Abstract
Spinal cord injury (SCI) results in lesions that destroy tissue and disrupt spinal tracts, producing deficits in locomotor and autonomic function. The majority of treatment strategies after SCI have concentrated on the damaged spinal cord, for example working to reduce lesion size or spread, or encouraging regrowth of severed descending axonal projections through the lesion, hoping to re-establish synaptic connectivity with caudal targets. In our work, we have focused on a novel target for treatment after SCI, surviving spinal motoneurons and their target musculature, with the hope of developing effective treatments to preserve or restore lost function following SCI. We previously demonstrated that motoneurons, and the muscles they innervate, show pronounced atrophy after SCI. Importantly, SCI-induced atrophy of motoneuron dendrites can be attenuated by treatment with gonadal hormones, testosterone and its active metabolites, estradiol and dihydrotestosterone. Similarly, SCI-induced reductions in muscle fiber cross-sectional areas can be prevented by treatment with androgens. Together, these findings suggest that regressive changes in motoneuron and muscle morphology seen after SCI can be ameliorated by treatment with gonadal hormones, further supporting a role for steroid hormones as neurotherapeutic agents in the injured nervous system.
Keywords: testosterone, dihydrotestosterone, estradiol, steroids, atrophy, neuroprotection, morphology, dendrites, muscle fibers, retrograde labeling
Introduction
Spinal cord injury (SCI) is a devastating medical problem with high mortality and long-term morbidity. The number of SCI patients in the US who were alive in 2017 is between 245,000–353,000, with an annual incidence of 17,500 new cases; the estimated lifetime cost of SCI is $1.6–4.8M per patient (National Spinal Cord Injury Statistical Center (NSCISC), 2018).
The pathophysiology of SCI is complex, and after the initial mechanical deformation, a protracted period of progressive damage occurs, causing spreading of the lesion and further segmental destruction (Liu et al., 1997). A variety of mechanisms contribute to this progressive secondary injury, including excitotoxicity (Liu et al., 1991), free radical generation (Diaz-Ruiz et al., 2002), protease activation (Wang et al., 1997), and inflammation (Ritz and Hausmann, 2008; Liu et al., 2009; Liu and Xu, 2010), resulting in the death of motoneurons, interneurons, and glial cells in the spinal cord (Liu et al., 1997; Liu and Xu, 2010). Similarly, damage to spinal nerves resulting in laceration and avulsion of spinal roots (e.g., cauda equina injury with high impact motor vehicle accidents; Moschilla et al., 2001) can lead to the death of motoneurons and preganglionic autonomic neurons in the spinal cord, resulting in autonomic and motor dysfunction (Hoang et al., 2003).
Surviving Motoneurons as a Treatment Target
The majority of treatment strategies after SCI have concentrated on the damaged spinal cord, for example working to reduce lesion size or spread, or encouraging regrowth of severed descending axonal projections through the lesion, hoping to re-establish synaptic connectivity with caudal targets. We have focused on a novel target for treatment after SCI, surviving spinal motoneurons and their target musculature. In contrast to the extensive studies on neuroprotection and axonal regeneration at the lesion site, the morphological and functional consequences of SCI for surviving motoneurons have been significantly understudied. The spinal motoneurons are the final common pathway for motor output to the effector muscles, and any impairment in these motoneurons can cause paralysis and muscle atrophy. Motoneurons in the lumbar spinal cord can be impaired by direct injury, but are far more commonly indirectly impaired after an “above-level injury”, where the injury occurs above the lumbar level; such above-level injuries account for 90% of all SCIs in human patients (National Spinal Cord Injury Statistical Center (NSCISC), 2018). Lesions caused by these injuries damage descending motor and propriospinal tracts, resulting in dendritic atrophy in the lumbar motoneurons, muscle atrophy, and concomitant locomotor deficits (Byers et al., 2012; Liu et al., 2014a, b; Sengelaub et al., 2018). Surviving motoneurons are thus a potential therapeutic target, and developing the ability to protect them from secondary atrophy is an important goal. As there are currently no effective treatments to preserve or restore lost function following SCI, identification of approaches that result in spared tissues/cell populations that may subsequently be the targets of regenerative therapy and/or rehabilitative plasticity interventions would be significant.
We reasoned that protecting spinal motoneurons from SCI-induced atrophy would have beneficial effects, and for the past several years we have been exploring novel treatment strategies to protect surviving motoneurons after SCI. In this review, we briefly summarize our work in a clinically relevant model in rats using steroid gonadal hormones as a powerful neurotherapeutic approach in the treatment of the secondary effects of spinal cord injury.
Neuroprotection with Androgens and Estrogens
Androgens and estrogens have been demonstrated to powerful neuroprotective effects after a wide variety of neural injuries (Foecking et al., 2015; Brotfain et al., 2016). For example, both testosterone and estradiol protect against cell death (Pike, 2001; Yune et al., 2004), promote functional recovery (Jones et al., 2001; Sribnick et al., 2010), and stimulate motoneuron axonal growth after peripheral nerve injury (Kujawa et al., 1989; Islamov, et al., 2003). The mechanisms through which androgens and estrogens act are multiple, and include regulation of apoptosis (Fargo et al., 2009; Kachadroka et al., 2010), injury-induced upregulation of glial fibrillary acidic protein (GFAP; Jones et al., 1997; Samantaray et al., 2016), and mediation of the glial response (Jones et al., 1999; Ritz and Hausmann, 2008). Proteins thought to be involved in neuroprotection are also regulated by androgens and estrogens, including proteins with antioxidant or pro-inflammatory functions (Ahlbom et al., 2001; Nilsen, 2008; Ritz and Hausmann, 2008) and the neurotrophin brain-derived neurotrophic factor (BDNF; Solum and Handa, 2002; Verhovshek et al., 2010).
Gonadal steroid hormones provide protection from many of the pathophysiological changes specifically seen after SCI, for example reducing the inflammation and free radical generation that contribute to progressive secondary injury. After SCI, treatment of rats with estradiol resulted in improved motor function, reduced inflammation, attenuated apoptotic cell death, reduced lesion size, increased white matter sparing, and earlier cytokine release and astroglial response (Yune et al., 2004; Sribnick et al., 2005, 2010; Ritz and Hausmann, 2008; Kachadroka et al., 2010; Brotfain et al., 2016; Samantaray et al., 2016). Similarly, treatment with testosterone improves motor function in spinal cord injury patients. Patients treated with testosterone had higher American Spinal Injury Association (ASIA) discharge motor scores, a result ascribed to either improved strength through the anabolic effects of testosterone on skeletal muscle or its neuroprotective effects (Clark et al., 2008).
Spinal Lesions
Consistent with previous studies, in our work we demonstrated that following contusion, the focal injuries delivered to the spinal cord developed into large lesions that spanned multiple thoracic spinal segments. Also consistent with previous studies (Yune et al., 2004; Sribnick et al., 2005; Chaovipoch et al., 2006; Ritz and Hausmann, 2008; Kachadroka et al., 2010; Siriphorn et al., 2012; Mosquera et al., 2014; Samantaray et al., 2016), treatment with estradiol was effective in reducing lesion volume; lesion volumes in animals treated only with estradiol were significantly smaller than those of all other groups (Sengelaub et al., 2018). This reduction in lesion size is thought to be the result of reducing inflammation, reactive astrogliosis, decreased immune response, apoptotic cell death, or reductions in oxidative stress (Yune et al., 2004; Ritz and Hausmann, 2008; Kachadroka et al., 2010; Siriphorn et al., 2012; Mosquera et al., 2014; Samantaray et al., 2016). Importantly, the reduction in lesion size we observed was produced through a physiological dose of estradiol, a result similar that reported by Samantary et al. (2016) with low doses of estradiol. The efficacy of low dosages indicates that estradiol could be a promising therapeutic agent for treating SCI (Samantaray et al., 2016). Furthermore, in our work, estradiol was administered after trauma, modeling a clinically relevant situation.
In contrast, treatment with androgens, either alone or when combined with estradiol, proved to be ineffective in reducing lesion size. Four weeks of treatment with testosterone, dihydrotestosterone, or dihydrotestosterone combined with estradiol had no effect on reducing lesion volume or increased tissue sparing (Byers et al., 2012; Sengelaub et al., 2018). Curiously, the effect of estradiol on decreasing lesion volume was not present when estradiol was co-administered with dihydrotestosterone. This negation of the protective effect of estradiol is similar to that reported by Hauben et al. (2002), wherein treatment of female rats with dihydrotestosterone prior to SCI impaired recovery. Given that androgens have been demonstrated to regulate many of the same neuroprotective effects seen with estradiol treatment, e.g., protecting against cell death (Pike, 2001), upregulating GFAP (Jones et al., 1997; Coers et al., 2002) or mediating the central glial response after injury (Jones et al., 1999), this negation with combined treatment after SCI warrants further study. One plausible mechanism for this negation with combined treatment could be through an androgen-mediated immunosuppression (Grossman, 1984). Regardless, given that testosterone is routinely metabolized into both estrogenic and androgenic metabolites, this negation could underlie the failure of testosterone treatment to affect SCI lesion volume we previously reported (Sengelaub et al., 2018).
Neuromuscular Protection after SCI
Although extensive, the spinal lesions produced in our studies did not extend into the lumbar spinal cord, thus sparing the gray matter and resident motoneurons. We selected lumbar motoneurons innervating the quadriceps muscle as our population of interest because of the major weight-bearing role this muscle plays. Counts of either Nissl-stained or retrogradely-labeled quadriceps motoneurons in SCI animals did not differ from those of sham animals, confirming that the lumbar motoneurons were not directly damaged by SCI-induced lesions. Similarly, soma size of quadriceps motoneurons was not significantly affected by SCI. Although quadriceps motoneuron number or soma size were unaffected after SCI, dendritic length in these motoneurons underwent marked dendritic atrophy (Figure 1). Dendritic length decreased by over 50% SCI animals compared to that of sham animals (Figure 2A). Reductions in dendritic length occurred throughout the radial distribution in SCI animals compared to sham animals, and were especially pronounced ventromedially where quadriceps motoneuron dendrites normally have a dense ramification into lamina VIII (Figure 2B). It is likely that the dendritic atrophy we observed following SCI in untreated animals reflects deafferentation resulting from the loss of descending motor and propriospinal tracts. Because both reticulospinal and propriospinal projections are concentrated in this area (Motorina, 1977; Jones and Yang, 1985; Menétey et al., 1985), the extensive lesions present after SCI could have produced a major denervation of dendrites in this area, resulting in the pronounced dendritic atrophy we observed. This loss is of particular significance after SCI, as descending reticulospinal fibers course through the ventral and lateral funiculi (Jones and Yang, 1985; Martin et al., 1985), and disruption of these tracts results in hindlimb motor deficits (Magnuson et al., 1999; Loy et al., 2002).
Figure 1.
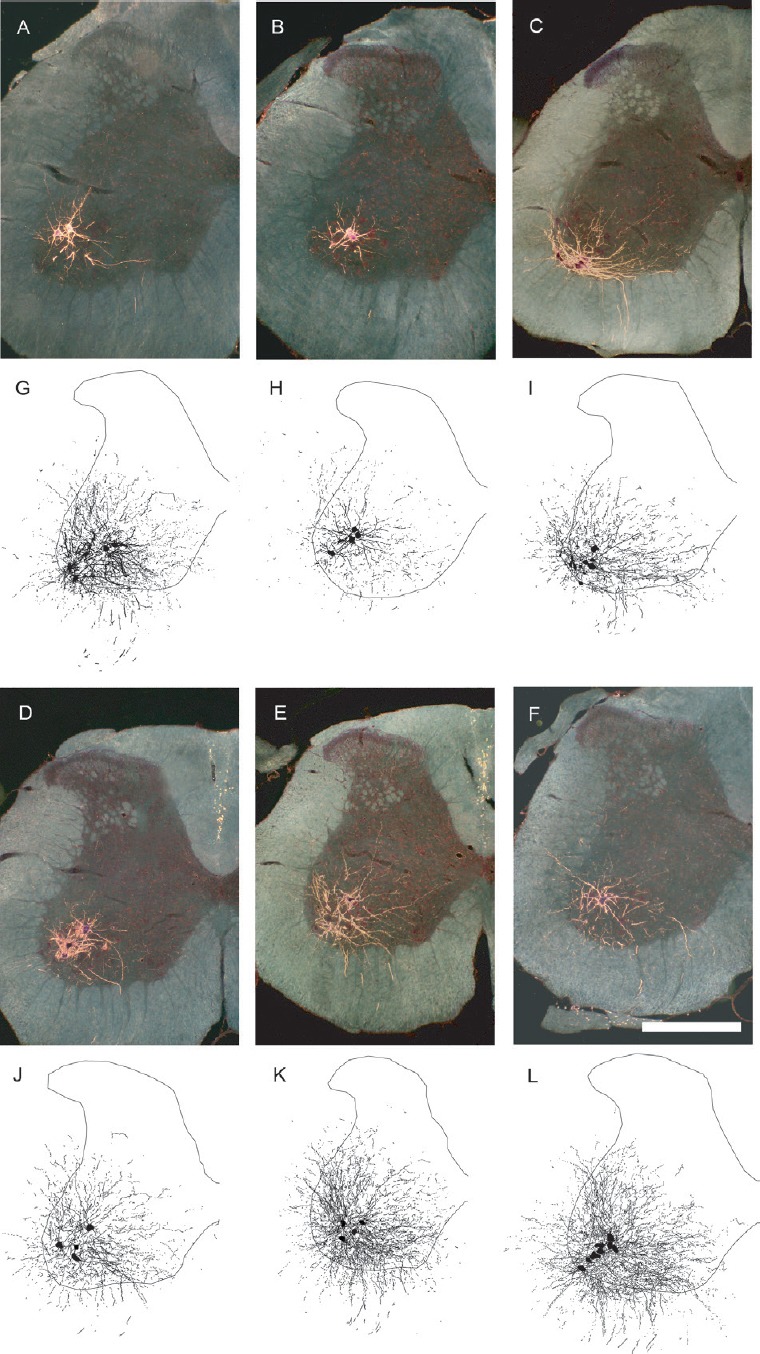
Motoneuron morphology is protected by gonadal hormones following spinal cord injury.
Darkfield digital micrographs and matching computer-generated composites of transverse hemisections through the lumbar spinal cords of a sham animal (A, G), an injured animal given a blank implant (SCI; B, H), an estradiol-treated injured animal (SCI + E; C, I), a dihydrotestosterone-treated injured animal (SCI + D; D, J), an injured animal treated with both hormones (SCI + E + D; E, K), and a testosterone-treated injured animal (SCI + T; F, L), after horseradish peroxidase conjugated to the cholera toxin B subunit (BHRP) injection into the left vastus lateralis muscle. Computer-generated composites of BHRP-labeled somata and processes were drawn at 480 μm intervals through the entire rostrocaudal extent of the quadriceps motor pool; these composites were selected because they are representative of their respective group average dendritic lengths. Scale bar: 500 µm. (Images from Byers et al. (2012) and Sengelaub et al. (2018).
Figure 2.
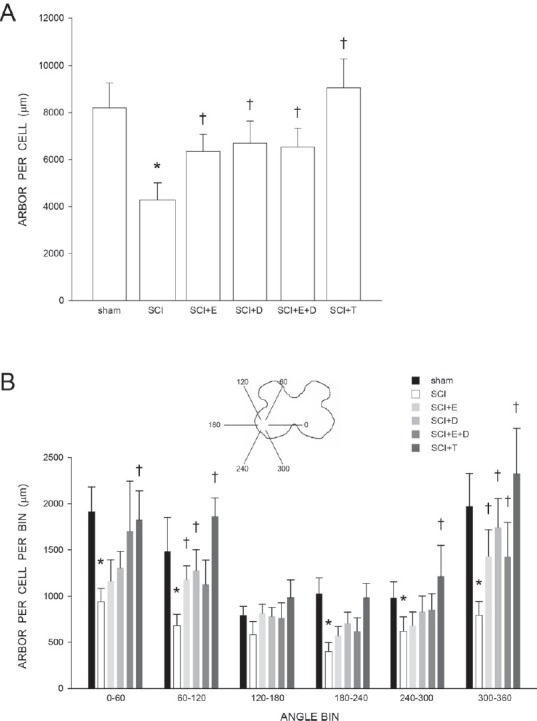
Motoneuron dendritic length and distribution is protected by gonadal hormones following spinal cord injury.
(A) Dendritic lengths of quadriceps motoneurons of sham animals and injured animals that were either untreated (SCI), or treated with estradiol (SCI + E), dihydrotestosterone (SCI + DHT), estradiol and dihydrotestosterone combined (SCI + E + DHT), or testosterone (SCI + T). Following contusion injury, surviving quadriceps motoneurons lost over 50% of their dendritic length. Treatment with hormones attenuated this dendritic atrophy. (B) Inset: Drawing of spinal gray matter divided into radial sectors for measure of quadriceps motoneuron dendritic distribution. Quadriceps motoneuron dendritic arbors normally display a non-uniform distribution, with the majority of the arbor located between 300° and 120°. Following contusion injury, surviving quadriceps motoneurons in untreated animals (SCI) had reduced dendritic lengths throughout the radial distribution, especially ventromedially (60%, 300° to 360°). Treatment with hormones attenuated these reductions. Bar heights represent the mean ± SEM. *indicates significantly different from sham animals, † indicates significantly different from untreated SCI. (Data from Byers et al. (2012) and Sengelaub et al. (2018).
We further demonstrated that SCI-induced atrophy of quadriceps motoneuron dendrites was attenuated in estradiol-, dihydrotestosterone-, estradiol combined with dihydrotestosterone-, and testosterone-treated animals, and dendritic lengths in hormone-treated SCI groups did not differ from those of sham animals. Dendritic lengths in hormone-treated SCI groups were also significantly longer than those of untreated SCI animals by at least 57%. Interestingly, similar effects on dendritic length were present after treatment with androgens alone or in combination with estradiol, despite there being no reductions in lesion size or increases in tissue sparing in these groups (see above).
Because these effects were seen independent of lesion size, our results suggest that these hormonal effects could potentially be the result of local action on spinal circuitry below the level of the lesion. It is likely that the attenuation in SCI-induced dendritic atrophy we observed could have been produced by a hormone-mediated sprouting of motoneuron dendrites locally onto remaining afferents. Sprouting could potentially maintain motor activation, and such an effect of hormones on attenuating dendritic atrophy and supporting motoneuron activation has in fact been directly demonstrated (Fargo et al., 2009; Little et al., 2009; Foecking et al., 2015). The mechanisms responsible for this sprouting are not clear, but gonadal hormones have been shown to regulate the expression of cytoskeletal proteins (e.g., β-tubulin, Jones and Oblinger, 1994; Matsumoto et al., 1994; Jones et al., 1999; Brown et al., 2001; actin and microtubule-associated protein 2, Hansberg-Pastor et al., 2015), as well as neuritin, a critical downstream mediator of the ability of gonadal hormones to increase neurite outgrowth (Marron et al., 2005; Fargo et al., 2008a, b). Sprouting could be driven by direct action on the motoneurons or via indirect action on afferents. Thus, it is possible that a hormone-mediated protection of local spinal circuitry below the level of the lesion could be responsible for the motoneurons dendritic protection we observed. One possible protected spinal population could be the short axon propriospinal neurons, which provide the largest source of input to lumbar spinal motoneurons (Szentagothai, 1951; Sterling and Kuypers, 1968; Rustioni et al., 1971). Changes in these afferents could underlie the regressive changes we have observed in motoneurons after SCI. Afferent input to motoneurons is important for the maintenance of their dendritic morphology, and deafferentation of motoneurons results in dendritic retraction (Bernstein and Standler, 1983; Bernstein et al., 1984; Standler and Bernstein, 1984); the rescue of the major afferent source to motoneurons could underlie the beneficial effects of hormone treatment on motoneuron dendrities we have observed.
Following SCI, we found that quadriceps muscle fiber cross-sectional area in untreated SCI animals was decreased by 25%, typical of muscles innervated by motoneurons below the level of the lesion, especially in weight-bearing muscles such as the quadriceps (Peckham et al., 1976; Giangregorio and McCartney, 2006; Figure 3). Muscle atrophy after SCI can result from either muscle denervation due to a loss of motoneurons or disuse consequent to decreases in muscle activation potentially due to the loss of synaptic input to remaining motoneurons (Gordan and Mao, 1994). The atrophy we observed in our work cannot be ascribed to an effect of denervation, as we observed no changes in quadriceps motoneuron number, or the number of horseradish peroxidase conjugated to the cholera toxin B subunit (BHRP)-labeled quadriceps motoneurons between sham animals and untreated SCI animals. Thus, the decreased fiber size we observed most likely reflects a disuse atrophy, potentially resulting after damage to descending and propriospinal projections and/or the reductions in quadriceps motoneuron dendritic length we observed. Such reductions in quadriceps motoneuron dendritic length result in attenuation of motor activation, reducing response amplitudes in the femoral nerve generated by dorsal root afferent stimulation (Little et al., 2009). Alternatively, disuse atrophy may also result from changes in muscle length or loading conditions that could decrease protein synthesis and increase protein degradation (Williams and Goldspink, 1973; Goldspink, 1978).
Figure 3.
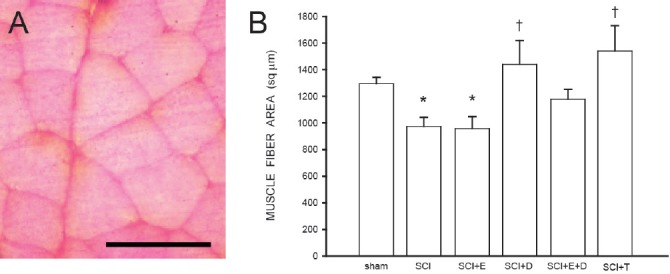
Muscle fiber area is protected by androgens following spinal cord injury.
(A) Cross-section through quadriceps muscle fibers. Scale bar: 100 µm. (B) SCI reduces muscle fiber area; treatment with estradiol (SCI + E) is ineffective, but this reduction is prevented by treatment with dihydrotestosterone (SCI + D), alone or in combination with estradiol (SCI + E + D), or testosterone (SCI + T). Bar heights represent means ± SEM. *significantly different from sham, † significantly different from untreated SCI. (Data from Byers et al. (2012) and Sengelaub et al. (2018).
We found that estradiol treatment was ineffective in preventing muscle fiber atrophy, with areas decreasing 26% after SCI. Although estrogens have a variety of effects in skeletal muscle (e.g., downregulation of proinflammatory cytokines, enhancing insulin-like growth factor-1 (IGF-1) expression, or satellite cell activation and proliferation; Tiidus et al., 2013), their effects on muscle fiber cross-sectional area vary in different muscles and in different directions. Estradiol replacement after ovariectomy has been reported to increase muscle fiber size in the gastrocnemius (Sciote et al., 2001), decrease it in the extensor digitorum longus (Suzuki and Yamamuro, 1985) and plantaris (Piccone et al., 2005), or either increase (Weigt et al., 2015) or decrease (Suzuki and Yamamuro, 1985) fiber size in the soleus.
In contrast, we also found that treatment with testosterone or dihydrotestosterone (either alone or in combination with estradiol) attenuated SCI-induced muscle fiber atrophy. These effects are consistent with the known protein anabolic effects of androgens on skeletal muscle tissue (Kochakian, 1975; Gao, 2010). Thus, treatment with androgens might have supported muscle protein synthesis and decreased protein degradation, and the resultant decrease in protein turnover could have prevented muscle atrophy. Alternatively, androgen treatment could have potentially altered mobility or activity in the treated animals, resulting in the preservation of both muscle as well as the related spinal cord circuitry and motoneuron dendritic morphology. This is quite plausible, as limb exercise after spinal cord transection during postnatal development has in fact been shown to prevent dendritic atrophy in spinal motoneurons (Gazula et al., 2004). Furthermore, exercise is known to elevate the expression of neurotrophic factors (e.g., BDNF) that can promote dendritic and axonal regrowth (Byers et al., 2012; Wilhelm et al., 2012; Sengelaub et al., 2018).
Summary
Overall, our results provided the first evidence of pronounced dendritic atrophy in spinal motoneurons caudal to a contusive injury. More importantly, such atrophy was prevented with treatment with gonadal hormones, supporting their protective role after SCI. Together, our results indicate that the use of gonadal hormones could be an effective treatment after SCI, directed by the particular therapeutic goals. We believe that our work will lead to developing sex-appropriate hormone treatments that will be effective in treating multiple sequelae of SCI.
Footnotes
Conflicts of interest: None declared.
Financial support: This work was supported by grants from Indiana Spinal Cord and Brain Injury Research Fund (ISCBIRF) and by IU's Office of the Vice Provost for Research through the Faculty Research Support Program to DRS, and NIH R01 NS103481, R01 NS100531, Department of Veterans Affairs I01 RX002356, I01 BX003705, Craig H Neilsen Foundation 296749, Indiana Department of Health 019919, ISCBIRF, and Mari Hulman George Endowment Fund to XMX.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Meng-Jen Lee, Chaoyang University of Technology, China; Paul Lu, University of California San Diego, USA.
Funding: This work was supported by grants from Indiana Spinal Cord and Brain Injury Research Fund (ISCBIRF) and by IU's Office of the Vice Provost for Research through the Faculty Research Support Program to DRS, and NIH R01 NS103481, R01 NS100531, Department of Veterans Affairs I01 RX002356, I01 BX003705, Craig H Neilsen Foundation 296749, Indiana Department of Health 019919, ISCBIRF, and Mari Hulman George Endowment Fund to XMX.
References
- Ahlbom E, Prins G, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892:255–262. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- Bernstein JJ, Standler N. Dendritic alteration of rat spinal motoneurons after dorsal horn mince: computer reconstruction of dendritic fields. Exp Neurol. 1983;82:532–540. doi: 10.1016/0014-4886(83)90078-x. [DOI] [PubMed] [Google Scholar]
- Bernstein JJ, Wacker W, Standler N. Spinal motoneuron dendritic alteration after spinal cord hemisection in the rat. Exp Neurol. 1984;83:548–554. doi: 10.1016/0014-4886(84)90122-5. [DOI] [PubMed] [Google Scholar]
- Brotfain E, Gruenbaum SE, Boyko M, Kutz R, Zlotnik A, Klein M. Neuroprotection by estrogen and progesterone in traumatic brain injury and spinal cord injury. Curr Neuropharmacol. 2016;14:641–653. doi: 10.2174/1570159X14666160309123554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TJ, Storer P, Oblinger M, Jones KJ. Androgenic enhancement of ßII-tubulin mRNA in spinal motoneurons following sciatic nerve injury. Rest Neurol Neurosci. 2001;18:191–198. [PubMed] [Google Scholar]
- Byers JS, Huguenard AL, Kuruppu D, Liu NK, Xu XM, Sengelaub DR. Neuroprotective effects of testosterone on muscle and motoneurons morphology following spinal cord injury. J Comp Neurol. 2012;520:2683–2696. doi: 10.1002/cne.23066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaovipoch P, Jelks KA, Gerhold LM, West EJ, Chongthammakun S, Floyd CL. 17 beta-estradiol is protective in spinal cord injury in post- and pre-menopausal rats. J Neurotrauma. 2006;23:830–852. doi: 10.1089/neu.2006.23.830. [DOI] [PubMed] [Google Scholar]
- Clark MJ, Petroski G, Mazurek MO, Hagglund KJ, Sherman AK, Lammy AB, Childers MK, Acuff ME. Testosterone replacement therapy and motor function in men with spinal cord injury. Am J Phys Med Rehabil. 2008;87:281–284. doi: 10.1097/PHM.0b013e318168bbec. [DOI] [PubMed] [Google Scholar]
- Coers S, Tanzer L, Jones KJ. Testosterone treatment attenuates the effects of facial nerve transection on glial fibrillary acidic protein (GFAP) levels in the hamster facial motor nucleus. Metab Brain Dis. 2002;17:55–63. doi: 10.1023/a:1015415226799. [DOI] [PubMed] [Google Scholar]
- Diaz-Ruiz A, Ibarra A, Perez-Severiano F, Guizar-Sahagun G, Grijalva I, Rios C. Constitutive and inducible nitric oxide synthase activities after spinal cord contusion in rats. Neurosci Lett. 2002;319:129–132. doi: 10.1016/s0304-3940(01)02540-x. [DOI] [PubMed] [Google Scholar]
- Fargo KN, Alexander TD, Tanzer L, Poletti A, Jones KJ. Androgen regulates neuritin mRNA in an in vivo model of steroid-enhanced peripheral nerve regeneration. J Neurotrauma. 2008a;25:561–566. doi: 10.1089/neu.2007.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargo KN, Foecking EM, Jones KJ, Sengelaub DR. Neuroprotective actions of androgens on motoneurons. Front Neuroendocrinol. 2009;30:130–141. doi: 10.1016/j.yfrne.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargo, KN, Galbiati M, Foecking EM, Poletti A, Jones KJ. Androgen regulation of axon growth and neurite extension in motoneurons. Horm Behav. 2008b;53:716–728. doi: 10.1016/j.yhbeh.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foecking EM, Fargo KN, Brown TJ, Sengelaub DR, Jones KJ. Gonadal steroids in regeneration and repair of neuromuscular systems. In: So KF, Xu XM, editors. Neural Regeneration. London: Elsevier; 2015. pp. 129–152. [Google Scholar]
- Gao W. Androgen receptor as a therapeutic target. Adv Drug Deliv Rev. 2010;62:1277–1284. doi: 10.1016/j.addr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Gazula VR, Roberts M, Luzzio C, Jawad AF, Kalb RG. Effects of limb exercise after spinal cord injury on motor neuron dendrite structure. J Comp Neurol. 2004;476:130–145. doi: 10.1002/cne.20204. [DOI] [PubMed] [Google Scholar]
- Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: Epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med. 2006;29:489–500. doi: 10.1080/10790268.2006.11753898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink DF. The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol (Lond) 1978;264:267–282. doi: 10.1113/jphysiol.1977.sp011667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordan T, Mao J. Muscle atrophy and procedures for training after spinal cord injury. Phys Ther. 1994;74:50–60. doi: 10.1093/ptj/74.1.50. [DOI] [PubMed] [Google Scholar]
- Grossman CJ. Regulation of the immune system by sex steroids. Endocr Rev. 1984;5:435–455. doi: 10.1210/edrv-5-3-435. [DOI] [PubMed] [Google Scholar]
- Hansberg-Pastor V, González-Arenas A, Piña-Medina AG, Camacho-Arroyo I. Sex hormones regulate cytoskeletal proteins involved in brain plasticity. Front Psychiatry. 2015;6:1–12. doi: 10.3389/fpsyt.2015.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauben E, Mizrahi T, Agranov E, Schwartz M. Sexual dimorphism in the spontaneous recovery from spinal cord injury: a gender gap in beneficial autoimmunity? Eur J Neurosci. 2002;16:1731–1740. doi: 10.1046/j.1460-9568.2002.02241.x. [DOI] [PubMed] [Google Scholar]
- Hoang TX, Nieto J, Tillakaratne NJK, Havton LA. Autonomic and motor neuron death is progressive and parallel in a lumbosacral ventral root avulsion model of cauda equina injury. J Comp Neurol. 2003;467:477–486. doi: 10.1002/cne.10928. [DOI] [PubMed] [Google Scholar]
- Islamov RR, Hendricks WA, Katwa LC, McMurray RJ, Pak ES, Spanier NS, Murashov AK. Effect of 17 beta-estradiol on gene expression in lumbar spinal cord following sciatic nerve crush injury in ovariectomized mice. Brain Res. 2003;966:65–75. doi: 10.1016/s0006-8993(02)04191-4. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Brown TJ, Damaser M. Neuroprotective effects of gonadal steroids on regenerating peripheral motoneurons. Brain Res Rev. 2001;37:372–382. doi: 10.1016/s0165-0173(01)00107-2. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Coers S, Storer PD, Tanzer L, Kinderman NB. Androgenic regulation of the central glia response following nerve damage. J Neurobiol. 1999;40:560–573. doi: 10.1002/(sici)1097-4695(19990915)40:4<560::aid-neu11>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Kinderman NB, Oblinger MM. Alterations in glial fibrillary acidic protein (GFAP) mRNA levels in the hamster facial motor nucleus: effects of axotomy and testosterone. Neurochem Res. 1997;22:1359–1366. doi: 10.1023/a:1022019106417. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Oblinger MM. Androgenic regulation of tubulin gene expression in axotomized hamster facial motoneurons. J Neurosci. 1994;14:3620–3627. doi: 10.1523/JNEUROSCI.14-06-03620.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KJ, Storer PD, Drengler SM, Oblinger MM. Differential regulation of cytoskeletal gene expression in hamster facial motoneurons: Effects of axotomy and testosterone treatment. J Neurosci Res. 1999;57:817–823. [PubMed] [Google Scholar]
- Jones BE, Yang TZ. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol. 1985;242:56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- Kachadroka S, Hall AM, Niedzielko TL, Chongthammakun S, Floyd CL. Effect of endogenous androgens on 17 beta-estradiol-mediated protection after spinal cord injury in male rats. J Neurotrauma. 2010;27:611–626. doi: 10.1089/neu.2009.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochakian CD. Definition of androgens and protein anabolic steroids. Pharmac Therap B. 1975;1:149–177. doi: 10.1016/0306-039x(75)90002-1. [DOI] [PubMed] [Google Scholar]
- Kujawa KA, Kinderman NB, Jones KJ. Testosterone-induced acceleration of recovery from facial paralysis following crush axotomy of the facial nerve in male hamsters. Exp Neurol. 1989;105:80–85. doi: 10.1016/0014-4886(89)90174-x. [DOI] [PubMed] [Google Scholar]
- Little CM, Coons KD, Sengelaub DR. Neuroprotective effects of testosterone on the morphology and function of somatic motoneurons following the death of neighboring motoneurons. J Comp Neurol. 2009;512:359–372. doi: 10.1002/cne.21885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Thangnipon W, McAdoo DJ. Excitatory amino acids rise to toxic levels upon impact injury to the rat spinal cord. Brain Res. 1991;547:344–348. doi: 10.1016/0006-8993(91)90984-4. [DOI] [PubMed] [Google Scholar]
- Liu NK, Byers JS, Lam T, Lu QB, Sengelaub DR, Xu XM. Inhibition of cPLA2 has neuroprotective effects on motoneuron and muscle atrophy following spinal cord injury. J Neurotrauma. 2014a doi: 10.1089/neu.2014.3690. doi:10.1089/neu.2014.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NK, Deng LX, Zhang YP, Lu QB, Wang XF, Hu JG, Oakes E, Shields CB, Xu XM. Cytosolic phospholipase A2 protein as a novel therapeutic target for spinal cord injury. Ann Neurol. 2014b;75:644–658. doi: 10.1002/ana.24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NK, Titsworth WL, Xu XM. Phospholipase A2 in CNS disorders: Implication on traumatic spinal cord and brain injuries. In: Lajtha A, editor. Handbook of Neurochemistry and Molecular Neurobiology. New York: Springer; 2009. pp. 321–341. [Google Scholar]
- Liu NK, Xu XM. Phospholipase A2 and its molecular mechanism after spinal cord injury. Mol Neurobiol. 2010;41:197–205. doi: 10.1007/s12035-010-8101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XZ, Xu XM, Hu R, Du C, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy DN, Talbott JF, Onifer SM, Mills MD, Burke DA, Dennison JB, Fajardo LC, Magnuson DSK, Whittemore SR. Both dorsal and ventral spinal cord pathways contribute to overground locomotion in the adult rat. Exp Neurol. 2002;177:575–580. doi: 10.1006/exnr.2002.7959. [DOI] [PubMed] [Google Scholar]
- Magnuson DSK, Trinder TC, Zhang YP, Burke D, Morassutti DJ, Shields CB. Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp Neurol. 1999;156:191–204. doi: 10.1006/exnr.1999.7016. [DOI] [PubMed] [Google Scholar]
- Marron TU, Guerini V, Rusmini P, Sau D, Brevini TA, Martini L, Poletti A. Androgen-induced neurite outgrowth is mediated by neuritin in motor neurons. J Neurochem. 2005;92:10–20. doi: 10.1111/j.1471-4159.2004.02836.x. [DOI] [PubMed] [Google Scholar]
- Martin GF, Vertes RP, Waltzer R. Spinal projections of the gigantocelluar reticular formation in the rat. Evidence for projections from different areas to laminae I and II and lamina IX. Exp Brain Res. 1985;58:154–162. doi: 10.1007/BF00238963. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y, Urano A, Hyodo S. Androgen regulates gene expression of cytoskeletal proteins in adult rat motoneurons. Horm Behav. 1994;28:357–366. doi: 10.1006/hbeh.1994.1032. [DOI] [PubMed] [Google Scholar]
- Menétey D, De Pommery J, Roudier F. Propriospinal fibers reaching the lumbar enlargement in the rat. Neurosci Lett. 1985;58:257–261. doi: 10.1016/0304-3940(85)90174-0. [DOI] [PubMed] [Google Scholar]
- Moschilla G, Song S, Chakera T. Post-traumatic lumbar nerve root avulsion. Australas Radiol. 2001;45:281–284. doi: 10.1046/j.1440-1673.2001.00921.x. [DOI] [PubMed] [Google Scholar]
- Mosquera L, Colon JM, Santiago JM, Torrado AI, Melendez M, Segarra AC, Rodriguez-Orengo JF, Miranda JD. Tamoxifen and estradiol improved locomotor function and increased spared tissues in rats after spinal cord injury: Their antioxidant effect and role of estrogen receptor alpha. Brain Res. 2014;1561:11–22. doi: 10.1016/j.brainres.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motorina MV. Distribution of reticulospinal fibers and their terminations in lumbar segments of the rat spinal cord. J Evol Biochem Physiol. 1977;12:520–527. [Google Scholar]
- National Spinal Cord Injury Statistical Center (NSCISC) (2018) Facts and Figures at a Glance. https://www.nscisc.uab.edu/
- Nilsen J. Estradiol and neurodegenerative oxidative stress. Front Neuroendocrinol. 2008;29:463–75. doi: 10.1016/j.yfrne.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Peckham PH, Mortimer JT, Marsolais EB. Alteration in the force and fatigability of skeletal muscle in quadriplegic humans following exercise induced by chronic electrical stimulation. Clin Orthop. 1976;114:326–333. [PubMed] [Google Scholar]
- Piccone CM, Brazeau GA, McCormick KM. Effect of oestrogen on myofibre size and myosin expression in growing rats. Exp Physiol. 2005;90:87–93. doi: 10.1113/expphysiol.2004.028373. [DOI] [PubMed] [Google Scholar]
- Pike CJ. Testosterone attenuates ß-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001;919:160–165. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- Ritz MF, Hausmann ON. Effect of 17B-estradiol on functional outcome, release of cytokines, astrocyte reactivity and inflammatory spreading after spinal cord injury in male rats. Brain Res. 2008;1203:177–188. doi: 10.1016/j.brainres.2008.01.091. [DOI] [PubMed] [Google Scholar]
- Rustioni A, Kuypers HG, Holstege G. Propiospinal projections from the ventral and lateral funiculi to the motoneurons in the lumbosacral cord of the cat. Brain Res. 1971;34:255–275. doi: 10.1016/0006-8993(71)90280-0. [DOI] [PubMed] [Google Scholar]
- Samantaray S, Das A, Matzelle DC, Yu SP, Wei L, Varma A, Ray SK, Banik NL. Administration of low dose estrogen attenuates gliosis and protects neurons in acute spinal cord injury in rats. J Neurochem. 2016;136:1064–1073. doi: 10.1111/jnc.13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciote JJ, Horton MJ, Zyman Y, Pascoe G. Differential effects of diminished oestrogen and androgen levels on development of skeletal muscle in hypogonadal mice. Acta Physiol Scand. 2001;172:179–187. doi: 10.1046/j.1365-201x.2001.00854.x. [DOI] [PubMed] [Google Scholar]
- Sengelaub DR, Han Q, Liu NK, Maczuga M, Szalavari V, Valencia SA, Xu XM. Protective effects of estradiol and dihydrotestosterone following spinal cord injury. J Neurotrauma. 2018;35:825–841. doi: 10.1089/neu.2017.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriphorn A, Dunham KA, Chompoopong S, Floyd CL. Postinjury administration of 17β-estradiol induces protection in the gray and white matter with associated functional recovery after cervical spinal cord injury in male rats. J Comp Neurol. 2012;520:2630–2646. doi: 10.1002/cne.23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci. 2002;22:2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sribnick EA, Samantaray S, Das A, Smith J, Matzelle DD, Ray SK, Banik NL. Postinjury estrogen treatment of chronic spinal cord injury improves locomotor function in rats. J Neurosci Res. 2010;88:1738–1750. doi: 10.1002/jnr.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sribnick EA, Wingrave JM, Matzelle DD, Wilford GG, Ray SK, Banik NL. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury rats. J Neurosci Res. 2005;82:283–293. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- Standler N, Bernstein JJ. Dendritic alteration of spinal motoneurons after ablation of somatomotor cortex. Exp Neurol. 1984;83:264–273. doi: 10.1016/S0014-4886(84)90097-9. [DOI] [PubMed] [Google Scholar]
- Sterling P, Kuypers HG. Anatomical organization of the brachial spinal cord of the cat. 3. The propriospinal connections. Brain Res. 1968;7:419–443. doi: 10.1016/0006-8993(68)90008-5. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Yamamuro T. Long-term effects of estrogen on rat skeletal muscle. Exp Neurol. 1985;87:291–299. doi: 10.1016/0014-4886(85)90219-5. [DOI] [PubMed] [Google Scholar]
- Szentagothai J. Short propriospinal neurons and intrinsic connections of the spinal gray. Acta Morphol Acad Sci Hung. 1951;1:81–94. [Google Scholar]
- Tiidus PM, Lowe DA, Brown MN. Estrogen replacement and skeletal muscle: mechanisms and population health. J Appl Physiol. 2013;115:569–578. doi: 10.1152/japplphysiol.00629.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhovshek T, Cai Y, Osborne MC, Sengelaub DR. Androgen regulates brain-derived neurotrophic factor in spinal motoneurons and their target musculature. Endocrinology. 2010;151:253–261. doi: 10.1210/en.2009-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CX, Olshowka JA, Wrathall JR. Increase of interleukin-1beta mRNA and protein in the spinal cord following experimental traumatic injury in the rat. Brain Res. 1997;759:190–196. doi: 10.1016/s0006-8993(97)00254-0. [DOI] [PubMed] [Google Scholar]
- Weigt C, Hertrampf T, Flenker U, Hülsemann F, Kurnaz P, Fritzemeier KH, Diel P. Effects of estradiol, estrogen receptor subtype-selective agonists and genistein on glucose metabolism in leptin resistant female Zucker diabetic fatty (ZDF) rats. J Steroid Biochem Mol Biol. 2015;154:12–22. doi: 10.1016/j.jsbmb.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Wilhelm JC, Xu M, Cucoranu D, Chmielewski S, Holmes T, Lau KS, Bassell GJ, English AW. Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J Neurosci. 2012;32:5002–5009. doi: 10.1523/JNEUROSCI.1411-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PE, Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibers. J Anat. 1973;116:45–55. [PMC free article] [PubMed] [Google Scholar]
- Yune TY, Kim SJ, Lee SM, Lee YK, Oh YJ, Kim YC, Markelonis GJ, Oh TH. Systemic administration of 17 beta-estradiol reduces apoptotic cell death and improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 2004;21:293–306. doi: 10.1089/089771504322972086. [DOI] [PubMed] [Google Scholar]


