See Coulthard and Love (doi:10.1093/brain/awy153) for a scientific commentary on this article.
The burden of co-pathologies across neurodegenerative diseases is unknown. Robinson et al. assess tau, Aβ, alpha-synuclein and TDP-43 proteinopathies in post-mortem individuals representing a spectrum of neurodegenerative disease. Co-pathologies are common, with age and APOE ɛ4 status affecting co-pathology prevalence. The findings have implications for clinical trials focusing on monotherapies.
Keywords: Alzheimer's disease, co-pathology, neurodegenerative disease, pathology
Abstract
Lewy bodies commonly occur in Alzheimer’s disease, and Alzheimer’s disease pathology is frequent in Lewy body diseases, but the burden of co-pathologies across neurodegenerative diseases is unknown. We assessed the extent of tau, amyloid-β, α-synuclein and TDP-43 proteinopathies in 766 autopsied individuals representing a broad spectrum of clinical neurodegenerative disease. We interrogated pathological Alzheimer’s disease (n = 247); other tauopathies (n = 95) including Pick’s disease, corticobasal disease and progressive supranuclear palsy; the synucleinopathies (n = 164) including multiple system atrophy and Lewy body disease; the TDP-43 proteinopathies (n = 188) including frontotemporal lobar degeneration with TDP-43 inclusions and amyotrophic lateral sclerosis; and a minimal pathology group (n = 72). Each group was divided into subgroups without or with co-pathologies. Age and sex matched logistic regression models compared co-pathology prevalence between groups. Co-pathology prevalence was similar between the minimal pathology group and most neurodegenerative diseases for each proteinopathy: tau was nearly universal (92–100%), amyloid-β common (20–57%); α-synuclein less common (4–16%); and TDP-43 the rarest (0–16%). In several neurodegenerative diseases, co-pathology increased: in Alzheimer’s disease, α-synuclein (41–55%) and TDP-43 (33–40%) increased; in progressive supranuclear palsy, α-synuclein increased (22%); in corticobasal disease, TDP-43 increased (24%); and in neocortical Lewy body disease, amyloid-β (80%) and TDP-43 (22%) increased. Total co-pathology prevalence varied across groups (27–68%), and was increased in high Alzheimer’s disease, progressive supranuclear palsy, and neocortical Lewy body disease (70–81%). Increased age at death was observed in the minimal pathology group, amyotrophic lateral sclerosis, and multiple system atrophy cases with co-pathologies. In amyotrophic lateral sclerosis and neocortical Lewy body disease, co-pathologies associated with APOE ɛ4. Lewy body disease cases with Alzheimer’s disease co-pathology had substantially lower Mini-Mental State Examination scores than pure Lewy body disease. Our data imply that increased age and APOE ɛ4 status are risk factors for co-pathologies independent of neurodegenerative disease; that neurodegenerative disease severity influences co-pathology as evidenced by the prevalence of co-pathology in high Alzheimer’s disease and neocortical Lewy body disease, but not intermediate Alzheimer’s disease or limbic Lewy body disease; and that tau and α-synuclein strains may also modify co-pathologies since tauopathies and synucleinopathies had differing co-pathologies and burdens. These findings have implications for clinical trials that focus on monotherapies targeting tau, amyloid-β, α-synuclein and TDP-43.
Introduction
The neuropathological diagnosis of nearly all major neurodegenerative diseases can be determined by the presence of one of four aggregated proteins each with a distinct morphology and distribution (Arnold et al., 2013). Tau-positive neurofibrillary tangles and amyloid-β-positive plaques define Alzheimer’s disease, while neuronal and glial tau inclusions define Pick’s disease (PiD), progressive supranuclear palsy (PSP), and corticobasal degeneration (CBD) (Montine et al., 2012; Irwin et al., 2015). α-Synuclein protein aggregates accumulate in multiple system atrophy (MSA) as glial cytoplasmic inclusions and the Lewy body diseases as neuronal Lewy bodies and neurites (Spillantini and Goedert, 2016). TAR DNA-binding protein 43 (TDP-43, encoded by TARDBP) positive neuronal inclusions define frontotemporal lobar degeneration with TDP-43 inclusions (FTLD-TDP, Brettschneider et al., 2014) and the majority of amyotrophic lateral sclerosis (ALS) cases (Brettschneider et al., 2013). While these pure proteinopathies define these neurodegenerative diseases, additional proteinopathies may accumulate as co-pathologies. Historically, before the molecular components of plaques, neurofibrillary tangles and Lewy bodies were identified as amyloid-β, tau and α-synuclein, respectively, Lewy bodies were seen in Alzheimer’s disease cases (Woodard, 1962) and Alzheimer’s disease pathology has long been included in the dementia with Lewy bodies criteria (McKeith et al., 2017). Beyond Alzheimer’s disease and Lewy body disease however, tau, amyloid-β, α-synuclein and TDP-43 co-pathologies in other neurodegenerative diseases are rarely reported (Spires-Jones et al., 2017).
The purpose of this study is to characterize the extent of co-pathology across a broad range of neurodegenerative disease. We examine four hypotheses to account for neurodegenerative disease co-pathologies. First, ageing itself associates with the incidental accumulation of neurodegenerative disease proteinopathies (Fîlfan et al., 2017). Recent studies have described the extent of pathological tau, amyloid-β, α-synuclein and TDP-43 in elderly individuals without cognitive impairment: tau was noted in 97–100%, amyloid-β in 46–69%, α-synuclein in 17–25%, and TDP-43 in 13–36% (Buchman et al., 2012; Kovacs et al., 2013; Elobeid et al., 2016; Abner et al., 2017). Second, co-pathologies may occur by proteopathic seeding when misfolded neurodegenerative disease proteins template normal counterpart proteins as well as cross-seed additional proteins (Clinton et al., 2010). Under this hypothesis, cases with more severe primary neurodegenerative disease proteinopathies have more co-pathologies. Third, genetic risk factors such as APOE ɛ4 that increase the likelihood of Alzheimer’s disease pathology in healthy ageing (Van Cauwenberghe et al., 2016) may also increase Alzheimer’s disease co-pathology across neurodegenerative disease. Fourth, while incidental co-pathologies may be relatively innocuous, more severe burdens should have clinical relevance as we have recently reported for Alzheimer’s disease co-pathology in Lewy body disease (Irwin et al., 2017) and others describe for comorbid cerebrovascular disease (Kapasi et al., 2017). In addition to Alzheimer’s disease, we interrogate the tauopathies including PiD, CBD and PSP; the synucleinopathies including MSA, Parkinson’s disease with and without dementia, and dementia with Lewy bodies; and the TDP-43 proteinopathies FTLD with TDP-43 and ALS using a cohort of autopsied subjects at the Center for Neurodegenerative Disease Research.
Materials and methods
Participants
Demographic, clinical, and neuropathological data were obtained for all autopsy cases conducted at the Center for Neurodegenerative Disease Research from January 2000 to December 2016 (Toledo et al., 2014). Most patients with neurodegenerative diseases were clinically evaluated longitudinally and invited to participate in the brain donation programme by clinicians at different clinical cores at the University of Pennsylvania, although 29 cases were evaluated clinically at the University of California San Francisco. Many of the patients without cognitive impairment were recruited at time of death due to other causes. Informed consent for autopsy was obtained in accordance with state laws and protocols approved by the University of Pennsylvania and University of California San Francisco.
Of 1210 accessioned cases, 766 met the following inclusion criteria:
Cases with primary clinicopathological diagnoses of either Alzheimer’s disease, PiD, CBD, PSP, MSA, Parkinson’s disease with and without dementia, dementia with Lewy bodies, FTLD-TDP, ALS, primary ageing-related tau (PART) (Crary et al., 2014), or unremarkable adult brains. We excluded rare disorders (n = 36) such as Creutzfeldt-Jakob disease, hereditary diffuse leukoencephalopathy with APP-positive spheroids, basophilic inclusion body disease, frontotemporal lobar degeneration with MAPT gene mutations, unclassifiable Lewy body diseases, unclassifiable tauopathies, SOD1-positive ALS, and FUS-positive ALS cases.
Cases with semiquantitative scores by immunohistochemistry for pathological tau, amyloid-β (or Thioflavin S histochemistry), and α-synuclein for 16 regions described below; and for pathological TDP-43 for a minimum of four regions: hippocampus, amygdala, mid-frontal cortex and superior temporal cortex. We excluded 408 cases (n = 408) primarily due to the lack of TDP-43 scores. Excluded cases were the majority of pre-2007 Alzheimer’s disease, PiD, CBD, PSP, MSA, Parkinson’s disease with and without dementia, dementia with Lewy bodies, PART, and unremarkable cases described before the discovery of TDP-43 as the primary proteinopathy in frontotemporal lobar degeneration and ALS (Neumann et al., 2006).
Neuropathological staging
Sixteen regions are routinely examined in the CNDR neuropathology evaluations as described in previous publications (Arnold et al., 2013; Toledo et al., 2014). Notably, since 2012, the occipital cortex has been routinely sampled as an additional neocortical region, as recommended in consensus criteria.
Each region was assigned a semiquantitative score i.e. none, rare, mild, moderate or severe for individual lesions (tau, amyloid-β, α-synuclein and TDP-43 positive pathologies). Tau-positive neurofibrillary tangles and amyloid-β plaques were assessed to determine the level of Alzheimer’s disease neuropathological change (ADNPC) (Montine et al., 2012). α-Synuclein positive Lewy pathology was classified into amygdala, brainstem, limbic or neocortical distributions (McKeith et al., 2017). TDP-43 pathology was assigned different stages depending on the pattern observed: a 4-point simplification of either ALS (Brettschneider et al., 2013) or behavioural variant FTLD-TDP (Brettschneider et al., 2014) stages, or a 3-point Alzheimer’s disease TDP-43 co-pathology stage (Josephs et al., 2014).
Pathological groups
Cases and were assigned to 1 of 13 neuropathological diagnostic groups: PiD, CBD, PSP, MSA, hAD, iAD, nLBD, lLBD, bLBD, ALS, FTLD-TDP, hTDP or minimal pathology group (defined below).
First, PiD, CBD, PSP, and MSA groups were assigned based on the presence their primary proteinopathy and morphology. Second, the severity of the primary proteinopathy determined assignment to the following groups: hAD for cases with a high level of ADNPC; iAD for cases with an intermediate level of ADNPC; nLBD for cases with neocortical α-synuclein; lLBD for cases with limbic α-synuclein; bLBD for cases with brainstem or amygdala α-synuclein; ALS for cases with ALS stages 1–3, FTLD-TDP for cases with frontotemporal lobar degeneration with TDP-43 stages 1–3 and hTDP for high burden, neuropathologically equivalent amyotrophic lateral sclerosis stage 3–4 and frontotemporal lobar degeneration with TDP-43 stage 3–4 cases. Alzheimer’s disease and Lewy body disease group assignment was resolved according to McKeith criteria (McKeith et al., 2017). Similarly, between Alzheimer’s disease and FTLD-TDP cases, cases with a high level of ADNPC were resolved in favour of hAD assignment. The remaining cases—with no ADNPC, low ADNPC, definite PART or possible PART—were assigned to the minimal pathology group.
Genetics
Genomic DNA was extracted from brain tissues using QIAamp® DNA mini kit (Qiagen). C9orf72 hexanucleotide repeat expansions (>30) were determined by repeat-primed PCR and capillary electrophoresis (n = 516) or genotyping of surrogate risk allele rs3849942 (n = 758) on frozen tissue derived DNA as previously described (Suh et al., 2015). PCR fragment size was analysed with GeneMapper software (Applied Biosystems). APOE allele status was defined using two single nucleotide polymorphisms—rs7412 and rs429358—which were genotyped by TaqMan® allelic discrimination assays (Thermo Fisher) (n = 698).
Clinical measures
The following test scores were analysed: Mini-Mental State Examination (MMSE) for Alzheimer’s disease, Lewy body disease and frontotemporal lobar degeneration with TDP-43 cases; ALS Functional Rating Scale (ALS FRS) scores for ALS cases; and Unified Parkinson’s Disease Rating Scale Part 3 (UPDRS-III) scores for Lewy body disease cases. All test scores were collected while ON medications.
Clinical severity measures
Clinical severity measures were obtained from tests performed during neurological examinations. MMSE scores were available for a combined Alzheimer’s disease group (172 of 247 from the hAD and iAD groups), a combined Lewy body disease group (54 of 138 from the bLBD, lLBD and nLBD groups) and a combined frontotemporal lobar degeneration group (67 of 188 from the ALS, FTLD-TDP and hTDP groups); ALS FRS scores were available for a combined ALS group (92 of 133 from the ALS and hTDP groups); UPDRS-III scores were available for a combined Lewy body disease group (49 of 138 from the bLBD, lLBD and nLBD groups). The median interval between last clinical test and date of death was: 3.5 years for Alzheimer’s disease MMSE scores; 1.3 years for Lewy body disease MMSE scores; 1.3 years for frontotemporal lobar degeneration MMSE scores; 0.2 years for ALS FRS scores; 1.3 years for UPDRS-III scores. Linear mixed-effects models were used to determine the annual rate of change of these clinical severity measures from the following median number of tests: four for Alzheimer’s disease MMSE scores; four for Lewy body disease MMSE scores; three for frontotemporal lobar degeneration MMSE scores; five for ALS FRS scores; four for UPDRS-III scores. Both random intercept and slope terms were included to account for the correlations among repeated measures of clinical severity outcomes, where the inclusion of the random slope terms was determined by the Akaike information criterion.
Statistics
Demographic characteristics were compared across groups using Pearson χ2 or post hoc t-tests with Excel (Microsoft, USA). All further analyses used logistic regression adjusting for age and sex, using SAS version 9.4 (SAS Institute Inc., USA). For greater statistical power, some frequency and severity measures were simplified to binary values. Clinical severity measures—MMSE, ALS FRS, and UPDRS-III—were analysed using linear mixed-effects models to estimate an annual rate of change for each measure. All statistical tests were two-sided, reporting degrees of freedom (df), with significance levels of ≤0.01 to reduce false positive findings in lieu of formal multiple testing adjustment due to the exploratory nature of the study (Bender and Lange, 2001).
Results
Neurodegenerative disease group characteristics
Seven hundred and sixty-six cases with staging data available for tau, amyloid-β, α-synuclein and TDP-43 pathology met the inclusion criteria described above. Cases were arranged by primary neurodegenerative disease proteinopathy and then divided into groups by neuropathology disease severity or morphology (Table 1). For the tauopathies, groups included PiD, CBD, and PSP. For cases with pathological amyloid-β and tau, iAD and hAD groups were assigned for cases with an intermediate or high level of ADPNC, respectively. For the synucleinopathies, groups included MSA, and nLBD, lLBD, and bLBD for cases with neocortical, limbic, and brainstem or amygdala patterns of Lewy pathology, respectively. For the TDP-43 proteinopathies, groups included ALS for cases at ALS stages 1–3, FTLD-TDP for cases at frontotemporal lobar degeneration with TDP-43 stages 1–3, and hTDP for cases with high burdens of amyotrophic lateral sclerosis or frontotemporal lobar degeneration with TDP-43 stages. Alzheimer’s disease and Lewy body disease group assignment was resolved according to the McKeith criteria (Montine et al., 2012; McKeith et al., 2017). The minimal pathology group comprised the remaining, low pathology cases.
Table 1.
Group characteristics
| Group | n | % Male | Age of onseta | Duration | Age at death | APOE ɛ4 frequencyb, % | APOE ɛ2 frequencyc, % | C9orf72 expansiond, % |
|---|---|---|---|---|---|---|---|---|
| Minimal | ||||||||
| MPG | 72 | 54 | 70e (16) | 9 (4) | 70 (12) | 31 | 22 | 0 |
| Amyloid-β | ||||||||
| iAD | 46 | 53 | 69 (11) | 11 (6) | 81 (11) | 42 | 11 | 0 |
| hAD | 201 | 48 | 66 (10) | 10 (4) | 76 (11) | 68 | 4 | 0.5f |
| Tau | ||||||||
| CBD | 29 | 38 | 60 (9) | 6 (3) | 65 (10) | 15 | 21 | 0 |
| PiD | 15 | 60 | 58 (12) | 9 (4) | 67 (13) | 15 | 13 | 0 |
| PSP | 51 | 63 | 68 (8) | 8 (4) | 76 (8) | 19 | 25 | 0 |
| TDP-43 | ||||||||
| ALS | 108 | 62 | 60 (11) | 5 (5) | 64 (10) | 22 | 13 | 15 |
| FTLD-TDP | 55 | 53 | 62 (8) | 8 (3) | 69 (9) | 21 | 18 | 24 |
| hTDP | 25 | 60 | 59 (14) | 6 (10) | 65 (10) | 29 | 16 | 29 |
| α-Synuclein | ||||||||
| MSA | 26 | 62 | 58 (11) | 8 (4) | 66 (9) | 22 | 8 | 0 |
| bLBD | 20 | 75 | 63 (8) | 13 (6) | 76 (7) | 37 | 20 | 0 |
| lLBD | 37 | 84 | 66 (9) | 14 (6) | 80 (7) | 22 | 11 | 0 |
| nLBD | 81 | 74 | 64 (10) | 13 (6) | 76 (8) | 52 | 4 | 0 |
aAge of onset of symptoms if known or applicable in years, mean (SD). n = 664. Not applicable to cases without symptoms.
bOne or more APOE ɛ4 alleles.
cOne or more APOE ɛ2 alleles.
dC9orf72 GGGGCC hexanucleotide repeat expansion size > 30.
en = 9 cases with clinical symptoms.
fn = 1. This C9orf72 expansion case was mixed Alzheimer’s disease/FTLD-TDP.
MPG = minimal pathology group.
Individuals were predominantly male (58% male) with a mean age at death of 71 years, and a mean age of disease onset of 63, with considerable variation (Table 1). In comparison to the minimal pathology group, several characteristics differed between groups (Supplementary Table 1). The minimal pathology group was 54% male and males were more frequent in lLBD and nLBD. The minimal pathology group had a mean age at death of 70, while several groups were older: iAD, hAD, PSP, lLBD, and nLBD; the ALS group was younger.
APOE ɛ4 is the major genetic risk factor for Alzheimer’s disease pathology, while APOE ɛ2 is protective (Van Cauwenberghe et al., 2016). One or more APOE ɛ4 alleles were present in 31% of the minimal pathology group and this frequency was statistically similar in most groups (Supplementary Table 1). However, APOE ɛ4 prevalence was higher in the hAD and nLBD groups. One or more APOE ɛ2 alleles was present in 22% of the minimal pathology group but several groups had a statistically lower frequency of the ɛ2 allele, including the iAD, hAD, ALS, MSA, lLBD and nLBD groups (Supplementary Table 1). The C9orf72 GGGGCC hexanucleotide repeat expansion is one of the major genetic causes of FTLD-TDP and ALS (DeJesus-Hernandez et al., 2011). The frequency of the C9orf72 expansion was 15% (17/107) in ALS, 24% (13/55) in FTLD-TDP, and 29% (7/24) in hTDP and did not associate with other groups, confirming the correlation between the C9orf72 expansion and the presence of primary TDP-43 proteinopathy.
Clinicopathological correlations
Each pathologically defined group showed variable sensitivity and specificity for the clinical diagnoses (Supplementary Table 2). The largest group was hAD. The hAD group had a 75% (117/155) sensitivity and 59% (119/201) specificity for possible or probable clinical Alzheimer’s disease. Fourteen per cent (28/201) had clinical frontotemporal degeneration representing a selection bias from the Penn FTD Center. Other groups had higher levels of clinicopathological correlation. The minimal pathology group had an 89% (64/72) sensitivity and 88% (64/73) specificity for individuals without a history of neurodegenerative disease. Similarly, the ALS group had a 92% (101/108) sensitivity and 86% (97/113) specificity for clinical ALS. The Lewy body disease groups had 74% (115/155) sensitivity and 83% (115/138) sensitivity for clinical Lewy body disease, which included dementia with Lewy bodies, Parkinson’s disease, Parkinson’s disease with mild cognitive impairment or Parkinson’s disease with dementia.
Frequency of neurodegenerative disease co-pathologies
Groups were defined by their primary proteinopathy—either tau, amyloid-β, α-synuclein or TDP-43—and each group had differing frequencies and severities of secondary co-pathologies (Fig. 1). Tau co-pathology was Alzheimer’s disease-type neurofibrillary tangles as the presence of non-Alzheimer’s disease tau pathology defined the CBD, PiD and PSP groups. Amyloid-β plaque co-pathology was staged by amyloid phases (Montine et al., 2012) across all groups; α-synuclein co-pathology was defined by the presence of Lewy pathology (McKeith et al., 2017) except in the MSA group; TDP-43 co-pathology was staged across all groups outside the primary TDP-43 proteinopathies (Josephs et al., 2014).
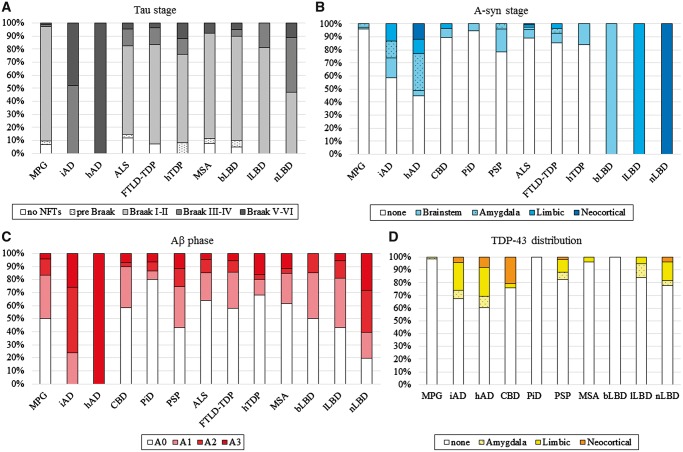
Figure 1.
Pathological tau, amyloid-β, α-synuclein and TDP-43 staging. Tau, amyloid-β, α-synuclein and TDP-43 pathologies were individually staged according to established criteria across 13 neuropathologically-defined groups. (A) Tau pathology principally took the form of Alzheimer’s disease-type neurofibrillary tangles, except in FTLD-Tau (i.e. PiD, CBD and PSP), allowing us to assign Braak stages and compare tau prevalence and severity (Montine et al., 2012). Co-pathological tau was nearly universal, and was commonly observed in the hippocampal formation (Braak I–II). (B) McKeith criteria staging of α-synuclein positive Lewy pathology was applied to all cases except the MSA group (McKeith et al., 2017). α-Synuclein (A-syn) co-pathological affected a minority of cases across neurodegenerative disease and was frequently limited to a brainstem or amygdala distribution except in the hAD group. (C) Amyloid phases were used to stage amyloid-β (Aβ) plaques (Montine et al., 2012). Amyloid-β plaque co-pathology was variably abundant across neurodegenerative disease except the nLBD group, which had an increased burden. (D) Distinct distributions of TDP-43 pathology defined the primary TDP-43 proteinopathies, but a common staging was possible for the remaining groups (Josephs et al., 2014). TDP-43 was rare to non-existent in several groups and frequently had a limbic distribution.
Thus, each neurodegenerative disease group can be divided into distinct subgroups: pure neurodegenerative disease with only primary pathology or neurodegenerative disease with single or multiple co-pathologies (Fig. 2). Tau was not included as a co-pathology for subsequent analysis because it was nearly universal in the cohort (Table 2) and, if included as a co-pathology, would severely limit the number of cases in each pure neurodegenerative disease subgroup.
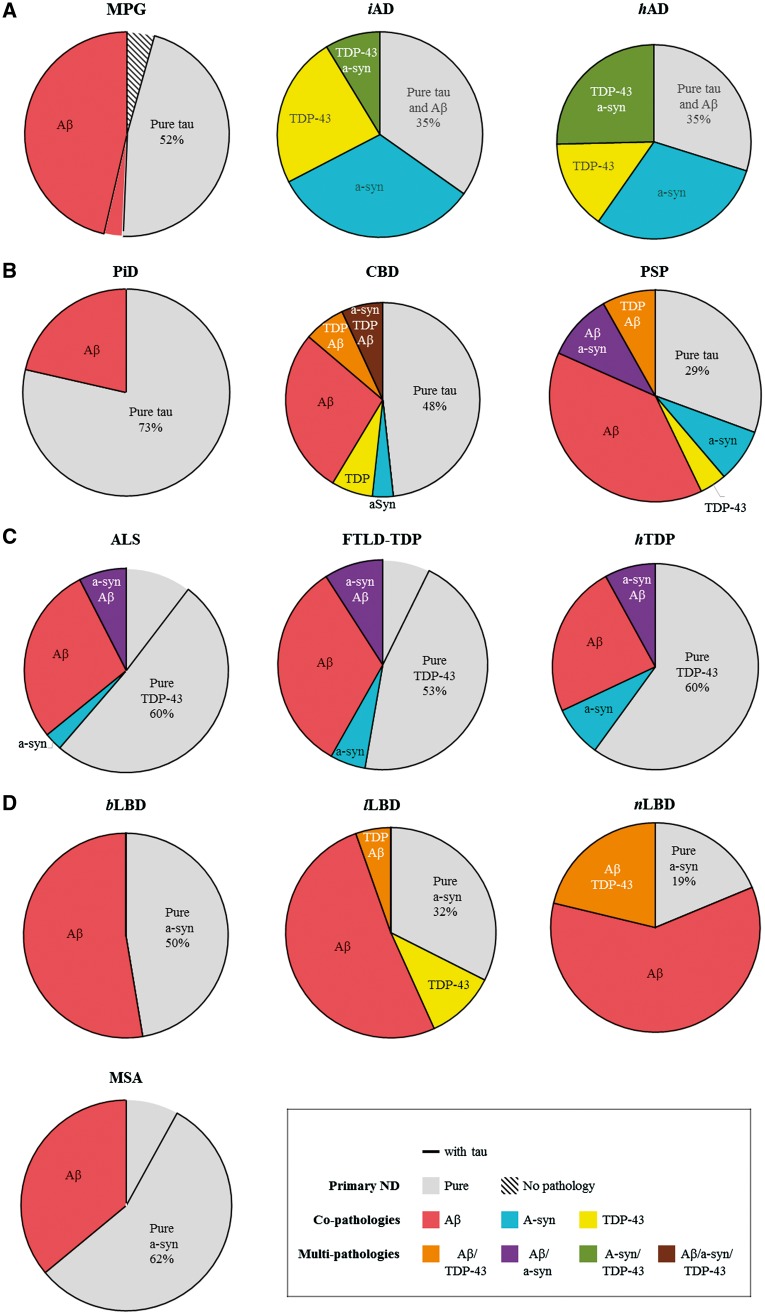
Figure 2.
Frequency of single and multiple neurodegenerative disease co-pathologies. Pie charts depict amyloid-β (Aβ), α-synuclein (a-syn) and TDP-43 co-pathologies and multiple co-pathologies in separate colours with each pure neurodegenerative disease indicated in grey. Tau—represented by a black border—was ubiquitously present with the other co-pathologies, and only rarely negative in pure neurodegenerative disease cases. Table 2 has the exact percentages for each co-pathology. (A) The minimal pathology group (MPG) was defined by a low level of ageing-related tau or amyloid-β. Pure iAD and hAD were in the minority as α-synuclein and TDP-43 co-pathologies were common in both groups. (B) In the tauopathy groups, PiD had the lowest level of total co-pathology, CBD had an increased burden of multiple co-pathologies—principally amyloid-β and TDP-43—and PSP had the highest level of co-pathology involving amyloid-β, α-synuclein and TDP-43. (C) Pathological amyloid-β and α-synuclein were similarly prevalent as co-pathologies and multiple co-pathologies across the TDP-43 proteinopathies. (D) In the synucleinopathy groups, amyloid-β was the only significant co-pathology in MSA and bLBD. Amyloid-β affected the majority of lLBD and nLBD cases while TDP-43 was present in a minority as well. Rare cases (n = 1) for single and multiple co-pathology combinations not depicted.
Table 2.
Prevalence of co-pathologies by neurodegenerative disease group
| Group | n | Proteinopathy | Co-pathology prevalence | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tau | Amyloid-β | α-Synuclein | TDP-43 | Single | Multiple | Total | |||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||
| Minimal | |||||||||||||||
| MPG | 72 | 67 | 93 | 36 | 50 | 3 | 4 | 1 | 1 | 33 | 46 | 2 | 3 | 35 | 48 |
| Amyloid-β | |||||||||||||||
| iAD | 46 | 46 | 100 | 46 | 100 | 19 | 41 | 15 | 33 | 26 | 57 | 4 | 9 | 30 | 65 |
| hAD | 201 | 201 | 100 | 201 | 100 | 111 | 55 | 81 | 40 | 90 | 45 | 51 | 25 | 141 | 70 |
| Tau | |||||||||||||||
| PiD | 15 | 15 | 100 | 3 | 20 | 1 | 7 | 0 | 0 | 4 | 27 | 0 | 0 | 4 | 27 |
| CBD | 29 | 29 | 100 | 12 | 41 | 3 | 10 | 7 | 24 | 10 | 34 | 5 | 17 | 15 | 52 |
| PSP | 51 | 51 | 100 | 29 | 57 | 11 | 22 | 8 | 16 | 25 | 49 | 11 | 22 | 36 | 71 |
| TDP-43 | |||||||||||||||
| ALS | 108 | 95 | 88 | 39 | 36 | 12 | 11 | 108 | 100 | 35 | 32 | 8 | 7 | 43 | 40 |
| FTLD-TDP | 55 | 51 | 93 | 23 | 42 | 8 | 15 | 55 | 100 | 21 | 38 | 5 | 9 | 26 | 47 |
| hTDP | 25 | 25 | 100 | 8 | 32 | 4 | 16 | 25 | 100 | 8 | 32 | 2 | 8 | 10 | 40 |
| α-Synuclein | |||||||||||||||
| MSA | 26 | 24 | 92 | 10 | 38 | 26 | 100 | 1 | 4 | 9 | 35 | 1 | 4 | 10 | 38 |
| bLBD | 20 | 19 | 95 | 10 | 50 | 20 | 100 | 0 | 0 | 10 | 50 | 0 | 0 | 10 | 50 |
| lLBD | 37 | 37 | 100 | 21 | 57 | 37 | 100 | 6 | 16 | 23 | 62 | 2 | 5 | 25 | 68 |
| nLBD | 81 | 81 | 100 | 65 | 80 | 81 | 100 | 18 | 22 | 49 | 60 | 17 | 21 | 59 | 81 |
MPG = minimal pathology group.
Minimal pathology group
In the minimal pathology group, tau as the primary proteinopathy affected 93% and amyloid-β was observed in 50% (Fig. 2A). α-Synuclein (4%) and TDP-43 (1%) were rare. Pure minimal pathology group with only one or no pathology accounted for 52% of all minimal pathology group cases (Table 2). Minimal pathology group co-pathology cases were observed at a 48% prevalence and multiple co-pathologies rarely observed.
Alzheimer’s disease
Pure Alzheimer’s disease represented a minority of cases and the majority had either α-synuclein, TDP-43, or both α-synuclein and TDP-43 co-pathologies. Compared to the minimal pathology group, α-synuclein prevalence increased to 41% in iAD (χ2 = 29.68, df = 1, P < 0.001) and 55% in hAD (χ2 = 15.91, df = 1, P < 0.001), reflecting a limbic burden of α-synuclein pathology in 47% (9/19) in iAD and 57% (63/111) in hAD cases with α-synuclein co-pathology. In addition, TDP-43 increased to 33% in iAD (χ2 = 7.58, df = 1, P = 0.006) and 40% in hAD (χ2 = 13.58, df = 1, P < 0.001) with limbic or neocortical TDP-43 present in 80% (12/15) in iAD and 77% (16/201) in hAD cases with TDP-43 pathology.
Compared to the minimal pathology group, iAD co-pathologies (χ2 = 1.02, df = 1, P = 0.93) and multiple co-pathologies (χ2 = 1.53, df = 1, P = 0.37) were similarly prevalent (Table 2), as were hAD co-pathologies (χ2 = 1.34, df = 1, P = 0.04) but hAD had a higher burden of multiple co-pathologies (χ2 = 3.21, df = 1, P = 0.002).
Tauopathies
The tauopathy groups—PiD, CBD and PSP—showed a range of amyloid-β, α-synuclein and TDP-43 co-pathologies (Fig. 2B). Pure PiD was common (73%); pure CBD was less common (48%); and pure PSP represented a minority (29%). In all tauopathy groups, amyloid-β was the principal co-pathology, noted in 20% of PiD, 41% of CBD, and 57% of PSP cases (Table 2). Compared to the minimal pathology group, α-synuclein prevalence did not increase in PiD (6%) or CBD (10%), but did increase to 22% in PSP (χ2 = 6.40, df = 1, P = 0.01). TDP-43 was absent in PiD, approached significance in 16% of PSP (χ2 = 5.43, df = 1, P = 0.02), and increased to 24% of CBD (χ2 = 7.56, df = 1, P = 0.006). Neocortical TDP-43 co-pathology was infrequent across neurodegenerative disease, as in PSP where only 12% (1/8) had a neocortical distribution (Fig. 1). However, 75% (6/8) of CBD cases with TDP-43 co-pathology had neocortical accumulations.
Compared to the minimal pathology group, PiD co-pathologies (χ2 = 0.61, df = 1, P = 0.13), CBD co-pathologies (χ2 = 1.17, df = 1, P = 0.51) and PSP co-pathologies (χ2 = 1.31, df = 1, P = 0.20) were similarly prevalent (Table 2). Multiple co-pathologies were absent in PiD and increased in both CBD (χ2 = 4.03, df = 1, P = 0.01) and PSP (χ2 = 3.63, df = 1, P = 0.006).
TDP-43 proteinopathies
The TDP-43 proteinopathies had similar rates of amyloid-β and α-synuclein co-pathologies across the ALS, FTLD-TDP and hTDP groups (Fig. 2C). Amyloid-β was the principal co-pathology (32–42%) with a similar prevalence to the minimal pathology group. α-Synuclein affected a smaller percentage (11–16%) and was notably increased only in hTDP (χ2 = 6.20, df = 1, P = 0.01). Interestingly, tau was absent in some of ALS (12%, 13/108) and FTLD-TDP cases (7%, 4/55). However, Braak III or higher stage tau pathology was also observed in 18% (19/108) of ALS, 16% (9/51) of FTLD-TDP, and 24% (6/25) of hTDP.
Compared to the minimal pathology group, co-pathology prevalence was similar in ALS (χ2 = 0.96, df = 1, P = 0.81), FTLD-TDP (χ2 = 0.72, df = 1, P = 0.72) and hTDP (χ2 = 0.92, df = 1, P = 0.76) (Table 2) while multiple co-pathologies approached significance in ALS (χ2 = 2.49, df = 1, P = 0.05), FTLD-TDP (χ2 = 2.31, df = 1, P = 0.08) and hTDP (χ2 = 4.27, df = 1, P = 0.06).
Synucleinopathies
The synucleinopathies exhibited differing prevalences of amyloid-β and TDP-43 co-pathologies (Fig. 2D). Pure neurodegenerative disease represented 62%, 45%, 32% and 19% of MSA, bLBD, lLBD and nLBD, respectively. Amyloid-β was the principal co-pathology, ranging from 38%, to 50%, to 57% and to 80% of MSA, bLBD, lLBD and nLBD, respectively. Across the synucleinopathies amyloid-β prevalence was statistically similar to the minimal pathology group, except in nLBD where amyloid-β deposits were more widespread (χ2 = 10.01, df = 1, P = 0.002) and more severe (χ2 = 13.69, df = 1, P < 0.001). TDP-43 was rare to absent in MSA (4%) and bLBD (0%), approached significance in 16% of lLBD (χ2 = 5.66, df = 1, P = 0.02) and increased to 22% of nLBD (χ2 = 7.42, P = 0.006). Tau pathology was frequently minimal, but the incidence of Braak stage III or higher stage increased with Lewy body disease stage and nLBD had significantly more tau pathology (53%, 43/81) than lLBD (19%, 7/37, χ2 = 15.05, df = 1, P < 0.001) and bLBD (10%, 2/20, χ2 = 13.39, df = 1, P < 0.001).
Compared to the minimal pathology group, co-pathology prevalence was similar in MSA (χ2 = 0.87, df = 1, P = 0.58), bLBD (χ2 = 0.87, df = 1, P = 0.63) and lLBD (χ2 = 1.11, df = 1, P = 0.69) but increased in nLBD (χ2 = 1.88, df = 1, P = 0.002) (Table 2). Multiple co-pathologies were absent in bLBD and were equally prevalent in MSA (χ2 = 1.83, df = 1, P = 0.40) and lLBD (χ2 = 1.50, df = 1, P = 0.64), and nLBD (χ2 = 1.83, df = 1, P = 0.27).
Incidence and severity of Alzheimer’s disease neuropathological change
Pathological tau and amyloid-β were the most frequent co-pathologies but the level of ADNPC was frequently limited to definite or possible PART (Fig. 3). However, compared to the minimal pathology group, there was a higher level of ADNPC in nLBD (χ2 = 18.48, df = 1, P < 0.001). The tauopathies and TDP-43 proteinopathies did not differ in their levels of ADNPC, but between the synucleinopathies, ADNPC was increased in nLBD (versus lLBD, χ2 = 38.03, df = 1, P < 0.001; versus bLBD, χ2 = 21.80, df = 1, P < 0.001; versus MSA, χ2 = 7.81, df = 1, P = 0.005).
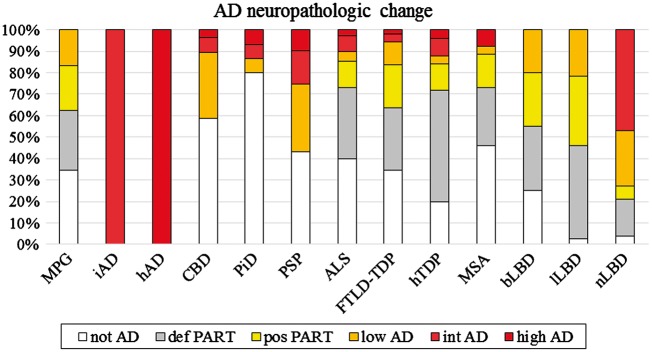
Figure 3.
Alzheimer’s disease neuropathological change co-pathology. The level of ADNPC was measured in all groups (Montine et al., 2012). Intermediate and high levels of ADNPC were rare except in the PSP and nLBD group. Definite and possible PART was common across the majority of groups (Crary et al., 2014). AD = Alzheimer’s disease; MPG = minimal pathology group.
Increased age at death associates with co-pathologies
Examination of age of onset, disease duration, and sex did not reveal an association with co-pathology versus pure neurodegenerative disease cases (data not shown), but for several groups, age at death was a significantly increased in co-pathology cases. An increased age at death associated with co-pathology versus pure neurodegenerative disease in the minimal pathology group (χ2 = 7.39, df = 1, P = 0.007), ALS (χ2 = 17.95, df = 1, P = 0.004), hTDP (χ2 = 9.66, df = 1, P = 0.002) and MSA (χ2 = 7.25, df = 1, P = 0.007). hAD co-pathology cases did not have an increased age at death compared to pure hAD cases (χ2 = 3.20, df = 1, P = 0.07), but hAD cases with TDP-43 co-pathology were older at death (χ2 = 7.49, df = 1, P = 0.006). Other measures were not significantly different. hAD cases with α-synuclein co-pathology did not differ in any measure.
APOE ɛ4 may influence co-pathology prevalence
While the incidence of APOE ɛ4 was common in all groups (Table 1), examination of APOE ɛ4 in relation to co-pathology (Table 3) revealed that APOE ɛ4 presence associated with co-pathology versus pure neurodegenerative disease only in ALS [odds ratio (OR) = 4.94, 95% confidence interval (CI) = 1.60–15.26] and nLBD (OR = 9.32, 95% CI = 2.12–40.95). In addition, CBD, PSP and MSA cases with co-pathologies had APOE ɛ4 positive cases, but pure CBD, PSP and MSA cases did not. Other groups did not show significant differences.
Table 3.
Association of APOE ɛ4 with co-pathology
| APOE ɛ4 | ORa | 95% CI | P-value | |
|---|---|---|---|---|
| Minimal | ||||
| MPG | 1.73 | 0.56 | 5.36 | 0.346 |
| Amyloid-β | ||||
| iAD | 0.71 | 0.17 | 2.95 | 0.639 |
| hAD | 0.93 | 0.48 | 1.82 | 0.832 |
| Tau | ||||
| PiDb | - | - | - | - |
| CBDc | - | - | - | - |
| PSPc | - | - | - | - |
| TDP-43 | ||||
| ALS | 4.94 | 1.60 | 15.26 | 0.006 |
| FTLD-TDP | 1.95 | 0.45 | 8.48 | 0.376 |
| hTDP | 7.51 | 0.301 | 187.309 | 0.212 |
| α-Synuclein | ||||
| MSAc | - | - | - | - |
| bLBD | 2.25 | 0.25 | 19.90 | 0.466 |
| lLBD | 8.69 | 0.70 | 107.39 | 0.092 |
| nLBD | 9.32 | 2.12 | 40.95 | 0.003 |
aPredictor is one or two copies of an APOE ɛ4 allele and covariates are gender and age.
bComparison not done since PiD cases with co-pathologies were all APOE ɛ4−.
cComparison not done since only APOE ɛ4 cases had co-pathologies.
MPG = minimal pathology group.
Since age and APOE ɛ4 and age associated with co-pathology in multiple groups, we also performed logistic regression within each group to assess if APOE ɛ2 carriers were protective against co-pathologies and if APOE ɛ4 carriers were younger. APOE ɛ2 carriers did not associate with co-pathologies, nor were they more likely to be free of co-pathologies and APOE ɛ4 carriers with co-pathologies did not differ in age at death than non-carriers (data not shown).
Clinical impact of co-pathologies
There were variable clinocopathological correlations across groups (Supplementary Table 2). We tested the hypothesis that cases with poor clinicopathological specificity—hAD cases with a clinical diagnosis of fronototemporal degeneration or Parkinson’s disease—would be more likely to have co-pathologies than cases with a strong clinocopathological correlation specificity. By logistic regression, poor clinicopathological specificity did not associate with co-pathology in any group (Supplementary Table 3).
A subset of the Alzheimer’s disease, ALS, FTLD-TDP and Lewy body disease patients were routinely administered the MMSE, ALS FRS and/or UPDRS-III tests (see ‘Materials and methods’ section) allowing us to more directly assess the clinical impact of co-pathologies (Table 4). MMSE scores declined over time in pure Alzheimer’s disease [t(127) = − 12.60, P < 0.001] and Alzheimer’s disease with co-pathologies [t(561) = − 16.61, P < 0.001], but the difference in decline between the two groups was not significant [F(1 561) = 2.17, P = 0.14]. MMSE scores also declined over time in the pure FTLD-TDP cases [t(118) = − 6.61, P < 0.001] and in the co-pathology FTLD-TDP cases [t(36) = − 8.58, P < 0.001], but the difference was not significant [F(1 118) = 0.13, P = 0.72]. However, this was not the case in the Lewy body disease groups (Fig. 4). MMSE scores declined slightly over time in pure Lewy body disease [t(240) = − 2.42, P = 0.016], but Lewy body disease with co-pathology associated with a greater annual decline in MMSE scores [t(62) = − 9.34, P < 0.001]. Between the two groups, this difference approached significance [F(1 240) = 5.52, P = 0.02] and was significantly increased for Lewy body disease with Alzheimer’s disease co-pathology [F(1 129) = 8.23, P = 0.005].
Table 4.
Annual rate of change in clinical severity measures by neurodegenerative disease group
| Measure | Estimate | SE | 95% CI | t | df | P-value | |
|---|---|---|---|---|---|---|---|
| MMSE | |||||||
| Pure AD | −2.581 | 0.201 | −2.993 | −2.168 | −12.60 | 127 | <0.0001a |
| AD co-pathology | −2.217 | 0.134 | −2.479 | −1.955 | −16.61 | 561 | <0.0001 |
| AD with a-synuclein | −2.389 | 0.164 | −2.711 | −2.068 | −14.60 | 445 | <0.0001 |
| AD with TDP | −2.381 | 0.198 | −2.771 | −1.991 | −12.01 | 395 | <0.0001 |
| Pure Lewy body disease | −0.470 | 0.194 | −0.085 | −0.088 | −2.42 | 240 | 0.016 |
| Lewy body disease co-pathology | −0.986 | 0.106 | −1.198 | −0.775 | −9.34 | 62 | <0.0001 |
| Lewy body disease with AD | −1.331 | 0.192 | −1.719 | −0.942 | −6.95 | 35 | <0.0001 |
| Pure FTLD-TDP | −2.973 | 0.445 | −3.864 | −2.082 | −6.61 | 118 | <0.0001 |
| FTLD-TDP co-pathology | −2.771 | 0.323 | −3.423 | −2.116 | −8.58 | 36 | <0.0001 |
| ALS FRS | |||||||
| Pure ALS | −13.064 | 1.109 | −15.246 | −10.882 | −11.78 | 341 | <0.0001a |
| ALS co-pathology | −10.892 | 1.471 | −13.824 | −7.959 | −7.40 | 72 | <0.0001 |
| UPDRS-III | |||||||
| Pure Lewy body disease | 1.989 | 0.610 | 0.755 | 3.223 | 3.26 | 39 | 0.002a |
| Lewy body disease co-pathology | 3.442 | 0.448 | 2.552 | 4.332 | 7.69 | 83 | <0.0001 |
aLinear mixed effect modeling of pure neurodegenerative disease groups without any co-pathologies showed MMSE declines in Alzheimer’s disease, ALS FRS in ALS and UPDRS-III in Lewy body disease.
AD = Alzheimer’s disease; MPG = minimal pathology group.
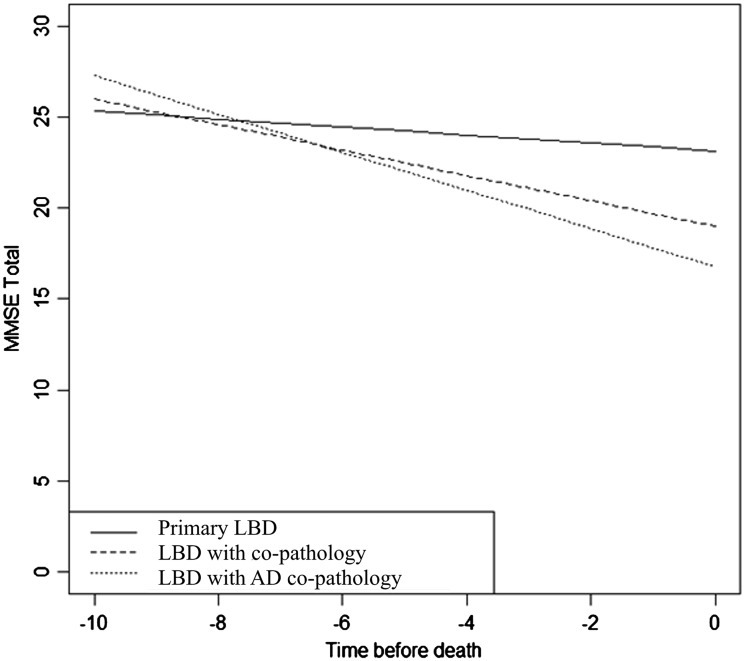
Figure 4.
Co-pathology in Lewy body disease results in a faster cognitive decline. MMSE test scores estimated from a linear mixed-effect model were plotted over a 10-year path. Primary Lewy body disease (LBD) cases without any co-pathologies (solid line) declined significantly over the course of disease (P-value < 0.001). Lewy body disease with co-pathologies (short dashes) had a slightly higher annual rate of decline. Cases with Lewy body disease with Alzheimer’s disease (AD) pathology (dotted line) declined significantly faster than pure Lewy body disease cases.
For ALS patients tested with the ALS FRS and Lewy body disease patients tested with the UPDRS-III test, pure ALS and Lewy body disease cases showed respective declines in ALS FRS [t(341) = − 11.78, P < 0.001] and UPDRS-III [t(39) = 3.26, P = 0.002] test scores, but co-pathology ALS [F(1 341) = 1.39, P = 0.24] and Lewy body disease [F(1,83) = 3.71, P = 0.06] cases did not decline more rapidly.
Discussion
In this study, we examined the prevalence the pathological tau, amyloid-β, α-synuclein and TDP-43 in the major neurodegenerative diseases. Pure neurodegenerative disease was the exception while neurodegenerative disease with co-pathologies was frequent. The ageing minimal pathology group had a co-pathology burden of 48% (Table 2). Similar percentages were observed in the majority of groups, with a higher co-pathology prevalence in the hAD, PSP and nLBD groups. Our data are consistent with previous studies that reported the prevalence of concomitant proteinopathies in large cohorts of elderly individuals with no cognitive or motor impairments (Buchman et al., 2012; Kovacs et al., 2013; Elobeid et al., 2016; Abner et al., 2017), and in Alzheimer’s disease and Lewy body disease (White et al., 2016; Irwin et al., 2017; Kapasi et al., 2017; Spires-Jones et al., 2017). It is now known that the incidental accumulation of pathologies is more prevalent than previously thought and that pure Alzheimer’s disease and Lewy body disease without co-pathologies represent the minority of diagnoses. Less is known about the prevalence of co-pathologies in the tauopathies (Dugger et al., 2014; Thal et al., 2015; Koga et al., 2017b; Tan et al., 2017), MSA (Koga et al., 2017a) and the TDP-43 proteinopathies. As such, our study is an important first step in understanding the extent of co-pathology across all neurodegenerative diseases.
Ageing, proteopathic seeding, strain and genetic factors may all actuate the presence of co-pathologies across neurodegenerative disease. In a recent study reporting a high frequency of co-pathology in frontotemporal lobar degeneration and Alzheimer’s disease, age, primary neurodegenerative disease, and genetics each impacted the overall prevalence of co-pathologies (Tan et al., 2017). We review the data and support for each of these hypotheses in our cohort.
First, in support of the ‘ageing’ hypothesis, it is well known that both pathological tau and amyloid-β occur with age independent of neurodegenerative disease (Braak et al., 2011) and both were common co-pathologies across the neurodegenerative disease studied here (Fig. 3). Age at death positively associated with co-pathologies versus pure neurodegenerative disease in the minimal pathology group, ALS, hTDP and MSA groups. The groups with the highest average age at death—including iAD and hAD, PSP, and lLBD and nLBD—also had the highest co-pathology frequencies (Tables 1 and 2). Not all groups had co-pathologies at older ages of death nor were all groups with younger ages at death free from co-pathologies. Nonetheless, neurodegenerative disease that develops late in life may develop or already has developed co-pathologies.
Second, co-pathologies may occur when the iterative seeding of neurodegenerative disease proteins directly cross-seeds or renders cellular systems vulnerable to additional protein accumulations (Clinton et al., 2010). In support of this ‘proteopathic seeding’ hypothesis, the Alzheimer’s disease and Lewy body disease groups with more severe primary pathology—but not the TDP-43 proteinopathy groups—exhibited higher prevalences of co-pathology (Table 2). The presence of multiple co-pathologies increased from 9% to 25% between iAD and hAD (χ2 = 1.91, df = 1, P = 0.02), and from 0% to 5% to 21% for bLBD, lLBD and nLBD, respectively (lLBD versus nLBD, χ2 = 2.37, df = 1, P = 0.03). Thus, primary pathological burden may influence co-pathology prevalence and severity. This was clinically relevant in Lewy body disease where cases with co-pathologies showed a greater rate of decline in MMSE scores than pure Lewy body disease (Fig. 4).
Third, our data support a model whereby different transmissible ‘strains’ of proteins in non-prion neurodegenerative disease may govern the types and frequencies of co-pathology seen (Boluda et al., 2015). Specifically, co-pathology prevalence differed among the tauopathies (Table 2). Within the tauopathies, co-pathology incidence ranged from a low of 27% in PiD to a high of 71% in PSP. More compellingly, CBD had a distinct pattern of TDP-43 inclusions with more neocortical TDP-43 inclusions than other tauopathies, while PSP had more amyloid-β and α-synuclein co-pathologies than PiD and CBD. These differing co-pathology prevalences and types may reflect the ability of different tau strains to cross-seed other neurodegenerative disease proteins.
Fourth, co-pathologies may be because of genetic risk factors such as APOE ɛ4. APOE ɛ4 is a major risk factor for Alzheimer’s disease pathology, and the CBD, PSP, and nLBD groups had the highest rates of ADNPC co-pathology (Fig. 3). Only CBD and PSP cases with co-pathologies were APOE ɛ4-positive and APOE ɛ4 statistically associated with co-pathologies in nLBD (Table 3).
There are several constraints that limit our study. First, our pathological approach is admittedly a simplification of the diverse neuropathological and clinical subtypes of neurodegenerative disease and while we provide some clinical correlations of our findings, a more detailed approach is warranted. For instance, the specific clinical measures of FTD behavioural and executive changes may be more revealing than the MMSE test scores analysed here. Second, although we report a surprisingly high burden of co-pathologies across a wide spectrum of neurodegenerative disease, examination of additional ageing and disease pathologies including hippocampal sclerosis, cerebrovascular lesions, amyloid angiopathy, ageing-related tau astrogliopathy inclusions would undoubtedly further increase co-pathology prevalence (Kapasi et al., 2017). Third, we report on the prevalence and severity of co-pathology across neurodegenerative diseases relative to the burden of co-pathology in the minimal pathology group. Another approach would be to threshold out the accumulation of age-associated incidental pathologies, thereby reporting more severe co-pathology prevalence that may be more clinically relevant. Fourth, while we examined the data available for 766 cases, some of our group sizes were small (Table 2) and the pure neurodegenerative disease and co-pathology subgroups were smaller still, limiting the analysis in some instances. Finally, cases may reflect a referral bias from tertiary academic centres limiting the generalizability of our results to greater population.
These limitations notwithstanding, our study demonstrates that pathological tau, amyloid-β, α-synuclein and TDP-43 affect most aged individuals across a full spectrum of clinical and neuropathological presentations. This has implications for the design of clinical trials that focus on monotherapies targeting amyloid-β and tau in Alzheimer’s disease, or α-synuclein in Lewy body disease (Perry et al., 2015). The prevalence of neurodegenerative disease co-pathology suggests that each disease may ultimately require combination therapy. Nonetheless, co-pathologies may occur later in disease processes and so approaches that treat neurodegenerative disease could be effective at earlier stages. Moreover, in matching trial participants to prospective therapies, cases may be better defined by their various proteinopathies than their clinical designations. Indeed, this may provide an additional motivation to develop the means of detecting specific proteinopathies in living patients.
Supplementary Material
Acknowledgements
We thank Terry Schuck and Katie Casalnova for their technical assistance in immunohistochemistry, and Young Baek, Rui Tong and Christopher Ernst for data management.
Glossary
Abbreviations
- ADNPC
Alzheimer’s disease neuropathological change
- ALS
amyoptrophic lateral sclerosis
- b/l/nLBD
brainstem or amygdala only/limbic/neocortical Lewy body disease
- CBD
corticobasal degeneration
- FRS
Functional rating Scale
- FTLD-TDP
frontotemporal lobar degeneration with TDP-43 inclusions
- hAD
high level of ADPNC
- hTDP
high level of TDP-43 inclusions
- iAD
intermediate level of ADPNC
- MMSE
Mini-Mental State Examination
- MSA
multiple system atrophy
- PART
primary ageing-related tau
- PiD
Pick’s disease
- PSP
progressive supranuclear palsy
- UPDRS
Unified Parkinson’s Disease Rating Scale
Funding
This work was supported by NIH grants P30 AG10124, PO1 AG17586, P50 NS053488, the Marian S. Ware Alzheimer Program, the Karen Cohen Segal, the Eleanor Margaret Kurtz Endowed Fund, the Mary Rasmus Endowed Fund for Alzheimer’s Research, Mrs. Gloria J. Miller and Arthur Peck, M.D.
Supplementary material
Supplementary material is available at Brain online.
References
- Abner EL, Kryscio RJ, Schmitt FA, Fardo DW, Moga DC, Ighodaro ET, et al. Outcomes after diagnosis of mild cognitive impairment in a large autopsy series. Ann Neurol 2017; 81: 549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SE, Toledo JB, Appleby DH, Xie SX, Wang LS, Baek Y, et al. Comparative survey of the topographical distribution of signature molecular lesions in major neurodegenerative diseases. J Comp Neurol 2013; 521: 4339–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R, Lange S. Adjusting for multiple testing–when and how? J Clin Epidemiol 2001; 54: 343–9. [DOI] [PubMed] [Google Scholar]
- Boluda S, Iba M, Zhang B, Raible KM, Lee VM, Trojanowski JQ. Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol 2015; 129: 221–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 2011; 70: 960–9. [DOI] [PubMed] [Google Scholar]
- Brettschneider J, Del Tredici K, Irwin DJ, Grossman M, Robinson JL, Toledo JB, et al. Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD). Acta Neuropathol 2014; 127: 423–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol 2013; 74: 20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Shulman JM, Nag S, Leurgans SE, Arnold SE, Morris MC, et al. Nigral Pathology and Parkinsonian signs in elders without Parkinson’s disease. Ann Neurol 2012; 71: 258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci 2010; 30: 7281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 2014; 128: 755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011; 72: 245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugger BN, Adler CH, Shill HA, Caviness J, Jacobson S, Driver-Dunckley E, et al. Concomitant pathologies among a spectrum of parkinsonian disorders. Parkinsonism Relat Disord 2014; 20: 525–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elobeid A, Libard S, Leino M, Popova SN, Alafuzoff I. Altered proteins in the aging brain. J Neuropathol Exp Neurol 2016; 75: 316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fîlfan M, Sandu RE, Zăvăleanu AD, GreşiŢă A, Glăvan DG, Olaru DG, et al. Autophagy in aging and disease. Rom J Morphol Embryol 2017; 58: 27–31. [PubMed] [Google Scholar]
- Irwin DJ, Cairns NJ, Grossman M, McMillan CT, Lee EB, Van Deerlin VM, et al. Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine. Acta Neuropathol 2015; 129: 469–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DJ, Grossman M, Weintraub D, Hurtig HI, Duda JE, Xie SX, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol 2017; 16: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Murray ME, Whitwell JL, Parisi JE, Petrucelli L, Jack CR, et al. Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol 2014; 127: 441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol 2017; 134: 171–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga S, Parks A, Uitti RJ, van Gerpen JA, Cheshire WP, Wszolek ZK, et al. Profile of cognitive impairment and underlying pathology in multiple system atrophy. Mov Disord 2017a; 32: 405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga S, Sanchez-Contreras M, Josephs KA, Uitti RJ, Graff-Radford N, van Gerpen JA, et al. Distribution and characteristics of transactive response DNA binding protein 43 kDa pathology in progressive supranuclear palsy. Mov Disord 2017b; 32: 246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, Milenkovic I, Wöhrer A, Höftberger R, Gelpi E, Haberler C, et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol 2013; 126: 365–84. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017; 89: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 2012; 123: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006; 314: 130–3. [DOI] [PubMed] [Google Scholar]
- Perry D, Sperling R, Katz R, Berry D, Dilts D, Hanna D, et al. Building a roadmap for developing combination therapies for Alzheimer’s disease. Expert Rev Neurother 2015; 15: 327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Goedert M. Synucleinopathies: past, present and future. Neuropathol Appl Neurobiol 2016; 42: 3–5. [DOI] [PubMed] [Google Scholar]
- Spires-Jones TL, Attems J, Thal DR. Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol 2017; 134: 187–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh E, Lee EB, Neal D, Wood EM, Toledo JB, Rennert L, et al. Semi-automated quantification of C9orf72 expansion size reveals inverse correlation between hexanucleotide repeat number and disease duration in frontotemporal degeneration. Acta Neuropathol 2015; 130: 363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan RH, Yang Y, Halliday GM. Multiple neuronal pathologies are common in young patients with pathologically proven Frontotemporal lobar degeneration. Neuropathol Appl Neurobiol 2017, in press. doi: 10.1111/nan.12455. [DOI] [PubMed] [Google Scholar]
- Thal DR, von Arnim CA, Griffin WS, Mrak RE, Walker L, Attems J, et al. Frontotemporal lobar degeneration FTLD-tau: preclinical lesions, vascular, and Alzheimer-related co-pathologies. J Neural Transm 2015; 122: 1007–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Van Deerlin VM, Lee EB, Suh E, Baek Y, Robinson JL, et al. A platform for discovery: The University of Pennsylvania integrated neurodegenerative disease biobank. Alzheimers Dement 2014; 10: 477–84.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauwenberghe C, Van Broeckhoven C, Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet Med 2016; 18: 421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LR, Edland SD, Hemmy LS, Montine KS, Zarow C, Sonnen JA, et al. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia Aging Studies. Neurology 2016; 86: 1000–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard JS. Concentric hyaline inclusion body formation in mental disease analysis of twenty-seven cases. J Neuropathol Exp Neurol 1962; 21: 442–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.