Abstract
OBJECTIVE:
To investigate the effects of myelotomy on locomotor recovery in rats subjected to spinal cord injury.
DATA SOURCES:
Electronic databases including PubMed, Science Citation Index, Cochrane Library, China National Knowledge Infrastructure, Chinese Journals Full-text Database, China Biology Medicine disc, and Wanfang Database were searched to retrieve related studies published before September 2017. The MeSH terms (the Medical Subject Headings) such as “myelotomy”, “spinal cord injuries”, “rats”, “randomized controlled trial” and all related entry terms were searched.
DATA SELECTION:
Randomized controlled trials using myelotomy for the treatment of acute spinal cord injury in rats were included. Basso, Beattie, and Bresnahan scores were adopted as the evaluation method. RevMan Software (version 5.3) was used for data processing. The χ2 and I2 tests were used to assess heterogeneity. Using a random-effects model, a subgroup analysis was conducted to analyze the source of the heterogeneity.
OUTCOME MEASURES:
Basso, Beattie, and Bresnahan scores were observed 1–6 weeks after spinal cord injury.
RESULTS:
Six animal trials were included, using a total of 143 lab rats. The included trials were divided into two subgroups by injury degrees (moderate or severe). The pooled results showed that, 1–6 weeks after spinal cord injury, the overall Basso, Beattie, and Bresnahan score was significantly higher in the myelotomy group than in the contusion group (weighted mean difference (WMD) = 0.60; 95% confidence interval (CI): 0.23–0.97; P = 0.001; WMD = 2.10; 95% CI: 1.56–2.64; P < 0.001; WMD = 2.65; 95% CI: 1.73–3.57; P < 0.001; WMD = 1.66; 95% CI: 0.80–2.52; P < 0.001; WMD = 2.09; 95% CI: 0.92–3.26, P < 0.001; WMD = 2.25; 95% CI: 1.06–3.44, P < 0.001). The overall heterogeneity was high (I2 = 85%; I2 = 95%; I2 = 94%; I2 = 88%; I2 = 91%; I2 = 89%). The results in the moderate injury subgroup showed that Basso, Beattie, and Bresnahan scores were significantly higher in the myelotomy group than in the contusion group (WMD = 0.91, 95% CI: 0.52–1.3, P < 0.001; WMD = 2.10; 95% CI: 1.56–2.64, P < 0.001; WMD = 2.65; 95% CI: 1.73–3.57, P < 0.001; WMD = 2.50, 95% CI: 1.72–3.28, P < 0.001; WMD = 3.29, 95% CI: 2.21–4.38, P < 0.001; WMD = 3.27; 95% CI: 2.31–4.23, P < 0.001). The relevant heterogeneity was low. However, there were no significant differences in Basso, Beattie, and Bresnahan scores between the myelotomy and contusion groups in the severe injury subgroup at 2 and 3 weeks after the injury (P = 0.75; P = 0.92).
CONCLUSION:
To date, this is the first attempt to summarize the potential effect of myelotomy on locomotor recovery in rats with spinal cord injury. Our findings conclude that myelotomy promotes locomotor recovery in rats with spinal cord injury, especially in those with moderate injury.
Keywords: nerve regeneration, spinal cord injury, myelotomy, locomotor recovery, rats, rehabilitation, moderate injury, randomized controlled trials, systematic review, meta-analysis, neural regeneration
Introduction
The incidence of spinal cord injury (SCI) is increasing year by year (Afsharipour et al., 2016; Ahuja et al., 2016; Amsters et al., 2016; Barman et al., 2016; Barthelemy et al., 2016; Berlowitz et al., 2016; Biglari et al., 2016; Da Silva et al., 2016; Arora et al., 2017; Baldea et al., 2017; Cortes et al., 2017). The pathophysiology of SCI involves a primary mechanical injury, which is due to rapid direct compression and contusion of the spinal cord, caused by bone dislocation that directly disrupts axons and blood vessels (El Tecle et al., 2016; Furlan et al., 2016; Grassner et al., 2016; He and Nan, 2016; Saadoun et al., 2016; Zhang et al., 2016; Piazza and Schuster, 2017). Following the primary injury, the secondary injury invades the central and peripheral regions of the spinal cord, and is characterized by edema, ischemia, cell death, and oxidative stress (Wu et al., 2016; Guizar-Sahagun et al., 2017). In addition, intramedullary hemorrhagic necrosis, which increases with time after the primary injury, greatly exacerbates the neurological function of the cord. The spinal cord is thus impaired not only by the mass impact of necrosis, but also by the secretions of toxic substances, such as metabolites and degradation products. As a result, SCI causes a wide range of severe problems. However, the treatment of SCI is still controversial.
Due to the severe damage of spinal cord tissues that is caused by the secondary injury, releasing pressure and removing hemorrhagic necrosis has long been a focus for the treatment of SCI. Some researchers insist that early surgical decompression targeting the dura mater is a pivotal therapeutic intervention for acute SCI because it relieves pressure and improves local microcirculation (Fehlings et al., 2001; Fehlings and Perrin, 2006; Fehlings and Arvin, 2009). However, decompression is not the best option because of incomplete removal of the pressure from the dura and intramedullary hemorrhage. In addition, the selection of ‘‘early’’ or ‘‘late’’ surgery has not been standardized to date (Yang et al., 2013; Gupta et al., 2015; El Tecle et al., 2016; Furlan et al., 2016; Grassner et al., 2016; Piazza and Schuster, 2017). Thus, surgical procedures, as well as the optimal time for decompression, still need to be discussed and unified. Pre-clinical studies into the use of bone marrow mesenchymal stem cells have consistently demonstrated beneficial outcomes and functional recovery after SCI (Boido et al., 2014; Shrestha et al., 2014). Nevertheless, the use of intraspinal transplantation of these stem cells is limited because of the implantation methods, which are difficult to apply in clinical trials, and which can easily lead to secondary injury (Sakamoto et al., 2003; Zhu et al., 2008; Smith et al., 2010; van Middendorp et al., 2013; Shrestha et al., 2014; Liu et al., 2016; Mattiassich et al., 2017). Therefore, effective therapies and measures to improve neurological outcomes after SCI are very important.
Allen (1911) first reported the modified decompression surgery, myelotomy, a century ago. This operation (involving a longitudinal midline incision in the dorsal cord) has been reported to be beneficial in preliminary and pre-clinical trials. He indicated that myelotomy can structurally improve the injured cord and improve function in injured animals and humans. More recent pre-clinical and clinical research has recognized the critical role played by myelotomy on the functional recovery of the central nervous system (Gunnarsson and Fehlings, 2003; Edmond, 2004; Zhu et al., 2008; Fehlings, 2009; Gupta et al., 2010; Smith et al., 2010; Chikuda et al., 2013; Grassner et al., 2016). In our previous studies, we treated the contusion site with a myelotomy procedure that reduced edema and promoted mobilization in rat models with SCI, and which decreased the likelihood of adverse events developing from secondary injuries (Yang et al., 2013; Hu et al., 2015). However, it is unclear whether the magnitude of integrative and protective effects is large enough to be meaningful.
This systematic review and meta-analysis of locomotor recovery in rat models with SCI was conducted by means of analyses between myelotomy and control groups. The aim of this review is to determine whether there is evidence to support myelotomy as a treatment for acute SCI.
Data and Methods
Search strategy and data extraction
We used the methodological recommendations of the Cochrane Collaboration and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. Electronic databases, including PubMed, Science Citation Index, Cochrane Library, China National Knowledge Infrastructure, Chinese Journals Full-text database, China Biology Medicine disc, and Wanfang Database, were searched to retrieve related studies published before September 2017. The MeSH terms (the Medical Subject Headings) including “myelotomy”, “spinal cord injuries”, “rats”, “randomized controlled trial” and all related entry terms were searched. No restrictions were established on language, publication data, or publication status.
Two independent authors screened citations and publications identified by the initial search, to select potentially relative titles, review their abstracts, and determine whether they were eligible. The reference lists in included studies were also screened for any relevant publications that were not identified by the primary search. If data were not available, the authors were contacted by email. Disagreements were solved by a debate and consensus between both reviewers.
Two authors independently abstracted data from the selected articles, recording the following information: First author's name, publication year, model used to induce SCI (contusion or compression), injury level of spinal cord, modeling parameters, operation time after injury, SCI degree (severe or moderate), the number of rats in each group, rat characteristics, myelotomy procedures, Basso, Beattie and Bresnahan (BBB) score, and other experimental results. For each comparison, data were collected for the mean outcome, standard deviation, and the number of animals per group. If any data were only shown in graphs, GetData Graph Digitizer software was used to estimate data.
Inclusion criteria
The inclusion criteria were established using the PICOS (Population, Intervention, Comparison, Outcomes, and Study design) method. The inclusion criteria were: 1) Randomized controlled animal trials; 2) lab rats had any type of acute SCI, such as compression, contusion, transection, and hemisection; 3) at least two different groups were set: myelotomy group (myelotomy after SCI) and contusion group (control group without treatment after SCI); 5) BBB score was adopted as the evaluation method; 6) similar surgical procedures of myelotomy were adopted.
Exclusion criteria
The exclusion criteria were: 1) No access to the full text; 2) review; 3) trials of low quality (both authors reached an agreement using the criteria outlined in the following section, titled Study quality assessment and evidence assessment); 4) no access to mean and standard deviations of BBB scores.
Study quality assessment and evidence assessment
The quality of included studies was evaluated on the basis of the Cochrane Handbook for Systematic Reviews of Interventions (version 5.3.0). The following six items were evaluated: 1) random sequence generation; 2) allocation concealment; 3) blinding of outcome assessment; 4) incomplete outcome data; 5) selective reporting; 6) other bias. Every study was assessed by two independent researchers and the judgment of every item was low risk, unclear, or high risk. Any divergence regarding eligibility during the extraction was resolved through a discussion. The GRADE methodology was then used to create, manage, and share summaries of research evidence (GRADE pro Guideline Development Tool; https://gradepro.org). The quality of evidence was judged as “high”, “moderate”, “low”, or “very low” for each outcome with six items: risk of bias, inconsistency, indirectness, imprecision, and publication bias. Any disagreement regarding evidence quality assessment was discussed and resolved.
Evaluation of locomotor recovery
Locomotor function was evaluated based on the open-field test. The 21-point BBB score was used to evaluate hindlimb locomotion. Normal function is rated as 21 points, and a lower score reflects a more impaired locomotor function (Scheff et al., 2002).
Statistical analysis
The meta-analysis was conducted using RevMan software (version 5.3; the Cochrane Collaboration, http://community.cochrane.org/help/tools-and-software/revman-5/revman-5-download. Pooled estimate was reported as weighted mean differences (WMDs) with 95% confidence intervals (CIs) for continuous outcomes. P-value < 0.05 was considered statistically significant. Statistical heterogeneity was evaluated using I2 and χ2 tests (for I2, 25% > I2 ≥ 0% means no heterogeneity, 50% > I2 ≥ 25% means slight heterogeneity, 75% > I2 ≥ 50% means moderate heterogeneity, I2 ≥ 75% means severe heterogeneity; for χ2, P value < 0.1 means heterogeneity, while P-value > 0.1 means no heterogeneity). A random-effects model was used to obtain summary WMDs. The subsequent subgroup analysis was based on the SCI degree (moderate or severe injuries). The BBB score was analyzed according to the weeks after SCI, from 1 week to 6 weeks. Subgroup analysis was used to analyze the source of heterogeneity.
Definitions of injury degree and myelotomy
In the included studies, all contusion models of SCI were produced using a New York University weight-drop device. According to Guizar-Sahagun et al. (2017), a 10 g rod was dropped from a height of 25 mm or 50 mm (for moderate or severe injuries, respectively) onto the exposed dura. This standard is used in the articles in our meta-analysis.
With respect to myelotomy, previous studies suggest that the critical step is the longitudinal midline incision in the spinal cord, together with the removal of “contused tissue”. However, there is no gold standard for myelotomy in animal trials, and surgical methods differ between centers, so small variations in the myelotomy procedure were accepted for this review.
Results
Study characteristics
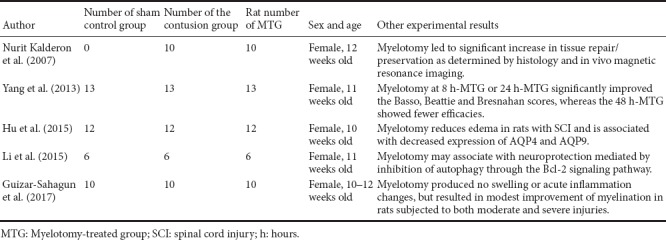
A total of 115 studies were initially identified from the literature search. After screening the studies using our filtering strategies, 110 studies were excluded. The final meta-analysis included six randomized controlled trials in five articles (Kalderon et al., 2007; Yang et al., 2013; Hu et al., 2015; Li, 2015; Guizar-Sahagun et al., 2017) that were published before September 2017 (Figure 1). A total of 143 Sprague-Dawley female rats were included in these studies. Detailed information about the studies is shown in Table 1. Locomotor performance of the rats was observed at 1–6 weeks after different intensities of SCI (Table 2). Five of the six comparisons contained three groups, including 1) sham control group: rats only underwent laminectomy; 2) contusion group: rats underwent laminectomy after contusion; 3) myelotomy group: rats underwent myelotomy after contusion and laminectomy. However, there were only two groups (contusion and myelotomy groups) in one study (Kalderon et al., 2007). The injury level of the spinal cord and the myelotomy procedure were also collected from each included study; the critical step of the myelotomy surgery was a longitudinal midline incision in the spinal cord together with the removal of necrotic tissue, although surgical instruments and incision depth differed between studies.
Figure 1.
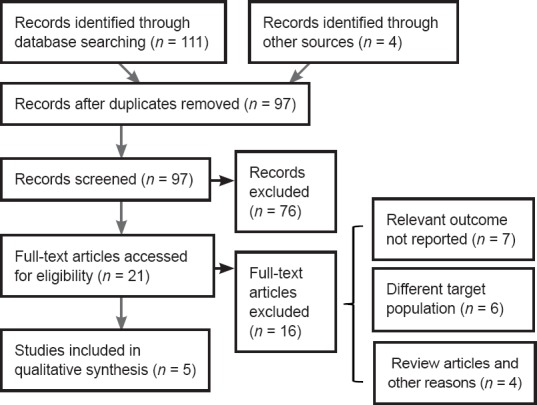
Selection of publications on myelotomy for spinal cord injury in rat models.
Table 1.
Description of studies
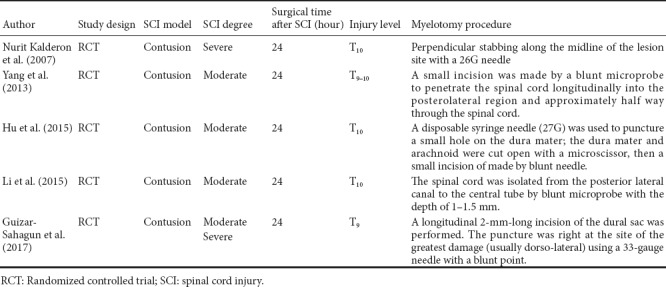
Table 2.
Observation time
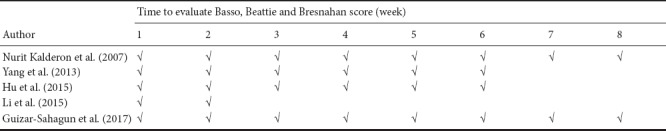
In Table 3, further details from the included studies are shown, such as the number of rats in each group, rat details, and other experimental results from each study. Notably, in these studies, myelotomy was found to reduce other undesirable pathological changes, nor it did not affect long-term spontaneous locomotor recovery by pathological and imaging tests.
Table 3.
Other details of included studies
Risk of bias in included studies
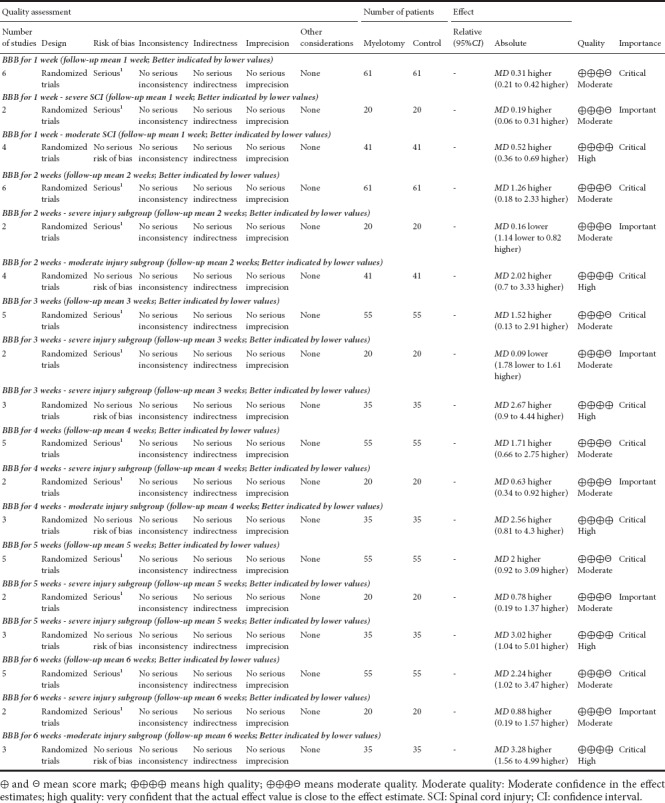
A risk of bias graph is shown in Figure 2, which reflects authors’ judgements about each risk of bias item, presented as percentages across all included studies. The evidence profile made by GRADE pro was used to summarize the evidence from systematic reviews for the subgroup analysis (Table 4). The results showed that the evidence level of the severe injury subgroup was moderate. In contrast, the evidence level was high in the moderate injury subgroup. In summary, the evidence quality was moderate or high and the risk of bias was regarded as low. Only one study did not clearly mention the blinding of outcome assessment, and one other study may have other biases.
Figure 2.
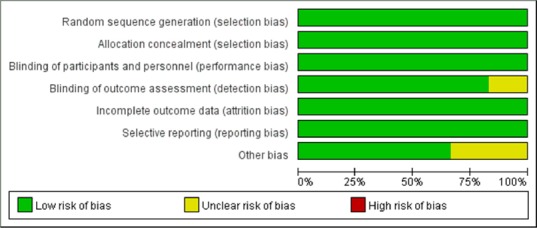
A risk of bias graph reviewed authors’ judgements about each risk of bias item, presented as percentages across all included studies.
The unclear risk of detection bias was found in the publication of “Kalederon et al., 2007”, because the description of blinding of Basso, Beattie and Bresnahan score assessment was not detailed. The unclear risk of other bias may exist in the publication of “Guizar et al., 2017”, because of the lack of some details.
Table 4.
GRADE evidence profile for creating, managing, and sharing summaries of research evidence using random effects
Level of evidence assessment
The GRADE evidence profiles are shown in Table 4. In terms of the severe injury subgroup, the GRADE level of evidence was moderate for locomotor recovery in rats at 1–6 weeks after acute SCI, and was high for locomotor recovery in rats in the moderate injury subgroup.
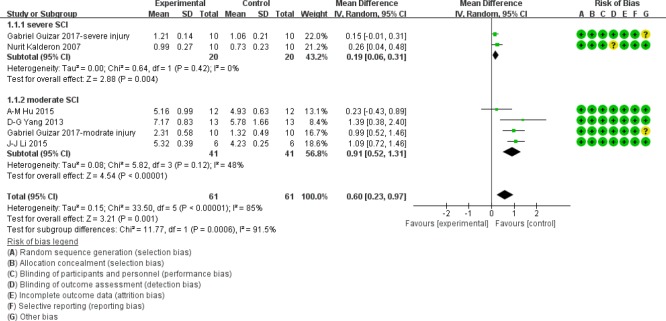
BBB score at 1 week after SCI
As shown in Figure 3, at 1 week after acute SCI, BBB scores in the moderate injury subgroup were significantly higher in myelotomy groups than in contusion control groups (WMD = 0.91; 95% CI: 0.52–1.3; P < 0.001), which suggests a protective effect of myelotomy. The heterogeneity was mild (I2 = 48%; P = 0.12). In the severe injury subgroup, BBB scores in myelotomy groups were also higher compared with contusion groups (WMD = 0.19; 95% CI: 0.06–0.31; P = 0.004). No relevant heterogeneity was found (I2 = 0%; P = 0.42). Accordingly, the overall BBB scores were significantly increased in myelotomy groups compared with contusion control groups (WMD = 0.60; 95% CI: 0.23–0.97; P = 0.001) and a high total heterogeneity existed (I2 = 85%; P < 0.001). Additionally, there was high heterogeneity describing subgroup differences (I2 = 91.5%).
Figure 3.
Subgroup analysis: forest plot shows that in moderate and severe injury subgroups, rats had a higher BBB score in myelotomy groups than in contusion groups, with significant differences at 1 week after SCI.
Random effects were used. SD: Standard deviation; CI: confidence interval; SCI: spinal cord injury.
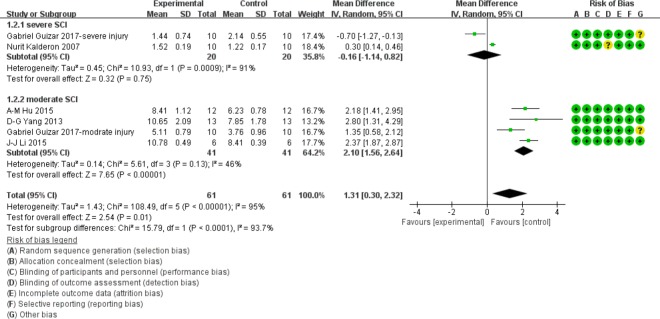
BBB score at 2 weeks after SCI
In the moderate injury subgroup at 2 weeks after injury, the BBB scores were significantly higher in the myelotomy groups compared with the contusion groups (WMD = 2.10; 95% CI: 1.56–2.64; P < 0.001), which suggests a protective effect of myelotomy. The relevant heterogeneity was mild (I2 = 46%; P = 0.13). In contrast, no significant difference in BBB score was found between the two comparison groups in the severe injury subgroup (P = 0.75). The relevant heterogeneity was high (I2 = 91%; P < 0.001). The overall BBB scores were significantly higher in myelotomy groups than in the contusion control groups (WMD = 1.31; 95% CI: 0.30–2.32; P = 0.01) and there was a high total heterogeneity (I2 = 95%; P < 0.001). There was high heterogeneity describing subgroup differences (I2 = 93.7%). All information is presented in Figure 4.
Figure 4.
Subgroup analysis: forest plot shows that in the moderate injury subgroup, rats had a higher BBB score in myelotomy groups than in contusion groups, with significant differences at 2 weeks after SCI.
No significant difference in BBB score was found between the two comparison groups in the severe injury subgroup. Random effects were used. SD: Standard deviation; CI: confidence interval; SCI: spinal cord injury.
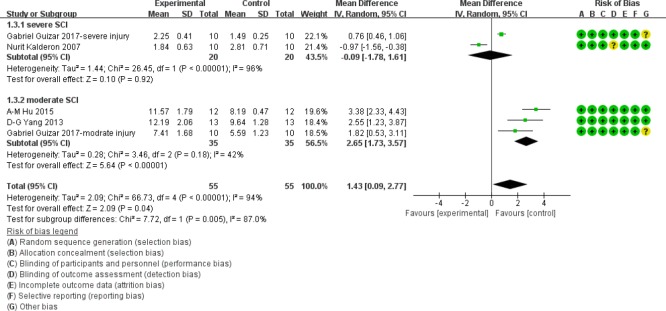
BBB score at 3 weeks after SCI
Similarly, at 3 weeks after injury, in the moderate injury subgroup, BBB scores were significantly higher in myelotomy groups than in contusion groups (WMD = 2.65; 95% CI: 1.73–3.57; P < 0.001), suggesting a protective effect of myelotomy. The relevant heterogeneity was mild (I2 = 42%; P = 0.18). However, there was no significant difference in BBB scores between the two comparison groups in the severe injury subgroup (P = 0.92). The relevant heterogeneity was high (I2 = 96%; P < 0.001). The overall BBB scores were significantly higher in the myelotomy groups than in the contusion control groups (WMD = 1.43; 95% CI: 0.09–2.77; P = 0.04) and there was a high total heterogeneity (I2 = 94%; P < 0.001). In addition, there was high heterogeneity describing subgroup differences (I2 = 87.0%). All information is presented in Figure 5.
Figure 5.
Subgroup analysis: forest plot shows that in the moderate injury subgroup, rats had a higher BBB score in myelotomy groups than in contusion groups, with significant differences at 3 weeks after SCI.
No significant difference in BBB score was found between the two comparison groups in the severe injury subgroup. Random effects were used. SD: Standard deviation; CI: confidence interval; SCI: spinal cord injury.
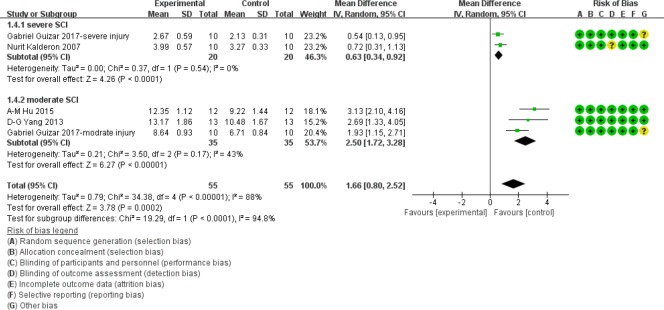
BBB score at 4 weeks after SCI
As shown in Figure 6, in the moderate injury subgroup at 4 weeks after acute SCI, BBB scores were significantly higher in myelotomy groups than contusion control groups (WMD = 2.50; 95% CI: 1.72–3.28; P < 0.001), which suggests a protective effect of myelotomy. The heterogeneity was mild (I2 = 43%; P = 0.17). In the severe injury subgroup, BBB scores in myelotomy groups were also significantly higher compared with contusion groups (WMD = 0.63; 95% CI: 0.34–0.92; P < 0.001). No relevant heterogeneity was found (I2 = 0%; P = 0.54). Accordingly, the overall BBB scores were significantly higher in myelotomy groups than in the contusion control groups (WMD = 1.66; 95% CI, 0.80–2.52; P < 0.001) and a high total heterogeneity existed (I2 = 88%; P < 0.001. Additionally, there was high heterogeneity describing subgroup differences (I2 = 94.8%).
Figure 6.
Subgroup analysis: forest plot shows that in moderate and severe injury subgroups, rats had a higher BBB score in myelotomy groups than in contusion groups, with significant differences at 4 weeks after SCI.
Random effects were used. SD: Standard deviation; CI: confidence interval; SCI: spinal cord injury.
BBB score at 5 weeks after SCI
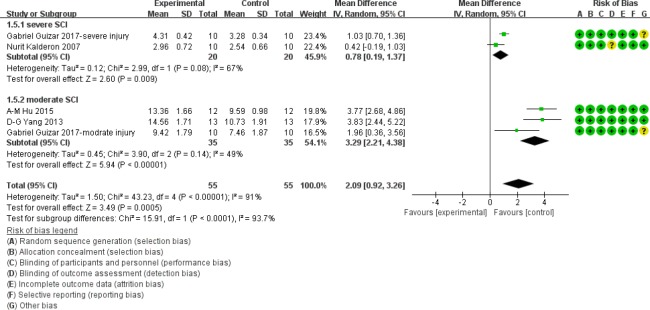
As displayed in Figure 7, at 5 weeks after acute SCI, BBB scores in the moderate injury subgroup were significantly higher in myelotomy groups than in the contusion control groups (WMD = 3.29; 95% CI: 2.21–4.38; P < 0.001), which suggests a protective effect of myelotomy. The heterogeneity was mild (I2 = 49%; P = 0.14). In the severe injury subgroup, BBB scores in myelotomy groups were also higher compared with the contusion groups (WMD = 0.78; 95% CI: 0.19–1.37; P = 0.009). There was a moderate relevant heterogeneity (I2 = 67%; P = 0.08). The overall BBB scores were significantly higher in the myelotomy groups than in the contusion control groups (WMD = 2.09; 95% CI: 0.92–3.26; P < 0.001) and there was a high total heterogeneity (I2 = 91%; P < 0.001). In addition, there was high heterogeneity describing subgroup differences (I2 = 93.7%).
Figure 7.
Subgroup analysis: forest plot shows that in moderate and severe injury subgroups, rats had a higher BBB score in myelotomy groups than in contusion groups, with significant differences at 5 weeks after SCI.
Random effects were used. SD: Standard deviation; CI: confidence interval; SCI: spinal cord injury.
BBB score at 6 weeks after SCI
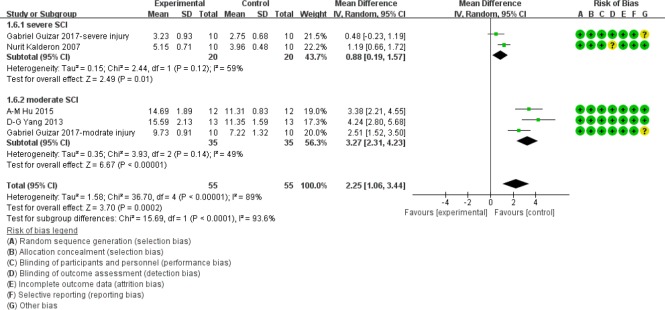
Similarly, at 6 weeks after injury, in the moderate injury subgroup, BBB scores were significantly higher in myelotomy groups than in contusion groups (WMD = 3.27; 95% CI: 2.31–4.23; P < 0.001), which suggests a protective effect of myelotomy. The relevant heterogeneity was mild (I2 = 49%; P = 0.14). In the severe injury subgroup, BBB scores in myelotomy groups were also significantly higher compared with the contusion groups (WMD = 0.88; 95% CI: 0.19–1.57; P = 0.01). There was moderate relevant heterogeneity (I2 = 59%; P = 0.12). The overall BBB scores were also significantly higher in myelotomy groups than in contusion control groups (WMD = 2.25; 95% CI: 1.06–3.44; P < 0.001) and there was a high total heterogeneity (I2 = 89%; P < 0.001). Additionally, there was high heterogeneity describing subgroup differences (I2 = 93.6%). All information is displayed in Figure 8.
Figure 8.
Subgroup analysis: forest plot shows that in moderate and severe injury subgroups, rats had a higher BBB score in myelotomy groups than in contusion groups, with significant differences at 6 weeks after SCI.
Random effects were used. SD: Standard deviation; CI: confidence interval; SCI: spinal cord injury.
Discussion
This systematic review of myelotomy on locomotor recovery is, to our knowledge, the first meta-analysis in this field. The results of our systematic review show that myelotomy promotes locomotor recovery in rats with SCI.
The results of this review are consistent with our previous publication, indicating that myelotomy is beneficial for motor function in rats subjected to SCI (Yang et al., 2013). Here, our systematic review centered on the effects of myelotomy on locomotor recovery after SCI in rats, and two subgroups were created based on the degree of injury (moderate or severe). Surgical decompression treatment after traumatic SCI remains controversial in spine surgery. At present, some researchers believe that releasing extradural elements is a substantial therapeutic strategy for SCI, due to neurological recovery (Nakamura et al., 2016; Richard-Denis et al., 2016; Takao et al., 2016; Aarabi et al., 2017; Agostinello et al., 2017; De la Garza Ramos et al., 2017; Gundogdu et al., 2017; Turtle et al., 2017). Indeed, early durotomy has been adopted to decompress from the meninges (Smith et al., 2010; Shrestha et al., 2014; Saadoun et al., 2016). However, it is insufficient, because decompression via durotomy cannot fully remove the constraint from the dura and pia maters. In addition, the accumulation of pathological changes induced by necrotic substances and edema may cause more impairments to functional outcomes (Li et al., 2016; Talekar et al., 2016; Zimering and Mesfin, 2016). Consequently, durotomy or pia incision should be considered as an incomplete decompression procedure or partial myelotomy. In contrast, myelotomy can remove hemorrhagic and necrotic tissues by opening the dura and swollen tissues. It is hoped to become be a promising clinical intervention for SCI, with precise localization of the lesion and avoidance of non-lesioned tissue (Hu et al., 2015; Inoue et al., 2017).
One discovery is worth noting in the subgroup analysis. In the moderate injury subgroup, BBB scores were remarkably higher in myelotomy groups than in corresponding contusion groups at all observed weeks (1–6 weeks) after the injury, and relevant heterogeneity was mild. In comparison, in the severe injury subgroup, at 1, 4, 5, and 6 weeks after the contusion, BBB scores in the myelotomy groups showed similar increases compared with the contusion groups. However, the differences in BBB score results were not seen at 2 and 3 weeks after the injury, and the relevant heterogeneity was quite high. These results imply that SCI rats undergoing myelotomy had good motor function in the moderate injury subgroup, but not in the severe injury subgroup. We thus analyze the possible reasons. On the one hand, it is presumed that a severe contusion has more of a passive impact on the spinal cord than a moderate contusion, because it induces more edema and intramedullary hemorrhagic necrosis. As a result, a severe acute inflammatory reaction occurs and hematoma accumulates, which may be detrimental to the enhanced myelination of the spinal cord. Moreover, myelotomy and other surgical decompression procedures can cause potential complications, which produce additional damage to the injured cord tissue, thereby exacerbating functional outcomes (Chung and Mortimer, 2016; Liu et al., 2016; Zhang et al., 2016). On the other hand, because of the high heterogeneity and the limited number of studies in the severe contusion group, some bias may exist. Thus, more studies are needed before justifying the results in the severe contusion group.
The current study also reviewed and summarized other pathological and imaging results, in addition to BBB scores, in the included publications. These data showed that myelotomy can not only reduce adverse pathological changes, but also produce full decompression to create intramedullary spaces without further damaging the injured spinal cord in rat models. These results are representative of the benefits of animal studies over human studies.
In this systematic review, all included studies used the BBB score to evaluate locomotor function in rats after SCI. The BBB score is a very sensitive and reliable tool to evaluate behavioral changes, as previously published (Basso et al., 1995; Sakamoto et al., 2003; Koopmans et al., 2005). Some researchers argue that if BBB scores are less than 8 points, it includes the spontaneous recovery at least (Dakson et al., 2017). However, in this review, the BBB scores of myelotomy groups are 2–4 points, which is higher than in the corresponding contusion groups. Thus, myelotomy benefits locomotor recovery in SCI rats.
Notably, GRADE provides explicit criteria for rating the quality of evidence, including study design, risk of bias, imprecision, inconsistency, indirectness, and magnitude of effect. However, insufficient details are given for the use of animal studies in the GRADE guidelines. Therefore, on the basis of “animal research reporting: in vivo experiment guidelines” and “gold standard publication checklist”, we attempted to reach a GRADE level of evidence in rat models.
These results have certain limitations. First, the numbers of included studies are limited, which may influence the results. Only six comparisons were selected for interpretation of data. Thus, with more relevant studies, a higher-quality meta-analysis could be achieved in the future. Second, the role of timing of surgical decompression after traumatic SCI is controversial (Jazayeri et al., 2015; Jalan et al., 2017). Although all included studies regarded 24 hours after SCI as the potential time window for myelotomy, there is still not enough evidence to support a definite time window. We speculated that a myelotomy procedure at 24 hours after SCI would show a positive effect on locomotor recovery in rat models; however, more studies regarding the optimal time window of myelotomy need to be carried out. Third, the authors classify the rodent studies as randomized controlled trials. The classification of a rodent study this way needs discussion; in animals, the genetic background is usually essentially identical. We thus suggest that other researches focus on other animal models to investigate the effects of myelotomy after SCI.
To date, this is the first attempt to summarize the potential effect of myelotomy on locomotor recovery in SCI rats. It concludes that myelotomy is an effective therapy for SCI in rat models. In addition, myelotomy promotes locomotor recovery especially in rats with moderate injury. However, more studies and meta-analyses should be conducted to validate our conclusions due to the limited study numbers.
Footnotes
Conflicts of interest: The authors declare no competing financial interests.
Financial support: This work was supported by the Special Fund for Basic Scientific Research of Central Public Research Institutes of China, No. 2015CZ-6, 2016CZ-4; a grant from the Beijing Institute for Brain Disorders, No. 201601. The conception, design, execution, and analysis of this article, as well as the preparation of and decision to publish this manuscript, were made independent of any funding organization. The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Reporting statement: This study follows the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of School of Rehabilitation Medicine of Capital Medical University, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by the Special Fund for Basic Scientific Research of Central Public Research Institutes of China, No. 2015CZ-6, 2016CZ-4; a grant from the Beijing Institute for Brain Disorders, No. 201601.
(Copyedited by Gardner B, Raye W, Wang J, Li CH, Qiu Y, Song LP, Zhao M)
References
- Aarabi B, Sansur CA, Ibrahimi DM, Simard JM, Hersh DS, Le E, Diaz C, Massetti J, Akhtar-Danesh N. Intramedullary lesion length on postoperative magnetic resonance imaging is a strong predictor of asia impairment scale grade conversion following decompressive surgery in cervical spinal cord injury. Neurosurgery. 2017;80:610–620. doi: 10.1093/neuros/nyw053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afsharipour B, Sandhu MS, Rasool G, Suresh NL, Rymer WZ. Using surface electromyography to detect changes in innervation zones pattern after human cervical spinal cord injury. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:3757–3760. doi: 10.1109/EMBC.2016.7591545. [DOI] [PubMed] [Google Scholar]
- Agostinello J, Battistuzzo CR, Skeers P, Bernard S, Batchelor PE. Early spinal surgery following thoracolumbar spinal cord injury: process of care from trauma to theater. Spine (Phila Pa 1976) 2017;42:E617–E623. doi: 10.1097/BRS.0000000000001903. [DOI] [PubMed] [Google Scholar]
- Ahuja CS, Martin AR, Fehlings M. Recent advances in managing a spinal cord injury secondary to trauma. F1000Res. 2016 doi: 10.12688/f1000research.7586.1. doi: 10.12688/f1000research.7586.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsters D, Schuurs S, Pershouse K, Power B, Harestad Y, Kendall M, Kuipers P. Factors which facilitate or impede interpersonal interactions and relationships after spinal cord injury: a scoping review with suggestions for rehabilitation. Rehabil Res Pract. 2016;2016:9373786. doi: 10.1155/2016/9373786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora M, Harvey LA, Glinsky JV, Chhabra HS, Hossain S, Arumugam N, Bedi PK, Lavrencic L, Hayes AJ, Cameron ID. Telephone-based management of pressure ulcers in people with spinal cord injury in low- and middle-income countries: a randomised controlled trial. Spinal Cord. 2017;55:141–147. doi: 10.1038/sc.2016.163. [DOI] [PubMed] [Google Scholar]
- Baldea KG, Blackwell RH, Vedachalam S, Kothari AN, Kuo PC, Gupta GN, Turk TMT. Outcomes of percutaneous nephrolithotomy in spinal cord injury patients as compared to a matched cohort. Urolithiasis. 2017;45:501–506. doi: 10.1007/s00240-016-0958-6. [DOI] [PubMed] [Google Scholar]
- Barman A, Sinha MK, Rao PB. Discovertebral (Andersson) lesion of the ankylosing spondylitis, a cause of autonomic dysreflexia in spinal cord injury. Spinal Cord Ser Cases. 2016;2:16008. doi: 10.1038/scsandc.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthelemy A, Gagnon DH, Duclos C. Gait-like vibration training improves gait abilities: a case report of a 62-year-old person with a chronic incomplete spinal cord injury. Spinal Cord Ser Cases. 2016;2:16012. doi: 10.1038/scsandc.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Berlowitz DJ, Wadsworth B, Ross J. Respiratory problems and management in people with spinal cord injury. Breathe (Sheff) 2016;12:328–340. doi: 10.1183/20734735.012616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biglari B, Child C, Yildirim TM, Swing T, Reitzel T, Moghaddam A. Does surgical treatment within 4 hours after trauma have an influence on neurological remission in patients with acute spinal cord injury? Ther Clin Risk Manag. 2016;12:1339–1346. doi: 10.2147/TCRM.S108856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boido M, Garbossa D, Fontanella M, Ducati A, Vercelli A. Mesenchymal stem cell transplantation reduces glial cyst and improves functional outcome after spinal cord compression. World Neurosurg. 2014;81:183–190. doi: 10.1016/j.wneu.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Chikuda H, Ohtsu H, Ogata T, Sugita S, Sumitani M, Koyama Y, Matsumoto M, Toyama Y, investigators O. Optimal treatment for spinal cord injury associated with cervical canal stenosis (OSCIS): a study protocol for a randomized controlled trial comparing early versus delayed surgery. Trials. 2013;14:245. doi: 10.1186/1745-6215-14-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K, Mortimer D. Effective treatment of decompression-related spinal cord injury with a novel hyperbaric oxygen treatment: a case report. Minn Med. 2016;99:57. [PubMed] [Google Scholar]
- Cortes M, Thickbroom GW, Elder J, Rykman A, Valls-Sole J, Pascual-Leone A, Edwards DJ. The corticomotor projection to liminally-contractable forearm muscles in chronic spinal cord injury: a transcranial magnetic stimulation study. Spinal Cord. 2017;55:362–366. doi: 10.1038/sc.2016.161. [DOI] [PubMed] [Google Scholar]
- Da Silva U, Villagra HA, Oliva LL, Marconi NF. EMG activity of upper limb on spinal cord injury individuals during whole-body vibration. Physiol Int. 2016;103:361–367. doi: 10.1556/2060.103.2016.3.10. [DOI] [PubMed] [Google Scholar]
- Dakson A, Brandman D, Thibault-Halman G, Christie SD. Optimization of the mean arterial pressure and timing of surgical decompression in traumatic spinal cord injury: a retrospective study. Spinal Cord. 2017;55:1033–1038. doi: 10.1038/sc.2017.52. [DOI] [PubMed] [Google Scholar]
- De la Garza Ramos R, Nakhla J, Nasser R, Jada A, Sciubba DM, Kinon MD, Yassari R. The impact of hospital teaching status on timing of intervention, inpatient morbidity, and mortality after surgery for vertebral column fractures with spinal cord injury. World Neurosurg. 2017;99:140–144. doi: 10.1016/j.wneu.2016.11.111. [DOI] [PubMed] [Google Scholar]
- Edmond P. Measurement in spinal cord injury. Spinal Cord. 2004;42:209–210. doi: 10.1038/sj.sc.3101557. [DOI] [PubMed] [Google Scholar]
- El Tecle NE, Dahdaleh NS, Hitchon PW. Timing of surgery in spinal cord injury. Spine (Phila Pa 1976) 2016;41:E995–E1004. doi: 10.1097/BRS.0000000000001517. [DOI] [PubMed] [Google Scholar]
- Fehlings MG. The impact of continued cord compression following traumatic spinal cord injury. J Neurosurg Spine. 2009;11:568–569. doi: 10.3171/2009.5.SPINE09417. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Perrin RG. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine (Phila Pa 1976) 2006;31:S28–S36. doi: 10.1097/01.brs.0000217973.11402.7f. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Arvin B. The timing of surgery in patients with central spinal cord injury. J Neurosurg Spine. 2009;10:1–2. doi: 10.3171/2008.10.SPI0822. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Sekhon LH, Tator C. The role and timing of decompression in acute spinal cord injury: what do we know? What should we do. Spine (Phila Pa 1976) 2001;26:S101–S110. doi: 10.1097/00007632-200112151-00017. [DOI] [PubMed] [Google Scholar]
- Furlan JC, Craven BC, Massicotte EM, Fehlings MG. Early versus delayed surgical decompression of spinal cord after traumatic cervical spinal cord injury: a cost-utility analysis. World Neurosurg. 2016;88:166–174. doi: 10.1016/j.wneu.2015.12.072. [DOI] [PubMed] [Google Scholar]
- Grassner L, Wutte C, Klein B, Mach O, Riesner S, Panzer S, Vogel M, Buhren V, Strowitzki M, Vastmans J, Maier D. Early decompression (< 8 h) after traumatic cervical spinal cord injury improves functional outcome as assessed by spinal cord independence measure after one year. J Neurotrauma. 2016;33:1658–1666. doi: 10.1089/neu.2015.4325. [DOI] [PubMed] [Google Scholar]
- Guizar-Sahagun G, Martinez-Cruz A, Franco-Bourland RE, Cruz-Garcia E, Corona-Juarez A, Diaz-Ruiz A, Grijalva I, Reyes-Alva HJ, Madrazo I. Creation of an intramedullary cavity by hemorrhagic necrosis removal 24 h after spinal cord contusion in rats for eventual intralesional implantation of restorative materials. PLoS One. 2017;12:e0176105. doi: 10.1371/journal.pone.0176105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundogdu I, Ozturk EA, Umay E, Karaahmet OZ, Unlu E, Cakci A. Implementation of a respiratory rehabilitation protocol: weaning from the ventilator and tracheostomy in difficult-to-wean patients with spinal cord injury. Disabil Rehabil. 2017;39:1162–1170. doi: 10.1080/09638288.2016.1189607. [DOI] [PubMed] [Google Scholar]
- Gunnarsson T, Fehlings MG. Acute neurosurgical management of traumatic brain injury and spinal cord injury. Curr Opin Neurol. 2003;16:717–723. doi: 10.1097/01.wco.0000102629.16692.ee. [DOI] [PubMed] [Google Scholar]
- Gupta DK, Vaghani G, Siddiqui S, Sawhney C, Singh PK, Kumar A, Kale SS, Sharma BS. Early versus delayed decompression in acute subaxial cervical spinal cord injury: A prospective outcome study at a Level I trauma center from India. Asian J Neurosurg. 2015;10:158–165. doi: 10.4103/1793-5482.161193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Bathen ME, Smith JS, Levi AD, Bhatia NN, Steward O. Advances in the management of spinal cord injury. J Am Acad Orthop Surg. 2010;18:210–222. doi: 10.5435/00124635-201004000-00004. [DOI] [PubMed] [Google Scholar]
- He B, Nan G. Neuronal regeneration after acute spinal cord injury in adult rats. Spine J. 2016;16:1459–1467. doi: 10.1016/j.spinee.2016.06.020. [DOI] [PubMed] [Google Scholar]
- Hu AM, Li JJ, Sun W, Yang DG, Yang ML, Du LJ, Gu R, Gao F, Li J, Chu HY, Zhang X, Gao LJ. Myelotomy reduces spinal cord edema and inhibits aquaporin-4 and aquaporin-9 expression in rats with spinal cord injury. Spinal Cord. 2015;53:98–102. doi: 10.1038/sc.2014.209. [DOI] [PubMed] [Google Scholar]
- Inoue T, Suzuki S, Endo T, Uenohara H, Tominaga T. Efficacy of early surgery for neurological improvement in spinal cord injury without radiographic evidence of trauma in the elderly. World Neurosurg. 2017;105:790–795. doi: 10.1016/j.wneu.2017.06.070. [DOI] [PubMed] [Google Scholar]
- Jalan D, Saini N, Zaidi M, Pallottie A, Elkabes S, Heary RF. Effects of early surgical decompression on functional and histological outcomes after severe experimental thoracic spinal cord injury. J Neurosurg Spine. 2017;26:62–75. doi: 10.3171/2016.6.SPINE16343. [DOI] [PubMed] [Google Scholar]
- Jazayeri SB, Beygi S, Shokraneh F, Hagen EM, Rahimi-Movaghar V. Incidence of traumatic spinal cord injury worldwide: a systematic review. Eur Spine J. 2015;24:905–918. doi: 10.1007/s00586-014-3424-6. [DOI] [PubMed] [Google Scholar]
- Kalderon N, Muruganandham M, Koutcher JA, Potuzak M. Therapeutic strategy for acute spinal cord contusion injury: cell elimination combined with microsurgical intervention. PLoS One. 2007;2:e565. doi: 10.1371/journal.pone.0000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans GC, Deumens R, Honig WM, Hamers FP, Steinbusch HW, Joosten EA. The assessment of locomotor function in spinal cord injured rats: the importance of objective analysis of coordination. J Neurotrauma. 2005;22:214–225. doi: 10.1089/neu.2005.22.214. [DOI] [PubMed] [Google Scholar]
- Li JA, Zan CF, Xia P, Zheng CJ, Qi ZP, Li CX, Liu ZG, Hou TT, Yang XY. Key genes expressed in different stages of spinal cord ischemia/reperfusion injury. Neural Regen Res. 2016;11:1824–1829. doi: 10.4103/1673-5374.194754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ. Effects of myelotomy on autophagy in injured spinal cord in rats. Zhongguo Kangfu Lilun yu Shijian. 2015;11:904–905. [Google Scholar]
- Liu JM, Long XH, Zhou Y, Peng HW, Liu ZL, Huang SH. Is urgent decompression superior to delayed surgery for traumatic spinal cord injury? A Meta-Analysis. World Neurosurg. 2016;87:124–131. doi: 10.1016/j.wneu.2015.11.098. [DOI] [PubMed] [Google Scholar]
- Mattiassich G, Gollwitzer M, Gaderer F, Blocher M, Osti M, Lill M, Ortmaier R, Haider T, Hitzl W, Resch H, Aschauer-Wallner S. Functional outcomes in individuals undergoing very early (< 5 h) and early (5-24 h) surgical decompression in traumatic cervical spinal cord injury: analysis of neurological improvement from the austrian spinal cord injury study. J Neurotrauma. 2017;34:3362–3371. doi: 10.1089/neu.2017.5132. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Inaba Y, Aota Y, Oba M, Machida J, Aida N, Kurosawa K, Saito T. New radiological parameters for the assessment of atlantoaxial instability in children with Down syndrome: the normal values and the risk of spinal cord injury. Bone Joint J. 2016;98-B:1704–1710. doi: 10.1302/0301-620X.98B12.BJJ-2016-0018.R1. [DOI] [PubMed] [Google Scholar]
- Piazza M, Schuster J. Timing of surgery after spinal cord injury. Neurosurg Clin N Am. 2017;28:31–39. doi: 10.1016/j.nec.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Richard-Denis A, Ehrmann Feldman D, Thompson C, Bourassa-Moreau E, Mac-Thiong JM. Costs and length of stay for the acute care of patients with motor-complete spinal cord injury following cervical trauma: the impact of early transfer to specialized acute sci center. Am J Phys Med Rehabil. 2016;96:449–456. doi: 10.1097/PHM.0000000000000659. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Werndle MC, Lopez de Heredia L, Papadopoulos MC. The dura causes spinal cord compression after spinal cord injury. Br J Neurosurg. 2016;30:582–584. doi: 10.3109/02688697.2016.1173191. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Kawaguchi M, Kurita N, Horiuchi T, Kakimoto M, Inoue S, Furuya H, Nakamura M, Konishi N. Long-term assessment of hind limb motor function and neuronal injury following spinal cord ischemia in rats. J Neurosurg Anesthesiol. 2003;15:104–109. doi: 10.1097/00008506-200304000-00007. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Saucier DA, Cain ME. A statistical method for analyzing rating scale data: the BBB locomotor score. J Neurotrauma. 2002;19:1251–1260. doi: 10.1089/08977150260338038. [DOI] [PubMed] [Google Scholar]
- Shrestha B, Coykendall K, Li Y, Moon A, Priyadarshani P, Yao L. Repair of injured spinal cord using biomaterial scaffolds and stem cells. Stem Cell Res Ther. 2014;5:91. doi: 10.1186/scrt480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Anderson R, Pham T, Bhatia N, Steward O, Gupta R. Role of early surgical decompression of the intradural space after cervical spinal cord injury in an animal model. J Bone Joint Surg Am. 2010;92:1206–1214. doi: 10.2106/JBJS.I.00740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao T, Okada S, Morishita Y, Maeda T, Kubota K, Ideta R, Mori E, Yugue I, Kawano O, Sakai H, Ueta T, Shiba K. Clinical influence of cervical spinal canal stenosis on neurological outcome after traumatic cervical spinal cord injury without major fracture or dislocation. Asian Spine J. 2016;10:536–542. doi: 10.4184/asj.2016.10.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talekar K, Poplawski M, Hegde R, Cox M, Flanders A. Imaging of spinal cord injury: acute cervical spinal cord injury, cervical spondylotic myelopathy, and cord herniation. Semin Ultrasound CT MR. 2016;37:431–447. doi: 10.1053/j.sult.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Turtle JD, Strain MM, Aceves M, Huang YJ, Reynolds JA, Hook MA, Grau JW. Pain input impairs recovery after spinal cord injury: Treatment with Lidocaine. J Neurotrauma. 2017;34:1200–1208. doi: 10.1089/neu.2016.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Middendorp JJ, Hosman AJ, Doi SA. The effects of the timing of spinal surgery after traumatic spinal cord injury: a systematic review and meta-analysis. J Neurotrauma. 2013;30:1781–1794. doi: 10.1089/neu.2013.2932. [DOI] [PubMed] [Google Scholar]
- Wu YJ, Hou YN, Zhang ZT, Liu ZP, Nie ZH, Fan GH. Early exercise training combined with neural stem cell transplantation improves hindlimb motor function after spinal cord injury in rats. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:876–882. [Google Scholar]
- Yang DG, Li JJ, Gu R, Yang ML, Zhang X, Du LJ, Sun W, Gao F, Hu AM, Wu YY, He JG, Feng YT, Chu HY. Optimal time window of myelotomy in rats with acute traumatic spinal cord injury: a preliminary study. Spinal Cord. 2013;51:673–678. doi: 10.1038/sc.2013.56. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang H, Zhang C, Li W. Intrathecal decompression versus epidural decompression in the treatment of severe spinal cord injury in rat model: a randomized, controlled preclinical research. J Orthop Surg Res. 2016;11:34. doi: 10.1186/s13018-016-0369-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Feng YP, Young W, You SW, Shen XF, Liu YS, Ju G. Early neurosurgical intervention of spinal cord contusion: an analysis of 30 cases. Chin Med J (Engl) 2008;121:2473–2478. [PubMed] [Google Scholar]
- Zimering JH, Mesfin A. Posterior reversible encephalopathy syndrome following elevated mean arterial pressures for cervical spinal cord injury. J Spinal Cord Med. 2016:1–4. doi: 10.1080/10790268.2016.1250030. [DOI] [PMC free article] [PubMed] [Google Scholar]