Abstract
Objective
Scientific evidence implicates anxiety sensitivity (AS) as a risk factor for poor smoking cessation outcomes. Integrated smoking cessation programs that target AS may lead to improved smoking cessation outcomes, potentially through AS reduction. Yet, little work has evaluated the efficacy of integrated smoking cessation treatment on smoking abstinence. The present study prospectively examined treatment effects of a novel AS reduction-smoking cessation intervention relative to a standard smoking cessation intervention on smoking abstinence.
Method
Participants (N=529; 45.9% male; Mage=38.23, SD=13.56) included treatment-seeking smokers who received either a 4-session integrated anxiety-reduction and smoking cessation intervention (Smoking Treatment and Anxiety Management Program; [STAMP]) or a 4-session standard smoking cessation program (SCP). The primary aims focused on examining the effects of STAMP on (1) AS reduction during treatment, (2) early and late smoking point prevalence abstinence, and (3) the mechanistic function of AS reduction on treatment effects across early and late smoking abstinence.
Results
Results indicated a significantly greater decline in AS in STAMP relative to SCP (B = −.72, p < .001). Treatment condition did not significantly directly predict early or late abstinence. However, the effect of STAMP on early abstinence was significantly mediated by reductions in AS (indirect = .16, 95% CI[.02, .40]).
Conclusions
Findings provide evidence for the efficacy of a novel, integrated anxiety and smoking cessation treatment to reduce AS. Moreover, the meditation pathway from STAMP to early abstinence through reductions in AS suggest that AS is a clinically important mechanism of change for smoking cessation treatment and research.
Keywords: Smoking Cessation, Anxiety Sensitivity, Mechanism, Point Prevalence Abstinence, Tobacco
Cigarette smoking is the leading cause of preventable death in the United States (U.S.), contributing to over 480,000 deaths each year (USDHHS, 2014). Although approximately 70% of current adult smokers report being motivated to quit, most established interventions are associated with relatively low long-term abstinence rates (15-35%; Simons, Dvorak, Batien, & Wray, 2010). Significant strides in helping smokers quit will likely be found in the ability to develop novel, specialized treatments that engage specific mechanisms of change. Experimental therapeutics is one promising approach to developing such treatments, as identified by the National Institutes of Health (e.g., National Cancer Institute Experimental Therapeutics Program) and others (Aan Het Rot, Zarate, Charney, & Mathew, 2012). Specifically, experimental therapeutics seeks to first identify a mechanistic target and subsequently engage that target for therapeutic change (Waldman, Kraft, Nelson, & Terzic, 2009).
One transdiagnostic mechanistic risk candidate for psychopathology and smoking, from an experimental therapeutics perspective, is anxiety sensitivity (AS). AS reflects a relatively stable individual difference factor that predisposes individuals to the development of anxiety/depressive problems (Taylor, 1999) by amplifying negative mood states (e.g., anxiety; Reiss, 1991; Zinbarg, Barlow, & Brown, 1997). To illustrate, when a person with high AS experiences physiological sensations (e.g., withdrawal symptoms due to smoking abstinence), she/he is likely to misinterpret these symptoms as signs of impending personal threat (e.g., “I’m going crazy”) and experience them as emotionally distressing (e.g., “I can’t stand this discomfort anymore”; Taylor, 1999). Thus, AS is an ‘amplifying factor,’ enhancing the aversiveness and need to escape/avoid negative affective or somatic experiences (Taylor, 1999; Zvolensky, Yartz, Gregor, Gonzalez, & Bernstein, 2008).
Numerous studies document the role of AS in a multitude of smoking maintenance and relapse processes (Leventhal & Zvolensky, 2015; Zvolensky et al., 2008). For example, prior work has found that smokers higher in AS are more motivated to use cigarettes to relieve negative affect, and to a lesser extent, because of the addictive and habitual properties (Leventhal & Zvolensky, 2015). Smokers higher in AS also expect reduced negative affect as a direct consequence of smoking (Battista et al., 2008; Farris, Leventhal, Schmidt, & Zvolensky, 2015; Gonzalez, Zvolensky, Vujanovic, Leyro, & Marshall, 2008; Gregor, Zvolensky, McLeish, Bernstein, & Morissette, 2008; Johnson, Farris, Schmidt, & Zvolensky, 2012; Leyro, Zvolensky, Vujanovic, & Bernstein, 2008). These subjective motives and expectations may be linked to actual smoking effects, as high AS smokers report greater smoking-induced reductions in subjective anxiety after stressful laboratory situations (Evatt & Kassel, 2010; Perkins, Karelitz, Giedgowd, Conklin, & Sayette, 2010) and more positive subjective benefits after smoking (Wong et al., 2013). Additionally, AS is related to the tendency to smoke when confronted with smoking-relevant thoughts, feelings, and sensations (e.g., bodily tension; Zvolensky, Farris, Schmidt, & Smits, 2014), as well as the subjective experience of more severe side effects for smoking cessation pharmacological aids (Zvolensky et al., 2017).
Other work has shown that AS is associated with the tendency to perceive quitting as more difficult (Langdon, Farris, Hogan, Grover, & Zvolensky, 2016; Zvolensky, Vujanovic, et al., 2007) and that periods of acute smoking deprivation will be personally threatening (Farris, Paulus, et al., 2015; Guillot, Leventhal, Raines, Zvolensky, & Schmidt, 2016). In fact, AS is related to greater negative affectivity, craving, and nicotine withdrawal symptoms (Johnson, Stewart, Rosenfield, Steeves, & Zvolensky, 2012; Langdon et al., 2013), greater likelihood of smoking lapse on days when negative affect is high (Langdon, Farris, Øverup, & Zvolensky, 2015), shorter time to lapse/relapse, and lower overall abstinence in aided (i.e. pharmacological and psychosocial treatment; Zvolensky, Stewart, Vujanovic, Gavric, & Steeves, 2009) and unaided quit attempts (i.e., self-guided quit attempt; Marshall, Johnson, Bergman, Gibson, & Zvolensky, 2009). Other studies demonstrate that AS mediates the relation between emotional disorders and severity of smoking behavior (Olvera et al., 2015; Powers et al., in press; Zvolensky, Farris, Leventhal, & Schmidt, 2014; Zvolensky et al., in press). Importantly, smokers respond to AS reduction treatment after a single intervention session (Feldner, Zvolensky, Babson, Leen-Feldner, & Schmidt, 2008). In this two-hour, single session group treatment, smokers received psychoeducation on anxiety and smoking, and completed interoceptive exposure exercises (Feldner et al., 2008). The goal of this treatment was to enhance motivation for smoking cessation, but not smoking abstinence per se. Thus, the extent that brief AS reduction-smoking cessation treatment impacts smoking behavior (abstinence) remains unknown.
Specialized treatment options to enhance AS reduction within the context of a smoking cessation have thus far focused on vigorous-intensity aerobic exercise or in-person psychosocial methods as means for delivering interoceptive exposure (i.e., intentional exposure to avoided internal bodily sensations) and cognitive re-structuring. For example, intensive vigorous-intensity aerobic exercise (15 sessions) for smokers with elevated AS has shown promise in enhancing cessation outcomes (Smits et al., 2016). Specifically, compared to standard group-based cognitive behavioral therapy (CBT) for smoking cessation, a group-based AS and smoking CBT treatment that included aerobic exercise (i.e., for interoceptive exposure) 3-times per week resulted in significantly higher point prevalence abstinence (PPA) and prolonged abstinence (PA) rates at end-of-treatment, 4-month follow-up, and 6-month follow-up (Smits et al., 2016). Furthermore, AS reduction via individual CBT intervention with interoceptive exposure (ranging from 8-16 sessions), including English and Spanish-language versions, have yielded positive smoking outcomes (Zvolensky, Bogiaizian, Salazar, Farris, & Bakhshaie, 2014; Zvolensky, Lejuez, Kahler, & Brown, 2003).
Extant integrated treatment options that address AS reduction in the context of smoking cessation, although promising, are limited by their time intensive nature and unlikely adoption in setting in which individuals seek help for quitting smoking (e.g., primary care settings; Verbiest et al., 2017). The time commitment required to complete 8 or more sessions of integrated anxiety and smoking cessation treatment may be overly burdensome and unrealistic. Structural barriers, including transportation issues, are often contributing factors to not seeking treatment or treatment drop out among individuals with mental health issues (Mojtabai et al., 2011). These are important consideration because AS is related to earlier treatment dropout (Langdon et al., 2016); thus, a briefer intervention may be critically important for these smokers. Therefore, a less time-intensive, yet theoretically and empirically valid treatment, may have the potential for greater client reach and increased public health impact. In an effort to address these limitations, we developed a theoretically-driven, empirically supported, 4-session integrated AS reduction-smoking cessation treatment within the context of a panic disorder prevention program for smokers. The treatment was presented to individuals as a novel smoking cessation program that could also improve their mental health, through management of anxiety-related symptoms and sensations without smoking. Thus, treatment incorporated core therapeutic tactics to address and bolster AS reduction and support smoking cessation (Schmidt, Raines, Allan, & Zvolensky, 2016). Our initial findings indicated that the AS reduction treatment compared to standard smoking counseling leads to improved AS and panic severity. Moreover, reduction in AS during treatment mediated treatment effects on panic symptom severity at quit week and 1-year follow-up (Schmidt et al., 2016). In the current study, we aimed to extend these findings and explore the indirect effect of treatment on smoking cessation outcomes through change in AS.
Overall, the present study aimed to fill an important gap in the existing literature by prospectively evaluating smoking outcomes within the context of a randomized controlled AS reduction-smoking cessation program. We examined the impact of an integrated Smoking Treatment and Anxiety Management Program (STAMP) compared to a Standard Cessation Program (SCP) on (a) reductions in AS during treatment, (b) early point-prevalence abstinence (PPA) trajectory (quit week to 2-weeks post-end-of-treatment [EOT]) and late PPA trajectory (1-month post-EOT to 1-year post-EOT), and (c) changes in AS as a mediator of the effects of treatment on PPA outcomes. Early and late PPA were examined separately to provide a more thorough understanding of the dynamic quit process post treatment. These outcomes are consistent with a phase-based process model of quitting (Baker et al., 2011), which purports phase-specific challenges that can interfere with smoking abstinence (e.g., high AS smokers struggle more earlier in the quit process). We hypothesized that STAMP would produce greater reductions in AS during treatment relative to SCP and would result in higher early and late PPA. Additionally, based on prior work showing AS is most relevant to the early phases of quitting (Brown, Kahler, Zvolensky, Lejuez, & Ramsey, 2001; Zvolensky, Bonn-Miller, Bernstein, & Marshall, 2006; Zvolensky et al., 2009), we hypothesized that the effect of STAMP on early PPA would be mediated by reductions in AS.
METHOD
Participants
The sample consisted of 529 (45.9% male; Mage = 38.23, SD = 13.56) treatment-seeking adult daily, cigarette smokers recruited from the community to participate in a large randomized controlled trial examining the efficacy of two smoking cessation interventions. All participants were recruited from two sites (University of Vermont and Florida State University; clinicaltrials.gov #NCT01753141). To be eligible for inclusion participants had to be 18 years of age or older, daily cigarette users (e.g., average ≥ 8 cigarettes per day for at least 1 year), and report a motivation to quit smoking (e.g., at least 5 on a 10-point scale). Additionally, individuals with a psychotic disorder, uncontrolled bipolar disorder, serious suicidal intent that warranted hospitalization or immediate treatment, or those using another smoking cessation program or tobacco product were excluded.
The sample was primarily Caucasian (75.4%) with 9.5% African American, 3.4% Hispanic, 0.9% Asian, 1.7% Other (e.g., biracial), and 9.1% failed to respond. At least one current (past year) Axis I diagnosis was endorsed by 43.7% of the sample. The most common primary diagnoses were social anxiety disorder (10.4%), generalized anxiety disorder (6.3%), current major depressive episode (4.7%), and posttraumatic stress disorder (3.0%). Regarding level of education, 5.3% completed some high school, 19.8% had a high school diploma or the equivalent, 30.2% completed some college, 9.3% graduated from a 2-year college, 13.4% graduated from a 4-year college, 4.9% completed professional school, 7.9% had a graduate degree, and 9.2% failed to respond. Finally, participants reported smoking 16.56 (SD = 9.55) cigarettes per day and had been smoking for an average of 19.66 years (SD = 13.44). According to the Fagerström Test for Cigarette Dependence (FTCD) measure, moderate level of tobacco dependence was observed in the sample (M = 5.2, SD = 2.3; Heatherton, Kozlowski, Frecker, & Fagerström, 1991).
Measures
Demographics Questionnaire
Demographic information collected included sex, age, and race. Items from this measure were used to describe the sample.
Structured Clinical Interview-Non-Patient Version for DSM-IV (SCID-I/NP)
Diagnostic assessments of past year Axis I psychopathology were conducted using the SCID-I/NP (First, Spitzer, Gibbon, & Williams, 1994). All SCID-I/NP interviews were administered by trained research assistants or doctoral level staff and supervised by independent doctoral-level professionals. Interviews were audio-taped and the reliability of a random selection of 12.5% of interviews was checked (MJZ) for accuracy; there were no cases of disagreement. Data from the SCID-I/NP was used to describe psychopathology among the sample.
Smoking History Questionnaire (SHQ)
The SHQ was used to assess smoking rate, years of daily smoking, and other characteristics (Brown, Lejuez, Kahler, & Strong, 2002). Smoking rate was obtained from the question, “Since you started regular daily smoking, what is the average number of cigarettes you smoked per day?” Furthermore, years as a daily smoker was assessed by the question, “For how many years, altogether, have you been a regular daily smoker?”.
Fagerström Test for Cigarette Dependence (FTCD)
The FTCD is a 6-item scale that assesses gradations in tobacco dependence (Fagerström, 2012; Heatherton et al., 1991). Scores range from 0-10, with higher scores reflecting high levels of physiological dependence on cigarettes. The FTCD has adequate internal consistency, positive relations with key smoking variables (e.g., saliva cotinine), and high test-retest reliability (Heatherton et al., 1991; Pomerleau, Carton, Lutzke, Flessland, & Pomerleau, 1994). In the current study, the FTCD total score was used to characterize tobacco dependence across the sample.
Positive and Negative Affect Schedule (PANAS)
The PANAS (Watson, Clark, & Tellegen, 1988) measured the extent to which participants experienced 20 different feelings and emotions on a scale ranging from 1 (Very slightly or not at all) to 5 (Extremely). The measure yields two factors, negative affect (NA) and positive affect (PA), and has strong documented psychometric properties (Watson et al., 1988). The NA subscale was utilized in the current study (α = .91).
Anxiety Sensitivity Index-3 (ASI-3)
The ASI-3 is an 18-item self-report measure of sensitivity to and fear of the potential negative consequences of anxiety-related symptoms and sensations (Taylor et al., 2007). Respondents are asked to indicate, on a 5-point Likert-type scale (0 = “very little” to 4 = “very much”), the degree to which they are concerned about these possible negative consequences (possible range 0-72). Average ASI-3 scores in adults from North America (n = 4,720) are 12.8 (SD = 10.6), whereas clinical samples of adults with anxiety disorders present with average ASI-3 scores ≥ 25 (Taylor et al., 2007). The ASI-3, derived in part from the original ASI (Reiss & McNally, 1985), has sound psychometric properties, including excellent internal consistency, predictive validity, and reliability among treatment-seeking smokers (Farris, DiBello, et al., 2015). The ASI-3 was administered at each treatment session (i.e., before quit week). In the present study, we utilized the total ASI-3 score (α = .92).
Abstinence
Self-reported smoking status was assessed in-person at Quit Week (EOT), 1-week post-EOT, 2-weeks post-EOT, 1-month post-EOT, 3-months post-EOT, 6-months post-EOT, and 1-year post-EOT. The Timeline Follow-Back (TLFB; Brown et al., 1998; Sobell & Sobell, 1992) procedure was used at all assessments to assess cigarette consumption at each day since the previous assessment. The assessment has demonstrated good reliability and validity with biochemical indices of smoking (Sobell & Sobell, 1996). Self-reported abstinence at every assessment was verified by expired carbon monoxide (CO) using a CMD/CO Series Carbon Monoxide Monitor (Model 3110; Spirometrics, Inc., Auburn, ME). Self-reported abstinence was overridden by a positive expired CO reading (> 4ppm) or saliva cotinine verification (>10 ng/mL). Self-reported abstinence served as the primary indicator of abstinence when neither expired CO nor cotinine levels were available. PPA was defined as self-reported no smoking, not even a puff, in the 7 days prior to any assessment and, when available, biochemical verification of abstinence at the time of the assessment.
Procedure
Data for the present study were collected during a multi-site randomized controlled clinical trial examining the efficacy of two interventions. Participants responding to study advertisements were scheduled by phone for an in-person baseline assessment session. Upon arriving at the clinic, participants provided written informed consent, were interviewed using the SCID-I/NP, and completed a computerized self-report assessment battery as well as biochemical verification of smoking status. Following these procedures, participants were evaluated for study eligibility as defined above. Eligible participants were randomly assigned to one of the two smoking cessation treatments and scheduled for their first treatment appointment approximately 1–2 weeks after the baseline assessment. The study statistician, who had no contact with participants, conducted the random assignment of individual participants using a random number generator for a fixed number of entries.
Smoking cessation treatment consisted of either (a) SCP or (b) STAMP. SCP included the standard of care treatment for standard smoking cessation as well as a review of general health information not specific to anxiety or smoking (to maintain equal contact time across the two conditions). Specifically, SCP was based on the most recent clinical practice guidelines from the USDHHS, Treating Tobacco Use and Dependence (Fiore et al., 2000) and consensus reports (Abrams & Niaura, 2003). SCP included elements such as: discussion of prior quit attempt, high-risk situation, social support, health risks of smoking, and perceived benefits of smoking (for a complete description of this treatment see Schmidt et al., 2016). STAMP integrated (1) interoceptive exposure, cognitive restructuring, and psychoeducation exercises developed for panic prevention and (2) standard of care treatment for standard smoking cessation. Importantly, the panic prevention components of STAMP were designed for a transdiagnostic, mechanism-focused approach. This approach was selected to address the heterogeneity of negative affective symptoms that may occur across disorders and is consistent with current experimental therapeutics (Wilamowska et al., 2010). The standard of care treatment consisted of relapse prevention counseling. Both treatment groups received nicotine replacement therapy via the transdermal nicotine patch, which was initiated at treatment session 4 (quit-day). Treatment consisted of four 60-minute weekly sessions conducted by trained doctoral-level graduate students. All treatment was supervised by principal investigators (MJZ and NBS) and checked for treatment fidelity by independent reviewers. Additional details regarding the interventions are described elsewhere (Schmidt et al., 2016). The ASI-3 was completed at all four-treatment sessions. Treatment participants were invited to complete seven follow-up assessment sessions as described above. All procedures were approved by the Institutional Review Board at both universities.
Data Analysis
Latent growth curve (LGC) analysis was used to examine the impact of treatment on AS growth from the first treatment session to quit week (with analyses centered on quit week), controlling for baseline levels of AS. LGC analysis was then used to examine the impact of treatment on early PPA trajectory (quit week to 2-weeks post-EOT) and late PPA trajectory (1-month post-EOT to 1-year post-EOT). Although assessment of AS and abstinence status were both collected on the same day during quit week, reduction in AS from the first treatment session to quit week was the primary predictor of interest in this model and occurred prior to assessments of smoking abstinence. Thus, examining AS reduction as a predictor of abstinence established temporal precedence for this model. PPA was treated as a categorical variable (coded 0 = smoking, 1 = abstinent). Early PPA models were centered on 2-weeks post-EOT and late PPA models were centered on 1-year post-EOT. In the AS and late PPA models, linear and quadratic growth models were compared using the χ2 difference test, with a significant difference indicating that the inclusion of a quadratic term improved model fit. Only linear growth models were examined for the early PPA model because only three-time points were available. Treatment condition (0 = SCP, 1 = STAMP) was included as a predictor of the intercept and the slope parameters in conditional models. All analyses were conducted in Mplus version 8 (Muthén & Muthén, 1998-2017) using robust maximum likelihood with the Yuan-Bentler (Y-B) scaled χ2 index to correct for data nonnormality and missing data.
Overall model fit was assessed using the Y-B χ2 value as well as additional χ2-based fit indices, including the comparative fit index (CFI), root mean square error of approximation (RMSEA) with accompanying 90% confidence intervals (CIs), and the standardized root mean square residual (SRMR). A nonsignificant χ2 value indicates good fit. CFI values greater than .95, RMSEA values below .05, and SRMR values below .08 suggest good fit. RMSEA lower bound CIs below .05 suggest that good fit cannot be ruled out and upper bound CIs above .10 suggest that poor fit cannot be ruled out. Model fit information was not provided in nonlinear models as means, variances, and covariances are not sufficient statistics for χ2 estimation (Edwards, Wirth, Houts, & Xi, 2012). For late smoking status models, linear and quadratic model fit was compared using -2LL values.
Following examination of direct effects, mediation models were conducted to examine the impact of treatment condition on early and late PPA through AS reductions during treatment. Mediation models were examined using bias-corrected bootstrapping with 5,000 resamples to provide asymmetric CIs (Hayes, 2013).
Results
Sample and Preliminary Analysis
The final sample included 529 participants, 296 (56%) were randomized to STAMP and 233 (44%) were randomized to SCP. Of the 529 participants assigned to the intervention, 218 (74%) of those in STAMP and 166 (71%) of those in SCP attended at least one treatment session. However, given we were interested in smoking cessation outcomes, participants were included only if they attended at least one session when PPA was assessed (i.e., quit week, 1-week post-EOT, or 2-weeks post-EOT). This resulted in a final sample of 290: 161 (55.5%) randomized to STAMP and 129 (44.5%) randomized to SCP. Primary psychiatric diagnoses in the sample are presented in Table 1 for descriptive purposes. Baseline demographics, ASI-3 scores, FTCD scores, and SHQ variables were compared across STAMP and SCP conditions (see Table 2). There were no statistically significant differences across any of the examined variables (all p’s > .05), although both age and baseline ASI-3 scores were marginally different across conditions (p’s < .10), and were therefore included as predictors of ASI-3 growth.
Table 1.
Primary Psychiatric Diagnosis at Baseline by Treatment Condition
| STAMP (N = 296) | SCP (N = 233) | |
|---|---|---|
| Axis I Disorders | Percentage | Percentage |
| Major Depressive Disorder | 4.75% | 4.72% |
| Dysthymic Disorder | 2.03% | 1.29% |
| Bipolar I Disorder | 0% | 0.43% |
| Bipolar II Disorder | 0.34% | 0% |
| Seasonal Depressive Disorder | 0% | 0.43% |
| Mood NOS | 0.68% | 1.00% |
| Alcohol Use Disorders | 4.05% | 4.30% |
| Substance Use Disorders | 2.37% | 3.01% |
| Social Anxiety Disorder | 9.46% | 11.59% |
| Specific Phobia | 6.08% | 4.73% |
| Obsessive-Compulsive Disorder | 1.35% | 1.29% |
| PostTraumatic Stress Disorder | 3.38% | 2.58% |
| Generalized Anxiety Disorder | 6.76% | 5.58% |
| Anxiety NOS | 0.68% | 2.58% |
| Anorexia Nervosa | 0% | 0.43% |
| Other | 1.01% | 1.29% |
| Any Disorder | 43.88% | 44.21% |
STAMP = Smoking Treatment and Anxiety Program. SCP = Standard Cessation Program. NOS = Not Otherwise Specified.
Table 2.
Comparison of Baseline Demographic Variables, ASI-3 Total Score, FTCD Score, and Smoking History across STAMP and SCP Conditions
| STAMP | SCP | Overall | |||||
|---|---|---|---|---|---|---|---|
| Baseline Variables | Mean | SD | Mean | SD | F | Mean | SD |
| Age (in years) | 37.48 | 14.38 | 40.23 | 12.98 | 2.84t1 | 38.70 | 13.82 |
| ASI-3 Total | 15.63 | 12.58 | 13.16 | 11.24 | 3.02t1 | 14.54 | 12.05 |
| FTCD | 5.06 | 2.20 | 5.18 | 2.39 | .192 | 5.11 | 2.28 |
| Average Cigarettes per Day | 16.92 | 9.01 | 15.95 | 8.08 | .862 | 16.16 | 8.68 |
| Years Daily Smoker | 19.21 | 14.00 | 21.33 | 13.29 | .202 | 20.15 | 13.70 |
| Session Attendance | 3.62 | .73 | 3.56 | .75 | .52 | 3.59 | .74 |
|
| |||||||
| % | N | % | N | χ2 (1 df) | |||
| Sex (% Female) | 55.28% | 89 | 50.78% | 65 | .45 | 53.10% | 154 |
Note. ASI-3 = Anxiety Sensitivity Index-3. FTCD = Fagerström Test for Cigarette Dependence. STAMP = Smoking Treatment and Anxiety Management Program. SCP = Standard Cessation Program.
p < .10.
F(1, 287),
F(1, 272).
Baseline demographics, ASI-3 scores, FTCD scores, average cigarettes per day, and years a daily smoker were compared between included (n = 290) and excluded (n = 239) cases. There were no significant differences in baseline ASI-3 scores, FTCD scores, age, or sex (p’s > .05). Of the 161 participants randomized to STAMP, 148 provided abstinence data at quit week, 153 at 1-week post-EOT, 151 at 2-weeks post-EOT, 127 at 1-month post-EOT, 89 at 3-months post-EOT, 78 at 6-months post-EOT, and 68 at 1-year post-EOT. Of the 129 participants randomized to SCP, 116 provided abstinence data at quit week, 124 at 1-week post-EOT, 122 at 2-weeks post-EOT, 97 at 1-month post-EOT, 74 at 3-months post-EOT, 57 at 6-months post-EOT, and 59 at 1-year post-EOT. Logistic regression was used to compare rates of attendance to the treatment and follow-up sessions. There was no significant differential attrition between conditions (p’s > .05). Multinomial regression was used to compare the number of treatment sessions attended across conditions. There was no significant difference in treatment sessions attended by condition (p > .05). Rates of abstinence across conditions were next examined (see Table 3). There were no significant differences in abstinence rates from quit week to 1-year post-quit.
Table 3.
Post-Intervention Abstinence Rates by Condition
| STAMP | SCP | ||||
|---|---|---|---|---|---|
|
|
|||||
| n | % | n | % | χ2 (1 df) | |
| Quit Week | 58 | 39.2% | 40 | 34.5% | .62 |
| 1-Week Post | 72 | 47.1% | 46 | 37.1% | 2.78 |
| 2-Week Post | 70 | 46.4% | 55 | 45.1% | .04 |
| 1-Month Post | 50 | 39.7% | 46 | 47.4% | 1.34 |
| 3-Month Post | 33 | 37.5% | 25 | 33.8% | .24 |
| 6-Month Post | 33 | 42.9% | 16 | 28.1% | 3.09 |
| 1-Year Post | 22 | 32.8% | 21 | 35.6% | .11 |
Note. STAMP = Smoking Treatment and Anxiety Management Program.
SCP = Standard Cessation Program. All χ2 p values > .05.
Latent Growth Curve Model Predicting ASI-3 Scores from Treatment Condition
The baseline unconditional LGC model for AS scores from session 1 to quit week (i.e., baseline, sessions 1-4) provided adequate fit to the data (Y-B χ2 = 14.70, df = 5, p = .01, RMSEA = .08, 90% CI [.04, .13], CFI = .98, SRMR = .03). The quadratic term was nonsignificant when included (p = .17). Therefore, the unconditional linear model was used in further analyses. The model including treatment condition and mean-centered baseline ASI-3 and age scores predicting ASI-3 scores from baseline to quit week provided adequate fit to the data (Y-B χ2 =23.88, df = 11, p = .01, RMSEA = .06, 90% CI [.03, .10], CFI = .99, SRMR = .02). Model parameters are provided in Table 4. The slope parameter was significant (slope = −.85, p < .001) as was the effect of the intervention on the slope (B = −.68, p < .001), indicating overall reductions in AS in both SCP and STAMP; however, a significantly greater decline in AS scores was found in STAMP as compared to SCP (Cohen’s d = .18). Figure 1 contains these trajectories from session 1 to quit week (session 4), adjusted for baseline ASI-3 and age.
Table 4.
Latent Growth Curve Parameters for ASI-3 Scores from Session One to Quit Week Predicted by Treatment Condition, Baseline ASI-3, and Age.
| ASI-3 Model Parameters | Estimate | SE | p |
|---|---|---|---|
| Intercept | 11.09 | .56 | <.001 |
| Intercept Variance | 37.93 | 5.64 | <.001 |
| Slope | −.85 | .15 | <.001 |
| Slope Variance | 1.98 | .70 | .01 |
| I-S Covariance | 4.95 | 1.65 | .003 |
|
| |||
| Covariate Effects (Intercept) | B | SE | p |
|
|
|||
| Condition | −1.06 | .78 | .17 |
| BL ASI-3 | 61 | .05 | <.001 |
| Age | .07 | .03 | .03 |
|
| |||
| Covariate Effects (Slope) | B | SE | p |
|
|
|||
| Condition | −.68 | .23 | .003 |
| BL ASI-3 | −.07 | .02 | <.001 |
| Age | .01 | .01 | .44 |
Note. ASI-3 = Anxiety Sensitivity Index-3. I-S = Intercept – Slope. BL = Baseline. Condition coded as 0 = Standard Cessation Program, 1 = Smoking Treatment and Anxiety Management Program. SE = Standard error.
Figure 1.
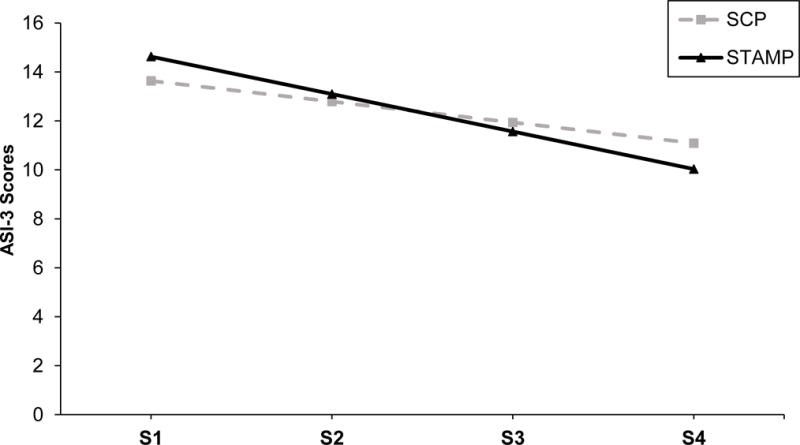
Adjusted (for baseline ASI-3 and age) ASI-3 scores from session one to quit week across STAMP (Smoking Treatment and Anxiety Management Program) and SCP (Smoking Cessation Program). S = Session. Session 4 was quit week.
Latent Growth Curve Models Predicting Early and Late PPA from Treatment Condition
Table 5 contains all parameters for these models. Model fit information was not available for PPA (0 = smoking, 1 = abstinent) due to the categorical nature of the data. The decline in PPA from quit week to 2-weeks post-EOT was not significant (p = .47) There were no significant effects of treatment condition on the intercept (p = .52) or slope values (p = .98).
Table 5.
Direct Effects of Treatment Condition on Early and Late Cigarette Smoking Outcomes
| Early Outcomes Quit Week to Week 2 | Late Outcomes Month 1 to Year 1 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| PPA | Estimate | SE | p | Estimate | SE | p |
|
|
||||||
| Intercept | .00 | .00 | – | .00 | .00 | – |
| Intercept Variance | 18.04 | 6.08 | .003 | 43.30 | 35.19 | .22 |
| Slope | .22 | .31 | .47 | −.06 | .04 | .11 |
| Slope Variance | 4.57 | 1.81 | .01 | .01 | .01 | .35 |
| I-S Covariance | 8.00 | 3.01 | .01 | .51 | .59 | .39 |
|
|
||||||
| Treatment Effects | B | SE | B | SE | ||
| Intercept | .44 | .69 | .52 | .59 | 1.41 | .68 |
| Slope | −.01 | .40 | .98 | .03 | .03 | .38 |
Note. Treatment coded as 0 = Standard Cessation Program, 1 = Smoking Treatment and Anxiety Management Program. PPA= Point-Prevalence Abstinence, coded as 0 = Smoking, 1 = Abstinent. SE = Standard error.
For the late PPA models, including quadratic growth terms did not improve the models (p = .053). Therefore, all models are reported as linear models. For PPA, the slope value was not significant (p = .11) and there were no significant effects of treatment condition on the intercept (p = .68) or slope (p = .38) values.
Mediation Models from Treatment Condition to Early and Late PPA through Declines in AS Scores
AS was examined as a mediator of the slope parameters of all early and late PPA models. The effect of the AS slope on early PPA slope was significant (B = .22, SE = .11, p = .04). The effect of the intervention on early PPA slope was significantly mediated by the AS slope (indirect = −.15, 95% CI [−.50, −.01]; see Figure 2). These results indicated that being assigned to STAMP predicted an increased rate of abstinence from quit week to 2-weeks post-EOT through observed reductions in AS across the treatment period. In contrast, the effect of the AS slope on late PPA slope was not significant (B = −.04, SE = .03, p = .11), nor was the indirect effect from intervention to late PPA, through AS slope (indirect = .03, 95% CI [−.004, .11]).1
Figure 2.
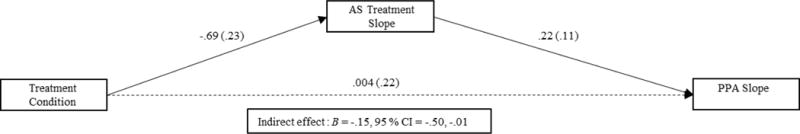
Mediation effects of treatment condition (0 = Standard Cessation Program, 1 = Smoking Treatment and Anxiety Management Program) on Early PPA (point-prevalence abstinence; 0 = Smoking, 1 = Abstinent) through reductions in AS (anxiety sensitivity) across treatment sessions (i.e., from session 1 to session 4).
Exploratory Analyses of Potential Moderators
In follow-up tests, we explored participant sex, baseline cigarette dependence, psychopathology status, and negative affectivity (PANAS-NA) as moderators of the direct effects of intervention on AS as well as the indirect effects of intervention on early and late PPA. There were no moderation effects on the intercept or slope parameters in the model of the direct effects of the intervention on AS (all p’s > .05). Additionally, there were no moderation effects of the direct effect of the intervention on early or late PPA. Finally, there were no moderation effects in moderated mediation models testing whether participant sex, baseline cigarette dependence, psychopathology status, or negative affectivity moderated the path from AS slope to early or late PPA (all p’s > .05).
Discussion
Following an experimental therapeutic model of treatment development, the current study evaluated the treatment effects of a novel transdiagnostic AS reduction-smoking cessation program (STAMP) to reduce AS and promote smoking abstinence relative to a standard smoking cessation program (SCP) among a sample of treatment-seeking smokers. Findings provided evidence for the efficacy of STAMP to reduce AS during treatment. Additionally, results indicated an indirect treatment effect of STAMP on early PPA, but not late PPA, through AS reduction during treatment. This finding is consistent with prior studies that report lower AS decreases susceptibility to early phase smoking lapse (e.g., Zvolensky, Bernstein, et al., 2007).
Extant literature on AS reduction has almost exclusively focused on symptoms change in various psychiatric symptoms and disorders (e.g., Timpano, Raines, Shaw, Keough, & Schmidt, 2016). Based on the documented linkages between AS and smoking, initial interventions studies, primarily open-trials, have examined and identified AS as a targetable risk factor in the context of intensive (e.g., 16 sessions) smoking cessation treatments (see Leventhal & Zvolensky, 2015). The present study provides novel evidence for the malleability of AS in response to a brief (4 session) smoking cessation treatment. Indeed, results indicated that AS was reduced in both STAMP and SCP, suggesting that smokers engaged in smoking cessation program (regardless of whether AS is directly targeted), experience reductions in AS. It is possible that smokers who receive general education about quitting smoking may feel more prepared and confident about their ability to quit and therefore be less likely to interpret internal anxiety-related symptoms (e.g., restlessness, somatic distress due to nicotine withdrawal) as harmful or dangerous. That is, AS may be malleable through SCP presumably due to the psychoeducation and preparedness for smoking cessation, including withdrawal management, symptom monitoring, and reduction in somatic arousal (Johnson, Farris, Schmidt, Smits, & Zvolensky, 2012; Johnson, Stewart, et al., 2012; Leventhal & Zvolensky, 2015). Overall, these findings align with the observation that AS is a malleable, clinically-relevant construct, particularly within the context of smoking cessation treatment (Leventhal & Zvolensky, 2015).
Although STAMP and SCP lead to reductions in AS across the treatment period, the rate of reduction was significantly greater for smokers in STAMP. Specifically, smokers who completed STAMP had a greater rate of reduction in AS over the course of treatment, which led to increased PPA during the early quit phase. The effects were specific to the early quit phase as they were not maintained through the late quit phase. These data are consistent with existing work on short-term interventions targeting AS. For example, Feldner and colleagues (2008) found that a one-session intervention that targeted motivation to quit smoking and AS reduction produced significant reductions in AS but not lasting effects on smoking behavior. Together, these data may suggest that if AS is reduced, it may produce its strongest therapeutic effect for smoking abstinence in the early quit phase relative to the later quit phase. It is worth noting that the indirect effects were reduced to marginal significance in the ITT analyses, which may suggest that the observed effect of AS reduction during STAMP on smoking abstinence only occurs when patients attend at least one treatment session.
These findings, however, should be interpreted in the context of observed sample characteristics. Average ASI-3 scores in the sample were 14.54 (SD = 12.05), which were slightly higher than average scores documented in North American nonclinical samples of adults (see Osman et al., 2010; Taylor et al., 2007), although markedly lower than ASI-3 scores typically documented in clinical samples. In addition, the reduction in AS during intervention was modest. Thus, despite the statistical significance that the rate of observed change in AS is strengthened when treatment directly targets AS via psychoeducation, cognitive restructuring, and interoceptive exposure, the clinical significance of this finding may be limited by the magnitude of the reduction. It is possible the modest-sized reductions reflect low baseline levels of AS and/or the relative “low dose” of STAMP treatment that was provided (4 sessions) and received (Msessions = 3.62, SD = .73). Also, if patients did not attend sessions wherein interoceptive exposure or cognitive restructuring were implemented, they may not have received a ‘sufficient dose.’ Nevertheless, this finding is important considering the current novel evidence that the rate of AS reduction during treatment mediated (explained) the effect of STAMP on early smoking abstinence outcomes.
Conceptually, smokers who receive treatment to specifically facilitate AS reduction in addition to general CBT for smoking cessation may experience an additive effect of the AS reduction treatment on associated symptoms. For example, smoking cessation treatment information may be more deeply encoded for smokers in an integrated treatment program and they may, therefore, develop a stronger psychological understanding and tolerance for aversive internal sensations. As a result, they may be more resilient to smoking cessation withdrawal symptoms, which commonly impede quitting success particularly in the early phase of quitting (West, Hajek, & Belcher, 1989). Ultimately, STAMP improves the rate at which AS is reduced, which in turn, supports increased likelihood of remaining abstinent during the early quit phase. Thus, present findings not only highlight AS as an important mechanism of change for treatment effects on early smoking abstinence, but also underscore the importance of considering the rate of change, not merely a static statistic of change such as a change score, in the context of integrated and standard smoking cessation treatments.
Neither treatment was directly related to abstinence across either the early or late quit phase. The lack of significance may be, in part, due to the relatively high rate of psychopathology observed in the current sample of smokers. For these smokers to achieve initial abstinence, underlying mechanisms that contribute to smoking behavior, such as AS, may need to change. Indeed, as suggested by the significant mediational pathway, it is not the mere treatment that impacts abstinence, but the impact of specialized treatment on AS that leads to (short-term) smoking cessation. In part, this finding is consistent with extant work that suggest lower AS is a protective factor against early cessation failure (Brown et al., 2001; Zvolensky et al., 2006; Zvolensky et al., 2009). Based on the present findings, however, longer-term abstinence may indeed require reduction in additional mechanistic factors related to smoking, such as dysphoric symptoms or distress tolerance (Garey, Bakhshaie, et al., 2016; Leventhal & Zvolensky, 2015). There also may be a need for interventions to reduce the reinforcing value of tobacco, including psychosocial and pharmacological therapy (e.g., varenicline). Relative to earlier generations of smokers, contemporary smokers are more tobacco dependent, more likely to die from a smoking-related disease, and have greater difficulty quitting, potentially as a result of psychiatric comorbidity (Sachs, Hodgkin, & Bostrom, 2009; Thun et al., 2013; Williams, Steinberg, Griffiths, & Cooperman, 2013), treatment efforts that target additional underlying mechanisms may be necessary to support long-term abstinence.
Clinically, the present findings suggest that it may be advisable for clinicians to directly target AS in the context of smoking cessation treatment, as reductions in AS appear to promote early smoking cessation likelihood. Data from the present study demonstrate the efficacy of as few as four sessions for producing a relatively modest reduction in AS. Considering that psychoeducation about AS coupled with interoceptive exposure appears to be the active ingredients to greater reductions in AS (Naragon-Gainey, 2010), this brief intervention could be broadly disseminated to clinicians (with proper training) (McHugh & Barlow, 2010). Ultimately, integrating AS reduction with smoking cessation would allow for a more individualized treatment plan that may promote greater early quit success. Indeed, early quit success is a necessary stepping-block that has the potential to influence longer-term quit behavior.
There are several study limitations. First, the sample consisted of predominately Caucasian, community-recruited, treatment-seeking daily cigarette smokers with moderate levels of tobacco dependence. Future studies would benefit from replicating findings among a more ethnically/racially diverse sample of smokers who exhibit both high and low tobacco dependence. Second, the lack of community norms of AS among smokers did not permit score comparisons across the presently observed baseline values and values from the community; however, ASI-3 scores in the current sample were slightly elevated relative to scores documented in North American adults (Taylor et al., 2007). This finding is consistent with extant research suggesting that smokers experience elevated AS, compared to non-smokers (McCabe et al., 2004; Morissette, Brown, Kamholz, & Gulliver, 2006). Nevertheless, to improve generalizability, work is needed to evaluate AS in a representative, community sample of smokers, as the degree of sample bias introduced by other characteristic (i.e., ethnicity and tobacco dependence) also may have an influence on AS. Third, the current analyses were conducted with data from participants who attended at least one treatment session. Scientific evidence supports unique differences across pre-treatment attrition and those who initiate treatment (Ahluwalia et al., 2002; Garey, Kauffman, Neighbors, Schmidt, & Zvolensky, 2016; MacPherson, Stipelman, Duplinsky, Brown, & Lejuez, 2008). Thus, it is possible that results may be biased by pre-treatment attrition. Future work is needed to further explore the impact of pre-treatment attrition on the currently investigated models.
Fourth, although not a limitation per se, the present study included only a relatively mild, single agent pharmacological component via the transdermal NRT patch. It is possible that the use of more robust pharmacological options, including combination NRT therapies or varenicline, would relate to increased and long-term abstinence. Therefore, future work is needed to replicate the present study wherein a more intensive pharmacological component is included. Fifth, future work may benefit by exploring moderators during a quit experience within the context of AS-smoking behavior, including autonomic nervous system arousal symptoms. If there is less arousal during cessation, for example, there may be fewer ‘activating’ stimuli for AS. Thus, autonomic arousal may be an important moderator of treatment outcome within AS-tobacco relations. Notably, in follow-up tests we explored sex, baseline cigarette dependence, psychopathology status, and negative affectivity as moderators of the present model. There was no evidence of any moderator effect for these factors. Future work is therefore needed on alternative moderating factors (i.e., autonomic arousal). Sixth, there were high rates of attrition, as only 42% of participants in STAMP and 51% of participants in SCP returned for the Year 1 follow-up as well as a large gap between early and late follow-up time periods. Although attrition rates observed here are within the observed range for long-term follow-up attrition across smoking cessation trials (Prochaska, Delucchi, & Hall, 2004), they may limit interpretability of the long-term follow-up findings. Thus, the stability and, by extension, replicability of the observed long-term findings are tenuous. These results should be interpreted with the utmost caution. To address this limitation, it may be beneficial for future work to incorporate added intervention doses (i.e., booster sessions) in the late quit phase to improve treatment retention and involvement. Moreover, it may be fruitful to evaluate outcomes more frequently during the follow-up period, such as monthly or bimonthly, to increase retention and enhance understanding of the impact of treatment on the dynamic post-cessation period within shorter windows of time.
Overall, the present investigation provides evidence of the initial efficacy of STAMP, a novel 4-session integrated AS reduction smoking cessation program. STAMP appears to engage its intended theoretical “target” mechanism (AS), which through modest reductions in this mechanism, can produce early smoking abstinence for smokers who attended at least one treatment session. Future work may benefit from exploring possible mechanisms that may aid in improving late smoking abstinence and should explore the efficacy of adding additional “booster” treatment sessions that continue to address AS which could promote longer-term cessation outcomes, or augment the treatment with a pharmacological aid (e.g., varenicline).
Public Health Significance Statement.
There is a high need for developing and testing brief integrated smoking cessation treatments. In the present efficacy study, treatment-seeking smokers who received a novel, integrated anxiety sensitivity-smoking cessation evinced decreased anxiety sensitivity during the course of treatment, which lead to increased likelihood of abstinence during the two weeks post-treatment. Findings underscore the importance and necessity for integrated treatments to address the unique needs of current smokers.
Acknowledgments
Funding: This study was funded by a National Institute of Mental Health (R01-MH076629) grant to MJZ and NBS.
Footnotes
Trial Registration: NCT01753141
Declarations of Interest: Dr. Smits receives royalties from Oxford University Press for books on exercise as a treatment for mood and anxiety disorders and is a paid consultant for MicroTransponder, Inc. All other authors report no financial relationships with commercial interests.
Intent-to-Treat Analyses (ITT): The direct and indirect effects of the intervention on PPA were also examined in an ITT model. All participants who were eligible for the study were included in the ITT analyses and missingness at follow-up was treated relapsed. There remained no direct effects of condition predicting early (p = .22) or late PPA (p = .82). The indirect effect of AS from intervention to early PPA slope was marginally significant (indirect = −.12, 95% CI [−.42, .00]) as was the indirect effect from intervention to late PPA slope (indirect −.03, 95% CI [−.03, .00]).
References
- Aan Het Rot M, Zarate CA, Charney DS, Mathew SJ. Ketamine for depression: where do we go from here? Biological psychiatry. 2012;72(7):537–547. doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams DB, Niaura R. The tobacco dependence treatment handbook: A guide to best practices. Guilford Press; 2003. [Google Scholar]
- Ahluwalia JS, Richter K, Mayo MS, Ahluwalia HK, Choi WS, Schmelzle KH, Resnicow K. African American Smokers Interested and Eligible for a Smoking Cessation Clinical Trial:: Predictors of not Returning for Randomization. Annals of epidemiology. 2002;12(3):206–212. doi: 10.1016/s1047-2797(01)00305-2. [DOI] [PubMed] [Google Scholar]
- Baker TB, Mermelstein R, Collins LM, Piper ME, Jorenby DE, Smith SS, Fiore MC. New methods for tobacco dependence treatment research. Annals of Behavioral Medicine. 2011;41(2):192–207. doi: 10.1007/s12160-010-9252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista SR, Stewart SH, Fulton HG, Steeves D, Darredeau C, Gavric D. A further investigation of the relations of anxiety sensitivity to smoking motives. Addictive Behaviors. 2008;33(11):1402–1408. doi: 10.1016/j.addbeh.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors. 1998;12(2):101. [Google Scholar]
- Brown RA, Kahler CW, Zvolensky MJ, Lejuez C, Ramsey SE. Anxiety sensitivity: Relationship to negative affect smoking and smoking cessation in smokers with past major depressive disorder. Addictive Behaviors. 2001;26(6):887–899. doi: 10.1016/s0306-4603(01)00241-6. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. J Abnorm Psychol. 2002;111(1):180. [PubMed] [Google Scholar]
- Edwards MC, Wirth R, Houts CR, Xi N. Categorical data in the structural equation modeling framework. Handbook of structural equation modeling. 2012:195–208. [Google Scholar]
- Evatt DP, Kassel JD. Smoking, arousal, and affect: the role of anxiety sensitivity. Journal of anxiety disorders. 2010;24(1):114–123. doi: 10.1016/j.janxdis.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerström Test for Cigarette Dependence. Nicotine & Tobacco Research. 2012;14(1):75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- Farris SG, DiBello AM, Allan NP, Hogan J, Schmidt NB, Zvolensky MJ. Evaluation of the Anxiety Sensitivity Index-3 among treatment-seeking smokers. Psychological assessment. 2015;27(3):1123. doi: 10.1037/pas0000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SG, Leventhal AM, Schmidt NB, Zvolensky MJ. Anxiety sensitivity and pre-cessation smoking processes: testing the independent and combined mediating effects of negative affect-reduction expectancies and motives. Journal of Studies on Alcohol and Drugs. 2015;76(2):317–325. doi: 10.15288/jsad.2015.76.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SG, Paulus DJ, Gonzalez A, Mahaffey BL, Bromet EJ, Luft BJ, Zvolensky MJ. Anxiety sensitivity mediates the association between post-traumatic stress symptom severity and interoceptive threat-related smoking abstinence expectancies among World Trade Center disaster-exposed smokers. Addictive Behaviors. 2015;51:204–210. doi: 10.1016/j.addbeh.2015.07.031. [DOI] [PubMed] [Google Scholar]
- Feldner MT, Zvolensky MJ, Babson K, Leen-Feldner EW, Schmidt NB. An integrated approach to panic prevention targeting the empirically supported risk factors of smoking and anxiety sensitivity: Theoretical basis and evidence from a pilot project evaluating feasibility and short-term efficacy. Journal of anxiety disorders. 2008;22(7):1227–1243. doi: 10.1016/j.janxdis.2008.01.005. http://doi.org/10.1016/j.janxdis.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, Lando HA. Treating tobacco use and dependence: clinical practice guideline. Rockville, MD: US Department of Health and Human Services; 2000. pp. 00–0032. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for Axis I DSM-IV Disorders - Patient Edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1994. [Google Scholar]
- Garey L, Bakhshaie J, Brandt CP, Langdon KJ, Kauffman BY, Schmidt NB, Zvolensky MJ. Interplay of dysphoria and anxiety sensitivity in relation to emotion regulatory cognitions of smoking among treatment-seeking smokers. The American Journal on Addictions. 2016;25(4):267–274. doi: 10.1111/ajad.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey L, Kauffman BY, Neighbors C, Schmidt NB, Zvolensky MJ. Treatment attrition: Associations with negative affect smoking motives and barriers to quitting among treatment-seeking smokers. Addictive Behaviors. 2016;63:165–171. doi: 10.1016/j.addbeh.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Zvolensky MJ, Vujanovic AA, Leyro TM, Marshall EC. An evaluation of anxiety sensitivity, emotional dysregulation, and negative affectivity among daily cigarette smokers: Relation to smoking motives and barriers to quitting. Journal of Psychiatric Research. 2008;43(2):138–147. doi: 10.1016/j.jpsychires.2008.03.002. http://dx.doi.org/10.1016/j.jpsychires.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor KL, Zvolensky MJ, McLeish AC, Bernstein A, Morissette S. Anxiety sensitivity and perceived control over anxiety-related events: Associations with smoking outcome expectancies and perceived cessation barriers among daily smokers. Nicotine & Tobacco Research. 2008;10(4):627–635. doi: 10.1080/14622200801978706. [DOI] [PubMed] [Google Scholar]
- Guillot CR, Leventhal AM, Raines AM, Zvolensky MJ, Schmidt NB. Anxiety sensitivity facets in relation to tobacco use, abstinence-related problems, and cognitions in treatment-seeking smokers. Addictive Behaviors. 2016;56:30–35. doi: 10.1016/j.addbeh.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press; 2013. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction to Alcohol and Other Drugs. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Farris SG, Schmidt NB, Smits JA, Zvolensky MJ. Panic attack history and anxiety sensitivity in relation to cognitive-based smoking processes among treatment-seeking daily smokers. Nicotine & Tobacco Research. 2012;15(1):1–10. doi: 10.1093/ntr/ntr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Farris SG, Schmidt NB, Zvolensky MJ. Anxiety sensitivity and cognitive-based smoking processes: Testing the mediating role of emotion dysregulation among treatment-seeking daily smokers. Journal of Addictive Diseases. 2012;31(2):143–157. doi: 10.1080/10550887.2012.665695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Stewart S, Rosenfield D, Steeves D, Zvolensky MJ. Prospective evaluation of the effects of anxiety sensitivity and state anxiety in predicting acute nicotine withdrawal symptoms during smoking cessation. Psychology of Addictive Behaviors. 2012;26(2):289–297. doi: 10.1037/a0024133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon KJ, Farris SG, Hogan JBD, Grover KW, Zvolensky MJ. Anxiety sensitivity in relation to quit day dropout among adult daily smokers recruited to participate in a self-guided cessation attempt. Addictive Behaviors. 2016;58:12–15. doi: 10.1016/j.addbeh.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon KJ, Farris SG, Øverup CS, Zvolensky MJ. Associations between anxiety sensitivity, negative affect, and smoking during a self-guided smoking cessation attempt. Nicotine & Tobacco Research. 2015;18(5):1188–1195. doi: 10.1093/ntr/ntv253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon KJ, Leventhal AM, Stewart S, Rosenfield D, Steeves D, Zvolensky MJ. Anhedonia and anxiety sensitivity: prospective relationships to nicotine withdrawal symptoms during smoking cessation. Journal of Studies on Alcohol & Drugs. 2013;74(3):469–478. doi: 10.15288/jsad.2013.74.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Zvolensky MJ. Anxiety, depression, and cigarette smoking: A transdiagnostic vulnerability framework to understanding emotion–smoking comorbidity. Psychological bulletin. 2015;141(1):176. doi: 10.1037/bul0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyro TM, Zvolensky MJ, Vujanovic AA, Bernstein A. Anxiety sensitivity and smoking motives and outcome expectancies among adult daily smokers: Replication and extension. Nicotine & Tobacco Research. 2008;10(6):985–994. doi: 10.1080/14622200802097555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson L, Stipelman BA, Duplinsky M, Brown RA, Lejuez C. Distress tolerance and pre-smoking treatment attrition: Examination of moderating relationships. Addictive Behaviors. 2008;33(11):1385–1393. doi: 10.1016/j.addbeh.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall EC, Johnson K, Bergman J, Gibson LE, Zvolensky MJ. Anxiety sensitivity and panic reactivity to bodily sensations: relation to quit-day (acute) nicotine withdrawal symptom severity among daily smokers making a self-guided quit attempt. Experimental and Clinical Psychopharmacology. 2009;17(5):356. doi: 10.1037/a0016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe RE, Chudzik SM, Antony MM, Young L, Swinson RP, Zolvensky MJ. Smoking behaviors across anxiety disorders. Journal of anxiety disorders. 2004;18(1):7–18. doi: 10.1016/j.janxdis.2003.07.003. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Barlow DH. The dissemination and implementation of evidence-based psychological treatments: a review of current efforts. American Psychologist. 2010;65(2):73. doi: 10.1037/a0018121. [DOI] [PubMed] [Google Scholar]
- Mojtabai R, Olfson M, Sampson NA, Jin R, Druss B, Wang PS, Kessler RC. Barriers to mental health treatment: results from the National Comorbidity Survey Replication. Psychological medicine. 2011;41(8):1751–1761. doi: 10.1017/S0033291710002291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette SB, Brown TA, Kamholz BW, Gulliver SB. Differences between smokers and nonsmokers with anxiety disorders. Journal of anxiety disorders. 2006;20(5):597–613. doi: 10.1016/j.janxdis.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide Eighth Edition. Los Angeles, CA: Muthen & Muthen; 1998–2017. [Google Scholar]
- Naragon-Gainey K. Meta-analysis of the relations of anxiety sensitivity to the depressive and anxiety disorders. Psychological bulletin. 2010;136(1):128. doi: 10.1037/a0018055. [DOI] [PubMed] [Google Scholar]
- Olvera H, Bakhshaie J, Garey L, Jardin C, Schmidt NB, Zvolensky MJ. The Role of Anxiety Sensitivity in the Relation Between Trait Worry and Smoking Behavior. Nicotine & Tobacco Research. 2015;17(6):682–689. doi: 10.1093/ntr/ntu233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman A, Gutierrez PM, Smith K, Fang Q, Lozano G, Devine A. The anxiety sensitivity index–3: analyses of dimensions, reliability estimates, and correlates in nonclinical samples. Journal of Personality Assessment. 2010;92(1):45–52. doi: 10.1080/00223890903379332. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA, Sayette MA. Differences in negative mood-induced smoking reinforcement due to distress tolerance, anxiety sensitivity, and depression history. Psychopharmacology. 2010;210(1):25–34. doi: 10.1007/s00213-010-1811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF. Reliability of the fagerstrom tolerance questionnaire and the fagerstrom test for nicotine dependence. Addictive Behaviors. 1994;19(1):33–39. doi: 10.1016/0306-4603(94)90049-3. http://dx.doi.org/10.1016/0306-4603(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Powers MP, Davis ML, Kauffman BY, Baird SO, Zvolensky MJ, Rosenfield D, Smits JAJ. Anxiety sensitivity, smoking variability, and nicotine addiction among treatment seeking smokers. Addictive Disorders and Their Treatment. doi: 10.1097/ADT.0000000000000075. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. Journal of consulting and clinical psychology. 2004;72(6):1144. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- Reiss S. Expectancy model of fear, anxiety, and panic. Clinical psychology review. 1991;11(2):141–153. [Google Scholar]
- Reiss S, McNally R. The expectancy model of fear. In: Reiss S, Bootzin RR, editors. Theoretical issues in behavior therapy. San Diego, CA: Academic Press; 1985. pp. 107–121. [Google Scholar]
- Sachs D, Hodgkin J, Bostrom A. COPD & Increasing Nicotine-Dependence Severity (1989–2006): Need To Change the Paradigm for Tobacco-Dependence Treatment in COPD C47. COPD EPIDEMIOLOGY. 2009:A4525. Am Thoracic Soc. [Google Scholar]
- Schmidt NB, Raines AM, Allan NP, Zvolensky MJ. Anxiety sensitivity risk reduction in smokers: a randomized control trial examining effects on panic. Behaviour research and therapy. 2016;77:138–146. doi: 10.1016/j.brat.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Dvorak RD, Batien BD, Wray TB. Event-level associations between affect, alcohol intoxication, and acute dependence symptoms: Effects of urgency, self-control, and drinking experience. Addictive Behaviors. 2010;35(12):1045–1053. doi: 10.1016/j.addbeh.2010.07.001. http://dx.doi.org/10.1016/j.addbeh.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Zvolensky MJ, Davis ML, Rosenfield D, Marcus BH, Church TS, Baird SO. The efficacy of vigorous-intensity exercise as an aid to smoking cessation in adults with high anxiety sensitivity: a randomized controlled trial. Psychosomatic medicine. 2016;78(3):354–364. doi: 10.1097/PSY.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back. In: Allen J, Litten RZ, editors. Measuring alcohol consumption: Psychosocial and Biological Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Sobell MB, Sobell LC. Problem drinkers: Guided self-change treatment. Guilford Press; 1996. [DOI] [PubMed] [Google Scholar]
- Taylor S. Anxiety sensitivity: Theory, research, and treatment of the fear of anxiety. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers; 1999. [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, Stewart SH. Robust dimensions of anxiety sensitivity: development and initial validation of the Anxiety Sensitivity Index-3. Psychological assessment. 2007;19(2):176. doi: 10.1037/1040-3590.19.2.176. [DOI] [PubMed] [Google Scholar]
- Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, Gapstur SM. 50-year trends in smoking-related mortality in the United States. New England Journal of Medicine. 2013;368(4):351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpano KR, Raines AM, Shaw AM, Keough ME, Schmidt NB. Effects of a brief anxiety sensitivity reduction intervention on obsessive compulsive spectrum symptoms in a young adult sample. Journal of Psychiatric Research. 2016;83:8–15. doi: 10.1016/j.jpsychires.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDHHS: United States Department of Health and Human Services. Surgeon General’s Report: The Health Consequences of Smoking—50 Years of Progress A Report of the Surgeon Genderal. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- Verbiest M, Brakema E, van der Kleij R, Sheals K, Allistone G, Williams S, Chavannes N. National guidelines for smoking cessation in primary care: a literature review and evidence analysis. NPJ primary care respiratory medicine. 2017;27 doi: 10.1038/s41533-016-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman SA, Kraft WK, Nelson TJ, Terzic A. Experimental therapeutics: a paradigm for personalized medicine. Clinical and translational science. 2009;2(6):436–438. doi: 10.1111/j.1752-8062.2009.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of personality and social psychology. 1988;54(6):1063. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P, Belcher M. Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychological medicine. 1989;19(4):981–985. doi: 10.1017/s0033291700005705. [DOI] [PubMed] [Google Scholar]
- Wilamowska ZA, Thompson-Hollands J, Fairholme CP, Ellard KK, Farchione TJ, Barlow DH. Conceptual background, development, and preliminary data from the unified protocol for transdiagnostic treatment of emotional disorders. Depression and Anxiety. 2010;27(10):882–890. doi: 10.1002/da.20735. [DOI] [PubMed] [Google Scholar]
- Williams JM, Steinberg ML, Griffiths KG, Cooperman N. Smokers with behavioral health comorbidity should be designated a tobacco use disparity group. American journal of public health. 2013;103(9):1549–1555. doi: 10.2105/AJPH.2013.301232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Krajisnik A, Truong L, Lisha NE, Trujillo M, Greenberg JB, Leventhal AM. Anxiety sensitivity as a predictor of acute subjective effects of smoking. Nicotine & Tobacco Research. 2013;15(6):1084–1090. doi: 10.1093/ntr/nts208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinbarg RE, Barlow DH, Brown TA. Hierarchical structure and general factor saturation of the Anxiety Sensitivity Index: Evidence and implications. Psychological assessment. 1997;9(3):277. [Google Scholar]
- Zvolensky M, Bonn-Miller M, Bernstein A, Marshall E. Anxiety sensitivity and abstinence duration to smoking. Journal of Mental Health. 2006;15(6):659–670. [Google Scholar]
- Zvolensky MJ, Bernstein A, Cardenas SJ, Colotla VA, Marshall EC, Feldner MT. Anxiety sensitivity and early relapse to smoking: A test among Mexican daily, low-level smokers. Nicotine & Tobacco Research. 2007;9(4):483–491. doi: 10.1080/14622200701239621. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Bogiaizian D, Salazar PL, Farris SG, Bakhshaie J. An anxiety sensitivity reduction smoking-cessation program for Spanish-speaking smokers (Argentina) Cognitive and Behavioral Practice. 2014;21(3):350–363. [Google Scholar]
- Zvolensky MJ, Farris SG, Leventhal AM, Schmidt NB. Anxiety sensitivity mediates relations between emotional disorders and smoking. Psychology of Addictive Behaviors. 2014;28(3):912. doi: 10.1037/a0037450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Farris SG, Schmidt NB, Smits JA. The role of smoking inflexibility/avoidance in the relation between anxiety sensitivity and tobacco use and beliefs among treatment-seeking smokers. Experimental and Clinical Psychopharmacology. 2014;22(3):229–237. doi: 10.1037/a0035306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Lejuez C, Kahler CW, Brown RA. Integrating an interoceptive exposure-based smoking cessation program into the cognitive-behavioral treatment of panic disorder: Theoretical relevance and case demonstration. Cognitive and Behavioral Practice. 2003;10(4):347–357. [Google Scholar]
- Zvolensky MJ, Paulus DJ, Garey L, Raines AM, Businelle M, Shankman SA, Schmidt NB. Anxiety Sensitivity and Nicotine Replacement Therapy Side Effects: Examining the Role of Emotion Dysregulation Among Treatment-Seeking Smokers. Journal of Studies on Alcohol and Drugs. 2017;78(6):877–883. doi: 10.15288/jsad.2017.78.877. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Rosenfield D, Garey L, Kauffman BY, Langdon KJ, Powers MB, Smits JAJ. Does exercise aid smoking cessation through reductions in anxiety sensitivity and dysphoria? Health Psychology. doi: 10.1037/hea0000588. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Stewart SH, Vujanovic AA, Gavric D, Steeves D. Anxiety sensitivity and anxiety and depressive symptoms in the prediction of early smoking lapse and relapse during smoking cessation treatment. Nicotine & Tobacco Research. 2009;11(3):323–331. doi: 10.1093/ntr/ntn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Vujanovic AA, Miller MOB, Bernstein A, Yartz AR, Gregor KL, Gibson LE. Incremental validity of anxiety sensitivity in terms of motivation to quit, reasons for quitting, and barriers to quitting among community-recruited daily smokers. Nicotine & Tobacco Research. 2007;9(9):965–975. doi: 10.1080/14622200701540812. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Yartz AR, Gregor K, Gonzalez A, Bernstein A. Interoceptive exposure-based cessation intervention for smokers high in anxiety sensitivity: A case series. Journal of Cognitive Psychotherapy. 2008;22(4):346–365. [Google Scholar]


