Abstract
Background
Pathways through which air pollution may impact cognitive function are poorly understood, particularly with regard to whether and how air pollution interacts with social and emotional factors to influence cognitive health.
Objective
To examine the association between air pollutant exposures and cognitive outcomes among older adults participating in the National Social Life, Health, and Aging Project (NSHAP) cohort study.
Methods
Measures of cognitive function, social connectedness, and physical and mental health were obtained for each NSHAP participant starting with Wave 1 of the study in 2005. Cognitive function was assessed using the Chicago Cognitive Function Measure (CCFM) for 3377 participants. Exposures to fine particles (PM2.5) were estimated for each participant using GIS-based spatio-temporal models, and exposures to nitrogen dioxide (NO2) were obtained from the nearest EPA monitors.
Results
In adjusted linear regression models, IQR increases in 1 to 7 year PM2.5 exposures were associated with a 0.22 (95% CI: -0.44, -0.01) to a 0.25 (95% CI: -0.43, -0.06) point decrease in CCFM scores, equivalent to aging 1.6 years, while exposures to NO2 were equivalent to aging 1.9 years. The impacts of PM2.5 on cognition were modified by stroke, anxiety, and stress, and were mediated by depression. The impacts of NO2 were mediated by stress and no effect modification for NO2 was found.
Conclusions
Exposures to long-term PM2.5 and NO2 were associated with decreased cognitive function in our cohort of older Americans, and individuals who experienced a stroke or elevated anxiety were more susceptible to the effects of PM2.5 on cognition. Additionally, mediation results suggest that PM2.5 may impact cognition through pathways related to mood disorders.
Keywords: Epidemiology, older adults, air pollution, cognitive function, mental health
1. Introduction
By the year 2030, an estimated 72 million people (1 out of every 5 Americans) will be over the age of 65.(Centers for Disease Control and Prevention (CDC) 2013) In this age group, cognitive disorders are one of the leading causes of death, with health care expenses for debilitating cognitive disorders like dementia and Alzheimer’s disease of $183 billion in 2011 and projected costs of $1.1 trillion in 2050 (in 2011 dollars) (Centers for Disease Control and Prevention (CDC) 2011; Sachs et al. 2011).
Risk factors that contribute to cognitive decline are several, with factors related to genetic predisposition and physical health status, such as cardiovascular heart disease, generally receiving the most attention (Breteler et al. 1994; Schram et al. 2007). Recently, however, the contribution of environmental risk factors, such as air pollution exposures, to cognitive decline in older adults has also been investigated. For example, in nationally representative samples of older adults, exposures to fine particulate matter (PM2.5, particles with aerodynamic diameters ≤2.5μm) were associated with worsening episodic memory in the Health and Retirement Survey (Ailshire and Crimmins 2014), decline in global cognition equivalent to aging approximately 2 years among participants in the Nurses Health Study (Weuve et al. 2012), and increased cognitive errors in the Americans Changing Lives Study (Ailshire and Clarke 2015). Similarly within an elderly cohort living in Boston, living closer to major roadways, used as a marker of traffic-related air pollution, was linked to poor cognitive outcomes, such as low scores on tests of verbal learning, memory, language, and executive function (Wellenius et al. 2012). In another Boston area study of older men participating in the Normative Aging Study, exposures to black carbon (BC), also a marker of traffic, were associated with decreased cognition, equivalent to aging nearly 2 years for each doubling in BC exposures (Power et al. 2011). Similar adverse impacts on cognitive function have been observed for PM2.5, and nitrogen dioxide (NO2) in Los Angeles adults (Gatto et al. 2014). Some of the studies showed evidence that certain groups may be more susceptible to air pollution exposures, as air pollution-associated cognition decrements were found to be greatest in smokers, individuals below 74 years of age, individuals who were obese, and those with at least some college education (Ailshire and Crimmins 2014; Power et al. 2011; Ranft et al. 2009; Wellenius et al. 2012).
While these studies provide important evidence that air pollution may harm cognition, they leave unanswered key questions regarding the pathways through which air pollution may impact cognition, particularly with regard to how an individual’s physical and emotional health may mediate the effect of air pollution exposures on cognition. Both physical and emotional health have been shown to impact cognitive function. For example, anxiety and depression were associated with cognitive impairment (Mantella et al. 2007; Yaffe et al. 1999). Correspondingly, both physical and emotional health have also been related to air pollution exposures (Mehta et al. 2015; Power et al. 2015; Pun et al. 2016), suggesting that both may be pathways through which air pollution affects cognitive function. To investigate the collective impacts of air pollution exposures and physical and emotional health on cognition, we used demographic, cognitive function, physical and emotional health, and social data obtained from older adults participating in the National Social Life, Health, and Aging Project (NSHAP).
2. Materials and methods
2.1. Study Participants
The NSHAP study is a nationally representative probability sample of community−dwelling persons, 57 to 85 years of age, from households across the United States, with oversampling of African-Americans, Hispanics, men, and the oldest persons (75 to 84 years of age at the time of screening). For each cohort member in NSHAP, numerous measures of health, wellness, and social factors were assessed in two waves using: 1) biological sample collection, 2) in-person interviews; and 3) leave-behind questionnaires. There were two data collection waves; Wave 1 was conducted from July 2005 to March 2006 with 3,005 participants, whereas Wave 2 was conducted from August 2010 to May 2011 with 3,377 participants (including 2,261 respondents from Wave 1). The present study focused on the 3,377 participants from Wave 2, for which cognitive measures were available, and Wave 1 information of the 2,261 return respondents was also extracted. Interviews were completed in English or Spanish as appropriate (O’Muircheartaigh et al. 2009). The protocol was approved by the Institutional Review Boards of Northeastern University and NORC at the University of Chicago. All respondents provided written informed consent.
2.2. Cognitive Measures
Cognitive function was assessed for each NSHAP participant in Wave 2 using a modified version of the Montreal Cognitive Assessment (MoCA) – known as the Chicago Cognitive Function Measure (CCFM). The CCFM measures overall global cognitive function across multiple domains such as executive function, visuo-construction skills, naming, memory, attention, language, abstract thinking, and orientation. The test has proven to be a valid and reliable test with a Cronbach’s α of 0.773 among NSHAP Wave 2 participants (Kotwal et al. 2015a). During Wave 2 the CCFM was administered to participants using Computer Assisted Personal Interviewing (CAPI) technology. Note that in Wave 1, cognitive function was assessed using the Short Portable Mental Status Questionnaire (SPMSQ). Findings from this test were not used to assess cognitive function in our health analyses, however, due to its poor sensitivity, with the vast majority of NSHAP participants receiving scores of 9 and 10 out of 10 possible points in the test. In mediation analyses (see below), we did control for SPMSQ.
2.3. Physical and Emotional Health Measures
Measures of height and weight were taken at the time of the interview to calculate to body mass index (BMI), with a BMI of over 30 kg/m2 defined as obese (Keys et al. 1972). Blood pressure was measured for each participant (2-3 consecutive times) during the home interview. Blood pressure was estimated as the average of the repeated measurements, with hypertension defined as having average systolic and diastolic measurement of greater than 140 mmHg or 90 mmHg, respectively (Pickering et al. 2005). Glycosylated hemoglobin (HbA1c) and C-Reactive Protein (CRP) were measured from dried blood samples and log transformed. Participants with HbA1c greater than or equal to 6.5% were considered to have elevated HbA1c. CRP was considered elevated if greater than 1 mg/L, and participants with CRP measurements greater than 40 mg/L were not included in CRP analyses, given the likelihood of an active infection (Hansson and Lindquist 1997). The amount of missing information on BMI, blood pressure (BP), HbA1C, and CRP, and was low with 186, 101, 340, and 32 missing measurements, respectively, for 3377 participants.
Functional health measures were obtained from participants via questionnaire. The ability for participants to conduct activities of daily living consisted of a series of 15 questions that asked about the following activities: preparing meals, taking medications, managing money, shopping for food, doing light work, using the phone, walking across a room, walking one block, dressing, bathing, eating, getting out of bed, using the toilet, driving during the day, or driving at night. If participants reported difficulty with any of these activities, they were defined as having impairment of activities of daily living. To assess long-term impairment, participants were instructed to exclude any difficulties that were expected to last less than 3 months.
Information on emotional health was also assessed via questionnaire. Social connectedness was determined by asking participants their frequency of socializing with friends and family in the past 12 months. We defined social connectedness as reporting the frequency of socializing as once per week or more. Loneliness was determined using the modified University of California, Los Angeles (UCLA) loneliness scale, and respondents reporting a score of 5 or greater on the 9-point scale were defined as being lonely. Depression was assessed using an 11-question version of the Center for Epidemiological Studies – Depression scale, CESD-11 (Kohout et al. 1993). A score of 9 or greater on the CESD-11 was used to identify individuals with “moderate-to-severe” depressive symptoms (Harada et al. 2012; Kohout et al. 1993). Elevated anxiety and stress were also measured and participants were defined as having clinically significant anxiety if they scored 8 or greater on the Hospital Anxiety and Depression scale (HADS, max 21 points) (Zigmond and Snaith 1983). Elevated stress was defined as having a score of 5 or greater on the modified 4-item shortened Perceived Stress Scale (PSS, max 12 points) (Cohen et al. 1983).
2.4. Air Pollution Exposure Assessment
Daily PM2.5 concentrations were estimated on a 6 kilometer (km) grid covering the conterminous United States from a set of five spatio-temporal generalized additive mixed models of daily PM2.5 mass levels fit separately to data from 1999–2001, 2002–2004, 2005–2007, 2008–2009, and 2010–2011 based on previous work from Yanosky et al. (Yanosky et al. 2014). PM2.5 data for the models were obtained primarily from the U.S. Environmental Protection Agency’s (EPA) Air Quality System (AQS) database and Interagency Monitoring of Protected Visual Environments network (Environmental Protection Agency (EPA) 2009; Interagency Monitoring of Protected Visual Environments (IMPROVE) 2013). The model included three meteorological covariates (i.e., wind speed, temperature, and total precipitation) that influence pollutant dispersion as well as several geospatial covariates such as smoothed county population density from the 2000 U.S. census, point-source PM2.5 emissions density within 7.5 km, proportion of urban land use within 1 km, elevation, and annual-average PM2.5 for 2002 from EPA’s Community Multiscale Air Quality model. Finally, the daily PM2.5 model includes traffic-related PM levels, represented as the output of a Gaussian line-source dispersion modeling approach. The line-source model uses ADMS-Roads software and associated spatially-smoothed traffic intensity and daily meteorological inputs to describe small-scale spatial gradients in primary PM concentrations near roadways. The daily PM2.5 model has undergone validation during development using cross-validation techniques, as in Yanosky et al. (Yanosky et al. 2014), and had a cross-validation R2 of 0.76. Exposures for study participants were estimated based on the grid value closest to their geocoded residential address for the wave of interest.
We assessed ambient air pollution exposures for NO2 using measurements from the EPA’s AQS. NO2 exposures were assessed for each participant and each wave using concentrations measured at the closest ambient monitor within 60 km of each NSHAP participant’s geocoded residential address. Seventy-two percent of NSHAP participants were matched to a monitor with available exposure estimates.
For both PM2.5 and NO2, exposures for relevant exposure windows, including 1 to 7 year moving averages from the date of interview, were calculated from daily values. Moving averages for each pollutant were considered valid if ≥ 75% of the daily values within each exposure window were available.
2.5. Statistical Analyses
To investigate the association between long-term air pollutant exposures and cognitive function, we analyzed the impact of moving averages for PM2.5 and NO2 on CCFM score using adjusted linear regression models as our main analysis. Potential confounders were selected a priori based on previous scientific findings for cognitive disorders and air pollution (Migliore and Coppedè 2009). Base models controlled for race/ethnicity (white, black, Hispanic non-black, other), gender, education (less than high school, completed high school or vocational school, college degree), and age. Adjusted models additionally controlled for season, current smoking status, geographic region (West, Midwest, Central, South, Northeast), and median household income of census tract. Missingness of variables included in the base and adjusted models were minimal with only 3 participants not included (0.1% missing), so no imputation was performed. All results are expressed as the change in CCFM score per interquartile range (IQR) increase in the pollutant exposures.
Given that inflammation and cardiovascular disease have been identified as pathways through which air pollution may impact cognition (Block and Calderón-Garcidueñas 2009), we investigated whether the association between air pollution and CCFM score was mediated via CRP and blood pressure. In addition, we also explored whether measures of depression, anxiety, stress, CRP, or blood pressure mediate the impact of air pollution on cognitive function. Following the methods of Cole and Maxwell, we restricted the analysis to 2,133 respondents who participated in both Wave 1 and 2 in order to longitudinally assess mediation (Cole and Maxwell 2003). As depicted in Figure 1, mediation was analyzed with linear regression models by first assessing the direct effect of PM2.5 or NO2 on the mediator in Wave 2 of the study (pathway a), controlling for the mediator in Wave 1 to account for change in participants’ status between waves. In the second step (pathway b), we examined the relationship between the mediator in Wave 1 and the outcome of CCFM score in Wave 2 of the study. The second step also assessed the direct effect between PM2.5 or NO2 and the outcome of CCFM in Wave 2 of the study (pathway c) while controlling for the mediator, which allowed for comparison to our main analysis assessing the association between pollution exposures and CCFM (Baron and Kenny 1986). Second step models controlled for the same potential confounders as in our fully adjusted models in our main analysis, namely race/ethnicity, gender, education, age, season, smoking, region, and median household income of census tract from Wave 2, and additionally controlled for the cognitive test from Wave 1, the SPMSQ, to account for low cognitive scores in Wave 1. Using both waves of data allowed for assessment of temporality in the exposure-mediator-outcome relationship (Cole and Maxwell 2003). The effect of mediators was assessed through use of the Sobel test (Sobel 1982). All effect modification and mediation analyses were performed using 1 year moving average PM2.5 and NO2 exposures as the exposure measure.
Figure 1. Conceptual Model for Mediation Analysis.
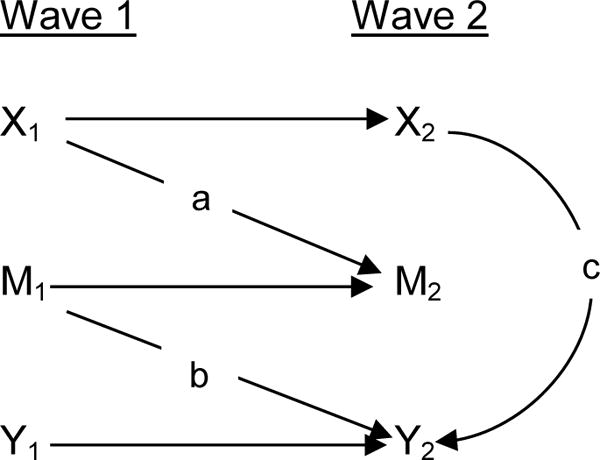
aPathway ‘a’ shows the effect of PM2.5 (X1) on mediators in Wave 2 (M2), controlling for mediators in Wave 1 (M1)
bPathway ‘b’ shows the mediators’ effects on CCFM (Y2), controlling for cognitive test results in Wave 1 (Y1)
cPathway ‘c’ shows the effect of PM2.5 on CCFM with mediators included
As sensitivity analyses, we fit logistic regression models with the outcome of low score, as defined by a score below the 25th percentile (11 points out of a maximum of 20) of all scores, since there is no clinically established cut-off score on the CCFM that indicates cognitive impairment. These analyses were also conducted using alternative definitions of low score based on the 10th (8 points) and 33rd percentile (12 points). Additional sensitivity analyses included two pollutant models that included both NO2 and PM2.5. As before, models adjusted for gender, age, race/ethnicity, education, season, current smoking status, region, and median household income.
All statistical analyses were conducted using SAS version 9.4 software (SAS Institute Inc., Cary, North Carolina).
3. Results
Our primary analyses included 3,374 individuals participating in Wave 2 of NSHAP (Table 1). Participants in Wave 2 were on average 72 years old, with approximately half female. The majority of participants were white (71.16%), had at least a high school education (80.90%), and exercised 1 or more times per week (56.32%). Nearly half of the participants had hypertension (46.40%), nearly a quarter reported ever having diabetes (23.67%), 13.33% reported being current smokers, and 9.36% of participants reported having a stroke within the last 5 years. The mean annual household income of the census tract of participants was $56,400. The mean score on the CCFM was 13.45 (± 4.05) out of a possible 20 points and was negatively correlated with age, with older individuals having lower CCFM scores. The questions and domains assessed in both the SPMSQ and the CCFM are shown in Table S1. During Wave 2, one year moving average pollutant concentrations equaled 10.23 (2.50) μg/m3 for PM2.5 and 10.13 (6.28) ppb for NO2. During Wave 1 the pollutant concentrations were higher with 13.07 (2.81) μg/m3 for PM2.5 and 14.92 (7.23) ppb for NO2. The Pearson correlation between PM2.5 and NO2 measurements was 0.30 (p-value <0.0001).
Table 1.
Characteristics of NSHAP Participants
| Characteristic | Wave 1 (Jul 2005–Mar 2006) |
Wave 2 (Aug 2010–Jun 2011) |
|---|---|---|
| Number of Participants | 3005 | 3377 |
| Age, years (SD) | 69.30 (7.85) | 72.38 (8.10) |
| Gender, n (%) | ||
| Women | 1551 (51.61%) | 1839 (54.46%) |
| Men | 1454 (48.39%) | 1538 (45.54%) |
| Race, n (%) | ||
| Non-Hispanic white | 2110 (70.22%) | 2403 (71.16%) |
| Non-Hispanic black | 509 (16.94%) | 517 (15.31%) |
| Hispanic non-black | 304 (10.12%) | 367 (10.87%) |
| Other | 82 (2.73%) | 90 (2.67%) |
| Education Level, n (%) | ||
| Less than High School | 699 (23.26%) | 645 (19.10%) |
| High School or vocational school | 1649 (54.88%) | 1905 (56.41%) |
| College degree or greater | 657 (21.86%) | 827 (24.49%) |
| Median Household Incomea, mean (SD) | 52.7 (25.1) | 56.4 (27.3) |
| Cognitive Testsb, n (%) | ||
| SPMSQ, mean (SD) | 9.03 (1.23) | |
| CCFM, mean (SD) | 13.45 (4.05) | |
| Diabetes, n (%) | 642 (12.36%) | 800 (23.67%) |
| Elevated HbA1c (HbA1c ≥ 6.5%), n (%) | 366 (12.18%) | 447 (13.24%) |
| Stroke, n (%) | 268 (8.92%) | 315 (9.36%) |
| Hypertension (>90 diastolic and/or >140 systolic), n (%) | 1367 (46.59%) | 1520 (46.40%) |
| CRP, mean (SD) | 3.20 (6.03) | 4.83 (10.38) |
| High CRP (>1), n (%) | 1207 (40.25%) | 2329 (69.63%) |
| BMI (kg/m2) | 29.10 (6.32) | 29.36 (6.33) |
| Obesity (>30 BMI), n (%) | 1054 (37.78%) | 1239 (38.83%) |
| Exercise (weekly or more), n (%) | 2306 (76.74%) | 1902 (56.32%) |
| Impaired Activities of Daily Livingc, n (%) | 1665 (55.43%) | 1989 (58.90%) |
| Current smoker, n (%) | 444 (14.79%) | 450 (13.33%) |
| Elevated depressiond, n (%) | 730 (24.32%) | 703 (20.82%) |
| Elevated anxietye, n (%) | 378 (13.50%) | 605 (21.31%) |
| Elevated stressf, n (%) | 391 (13.98%) | 969 (34.25%) |
| Social Connectednessg, n (%) | 1299 (43.23%) | 1489 (44.09%) |
| Loneliness, n (%) | 731 (24.33%) | 747 (22.12%) |
| Pollutantsh, mean (SD) | ||
| PM2.5 (μg/m3) | 13.07 (2.81) | 10.23 (2.50) |
| NO2 (ppb) | 14.92 (7.23) | 10.13 (6.28) |
Median household income is from census tract of each participant, results given in thousands of dollars.
SPMSQ = Short Portable Mental Status Questionnaire used in Wave 1 with a maximum score of 10; CCFM = Chicago Cognitive Function Measure used in Wave 2 with a maximum score of 20, 3362 participants completed the CCFM in Wave 2
Impaired Activities of Daily Living defined as participants reporting difficulty in any of the following activities: preparing meals, taking medications, managing money, shopping for food, doing light work, using the phone, walking across a room, walking one block, dressing, bathing, eating, getting out of bed, using the toilet, driving during the day, or driving at night
Elevated depression defined as a score of 9 or greater on the Center for Epidemiological Studies Scale (CESD-11, max 33 points)
Elevated anxiety defined as a score of 8 or greater on Hospital Anxiety and Depression Scale (HADS, max 21 points)
Elevated stress defined as a score of 5 or greater on the Perceived Stress Scale (PSS, max 12 points)
Social connectedness defined as socializing with friends and family 1/week or more
PM2.5 = Particulate matter < 2.5μm in diameter; NO2 = Nitrogen dioxide; all pollutant values given are yearly averages based on interview date
Associations of 1 to 7 year moving averages of ambient pollutant exposures and the change in CCFM score are presented in Table 2. In bivariate analysis, an IQR increase in all PM2.5 moving averages was associated with a decrease in CCFM score (1 year moving average −1.10 95% CI: −1.35, −0.85). In base models, adjusting for gender, age, race/ethnicity, and education, an IQR increase in PM2.5 was associated with relatively constant decreases in CCFM scores for all examined exposure windows. For example, an IQR increase in either 1 or 7 year PM2.5 was associated with a 0.27 (1 year 95% CI: −0.47, −0.07; 7 year 95% CI: −0.45, −0.08) reduction in CCFM scores. Models additionally adjusting for season, current smoking status, region, and median household income (“Fully Adjusted”) resulted in slightly attenuated decreases in CCFM scores for all moving averages, with the greatest effect in PM2.5 associated risks observed for the 7 year moving average exposure (−0.25, 95% CI: −0.43, -0.06).
Table 2.
Change in CCFM Score per IQR Increase in Ambient Air Pollutants over 1–7 year Moving Average Exposuresa
| PM2.5b (n=3374) | NO2c (n=2106) | |||||
|---|---|---|---|---|---|---|
| Bivariated | Basee | Fully Adjusted f | Bivariated | Basee | Fully Adjustedf | |
| 1 year | −1.10 (−1.35, −0.85) | −0.27 (−0.47, −0.07)* | −0.22 (−0.44, −0.01)* | −0.41 (−0.67, −0.15)* | −0.06 (−0.24, 0.12) | −0.13 (−0.34, 0.08) |
| 2 years | −1.11 (−1.35, −0.87) | −0.23 (−0.43,−0.04)* | −0.22 (−0.42, −0.01)* | −0.47 (−0.68, −0.26)* | −0.13 (−0.29, 0.04) | −0.26 (−0.45, −0.06)* |
| 3 years | −1.00 (−1.23, −0.78) | −0.21 (−0.40, −0.03)* | −0.21 (−0.40, −0.02)* | −0.43 (−0.64, −0.22)* | −0.13 (−0.30, 0.03) | −0.27 (−0.46, −0.07)* |
| 4 years | −0.95 (−1.17, −0.73) | −0.23 (−0.41, −0.05)* | −0.22 (−0.40, −0.04)* | −0.42 (−0.63, −0.21)* | −0.14 (−0.31, 0.02) | −0.29 (−0.48, −0.09)* |
| 5 years | −0.96 (−1.19, −0.74) | −0.25 (−0.43, −0.07)* | −0.23 (−0.42, −0.05)* | −0.49 (−0.70, −0.27)* | −0.17 (−0.34, −0.01)* | −0.32 (−0.52, −0.12)* |
| 6 years | −0.95 (−1.17, −0.73) | −0.26 (−0.43, −0.08)* | −0.24 (−0.42, −0.06)* | −0.50 (−0.73, −0.28)* | −0.18 (−0.35, −0.00)* | −0.33 (−0.54, −0.12)* |
| 7 years | −0.95 (−1.18, −0.73) | −0.27 (−0.45, −0.08)* | −0.25 (−0.43, −0.06)* | −0.48 (−0.71, −0.25)* | −0.15 (−0.32, 0.03) | −0.27 (−0.48, −0.07)* |
Contains participants in Wave 2 who completed CCFM cognitive test
PM2.5 1yr IQR=4.25 ug/m3, 2yr IQR=4.03 ug/m3, 3 yr IQR=3.93 ug/m3, 4 yr IQR=3.99 ug/m3, 5yr IQR=4.10 ug/m3, 6 yr IQR=4.18 ug/m3, 7 yr IQR=4.33 ug/m3
NO2 1yr IQR=8.37 ppb, 2yr IQR =6.66 ppb, 3 yr IQR=6.68 ppb, 4 yr IQR=6.90 ppb, 5 yr IQR=6.99 ppb, 6 yr IQR=7.28 ppb, 7yr IQR=7.42 ppb
Bivariate analysis includes pollutants only
Base model includes adjustment for gender, age, race/ethnicity, education
Fully adjusted model includes adjustment for gender, age, race/ethnicity, education, season, smoking, region, median household income of census tract
p<0.05
Similarly, IQR increases in 2 year (−0.26, 95% CI: −0.45, −0.06) to 7 year (−0.27, 95% CI: −0.48, −0.07) moving averages of NO2 exposures were also associated with decreased CCFM scores in fully adjusted models. By comparing the results for PM2.5 and NO2 to the changes in cognition for every one year increase in age, it was determined that increased IQR exposures were associated with significant decreases in cognitive function, equivalent to aging 1.6 years for PM2.5 and 1.9 years for NO2. Two pollutant fully adjusted models that included PM2.5 and NO2 showed similar effect estimates for all pollutants (Table S2).
Results for the mediation analysis for PM2.5 are shown in Table 3, and the mediation analysis for NO2 is shown in Table S4.
Table 3.
Mediation Analysis: Mediators of the Association Between PM2.5 and Cognitive Functiona
| Mediators | |||||
|---|---|---|---|---|---|
| Depression | Anxiety | Stress | CRPe | Systolic BP | |
| Effect of PM2.5 on Mediatorsb | 0.42 (0.16, 0.68) | 0.02 (−0.21, 0.25) | −0.04 (−0.21, 0.14) | 0.02 (−0.05, 0.09) | −0.84 (−2.09, 0.41) |
| Mediators’ effects on CCFMc | −0.09 (−0.11, −0.06) | −0.12 (−0.16, −0.08) | −0.21 (−0.27, −0.15) | 0.15 (0.00, 0.29) | −0.01 (−0.02, −0.001) |
| Effect of PM2.5 on CCFM with mediators includedd | −0.16 (−0.42, 0.10)* | −0.13 (−0.40, 0.13) | −0.11 (−0.37, 0.16) | −0.16 (−0.48, 0.16) | −0.21 (−0.47, 0.06) |
Contains participants in Wave 1 and Wave 2 who completed CCFM and SPMSQ cognitive tests and had available mediator data; models include adjustment for race/ethnicity, gender, education (<H.S., H.S., College), age, season, smoking, region, median household income of census tract
Mediator test run on mediators in Wave 2, controlling for mediators in Wave 1, results per IQR increase in 1 year moving average PM2.5 from Wave 1
Test run between mediator in Wave 1 and CCFM outcome in Wave 2, controlling for SPMSQ results in Wave 1
Results per IQR increase in 1 year moving average PM2.5 in Wave 2, controlling for SPMSQ results in Wave 1
CRP values log transformed
p<0.05 for value of Sobel test
In the subset of individuals participating in both waves of NSHAP, depression was the only mediator that showed significant mediation of the relationship between PM2.5 and CCFM with the Sobel test. There were significant indirect effects, where increased PM2.5 exposure was related to increased depression (0.42, 95% CI: 0.12, 0.68), which, in turn, was associated with decreased CCFM scores (−0.09, 95% CI: −0.11, -0.06). When controlling for depression, the effect of an IQR increase in 1-year averaged PM2.5 exposures on CCFM dropped to −0.16 (95% CI: −0.42, 0.10). In contrast, stress, anxiety, CRP and blood pressure were not found to be mediators of the association between PM2.5 and CCFM as no indirect effect was found between PM2.5 and the mediators in Wave 2 ((0.02 (95% CI: −0.21, 0.25) for anxiety, (−0.04 (95% CI: −0.21, 0.14) for stress, (0.02 (95% CI: −0.05, 0.09) for CRP, and −0.84 (95% CI: −2.09, 0.41) for systolic BP). Similar null results were found for diastolic BP and hypertension (not shown)). Stress was found to be a significant mediator for the effect between NO2 and CCFM scores with the indirect effect of NO2 found to increase stress (0.11 (95% CI: 0.02, 0.19)). Null results were found for the other mediators investigated with NO2, including depression, anxiety, CRP, and systolic BP (Table S4).
Table 4 shows effect modification results for the relationship between CCFM score and PM2.5 and between CCFM score and NO2 levels averaged over 1 year. Participants with elevated anxiety had significantly lower decreases in CCFM score with IQR increases in PM2.5 exposure compared to those without anxiety (-0.15 (95%CI: -0.75, 0.44) compared to -0.41 (95%CI: -0.67, -0.19)), as did participants with elevated stress (-0.19 (95%CI: -0.70, 0.32) compared to -0.35 (95% CI: -0.59, -0.11)). In contrast, participants who had not experienced a stroke had higher decreases in CCFM score of -0.48 (95%CI: -0.82, -0.13) compared to subjects who did not experience a stroke (-0.03 (95% CI: -0.82, 0.77)). While not reaching nominal statistical significance for the effect of interaction terms, individuals who were diabetic, had elevated HbA1C, were current smokers, and had elevated depression showed larger decreases in CCFM scores per IQR increase in PM2.5. Results were similar for NO2 with individuals who were diabetic, had elevated HbA1C, were current smokers, and had elevated depression showing larger decreases in CCFM scores per IQR increase in NO2. With the exception of impaired activities of daily living where individuals with impairment had an increase in CCFM score compared to a decrease in those without impairment (0.86 (95%CI: 0.54, 1.18) compared to -0.15 (95%CI: -0.36, 0.06)), no other modifiers for NO2 reached statistical significance.
Table 4.
Change in CCFM Score (95% CI) per IQR Increment in 1 year Ambient Air Pollutant Levels in Multivariate Models: Modification by Participant Characteristicsa
| Effect modifier | PM2.5 | p-value | NO2 | p-value |
|---|---|---|---|---|
| Age | 0.28 | 0.29 | ||
|
| ||||
| <70 | −0.19 (−0.41, 0.02) | −0.13 (−0.34, 0.09) | ||
| >70 | 0.47 (−0.09, 1.02) | −0.11 (−0.59, 0.38) | ||
|
| ||||
| BMI | 0.47 | 0.85 | ||
|
| ||||
| <30 | −0.15 (−0.38, 0.07) | −0.12 (−0.34, 0.10) | ||
| ≥30 | −0.15 (−0.63, 0.34) | 0.01 (−0.31, 0.33) | ||
|
| ||||
| CRP | 0.28 | 0.11 | ||
|
| ||||
| Low (<1) | −0.23 (−0.71, 0.29) | −0.14 (−0.36, 0.08) | ||
| Elevated (>1) | −0.21 (−0.46, −0.00) | −0.26 (−0.60, 0.07) | ||
|
| ||||
| Diabetes | 0.44 | 0.60 | ||
|
| ||||
| No | −0.16 (−0.41, 0.09) | −0.01 (−0.34, 0.14) | ||
| Yes | −0.41 (−0.97, 0.16) | −0.30 (−0.67, 0.07) | ||
|
| ||||
| HbA1c | 0.32 | 0.91 | ||
|
| ||||
| <6.5 | −0.10 (−0.41, 0.20) | −0.11 (−0.39, 0.18) | ||
| ≥6.5 | −0.72 (−1.45, 0.00) | −0.62 (−1.07, −0.17) | ||
|
| ||||
| Hypertensionb | 0.15 | 0.66 | ||
|
| ||||
| No | −0.23 (−0.45, −0.01) | −0.01 (−0.31, 0.12) | ||
| Yes | −0.30 (−0.16, 0.77) | −0.08 (−0.39, −0.23) | ||
|
| ||||
| Stroke | 0.046* | 0.62 | ||
|
| ||||
| No | −0.48 (−0.82, −0.13) | −0.18 (−0.53, 0.17) | ||
| Yes | −0.03 (−0.82, 0.77) | −0.54 (−1.07, −0.01) | ||
|
| ||||
| Current Smoker | 0.10 | 0.08 | ||
|
| ||||
| No | −0.04 (−0.34, 0.26) | 0.08 (−0.22, 0.38) | ||
| Yes | −0.86 (−1.55, −0.17) | −0.30 (−0.74, 0.15) | ||
|
| ||||
| Physical activity | 0.73 | 0.63 | ||
|
| ||||
| None | −0.21 (−0.43, 0.01) | −0.12 (−0.33, 0.09) | ||
| ≥1/ week | −0.20 (−0.67, 0.27) | −1.19 (−1.66, −0.71) | ||
|
| ||||
| Impaired Activities of Daily Livingc | 0.42 | 0.047* | ||
|
| ||||
| No | −0.23 (−0.45, −0.01) | −0.15 (−0.36, 0.06) | ||
| Yes | 0.94 (0.47, 1.41) | 0.86 (0.54, 1.18) | ||
|
| ||||
| Elevated Depressiond | 0.94 | 0.053 | ||
|
| ||||
| No | −0.19 (−0.44, 0.07) | 0.02 (−0.23, 0.26) | ||
| Yes | −0.90 (−1.49, −0.32) | −1.09 (−1.46, −0.71) | ||
|
| ||||
| Elevated Anxietye | 0.03* | 0.38 | ||
|
| ||||
| No | −0.41 (−0.67, −0.19) | −0.12 (−0.38, 0.13) | ||
| Yes | −0.15 (−0.75, 0.44) | −0.71 (−0.10, −0.31) | ||
|
| ||||
| Elevated Stressf | 0.01* | 0.93 | ||
|
| ||||
| No | −0.35 (−0.59, −0.11) | −0.18 (−0.41, 0.04) | ||
| Yes | −0.19 (−0.70, 0.32) | −0.83 (−1.17, −0.48) | ||
|
| ||||
| Social Connectednessg | 0.15 | 0.35 | ||
|
| ||||
| No | 0.05 (−0.33, 0.43) | −0.02 (−0.40, 0.36) | ||
| Yes | −0.36 (−1.03, 0.30) | 0.27 (−0.16, 0.69) | ||
|
| ||||
| Loneliness | 0.06 | 0.27 | ||
|
| ||||
| No | −0.22 (−0.56, 0.11) | −0.04 (−0.28, 0.21) | ||
| Yes | −0.22 (−1.12, 0.69) | −0.22 (−0.59, 0.15) | ||
Multivariate models include adjustment for gender, race/ethnicity, education, median household income of census tract, age, region, smoking, season
Hypertension defined as >90 diastolic and/or >140 systolic, average of repeated blood pressure readings used
Impaired Activities of Daily Living defined as participants reporting difficulty in any of the following activities: preparing meals, taking medications, managing money, shopping for food, doing light work, using the phone, walking across a room, walking one block, dressing, bathing, eating, getting out of bed, using the toilet, driving during the day, or driving at night
Elevated depression defined as a score of 9 or greater on the Center for Epidemiological Studies Scale (CESD-11, max 33 points)
Elevated anxiety defined as a score of 8 or greater on Hospital Anxiety and Depression Scale (HADS, max 21 points)
Elevated stress defined as a score of 5 or greater on the Perceived Stress Scale (PSS, max 12 points)
Social connectedness defined as socializing with friends and family 1/week or more
p for interaction <0.05
In our sensitivity analyses, results from logistic models that analyzed the odds of low CCFM score (as defined by scoring below the 25th percentile, <=10 points) are shown in Table S3. An IQR increase in 1 year moving average PM2.5 exposure corresponded to an adjusted odds ratio of 1.12 (95% CI: 0.92, 1.36) for having a low CCFM score, with consistent results across all moving averages. NO2 results were also similar with an adjusted odds ratio of 1.15 (95% CI: 0.93, 1.43) per IQR increase in 1 year moving average. Sensitivity analyses using the 10th (8 of 20 points) and 33rd (12 of 20 points) percentiles produced stronger and weaker associations, respectively (results not shown).
4. Discussion
Our findings join a small but growing body of literature that provides evidence of the effects of individual-specific long-term air pollution exposures on cognitive outcomes while providing new information on factors that modify these impacts and on potential pathways through which air pollution can affect cognition. We did so using a nationally representative study of 3374 older adults, showing that increased PM2.5 and NO2 exposures are associated with significant decreases in cognitive function, equivalent to aging 1.6 and 1.9 years, respectively. These deficits are comparable to those found in the Nurses’ Health Study and in the Health and Retirement Study, showing effects to be equivalent to aging approximately 2 years (Weuve et al. 2012) and between 1.7 and 2.8 years (Ailshire and Crimmins 2014), respectively. Further, our findings are consistent with other studies that show associations between PM2.5 and lower verbal scores among older adults in Los Angeles (Gatto et al. 2014), decline in global cognition among older women across the U.S. (Weuve et al. 2012), and worsening reasoning and memory among older adults in London (Tonne et al. 2014). For the gaseous pollutants, our NO2 findings are similar to those from Gatto et al of older adults in Los Angeles, which showed that yearly exposures to NO2 were associated with lower logical memory scores (Gatto et al. 2014). They are also consistent with studies of BC, which like NO2 is considered a proxy of traffic exposures (Beckerman et al. 2008), that linked BC exposures to increased risk of lower cognitive function as measured by the Mini-Mental State Examination (MMSE) (Power et al. 2011; Wellenius et al. 2012).
Notably we found that the impacts of PM2.5 and NO2 on cognitive function are mediated by depression and stress, respectively, suggesting the importance of mental illness as a pathway to cognitive deficits. While no studies have investigated mediation of air pollution and cognition by mood disorders, mediation by mental health disorders is consistent with results from studies showing relationships between (1) PM2.5 and stress among older men (Mehta et al. 2015), anxiety among a cohort of nurses (Power et al. 2015), and depression and anxiety in our NSHAP cohort (Pun et al. 2016) and (2) mood disorders and cognitive decline (Copeland et al. 2003; Mantella et al. 2007; Modrego and Ferrández 2004; Yaffe et al. 1999). Additionally, it has been shown that individuals who are experiencing depression, stress, or anxiety may experience changes in behaviors, such as limited physical activity and social isolation (Roshanaei-Moghaddam et al. 2009), that subsequently lead to cognitive decline (Hillman et al. 2006; Wilson et al. 2007).
Correspondingly, both PM2.5-related impacts on mood disorders and cognitive function are thought to occur through similar pathways related to oxidative stress, neuroinflammation, cerebrovascular damage, and neurodegeneration (Block and Calderón-Garcidueñas 2009; MohanKumar et al. 2008), which can lead to dopamine neurotoxicity (Block et al. 2004). PM2.5 pollution may also harm cognitive and mental health by increasing markers of glucocorticoid activity and levels of the stress hormone cortisol (Thomson et al. 2013; Tomei et al. 2003) or by contributing to respiratory or cardiac disease (Power et al. 2015; Wang et al. 2014), which have in turn been associated with cognitive deficits and mood disorders (Aben et al. 2003; Breteler et al. 1994; Scott et al. 2007). Contrary to these presumed biological pathways, however, we did not find CRP or hypertension, often used as markers of inflammation and vascular dysfunction (Budhiraja et al. 2004; Windgassen et al. 2015), respectively, to be mediators of the relationship between air pollution and cognitive function, possibly reflective of their non-specificity, which make them imperfect indicators of their respective conditions (Du Clos 2000). Given observed associations between PM2.5 and mood disorders (Mehta et al. 2015; Power et al. 2015; Pun et al. 2016), and between mood disorders and cognitive decline (Copeland et al. 2003; Mantella et al. 2007; Modrego and Ferrández 2004; Yaffe et al. 1999), it follows that mood disorders are a logical intermediate on the pathway between air pollution and cognitive decline. To our knowledge only one other study has investigated mediation of the association between air pollution and cognition by respiratory and cardiovascular disease, with preliminary evidence pointing to null findings (Weuve et al. 2012). However, to date no studies have investigated mediation by mood disorders.
We also showed that susceptibility to cognitive deficits as the result of PM2.5 exposures varies by mood disorders, where, for example, individuals without anxiety and stress had significantly greater cognitive deficits as compared to those with anxiety and stress, both comparable to a 3% decrease in mean CCFM scores. While higher air pollution-associated risks of cognitive impairment in individuals without mood disorders was somewhat unexpected, it is possible that the residual effects of PM2.5 are less pronounced in individuals who are already experiencing anxiety and stress.
Our study has potential limitations that warrant discussion. First, exposures for PM2.5 were estimated using spatio-temporal models created from ambient monitor measurements that are imperfect measures of personal exposures to ambient air pollution. However, our exposure assessment method minimized exposure error resulting from spatial variation in PM2.5 concentrations, as the distance of the grid point estimates to residential addresses were minimal and substantially less than would otherwise occur if more traditional closest stationary ambient monitor concentrations were used to assess exposures. In contrast, exposures for NO2 were measured using the closest stationary ambient monitor measurements within 60 km, resulting in greater exposure error. Exposure error for PM2.5 and NO2 would likely bias our results towards the null, as shown by previous studies that show that chronic health risks are underestimated using nearest monitor exposures (Kioumourtzoglou et al. 2014; Paciorek et al. 2009; Suh and Zanobetti 2010). Secondly, the NSHAP study consisted of community dwelling older adults, limiting its generalizability to institutionalized or older adults in the youngest age range. Additionally, there are a number of confounding variables that were not available for all participants in this study that could be included in future studies. Examples include an improved measure of individual socio-economic status in addition to our inclusion of education and median household income of the census tract. However, based on recent analysis of similar studies, further adjustment of using these improved measures of SES is unlikely to substantially affect our estimates of associations between PM2.5 and cognitive decline (Power et al. 2016). Lastly, there are only two waves of data with which to assess mediation and only one wave of data with the CCFM test to assess cognition. Ideally, mediation analysis would be assessed longitudinally with three waves or more of available data. With only two waves of data, this study is limited in its ability to assess temporality between the exposure, mediator, and outcome.
Important strengths of this study include our NSHAP population, which is a nationally representative study of older adults. Data was available on measures of mental health, social variables, functional health, and underlying health conditions not previously included in studies of air pollution and cognitive decline. The rich participant data available in NSHAP allowed for comprehensive characterization of each participant and investigation of potential confounders, the possibility of effect modification, and analysis of mediation. An understanding of mediation leads to knowledge about the biological pathways that lead to disease. Use of the multidimensional CCFM test allowed for robust testing of eight cognitive domains with demonstrated reliability (Kotwal et al. 2015b). The CCFM can be considered superior to the MMSE used in previous studies, which has been shown to a poor tool to assess mild degrees of cognitive impairment especially across individuals with varying age, education, and cultural backgrounds (Tombaugh and McIntyre 1992). Additionally, we assessed PM2.5 exposures for each participant using Geographic Information System (GIS) based spatio-temporal models, which reduced exposure error in our exposure estimates. Importantly, our findings were robust to our method of analysis, with results from several sensitivity analyses being qualitatively similar to those for our main analysis.
5. Conclusions
In summary we found that increased long-term PM2.5 and NO2 exposures are associated with significant decreases in cognitive function, equivalent to aging 1.6 and 1.9 years. We also found that the effect of PM2.5 on CCFM is mediated by depression and the effect of NO2 on CCFM is mediated by stress, suggesting mental health pathways through which PM2.5 and NO2 affect cognition. These results suggest that limiting exposure to PM2.5 and NO2 and addressing mental health issues among older adults may prevent deterioration of cognitive function that affects an increasing number of individuals.
Supplementary Material
Highlights.
Associations between air pollutant exposures and cognitive outcomes were assessed.
Increases in PM2.5 and NO2 exposures were associated with lower cognitive scores.
Impacts of PM2.5 on cognition were modified by stroke, anxiety, and stress.
Depression mediated the impacts of PM2.5 on cognition, and the effect of NO2 on CCFM is mediated by stress
Mediation results suggest new pathways through which PM2.5 and NO2 may impact cognition.
Acknowledgments
We acknowledge Dr. Jeffrey Yanosky from Penn State University for providing daily PM2.5 grid data. We also thank Dr. Murray A. Mittleman, Dr. Marianthi-Anna Kioumourtzoglou, and Dr. Brent Coull for providing guidance on this work.
Funding: This work was funded by NIEHS grant R01ES022657-01A1. Development of PM2.5 GIS-based spatio-temporal models was supported by National Institutes of Health grant R01 ES019168. NSHAP is supported by the National Institutes of Health, including the National Institute on Aging (R37AG030481; R01AG033903), the Office of Women’s Health Research, the Office of AIDS Research, and the Office of Behavioral and Social Sciences Research (R01AG021487), and by NORC which was responsible for the data collection.
Abbreviations
- AQS
Air Quality System
- BC
Black Carbon
- BMI
Body Mass Index
- BP
blood pressure
- CAPI
Computer Assisted Personal Interviewing
- CESD
Center for Epidemiologic Studies Depression scale
- CDC
Centers for Disease Control and Prevention
- CCFM
Chicago Cognitive Function Measure
- CI
confidence interval
- CRP
C-reactive protein
- EPA
US Environmental Protection Agency
- HADS
Hospital Anxiety and Depression Scale
- HbA1c
glycosylated hemoglobin
- IMPROVE
Interagency Monitoring of Protected Visual Environments
- IQR
interquartile range
- MMSE
Mini-Mental State Examination
- MoCA
Montreal Cognitive Assessment
- NO2
nitrogen dioxide
- NSHAP
National Social
- Life
Health, and Aging Project
- PM2.5
particulate matter with an aerodynamic diameter of ≤2.5μm
- ppb
parts per billion
- PSS
Perceived Stress Scale
- SD
standard deviation
- SPMSQ
Short Portable Mental Status Questionnaire
- UCLA
University of California, Los Angeles
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- Aben I, Verhey F, Strik J, Lousberg R, Lodder J, Honig A. A comparative study into the one year cumulative incidence of depression after stroke and myocardial infarction. J Neurol Neurosurg Psychiatry. 2003;74:581–5. doi: 10.1136/jnnp.74.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailshire JA, Clarke P. Fine particulate matter air pollution and cognitive function among U.S. older adults. J Gerontol B Psychol Sci Soc Sci. 2015;70:322–8. doi: 10.1093/geronb/gbu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailshire JA, Crimmins EM. Fine particulate matter air pollution and cognitive function among older US adults. Am J Epidemiol. 2014;180:359–66. doi: 10.1093/aje/kwu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beckerman B, Jerrett M, Brook JR, Verma DK, Arain MA, Finkelstein MM. Correlation of nitrogen dioxide with other traffic pollutants near a major expressway. Atmos Environ. 2008;42:275–90. [Google Scholar]
- Block ML, Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32:506–16. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Wu X, Pei Z, Li G, Wang T, Qin L, et al. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004;18:1618–20. doi: 10.1096/fj.04-1945fje. [DOI] [PubMed] [Google Scholar]
- Breteler MM, Claus JJ, Grobbee DE, Hofman A. Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam Study. BMJ. 1994;308:1604–8. doi: 10.1136/bmj.308.6944.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–65. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) The state of aging and health in America. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2013. p. 2013. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) The CDC Health Brain Initiative: Progress 2006–2011. National Center for Chronic Disease Prevention and Health Promotion; 2015. p. 2011. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983:385–96. [PubMed] [Google Scholar]
- Cole DA, Maxwell SE. Testing mediational models with longitudinal data: questions and tips in the use of structural equation modeling. J Abnorm Psychol. 2003;112:558. doi: 10.1037/0021-843X.112.4.558. [DOI] [PubMed] [Google Scholar]
- Copeland MP, Daly E, Hines V, Mastromauro C, Zaitchik D, Gunther J, et al. Psychiatric symptomatology and prodromal Alzheimer's disease. Alzheimer Disease & Associated Disorders. 2003;17:1–8. doi: 10.1097/00002093-200301000-00001. [DOI] [PubMed] [Google Scholar]
- Du Clos TW. Function of C-reactive protein. Ann Med. 2000;32:274–8. doi: 10.3109/07853890009011772. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency (EPA) EPA Air Quality System. 2009;2015 [Google Scholar]
- Gatto NM, Henderson VW, Hodis HN, John JAS, Lurmann F, Chen J, et al. Components of air pollution and cognitive function in middle-aged and older adults in Los Angeles. Neurotoxicology. 2014;40:1–7. doi: 10.1016/j.neuro.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson L, Lindquist L. C-reactive protein: its role in the diagnosis and follow-up of infectious diseases. Curr Opin Infect Dis. 1997;10:196–201. [Google Scholar]
- Harada N, Takeshita J, Ahmed I, Chen R, Petrovitch H, Ross GW, et al. Does cultural assimilation influence prevalence and presentation of depressive symptoms in older Japanese American men? The Honolulu-Asia Aging Study. The American Journal of Geriatric Psychiatry. 2012;20:337–45. doi: 10.1097/JGP.0b013e3182107e3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman CH, Motl RW, Pontifex MB, Posthuma D, Stubbe JH, Boomsma DI, et al. Physical activity and cognitive function in a cross-section of younger and older community-dwelling individuals. Health psychology. 2006;25:678. doi: 10.1037/0278-6133.25.6.678. [DOI] [PubMed] [Google Scholar]
- Interagency Monitoring of Protected Visual Environments (IMPROVE) IMPROVE Homepage. 2013;2015 [Google Scholar]
- Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis. 1972;25:329–43. doi: 10.1016/0021-9681(72)90027-6. [DOI] [PubMed] [Google Scholar]
- Kioumourtzoglou M, Spiegelman D, Szpiro AA, Sheppard L, Kaufman JD, Yanosky JD, et al. Exposure measurement error in PM2. 5 health effects studies: A pooled analysis of eight personal exposure validation studies. Environ Health. 2014;13:2. doi: 10.1186/1476-069X-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–93. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- Kotwal AA, Schumm P, Kern DW, McClintock MK, Waite LJ, Shega JW, et al. Evaluation of a Brief Survey Instrument for Assessing Subtle Differences in Cognitive Function Among Older Adults. Alzheimer Dis Assoc Disord. 2015a;29:317–24. doi: 10.1097/WAD.0000000000000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwal AA, Schumm P, Kern DW, McClintock MK, Waite LJ, Shega JW, et al. Evaluation of a Brief Survey Instrument for Assessing Subtle Differences in Cognitive Function Among Older Adults. Alzheimer Dis Assoc Disord. 2015b;29:317–24. doi: 10.1097/WAD.0000000000000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantella RC, Butters MA, Dew MA, Mulsant BH, Begley AE, Tracey B, et al. Cognitive impairment in late-life generalized anxiety disorder. The American Journal of Geriatric Psychiatry. 2007;15:673–9. doi: 10.1097/JGP.0b013e31803111f2. [DOI] [PubMed] [Google Scholar]
- Mehta AJ, Kubzansky LD, Coull BA, Kloog I, Koutrakis P, Sparrow D, et al. Associations between air pollution and perceived stress: the Veterans Administration Normative Aging Study. Environ Health. 2015;14:1. doi: 10.1186/1476-069X-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore L, Coppedè F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2009;674:73–84. doi: 10.1016/j.mrgentox.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Modrego PJ, Ferrández J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch Neurol. 2004;61:1290–3. doi: 10.1001/archneur.61.8.1290. [DOI] [PubMed] [Google Scholar]
- MohanKumar SM, Campbell A, Block M, Veronesi B. Particulate matter, oxidative stress and neurotoxicity. Neurotoxicology. 2008;29:479–88. doi: 10.1016/j.neuro.2007.12.004. [DOI] [PubMed] [Google Scholar]
- O’Muircheartaigh C, Eckman S, Smith S. Statistical Design and Estimation for the National Social Life, Health, and Aging Project. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2009;64B:i12–9. doi: 10.1093/geronb/gbp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek CJ, Yanosky JD, Puett RC, Laden F, Suh HH. Practical large-scale spatio-temporal modeling of particulate matter concentrations. The Annals of Applied Statistics. 2009:370–97. [Google Scholar]
- Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–61. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- Power MC, Adar SD, Yanosky JD, Weuve J. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: A systematic review of epidemiologic research. Neurotoxicology. 2016 doi: 10.1016/j.neuro.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power MC, Weisskopf MG, Alexeeff SE, Coull BA, Spin A, III, Schwartz J. Traffic-related air pollution and cognitive function in a cohort of older men. Environ Health Perspect. 2011;19:682. doi: 10.1289/ehp.1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power MC, Kioumourtzoglou MA, Hart JE, Okereke OI, Laden F, Weisskopf MG. The relation between past exposure to fine particulate air pollution and prevalent anxiety: observational cohort study. BMJ. 2015;350:h1111. doi: 10.1136/bmj.h1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun VC, Manjourides J, Suh H. Association of Ambient Air Pollution with Depressive and Anxiety Symptoms in Older Adults: Results from the NSHAP Study. Environ Health Perspect. 2016 doi: 10.1289/EHP494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranft U, Schikowski T, Sugiri D, Krutmann J, Krämer U. Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. Environ Res. 2009;109:1004–11. doi: 10.1016/j.envres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Roshanaei-Moghaddam B, Katon WJ, Russo J. The longitudinal effects of depression on physical activity. Gen Hosp Psychiatry. 2009;31:306–15. doi: 10.1016/j.genhosppsych.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Sachs GA, Carter R, Holtz LR, Smith F, Stump TE, Tu W, et al. Cognitive impairment: an independent predictor of excess mortality: a cohort study. Ann Intern Med. 2011;155:300–8. doi: 10.7326/0003-4819-155-5-201109060-00007. [DOI] [PubMed] [Google Scholar]
- Schram MT, Euser SM, De Craen AJ, Witteman JC, Frölich M, Hofman A, et al. Systemic markers of inflammation and cognitive decline in old age. J Am Geriatr Soc. 2007;55:708–16. doi: 10.1111/j.1532-5415.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- Scott KM, Von Korff M, Ormel J, Zhang M, Bruffaerts R, Alonso J, et al. Mental disorders among adults with asthma: results from the World Mental Health Survey. Gen Hosp Psychiatry. 2007;29:123–33. doi: 10.1016/j.genhosppsych.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociological methodology. 1982;13:290–312. [Google Scholar]
- Suh HH, Zanobetti A. Exposure error masks the relationship between traffic-related air pollution and heart rate variability. J Occup Environ Med. 2010;52:685–92. doi: 10.1097/JOM.0b013e3181e8071f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson EM, Vladisavljevic D, Mohottalage S, Kumarathasan P, Vincent R. Mapping acute systemic effects of inhaled particulate matter and ozone: multiorgan gene expression and glucocorticoid activity. Toxicol Sci. 2013;135:169–81. doi: 10.1093/toxsci/kft137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- Tomei F, Rosati MV, Ciarrocca M, Baccolo TP, Gaballo M, Caciari T, et al. Plasma cortisol levels and workers exposed to urban pollutants. Ind Health. 2003;41:320–6. doi: 10.2486/indhealth.41.320. [DOI] [PubMed] [Google Scholar]
- Tonne C, Elbaz A, Beevers S, Singh-Manoux A. Traffic-related air pollution in relation to cognitive function in older adults. Epidemiology. 2014;25:674–81. doi: 10.1097/EDE.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Eliot MN, Koutrakis P, Gryparis A, Schwartz JD, Coull BA, et al. Ambient air pollution and depressive symptoms in older adults: results from the MOBILIZE Boston study. 2014 doi: 10.1289/ehp.1205909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenius GA, Boyle LD, Coull BA, Milberg WP, Gryparis A, Schwartz J, et al. Residential Proximity to Nearest Major Roadway and Cognitive Function in Community- Dwelling Seniors: Results from the MOBILIZE Boston Study. J Am Geriatr Soc. 2012;60:2075–80. doi: 10.1111/j.1532-5415.2012.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med. 2012;172:219–27. doi: 10.1001/archinternmed.2011.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64:234–40. doi: 10.1001/archpsyc.64.2.234. [DOI] [PubMed] [Google Scholar]
- Windgassen EB, Funtowicz L, Lunsford TN, Harris LA, Mulvagh SL. C-reactive protein and high-sensitivity C-reactive protein: an update for clinicians. Postgrad Med. 2015 doi: 10.3810/pgm.2011.01.2252. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Blackwell T, Gore R, Sands L, Reus V, Browner WS. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry. 1999;56:425–30. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]
- Yanosky JD, Paciorek CJ, Laden F, Hart JE, Puett RC, Liao D, et al. Spatio-temporal modeling of particulate air pollution in the conterminous United States using geographic and meteorological predictors. Environ Health. 2014;13:63. doi: 10.1186/1476-069X-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


