Abstract
Surgical brain injury may result in irreversible neurological deficits. Our previous report showed that partial regeneration of a traumatic brain lesion is achieved by implantation of collagen glycosaminoglycan (CGM). Matrix metalloproteinases (MMPs) may play an important role in neurogenesis but there is currently a lack of studies displaying the relationship between the stimulation of MMPs and neurogenesis after collagen glycosaminoglycan implantation following surgical brain trauma. The present study was carried out to further examine the expression of MMP2 and MMP9 after implantation of collagen glycosaminoglycan (CGM) following surgical brain trauma. Using the animal model of surgically induced brain lesion, we implanted CGM into the surgical trauma. Rats were thus divided into three groups: (1) sham operation group: craniotomy only; (2) lesion (L) group: craniotomy + surgical trauma lesion; (3) lesion + CGM (L + CGM) group: CGM implanted following craniotomy and surgical trauma lesion. Cells positive for SOX2 (marker of proliferating neural progenitor cells) and matrix metalloproteinases (MMP2 and MMP9) in the lesion boundary zone were assayed and analyzed by immunofluorescence and ELISA commercial kits, respectively. Our results demonstrated that following implantation of CGM after surgical brain trauma, significant increases in MMP2+/SOX2+ cells and MMP9+/SOX2+ cells were seen within the lesion boundary zone in the L + CGM group. Tissue protein concentrations of MMP2 and MMP9 also increased after CGM scaffold implantation. These findings suggest that implantation of a CGM scaffold alone after surgical brain trauma can enhance the expression of MMP2 and MMP9 accompanied by neurogenesis.
Keywords: collagen glycosaminoglycan, matrix metalloproteinases, surgical brain trauma, neurogenesis, neural regeneration
Introduction
Surgical or traumatic brain injury may lead to serious and irreversible neurological deficits. Surgical brain trauma (SBT) is still inevitable and frequently associated with postoperative neurological deficits (Fugate, 2015). Strategies to increase neuroregeneration after traumatic or surgical brain injury have been reviewed but there is still a lack of effective medical approaches to promote nerve tissue regeneration following surgical brain trauma. In the damaged areas of the brain, both the complicated molecular and cellular environment could contribute to limitations in neural regeneration. Using the animal model of surgical brain injury, our previous study demonstrated that biodegradable collagen glycosaminoglycan matrix (CGM) scaffolds (an extracellular matrix (ECM) analogue) not only offer physical support but also have beneficial effects in serving as a regulator of cell behaviors including adhesion, proliferation, and differentiation, the lesions get smaller over time and it also contains more neurons in the matrix group compared to the injured group (Huang et al., 2012).
The ECM has been demonstrated to play an essential role in preservation of the microenvironment in adult neuronal networks and implantation of its analogue, CGM, can have a favorable effect on adaptation of connectivity and nervous network architecture (Bikbaev et al., 2015). Accumulating evidence illustrates the association of the behavior of both ECM components and adhesion molecules, which provide a microenvironment that may be advantageous for neurogenesis (Bikbaev et al., 2015). ECM molecules have been shown to construct an microenvironment allowing neurogenesis-associated processes and ECM remodeling that is principally regulated by matrix metalloproteinases (MMPs) (Sirbulescu et al., 2015). Earlier reports also revealed that MMPs, especially MMP2 and MMP9 (Barkho et al., 2008; Gueye et al., 2011; Wojcik-Stanaszek et al., 2011; Lei et al., 2013; Verslegers et al., 2013) have been correlated with neurogenesis. Furthermore, there is evidence that a three-dimensional scaffold with controlled porous size and stiffness can offer an ideal extracellular environment for wound healing (Hsu et al., 2000; Harley et al., 2008; Hsu et al., 2008; Huang et al., 2012). However, there is a lack of studies displaying the relationship between the stimulation of MMPs and neuronal regeneration such as proliferation and/or further differentiation after CGM implantation following surgical brain trauma.
MMPs are zinc-dependent proteases and play essential roles in both cell development and pathogenesis. In addition, ECM modifying is also regulated by MMPs. Reports also revealed that both MMP2 and MMP9 contribute to the cellular response which is important for neural maturity. Furthermore, MMP2 is mainly involved in regulation of the function of neural precursor cells (NPCs), whereas MMP9 facilitates migration of the NPCs (Sirbulescu et al., 2015). However, there are still few studies demonstrating the role of MMPs in neurogenesis-associated processes such as proliferation and further differentiation. The aim of the current study is to investigate the role of MMP2 and MMP9 in neurogenesis associated processes after CG matrix implantation following surgical brain trauma.
Materials and Methods
Preparation of CGM
According to the previously described method (Huang et al., 2012), we synthesized a biodegradable collagen matrix (1% collagen (C)/0.02% glycosaminoglycan (GAG) copolymer; Taiwan, China as formerly defined) (Yannas et al., 1989; Hsu et al., 2000; Harley et al., 2008). We prepared type I CGM as a slightly acidic solution and mixed at high speed in order to create a mud-like substance. Via thermal dehydration in a vacuum, it was cross-linked after lyophilization and then exposed to ultraviolet light (Wu et al., 2008). Before implantation, we cut the C-GAG copolymer matrix scaffold into 6 mm× 4 mm× 3 mm (72 mm3) blocks. Optimal degradation time (around 28 days) was designated based on the reported time course of endogenous neural stem cell (NSC) proliferation and differentiation (Kernie and Parent, 2010).
Animal models of surgically induced brain trauma and CGM implantation
All of the animal experimental procedures were approved by the Animal Care and Use Committee of Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, China (No. 103-IACUC-002) and conformed to the Guiding Principles in the Care and Use of Animals of the American Physiology Society. Sprague-Dawley rats (adult male, weighing 300–350 g, aged 6–8 weeks) were anesthetized by intraperitoneal injection of pentobarbital (65 mg/kg) and then fixed in a stereotaxic apparatus (Sigma, St. Louis, MO, USA) after being placed in a prone position. The operation was performed under sterile conditions and the animals were randomly assigned into three groups: (1) Sham group: animals had craniotomy only. (2) Lesion (L) group: rats had a right frontal-parietal craniotomy with exposure of the underlying brain and a lesion was created directly by suction and surgical clamps. The location was confirmed by the stereotactic apparatus mentioned previously at 1.0 mm anterior to and 4.0 mm posterior to the bregma and 1.0 to 5.0 mm lateral to the midline, with a depth of 3 mm from the brain surface. (3) Lesion + CGM (L + CGM) group, where a 6 mm × 4 mm × 3 mm block of CGM scaffold was implanted into the created lesion cavity of the surgical brain injury rats. In each group, the rat number was 10 (5 rats used for immunofluorescence staining and 5 rats for enzyme-linked immunosorbent assay (ELISA)). After all operations, the skin was closed with 3-0 silk (Ethicon, Taiwan, Taipei, China). We monitored vital signs throughout the procedures.
Double immunofluorescence staining
We perfused all rats (after anesthesia) transcardially with phosphate buffered saline and paraformaldehyde (4%). We removed the brains, put them in paraformaldehyde (4%) overnight and fixed them firmly in paraffin blocks. Serial section was done every 6 μm. Section area was analyzed in the same cross section (2.0 mm anterior to the bregma).
We treated each section with 4% paraformaldehyde in phosphate-buffered saline (PBS) at room temperature for 1 hour, washed twice with PBS, and then incubated in 2 M HCl at 37°C for 1 hour for double immunofluorescence labeling. Then, sections were incubated in blocking solution with primary antibodies, either (1) a rabbit polyclonal MMP-2 antibody (1:500; Abcam, Cambridge, UK), and a mouse monoclonal SOX2 antibody (1:100; Sigma); (2) a rabbit polyclonal MMP-9 antibody (1:250; Abcam), and a mouse monoclonal SOX2 antibody (1:100; Sigma); (3) a rabbit polyclonal MMP2 antibody (1:500; Abcam), and a mouse monoclonal anti-GFAP antibody (1:2000; Sigma); or (4) a rabbit polyclonal MMP-9 antibody (1:250; Abcam), and a mouse monoclonal anti-GFAP antibody (1:2000; Sigma) at 4°C overnight and with secondary antibodies (Alexa Fluor-488 goat anti-mouse immunoglobulin G (IgG; 1:200; Invitrogen, Carlsbad, CA, USA) and DyeLight 549 anti-rabbit IgG (1:200; Jackson ImmunoResearch, West Grove, PA, USA) at room temperature for 2 hours. Sections were mounted with Mounting Medium H-1000 (Vector Laboratories, Burlingame, CA, USA). Nonspecific staining was visualized by omitting the primary antibody and was negative. A Nikon ellipse 80i microscope (Nikon Optical, Tokyo, Japan) and a Nikon Digital Sight DS-5M camera using NIS-Elements F 2.30 software (Nikon) were used to obtain the fluorescent microscopic images, followed with digital image processing performed by Image-pro Plus, version 5.1 (Media Cybernetics, Silver Spring, MD, USA).
After double immunofluorescence staining, the number of positively stained cells in the intra-matrix zone (IMZ) and lesion boundary zone (LBZ) were counted manually in three to five different fields per section of each rat brain (using an eyepiece grid covering an area of 0.0625 mm2) on day 7 (D7), D14, D21, and D28 following implantation of CGM by an individual who was unaware of the experimental design. Blood cells and vessels were excluded. The sections were observed, and images were recorded using a Nikon epifluorescent microscope. Cells in the IMZ and LBZ were visualized by staining at 20× magnification using Openlab software (Improvision, Cambridge, MA, USA). Cells were visualized as fluorescent red (DL 549), and fluorescent green (Alexa 488) in the IMZ and LBZ at 20× magnification, while double-positive cells were visualized as yellow. For double immunofluorescence staining, double-positive cells were manually counted in the same manner as mentioned above. Finally, we presented the results as the number of immunopositive cells per field.
Enzyme-linked immunosorbent assay (ELISA) for measurement of tissue concentrations of MMP2 and MMP9
Brain samples were removed after cervical dislocation from the animals on D7, D14, D21, and D28 after surgery. A 3-mm coronal section was taken from the injured area over the parietal cortex, snap-frozen in liquid nitrogen, and then stored at –70°C until needed. All brain samples were homogenized in buffer consisting of 0.05 M Tris HCl, 0.15 M NaCl, 0.1% Nonidet 40, 0.5 M phenylmethylsuplhonyl fluoride, 50 mg/mL aprotinin, 10 mg/mL leupeptin, 50 mg/mL pepstatin, 4 mM sodium orthovanadate, 10 mM sodium fluoride, and 10 mM sodium pyrophosphate. Homogenates were centrifuged at 4°C and 12,000 × g for 15 minutes. Then the supernatants were removed and assayed in duplicate using MMP2 and MMP9 assay kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's guidelines. Concentrations of tissue proteins (MMP2 and MMP9) were expressed as picograms of antigen per milligram of protein. In our experiments, the concentration of MMP2 and MMP9 from tissue samples was measured on D7, D14, D21, and D28 with an ELISA kit (R&D Systems).
Statistical analysis
Comparisons between multiple groups were conducted using a one-way analysis of variance (ANOVA) with the Bonferonni correction. All statistical analyses were performed using Sigma Stat Version 2.0 (Jandel Scientific, San Diego, CA, USA). Data are expressed as the mean ± standard deviation (SD). Differences were considered significant as P < 0.05.
Results
Increased MMP2+/SOX2+ cells within the LBZ of rats following implantation of CGM
Representative photomicrographs show double immunofluorescence staining with antibodies against MMP2, and SOX2 (a marker of the proliferative neural progenitor cell) of brain sections from L+ CGM group rats on D14 following injury (Figure 1A–C). Further, we counted the density (cells/mm2) of MMP2+/SOX2+ cells in the LBZ of the Sham, L, and L + CGM groups of rats at various time points. The L + CGM group also showed a significant increase in MMP2+/SOX2+ cells on D7 after the surgical brain lesion (P < 0.001), with levels sustained and peaking with a slight increase on D21 (Figure 1D). CGM implantation promoted proliferative neural progenitor cells with immunoreactivity of MMP2 in the LBZ of surgical brain lesions after CGM implantation.
Figure 1.
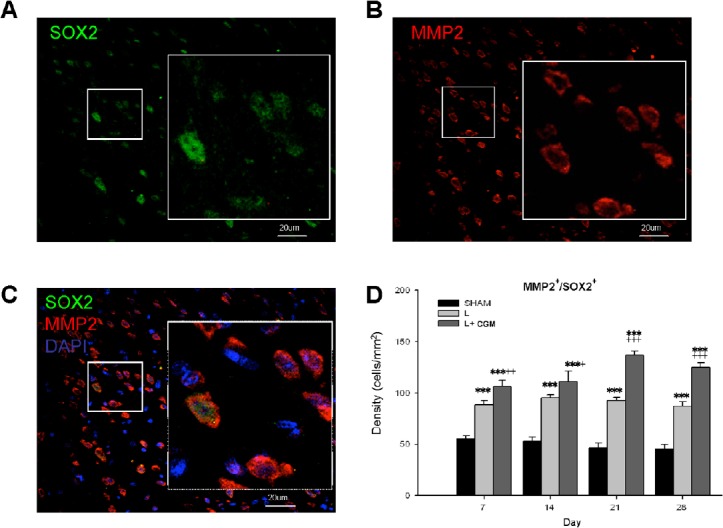
Proliferating neural progenitor cells (SOX2+) with MMP2 immunoreactivity in the LBZ of rats on day 21 following implantation of CGM.
(A–C) Representative microphotographs of double immunofluorescence staining of SOX and MMP2 in representative brain sections from L + CGM group rats on day 21 following surgical brain trauma. (A) Image of immunoreactivity of SOX2 (green; marker of proliferating neural progenitor cells), and (B) MMP2 (red) positive cells and (C) merged image in the LBZ of rats in the L + CGM group. (D) Numbers of MMP2+/SOX2+ cells in the LBZ from the brain sections of sham (SHAM), L and L + CGM groups on days 7, 14, 21 and 28 after surgery. Data are expressed as the mean ± SD. ***P < 0.001, vs. SHAM group; +P < 0.05, ++P < 0.01 and +++P < 0.001, vs. L group (one-way analysis of variance with the Bonferonni correction). MMP2: Matrix metalloproteinase-2; CGM: collagen glycosaminoglycan matrix; LBZ: lesion boundary zone; DAPI: 4′,6-diamidino-2-phenylindole.
Increased MMP9+/SOX2+ cells within the LBZ of rats following implantation of CGM
Representative photomicrographs show double immunofluorescence staining with antibodies against MMP9, and SOX2 of brain sections from L + CGM group rats on D14 following injury (Figure 2A–C). Further, we counted the density (cells/mm2) of MMP9+/SOX2+ cells in the LBZ of the Sham, L, and L + CGM groups of rats at various time points. The L + CGM group also showed a significant increase in MMP9+/SOX2+ cells on D7 after the surgical brain lesion (P < 0.001), with levels sustained and peaking with a slight increase on D28 (Figure 2D). CGM implantation promoted proliferative neural progenitor cells with immunoreactivity of MMP9 in the LBZ of surgical brain lesions after implantation.
Figure 2.
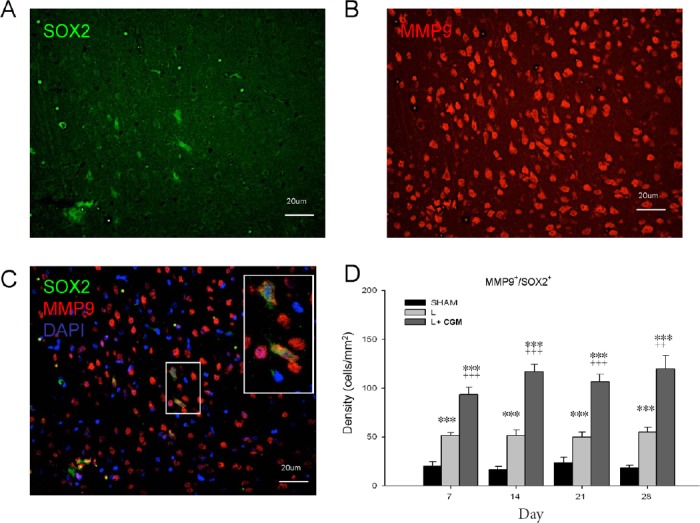
Proliferating neural progenitor cells (SOX2+) with MMP9 immunoreactivity in the LBZ of surgical brain lesion on day 14 following implantation of CGM.
(A–C) Representative microphotographs of double immunofluorescence staining of representative brain sections from L + CGM group rats on day 14 following surgical brain trauma. (A) Image of SOX2+ (green; marker of proliferating neural progenitor cells), and (B) MMP9+ (red) cells, and (C) merged image in LBZ of rats in the L + CGM group. (D) Numbers of MMP9+/SOX2+ cells in the LBZ from the brain sections of rats in the sham (SHAM), L and L + CGM groups on days 7, 14, 21 and 28 after surgery. Data are expressed as the mean ± SD. ***P < 0.001, vs. SHAM group; ++P < 0.01, +++P < 0.001, vs. L group (one-way analysis of variance with the Bonferonni correction). MMP9: Matrix metalloproteinase-9; CGM: collagen glycosaminoglycan matrix; LBZ: lesion boundary zone; DAPI: 4′,6-diamidino-2-phenylindole.
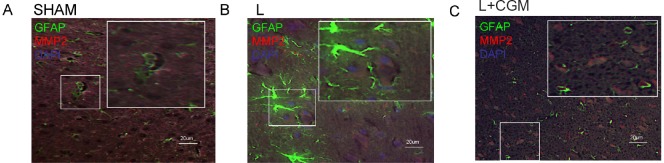
MMP2- and MMP-immunoreactive cells were not astrocytes (GFAP+)
We further investigated whether the astrocytes with MMP2 or MMP9 immunoreactivity exist or not. Double immunofluorescent staining of GFAP/MMP2 or GFAP/MMP9 showed no double-staining cells were noted in the LBZ of rats in the sham, L and L + CGM groups (Figures 3 and 4).
Figure 3.
Merged image of GFAP+ cells and MMP2+ cells in the lesion boundary zone of surgical brain lesion following implantation of CGM.
(A–C) Representative microphotographs of double immunofluorescence staining of representative brain sections from the L + CGM group rats on day 14 following surgical brain trauma. Merged image of GFAP+ (green; astrocyte marker), and MMP2+ (red) cells in the sham (SHAM; A), L (B) and L + CGM (C) groups. GFAP: Glial fibrillary acidic protein; MMP2: matrix metalloproteinase-2; CGM: collagen glycosaminoglycan matrix.
Figure 4.
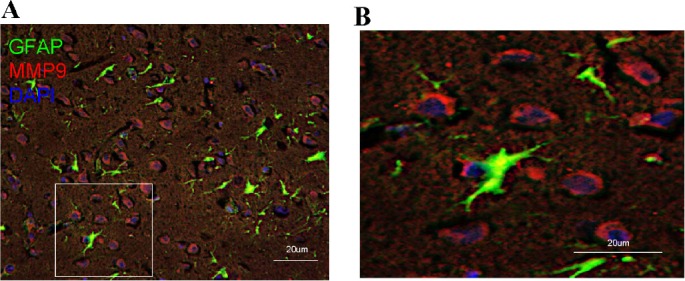
Merged image of GFAP+ cells and MMP9+ cells in the lesion boundary zone of surgical brain lesion following implantation of CGM.
(A, B) Representative microphotographs of double immunofluorescence staining for GFAP (green; astrocyte marker)/MMP9 (red) in representative brain sections of rats from the L + CGM group on day 14 following surgical brain trauma. B is a magnified image from the box in A. GFAP: Glial fibrillary acidic protein; DAPI: 4′,6-diamidino-2-phenylindole; MMP9: matrix metalloproteinase-9; CGM: collagen glycosaminoglycan matrix.
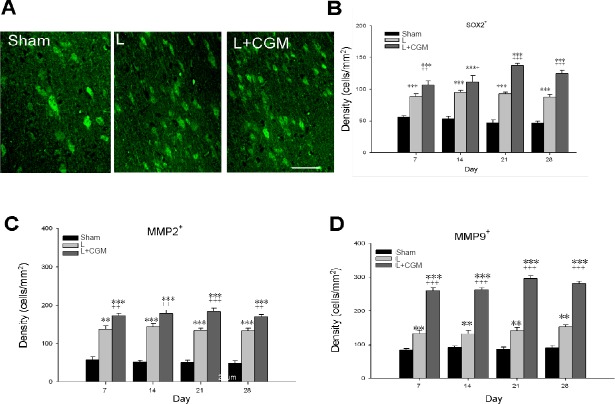
Increased Sox2-, MMP2-, and MMP9-immunoreactive cells within the LBZ of rats following implantation of CGM
The L + CGM group also showed a significant increase in Sox2-, MMP2-, and MMP9-immunoreactive cells on day 7 after the surgical brain lesion compared with the L group (P < 0.01 or P < 0.001), with levels sustained and peaking with a slight increase on day 21 (Figure 5).
Figure 5.
Increased SOX2-, MMP2-, and MMP9-immunoreactive cells in the LBZ of surgical brain lesion rats following implantation of CGM.
(A) Representative microphotographs of SOX2-immunoreactive cells (immunofluorescence staining: green) in the LBZ of representative brain sections from Sham, L and L + CGM rats on day 14 following surgical brain trauma. (B–D) Numbers of Sox2- (B), MMP2- (C), and MMP9-immunoreactive (D) cells in the LBZ of brain sections of rats in the sham, L and L + CG groups on days 7, 14, 21 and 28 after surgery. Data are expressed as the mean ± SD. **P < 0.01, ***P < 0.001, vs. SHAM group; +P < 0.05, ++P < 0.01 and +++P < 0.001, vs. L group (one-way analysis of variance with the Bonferonni correction). MMP2: Matrix metalloproteinase-2; MMP9: matrix metalloproteinase-9; LBZ: lesion boundary zone; CGM: collagen glycosaminoglycan matrix.
Increased tissue concentration of MMP2, MMP9 in the LBZ after implantation of CGM following surgical brain trauma
Using ELISA, we also measured the tissue concentrations of MMP2 and MMP9 in the LBZ of rats in the sham, L and L + CGM groups on days 7, 14, 21, 28. The L + CGM group showed significant and sustained increased in the tissue concentration of MMP2 and MMP9 in the L + CGM group compared with the L group (P < 0.001), with levels peaking on D14 and sustained up to D28 (Figure 6A, B).
Figure 6.
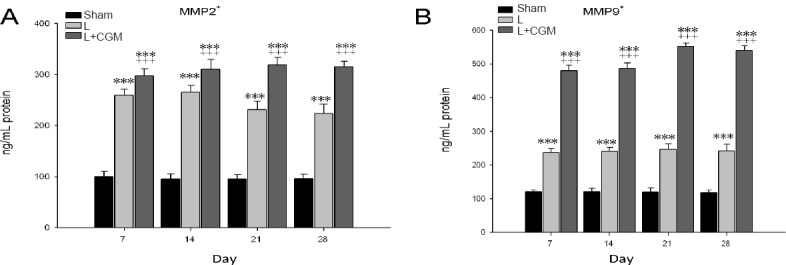
Increase of tissue concentrations of MMP2 and MMP9 in the lesion boundary zone in the surgical brain lesion rats following implantation of CGM.
MMP2 and MMP9 protein concentrations as measured by ELISA in the surgical brain lesion rats from the sham (SHAM), L and L + CGM groups. Data are expressed as the mean ± SD. ***P < 0.001, vs. SHAM group; +++P < 0.001, vs. L group (one-way analysis of variance with the Bonferonni correction). MMP2: Matrix metalloproteinase-2; MMP9: matrix metalloproteinase-9; CGM: collagen glycosaminoglycan matrix.
Discussion
We demonstrated increased MMP2 and MMP9 accompanied by neurogenesis after CGM implantation in a rat surgical brain lesion model. We showed histological findings of proliferative neural progenitor (SOX2+), MMP2+, and MMP9+ cells in the LBZ of rats following implantation of CGM. We also demonstrated increased MMP2+/SOX2+ cells and MMP9+/SOX2+ cells within the LBZ of rats following implantation of CGM. Tissue protein concentrations of MMP2 and MMP9 also increased in the injured brain of rats after CGM scaffold implantation. These findings confirmed that both MMP2 and MMP9 contribute to neurogenesis following surgical brain trauma through implantation of CGM.
Several recent studies revealed that ECM plays important roles in modifying cell differentiation, migration, and proliferation (Suzuki et al., 2003; Farrell et al., 2006; Reilly and Engler, 2010). Depending on its composition ECM can regulate cell behavior in different ways. It is known that ECM has an essential influence on both migration and proliferation of adult rodent cerebellar NPCs (Murase and Horwitz, 2002). An in vitro study has revealed the role of ECM in the modulation of the cortical neural progenitor cells development as well as proliferation in cell culture (Aizman et al., 2013). ECM might also play a role as a controller of neurogenesis in the development stage of the central nervous system (Aizman et al., 2014; Heikkinen et al., 2014; Shin et al., 2014; Faissner and Reinhard, 2015). There is accumulating evidence that in some conditions, ECM proteins can have correlation with growth factors in the modulation of neurogenesis (Aizman et al., 2014; Heikkinen et al., 2014; Shin et al., 2014; Faissner and Reinhard, 2015) and that the process of degradation of ECM may also result in an elevation in some growth factors. Therefore, the biodegradation and fragmentation of molecules within the ECM is essential for the process of neurogenesis. Some enzymes, such as MMP2 and MMP9, further degrade the ECM and modify the cell adhesion components (Ethell and Ethell, 2007). These enzymes may have the physiological significances of correlation with neurogenesis by surgical trauma and while implantation of CGM (analogue of ECM) may further promoted the activity of these enzymes.
These MMPs, by managing the diversity of many extracellular components involving extracellular matrix proteins and many growth factors, contribute to various physiological and pathological activities (Wojcik-Stanaszek et al., 2011; Brkic et al., 2015). Whereas MMPs have often been studied in the context of their harmful role in brain injuries, other reports indicate that they are also favorable for the promotion of NPCs and expression of MMPs was abundant in neural stem cells obtained from the central nervous system (Fujioka et al., 2012). They play an essential role in the regulation of the proliferation of NPCs throughout their development, so it is persuasive to imagine the contribution of these enzymes to traumatic injury recovery, perhaps in their support of the migration of NPCs to the lesion area to replenish damaged cells. Consistent with this hypothesis, recent reports also revealed the association of MMPs with neurogenesis and predominantly in neuroblast migration (Lei et al., 2013; Saftig and Bovolenta, 2015; Abdul-Muneer et al., 2016). These results also confirm the regulatory roles of MMPs in the proliferation of NPCs in the central nervous system both in humans and rodents (Sellebjerg and Sorensen, 2003; Barkho et al., 2008; Barkho and Zhao, 2011). The most probable situation, consistent with the recognized role of MMPs, is the biodegradation and thus remodeling of ECM with modulation of several molecules required for NPCs migration, differentiation, and proliferation (Wojcik-Stanaszek et al., 2011). Furthermore, these products of the biodegradation of ECM in turn facilitate modifications in interaction between cells and these ECM proteins could release some soluble growth factors that benefit migration. In addition, MMPs can stimulate proliferation and differentiation of NPCs (Reilly and Engler, 2010) and, while MMPs would ordinarily be expected to promote neural precursor maturation, MMP-facilitated alteration of numerous trophic factors interacting with the ECM may also yield signals associated with neurogenesis (Abdul-Muneer et al., 2016). CGM and, although our study data is consistent with other research, a deeper understanding of this mechanism would be beneficial to understanding neurogenesis in the adult brain following trauma. The increased expression of MMP2 and MMP9 after implantation of CGM following surgical brain trauma may be due to these products of the biodegradation of ECM and thus leads to the promotion of neurogenesis.
Conclusions
This report demonstrates that implantation of CGM following surgical brain injury motivates neurogenesis, and this reaction is accompanied by a significant increase in extracellular metalloproteinases. This reveals that a significant increase in MMP2 and MMP9 is involved in neurogenesis correlating activities. This study verifies the association between MMP2 and MMP9 and neurogenesis following CGM implantation after surgical brain trauma.
Acknowledgments
The authors would like to thank Life Spring Biotech, Horien Biochemical Technology, and the Core Lab, Taipei Tzu-Chi Hospital, Buddhist Tzu Chi Medical Foundation, for laboratory and technical support of this study.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
Financial support: This study was supported by grants from the National Science Council of China (NSC102-2314-B-303-004) and the Tzu Chi Medical Mission Project 105-06, Buddhist Tzu Chi Medical Foundation. None of the funding bodies play any role in the study other than to provide funding.
Institutional review board statement: This study was approved by the Animal care and Use Committee of Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (No. 103-IACUC-002) and conformed to the Guiding Principles in the Care and Use of Animals of the American Physiology Society.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by grants from the National Science Council of China (NSC 102-2314-B-303-004) and the Tzu Chi Medical Mission Project 105-06, Buddhist Tzu Chi Medical Foundation.
(Copyedited by Li CH, Song LP, Zhao M)
References
- Abdul-Muneer PM, Pfister BJ, Haorah J, Chandra N. Role of matrix metalloproteinases in the pathogenesis of traumatic brain injury. Mol Neurobiol. 2016;53:6106–6123. doi: 10.1007/s12035-015-9520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizman I, McGrogan M, Case CC. Quantitative microplate assay for studying mesenchymal stromal cell-induced neuropoiesis. Stem Cells Transl Med. 2013;2:223–232. doi: 10.5966/sctm.2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizman I, Tirumalashetty BJ, McGrogan M, Case CC. Comparison of the neuropoietic activity of gene-modified versus parental mesenchymal stromal cells and the identification of soluble and extracellular matrix-related neuropoietic mediators. Stem Cell Res Ther. 2014;5:29. doi: 10.1186/scrt418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkho BZ, Zhao X. Adult neural stem cells: response to stroke injury and potential for therapeutic applications. Curr Stem Cell Res Ther. 2011;6:327–338. doi: 10.2174/157488811797904362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkho BZ, Munoz AE, Li X, Li L, Cunningham LA, Zhao X. Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. Stem Cells. 2008;26:3139–3149. doi: 10.1634/stemcells.2008-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikbaev A, Frischknecht R, Heine M. Brain extracellular matrix retains connectivity in neuronal networks. Sci Rep. 2015;5:14527. doi: 10.1038/srep14527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brkic M, Balusu S, Libert C, Vandenbroucke RE. Friends or foes: matrix metalloproteinases and their multifaceted roles in neurodegenerative diseases. Mediators Inflamm. 2015;2015:620581. doi: 10.1155/2015/620581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J Neurosci Res. 2007;85:2813–2823. doi: 10.1002/jnr.21273. [DOI] [PubMed] [Google Scholar]
- Faissner A, Reinhard J. The extracellular matrix compartment of neural stem and glial progenitor cells. Glia. 2015;63:1330–1349. doi: 10.1002/glia.22839. [DOI] [PubMed] [Google Scholar]
- Farrell E, O’Brien FJ, Doyle P, Fischer J, Yannas I, Harley BA, O’Connell B, Prendergast PJ, Campbell VA. A collagen-glycosaminoglycan scaffold supports adult rat mesenchymal stem cell differentiation along osteogenic and chondrogenic routes. Tissue Eng. 2006;12:459–468. doi: 10.1089/ten.2006.12.459. [DOI] [PubMed] [Google Scholar]
- Fugate JE. Complications of Neurosurgery. Continuum (Minneap Minn) 21(5 Neurocritical Care) 2015:1425–1444. doi: 10.1212/CON.0000000000000227. [DOI] [PubMed] [Google Scholar]
- Fujioka H, Dairyo Y, Yasunaga K, Emoto K. Neural functions of matrix metalloproteinases: plasticity, neurogenesis, and disease. Biochem Res Int 2012. 2012:789083. doi: 10.1155/2012/789083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueye Y, Ferhat L, Sbai O, Bianco J, Ould-Yahoui A, Bernard A, Charrat E, Chauvin JP, Risso JJ, Feron F, Rivera S, Khrestchatisky M. Trafficking and secretion of matrix metalloproteinase-2 in olfactory ensheathing glial cells: A role in cell migration. Glia. 2011;59:750–770. doi: 10.1002/glia.21146. [DOI] [PubMed] [Google Scholar]
- Harley BA, Kim HD, Zaman MH, Yannas IV, Lauffenburger DA, Gibson LJ. Microarchitecture of three-dimensional scaffolds influences cell migration behavior via junction interactions. Biophys J. 2008;95:4013–4024. doi: 10.1529/biophysj.107.122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen A, Pihlajaniemi T, Faissner A, Yuzaki M. Neural ECM and synaptogenesis. Prog Brain Res. 2014;214:29–51. doi: 10.1016/B978-0-444-63486-3.00002-5. [DOI] [PubMed] [Google Scholar]
- Hsu WC, Spilker MH, Yannas IV, Rubin PA. Inhibition of conjunctival scarring and contraction by a porous collagen-glycosaminoglycan implant. Invest Ophthalmol Vis Sci. 2000;41:2404–2411. [PubMed] [Google Scholar]
- Hsu WC, Ritch R, Krupin T, Chen HS. Tissue bioengineering for surgical bleb defects: an animal study. Graefes Arch Clin Exp Ophthalmol. 2008;246:709–717. doi: 10.1007/s00417-007-0744-9. [DOI] [PubMed] [Google Scholar]
- Huang KF, Hsu WC, Chiu WT, Wang JY. Functional improvement and neurogenesis after collagen-GAG matrix implantation into surgical brain trauma. Biomaterials. 2012;33:2067–2075. doi: 10.1016/j.biomaterials.2011.11.040. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Parent JM. Forebrain neurogenesis after focal ischemic and traumatic brain injury. Neurobiol Dis. 2010;37:267–274. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei C, Lin S, Zhang C, Tao W, Dong W, Hao Z, Liu M, Wu B. Activation of cerebral recovery by matrix metalloproteinase-9 after intracerebral hemorrhage. Neuroscience. 2013;230:86–93. doi: 10.1016/j.neuroscience.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Murase S, Horwitz AF. Deleted in colorectal carcinoma and differentially expressed integrins mediate the directional migration of neural precursors in the rostral migratory stream. J Neurosci. 2002;22:3568–3579. doi: 10.1523/JNEUROSCI.22-09-03568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly GC, Engler AJ. Intrinsic extracellular matrix properties regulate stem cell differentiation. J Biomech. 2010;43:55–62. doi: 10.1016/j.jbiomech.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Saftig P, Bovolenta P. Proteases at work: cues for understanding neural development and degeneration. Front Mol Neurosci. 2015;8:13. doi: 10.3389/fnmol.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellebjerg F, Sorensen TL. Chemokines and matrix metalloproteinase-9 in leukocyte recruitment to the central nervous system. Brain Res Bull. 2003;61:347–355. doi: 10.1016/s0361-9230(03)00097-2. [DOI] [PubMed] [Google Scholar]
- Shin Y, Yang K, Han S, Park HJ, Seok Heo Y, Cho SW, Chung S. Reconstituting vascular microenvironment of neural stem cell niche in three-dimensional extracellular matrix. Adv Healthc Mater. 2014;3:1457–1464. doi: 10.1002/adhm.201300569. [DOI] [PubMed] [Google Scholar]
- Sirbulescu RF, Ilies I, Zupanc GK. Matrix metalloproteinase-2 and -9 in the cerebellum of teleost fish: Functional implications for adult neurogenesis. Mol Cell Neurosci. 2015;68:9–23. doi: 10.1016/j.mcn.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Iwama A, Miyashita H, Nakauchi H, Taniguchi H. Role for growth factors and extracellular matrix in controlling differentiation of prospectively isolated hepatic stem cells. Development. 2003;130:2513–2524. doi: 10.1242/dev.00459. [DOI] [PubMed] [Google Scholar]
- Verslegers M, Van Hove I, Buyens T, Dekeyster E, Knevels E, Moons L. Identification of MMP-2 as a novel enhancer of cerebellar granule cell proliferation. Mol Cell Neurosci. 2013;57:63–72. doi: 10.1016/j.mcn.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Wojcik-Stanaszek L, Sypecka J, Szymczak P, Ziemka-Nalecz M, Khrestchatisky M, Rivera S, Zalewska T. The potential role of metalloproteinases in neurogenesis in the gerbil hippocampus following global forebrain ischemia. PLoS One. 2011;6:e22465. doi: 10.1371/journal.pone.0022465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WC, Lai CC, Chen HS, Sun MH, Lee LM, Shih CP, Lee HW, Hsu WC. Efficacy and safety of biodegradable collagen-glycosaminoglycan polymer as a material for scleral buckling. Invest Ophthalmol Vis Sci. 2008;49:2673–2678. doi: 10.1167/iovs.07-1594. [DOI] [PubMed] [Google Scholar]
- Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci U S A. 1989;86:933–937. doi: 10.1073/pnas.86.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]