Abstract
Background & objectives:
Different formulations of Bacillus thuringiensis var. israelensis (Bti) have been tested against different mosquito vectors and other insects for their residual activity. In the present study, the efficacy and residual activity of a new formulation of Bti (Bactivec Suspension Concentrate) were evaluated against immature stages of Anopheles stephensi Liston (Diptera: Culicidae), Aedes aegypti Linnaeus (Diptera: Culicidae) and Culex quinquefasciatus Say (Diptera: Culicidae), in natural habitats in Phase II and Phase III in Bengaluru, India.
Methods:
Preferential breeding habitats of the mosquito species were selected and four dosages (0.25, 0.5, 1 and 2 ml/50 l) were tested in Phase II trial. Two most effective dosages, 0.5 and 1 ml/50 l were selected for Phase III trial. The evaluation was carried out essentially following the guidelines of the World Health Organization Pesticide Evaluation Scheme. Pre-treatment and post-treatment densities were recorded at regular intervals, and >80 per cent reduction in pupae was taken as the duration of effectiveness.
Results:
Bactivec SC treated at the dosage of 1 ml/50 l could produce 10-17 days efficacy (>80% reduction in pupae) in clean water habitats tested, whereas 0.5 ml/50 l dosage showed residual activity from 7 to 14 days against Ae. aegypti and An. stephensi in Phase III studies. In polluted water habitats, 4-7 days efficacy could be recorded against Cx. quinquefasciatus in Phase III.
Interpretation & conclusions:
The Bactivec SC formulation was operationally feasible and easy to handle. For the control of Anopheles and Aedes mosquitoes in freshwater habitats, 1 ml/50 l dosage was found effective, whereas in polluted water habitats against Cx. quinquefasciatus 5 ml/m2 was found effective.
Keywords: Aedes aegypti, Anopheles stephensi, Bacillus thuringiensis var. israelensis, Bactivec, Culex quinquefasciatus, larvicidal efficacy
Mosquito-borne diseases are of major public health concern in the world. Among these dengue, malaria, chikungunya, filariasis, etc. are causing high morbidity and mortality in many countries of the world1. Adult mosquito control and larval control are being undertaken by many vector control programmes to contain these diseases in many countries. Bacillus thuringiensis var. israelensis (Bti) is a Gram-positive, spore-forming bacterium, and toxins secreted by it are being used as biolarvicide against caterpillars, beetles, and flies, including mosquitoes and black flies2. The spores enter into the gut of the mosquito and disrupt the midgut endothelium, thereby causing the death of the larvae. These toxins are only effective against the feeding aquatic stages of mosquitoes. Two Bti formulations, namely WG, water-dispersible granule; and DT, ready-to-use tablet have been evaluated using the World Health Organization Pesticide Evaluation Scheme (WHOPES) and recommended as mosquito larvicides3,4.
Much emphasis has been given by the pesticide manufacturers to produce formulations that are safe in storage, handling and spraying operations in the field and may substantially influence effectiveness and safety5. Controlled release formulations such as microencapsulation, wettable granules, capsule suspensions and suspension concentrates have been developed to minimize the exposure during spray preparations, handling, slow release to extend the bioavailability of the insecticide on the surface, extended efficacy and to minimize the environmental contamination, operational feasibility, storage, etc6.
Bactivec SC is recommended for the control of mosquitoes such as Anopheles stephensi and Aedes aegypti (Diptera: Culicidae). In a study conducted at Nova Igua, Brazil, Bactivec resulted in a reduction in ovitrap positivity and also the highest house index reduction in Ae. aegypti when compared to Vectobac Granular (G) and Vectobac water dispersible granules (WDGs) formulations7. In another study conducted by Harwood et al8, different formulations of Bt showed efficacy in controlling mosquito immatures of Aedes vector mosquitoes in simulated tree holes and other aquatic habitats. In another study9, Vectomax Water Soluble Pouch, a formulation containing Bti and Bacillus sphaericus was found to be effective in controlling third and fourth instar larvae of Culex pipiens (Diptera: Culicidae) in septic tanks. In a study carried out by Tamilselvan et al10, using Bti with fly ash based formulation for the control of Culex quinquefasciatus (Diptera: Culicidae) larvae in their natural breeding habitats reported high efficacy in causing mortality in immature stages. Terbot et al11 emphasized the efficacy of Bti formulation in the control of the larval population of mosquitoes. A few other studies also reported the efficacy of different Bti formulations in different settings12,13,14,15,16,17,18,19,20. In the present study, the efficacy and residual activity of Bactivec SC were tested in different breeding habitats in Phase II and Phase III manner against immature stages of An. stephensi, Cx. quinquefasciatus and Ae. aegypti in Bengaluru city, India.
Material & Methods
Bengaluru, the capital of Karnataka State, India is divided into 198 municipal wards. The population of the city is approximately 8.5 million, with a 1-1.2 million floating population (www.bbmp.gov.in). The temperature in Bengaluru ranges from 21 to 35°C. The average annual rainfall is about 970 mm. The study was undertaken during the period of June-December 2015 in north and east Bengaluru, covering 40 wards where An. stephensi, Cx. quinquefasciatus and Ae. aegypti were reported.
Test product:Bactivec® SC supplied by M/s Labiofam Enterprise Group, La Habana, Cuba in 1 l bottles, is a biological larvicide that targets immature stages of mosquitoes and kills larvae at all the stages. The test formulation Bactivec SC contains Bti serotype H-14, strain 266/2 as active ingredient (6 g/l insecticidal toxins and spores; and 994 g/l other ingredients). The biopotency of the compound is >1200 International toxic units/mg (ITU/mg). According to its material data safety sheet, it is classified as slightly hazardous Class III. The product is unstable at 4≤ pH ≥10. The compound is water soluble, non-toxic to persons, warm-blooded animals or hydrobionts. It is biodegradable and ecosystem friendly21.
Phase II field trial in natural breeding habitats small scale): The study was conducted according to the WHO standard guidelines for small scale field trials of biological larvicides22. Field surveys were undertaken in the northern and eastern parts of Bengaluru City for identifying potential natural breeding sources of mosquitoes. Larval and pupal samples were collected from different breeding habitats and placed in separate containers. These were maintained in insectary of National Institute of Malaria Research Field Unit, Bengaluru, for adult emergence for identification of species. The habitats which were supporting the breeding of An. stephensi, Ae. aegypti and Cx. quinquefasciatus were selected for the study. Plastic containers, plastic tanks used for storing water, flower pots (both earthen and cement make), and domestic cement tanks which are the most preferred breeding habitats of Aedes mosquitoes in Bengaluru were included. For Anopheles species cement tanks and flower pots were included. Polluted stagnant drains, cement tanks and pools (both polluted and clean water) were included for testing on Cx. quinquefasciatus species. Water temperature and pH of the habitats were noted and the habitats having pH <4 and >10 were excluded preferably. In all, 4-6 habitats were selected for each type of habitat in Phase II study.
Larval sampling: Larval densities of all Stages I + II and III + IV instars and pupal counts were monitored in the habitats by dipping method (300 ml enamel dipper). Where dipping was not possible in small containers, the contents were poured into a tray and developmental stages were counted and the entire contents were returned to the habitats. Five dips were taken in each habitat to assess the density per dip as described elsewhere22. After assessing the densities in all the habitats, these were allocated to five arms of four each. Four dosages were tested in Phase II trial, i.e. 0.25, 0.5, 1 and 2 ml/50 l in clean water habitats. Where possible, the volume of the water was calculated. Habitats from each type with comparable pre-treatment densities were assigned to either treatment or control groups.
For large habitats such as wastewater drains and pools, the drain at its entire length/entire pool was treated with one dosage and each segment of 10-12 m2 was considered as a replicate. Separate drains were selected for each dosage as well as for control. Four to six replicates were tested per dose/control for each habitat. The dosages tested as per surface area were 2, 3, 5 and 7 ml/m2. The habitats where observations were made continuously for a minimum of 10 days were only considered for analysis. Habitats that lost for reasons such as emptying by owners, becoming dry and diluted due to rains were excluded.
Treatment procedure: Large water bodies (more than 500 l) were treated with hand atomizer sprayer (2 l capacity) with respective dosages according to the surface area. For containers containing <500 l of water manual application of the insecticide using graded pipette was done.
Monitoring and evaluation of impact: Mosquito larvae/pupae were sampled using enamel dippers, counted by stage and returned to their habitats. Post-treatment monitoring of the density of mosquitoes larvae/pupae was done on 1, 3, 7, 10, 14, 17, 20 and 24th day post-application until the pupal density in the treated habitats reached the level comparable to the untreated habitats. The criterion of 80 per cent reduction in larval/pupal counts as per the WHOPES larvicide guidelines22 was used to determine the performance of the test dose for each habitat. The percentage reductions in I-II instars, III-IV instar larvae and pupae were calculated as per Mulla's formula23.
Phase III field trial in natural breeding habitats (large scale): This study was designed in accordance with the WHO standard guidelines for the large-scale field testing of biolarvicides22.
Habitat identification: The field trials were conducted with natural populations of anopheline and culicine larvae in native habitats. Concurrent control replicates were maintained for comparison. Care was taken to select the type of habitats generally preferred by these species in nature for breeding. The selected natural habitats were treated with the Bactivec SC formulation at two effective field application dosages determined in the Phase II field trial for clean and polluted water habitats, i.e. 0.5 and 1.0 ml/50 l dosages for clean water habitats and 5 and 7 ml/m2 for larger water bodies and polluted habitats. The treatment procedure, larval and pupal assessments described in Phase II testing were followed as specified for respective breeding habitats. In all, about 25-40 habitats were selected for each type of habitat and dosage.
Statistical analysis: The per cent reduction was calculated as per Mulla's formula23. The percentage data were Log10 transformed in case of positive values and in the case of negative percentages additional Log Modulus transformation was used. Log10 transformed data were used for two-way analysis of variance keeping days of observation and dosages as independent variables. Student's t test was used for comparison between two dosages in Phase III trial. Only percentage data were used for presentation of results.
Results
Phase II: Small scale field trial: Phase II studies were conducted with four dosages of Bactivec SC, i.e. 0.25, 0.5, 1.0 and 2 ml/50 l in clean water habitats and 2, 3, 5 and 7 ml/m2 in polluted water habitats against three mosquito vector species. In most of the habitats tested, >80 per cent reduction in pupal density was observed up to 10 days with all the dosages against Ae. aegypti and An. stephensi, except 0.25 ml/50 l dose, where >80 per cent reduction was observed up to seven days in cement tanks and plastic containers (Table I). Based on the results, 0.5 ml/50 l and 1.0 ml/50 l dosages were selected for large-scale Phase III trial in clean water habitats.
Table I.
Duration of effectiveness of different dosages of Bactivec suspension concentrate (>80% reduction in pupal densities) against three mosquito species in different breeding habitats in Phase II trial
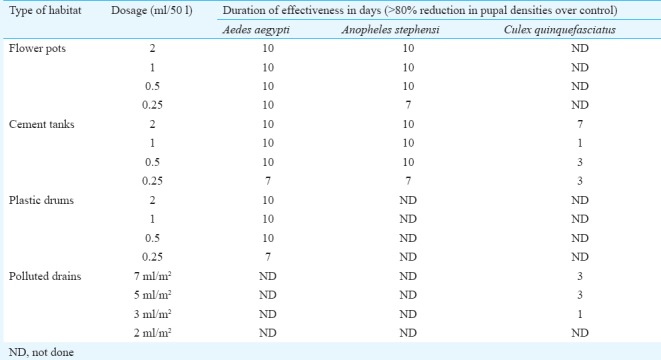
In case of Cx. quinquefasciatus, residual activity was observed up to a maximum of seven days only in cemented containers treated with 2 ml/50 l dosage. In cemented clean water habitats, all the tested dosages produced >80 per cent reduction up to three days only. In polluted habitats, i.e. drains >80 per cent reduction in pupal and 3-4 instar larval densities was observed up to three days in case of 5 and 7 ml/m2 dosages and 0 to 1 day in case of 2 and 3 ml/m2 dosages. No significant difference could be observed when per cent reductions were compared among the dosages. Although results of Cx. quinquefasciatus were not conclusive, 5 and 7 ml/m2 dosages were chosen for Phase III trial.
Phase III: Large scale trial
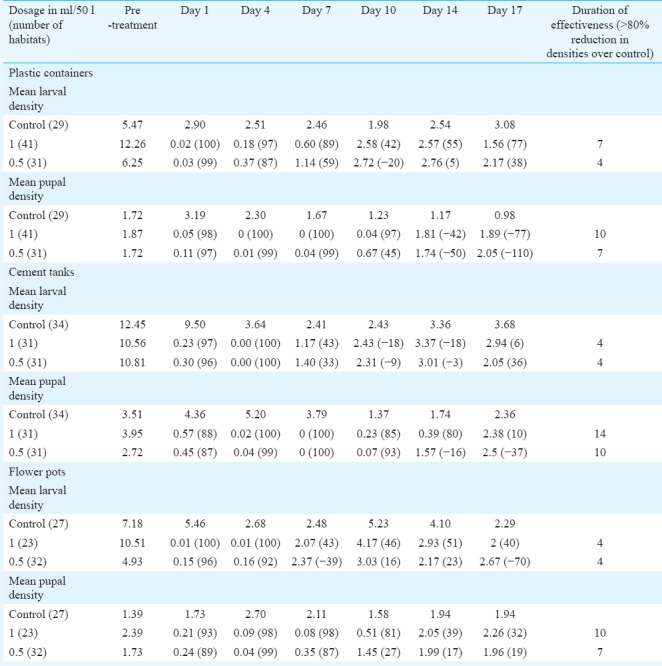
Aedes aegypti: Mean larval and pupal densities of Ae. aegypti and per cent reductions over control in habitats treated with 0.5 and 1 ml/50 l are shown in Table II. The results revealed that duration of effectiveness in causing >80 per cent reduction in pupal densities in treated habitats was 10-14 days for the 1 ml/50 l dosage and 7-10 days for 0.5 ml/50 l dosage in all the three habitats tested. There was no significant difference in per cent reductions when compared between the two dosages tested for early, late instars, and pupae also in all the three types of habitats tested. From the results, it was observed that 1 ml/50 l dosage was effective up to 10-14 days and 0.5 ml/50 l was effective up to 7-10 days.
Table II.
Mean larval and pupal densities per dip (% reduction over control) of Aedes aegypti in different habitats in Phase III evaluation
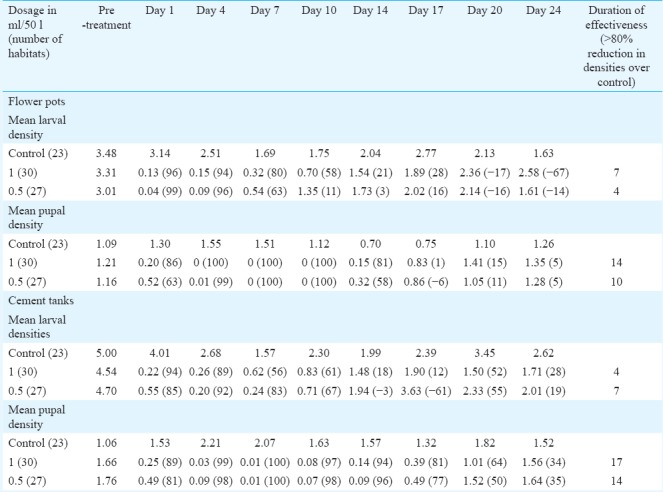
Anopheles stephensi: Mean larval and pupal densities of An. stephensi and per cent reductions over control in habitats treated with 0.5 and 1 ml/50 l are shown in Table III. The results revealed that the residual activity (>80% reduction in pupal density) was 10-14 days in cement tanks and flower pots treated with 0.5 ml dosage, whereas 14-17 days in case of 1 ml/50 l dosage. There was no significant difference between the two dosages in reducing the density of larvae as well as pupae in both types of habitats tested, indicating the equal effectiveness of both the dosages in controlling the immatures.
Table III.
Mean larval and pupal densities per dip (% reduction over control) of Anopheles stephensi in treated habitats in Phase III evaluation
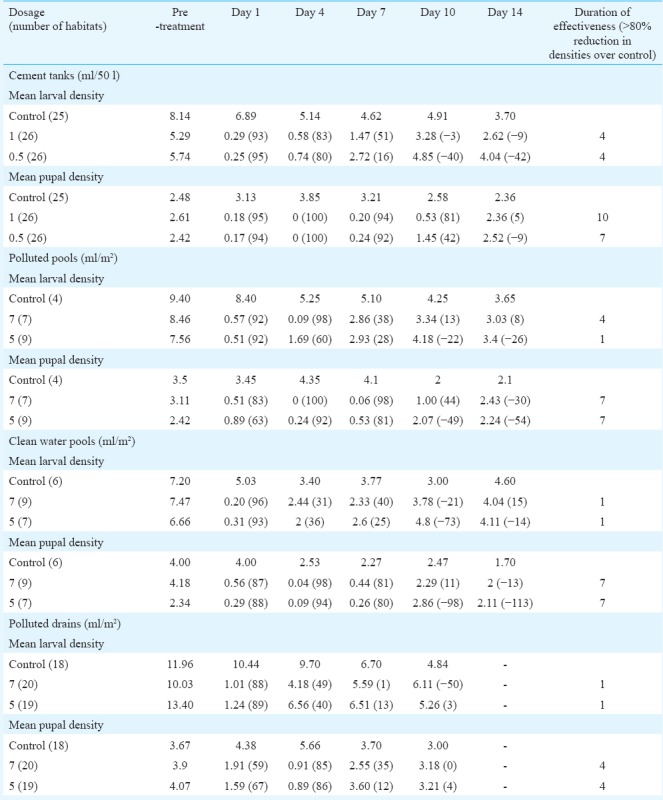
Culex quinquefasciatus: Mean larval and pupal densities of Cx. quinquefasciatus and per cent reductions over control in habitats treated with different dosages are shown in Table IV. Cement tanks, drains, polluted, and clean water pools were selected for assessing the efficacy against Culex larvae. The results showed that in clean water habitats such as cemented containers and pools, >80 per cent reduction was seen up to 10 days in 1 ml/50 l dosage, seven days in case of lower dosage 0.5 ml/50 l in cement tanks. In pools with clean water, seven days efficacy was observed with both the dosages. In polluted drains, only four days residual activity was observed with both dosages of 5 ml and 7 ml/m2. In contrast, in polluted pools, seven days residual activity was observed. There was no significant difference in between the dosages in reducing the densities of either larvae or pupae, inferring that both the dosages are equally effective.
Table IV.
Mean larval and pupal densities per dip (% reduction over control) of Culex quinquefasciatus in different habitats in Phase III evaluation
Discussion
In the present study, suspension concentrate of Bti was tested against aquatic stages of mosquitoes in their natural breeding habitats. The duration of effectiveness ranged from 7 to 17 days in different clean water habitats and up to 4-7 days in polluted water habitats. The formulation was found effective in killing the immature stages with all the dosages tested. The results were in conformity with other studies that reported the efficacy of Bti in reducing the larval density in many habitats. Harwood et al8 in their study on different formulations of Bti reported the effectiveness in controlling mosquito larvae in tree holes and other habitats. Cetin et al9 reported 24 days efficacy of Bti + Bs combination in controlling the larvae of Cx. pipiens in septic tanks. Dambach et al24 also reported the efficacy of Bti in controlling Anopheles mosquito larvae in natural conditions in sub-Saharan Africa. Li et al25 reported the efficacy of Bti WP formulations in controlling the mosquito immatures of Aedes, Anopheles and Culex immatures. A study on two formulations of Bti, WDGs and an extruded pellet against Ae. albopictus reported 100 per cent reduction in mosquito larvae and about three weeks residual efficacy26.
The efficacy of Vectobac GR (potency 200 ITU/mg), a new formulation of bacterial larvicide Bt var. israelensis Strain AM65-52, was tested against An. gambiae and Cx. quinquefasciatus in the simulated field and natural habitats in Benin and an efficacy of 2-3 days against larvae and up to 10 days against pupae in natural habitats was reported27. Guidi et al28 in their evaluation of a commercial biolarvicide based on Bt var. israelensis and Lysinibacillus sphaericus to control mosquitoes breeding in catch basins in southern Switzerland reported >97 per cent of reduction of late instars (3rd and 4th instars) and pupae for four weeks. In contrast, Gezelbash et al16 reported the low efficacy of Bti MH-14 (Bioflash) in laboratory and field trials against Anopheles larvae. In the present study, low residual activity was reported against Cx. quinquefasciatus probably due to the fast settling of the spores of the formulation, bacterial degradation and toxic effluents in the drainage pools.
In conclusion, in our study Bactivec SC, a biological larvicide showed promising efficacy in reducing the larval and pupal densities within 24 h post-treatment in the majority of the habitats and at low dosages. The product was easy to handle and operationally feasible for the application. For comprehensive control in clean water habitats 1 ml/50 l dosage could be effective up to two weeks against Anopheles and Aedes larvae. For the control of Cx. quinquefasciatus in polluted habitats, 5 ml/m2 dosage was found to be effective for a week.
Acknowledgment
The authors thank the staff of the National Institute of Malaria Research Field Unit, Bengaluru for their assistance in Field work, and to Dr Mehul Chaurasia and Shrimati Mala Selvam for their suggestions in the statistical analysis of the data.
Footnotes
Financial support & sponsorship: This study was sponsored by World Health Organization Pesticide Evaluation Scheme (WHOPES)
Conflicts of Interest: None.
References
- 1.World Health Organization. Vector Borne Diseases. [accessed on May 23, 2016]. Available from: http://www.who.int/mediacentre/factsheets/fs387/en/,
- 2.World Health Organization. Geneva: WHO; 2009. [accessed on May 23, 2016]. Bacillus thuringiensis israelensis (Bti) in drinking-water Background document for development of WHO guidelines for drinking-water quality. Available from: http://www.who.int/water_sanitation_health/gdwqrevision/RevisedFourthEdition Bacillusthuringiensis_Bti_July272009_2pdf . [Google Scholar]
- 3.World Health Organization. Geneva: WHO; 2004. [accessed on February 23, 2016]. Report of the seventh WHOPES working group meeting, Geneva 2-4 December 2003. Available from: http://www.who.int/whopes/resources/who_cds_whopes_20048/en/, [Google Scholar]
- 4.World Health Organization. Geneva: WHO; 2006. [accessed on February 20, 2010]. Report of the ninth WHOPES working group meeting, 5-9 December 2005. Available from: http://www.who.int/whopes/resources/who_cds_ntd_whopes_20062/en/, [Google Scholar]
- 5.Knowles A. Recent developments of safer formulations of agrochemicals. Environmentalist. 2008;28:35–44. [Google Scholar]
- 6.Scher HB. New York: Marcel Decker Inc; 1999. Controlled release delivery systems for pesticides; pp. 1–331. [Google Scholar]
- 7.Filho UGA, Silva WA. Application of Bacillus thuringiensis var.israelensis SH-14 formulations against Aedes (S) aegypti. Rev Cubana Med Trop. 2004;56:163–6. [Google Scholar]
- 8.Harwood JF, Farooq M, Turnwall BT, Richardson AG. Evaluating liquid and granular Bacillus thuringiensis var.israelensis broadcast applications for controlling vectors of dengue and chikungunya viruses in artificial containers and tree holes. J Med Entomol. 2015;52:663–71. doi: 10.1093/jme/tjv043. [DOI] [PubMed] [Google Scholar]
- 9.Cetin H, Oz E, Yanikoglu A, Cilek JE. Operational evaluation of Vectomax® WSP (Bacillus thuringiensis subsp. israelensis + Bacillus sphaericus) against larval Culex pipiens in septic tanks. J Am Mosq Control Assoc. 2015;31:193–5. doi: 10.2987/15-6480R. [DOI] [PubMed] [Google Scholar]
- 10.Tamilselvan S, Jambulingam P, Manoharan V, Shanmugasundaram R, Vivekanandan G, Manonmani AM, et al. Fly ash based Bacillus thuringiensis var. israelensis formulation for use against Culex quinquefasciatus, the vector of filariasis in natural ecosystems. J Vector Borne Dis. 2015;52:193–200. [PubMed] [Google Scholar]
- 11.Terbot JW, 2nd, Nikbakhtzadeh MR, Foster WA. Evaluation of Bacillus thuringiensis israelensis as a control agent for adult anopheles Gambiae. J Am Mosq Control Assoc. 2015;31:258–61. doi: 10.2987/moco-31-03-258-261.1. [DOI] [PubMed] [Google Scholar]
- 12.Baldacchino F, Caputo B, Chandre F, Drago A, Della Torre A, Montarsi F, et al. Control methods against invasive Aedes mosquitoes in Europe: A review. Pest Manag Sci. 2015;71:1471–85. doi: 10.1002/ps.4044. [DOI] [PubMed] [Google Scholar]
- 13.El-kersh TA, Al-akeel RA, Al-sheikh YA, Alharbi SA. Isolation and distribution of mosquito-larvicidal cry genes in Bacillus thuringiensis strains native to Saudi Arabia. Trop Biomed. 2014;31:616–32. [PubMed] [Google Scholar]
- 14.Palma L, Muñoz D, Berry C, Murillo J, Caballero P. Bacillus thuringiensis toxins: An overview of their biocidal activity. Toxins (Basel) 2014;6:3296–325. doi: 10.3390/toxins6123296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams GM, Faraji A, Unlu I, Healy SP, Farooq M, Gaugler R, et al. Area-wide ground applications of Bacillus thuringiensis var. israelensis for the control of Aedes albopictus in residential neighborhoods: From optimization to operation. PLoS One. 2014;9:e110035. doi: 10.1371/journal.pone.0110035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gezelbash Z, Vatandoost H, Abai MR, Raeisi A, Rassi Y, Hanafi-Bojd AA, et al. Laboratory and field evaluation of two formulations of Bacillus thuringiensis M-H-14 against mosquito larvae in the Islamic republic of Iran, 2012. East Mediterr Health J. 2014;20:229–35. [PubMed] [Google Scholar]
- 17.Jude PJ, Tharmasegaram T, Sivasubramaniyam G, Senthilnanthanan M, Kannathasan S, Raveendran S, et al. Salinity-tolerant larvae of mosquito vectors in the tropical coast of Jaffna, Sri Lanka and the effect of salinity on the toxicity of Bacillus thuringiensis to Aedes aegypti larvae. Parasit Vectors. 2012;5:269. doi: 10.1186/1756-3305-5-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.González A, Díaz R, Díaz M, Borrero Y, Bruzón RY, Carreras B, et al. Characterization of Bacillus thuringiensis soil isolates from Cuba, with insecticidal activity against mosquitoes. Rev Biol Trop. 2011;59:1007–16. [PubMed] [Google Scholar]
- 19.Marcombe S, Darriet F, Agnew P, Etienne M, Yp-Tcha MM, Yébakima A, et al. Field efficacy of new larvicide products for control of multi-resistant Aedes aegypti populations in Martinique (French West Indies) Am J Trop Med Hyg. 2011;84:118–26. doi: 10.4269/ajtmh.2011.10-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacey LA. Bacillus thuringiensis serovariety israelensis and Bacillus sphaericus for mosquito control. J Am Mosq Control Assoc. 2007;23:133–63. doi: 10.2987/8756-971X(2007)23[133:BTSIAB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Geneva: WHO; 2016. [accessed on January 26, 2016]. Report of the nineteenth WHOPES working group meeting: WHO/HQ, Geneva, 8-11 February 2016. Available from: http://www.who.int/whopes/resources/9789241510400/en/ [Google Scholar]
- 22.World Health Organization. Geneva: WHO; 2005. [accessed on June 20, 2011]. WHO Pesticide Evaluation Scheme (WHOPES) Guidelines for laboratory and field testing of mosquito larvicides. Available from: http://www.who.int/iris/handle/10665/69101, [Google Scholar]
- 23.Mulla MS, Darwazeh HA. Activity and longevity of insect growth regulators against mosquitoes. J Econ Entomol. 1975;68:791–4. doi: 10.1093/jee/68.6.791. [DOI] [PubMed] [Google Scholar]
- 24.Dambach P, Louis VR, Kaiser A, Ouedraogo S, Sié A, Sauerborn R, et al. Efficacy of Bacillus thuringiensis var.israelensis against malaria mosquitoes in Northwestern Burkina Faso. Parasit Vectors. 2014;7:371. doi: 10.1186/1756-3305-7-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li JL, Zhu GD, Zhou HY, Tang JX, Cao J. Experimental observation of toxic effect of Bacillus thuringiensis var. israelensis against Aedes, Culex and anopheles larvae. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2014;26:67–8. [PubMed] [Google Scholar]
- 26.Farajollahi A, Williams GM, Condon GC, Kesavaraju B, Unlu I, Gaugler R, et al. Assessment of a direct application of two Bacillus thuringiensis israelensis formulations for immediate and residual control of Aedes albopictus. J Am Mosq Control Assoc. 2013;29:385–8. doi: 10.2987/13-6332.1. [DOI] [PubMed] [Google Scholar]
- 27.Djènontin A, Pennetier C, Zogo B, Soukou KB, Ole-Sangba M, Akogbéto M, et al. Field efficacy of Vectobac GR as a mosquito larvicide for the control of anopheline and culicine mosquitoes in natural habitats in Benin, West Africa. PLoS One. 2014;9:e87934. doi: 10.1371/journal.pone.0087934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guidi V, Lüthy P, Tonolla M. Comparison between diflubenzuron and a Bacillus thuringiensis israelensis- and Lysinibacillus sphaericus-based formulation for the control of mosquito larvae in urban catch basins in Switzerland. J Am Mosq Control Assoc. 2013;29:138–45. doi: 10.2987/12-6301R.1. [DOI] [PubMed] [Google Scholar]


