.1038/ni.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mosser D. M., Edwards J. P. Exploring the full spectrum of macrophage activation. Nature Reviews. Immunology. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Q. Z., Su W. R., Shi S. H., et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28(10):1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selleri S., Bifsha P., Civini S., et al. Human mesenchymal stromal cell-secreted lactate induces M2-macrophage differentiation by metabolic reprogramming. Oncotarget. 2016;7(21):30193–30210. doi: 10.18632/oncotarget.8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi C., Jia T., Mendez-Ferrer S., et al. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34(4):590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ciccocioppo R., Bernardo M. E., Sgarella A., et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut. 2011;60(6):788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 58.Chaturvedi P., Gilkes D. M., Takano N., Semenza G. L. Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(20):E2120–E2129. doi: 10.1073/pnas.1406655111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu W., Zhang S., Gu S., Sang L., Dai C. Mesenchymal stem cells recruit macrophages to alleviate experimental colitis through TGFβ1. Cellular Physiology and Biochemistry. 2015;35(3):858–865. doi: 10.1159/000369743. [DOI] [PubMed] [Google Scholar]
- 60.Kaech S. M., Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nature Reviews Immunology. 2012;12(11):749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soroosh P., Doherty T. A. Th9 and allergic disease. Immunology. 2009;127(4):450–458. doi: 10.1111/j.1365-2567.2009.03114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8(3):275–283. doi: 10.1016/S1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 63.Dimeloe S., Burgener A. V., Grählert J., Hess C. T-cell metabolism governing activation, proliferation and differentiation: a modular view. Immunology. 2017;150(1):35–44. doi: 10.1111/imm.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ren G., Zhao X., Zhang L., et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. Journal of Immunology. 2010;184(5):2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Di Nicola M., Carlo-Stella C., Magni M., et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.V99.10.3838. [DOI] [PubMed] [Google Scholar]
- 66.Akiyama K., Chen C., Wang D. D., et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10(5):544–555. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chinnadurai R., Copland I. B., Garcia M. A., et al. Cryopreserved mesenchymal stromal cells are susceptible to T-cell mediated apoptosis which is partly rescued by IFNγ licensing. Stem Cells. 2016;34(9):2429–2442. doi: 10.1002/stem.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Renner P., Eggenhofer E., Rosenauer A., et al. Mesenchymal stem cells require a sufficient, ongoing immune response to exert their immunosuppressive function. Transplantation Proceedings. 2009;41(6):2607–2611. doi: 10.1016/j.transproceed.2009.06.119. [DOI] [PubMed] [Google Scholar]
- 69.Rashedi I., Gómez-Aristizábal A., Wang X. H., Viswanathan S., Keating A. TLR3 or TLR4 activation enhances mesenchymal stromal cell-mediated Treg induction via notch signaling. Stem Cells. 2017;35(1):265–275. doi: 10.1002/stem.2485. [DOI] [PubMed] [Google Scholar]
- 70.Gratz I. K., Rosenblum M. D., Abbas A. K. The life of regulatory T cells. Annals of the New York Academy of Sciences. 2013;1283(1):8–12. doi: 10.1111/nyas.12011. [DOI] [PubMed] [Google Scholar]
- 71.De Silva N. S., Klein U. Dynamics of B cells in germinal centres. Nature Reviews. Immunology. 2015;15(3):137–148. doi: 10.1038/nri3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Depoil D., Weber M., Treanor B., et al. Early events of B cell activation by antigen. Science Signaling. 2009;2(63, Part 1) doi: 10.1126/scisignal.263pt1. [DOI] [PubMed] [Google Scholar]
- 73.O’Connor B. P., Vogel L. A., Zhang W., et al. Imprinting the fate of antigen-reactive B cells through the affinity of the B cell receptor. Journal of Immunology. 2006;177(11):7723–7732. doi: 10.4049/jimmunol.177.11.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Augello A., Tasso R., Negrini S. M., et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. European Journal of Immunology. 2005;35(5):1482–1490. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 75.Feng X., Che N., Liu Y., et al. Restored immunosuppressive effect of mesenchymal stem cells on B cells after olfactory 1/early B cell factor-associated zinc-finger protein down-regulation in patients with systemic lupus erythematosus. Arthritis & Rhematology. 2014;66(12):3413–3423. doi: 10.1002/art.38879. [DOI] [PubMed] [Google Scholar]
- 76.Fan L., Hu C., Chen J., Cen P., Wang J., Li L. Interaction between mesenchymal stem cells and B-cells. International Journal of Molecular Sciences. 2016;17(5) doi: 10.3390/ijms17050650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saparov A., Ogay V., Nurgozhin T., Jumabay M., Chen W. C. W. Preconditioning of human mesenchymal stem cells to enhance their regulation of the immune response. Stem Cells International. 2016;2016:10. doi: 10.1155/2016/3924858.3924858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krampera M., Cosmi L., Angeli R., et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24(2):386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 79.Bernardo M. E., Locatelli F., Fibbe W. E. Mesenchymal stromal cells. Annals of the New York Academy of Sciences. 2009;1176(1):101–117. doi: 10.1111/j.1749-6632.2009.04607.x. [DOI] [PubMed] [Google Scholar]
- 80.Frenette P. S., Pinho S., Lucas D., Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annual Review of Immunology. 2013;31(1):285–316. doi: 10.1146/annurev-immunol-032712-095919. [DOI] [PubMed] [Google Scholar]
- 81.Nauta A. J., Fibbe W. E. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 82.Tang K. C., Trzaska K. A., Smirnov S. V., et al. Down-regulation of MHC II in mesenchymal stem cells at high IFN-gamma can be partly explained by cytoplasmic retention of CIITA. Journal of Immunology. 2008;180(3):1826–1833. doi: 10.4049/jimmunol.180.3.1826. [DOI] [PubMed] [Google Scholar]
- 83.Németh K., Leelahavanichkul A., Yuen P. S. T., et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nature Medicine. 2009;15(1):42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spaggiari G. M., Capobianco A., Abdelrazik H., Becchetti F., Mingari M. C., Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111(3):1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 85.Jarvinen L., Badri L., Wettlaufer S., et al. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. Journal of Immunology. 2008;181(6):4389–4396. doi: 10.4049/jimmunol.181.6.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ge W., Jiang J., Arp J., Liu W., Garcia B., Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. 2010;90(12):1312–1320. doi: 10.1097/TP.0b013e3181fed001. [DOI] [PubMed] [Google Scholar]
- 87.Ren G., Su J., Zhang L., et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27(8):1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 88.Wisniewski H. G., Vilcek J. TSG-6: an IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine & Growth Factor Reviews. 1997;8(2):143–156. doi: 10.1016/S1359-6101(97)00008-7. [DOI] [PubMed] [Google Scholar]
- 89.Choi E. W., Shin I. S., Park S. Y., et al. Reversal of serologic, immunologic, and histologic dysfunction in mice with systemic lupus erythematosus by long-term serial adipose tissue-derived mesenchymal stem cell transplantation. Arthritis and Rheumatism. 2012;64(1):243–253. doi: 10.1002/art.33313. [DOI] [PubMed] [Google Scholar]
- 90.Sato K., Ozaki K., Oh I., et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109(1):228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 91.Lukacs-Kornek V., Malhotra D., Fletcher A. L., et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nature Immunology. 2011;12(11):1096–1104. doi: 10.1038/ni.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barrachina L., Remacha A. R., Romero A., et al. Priming equine bone marrow-derived mesenchymal stem cells with proinflammatory cytokines: implications in immunomodulation-immunogenicity balance, cell viability, and differentiation potential. Stem Cells and Development. 2017;26(1):15–24. doi: 10.1089/scd.2016.0209. [DOI] [PubMed] [Google Scholar]
- 93.Yang S. H., Park M. J., Yoon I. H., et al. Soluble mediators from mesenchymal stem cells suppress T cell proliferation by inducing IL-10. Experimental & Molecular Medicine. 2009;41(5):315–324. doi: 10.3858/emm.2009.41.5.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weil B. R., Manukyan M. C., Herrmann J. L., et al. Mesenchymal stem cells attenuate myocardial functional depression and reduce systemic and myocardial inflammation during endotoxemia. Surgery. 2010;148(2):444–452. doi: 10.1016/j.surg.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 95.Rafei M., Campeau P. M., Aguilar-Mahecha A., et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. Journal of Immunology. 2009;182(10):5994–6002. doi: 10.4049/jimmunol.0803962. [DOI] [PubMed] [Google Scholar]
- 96.Lotfinia M., Kadivar M., Piryaei A., et al. Effect of secreted molecules of human embryonic stem cell-derived mesenchymal stem cells on acute hepatic failure model. Stem Cells and Development. 2016;25(24):1898–1908. doi: 10.1089/scd.2016.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brown J. M., Nemeth K., Kushnir-Sukhov N. M., Metcalfe D. D., Mezey E. Bone marrow stromal cells inhibit mast cell function via a COX2-dependent mechanism. Clinical and Experimental Allergy. 2011;41(4):526–534. doi: 10.1111/j.1365-2222.2010.03685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li F., Zhao S. Z. Control of cross talk between angiogenesis and inflammation by mesenchymal stem cells for the treatment of ocular surface diseases. Stem Cells International. 2016;2016:8. doi: 10.1155/2016/7961816.7961816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cho K. A., Ju S. Y., Cho S. J., et al. Mesenchymal stem cells showed the highest potential for the regeneration of injured liver tissue compared with other subpopulations of the bone marrow. Cell Biology International. 2009;33(7):772–777. doi: 10.1016/j.cellbi.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 100.Qian H., Yang H., Xu W., et al. Bone marrow mesenchymal stem cells ameliorate rat acute renal failure by differentiation into renal tubular epithelial-like cells. International Journal of Molecular Medicine. 2008;22(3):325–332. [PubMed] [Google Scholar]
- 101.Rose R. A., Jiang H., Wang X., et al. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells. 2008;26(11):2884–2892. doi: 10.1634/stemcells.2008-0329. [DOI] [PubMed] [Google Scholar]
- 102.Munir H., McGettrick H. M. Mesenchymal stem cell therapy for autoimmune disease: risks and rewards. Stem Cells and Development. 2015;24(18):2091–2100. doi: 10.1089/scd.2015.0008. [DOI] [PubMed] [Google Scholar]
- 103.Wohn D. Y. Korea okays stem cell therapies despite limited peer-reviewed data. Nature Medicine. 2012;18(3):p. 329. doi: 10.1038/nm0312-329a. [DOI] [PubMed] [Google Scholar]
- 104.Wolf D., Reinhard A., Wolf D., et al. Regenerative capacity of intravenous autologous, allogeneic and human mesenchymal stem cells in the infarcted pig myocardium-complicated by myocardial tumor formation. Scandinavian Cardiovascular Journal. 2009;43(1):39–45. doi: 10.1080/14017430802100280. [DOI] [PubMed] [Google Scholar]
- 105.Hare J. M., Fishman J. E., Gerstenblith G., et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308(22):2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Le Blanc K., Rasmusson I., Sundberg B., et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. The Lancet. 2004;363(9419):1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 107.Levine J. E., Paczesny S., Sarantopoulos S. Clinical applications for biomarkers of acute and chronic graft-versus-host disease. Biology of Blood and Marrow Transplantation. 2012;18(1):S116–S124. doi: 10.1016/j.bbmt.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Szyska M., Na I. K. Bone marrow GvHD after allogeneic hematopoietic stem cell transplantation. Frontiers in Immunology. 2016;7:p. 118. doi: 10.3389/fimmu.2016.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Götherström C., Ringdén O., Tammik C., Zetterberg E., Westgren M., le Blanc K. Immunologic properties of human fetal mesenchymal stem cells. American Journal of Obstetrics and Gynecology. 2004;190(1):239–245. doi: 10.1016/j.ajog.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 110.Leung Y., Geddes M., Storek J., Panaccione R., Beck P. L. Hematopoietic cell transplantation for Crohn’s disease; is it time? World Journal of Gastroenterology. 2006;12(41):6665–6673. doi: 10.3748/wjg.v12.i41.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rebeiro P., Moore J. The role of autologous haemopoietic stem cell transplantation in the treatment of autoimmune disorders. Internal Medicine Journal. 2016;46(1):17–28. doi: 10.1111/imj.12944. [DOI] [PubMed] [Google Scholar]
- 112.Liechty K. W., MacKenzie T. C., Shaaban A. F., et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nature Medicine. 2000;6(11):1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 113.Wang D., Zhang H., Liang J., et al. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplantation. 2013;22(12):2267–2277. doi: 10.3727/096368911X582769c. [DOI] [PubMed] [Google Scholar]
- 114.Ibraheim H., Giacomini C., Kassam Z., Dazzi F., Powell N. Advances in mesenchymal stromal cell therapy in the management of Crohn’s disease. Expert Review of Gastroenterology & Hepatology. 2018;12(2):141–153. doi: 10.1080/17474124.2018.1393332. [DOI] [PubMed] [Google Scholar]
- 115.Carnegie P. R. Amino acid sequence of the encephalitogenic basic protein from human myelin. The Biochemical Journal. 1971;123(1):57–67. doi: 10.1042/bj1230057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zappia E., Casazza S., Pedemonte E., et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106(5):1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 118.Bai L., Lennon D. P., Eaton V., et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57(11):1192–1203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thanunchai M., Hongeng S., Thitithanyanont A. Mesenchymal stromal cells and viral infection. Stem Cells International. 2015;2015:8. doi: 10.1155/2015/860950.860950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Steffenhagen C., Dechant F. X., Oberbauer E., et al. Mesenchymal stem cells prime proliferating adult neural progenitors toward an oligodendrocyte fate. Stem Cells and Development. 2012;21(11):1838–1851. doi: 10.1089/scd.2011.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Connick P., Kolappan M., Patani R., et al. The mesenchymal stem cells in multiple sclerosis (MSCIMS) trial protocol and baseline cohort characteristics: an open-label pre-test: post-test study with blinded outcome assessments. Trials. 2011;12(1):p. 62. doi: 10.1186/1745-6215-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rathmann W., Giani G. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(10):2568–2569. doi: 10.2337/diacare.27.10.2568. [DOI] [PubMed] [Google Scholar]
- 123.Voltarelli J. C., Couri C. E. B., Stracieri A. B. P. L., et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297(14):1568–1576. doi: 10.1001/jama.297.14.1568. [DOI] [PubMed] [Google Scholar]
- 124.The DIAMOND Project Group. Incidence and trends of childhood type 1 diabetes worldwide 1990-1999. Diabetic Medicine. 2006;23(8):857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 125.Dazzi F., Horwood N. J. Potential of mesenchymal stem cell therapy. Current Opinion in Oncology. 2007;19(6):650–655. doi: 10.1097/CCO.0b013e3282f0e116. [DOI] [PubMed] [Google Scholar]
- 126.Wu H., Mahato R. I. Mesenchymal stem cell-based therapy for type 1 diabetes. Discovery Medicine. 2014;17(93):139–143. [PubMed] [Google Scholar]
- 127.Aikawa E., Fujita R., Asai M., Kaneda Y., Tamai K. Receptor for advanced glycation end products-mediated signaling impairs the maintenance of bone marrow mesenchymal stromal cells in diabetic model mice. Stem Cells and Development. 2016;25(22):1721–1732. doi: 10.1089/scd.2016.0067. [DOI] [PubMed] [Google Scholar]
- 128.Urbán V. S., Kiss J., Kovács J., et al. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 2008;26(1):244–253. doi: 10.1634/stemcells.2007-0267. [DOI] [PubMed] [Google Scholar]
- 129.Tsokos G. C. Systemic lupus erythematosus. The New England Journal of Medicine. 2011;365(22):2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 130.Jordan N. P., D’Cruz D. P. Efficacy, pharmacokinetic and pharmacodynamic profile of belimumab for systemic lupus erythematosus. Expert Opinion on Drug Metabolism & Toxicology. 2015;11(10):1635–1645. doi: 10.1517/17425255.2015.1077808. [DOI] [PubMed] [Google Scholar]
- 131.Sharma J., Hampton J. M., Valiente G. R., et al. Therapeutic development of mesenchymal stem cells or their extracellular vesicles to inhibit autoimmune-mediated inflammatory processes in systemic lupus erythematosus. Frontiers in Immunology. 2017;8:p. 526. doi: 10.3389/fimmu.2017.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Le Blanc K., Frassoni F., Ball L., et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. The Lancet. 2008;371(9624):1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 133.Elabd C., Centeno C. J., Schultz J. R., Lutz G., Ichim T., Silva F. J. Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: a long-term safety and feasibility study. Journal of Translational Medicine. 2016;14(1):p. 253. doi: 10.1186/s12967-016-1015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Freitag J., Bates D., Boyd R., et al. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy – a review. BMC Musculoskeletal Disorders. 2016;17(1):p. 230. doi: 10.1186/s12891-016-1085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rubio D., Garcia S., Paz M. F., et al. Molecular characterization of spontaneous mesenchymal stem cell transformation. PLoS One. 2008;3(1):p. e1398. doi: 10.1371/journal.pone.0001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mao F., Tu Q., Wang L., et al. Mesenchymal stem cells and their therapeutic applications in inflammatory bowel disease. Oncotarget. 2017;8(23):38008–38021. doi: 10.18632/oncotarget.16682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10(6):709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 138.Rengasamy M., Gupta P. K., Kolkundkar U., et al. Preclinical safety & toxicity evaluation of pooled, allogeneic human bone marrow-derived mesenchymal stromal cells. The Indian Journal of Medical Research. 2016;144(6):852–864. doi: 10.4103/ijmr.IJMR_1842_15. [DOI] [PMC free article] [PubMed] [Google Scholar]


