Abstract
Signaling networks that regulate cellular proliferation often involve complex interactions between several signaling pathways. In this manuscript we review the crosstalk between the Casein Kinase II (CK2) and Glycogen Synthase Kinase-3 (GSK-3) pathways that plays a critical role in the regulation of cellular proliferation in leukemia. Both CK2 and GSK-3 are potential targets for anti-leukemia treatment. Previously published data suggest that CK2 and GSK-3 act synergistically to promote the phosphatidylinositol-3 kinase (PI3K) pathway via phosphorylation of PTEN. More recent data demonstrate another mechanism through which CK2 promotes the PI3K pathway – via transcriptional regulation of PI3K pathway genes by the newly-discovered CK2-Ikaros axis. Together, these data suggest that the CK2 and GSK-3 pathways regulate AKT/PI3K signaling in leukemia via two complementary mechanisms: a) direct phosphorylation of PTEN and b) transcriptional regulation of PI3K-promoting genes. Functional interactions between CK2, Ikaros and GSK3 define a novel signaling network that regulates proliferation of leukemia cells. This regulatory network involves both direct posttranslational modifications (by CK and GSK-3) and transcriptional regulation (via CK2-mediated phosphorylation of Ikaros). This information provides a basis for the development of targeted therapy for leukemia.
Keywords: GSK-3, Ikaros, Casein kinase II (CK2), Leukemia, CX4945, Phosphorylation, Targeted therapy
1. Introduction
Casein kinase II (CK2) and Glycogen Synthase Kinase-3 (GSK-3) are two ubiquitous, highly expressed serine/threonine kinases that are involved in the regulation of multiple pathways (Pinna, 1994; Woodgett, 1990). Over the last several decades, tremendous advances have been made in understanding the biochemical and biological functions of these proteins in physiological and pathological conditions. Although these studies produced a wealth of knowledge and provided novel insights into a number of physiological processes, there are many unanswered questions regarding the roles of these enzymes in health and disease. More recently, the development of inhibitors for these kinases provided the opportunity to modify their activity as a therapeutic strategy for various diseases. Since both CK2 and GSK-3 regulate pathways that are essential for cellular proliferation, it is not surprising that inhibitors of these enzymes were tested first as potential therapeutic agents for malignant diseases. The initial success of these inhibitors in preclinical studies further enhanced interest in the function of CK2 and GSK-3 in regulating cellular proliferation. The recent discovery of a novel CK2-Ikaros signaling axis and its role in the regulation of the phosphatidylinositol 3-kinase (PI3K) pathway in leukemia (Song et al., 2015), along with the known role of GSK-3 in regulating the function of key proteins in PI3K pathway (Al-Khouri et al., 2005; Cordier et al., 2012; Maccario et al., 2007; McCubrey et al., 2015), shed new light on the role of CK2 and GSK-3 in cellular proliferation in leukemia. The purpose of this review is to briefly summarize current knowledge of the function of CK2 and GSK-3, and to highlight several interactions between CK2 and GSK-3-regulated signaling pathways that are relevant for malignant diseases with an emphasis on novel discoveries regarding the role of the CK2-Ikaros axis and GSK-3 in regulating the PI3K pathway in leukemia.
2. Casein Kinase II (CK2)
Casein Kinase II (CK2) is a ubiquitous serine/threonine-selective pro-oncogenic protein kinase that has become a more prominent target for research due to its effects and key role in tumorigenesis (Gowda, C. et al., 2017a; Pinna, 2002). CK2, a well-conserved, pleiotropic kinase, phosphorylates a variety of substrates that are implicated in gene expression, signal transduction, and other nuclear functions (Meggio and Pinna, 2003). Casein Kinase II was initially identified as an essential protein that phosphorylates casein in vitro in 1954, but it was later shown that casein is not one of its immediate physiological substrates as previously thought (Pinna, 1994). CK2 kinase has a unique structure and is the only protein in the kinase family to have more than three consecutive basic amino acids; interestingly, it has a stretch of six basic amino acids that are responsible for the binding of CK2β (Pinna, 1990).
2.1. CK2 function and structure
In humans, CK2 is encoded at 4 genomic loci, however, one of the loci is not transcribed; the 3 transcribed genomic loci are located on chromosomes 20, 16, and 6. The subunits encoded on these chromosomes, respectively, are referred to as the α subunit, the α′ subunit (catalytic subunits), and the β subunit, (regulatory subunit) (Ackermann et al., 2005). Potential unique roles of the α and α′ catalytic subunits have not been widely studied, however they are approximately 75% identical (Cozza et al., 2013).
CK2 is comprised of a tetramer consisting of two catalytic α and/or α′ subunits and two regulatory β subunits. The catalytic α and α′ subunits are 391 and 350 amino acids long (130 kDa), respectively, compared to the regulatory β subunit (25 kDa), which is only 215 amino acids long (Krehan et al., 1998). Three permutations of the catalytic and regulatory subunits, α2β2, αα′β2, and α′2 β2, can be utilized to form the heterotrimeric complexes (Cozza et al., 2013).
Although CK2 activity does not depend on small molecules involved in second-messenger kinase activation, specific negatively charged compounds, such as heparin, can inhibit CK2, while positively charged compounds, such as polyamines, activate CK2 (Shore et al., 1997; Tuazon and Traugh, 1991).
2.2. CK2 in oncogenesis
The ubiquitous and pleiotropic nature of CK2 suggest its physiological significance (Meggio and Pinna, 2003; Ruzzene et al., 2017), and have encouraged a number of studies focused on the identification of potential substrates that play important roles in the progression and processes of various diseases, particularly cancer.
Previous in vitro studies have confirmed the role of CK2 protein substrates as key regulators of gene expression, and protein synthesis, as well as DNA repair (Meggio and Pinna, 2003). CK2 has been shown to control cell growth and proliferation by regulating cell cycle progression (Pinna and Meggio, 1997). CK2 also phosphorylates key proteins that possess anti-apoptotic functions, thus suppressing cellular apoptosis (Gray et al., 2014). The ability of CK2 to override cellular apoptotic signaling suggests a role in tumorigenesis and CK2 has been shown to aggressively increase tumor growth in most, if not all, cancer cells tested thus far (Guerra and Issinger, 1999; Meggio and Pinna, 2003; Nelson et al., 2017).
Consistent with a role for CK2 in oncogenesis, increased levels of CK2 have been widely observed in various tumors, including lung, breast, prostate, head and neck, and colon cancers (Bliesath et al., 2012; Gray et al., 2014; Ruzzene et al., 2017; Wang et al., 2006; Yu et al., 2006; Zhang et al., 2009).
2.3. CK2 in leukemia
Overexpression of CK2α and CK2β in hematological malignancies, has been confirmed in several malignancies including B-precursor lymphoblastic leukemia (B-ALL), chronic lymphocytic leukemia (CLL), acute myeloid leukemia (AML), and mature B-cell neoplasms (Ge et al., 2016b; Martins et al., 2010; Pizzi et al., 2015; Quotti Tubi et al., 2013; Scaglioni et al., 2008). In all of the preceding tumors mentioned, further analysis showed a strong correlation between increased level of CK2α and poor clinical outcome. Moreover, in patients with karyotypically normal AML, levels of CK2α provide a prognostic indicator (Kim et al., 2007).
2.4. CK2 as a therapeutic target in leukemia and other cancers
CK2 inhibition, via CK2-specific inhibitors, such 4,5,6,7-tetrabromobenzotriazole (TBB), CX-4945, apigenin, and molecular degeneration utilizing antisense CK2 and siRNA, has been shown to significantly decrease death receptor ligands in tumor cells of various cancers, such as breast, prostate, and lung cancer, as well as leukemia (Okoumassoun et al., 2007; Wang et al., 2005).
CX-4945 (Silmitasertib) is a potent and selective CK2-specific inhibitor that can be orally administered to patients. CX-49495 serves as an ATP-competitive inhibitor for catalytic CK2α and CK2α′ subunits (Battistutta et al., 2011; Pierre et al., 2011; Siddiqui-Jain et al., 2010). Leukemia cells treated with CX-49495 are observed to display a significant decrease in cellular growth and in vivo preclinical models of leukemia show prolonged survival with CX-4945 treatment (Chon et al., 2015; Pierre et al., 2011; Song et al., 2015). Furthermore, the inhibition of CK2 by CX-4945 enhances the regulatory functions of the Ikaros tumor (Pierre et al., 2011; Siddiqui-Jain et al., 2012) suppressor which will be further discussed below.
The treatment of CML cells with CX-4945 inhibits the interaction between CK2 and BCR-ABL1, an oncoprotein expressed in hematopoietic stems cells and differentiates myeloid and lymphoid progeny, which suggests its strong efficacy as a monotherapy (Mishra et al., 2007). Interestingly, CX-4945 has been identified to express synergism in combination with several drugs including imatinib, a protein-tyrosine kinase inhibitor, to significantly decrease cell viability (Mishra et al., 2007). The overexpression of CK2 is becoming a hallmark of ALL and therefore serves as an important therapeutic target, via CK2-specific inhibitors, in T-cell and B-cell ALL (Buontempo et al., 2014; Ge et al., 2017; Gowda, C. et al., 2017a; Li et al., 2012; Song et al., 2015).
3. GSK-3
Glycogen synthase kinase-3 (GSK-3) is a ubiquitous serine/threonine kinase that is involved in multiple signaling pathways that are crucial for cellular metabolism and proliferation (Doble and Woodgett, 2003; Frame and Cohen, 2001; Grimes and Jope, 2001; Woodgett, 1990). GSK-3 is known to directly phosphorylate at least 40 substrates, although the actual number of substrates is probably much larger (Linding et al., 2007; Sutherland, 2011). Advances in understanding the role of GSK-3 in cellular regulation revealed that this kinase has an important role in the regulation of Wnt, Notch, hedghog, nuclear factor of activated T cells (NF-AT), cyclic adenosine monophosphate (cAMP) and phosphatidylinositol 3-kinase (PI3K) (Frame and Cohen, 2001; Grimes and Jope, 2001). GSK-3 protein can exist in two forms (GSK-3α and GSK-3β) that are encoded by two separate genes (Woodgett, 1990). These GSK-3 proteins can be regulated by distinct post-translational mechanisms involving amino acids that are unique for each protein. Phosphorylation of GSK-3β at serine 389 (S389) by p38 mitogen-activated protein kinase (MAPK) in response to DNA double-strand breaks, inhibits activity of this protein in thymus (Thornton et al., 2008, 2016). Since S389 is absent in GSK-3α, these data reveal distinct functions and regulatory networks for the two GSK3 proteins. Further, GSK-3α, but not GSK-3β has been shown to function as a suppressor of aging and plays a role in atherosclerosis (Banko et al., 2014; Zhou et al., 2013).
3.1. GSK3 in cancer
The role of GSK-3 in cancer is much less clear than that of CK2. While CK2 is a known oncogene, it appears that GSK-3 can act both as a tumor suppressor or an oncogene, depending on context (Beurel et al., 2004; Fitzgerald et al., 2015; McCubrey et al., 2014; Takahashi-Yanaga, 2013). Early results showed that GSK3 negatively regulates the Wnt signaling pathway (Patel et al., 2004). Due to a proven role for Wnt in carcinogenesis, GSK-3 was considered to function as a tumor suppressor (Ruvolo, 2017). However, the discovery that GSK-3β knockout mice die in utero due to impaired NF-kB activation leading to apoptosis of liver cells (Hoeflich et al., 2000), revealed a role for GSK-3β in NF-kB activation and in apoptosis. Further studies in hematopoietic malignancies, like B-cell chronic lymphocytic leukemia (B-CLL) and acute myeloid leukemia (AML), established that GSK-3β has a pro-survival function and positively regulates cellular proliferation and drug resistance in leukemia (Beurel et al., 2004, 2010; De Toni et al., 2006; Ougolkov et al., 2007).
The above results led to the development of several inhibitors of GSK-3 and their testing against diseases with known dysregulation of GSK-3 (Licht-Murava et al., 2016; Plotkin et al., 2003; Ricciardi et al., 2017). While most of the positive results have been achieved in the preclinical setting, one compound (tideglusib) has reached Phase II trial for Alzheimer’s disease (Lovestone et al., 2015). Clearly, due to the complex function of GSK-3 in malignant diseases, testing of this type of drug will proceed slowly and with additional precautions. However, strong pre-clinical data support further studies of uses for GSK-3 inhibitors in the treatment of malignant diseases.
3.2. Cross-talk between CK2 and GSK-3 signaling pathways in malignancy
Wnt/β-catenin. Since both CK2 and GSK-3 are abundant, highly promiscuous kinases, it is of little surprise that many pathways regulated by these kinases interact with each other, forming a complex regulatory network. Due to the complex function of GSK-3, in some of these pathways CK2 and GSK-3 act synergistically, while in the others they have an opposing effect. One of the first pathways shown to be highly relevant for malignant transformation and cellular proliferation was the Wnt signaling pathway. The work of Seldin’s group showed that the Wnt pathway is positively regulated by CK2 (Song et al., 2003). Specifically, the Wnt pathway intermediate Phosphoprotein ‘Dishevelled’ (Dsh) is a direct substrate for CK2 (Song et al., 2003). A major component of the Wnt/β-catenin pathway, β-catenin, is directly phosphorylated by CK2 in vivo (Song et al., 2000). Functional studies using a β-catenin mutant that is resistant to CK2 phosphorylation showed that phosphorylation by CK2 promotes β-catenin protein stability (Song et al., 2000). These data provided mechanistic support for a positive regulatory role for CK2 in Wnt/β-catenin signaling. Since it has been previously demonstrated that GSK-3 suppresses Wnt/β-catenin pathway, this is an example of an opposing effect of CK2 and GSK-3 on malignant transformation. The role of both the Wnt/β-catenin pathway and CK2 in mammary carcinoma has been strongly suggested by experimental data (Landesman-Bollag et al., 2001a, 2001b; Rosner et al., 2002). Thus, in mammary gland carcinogenesis, GSK-3 will have a tumor suppressor role and oppose the oncogenic effect of CK2 via the Wnt/β-catenin pathway.
3.3. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN)
PTEN is a dual-specificity protein phosphatase (Maehama and Dixon, 1998). The main function of PTEN is to catalyze dephosphorylation of phosphatidylinositol 3,4,5-triphosphate (PIP3) into phosphatidylinositol 4,5-biphosphate (PIP2) (Maehama and Dixon, 1998; Stambolic et al., 1998). This process directly opposes the PI3K/AKT signal transduction pathway. The PI3K pathway is a major driver of cellular proliferation and survival, and has an important role in regulating differentiation, and cellular metabolism (Follo et al., 2015; Luo et al., 2003; Song et al., 2012; Vivanco and Sawyers, 2002). Mutations resulting in the loss of PTEN functions have been detected in various types of human malignancies (Fragoso and Barata, 2014; Li et al., 1997; Sansal and Sellers, 2004; Steck et al., 1997). Mice that are heterozygous for the loss of PTEN develop various malignancies, along with T-cell leukemia and lymphoma (Di Cristofano et al., 1998; Jotta et al., 2010; Podsypanina et al., 1999; Suzuki et al., 1998). The function of PTEN protein is regulated by posttranslational modifications that include phosphorylation by multiple kinases (Al-Khouri et al., 2005; Leslie et al., 2012; Torres and Pulido, 2001; Vazquez et al., 2000). CK2 phosphorylates PTEN at 5 different amino acids (Torres and Pulido, 2001). CK2-mediated phosphorylation of PTEN results in increased stability of the PTEN protein, but also in reduced PTEN activity toward PIP3 (Miller et al., 2002). CK2-mediated phosphorylation of PTEN also occurs during T-cell receptor (TCR) response resulting in reduced PTEN activity and increased protein stability (Patsoukis et al., 2013). A series of elegant experiments done by Barata’s group showed that the increased expression and activity of CK2 that is observed in leukemia results in inhibition of PTEN activity and in increased activation of the PI3K/AKT signaling pathway (Leslie et al., 2012; Martins et al., 2014a; Silva et al., 2010; Silva et al., 2008). The use of a specific CK2 inhibitor negatively regulates PI3K/AKT signaling (Barata, 2011; Gomes et al., 2014; Martins et al., 2014a, 2010a, 2014b).
A separate experimental approach identified PTEN as a direct substrate for GSK-3 kinase (Al-Khouri et al., 2005). GSK3β phosphorylates PTEN at two residues that do not overlap with the 5 residues that are phosphorylated by CK2 (Al-Khouri et al., 2005; Maccario et al., 2007). Functional studies determined that GSK-3-mediated phosphorylation of PTEN reduces its activity, although its effect on PTEN protein stability is less clear and might be cell-specific (Al-Khouri et al., 2005; Maccario et al., 2007). Experiments that utilized NMR spectroscopy, determined that CK2 and GSK3β likely phosphorylate PTEN synergistically and that the phosphorylation cascade is dependent on the activity of both kinases (Cordier et al., 2012). Considering the recently-discovered oncogenic role of GSK3β in leukemia, it is quite possible that both CK2 and GSK3β have synergistic, oncogenic roles in leukemia (contrary to the opposing role in mammary carcinogenesis described above). These examples illustrate the complexity of signaling networks regulated by CK2 and GSK-3 and indicate that more studies are necessary to understand the role of these kinases in human malignancies.
4. IKZF1 (Ikaros)
The IKZF1 gene encodes Ikaros, a kruppel-like zinc finger DNA-binding protein that functions as a master regulator of hematopoiesis (Georgopoulos et al., 1992, 1994; Lo et al., 1991). The absence of Ikaros has a detrimental effect on normal hematopoiesis as evidenced by the loss of B, NK, and dendritic cells as well as reduced T cells (Cortes et al., 1999; Georgopoulos et al., 1994). The critical role of Ikaros in the immune system (Avitahl et al., 1999; Ernst et al., 1993), as well as in myeloid differentiation (Dumortier et al., 2003) has been proven. The role of Ikaros, as a tumor suppressor was first identified in 1994 in Ikaros haplo-knockout mice (Georgopoulos et al., 1994; Winandy et al., 1995). Mice that are missing one copy of Ikaros develop T-cell leukemia with 100% penetrance (Winandy et al., 1995). Reintroduction of Ikaros into these leukemia cells results in cessation of cell growth and partial induction of T-cell differentiation (Kathrein et al., 2005).
The deletion of Ikaros in humans has been directly associated with the development of high-risk leukemia (Mullighan et al., 2007) and primary immunodeficiency diseases. IKZF1 deletion has been linked with an increase in relapse rate of up to 12-fold in acute lymphoblastic leukemia (Kuiper et al., 2010). Among B-ALL, a deletion of one IKZF1 alelle is found in approximately 80% of BCR-ABL1+ ALL (Mullighan et al., 2008) and Ph-like ALL (Den Boer et al., 2009) as well as ~20% of patients that are BCR-ABL negative (Mullighan et al., 2007). Approximately 9% of T-cell ALL (Zhang et al., 2012) and 11% of early precursor T cell ALL (ETP-ALL) show mutation or inactivation of one IKZF1 allele (Zhang et al., 2012). Germline mutation of IKZF1 has also been associated with congenital pancytopenia (Goldman et al., 2012).
Through the process of alternative splicing, the IKZF1 gene is capable of encoding a large number of Ikaros isoforms (Molnar et al., 1996). Some of these isoforms were shown to have distinct functions (Li et al., 2011; Ronni et al., 2007). Ikaros protein contains four zinc fingers at the N-terminus that directly interacts with DNA and determine DNA-binding affinity and specificity of Ikaros, and two zinc fingers at the C-terminus that participate in protein-protein interactions (Molnár and Georgopoulos, 1994). The protein-protein interactions include the formation of dimers with other Ikaros isoforms or the other members of Ikaros family proteins (Li et al., 2011; Molnár and Georgopoulos, 1994). Isoforms of Ikaros that lack DNA-binding zinc fingers can form a functionally inactive complex that can impair function of the full-length Ikaros (Sun et al., 1996).
Ikaros utilizes chromatin remodeling to activate or repress the transcription of its target genes (Su et al., 2004). Ikaros directly associates with histone deacetylases HDAC1 and HDAC2 and can recruit them to the upstream regulatory elements of its target genes (Kim et al., 1999; Koipally et al., 1999a, 1999b). The ability of Ikaros to regulate transcription of its target genes is often dependent on its ability to localize to pericetromeric heterochromatin (Brown et al., 1997; Cobb et al., 2000; Liberg et al., 2003). Ikaros binds to the upstream regulatory element (URE) of its target genes and assists in their recruitment to pericentromeric heterochromatin (Brown et al., 1997).
4.1. Regulation of Ikaros by phosphorylation
Ikaros protein is phosphorylated at multiple sites (Dovat et al., 2002). The functional significance of Ikaros phosphorylation was first demonstrated by analysis of Ikaros phosphorylation during the cell cycle. These data showed that Ikaros undergoes hyperphosphorylation during mitosis at an evolutionarily-conserved linker that connects DNA-binding zinc finger motifs (Dovat et al., 2002). Phosphorylation during mitosis inhibits Ikaros’ ability to bind DNA and is likely a global control mechanism for DNA binding of C2H2 zinc finger proteins during mitosis. Subsequent analyses revealed that Ikaros is extensively phosphorylated at multiple, evolutionarily-conserved serine and threonine residues by Casein Kinase II (CK2) (Gurel et al., 2008). Functional analysis using Ikaros mutants with phosphomimetic and phosphoresistant mutations at CK2 target sites demonstrated that phosphorylation by CK2 impairs Ikaros DNA-binding affinity and localization to pericentromeric heterochromatin (Gurel et al., 2008). Since both of these Ikaros abilities are part of its function as a transcriptional regulator, these data suggest that CK2 has an important role in controlling Ikaros function in regulating transcripltion and as a tumor suppressor. CK2-mediated phosphorylation of Ikaros is cell cycle specific, suggesting a role for CK2 in regulating Ikaros function during G1/S transition (Gomez-del Arco et al., 2004), as well as during S phase in human leukemia (Li et al., 2012). Phosphorylation of Ikaros by CK2 was demonstrated to play a critical role in transcriptional regulation of the terminal deoxytransferase (TdT) gene during thymocyte differentiation (Wang et al., 2014).
The role of phosphorylation in regulating Ikaros function was further supported by the discovery that Ikaros is a substrate for protein phosphatase 1 (PP1), a tumor suppressor, that has strong activity in the nucleus and that can regulate chromatin remodeling (Popescu et al., 2009). An Ikaros mutant that cannot associate with PP1, undergoes hyperphosphorylation, resulting in a complete loss of its DNA binding ability, loss of pericentromeric localization and increased protein degradation via the ubiquitin pathway. The introduction of phosphoresistant mutations at CK2 sites, enhances Ikaros protein stability, partially restores its DNA-binding affinity and restores its localization to pericentromeric heterochromatin (Popescu et al., 2009). These experiments demonstrate the significance of CK2-mediated phosphorylation in Ikaros function and established CK2 ad PP1 as proteins that have important roles in regulating Ikaros activity in cells (Song et al., 2011). Given that 1) CK2 is strongly overexpressed in human malignancies, including leukemia, and 2) CK2 overexpression in the T-cell lineage results in a T-ALL, that is phenotypically similar to T-ALL in Ikaros haplo-knockout mice, it was hypothesized that CK2 might be one of the primary regulators of Ikaros function in leukemia (Dovat et al., 2011; Payne and Dovat, 2011; Song et al., 2011).
4.2. CK2-Ikaros axis in leukemia
To understand the role of Ikaros in regulating gene expression, extensive studies were done to identify Ikaros target genes in leukemia. Global Ikaros DNA-binding was determined using chromatin immunoprecipitation followed by next-generation sequencing (ChIP-seq) in primary B-ALL cells and a B-ALL cell line (Song et al., 2015). Results showed that Ikaros binds to promoter regions of a large number of genes that regulate cellular proliferation, including multiple genes that promote cell cycle progression (Song et al., 2015). Detailed functional analyses demonstrated that Ikaros can directly activate or represses its target genes that function as tumor suppressors or oncogenes, and that Ikaros-mediated transcriptional regulation of gene expression controls cellular proliferation. Additional analyses showed that Ikaros regulates expression of its target genes via chromatin remodeling, which in some cases involves recruitment of histone deacetylase 1 (HDAC1) to promoter regions of its target genes (Song et al., 2016).
Somewhat unexpectedly, ChIP-seq analysis revealed that Ikaros binds to the promoters of many genes that have a critical role in the PI3K pathway (Song et al., 2015). Gain-of-function and loss-of-function experiments showed that Ikaros directly represses a large number of genes that promote the PI3K pathway (e.g. PIK3CD, PI4KB etc.) at multiple distinct steps, while it activates expression of the gene that opposes the PI3K pathway (INPPD5). The overexpression of Ikaros in leukemia cells results in inhibition of the AKT-PI3K pathway, which was evidenced by reduced phosphorylation of AKT (Song et al., 2015). In fact, overexpression of Ikaros reduced AKT phosphorylation in leukemia cells equally well as treatment with Imatinib. These data provide strong support for Ikaros function as a repressor of the AKT/PI3K pathway.
Since it was known that CK2 impairs Ikaros function by direct phosphorylation (summarized above), and CK2 is over-expressed in B-ALL, the effect of CK2 inhibition on expression of Ikaros target genes was analyzed. Results showed that both molecular and pharmacological inhibition of CK2 has the same effect on expression of Ikaros target genes (activating or repressing) as does the overexpression of Ikaros. The critical question was whether the effect of CK2 inhibition on expression of Ikaros target genes is dependent on Ikaros function. Data showed that Ikaros knock-down with shRNA abolished the ability of CK2 inhibitors to regulate expression of Ikaros target genes both in vitro and in vivo (Song et al., 2015). These data proved that transcriptional regulation of a large number of PI3K genes by CK2 inhibitors in leukemia is Ikaros-dependent. This established the existence of a novel CK2-Ikaros signaling axis as a regulator of the PI3K pathway in leukemia. The ability of CK2-Ikaros axis to regulate expression of Ikaros target genes was demonstrated even in high-risk B-ALL that are Ikaros haploinssufficient due to deletion of one Ikaros allele. Ikaros activity in B-ALL with deletion of one Ikaros allele is severely impaired. However, the treatment of Ikaros-haploinsuffucient B-All cells with CK2 specific inhibitor restores Ikaros function as transcriptional regulator of its target genes and inhibits the PI3K pathway (Song et al., 2015).
Subsequent work confirmed the presence of the CK2-Ikaros signaling axis, and its role in regulating expression of a large number of tumor promoters and suppressors in pediatric and adult leukemia (Ge et al., 2015, 2016a, 2016b, 2016c, 2016d, 2017; Wang et al., 2016). Epigenetic analysis of the promoters of Ikaros target genes showed that CK2 regulates not only Ikaros DNA-binding affinity to promoters of its target genes, but also Ikaros-mediated recruitment of HDAC1, as well as the epigenetic signature at regulatory elements of Ikaros targets (Song et al., 2016).
The above-described studies demonstrate the existence of a novel signal transduction pathway – a CK2-Ikaros axis that regulates cell cycle, the PI3K pathway, expression of individual oncogenes and tumor suppressors, and the epigenetic landscape (Gowda et al., 2017b; Gowda, C.S. et al., 2016). These results revealed additional mechanisms through which CK2 regulates the PI3K pathway – by regulating expression of genes that are critical for the PI3K pathway, via the CK2-Ikaros axis. Future experiments aimed at determining additional gene targets for the CK-Ikaros axis, as well as the other cellular functions that might be regulated by this pathway, will be important for the development of targeted combination therapies for the treatment of leukemia.
5. Conclusion
Interactions between different signaling pathways are often complex and dependent on physiological condition and cell type. The cross-talk between CK2 and GSK-3 pathways are similarly complicated. Recent data concerning the role of CK2 and GSK3 in the regulation of PTEN and particularly in regulating the expression of PI3K-promoting genes by the CK2-Ikaros axis, have provided new insights into the regulation of cellular proliferation by CK2 and GSK-3. Interestingly, it appears that two distinct signaling pathways, CK2 and GSK-3, converge to regulate a third pathway, the AKT/PI3K pathway, using two distinct and complementary mechanisms.
The first mechanism involves direct phosphorylation of target proteins (phosphorylation of PTEN by CK2 and GSK-3). The second mechanism involves transcriptional regulation of PI3K-promoting genes (via CK2-mediated phosphorylation of Ikaros) (Fig. 1). The role of Ikaros in regulating the PI3K pathway illustrates a major distinction from the typical cross-talk between two signaling pathways that involves only posttranslational modifications of the same target proteins.
Fig. 1.
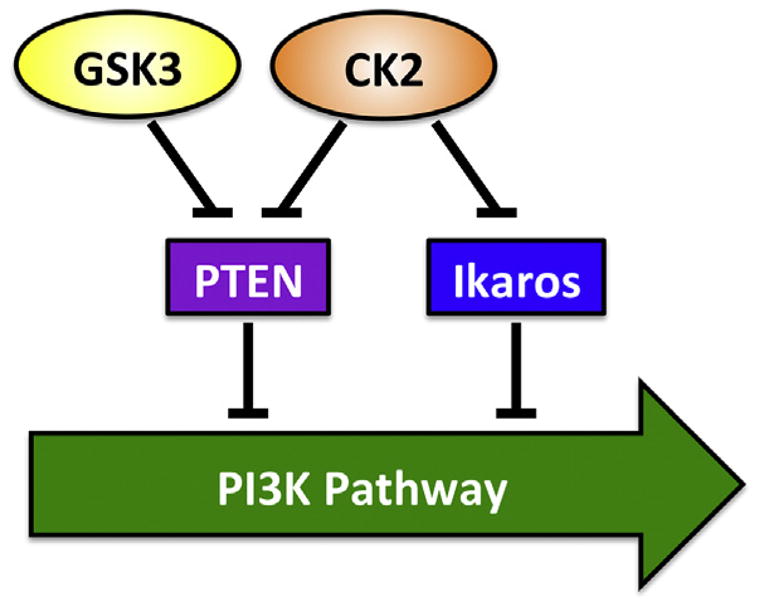
Current model of CK2, Ikaros, and GSK-3 network in the regulation of the PI3K pathway in leukemia.
The translational significance of this complex interaction is multiple: 1) It provides a mechanistic explanation for why Ikaros haploinsufficiency in high-risk leukemia results in aggressive disease; 2) It provides a rationale for the use of CK2 inhibitors in hematopoietic malignancies that have deletion and/or mutation of PTEN, since restoration of Ikaros function following CK2 inhibition will result in suppression of the PI3K pathway; and 3) Functional interaction between CK2, Ikaros and GSK-3 represents a paradigm of a more complex signaling network that is not limited to “classic” signaling proteins, but also involves transcriptional regulation, along with epigenetic modifications. As technical advances allow detailed studies of gene expression, we can expect to see additional examples of signaling interactions that involve different levels of cross-talk that include transcriptional factors. This will advance our understanding of the signaling mechanisms that regulate cellular proliferation and cellular metabolism, which will help in designing targeted therapies for human diseases.
Acknowledgments
This work has been supported by Hyundai Hope on Wheels Scholar Grant Award, Alex’s Lemonade Stand Grant, Bear Necessities Pediatric Cancer Foundation, the Four Diamonds Fund of the Pennsylvania State University, College of Medicine, and the John Wawrynovic Leukemia Research Scholar Endowment (SD); by NIH R01 CA209829 (SD and KJP), by St. Baldrick’s Foundation Fellows Award and Hyundai Hope on Wheels Fellowship Grant Award (CG).
Footnotes
Conflict of interest
Authors declare no financial or research conflict of interest related to this work.
References
- Ackermann K, Neidhart T, Gerber J, Waxmann A, Pyerin W. The catalytic subunit alpha’ gene of human protein kinase CK2 (CSNK2A2): genomic organization, promoter identification and determination of Ets1 as a key regulator. Mol Cell Biochem. 2005;274(1–2):91–101. doi: 10.1007/s11010-005-3076-2. [DOI] [PubMed] [Google Scholar]
- Al-Khouri AM, Ma Y, Togo SH, Williams S, Mustelin T. Cooperative phosphorylation of the tumor suppressor phosphatase and tensin homologue (PTEN) by casein kinases and glycogen synthase kinase 3beta. J Biol Chem. 2005;280(42):35195–35202. doi: 10.1074/jbc.M503045200. [DOI] [PubMed] [Google Scholar]
- Avitahl N, Winandy S, Friedrich C, Jones B, Ge Y, Georgopoulos K. Ikaros sets thresholds for T cell activation and regulates chromosome propagation. Immunity. 1999;10(3):333–343. doi: 10.1016/s1074-7613(00)80033-3. [DOI] [PubMed] [Google Scholar]
- Banko NS, McAlpine CS, Venegas-Pino DE, Raja P, Shi Y, Khan MI, Werstuck GH. Glycogen synthase kinase 3alpha deficiency attenuates atherosclerosis and hepatic steatosis in high fat diet-fed low density lipoprotein receptor-deficient mice. Am J Pathol. 2014;184(12):3394–3404. doi: 10.1016/j.ajpath.2014.07.028. [DOI] [PubMed] [Google Scholar]
- Barata JT. The impact of PTEN regulation by CK2 on PI3K-dependent signaling and leukemia cell survival. Adv Enzyme Regul. 2011;51(1):37–49. doi: 10.1016/j.advenzreg.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Battistutta R, Cozza G, Pierre F, Papinutto E, Lolli G, Sarno S, O’Brien SE, Siddiqui-Jain A, Haddach M, Anderes K, Ryckman DM, Meggio F, Pinna LA. Unprecedented selectivity and structural determinants of a new class of protein kinase CK2 inhibitors in clinical trials for the treatment of cancer. Biochemistry. 2011;50(39):8478–8488. doi: 10.1021/bi2008382. [DOI] [PubMed] [Google Scholar]
- Beurel E, Kornprobst M, Blivet-Van Eggelpoel MJ, Ruiz-Ruiz C, Cadoret A, Capeau J, Desbois-Mouthon C. GSK-3beta inhibition by lithium confers resistance to chemotherapy-induced apoptosis through the repression of CD95 (Fas/APO-1) expression. Exp Cell Res. 2004;300(2):354–364. doi: 10.1016/j.yexcr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3) Trends Immunol. 2010;31(1):24–31. doi: 10.1016/j.it.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliesath J, Huser N, Omori M, Bunag D, Proffitt C, Streiner N, Ho C, Siddiqui-Jain A, O’Brien SE, Lim JK, Ryckman DM, Anderes K, Rice WG, Drygin D. Combined inhibition of EGFR and CK2 augments the attenuation of PI3K-Akt-mTOR signaling and the killing of cancer cells. Cancer Lett. 2012;322(1):113–118. doi: 10.1016/j.canlet.2012.02.032. [DOI] [PubMed] [Google Scholar]
- Den Boer ML, van Slegtenhorst M, De Menezes RX, Cheok MH, Buijs-Gladdines JG, Peters ST, Van Zutven LJ, Beverloo HB, Van der Spek PJ, Escherich G, Horstmann MA, Janka-Schaub GE, Kamps WA, Evans WE, Pieters R. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10(2):125–134. doi: 10.1016/S1470-2045(08)70339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KE, Guest SS, Smale ST, Hahm K, Merkenschlager M, Fisher AG. Association of transcriptionally silent genes with Ikaros complexes at centromeric heterochromatin. Cell. 1997;91(6):845–854. doi: 10.1016/s0092-8674(00)80472-9. [DOI] [PubMed] [Google Scholar]
- Buontempo F, Orsini E, Martins LR, Antunes I, Lonetti A, Chiarini F, Tabellini G, Evangelisti C, Evangelisti C, Melchionda F, Pession A, Bertaina A, Locatelli F, McCubrey JA, Cappellini A, Barata JT, Martelli AM. Cytotoxic activity of the casein kinase 2 inhibitor CX-4945 against T-cell acute lymphoblastic leukemia: targeting the unfolded protein response signaling. Leukemia. 2014;28(3):543–553. doi: 10.1038/leu.2013.349. [DOI] [PubMed] [Google Scholar]
- Chon HJ, Bae KJ, Lee Y, Kim J. The casein kinase 2 inhibitor, CX-4945, as an anti-cancer drug in treatment of human hematological malignancies. Front Pharmacol. 2015;6:70. doi: 10.3389/fphar.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb BS, Morales-Alcelay S, Kleiger G, Brown KE, Fisher AG, Smale ST. Targeting of Ikaros to pericentromeric heterochromatin by direct DNA binding. Genes Dev. 2000;14:2146–2160. doi: 10.1101/gad.816400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordier F, Chaffotte A, Terrien E, Prehaud C, Theillet FX, Delepierre M, Lafon M, Buc H, Wolff N. Ordered phosphorylation events in two independent cascades of the PTEN C-tail revealed by NMR. J Am Chem Soc. 2012;134(50):20533–20543. doi: 10.1021/ja310214g. [DOI] [PubMed] [Google Scholar]
- Cortes M, Wong E, Koipally J, Georgopoulos K. Control of lymphocyte development by the Ikaros gene family. Curr Opin Immunol. 1999;11(2):167–171. doi: 10.1016/s0952-7915(99)80028-4. [DOI] [PubMed] [Google Scholar]
- Cozza G, Pinna LA, Moro S. Kinase CK2 inhibition: an update. Curr Med Chem. 2013;20(5):671–693. doi: 10.2174/092986713804999312. [DOI] [PubMed] [Google Scholar]
- De Toni F, Racaud-Sultan C, Chicanne G, Mas VM, Cariven C, Mesange F, Salles JP, Demur C, Allouche M, Payrastre B, Manenti S, Ysebaert L. A crosstalk between the Wnt and the adhesion-dependent signaling pathways governs the chemosensitivity of acute myeloid leukemia. Oncogene. 2006;25(22):3113–3122. doi: 10.1038/sj.onc.1209346. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19(4):348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116(Pt 7):1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovat S, Ronni T, Russell D, Ferrini R, Cobb BS, Smale ST. A common mechanism for mitotic inactivation of C2H2 zinc finger DNA-binding domains. Genes Dev. 2002;16(23):2985–2990. doi: 10.1101/gad.1040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovat S, Song C, Payne KJ, Li Z. Ikaros, CK2 kinase, and the road to leukemia. Mol Cell Biochem. 2011;356(1–2):201–207. doi: 10.1007/s11010-011-0964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumortier A, Kirstetter P, Kastner P, Chan S. Ikaros regulates neutrophil differentiation. Blood. 2003;101(6):2219–2226. doi: 10.1182/blood-2002-05-1336. [DOI] [PubMed] [Google Scholar]
- Ernst P, Hahm K, Smale ST. Both LyF-1 and an Ets protein interact with a critical promoter element in the murine terminal transferase gene. Mol Cell Biol. 1993;13(5):2982–2992. doi: 10.1128/mcb.13.5.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald TL, Lertpiriyapong K, Cocco L, Martelli AM, Libra M, Candido S, Montalto G, Cervello M, Steelman L, Abrams SL, McCubrey JA. Roles of EGFR and KRAS and their downstream signaling pathways in pancreatic cancer and pancreatic cancer stem cells. Adv Biol Regul. 2015;59:65–81. doi: 10.1016/j.jbior.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Follo MY, Manzoli L, Poli A, McCubrey JA, Cocco L. PLC and PI3K/Akt/mTOR signalling in disease and cancer. Adv Biol Regul. 2015;57:10–16. doi: 10.1016/j.jbior.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Fragoso R, Barata JT. PTEN and leukemia stem cells. Adv Biol Regul. 2014;56:22–29. doi: 10.1016/j.jbior.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359(Pt 1):1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Song EJ, Imamura Kawasawa Y, Li J, Dovat S, Song C. WDR5 high expression and its effect on tumorigenesis in leukemia. Oncotarget. 2016d Jun 21;7(25):37740–37754. doi: 10.18632/oncotarget.9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Guo X, Li J, Hartman M, Kawasawa YI, Dovat S, Song C. Clinical significance of high c-MYC and low MYCBP2 expression and their association with Ikaros dysfunction in adult acute lymphoblastic leukemia. Oncotarget. 2015;6(39):42300–42311. doi: 10.18632/oncotarget.5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Gu Y, Han Q, Zhao G, Li M, Li J, Chen B, Sun T, Dovat S, Gale RP, Song C. Targeting high Dynamin-2 (DNM2) expression by restoring ikaros function in acute lymphoblastic leukemia. Sci Rep. 2016a;6:38004. doi: 10.1038/srep38004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Gu Y, Xiao L, Han Q, Li J, Chen B, Yu J, Kawasawa YI, Payne KJ, Dovat S, Song C. Co-existence of IL7R high and SH2B3 low expression distinguishes a novel high-risk acute lymphoblastic leukemia with Ikaros dysfunction. Oncotarget. 2016b;7(29):46014–46027. doi: 10.18632/oncotarget.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Gu Y, Zhao G, Li J, Chen B, Han Q, Guo X, Liu J, Li H, Yu MD, Olson J, Steffens S, Payne KJ, Song C, Dovat S. High CRLF2 expression associates with IKZF1 dysfunction in adult acute lymphoblastic leukemia without CRLF2 rearrangement. Oncotarget. 2016c;7(31):49722–49732. doi: 10.18632/oncotarget.10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Zhou X, Gu Y, Han Q, Li J, Chen B, Ge Q, Dovat E, Payne JL, Sun T, Song C, Dovat S. Ikaros regulation of the BCL6/BACH2 axis and its clinical relevance in acute lymphoblastic leukemia. Oncotarget. 2017;8(5):8022–8034. doi: 10.18632/oncotarget.14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos K, Moore DD, Derfler B. Ikaros, an early lymphoid-specific transcription factor and a putative mediator for T cell commitment. Science. 1992;258(5083):808–812. doi: 10.1126/science.1439790. [DOI] [PubMed] [Google Scholar]
- Georgopoulos K, Bigby M, Wang JH, Molnar A, Wu P, Winandy S, Sharpe A. The Ikaros gene is required for the development of all lymphoid lineages. Cell. 1994;79(1):143–156. doi: 10.1016/0092-8674(94)90407-3. [DOI] [PubMed] [Google Scholar]
- Goldman FD, Gurel Z, Al-Zubeidi D, Fried AJ, Icardi M, Song C, Dovat S. Congenital pancytopenia and absence of B lymphocytes in a neonate with a mutation in the Ikaros gene. Pediatr Blood Cancer. 2012;58(4):591–597. doi: 10.1002/pbc.23160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AM, Soares MV, Ribeiro P, Caldas J, Povoa V, Martins LR, Melao A, Serra-Caetano A, de Sousa AB, Lacerda JF, Barata JT. Adult B-cell acute lymphoblastic leukemia cells display decreased PTEN activity and constitutive hyperactivation of PI3K/Akt pathway despite high PTEN protein levels. Haematologica. 2014;99(6):1062–1068. doi: 10.3324/haematol.2013.096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-del Arco P, Maki K, Georgopoulos K. Phosphorylation controls Ikaros’s ability to negatively regulate the G(1)-S transition. Mol Cell Biol. 2004;24(7):2797–2807. doi: 10.1128/MCB.24.7.2797-2807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda C, Sachdev M, Muthisami S, Kapadia M, Petrovic-Dovat L, Hartman M, Ding Y, Song C, Payne JL, Tan BH, Dovat S. Casein kinase II (CK2) as a therapeutic target for hematological malignancies. Curr Pharm Des. 2017c;23(1):95–107. doi: 10.2174/1381612822666161006154311. [DOI] [PubMed] [Google Scholar]
- Gowda CS, Song C, Ding Y, Kapadia M, Dovat S. Protein signaling and regulation of gene transcription in leukemia: role of the Casein Kinase II-Ikaros axis. J Investig Med. 2016;64(3):735–739. doi: 10.1136/jim-2016-000075. [DOI] [PubMed] [Google Scholar]
- Gowda C, Song C, Kapadia M, Payne JL, Hu T, Ding Y, Dovat S. Regulation of cellular proliferation in acute lymphoblastic leukemia by Casein Kinase II (CK2) and Ikaros. Adv Biol Regul. 2017b;63:71–80. doi: 10.1016/j.jbior.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray GK, McFarland BC, Rowse AL, Gibson SA, Benveniste EN. Therapeutic CK2 inhibition attenuates diverse prosurvival signaling cascades and decreases cell viability in human breast cancer cells. Oncotarget. 2014;5(15):6484–6496. doi: 10.18632/oncotarget.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65(4):391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Guerra B, Issinger OG. Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis. 1999;20(2):391–408. doi: 10.1002/(SICI)1522-2683(19990201)20:2<391::AID-ELPS391>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Gurel Z, Ronni T, Ho S, Kuchar J, Payne KJ, Turk CW, Dovat S. Recruitment of ikaros to pericentromeric heterochromatin is regulated by phosphorylation. J Biol Chem. 2008;283(13):8291–8300. doi: 10.1074/jbc.M707906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406(6791):86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- Jotta PY, Ganazza MA, Silva A, Viana MB, da Silva MJ, Zambaldi LJ, Barata JT, Brandalise SR, Yunes JA. Negative prognostic impact of PTEN mutation in pediatric T-cell acute lymphoblastic leukemia. Leukemia. 2010;24(1):239–242. doi: 10.1038/leu.2009.209. [DOI] [PubMed] [Google Scholar]
- Kathrein KL, Lorenz R, Innes AM, Griffiths E, Winandy S. Ikaros induces quiescence and T-cell differentiation in a leukemia cell line. Mol Cell Biol. 2005;25(5):1645–1654. doi: 10.1128/MCB.25.5.1645-1654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Sif S, Jones B, Jackson A, Koipally J, Heller E, Winandy S, Viel A, Sawyer A, Ikeda T, Kingston R, Georgopoulos K. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity. 1999;10(3):345–355. doi: 10.1016/s1074-7613(00)80034-5. [DOI] [PubMed] [Google Scholar]
- Kim JS, Eom JI, Cheong JW, Choi AJ, Lee JK, Yang WI, Min YH. Protein kinase CK2alpha as an unfavorable prognostic marker and novel therapeutic target in acute myeloid leukemia. Clin Cancer Res. 2007;13(3):1019–1028. doi: 10.1158/1078-0432.CCR-06-1602. [DOI] [PubMed] [Google Scholar]
- Koipally J, Kim J, Jones B, Jackson A, Avitahl N, Winandy S, Trevisan M, Nichogiannopoulou A, Kelley C, Georgopoulos K. Ikaros chromatin remodeling complexes in the control of differentiation of the hemolymphoid system. Cold Spring Harb Symp Quant Biol. 1999a;64:79–86. doi: 10.1101/sqb.1999.64.79. [DOI] [PubMed] [Google Scholar]
- Koipally J, Renold A, Kim J, Georgopoulos K. Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes. EMBO J. 1999b;18(11):3090–3100. doi: 10.1093/emboj/18.11.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krehan A, Meggio F, Pipkorn R, Pinna LA, Pyerin W. Identification of structural elements of subunit beta of human protein kinase CK2 participating in tight physical alpha-beta intersubunit contacts directly adjacent to a surface-oriented region. Eur J Biochem. 1998;251(3):667–672. doi: 10.1046/j.1432-1327.1998.2510667.x. [DOI] [PubMed] [Google Scholar]
- Kuiper RP, Waanders E, van der Velden VH, van Reijmersdal SV, Venkatachalam R, Scheijen B, Sonneveld E, van Dongen JJ, Veerman AJ, van Leeuwen FN, van Kessel AG, Hoogerbrugge PM. IKZF1 deletions predict relapse in uniformly treated pediatric precursor B-ALL. Leukemia. 2010;24(7):1258–1264. doi: 10.1038/leu.2010.87. [DOI] [PubMed] [Google Scholar]
- Landesman-Bollag E, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC. Protein kinase CK2 in mammary gland tumorigenesis. Oncogene. 2001a;20(25):3247–3257. doi: 10.1038/sj.onc.1204411. [DOI] [PubMed] [Google Scholar]
- Landesman-Bollag E, Song DH, Romieu-Mourez R, Sussman DJ, Cardiff RD, Sonenshein GE, Seldin DC. Protein kinase CK2: signaling and tumorigenesis in the mammary gland. Mol Cell Biochem. 2001b;227(1–2):153–165. [PubMed] [Google Scholar]
- Leslie NR, Dixon MJ, Schenning M, Gray A, Batty IH. Distinct inactivation of PI3K signalling by PTEN and 5-phosphatases. Adv Biol Regul. 2012;52(1):205–213. doi: 10.1016/j.advenzreg.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275(5308):1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Li Z, Perez-Casellas LA, Savic A, Song C, Dovat S. Ikaros isoforms: the saga continues. World J Biol Chem. 2011;2(6):140–145. doi: 10.4331/wjbc.v2.i6.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Song C, Ouyang H, Lai L, Payne KJ, Dovat S. Cell cycle-specific function of Ikaros in human leukemia. Pediatr Blood Cancer. 2012;59(1):69–76. doi: 10.1002/pbc.23406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberg D, Smale ST, Merkenschlager M. Upstream of ikaros. Trends Immunol. 2003;24(11):567–570. doi: 10.1016/j.it.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Licht-Murava A, Paz R, Vaks L, Avrahami L, Plotkin B, Eisenstein M, Eldar-Finkelman H. A unique type of GSK-3 inhibitor brings new opportunities to the clinic. Sci Signal. 2016;9(454):ra110. doi: 10.1126/scisignal.aah7102. [DOI] [PubMed] [Google Scholar]
- Linding R, Jensen LJ, Ostheimer GJ, van Vugt MA, Jorgensen C, Miron IM, Diella F, Colwill K, Taylor L, Elder K, Metalnikov P, Nguyen V, Pasculescu A, Jin J, Park JG, Samson LD, Woodgett JR, Russell RB, Bork P, Yaffe MB, Pawson T. Systematic discovery of in vivo phosphorylation networks. Cell. 2007;129(7):1415–1426. doi: 10.1016/j.cell.2007.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo K, Landau NR, Smale ST. LyF-1, a transcriptional regulator that interactswith a novel class of promoters for lymphocyte-specific genes. Mollecular Cell Biol. 1991;11:5229–5243. doi: 10.1128/mcb.11.10.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovestone S, Boada M, Dubois B, Hull M, Rinne JO, Huppertz HJ, Calero M, Andres MV, Gomez-Carrillo B, Leon T, del Ser T investigators A. A phase II trial of tideglusib in Alzheimer’s disease. J Alzheimers Dis. 2015;45(1):75–88. doi: 10.3233/JAD-141959. [DOI] [PubMed] [Google Scholar]
- Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4(4):257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- Maccario H, Perera NM, Davidson L, Downes CP, Leslie NR. PTEN is destabilized by phosphorylation on Thr366. Biochem J. 2007;405(3):439–444. doi: 10.1042/BJ20061837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273(22):13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Martins LR, Lucio P, Silva MC, Anderes KL, Gameiro P, Silva MG, Barata JT. Targeting CK2 overexpression and hyperactivation as a novel therapeutic tool in chronic lymphocytic leukemia. Blood. 2010;116(15):2724–2731. doi: 10.1182/blood-2010-04-277947. [DOI] [PubMed] [Google Scholar]
- Martins LR, Lucio P, Melao A, Antunes I, Cardoso BA, Stansfield R, Bertilaccio MT, Ghia P, Drygin D, Silva MG, Barata JT. Activity of the clinical-stage CK2-specific inhibitor CX-4945 against chronic lymphocytic leukemia. Leukemia. 2014a;28(1):179–182. doi: 10.1038/leu.2013.232. [DOI] [PubMed] [Google Scholar]
- Martins LR, Perera Y, Lucio P, Silva MG, Perea SE, Barata JT. Targeting chronic lymphocytic leukemia using CIGB-300, a clinical-stage CK2-specific cell-permeable peptide inhibitor. Oncotarget. 2014b;5(1):258–263. doi: 10.18632/oncotarget.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey JA, Davis NM, Abrams SL, Montalto G, Cervello M, Basecke J, Libra M, Nicoletti F, Cocco L, Martelli AM, Steelman LS. Diverse roles of GSK-3: tumor promoter-tumor suppressor, target in cancer therapy. Adv Biol Regul. 2014;54:176–196. doi: 10.1016/j.jbior.2013.09.013. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Abrams SL, Fitzgerald TL, Cocco L, Martelli AM, Montalto G, Cervello M, Scalisi A, Candido S, Libra M, Steelman LS. Roles of signaling pathways in drug resistance, cancer initiating cells and cancer progression and metastasis. Adv Biol Regul. 2015;57:75–101. doi: 10.1016/j.jbior.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? Faseb J. 2003;17(3):349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- Miller SJ, Lou DY, Seldin DC, Lane WS, Neel BG. Direct identification of PTEN phosphorylation sites. FEBS Lett. 2002;528(1–3):145–153. doi: 10.1016/s0014-5793(02)03274-x. [DOI] [PubMed] [Google Scholar]
- Mishra S, Pertz V, Zhang B, Kaur P, Shimada H, Groffen J, Kazimierczuk Z, Pinna LA, Heisterkamp N. Treatment of P190 Bcr/Abl lymphoblastic leukemia cells with inhibitors of the serine/threonine kinase CK2. Leukemia. 2007;21(1):178–180. doi: 10.1038/sj.leu.2404460. [DOI] [PubMed] [Google Scholar]
- Molnár A, Georgopoulos K. The Ikaros gene encodes a family of functionally diverse zinc finger DNA-binding proteins. Mol Cell Biol. 1994;14(12):8292–8303. doi: 10.1128/mcb.14.12.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A, Wu P, Largespada DA, Vortkamp A, Scherer S, Copeland NG, Jenkins NA, Bruns G, Georgopoulos K. The Ikaros gene encodes a family of lymphocyte-restricted zinc finger DNA binding proteins, highly conserved in human and mouse. J Immunol. 1996;156(2):585–592. [PubMed] [Google Scholar]
- Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD, Girtman K, Mathew S, Ma J, Pounds SB, Su X, Pui CH, Relling MV, Evans WE, Shurtleff SA, Downing JR. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446(7137):758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- Mullighan CG, Miller CB, Radtke I, Phillips LA, Dalton J, Ma J, White D, Hughes TP, Le Beau MM, Pui CH, Relling MV, Shurtleff SA, Downing JR. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453(7191):110–114. doi: 10.1038/nature06866. [DOI] [PubMed] [Google Scholar]
- Nelson N, Szekeres K, Iclozan C, Rivera IO, McGill A, Johnson G, Nwogu O, Ghansah T. Apigenin: selective CK2 inhibitor increases Ikaros expression and improves T cell homeostasis and function in murine pancreatic cancer. PloS One. 2017;12(2):e0170197. doi: 10.1371/journal.pone.0170197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoumassoun LE, Russo C, Denizeau F, Averill-Bates D, Henderson JE. Parathyroid hormone-related protein (PTHrP) inhibits mitochondrial-dependent apoptosis through CK2. J Cell Physiol. 2007;212(3):591–599. doi: 10.1002/jcp.21055. [DOI] [PubMed] [Google Scholar]
- Ougolkov AV, Bone ND, Fernandez-Zapico ME, Kay NE, Billadeau DD. Inhibition of glycogen synthase kinase-3 activity leads to epigenetic silencing of nuclear factor kappaB target genes and induction of apoptosis in chronic lymphocytic leukemia B cells. Blood. 2007;110(2):735–742. doi: 10.1182/blood-2006-12-060947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Doble B, Woodgett JR. Glycogen synthase kinase-3 in insulin and Wnt signalling: a double-edged sword? Biochem. Soc Trans. 2004;32(Pt 5):803–808. doi: 10.1042/BST0320803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsoukis N, Li L, Sari D, Petkova V, Boussiotis VA. PD-1 increases PTEN phosphatase activity while decreasing PTEN protein stability by inhibiting casein kinase 2. Mol Cell Biol. 2013;33(16):3091–3098. doi: 10.1128/MCB.00319-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne KJ, Dovat S. Ikaros and tumor suppression in acute lymphoblastic leukemia. Crit Rev Oncog. 2011;16(1–2):3–12. doi: 10.1615/critrevoncog.v16.i1-2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre F, Chua PC, O’Brien SE, Siddiqui-Jain A, Bourbon P, Haddach M, Michaux J, Nagasawa J, Schwaebe MK, Stefan E, Vialettes A, Whitten JP, Chen TK, Darjania L, Stansfield R, Bliesath J, Drygin D, Ho C, Omori M, Proffitt C, Streiner N, Rice WG, Ryckman DM, Anderes K. Pre-clinical characterization of CX-4945, a potent and selective small molecule inhibitor of CK2 for the treatment of cancer. Mol Cell Biochem. 2011;356(1–2):37–43. doi: 10.1007/s11010-011-0956-5. [DOI] [PubMed] [Google Scholar]
- Pinna LA. Casein kinase 2: an ‘eminence grise’ in cellular regulation? Biochim Biophys Acta. 1990;1054(3):267–284. doi: 10.1016/0167-4889(90)90098-x. [DOI] [PubMed] [Google Scholar]
- Pinna LA. A historical view of protein kinase CK2. Cell Mol Biol Res. 1994;40(5–6):383–390. [PubMed] [Google Scholar]
- Pinna LA. Protein kinase CK2: a challenge to canons. J Cell Sci. 2002;115(Pt 20):3873–3878. doi: 10.1242/jcs.00074. [DOI] [PubMed] [Google Scholar]
- Pinna LA, Meggio F. Protein kinase CK2 (“casein kinase-2”) and its implication in cell division and proliferation. Prog Cell Cycle Res. 1997;3:77–97. doi: 10.1007/978-1-4615-5371-7_7. [DOI] [PubMed] [Google Scholar]
- Pizzi M, Piazza F, Agostinelli C, Fuligni F, Benvenuti P, Mandato E, Casellato A, Rugge M, Semenzato G, Pileri SA. Protein kinase CK2 is widely expressed in follicular, Burkitt and diffuse large B-cell lymphomas and propels malignant B-cell growth. Oncotarget. 2015;6(9):6544–6552. doi: 10.18632/oncotarget.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin B, Kaidanovich O, Talior I, Eldar-Finkelman H. Insulin mimetic action of synthetic phosphorylated peptide inhibitors of glycogen synthase kinase-3. J Pharmacol Exp Ther. 2003;305(3):974–980. doi: 10.1124/jpet.102.047381. [DOI] [PubMed] [Google Scholar]
- Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, Cordon-Cardo C, Catoretti G, Fisher PE, Parsons R. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci U S A. 1999;96(4):1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu M, Gurel Z, Ronni T, Song C, Hung KY, Payne KJ, Dovat S. Ikaros stability and pericentromeric localization are regulated by protein phosphatase 1. J Biol Chem. 2009;284(20):13869–13880. doi: 10.1074/jbc.M900209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quotti Tubi L, Gurrieri C, Brancalion A, Bonaldi L, Bertorelle R, Manni S, Pavan L, Lessi F, Zambello R, Trentin L, Adami F, Ruzzene M, Pinna LA, Semenzato G, Piazza F. Inhibition of protein kinase CK2 with the clinical-grade small ATP-competitive compound CX-4945 or by RNA interference unveils its role in acute myeloid leukemia cell survival, p53-dependent apoptosis and daunorubicin-induced cytotoxicity. J Hematol Oncol. 2013;6:78. doi: 10.1186/1756-8722-6-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi MR, Mirabilii S, Licchetta R, Piedimonte M, Tafuri A. Targeting the Akt, GSK-3, Bcl-2 axis in acute myeloid leukemia. Adv Biol Regul. 2017 May 19; doi: 10.1016/j.jbior.2017.05.002. pii: S2212-4926(17)30089-1. doi: http://dx.doi.org/10.1016/j.jbior.2017.05.002 Epub ahead of print. [DOI] [PubMed]
- Ronni T, Payne KJ, Ho S, Bradley MN, Dorsam G, Dovat S. Human Ikaros function in activated T cells is regulated by coordinated expression of its largest isoforms. J Biol Chem. 2007;282(4):2538–2547. doi: 10.1074/jbc.M605627200. [DOI] [PubMed] [Google Scholar]
- Rosner A, Miyoshi K, Landesman-Bollag E, Xu X, Seldin DC, Moser AR, MacLeod CL, Shyamala G, Gillgrass AE, Cardiff RD. Pathway pathology: histological differences between ErbB/Ras and Wnt pathway transgenic mammary tumors. Am J Pathol. 2002;161(3):1087–1097. doi: 10.1016/S0002-9440(10)64269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvolo PP. GSK-3 as a novel prognostic indicator in leukemia. Adv Biol Regul. 2017 May 8; doi: 10.1016/j.jbior.2017.05.001. pii: S2212-4926(17)30086-6. doi: http://dx.doi.org/10.1016/j.jbior.2017.05.001 Epub ahead of print. [DOI] [PubMed]
- Ruzzene M, Bertacchini J, Toker A, Marmiroli S. Cross-talk between the CK2 and AKT signaling pathways in cancer. Adv Biol Regul. 2017 May;64:1–8. doi: 10.1016/j.jbior.2017.03.002. doi: http://dx.doi.org/10.1016/j.jbior.2017.03.002. Epub 2017 Mar 28. [DOI] [PubMed] [Google Scholar]
- Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22(14):2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- Scaglioni PP, Yung TM, Choi S, Baldini C, Konstantinidou G, Pandolfi PP. CK2 mediates phosphorylation and ubiquitin-mediated degradation of the PML tumor suppressor. Mol Cell Biochem. 2008;316(1–2):149–154. doi: 10.1007/s11010-008-9812-7. [DOI] [PubMed] [Google Scholar]
- Shore LJ, Soler AP, Gilmour SK. Ornithine decarboxylase expression leads to translocation and activation of protein kinase CK2 in vivo. J Biol Chem. 1997;272(19):12536–12543. doi: 10.1074/jbc.272.19.12536. [DOI] [PubMed] [Google Scholar]
- Siddiqui-Jain A, Drygin D, Streiner N, Chua P, Pierre F, O’Brien SE, Bliesath J, Omori M, Huser N, Ho C, Proffitt C, Schwaebe MK, Ryckman DM, Rice WG, Anderes K. CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy. Cancer Res. 2010;70(24):10288–10298. doi: 10.1158/0008-5472.CAN-10-1893. [DOI] [PubMed] [Google Scholar]
- Siddiqui-Jain A, Bliesath J, Macalino D, Omori M, Huser N, Streiner N, Ho CB, Anderes K, Proffitt C, O’Brien SE, Lim JK, Von Hoff DD, Ryckman DM, Rice WG, Drygin D. CK2 inhibitor CX-4945 suppresses DNA repair response triggered by DNA-targeted anticancer drugs and augments efficacy: mechanistic rationale for drug combination therapy. Mol Cancer Ther. 2012;11(4):994–1005. doi: 10.1158/1535-7163.MCT-11-0613. [DOI] [PubMed] [Google Scholar]
- Silva A, Yunes JA, Cardoso BA, Martins LR, Jotta PY, Abecasis M, Nowill AE, Leslie NR, Cardoso AA, Barata JT. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest. 2008;118(11):3762–3774. doi: 10.1172/JCI34616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A, Jotta PY, Silveira AB, Ribeiro D, Brandalise SR, Yunes JA, Barata JT. Regulation of PTEN by CK2 and Notch1 in primary T-cell acute lymphoblastic leukemia: rationale for combined use of CK2- and gamma-secretase inhibitors. Haematologica. 2010;95(4):674–678. doi: 10.3324/haematol.2009.011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DH, Sussman DJ, Seldin DC. Endogenous protein kinase CK2 participates in Wnt signaling in mammary epithelial cells. J Biol Chem. 2000;275(31):23790–23797. doi: 10.1074/jbc.M909107199. [DOI] [PubMed] [Google Scholar]
- Song DH, Dominguez I, Mizuno J, Kaut M, Mohr SC, Seldin DC. CK2 phosphorylation of the armadillo repeat region of beta-catenin potentiates Wnt signaling. J Biol Chem. 2003;278(26):24018–24025. doi: 10.1074/jbc.M212260200. [DOI] [PubMed] [Google Scholar]
- Song C, Li Z, Erbe AK, Savic A, Dovat S. Regulation of Ikaros function by casein kinase 2 and protein phosphatase 1. World J Biol Chem. 2011;2(6):126–131. doi: 10.4331/wjbc.v2.i6.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13(5):283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- Song C, Gowda C, Pan X, Ding Y, Tong Y, Tan BH, Wang H, Muthusami S, Ge Z, Sachdev M, Amin SG, Desai D, Gowda K, Gowda R, Robertson GP, Schjerven H, Muschen M, Payne KJ, Dovat S. Targeting casein kinase II restores Ikaros tumor suppressor activity and demonstrates therapeutic efficacy in high-risk leukemia. Blood. 2015;126(15):1813–1822. doi: 10.1182/blood-2015-06-651505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Pan X, Ge Z, Gowda C, Ding Y, Li H, Li Z, Yochum G, Muschen M, Li Q, Payne KJ, Dovat S. Epigenetic regulation of gene expression by Ikaros, HDAC1 and Casein Kinase II in leukemia. Leukemia. 2016;30(6):1436–1440. doi: 10.1038/leu.2015.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95(1):29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15(4):356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- Su RC, Brown KE, Saaber S, Fisher AG, Merkenschlager M, Smale ST. Dynamic assembly of silent chromatin during thymocyte maturation. Nat Genet. 2004;36(5):502–506. doi: 10.1038/ng1351. [DOI] [PubMed] [Google Scholar]
- Sun L, Liu A, Georgopoulos K. Zinc finger-mediated protein interactions modulate Ikaros activity, a molecular control of lymphocyte development. EMBO J. 1996;15(19):5358–5369. [PMC free article] [PubMed] [Google Scholar]
- Sutherland C. What Are the bona fide GSK3 Substrates? Int. J Alzheimers Dis. 2011;2011:505607. doi: 10.4061/2011/505607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, del Barco Barrantes I, Ho A, Wakeham A, Itie A, Khoo W, Fukumoto M, Mak TW. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998;8(21):1169–1178. doi: 10.1016/s0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- Takahashi-Yanaga F. Activator or inhibitor? GSK-3 as a new drug target. Biochem Pharmacol. 2013;86(2):191–199. doi: 10.1016/j.bcp.2013.04.022. [DOI] [PubMed] [Google Scholar]
- Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, Rincon M. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320(5876):667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton TM, Delgado P, Chen L, Salas B, Krementsov D, Fernandez M, Vernia S, Davis RJ, Heimann R, Teuscher C, Krangel MS, Ramiro AR, Rincon M. Inactivation of nuclear GSK3beta by Ser(389) phosphorylation promotes lymphocyte fitness during DNA double-strand break response. Nat Commun. 2016;7:10553. doi: 10.1038/ncomms10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276(2):993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- Tuazon PT, Traugh JA. Casein kinase I and II–multipotential serine protein kinases: structure, function, and regulation. Adv Sec Messenger Phosphoprot Res. 1991;23:123–164. [PubMed] [Google Scholar]
- Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. 2000;20(14):5010–5018. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nature reviews Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Wang G, Ahmad KA, Ahmed K. Modulation of death receptor-mediated apoptosis by CK2. Mol Cell Biochem. 2005;274(1–2):201–205. doi: 10.1007/s11010-005-2952-0. [DOI] [PubMed] [Google Scholar]
- Wang G, Ahmad KA, Ahmed K. Role of protein kinase CK2 in the regulation of tumor necrosis factor-related apoptosis inducing ligand-induced apoptosis in prostate cancer cells. Cancer Res. 2006;66(4):2242–2249. doi: 10.1158/0008-5472.CAN-05-2772. [DOI] [PubMed] [Google Scholar]
- Wang H, Song C, Gurel Z, Song N, Ma J, Ouyang H, Lai L, Payne KJ, Dovat S. Protein phosphatase 1 (PP1) and casein kinase II (CK2) regulate ikaros-mediated repression of TdT in thymocytes and T-cell leukemia. Pediatr Blood Cancer. 2014;61(12):2230–2235. doi: 10.1002/pbc.25221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Song C, Ding Y, Pan X, Ge Z, Tan BH, Gowda C, Sachdev M, Muthusami S, Ouyang H, Lai L, Francis OL, Morris CL, Abdel-Azim H, Dorsam G, Xiang M, Payne KJ, Dovat S. Transcriptional regulation of JARID1B/KDM5B histone demethylase by ikaros, histone deacetylase 1 (HDAC1), and casein kinase 2 (CK2) in B-cell acute lymphoblastic leukemia. J Biol Chem. 2016;291(8):4004–4018. doi: 10.1074/jbc.M115.679332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winandy S, Wu P, Georgopoulos K. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell. 1995;83(2):289–299. doi: 10.1016/0092-8674(95)90170-1. [DOI] [PubMed] [Google Scholar]
- Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9(8):2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Yeh J, Van Waes C. Protein kinase casein kinase 2 mediates inhibitor-kappaB kinase and aberrant nuclear factor-kappaB activation by serum factor(s) in head and neck squamous carcinoma cells. Cancer Res. 2006;66(13):6722–6731. doi: 10.1158/0008-5472.CAN-05-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nature reviews Cancer. 2009;9(1):28–39. doi: 10.1038/nrc2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ding L, Holmfeldt L, Wu G, Heatley SL, Payne-Turner D, Easton J, Chen X, Wang J, Rusch M, Lu C, Chen SC, Wei L, Collins-Underwood JR, Ma J, Roberts KG, Pounds SB, Ulyanov A, Becksfort J, Gupta P, Huether R, Kriwacki RW, Parker M, McGoldrick DJ, Zhao D, Alford D, Espy S, Bobba KC, Song G, Pei D, Cheng C, Roberts S, Barbato MI, Campana D, Coustan-Smith E, Shurtleff SA, Raimondi SC, Kleppe M, Cools J, Shimano KA, Hermiston ML, Doulatov S, Eppert K, Laurenti E, Notta F, Dick JE, Basso G, Hunger SP, Loh ML, Devidas M, Wood B, Winter S, Dunsmore KP, Fulton RS, Fulton LL, Hong X, Harris CC, Dooling DJ, Ochoa K, Johnson KJ, Obenauer JC, Evans WE, Pui CH, Naeve CW, Ley TJ, Mardis ER, Wilson RK, Downing JR, Mullighan CG. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481(7380):157–163. doi: 10.1038/nature10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Freeman TA, Ahmad F, Shang X, Mangano E, Gao E, Farber J, Wang Y, Ma XL, Woodgett J, Vagnozzi RJ, Lal H, Force T. GSK-3alpha is a central regulator of age-related pathologies in mice. J Clin Invest. 2013;123(4):1821–1832. doi: 10.1172/JCI64398. [DOI] [PMC free article] [PubMed] [Google Scholar]


