Abstract
Whether elevated uric acid (UA) is an independent risk factor for chronic kidney disease (CKD) is not well established. The authors evaluated the relationship of UA with rapid kidney function decline (RKFD) and incident CKD among 3702 African Americans (AAs) in the Jackson Heart Study with serum UA levels measured at baseline exam (2000‐2004). RKFD was defined as ≥ 30% eGFR loss and incident CKD as development of eGFR < 60 mL/min/1.73 m2 with a ≥ 25% decline in eGFR between baseline and exam 3 (2009‐2013). RKFD and CKD were found in 11.4% and 7.5% of the participants, respectively. In a fully adjusted model, the odds of RKFD (OR, 1.8; 95% CI, 1.25‐2.49) and incident CKD (OR, 2.00; 95% CI, 1.31‐3.06) were significantly higher among participants in the top UA quartile vs bottom quartile. In the JHS, elevated UA was significantly associated with RKFD and incident CKD.
Keywords: African American, albuminuria, chronic kidney disease, CKD progression, estimated glomerular filtration rate, rapid kidney function decline, uric acid
1. INTRODUCTION
The pathogenic role of uric acid (UA) for kidney disease and hypertension is well accepted in animal models; however, conflicting reports exist on the impact of elevated UA on kidney outcomes in humans.1 Previous studies have reported an association between elevated UA levels and the increased risk of hypertension, cardiovascular disease, and kidney disease outcomes and progression.2, 3, 4 Whether elevated UA represents an independent risk factor for chronic kidney disease (CKD) may be dependent on the particular sociocultural context and the characteristics of the population studied. Studies of Asian and Australian participants have reported UA to be independently associated with the development of CKD.5, 6, 7, 8, 9, 10, 11 However, neither the Framingham Heart Study3 and later the Modification of Diet in Renal Disease2 nor the Mild to Moderate Kidney Disease studies from North America provided evidence for an independent association between higher UA levels and kidney disease progression.2, 12 A pooled cohort analysis of Atherosclerosis Risks in Communities (ARIC) and the Cardiovascular Health Study (CHS) participants showed that although increased UA levels might lead to incident CKD in participants with normal kidney function in general, such an effect was not evident among African American (AA) participants. In a separate longitudinal analysis for CHS participants, a weak association was reported between UA and kidney disease progression in AAs.13, 14
The reported prevalence of elevated UA levels (UA ≥ 7.0 mg/dL among men and ≥ 5.7 mg/dL among women) in the United States is high (25%), and 25.7% of AAs are considered to be hyperuricemic.1, 15 Studying potential associations of UA with adverse kidney outcomes in AAs is particularly important, as AAs are a population with a substantially greater risk for CKD‐related outcomes compared to whites.16 Using the Jackson Heart Study (JHS), we evaluated a large cohort composed exclusively of AAs to investigate the relationship between UA and CKD progression with focus on rapid kidney function decline (RKFD) and the development of new‐onset CKD.
2. MATERIALS AND METHODS
2.1. Study population
The present analysis was based on data from JHS, a single‐site prospective cohort study designed to investigate risk factors for cardiovascular disease (CVD) in AAs. From September 2000 to March 2004 (exam 1 clinic visit), JHS enrolled 5306 AAs aged 21‐94 years from the Jackson, Mississippi tricounty metropolitan area (Hinds, Madison, and Rankin).17 After completing exam 1 clinic visit, participants returned for 2 additional visits, exam 2 (October 2005 to December 2008) and exam 3 (February 2009 to January 2013). The study was approved by the institutional review boards of the University of Mississippi Medical Center, Jackson State University, and Tugaloo College. All participants provided their informed consent in writing.
2.2. Analysis population
Participants with complete UA measure at exam 1, N = 5211 (excluding participants missing UA, n = 95) were included in the current analysis. We excluded participants who self‐reported being on dialysis (n = 23) or were missing serum creatinine at exam 1 (n = 1). We also excluded participants who did not return for exam 3 (n = 1432) or who did not have serum creatinine recorded at exam 3 (n = 53). Following these exclusions, there were 3702 participants available for analysis of RKFD. For incident CKD analysis, an additional 146 participants with reduced eGFR (eGFR < 60 mL/min/per 1.73 m2) at exam 1 were excluded, leaving 3556 participants in the final analytic sample (a consort diagram, Figure 1).
Figure 1.
CONSORT flow diagram for the analysis of uric acid and rapid kidney function decline (RKFD) and incident chronic kidney disease (CKD). JHS, Jackson Heart Study; RKFD, rapid kidney function decline; CKD, chronic kidney disease
2.3. Exposure and covariate measurements
At exam 1, UA was measured using the uricase method with the Vitro 5.1 analytical system (Jackson, MS, USA) at the University of Mississippi Medical Center Lab (Jackson, MS, USA). An in‐home interview and clinic visit were used to collect demographic information, including age, sex, education, smoking, and alcohol use. The modification of the Baecke questionnaire was used to record the duration, frequency, and intensity of physical activity during active living, work, home life, and sports.18 American Heart Association (AHA) criteria were then used to contrast physical activity with ideal health (yes/no, defined as ≥ 150 minutes of moderate physical activity, or ≥ 75 minutes of vigorous physical activity, or ≥ 150 minutes of combined moderate and vigorous physical activity per week).
During the clinic visit, a group of trained staff measured blood pressure, height, and weight; recorded the names of prescription and over‐the‐counter medications taken within the period of 2 weeks prior to the study visit; and collected blood and urine samples. Initially, 24‐hour urine samples were collected from participants, which was later followed by the collection of random spot urine samples. Sitting blood pressure was measured in a resting state and determined by the average of 2 measurements with a Hawksley random zero sphygmomanometer (Hawksley and Sons Ltd, Langing, UK) equipped with 1 of 4 cuff sizes selected based on arm circumference.19, 20
An enzymatic method on a Vitros 950 or 250 Ortho‐Clinical Diagnosis analyzer (Raritan, NJ) was used for measuring fasting blood glucose, serum, and urine creatinine levels. Vitros Ortho‐Clinical Diagnostics Analyzer and later Roche Chemistry analyzer (Roche Diagnostics, Indianapolis, IN, USA) were used to measure serum creatinine.21 Urinary albumin was measured on a Dade‐Behring BN II nephelometer (Dade Behring, Newark, DE, USA), and the lipid profile and hemoglobin A1c (HbA1c) were assayed by the oxidase method on a Roche COBAS Fara analyzer (Jackson, MS, USA) and TOSOH (Jackson, MS, USA) high‐performance liquid chromatography system, respectively.
Prevalent diabetes was defined according to the American Diabetes Association criteria as fasting (≥ 8 hours) glucose ≥ 126 mg/dL, hemoglobin A1c (HbA1C) ≥ 6.5% and by the use of antidiabetic medication.22 Hypertension was defined as SBP ≥ 140 mm Hg, DBP ≥ 90 mm Hg, or by the use of blood pressure medication. Albuminuria was defined as the urinary‐albumin‐to‐urinary‐creatinine ratio of (UACR) ≥ 30 mg/g. If 24‐hour UACR data were missing, then random spot UACR was used, which has been validated in this population.23 Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.
2.4. Outcomes
Renal outcomes included RKFD and incident CKD, evaluated at exam 3 as serum creatinine was not measured at the exam 2. Serum creatinine measured at exam 1 using enzymatic method was then calibrated to the isotope‐dilution mass spectrometry‐traceable method used at exam 3.24 Both endpoints were defined based on estimated glomerular filtration rate (eGFR) change in percentage between exams 1 and 3. Estimated GFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) creatinine equation.25 RKFD was defined as a decline of eGFR ≥ 30% during the period of longitudinal follow‐ups (between exams 1 and 3).23, 26, 27 The percentage change in eGFR was calculated as 100*(eGFR at exam 1 minus eGFR at exam 3) divided by eGFR at exam 1. Incident CKD was defined as eGFR ≥ 60 mL/min/per 1.73 m2 at exam 1 (ie, no evidence of stage‐3 CKD at exam 1) and eGFR < 60 mL/min/per 1.73 m2 at exam 3 and a decline in eGFR ≥ 25% from exam 1 to exam 3.28 Any eGFR > 120 mL/min/1.73 m2 was truncated to 120 mL/min/1.73 m2 to avoid large changes in those with high normal eGFRs.29, 30
2.5. Statistical analyses
Quartiles of UA were derived (quartile [Q] 1, mean 3.71 mg/dL; Q2, mean 4.89 mg/d; Q3, mean 5.89 mg/dL, and Q4, mean 7.45 mg/dL). Participants’ characteristics at exam 1 were compared across quartiles of UA using a 2‐sample t test for continuous variables and a chi‐square test for categorical variables. The proportions of RKFD and CKD by UA level were calculated for each quartile. For the dichotomous outcomes of RKFD and incident CKD, logistic regression was used. Odds ratios (OR) and 95% confidence intervals (CI) were calculated comparing the odds of incident renal outcomes among participants with different levels of UA. Multivariable analyses were conducted using nested logistic regression models to assess the associations between quartiles of UA and renal outcomes independent of exam 1 covariates. Four nested models were estimated: Model 1, unadjusted model; Model 2, adjusted for age and sex; Model 3, adjusted for variables in Model 2 and eGFR (for RKFD), gout medicine, loop diuretics, thiazide diuretics, and potassium sparing diuretics; and Model 4, adjusted for variables in Model 3 and BMI, diabetes, total cholesterol, and high sensitive C‐reactive protein (hsCRP). Missing covariates (34.8% of the participants did not have UACR and an additional 15.8% of the participants had no other covariates) were imputed using the Markov Chain Monte Carlo method for imputing missing values with arbitrary missing patterns.31 Because of differences in UA distributions between male and female participants, we performed multivariable logistic regression analyses by sex and tested for correlations between UA and sex. In a final analysis, risk for RKFD and incident CKD were calculated stratified by the use of UA‐lowering therapy. Participants reported to be using gout medications were excluded.
Using chi‐square test, a study sample size of 3702, an exposure of 50%, 10% event rate in the control group, and 80% power, a 5% 2‐sided type I error would yield a minimum detectable OR of 1.33. Statistical analyses were performed using the SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC, USA). OR and 95% CI and P‐values < .05 were considered statistically significant.
3. RESULTS
3.1. Characteristics of the study population
The demographic and clinical characteristics of JHS participants are presented in Table 1 both for the whole sample and stratified by UA quartiles. At exam 1, study participants had an average age of 55.3 (12.4) years and 64.5% were female. The mean UA level was 5.4 (1.6) mg/dL (5.0 ± 1.5 mg/dL in women and 6.2 ± 1.5 mg/dL in men). Among participants, 588 (15.9%) had a UA level of > 7 mg/dL (27.5% men and 30.8% women). Mean eGFR at exam 1 was 95.9 (19.9) mL/min/per 1.73 m2. Participants with higher UA levels were more likely to be older (57.7 vs 50.9), male, had a higher BMI, suffered from diabetes (DM), hypertension (HTN), and were likely to report the use of blood pressure medications. In addition, participants with higher UA levels also reported the use of UA‐altering medications, including antihyperlipidemics, thiazide diuretics, loop diuretics, angiotensin II receptor blockers (ARBs), and gout medications (Table 1).
Table 1.
Characteristics of Jackson Heart Study participants by mean uric acid (mg/dL) quartiles (N = 3702)
| Characteristics | Mean (SD)/count (%) (overall) | Mean (SD)/count (%) In Q1 | Mean (SD)/count (%) In Q2 | Mean (SD)/count (%) In Q3 | Mean (SD)/count (%) In Q4 | P valuea |
|---|---|---|---|---|---|---|
| N | 3702 | 951 | 960 | 906 | 885 | |
| Mean uric acid, mg/dL | 5.44 (1.59) | 3.71 (0.76) | 4.89 (0.64) | 5.89 (0.69) | 7.45 (1.15) | |
| Age, years | 55.25 (12.40) | 50.88 (12.18) | 53.48 (11.90) | 55.65 (11.68) | 57.74 (11.55) | <.001 |
| Female | 2349 (64.45) | 605 (25.76) | 610 (25.97) | 563 (23.97) | 571 (24.31) | <.001 |
| Education ≥ high school | 3084 (83.51) | 840 (27.24) | 811 (26.30) | 760 (24.64) | 673 (21.82) | <.001 |
| Body mass index (BMI), kg/m2 | 31.79 (7.11) | 28.87 (6.08) | 31.30 (6.49) | 33.05 (7.15) | 34.20 (7.56) | <.001 |
| Physical activity | 755 (20.41) | 219 (29.01) | 186 (24.64) | 190 (25.17) | 160 (21.19) | .0522 |
| Alcohol use | 1761 (47.76) | 488 (27.71) | 451 (25.61) | 451 (25.61) | 371 (21.07) | .0004 |
| Diabetes | 692 (18.71) | 129 (18.64) | 151 (21.82) | 175 (25.29) | 237 (34.25) | <.001 |
| Hypertension | 1974 (53.02) | 313 (15.86) | 456 (23.10) | 520 (26.34) | 685 (34.70) | <.001 |
| Systolic blood pressure, mm Hg | 126.30 (15.94) | 122.88 (15.11) | 126.42 (15.65) | 127.35 (15.94) | 128.75 (16.52) | <.001 |
| Diastolic blood pressure, mm Hg | 75.89 (8.64) | 75.07 (8.49) | 76.19 (8.40) | 76 .11 (8.56) | 76.22 (9.08) | .0089 |
| HbA1c (%) | 5.89 (1.4) | 5.73 (1.27) | 5.82 (1.11) | 5.92 (1.16) | 6.09 (1.09) | <.001 |
| Fasting total cholesterol, mg/dL | 199.33 (39.44) | 190.95 (35.80) | 198.90 (38.67) | 201.19 (37.79) | 206.67 (43.78) | <.001 |
| Fasting HDL‐C, mg/Dl | 51.79 (14.31) | 54.11 (13.91) | 52.88 (14.73) | 50.32 (13.52) | 49.70 (14.62) | <.001 |
| Fasting LDL‐C, mg/dL | 126.97 (36.23) | 120.44 (33.20) | 126.91 (36.21) | 129.33 (35.22) | 131.51 (39.36) | <.001 |
| Fasting triglycerides, mg/dL | 103.67 (70.12) | 82.13 (48.70) | 96.93 (72.08) | 107.95 (54.52) | 129.23 (90.08) | <.001 |
| UACR ≥ 30 mg/g | 32.79 (18.47) | 45 (18.91) | 43 (18.07) | 60 (25.21) | 90 (37.82) | <.001 |
| HsCRP > 3.0 mg/dL | 1679 (45.64) | 320 (19.06) | 414 (24.66) | 471 (28.05) | 474 (28.23) | <.001 |
| eGFR, mL/min/1.73 m2 | 95.94 (19.89) | 104.32 (17.40) | 99.36 (17.97) | 94.03 (18.08) | 85.17 (29.94) | <.001 |
| Antihypertensive medication use | 1715 (47.14) | 264 (15.39) | 380 (22.16) | 440 (25.66) | 631 (37.79) | <.001 |
| Antihyperlipidemic | 416 (11.24) | 79 (18.99) | 90 (21.63) | 123 (29.57) | 124 (29.81) | <.001 |
| Gout medication | 39 (1.30) | 7 (17.95) | 9 (23.08) | 6 (15.38) | 17 (43.59) | .0649 |
| ARB | 153 (4.13) | 32 (20.92) | 35 (22.88) | 33 (21.57) | 53 (34.64) | .0167 |
| Loop diuretic | 156 (5.19) | 20 (12.82) | 28 (17.95) | 39 (25.00) | 69 (44.23) | <.001 |
| Thiazide‐type diuretic | 367 (12.22) | 44 (11.99) | 62 (16.89) | 95 (25.89) | 166 (45.23) | <.001 |
| Potassium‐sparing diuretic | 48 (1.60) | 8 (16.67) | 10 (20.83) | 5 (10.42) | 25 (52.08) | .0003 |
ARB, angiotensin‐receptor blocker; BP, blood pressure; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; HSCRP, high‐sensitive protein; UACR, urinary‐albumin creatinine ratio; eGFR, estimated glomerular filtration rate.
Values in the table represented as either percentages or mean ± SD.
Determined using χ2 and t tests, as appropriate.
3.2. Uric acid quartiles and renal outcomes
During a median follow‐up of 8.1 years (range: 5.9‐12.2 years) between exams 1 and 3, the cohort's eGFR declined by 10.4% on average (median: 8.2%, interquartile range: 0.0%‐18.8%). A total of 420 (11.4%) participants experienced RKFD. In a fully adjusted model, participants in the highest UA quartile had significantly greater odds of experiencing RKFD compared to those in the reference quartile (OR 1.8; 95% CI, 1.25‐2.49; P = .001; Table 2). Figure 2 suggests a nonlinear increased risk for decline in kidney function with a rapid rise in UA level above ~8.5 mg/dL.
Table 2.
Odds ratios (ORs) for rapid kidney function decline (RKFD) associated with quartiles of uric acid (mg/dL) among Jackson Heart Study participants (N = 3702)
| Serum uric acid mg/dL | Cases/# at risk (%) | Odds Ratio (95% CI) | |||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| Panel A: Overall population | |||||
| ≤ 4.30 | 63/951 (6.63) | Ref | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| 4.40‐5.30 | 101/960 (10.52) | 1.66 (1.19, 2.30) | 1.48 (1.06, 2.07) | 1.44 (1.03, 2.03) | 1.43 (1.13, 2.02) |
| 5.40‐6.50 | 105/906 (11.59) | 1.85 (1.33, 2.56) | 1.49 (1.07, 2.08) | 1.39 (0.99, 1.96) | 1.36 (0.96, 1.94) |
| ≥ 6.60 | 153/885 (17.29) | 2.95 (2.16, 4.01) | 2.18 (1.59, 2.99) | 1.97 (1.41, 2.74) | 1.77 (1.25, 2.49) |
| Panel B: Female participants | |||||
| ≤ 4.30 | 34/605 (5.62) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| 4.40‐5.30 | 68/610 (11.15) | 2.11 (1.37, 3.23) | 1.74 (1.12, 2.70) | 1.69 (1.08, 2.65) | 1.69 (1.06, 2.72) |
| 5.40‐6.50 | 70/563 (12.43) | 2.38 (1.56, 3.65) | 1.67 (1.08, 2.59) | 1.59 (1.00, 2.55) | 1.50 (0.63, 2.44) |
| ≥ 6.60 | 113/571 (19.79) | 4.14 (2.77, 6.19) | 2.56 (1.69, 3.89) | 2.31 (1.44, 3.71) | 2.06 (1.25, 3.37) |
| Panel C: Male participants | |||||
| ≤ 4.30 | 29/346 (8.38) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| 4.40‐5.30 | 33/350 (9.43) | 1.14 (0.68, 1.92) | 1.13 (0.67, 1.91) | 1.21 (0.70, 2.09) | 1.27 (0.72, 2.26) |
| 5.40‐6.50 | 35/343 (10.20) | 1.24 (0.74, 2.08) | 1.21 (0.72, 2.04) | 1.16 (0.67, 2.03) | 1.27 (0.70, 2.30) |
| ≥ 6.60 | 40/314 (12.74) | 1.59 (0.96, 2.64) | 1.53 (0.92, 2.55) | 1.46 (0.81, 2.62) | 1.29 (0.69, 2.41) |
Rapid kidney function decline (RKFD) is defined as a decline in estimated glomerular filtration rate (eGFR) ≥ 30% from exam 1 to 3 (8.1 median years).
Model 1: Unadjusted.
Model 2: Adjusted for age and sex.
Model 3: Adjusted for age, sex, body mass index, gout medications, loop diuretics, thiazide diuretics, potassium‐sparing diuretics, and antihyperlipidemics.
Model 4: Adjusted for age, sex, body mass index, gout medications, loop diuretics, thiazide diuretics, potassium‐sparing diuretics, antihyperlipidemics, diabetes, total cholesterol, C‐reactive protein, and urine albumin‐creatinine ratio (UACR).
Results for Models 3 and 4 are based on multiple imputations.
Figure 2.
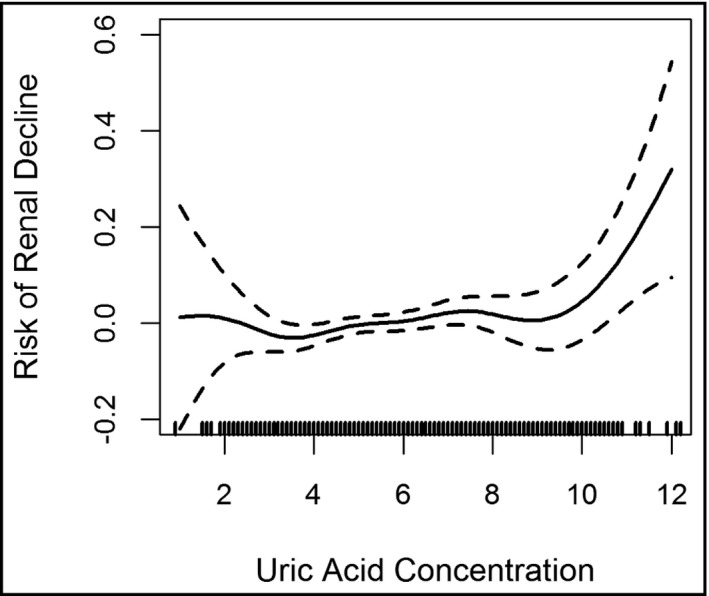
Penalized spline smoother of the relationship between the risk of rapid kidney function decline and uric acid concentration levels. We observed a nonlinear relationship between the concentration of uric acid levels and the risk of decline in kidney function with a sharp increase when uric acid is ~8.5 mg/dL and above
Of those without CKD at exam 1, 268 (7.5%) developed CKD at follow‐up. Compared with participants in the reference quartile, those in the highest UA quartile had significantly greater odds (OR, 2.00; 95% CI, 1.31‐3.06; P < .0001) of developing CKD after multivariable adjustments (Table 3).
Table 3.
Odds ratios (ORs) of incident chronic kidney disease (CKD) associated with quartiles of uric acid (mg/dL) among Jackson Heart Study participants (N = 3556)
| Serum uric acid mg/dL | Cases/# at risk (%) | Odds Ratio (95% CI) | |||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| Panel A: Overall population | |||||
| ≤ 4.30 | 39/943 (4.14) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| 4.40‐5.30 | 49/946 (5.18) | 1.23 (0.82, 1.95) | 1.09 (0.70, 1.69) | 1.06 (0.68, 1.65) | 1.01 (0.63, 1.59) |
| 5.40‐6.50 | 79/883 (8.95) | 2.28 (1.53, 3.38) | 1.76 (1.17, 2.64) | 1.62 (1.06, 2.45) | 1.59 (1.04, 2.44) |
| ≥ 6.60 | 101/784 (12.88) | 3.43 (2.34, 5.03) | 2.42 (1.63, 3.59) | 2.19 (1.44, 3.31) | 2.00 (1.31, 3.06) |
| Panel B: Female participants | |||||
| ≤ 4.30 | 22/602 (3.65) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| 4.40‐5.30 | 27/600 (4.50) | 1.24 (0.69, 2.21) | 0.93 (0.51, 1.68) | 0.89 (0.49, 1.64) | 0.86 (0.46, 1.61) |
| 5.40‐6.50 | 51/545 (9.36) | 2.72 (1.63, 4.55) | 1.69 (0.99, 2.90) | 1.57 (0.90, 2.73) | 1.52 (0.86, 2.69) |
| ≥ 6.60 | 71/488 (14.55) | 4.49 (2.74, 7.36) | 2.48 (1.48, 4.15) | 2.15 (1.25, 2.55) | 1.96 (1.11, 3.46) |
| Panel C: Male participants | |||||
| ≤ 4.30 | 17/341 (4.99) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| 4.40‐5.30 | 22/346 (6.36) | 1.29 (0.68, 2.48) | 1.29 (0.67, 2.49) | 1.30 (0.66, 2.55) | 1.33 (0.65, 2.71) |
| 5.40‐6.50 | 28/338 (8.28) | 1.72 (0.92, 3.98) | 1.69 (0.89, 3.18) | 1.54 (0.80, 2.96) | 1.74 (0.87, 3.46) |
| ≥ 6.60 | 30/296 (10.14) | 2.15 (1.16, 3.98) | 2.06 (1.10, 3.86) | 2.03 (1.06, 3.91) | 1.81 (0.89, 3.68) |
Incident chronic kidney disease (CKD): Among individuals with estimated glomerular filtration rate (eGFR) ≥ 60 mL/min per 1.73 at exam 1, a decline to an EGFR < 60 mL/min per 1.73 m2 accompanied with ≥ 25% eGFR decline from exam 1 to 3 (8.1 median year follow‐up).
Model 1: Unadjusted.
Model 2: Adjusted for age and sex.
Model 3: Adjusted for age, sex, body mass index, gout medications, loop diuretics, thiazide diuretics, potassium‐sparing diuretics, and antihyperlipidemics.
Model 4: Adjusted for age, sex, body mass index, estimated glomerular filtration rate (eGFR), gout medications, loop diuretics, thiazide diuretics, potassium‐sparing diuretics, antihyperlipidemics, diabetes, total cholesterol, C‐reactive protein, and urine albumin‐creatinine ratio (UACR).
Results for Models 3 and 4 are based on multiple imputations.
No correlation between sex and UA was identified for both outcomes (RKFD, P = .12; incident CKD, P = .70). However, in a sex‐specific analyses, a strong association between higher UA level and RKFD was observed in women participants (OR, 2.06; 95% CI, 1.25‐3.37; P = .004); however, such an association was not seen in male participants (OR 1.29; 95% CI, 0.69‐2.41; P = .42). As regards incident CKD outcome, the multivariable adjusted model yielded statistically significant association between UA and incident CKD in female participants (OR, 1.96; 95% CI, 1.11‐3.46; P = .02) but not male participants (OR, 1.81; 95% CI, 0.89‐3.68; P = .10) participants.
3.3. Subgroup analysis
The multivariate adjusted ORs for RKFD comparing participants in the highest quartile UA levels vs participants in the bottom UA quartile levels were 1.15 (95% CI, 0.63‐2.06) and 2.07 (95% CI, 1.30‐3.29) among participants taking and not taking uric acid‐lowering therapy, respectively (Table 4). The adjusted ORs for incident CKD comparing participants in the highest UA quartile levels vs participants in the bottom UA quartile levels were 1.47 (95% CI, 0.72‐3.02) and 1.87 (95% CI, 1.05‐3.33) among participants taking and not taking uric acid‐lowering therapy, respectively. Interaction between UA‐lowering therapy and level of UA observed was not statistically significant.
Table 4.
Association of uric acid level with rapid kidney function decline (RKFD) and incident chronic kidney disease (CKD) stratified by the use of uric acid‐lowering therapy (excluding participants using gout medications)
| Serum uric acid, mg/dL | Cases/# at risk | Multivariate Adjusted Odds Ratio (95% CI) | Cases/# at risk | Multivariable Adjusted Odds Ratio (95% CI) |
|---|---|---|---|---|
| Participants taking uric acid‐lowering therapy | Participants not taking uric acid‐lowering therapy | |||
| Panel A: Rapid kidney function decline | ||||
| ≤ 4.30 | 21/140 | 1 (ref) | 37/587 | 1 (ref) |
| 4.40‐5.30 | 33/173 | 1.14 (0.60, 2.14) | 50/573 | 1.33 (0.83, 2.11) |
| 5.40‐6.50 | 51/243 | 1.35 (0.74, 2.46) | 47/499 | 1.28 (0.79, 2.07) |
| ≥ 6.60 | 68/328 | 1.15 (0.63, 2.06) | 71/422 | 2.07 (1.30, 3.29) |
| Panel B: Incident CKD | ||||
| ≤ 4.30 | 13/138 | 1 (ref) | 24/583 | 1 (ref) |
| 4.40‐5.30 | 16/169 | 0.75 (0.33, 1.69) | 27/566 | 1.00 (0.55, 1.82) |
| 5.40‐6.50 | 43/234 | 1.87 (0.92, 3.78) | 29/488 | 1.05 (0.57, 1.93) |
| ≥ 6.60 | 44/276 | 1.47 (0.72, 3.02) | 44/383 | 1.87 (1.05, 3.33) |
Rapid kidney function decline (RKFD) was defined as a decline in estimated glomerular filtration rate (eGFR) ≥ 30% from exam 1 (2000‐2004) to exam 3 (2009‐2013), a median follow‐up duration, 8.1 years.
Incident chronic kidney disease (CKD) was defined as a decline from eGFR ≥ 60 mL/min/1.73 m2 at exam1 (2000‐2004) to eGFR < 60 mL/min/1.73 m2 at exam 3 (2009‐2013) follow‐up (median follow‐up duration, 8.1 years).
Multivariate model for the estimation of ORs were adjusted for age, sex, body mass index, baseline estimated glomerular filtration rate ([eGFR] for incident CKD), diabetes, total cholesterol, C‐reactive protein, and urine albumin‐creatinine ratio (UACR).
Results for the final model were based on multiple imputations.
4. DISCUSSION
In this sample of AAs in a community‐based cohort, higher UA level (UA ≥ 7.0 mg/dL for men and UA ≥ 5.7 mg/dL for women) was observed among 27.5% of males and 28.3% of females and were significantly associated with an increased risk for RKFD (≥ 30% eGFR decline) and the development of CKD. The association between higher UA level and RKFD remained statistically significant after multivariable adjustment. Women were more likely than men to have RKFD and incident CKD when sex‐specific cut‐offs for elevated UA were used. Elevated UA levels were significantly associated with the risk of RKFD and incident CKD among participants not taking UA‐lowering therapy only.
Previous studies have reported mixed results regarding the potential link between elevated UA and the decline in kidney function or the development of CKD.32 In a pooled analysis involving 13 338 participants in the ARIC Study and CHS, 2 community‐based longitudinal studies, participants with intact kidney function at baseline were followed for 8.5 years to assess UA effect in reduced kidney function. UA measured at baseline was found to be independently associated with incident CKD but no significant association was observed for AA participants (OR 1.22, 95% CI, 0.89‐1.68).13 When compared to JHS, participants in the ARIC‐CHS pooled cohort analyses were slightly older (57.4 vs 55.3 mean year) and had lower mean baseline eGFR (90.4 vs 95.9 mL/min per 1.73 m2), which might have been a factor in the less pronounced association. In a separate longitudinal analyses composed of CHS participants only, a weak association was observed between baseline UA and the progression of kidney disease (OR, 1.49; 95% CI, 1.00‐2.22), whereas no significant association was found between UA levels and incident CKD (OR, 1.00; 95% CI, 0.89‐1.14).14 In 2 separate longitudinal studies involving Asian populations, where participants were followed for 5 years to assess the effect of baseline UA on kidney dysfunction (eGFR < 60 mL/min/1.73 m2 or UACR ≥ 30 mg/dL) and kidney failure (eGFR < 15 mL/min/1.73 m2), participants with baseline UA levels ≥ 6 mg/dL had a more significant eGFR decline with evidence of an increasing dose response when compared to the lowest UA quartile.6, 33 Similar results were reported in the Jerusalem Lipid Research Clinic's study involving 2449 participants and a Japanese study of 6403 individuals with normal kidney function at baseline where participants were followed for 24‐28 months and 2 years34, 35 respectively. The evidence from these different studies suggest that UA may be an independent risk factor for CKD development.10
In the present analysis, the risk of kidney function decline as depicted by a spline model, Figure 2, is nonlinear, relatively stable between approximately 8‐9 mg/dL UA levels with associated risk increasing rapidly above these levels. This is consistent with what has been previously reported in The Vienna Health Screening Project, a prospective cohort study (mean follow‐up period: 7.4 year). In this healthy Austrians cohort study, the investigators found that a slightly elevated UA was independently associated with nearly doubled risks for the development of CKD. The spline model depicting risk for kidney function observed in this cohort, increase roughly linearly with UA level of approximately 7.0‐8.9 mg/dL with a sharp increase in CKD‐associated risks above these levels.8 A number of factors associated with the observed higher UA levels are prevalent among JHS participants. These include obesity, metabolic syndrome,36 hypertension, and diet high in added sugar.37 Genetic polymorphism in the anion transporter is another factor that is known to lead to elevated UA.38 These may explain the elevated UA level and its strong association with CKD observed in this cohort.
Previous studies have proposed mechanisms that link UA and CKD. These include direct toxicity to the kidney, including deposition of UA and potential exacerbation of other risk factors for kidney disease, specifically hypertension. The overlap with obstructive sleep apnea is another possibility.39 In animal studies, it was found that mild elevation of UA induces oxidative stress and endothelial dysfunction, which results in the development of glomerular hypertension and arteriolosclerosis, manifested by renal vasoconstriction. This may happen via renin‐angiotensin system (RAS), mitochondria dysfunction, epithelial‐mesenchymal transition, and endothelial dysfunction among others13, 40 Some authors have raised the possibility of intrarenal vasoconstriction due to UA, an effect significant after adjustment for multiple confounders, including eGFR and albuminuria.41 Both direct kidney injury and preexisting systemic hypertension may lead to reduced kidney function and kidney disease. However, considering the multifactorial nature of systemic hypertension, others have argued that UA may be a marker of kidney risk rather than a direct contributor to kidney injury.
Although this study and other observational studies show that asymptomatic UA is associated with incident CKD and CKD progression, challenges remain on conducting adequately powered randomized clinical trials (RCT) to assess beneficial effects of UA‐lowering therapy. Currently, few data exist examining the effects of UA‐lowering therapy on renal outcomes.42, 43 A meta‐analysis of 8 RCT composed of 476 participants evaluating the benefits and risks of UA‐lowering therapy indicated insufficient evidence to currently recommend widespread use of UA‐lowering therapy to slow progression of CKD.
The current study has several strengths. We studied a large community‐based cohort of AAs, a population at an increased risk of CKD. This provides us with an opportunity to make generalized observations about an important vulnerable group. Another strength is the collection and availability of repeated measures of kidney function over time and availability of deep phenotype data, which allowed for the control of numerous potential confounders, such as medications and comorbid conditions, known to alter UA levels. This study has a few limitations. Serum creatinine values were available at only 2 points in time approximately 8 years apart, with no intervening measurement of renal function, thus limiting the accurate assessment of the annual rate of decline in kidney function. Although all known measured confounders were accounted for and taken into consideration during this analysis, possible residual confounding could exist in the pathways predicting these outcomes, considering covariates variation across UA quartiles. Furthermore, our results were obtained from an AA cohort and may not be generalizable to other ethnic groups or other communities of AAs.
In summary, our findings show that elevated UA levels were prevalent and associated with progressive renal function impairment and development of incident CKD among JHS participants over time. Thus, in addition to being an indicator of reduced renal excretion, elevated UA levels may play a pathogenic role in CKD progression and provide an opportunity for intervention.
CONFLICT OF INTEREST
None of the authors have any conflicts of interest to disclose.
DISCLAIMER
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; and the US Department of Health and Human Services. The US Department of Veterans Affairs does not endorse any of the statements or opinions advocated by this article.
ACKNOWLEDGMENTS
The authors thank the participants and data collection staff of the Jackson Heart Study. The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, and HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. This work was supported in part by Dr Young's NIH National Institute of Diabetes, Digestive, and Kidney Disease Grant, R01DK102134‐01. Dr. Young is also supported in part by funding from Veterans Affairs Puget Sound Health Care System. This paper represents a mentored research project developed under the equal supervision of Drs Tibor Fülöp and Bessie A. Young. We sincerely appreciated the assistance of Mr Attila Lénárt‐Muszka during editing and grammar review. We also want to thank Dr. Yuan‐I Min for providing analytical support and guidance for this manuscript.
Mwasongwe SE, Fülöp T, Katz R, et al. Relation of uric acid level to rapid kidney function decline and development of kidney disease: The Jackson Heart Study. J Clin Hypertens. 2018;20:775–783. 10.1111/jch.13239
REFERENCES
- 1. Bellomo G. The relationship between uric acid, allopurinol, cardiovascular events, and kidney disease progression: a step forward. Am J Kidney Dis. 2015;65:525‐527. [DOI] [PubMed] [Google Scholar]
- 2. Kang DH, Chen W. Uric acid and chronic kidney disease: new understanding of an old problem. Semin Nephrol. 2011;31:447‐452. [DOI] [PubMed] [Google Scholar]
- 3. Wen CP, David Cheng TY, Chan HT, et al. Is high serum uric acid a risk marker or a target for treatment? Examination of its independent effect in a large cohort with low cardiovascular risk. Am J Kidney Dis. 2010;56:273‐288. [DOI] [PubMed] [Google Scholar]
- 4. Cagli K, Turak O, Canpolat U, et al. Association of serum uric acid level with blood pressure variability in newly diagnosed essential hypertension. J Clin Hypertens (Greenwich). 2015;17:929‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuwabara M, Bjornstad P, Hisatome I, et al. Elevated serum uric acid level predicts rapid decline in kidney function. Am J Nephrol. 2017;45:330‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsai CW, Lin SY, Kuo CC, Huang CC. Serum uric acid and progression of kidney disease: a longitudinal analysis and mini‐review. PLoS One. 2017;12:e0170393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Domrongkitchaiporn S, Sritara P, Kitiyakara C, et al. Risk factors for development of decreased kidney function in a southeast Asian population: a 12‐year cohort study. J Am Soc Nephrol. 2005;16:791‐799. [DOI] [PubMed] [Google Scholar]
- 8. Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser‐Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol. 2008;19:2407‐2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sonoda H, Takase H, Dohi Y, Kimura G. Uric acid levels predict future development of chronic kidney disease. Am J Nephrol. 2011;33:352‐357. [DOI] [PubMed] [Google Scholar]
- 10. Wang S, Shu Z, Tao Q, Yu C, Zhan S, Li L. Uric acid and incident chronic kidney disease in a large health check‐up population in Taiwan. Nephrology (Carlton). 2011;16:767‐776. [DOI] [PubMed] [Google Scholar]
- 11. Mok Y, Lee SJ, Kim MS, Cui W, Moon YM, Jee SH. Serum uric acid and chronic kidney disease: the Severance cohort study. Nephrol Dial Transplant. 2012;27:1831‐1835. [DOI] [PubMed] [Google Scholar]
- 12. Sturm G, Kollerits B, Neyer U, Ritz E, Kronenberg F. Uric acid as a risk factor for progression of non‐diabetic chronic kidney disease? The Mild to Moderate Kidney Disease (MMKD) Study. Exp Gerontol. 2008;43:347‐352. [DOI] [PubMed] [Google Scholar]
- 13. Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008;19:1204‐1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chonchol M, Shlipak MG, Katz R, et al. Relationship of uric acid with progression of kidney disease. Am J Kidney Dis. 2007;50:239‐247. [DOI] [PubMed] [Google Scholar]
- 15. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007‐2008. Arthritis Rheum. 2011;63:3136‐3141. [DOI] [PubMed] [Google Scholar]
- 16. Tarver‐Carr ME, Powe NR, Eberhardt MS, et al. Excess risk of chronic kidney disease among African‐American versus white subjects in the United States: a population‐based study of potential explanatory factors. J Am Soc Nephrol. 2002;13:2363‐2370. [DOI] [PubMed] [Google Scholar]
- 17. Mwasongwe S, Gao Y, Griswold M, et al. Leukocyte telomere length and cardiovascular disease in African Americans: the Jackson Heart Study. Atherosclerosis. 2017;266:41‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dubbert PM, Carithers T, Ainsworth BE, Taylor HA Jr, Wilson G, Wyatt SB. Physical activity assessment methods in the Jackson Heart Study. Ethn Dis. 2005;15(4)(suppl 6):S6‐S56‐61. [PubMed] [Google Scholar]
- 19. Hickson DA, Diez Roux AV, Wyatt SB, et al. Socioeconomic position is positively associated with blood pressure dipping among African‐American adults: the Jackson Heart Study. Am J Hypertens. 2011;24:1015‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor HA Jr. The Jackson Heart Study: an overview. Ethn Dis. 2005;15(4)(suppl 6):S6‐1‐3. [PubMed] [Google Scholar]
- 21. Carpenter MA, Crow R, Steffes M, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328:131‐144. [DOI] [PubMed] [Google Scholar]
- 22. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62‐S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Young BA, Katz R, Boulware LE, et al. Risk factors for rapid kidney function decline among African Americans: the Jackson Heart Study (JHS). Am J Kidney Dis. 2016;68:229‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang W, Young BA, Fulop T, et al. Effects of serum creatinine calibration on estimated renal function in African Americans: the Jackson Heart Study. Am J Med Sci. 2015;349:379‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end‐stage renal disease and mortality. JAMA. 2014;311:2518‐2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shlipak MG, Katz R, Kestenbaum B, et al. Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol. 2009;20:2625‐2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bash LD, Coresh J, Kottgen A, et al. Defining incident chronic kidney disease in the research setting: the ARIC Study. Am J Epidemiol. 2009;170:414‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hiramoto JS, Katz R, Peralta CA, et al. Inflammation and coagulation markers and kidney function decline: the Multi‐Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 2012;60:225‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stevens LA, Schmid CH, Greene T, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD‐EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2 . Am J Kidney Dis. 2010;56:486‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yuan Y. Multiple imputation using SAS software. J Stat Softw. 2011;45:1‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jalal DI, Chonchol M, Chen W, Targher G. Uric acid as a target of therapy in CKD. Am J Kidney Dis. 2013;61:134‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takae K, Nagata M, Hata J, et al. Serum uric acid as a risk factor for chronic kidney disease in a Japanese Community—The Hisayama Study. Circ J. 2016;80:1857‐1862. [DOI] [PubMed] [Google Scholar]
- 34. Ben‐Dov IZ, Kark JD. Serum uric acid is a GFR‐independent long‐term predictor of acute and chronic renal insufficiency: the Jerusalem Lipid Research Clinic cohort study. Nephrol Dial Transplant. 2011;26:2558‐2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iseki K, Oshiro S, Tozawa M, Iseki C, Ikemiya Y, Takishita S. Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens Res. 2001;24:691‐697. [DOI] [PubMed] [Google Scholar]
- 36. Musani SK, Vasan RS, Bidulescu A, et al. Aldosterone, C‐reactive protein, and plasma B‐type natriuretic peptide are associated with the development of metabolic syndrome and longitudinal changes in metabolic syndrome components: findings from the Jackson Heart Study. Diabetes Care. 2013;36:3084‐3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu J, Hickson DA, Musani SK, et al. Dietary patterns, abdominal visceral adipose tissue, and cardiometabolic risk factors in African Americans: the Jackson Heart Study. Obesity (Silver Spring). 2013;21:644‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Feig DI. The role of uric acid in the pathogenesis of hypertension in the young. J Clin Hypertens (Greenwich). 2012;14:346‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hirotsu C, Tufik S, Guindalini C, Mazzotti DR, Bittencourt LR, Andersen ML. Association between uric acid levels and obstructive sleep apnea syndrome in a large epidemiological sample. PLoS One. 2013;8:e66891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnson RJ, Nakagawa T, Jalal D, Sanchez‐Lozada LG, Kang DH, Ritz E. Uric acid and chronic kidney disease: which is chasing which? Nephrol Dial Transplant. 2013;28:2221‐2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Geraci G, Mule G, Mogavero M, Geraci C, Nardi E, Cottone S. Association between uric acid and renal hemodynamics: pathophysiological implications for renal damage in hypertensive patients. J Clin Hypertens (Greenwich). 2016;18:1007‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bose B, Badve SV, Hiremath SS, et al. Effects of uric acid‐lowering therapy on renal outcomes: a systematic review and meta‐analysis. Nephrol Dial Transplant. 2014;29:406‐413. [DOI] [PubMed] [Google Scholar]
- 43. Badve SV, Brown F, Hawley CM, et al. Challenges of conducting a trial of uric‐acid‐lowering therapy in CKD. Nat Rev Nephrol. 2011;7:295‐300. [DOI] [PubMed] [Google Scholar]


