Ventral intermediate thalamic stimulation is effective in treating essential tremor and tremor-dominant Parkinson’s disease, but its precise mechanism of action is unclear. Milosevic et al. show that thalamic inhibition of neuronal firing is necessary for tremor reduction, suggesting that the thalamus acts as a filter for uncoupling central and peripheral tremor networks.
Keywords: clinical neurophysiology, deep brain stimulation, neurosurgery, tremor, Parkinson’s disease
Abstract
Ventral intermediate thalamic deep brain stimulation is a standard therapy for the treatment of medically refractory essential tremor and tremor-dominant Parkinson’s disease. Despite the therapeutic benefits, the mechanisms of action are varied and complex, and the pathophysiology and genesis of tremor remain unsubstantiated. This intraoperative study investigated the effects of high frequency microstimulation on both neuronal firing and tremor suppression simultaneously. In each of nine essential tremor and two Parkinson’s disease patients who underwent stereotactic neurosurgery, two closely spaced (600 µm) microelectrodes were advanced into the ventral intermediate nucleus. One microelectrode recorded action potential firing while the adjacent electrode delivered stimulation trains at 100 Hz and 200 Hz (2–5 s, 100 µA, 150 µs). A triaxial accelerometer was used to measure postural tremor of the contralateral hand. At 200 Hz, stimulation led to 68 ± 8% (P < 0.001) inhibition of neuronal firing and a 53 ± 5% (P < 0.001) reduction in tremor, while 100 Hz reduced firing by 26 ± 12% (not significant) with a 17 ± 6% (P < 0.05) tremor reduction. The degree of cell inhibition and tremor suppression were significantly correlated (P < 0.001). We also found that the most ventroposterior stimulation sites, closest to the border of the ventral caudal nucleus, had the best effect on tremor. Finally, prior to the inhibition of neuronal firing, microstimulation caused a transient driving of neuronal activity at stimulus onset (61% of sites), which gave rise to a tremor phase reset (73% of these sites). This was likely due to activation of the excitatory glutamatergic cortical and cerebellar afferents to the ventral intermediate nucleus. Temporal characteristics of the driving responses (duration, number of spikes, and onset latency) significantly differed between 100 Hz and 200 Hz stimulation trains. The subsequent inhibition of neuronal activity was likely due to synaptic fatigue. Thalamic neuronal inhibition seems necessary for tremor reduction and may function in effect as a thalamic filter to uncouple thalamo-cortical from cortico-spinal reflex loops. Additionally, our findings shed light on the gating properties of the ventral intermediate nucleus within the cerebello-thalamo-cortical tremor network, provide insight for the optimization of deep brain stimulation technologies, and may inform controlled clinical studies for assessing optimal target locations for the treatment of tremor.
Introduction
Tremor is characterized by involuntary rhythmic muscle contractions that can occur in one or more body parts. It can occur alone as in essential tremor, or with other motor symptoms as in Parkinson’s disease and occasionally dystonia. Essential tremor is currently the most prevalent movement disorder in man (Louis et al., 1998), and three of four patients with Parkinson’s disease develop tremor at some point during the disease process (Hughes et al., 1993). In Parkinson’s disease, tremor is typically present at rest, while essential tremor patients possess postural or kinetic tremor (Deuschl et al., 1998; Elble and Deuschl, 2009). Tremor is regarded as the most difficult to treat symptom of Parkinson’s disease as it may not respond well to dopamine replacement therapy, and essential tremor has also proven quite intractable to treat pharmaceutically in a subset of patients (Goldman et al., 1992; Koller et al., 1994; Ondo et al., 1998; Fishman, 2008). Deep brain stimulation (DBS) of the thalamic ventral intermediate nucleus (Vim) is an efficacious and reversible standard of care that has largely replaced Vim thalamotomy for the amelioration of tremor (Benabid et al., 1991, 1993, 1996; Nguyen and Degos, 1993; Deiber et al., 1993). Numerous studies have supported the central origin of tremor by hypothesizing the presence of a single pathological oscillation frequency between 4 and 6 Hz (Rajput et al., 1991; Deuschl et al., 1998; Llinás et al., 2005).
In Parkinson’s disease, an early thalamo-centric theory of tremor genesis stated that 12–15 Hz oscillations in pallidal output found in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine monkeys were converted into 4–6 Hz tremor oscillations by intrinsic thalamic membrane hysteresis (Llinás and Paré, 1995). A more recent pallido-centric theory (Helmich et al., 2011), termed the dimmer-switch hypothesis, suggests that Parkinson’s disease tremor is initiated by the basal ganglia (the switch) and its amplitude is modulated by the cerebello-thalamo-cortical network (the dimmer). Indeed, single neurons with 4–6 Hz tremor oscillations are present in the human globus pallidus internus (GPi; Hutchison et al., 1997). This theory suggests that the GPi sends tremorgenic output to the thalamus, which then ascends though the thalamo-cortical network. However, that would suggest a predominant role for the pallidal thalamic input nuclei, ventral oral anterior and posterior (Voa, Vop), in tremor-genesis, but this does not fit with DBS intraoperative findings, which show that intervention of the cerebellar thalamus (Vim) is superior for treating tremor (Atkinson, et al., 2002), or that there are more ‘tremor cells’ in the Vim than in Vop/Voa (Magnin et al., 2000). However, studies (reviewed in Duval, et al., 2016) suggest that bursting activity can propagate to different nuclei within the thalamus by way of relay nuclei that can either induce bursting activity in neighbouring neurons, or simply relay bursting activity that is already present. Furthermore, burst firing of thalamic neurons has been demonstrated to provide a non-linear amplification of sensory signals (Guido and Weyand, 1995). Thus, periodic oscillations at tremor frequency could be amplified in cortical regions. The same cortical regions that receive this thalamic input exhibit oscillatory tremor-related activity, and send projections to the striatum (Volkmann et al., 1996), as well as direct projections to the subthalamic nucleus (STN; Monakow et al., 1978; Nambu et al., 1996; Mathai and Smith, 2011), which could explain the presence of tremor-related oscillations within the basal ganglia.
Essential tremor is regarded as a disorder of the cerebellum. Post-mortem studies have described various levels of neurodegeneration in essential tremor patients including Purkinje cell loss and Purkinje cell axonal swelling in the neocerebellum and vermis (Louis et al., 2007; Axelrad et al., 2008; Shill et al., 2008; Louis et al., 2011; Yu et al., 2012). However, other studies have not found neurodegenerative changes, rather that there is neurophysiological evidence of a reduction in GABAergic tone. In the dentate nucleus of essential tremor patients, post-mortem studies have revealed lower levels of GABA-A and GABA-B receptors compared to control subjects (Paris-Robidas et al., 2012). Thus, the restricted inhibitory influence of Purkinje cells may result in increased disinhibition of deep cerebellar neurons, and the subsequent overactivity may spread through the cerebello-thalamo-cortical network. Indeed, the Vim has a distinct role within essential tremor pathophysiology. DBS studies have demonstrated tremor-related local field potential clusters (Pedrosa et al., 2012) and intraoperative studies have shown single-unit tremor-related discharges (tremor cells; Lenz et al., 1988; Takahashi et al., 1998) in Vim that are coherent with tremor. What drives these oscillatory networks is still unsubstantiated. Early theories hypothesize that unique ion channel dynamics in the thalamus, inferior olive, and cerebellum can generate oscillations (Jahnsen and Llinas 1984a, b; Llinás, 1988). Movement-related activation of nucleo-olivary cells may cause Purkinje cells to synchronously inhibit deep cerebellar nuclei, which generate oscillatory rebound potentials (inhibition-induced excitation) that make their way through the cerebello-thalamo-cortical network. However, studies (reviewed in Helmich et al., 2013) have moved away from single oscillator hypotheses, and suggest that there may be shifting modes of cooperation in all nodes of the tremor network, and that all components are capable of acting as resonators and entraining each other.
In this study, we set out to elucidate how electrical stimulation interacts with the brain on a physiological level during therapeutic high-frequency stimulation (HFS) and how it leads to clinical benefit. While modelling studies (Meijer et al., 2011; Kuncel et al., 2012; Birdno et al., 2014) have been used to predict the effects of thalamic DBS on neuronal firing, our unique intraoperative dual-microelectrode assembly allows us to record the activity of single neurons during stimulation from a nearby electrode while simultaneously quantifying effects on tremor. Our findings suggest that tremor reduction was associated with inhibition of neuronal firing, which occurred after a transient driving of neuronal activity. Additionally, our findings shed light on the complex pathophysiology of tremor-genesis, and could also provide insight for the optimization of DBS technology for the treatment of tremor.
Methods and materials
Patients
A total of 21 Vim sites were investigated during microelectrode-guided placement of DBS electrodes in 11 patients; nine with essential tremor and two with Parkinson’s disease (who had an additional postural tremor component). The experiment conformed to the guidelines set by the Tri-Council Policy on Ethical Conduct for Research Involving Humans and were approved by the University Health Network Research Ethics Board. Furthermore, all of the patients in this study provided written, informed consent prior to taking part in the study.
Data acquisition
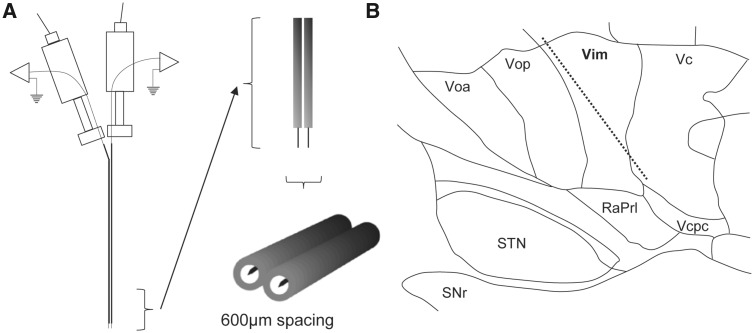
Two independently driven microelectrodes (25 μm tip lengths, 600 μm apart, 0.2–0.4 MΩ impedances, sampled at 12.5 kHz), which share a common ground on a stainless-steel intracranial guide tube, were used for recordings and microstimulation (Fig. 1A). Open filter recordings (5–3000 Hz) were amplified 5000 times using two Guideline System GS3000 amplifiers (Axon Instruments), digitized using a CED 1401 data acquisition system (Cambridge Electronic Design), and monitored using Spike2 software (Cambridge Electronic Design). Microstimulation was done using one of the two isolated constant-current stimulators (Neuro-Amp1A, Axon Instruments) with square wave, 0.3 ms biphasic pulses (cathodal followed by anodal).
Figure 1.
Experimental setup. (A) Our custom dual-microelectrode recording assembly with ∼600 µm mediolateral spacing between adjacent microelectrodes. Upon locating a well isolated spike on one microelectrode, the adjacent microelectrode was used to deliver stimulation trains at the same depth. (B) Representative microelectrode track of the Vim and surrounding structures, thalamic sub-nuclei, and fibres. RaPrl = prelemniscal radiations; Vcpc = ventral caudal parvocellular.
Microelectrode recording procedure
Techniques used for intraoperative electrophysiological identification of Vim have been published previously (Lenz et al., 1988; Ohye et al., 1989). Briefly, stereotactic coordinates of the anterior commissure and posterior commissure were determined using a T1–T2 fusion MRI (Signa, 1.5 T or 3 T, General Electric) on a surgical neuronavigation workstation (Mach 4.1, StealthStation, Medtronic, Minneapolis, USA), in addition to an estimation of the location of Vim based on the 14.5 mm sagittal section of the Schaltenbrand and Wahren (1977) standard atlas. The two microelectrodes were advanced through a tentative trajectory through the thalamus in an anterodorsal to ventroposterior direction towards coordinates of x = 14.5 mm (or 11 mm lateral to the third ventricle), y = 6 mm anterior to the posterior commissure and z = 0 mm from the mid-commissural point (Fig. 1B). Several techniques were used for the delineation of thalamic sub-nuclei. Single units were tested for responses to passive and active movements of the wrist, elbow, and shoulder. Units with movement-related responses were considered cells of the motor thalamus: Vop/Vim (Molnar et al., 2005). Microstimulation (100–200 Hz, 100 µA, 2–5 s, 0.3 ms pulse width) was delivered every 1 mm along the trajectory to coarsely delineate Vim from Vop based on tremor reduction or tremor arrest. The first site along the trajectory with stimulation-induced paraesthesia was considered to be in the vicinity of the anterior border of the ventral caudal nucleus (Vc). We also confirm Vim recording sites by the presence of beta oscillatory activity in the absence of tremor, which is not otherwise found in surrounding structures (Basha et al., 2014).
Experimental protocol
Based on the above criteria, the protocol was undertaken in recording sites that were determined to be in the Vim (maximum 5 mm away from Vc). Upon locating a well isolated single unit (cell), patients were asked to maintain a tremorgenic posture by holding up a bottle of isopropyl alcohol (filled to ∼150 ml), while a triaxial accelerometer (Crossbow Technology) was used to measure the scalar sum of accelerations on the wrist of the contralateral hand. In two patients we also obtained EMG (Intronix Technologies) from the wrist extensor muscle. When stable tremor was present, stimulation trains at 100 Hz and 200 Hz were delivered (2–5 s, 100 μA, 150 µs) from the adjacent microelectrode (600 μm away in the mediolateral direction). A total of 88 stimulation trains were delivered (40 at 100 Hz and 48 at 200 Hz, at least one of each per stimulation site). At three recording sites only tremor reduction was measured as the units were lost (excluded from correlations).
Offline analyses and statistics
To measure firing rates during stimulation trains, stimulus artefacts (0.3 ms pulse duration) were removed offline from the signal starting at the onset of the stimulation pulse to its end. Single units were discriminated using the waveform template matching tool in Spike2. Cell inhibition was measured as the ratio of the firing rate during the stimulation train to a 10-s pre-stimulation baseline firing rate of the cell. This value was subtracted from 1 and multiplied by 100 to get ‘% cell inhibition’ (i.e. a value of 100 represents complete inhibition). In recordings sites that had an initial transient driving of neuronal activity at stimulation onset (Fig. 5), the cell inhibition was measured after the initial burst. In these recording sites, we measured the burst duration (ms), firing rate (Hz), number of spikes, and onset latency (ms; from the first pulse of the stimulation train). For tremor reduction, the root mean square amplitude (0.2 s time constant) of the accelerometer signal was measured. A ratio was taken between the waveform averages during the tremor reduction period compared to a pre-stimulation baseline period immediately before the stimulation train. This value was subtracted from 1 and multiplied by 100 to get ‘% tremor reduction’ (i.e. a value of 100 represents complete tremor arrest). The duration of both the tremor reduction period and pre-stimulation baseline were equivalent to the duration of the stimulation train. However, we measured the maximal tremor reduction period, which always had a delay with respect to the stimulation train onset, as seen in Fig. 2. The average delay between stimulation onset and maximal tremor reduction period was 466 ± 24 ms [average ± standard error (SE)]. Tremor phase resets were determined by comparing the instantaneous frequency of each phase of the tremor cycle before stimulation, to the instantaneous frequency immediately after onset of the stimulation (Fig. 6). Paired sample t-tests (two-tailed) were used to determine whether stimulation trains had a significant effect on tremor reduction and neuronal inhibition compared to baseline for each of the frequencies. To compare the effect of stimulation frequency on cell inhibition, tremor reduction, and the transient driving response variables (listed above), paired sample t-tests (one-tailed) were used, under the hypothesis that 200 Hz had a greater effect on each of the parameters than 100 Hz. A second-order polynomial regression line was fit to the correlation between cell inhibition and tremor reduction, and a Pearson’s coefficient of correlation was calculated. To determine the effect of tremor reduction as a function of depth though the trajectory at 100 Hz and 200 Hz, linear regression lines were fit and Pearson’s coefficients of correlation were calculated.
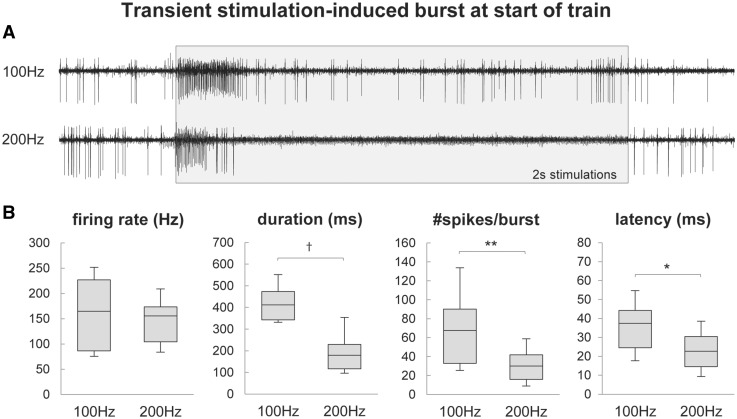
Figure 5.
Transient stimulation-induced driving of neuronal activity. (A) Representative example of the transient driving of neuronal activity at the start of a 100 Hz and 200 Hz stimulation train at a recording site in a single patient (with stimulus artefacts removed and represented with shaded box). (B) Box-and-whisker plots describing the transient driving responses. The figures show the 10th and 90th percentiles, first and third quartiles, and median of the firing rate, duration, number of spikes, and onset latency of the driving responses. There was a significant difference in all values except firing rate. *P < 0.05, **P < 0.05, †P < 0.001.
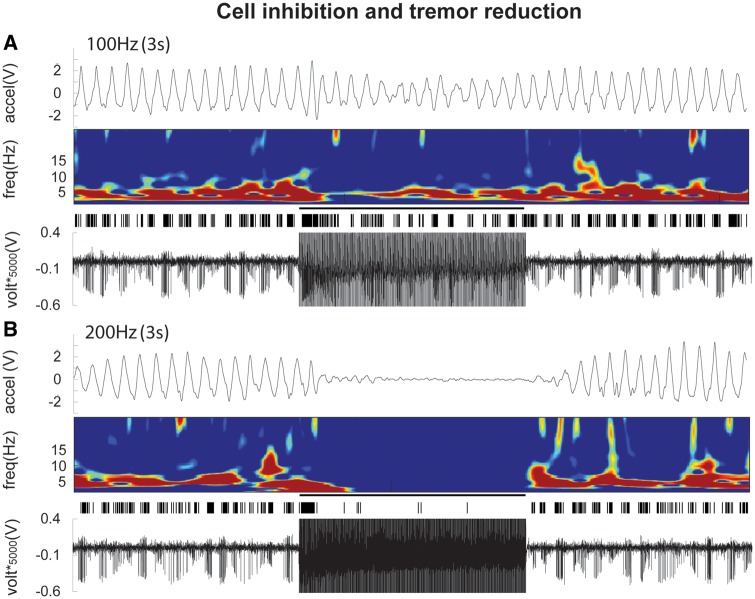
Figure 2.
Sample data during 100 Hz (A) and 200 Hz (B) stimulations from a single patient. Collectively, the figures show that 200 Hz stimulation led to near complete cell inhibition and tremor reduction, while 100 Hz was insufficient for achieving these phenomena. The bottom trace in each panel is a raw microelectrode recording during stimulation from the adjacent microelectrode. Above that is the artefact-removed, template-matched spike, which shows the neuronal activity during the stimulation train. The spectrogram demonstrates the frequency of the spike bursting (depicting a 5 Hz synchronous discharge of the neuronal firing; tremor cell), and shows that at 200 Hz (when spike firing is mostly inhibited) the 5 Hz tremor-related activity is desynchronized, but at 100 Hz (when spike firing is persistent) the 5 Hz activity is still present. The top trace in each panel is the accelerometer signal during postural tremor of the contralateral hand.
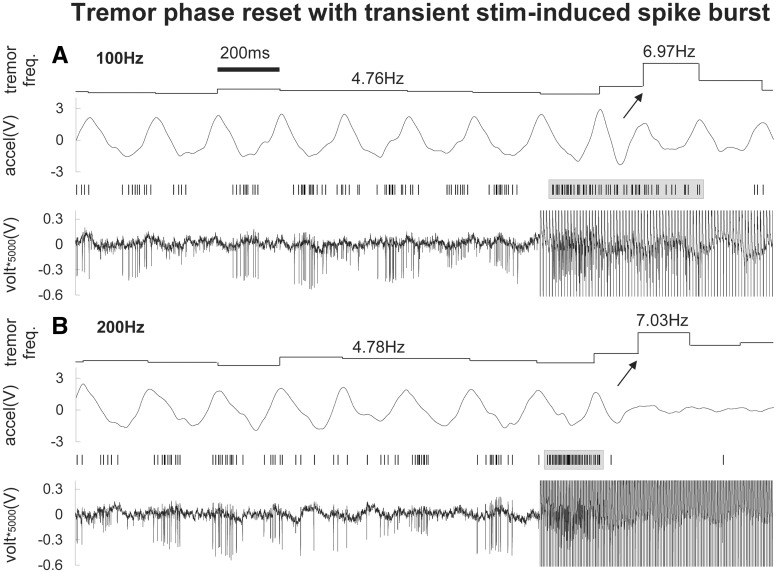
Figure 6.
Representative example of tremor phase resets at the start of a 100 Hz (A) and 200 Hz (B) stimulation train. A tremor phase reset is present at the start of the stimulation train, which closely follows the initial stimulation-induced neuronal driving response of the cell. This is likely due to a thalamo-cortical activation of motor cortical areas during the driving response, before the subsequent neuronal inhibition (and tremor suppression) occurs.
Results
Ventral intermediate nucleus recording sites
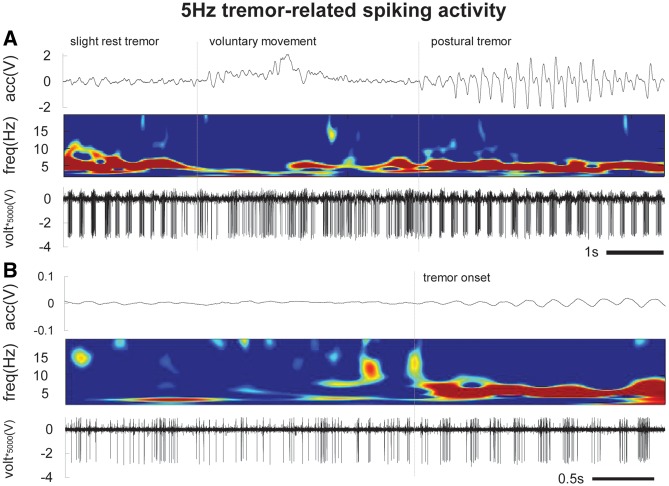
The average pre-stimulation baseline firing rate of all recorded neurons was 48 ± 8 Hz (average ± SE). Of the recorded neurons, 56% (10/18) were tremor cells that exhibited 4–6 Hz tremor-related burst firing (with an average intraburst firing rate of 88 ± 12 Hz) and movement-related responses (Fig. 7). In 61% (11/18) of the neurons, we recorded transient stimulation-induced driving of neuronal activity that was limited to the start of the stimulation trains (Fig. 5A). In 57% (12/21) of all recordings sites, a tremor phase reset occurred at the start of the stimulation trains (Fig. 6). Eight of the 11 (73%) neurons with transient driving responses had phase resets. Our EMG recordings from the wrist extensor muscles showed an average fast latency muscle activation of 62 ± 4 ms from the start of the stimulation train during phase resets.
Figure 7.
Tremor and movement-related spiking in Vim in a single neuron. (A) 5 Hz spiking activity is present during a slight rest tremor. When the patient was asked to raise their arm (to begin our experimental protocol), there is a voluntary movement-related kinaesthetic response and an interruption in the 5 Hz tremor-related activity. This is followed by a re-emergence of tremor bursting with the maintenance of a tremorgenic posture (postural tremor). (B) At rest with no tremor, the neuron had irregular tonic firing, and the emergence of 5 Hz bursting was robustly measurable even with the slightest tremor onset.
Tremor reduction and cell inhibition during stimulation
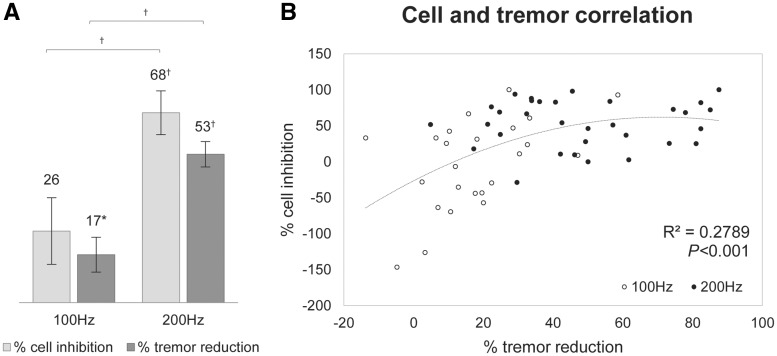
Higher neuronal inhibition was associated with improved tremor reduction, which was more prominent at 200 Hz. Figure 3A shows that 200 Hz stimulation led to 68 ± 8% (P < 0.001) inhibition of neuronal firing compared to baseline, and a 53 ± 5% (P < 0.001) reduction in tremor, while 100 Hz only reduced firing by 26 ± 12% (not significant) with a 17 ± 6% (P < 0.05) tremor reduction. At 200 Hz, both the cell inhibition (P < 0.001) and tremor reduction (P < 0.001) were significantly higher than at 100 Hz. Figure 3B shows that the degree of neuronal inhibition and tremor reduction were significantly correlated with a second-order polynomial fit (R2 = 0.28, P < 0.001), most representative of the relationship. There was also a significant linear correlation (R2 = 0.28, P < 0.001; not shown in the figure).
Figure 3.
Neuronal inhibition and tremor reduction. (A) The degree of cell inhibition and tremor reduction during stimulation trains at 100 Hz and 200 Hz compared to baseline for stimulations across all recording sites. At 200 Hz, there was significantly more cell inhibition and tremor reduction compared to 100 Hz. (B) The correlation between cell inhibition and tremor reduction across all recording sites, fitted with a second order polynomial. *P < 0.05, †P < 0.001.
Spatial distribution of tremor reduction
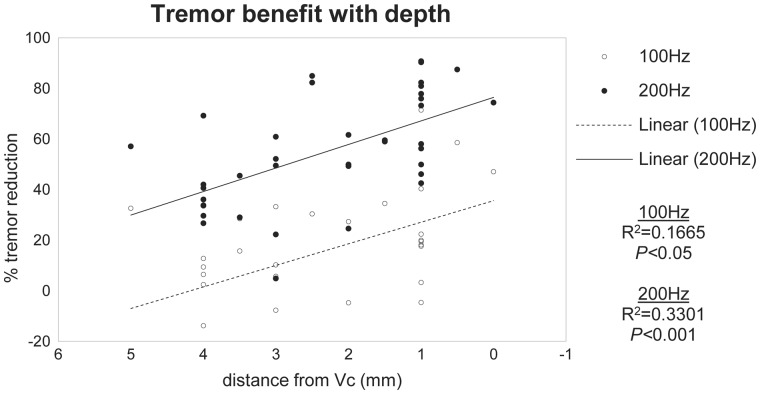
The most ventroposterior stimulation sites, closest to the Vim-Vc border, had the best effect on tremor. Figure 4 shows that tremor reduction and proximity to the Vim-Vc border were significantly correlated at both 100 Hz (R2 = 0.17, P < 0.05) and 200 Hz (R2 = 0.33, P < 0.001). At 200 Hz, stimulation sites within 1 mm of the Vim-Vc border led to a tremor reduction of 70 ± 4%.
Figure 4.
Tremor reduction with respect to distance from ventral caudal nucleus. The correlation suggests that clinical benefit was maximal at recording sites closest to the Vim-Vc border. The 0-mm mark is the first location with patient-reported paraesthesia. This does not imply that the recorded neuron at that site was in Vc, but rather that the stimulation has begun to spread into Vc.
Transient stimulation-induced driving of neuronal activity
In all recording sites with transient driving responses, the bursts were present during both 100 Hz and 200 Hz stimulations. Figure 5B shows that the duration of the bursts at 100 Hz (421 ± 24 ms) was significantly longer (P < 0.001) than at 200 Hz (194 ± 21 ms), there were significantly more (P < 0.01) spikes per burst at 100 Hz (71 ± 11) compared to 200 Hz (30 ± 4), the latency from stimulation onset to burst onset was significantly longer (P < 0.05) at 100 Hz (36 ± 4 ms) compared to 200 Hz (24 ± 3 ms), but there was no significant difference between the burst firing rates between 100 Hz (166 ± 21 Hz) and 200 Hz (154 ± 16 Hz), likely due to the refractory period of spike firing.
Discussion
A major finding of the present study is that—following an initial transient driving response—both the firing of Vim neurons and contralateral hand tremor were strongly suppressed during 200 Hz microstimulation, and not affected or only partially reduced during 100 Hz. Therefore, thalamic neuronal inhibition seems necessary for tremor reduction and may function as a thalamic filter to uncouple thalamo-cortical from cortico-spinal reflex loops.
The likely reason for this pattern of brief excitation followed by inhibition is the activation of afferent inputs to the neurons. The Vim is primarily innervated by excitatory glutamatergic projections from both the dentate nucleus of the cerebellum (Asanuma et al., 1983; Anderson and Turner, 1991; Kultas-Ilinsky and Ilinsky, 1991; Kuramoto et al., 2011) and the cerebral cortex (Bromberg et al., 1981; Sherman and Guillery, 1996). The less prominent afferent inputs are the inhibitory GABAergic thalamic reticular projections (Ambardekar et al., 1999; Ilinsky et al., 1999; Kuramoto et al., 2011). The activation of glutamatergic presynaptic terminals by electrical stimulation would explain why the somadendritic part of the neurons produced the initial burst of action potentials. It may also explain why Vim neurons were not as prone to inhibition compared to neurons in the STN, substantia nigra pars reticulata (SNr), and GPi that we have previously studied (Liu et al., 2012; Milosevic et al., 2017). The predominant afferent inputs of these basal ganglia structures are GABAergic (Rinvik and Ottersen, 1993; Parent and Hazrati, 1995a,b), and we found that 100 Hz stimulation was effective at completely silencing neuronal firing in the STN, while SNr and GPi could be silenced with an even lower frequency of 50 Hz. Furthermore, neither transient nor tonic excitatory responses occurred in those structures, unlike in Vim. This suggests that the mechanism of action of electrical stimulation is dependent on the underlying microcircuit anatomy of the target structure.
Initial burst and subsequent inhibition during high frequency stimulation
A modelling study by Kuncel et al. (2012) predicted that with 125 Hz Vim-DBS, neuronal firing is either entirely inhibited, or exhibits a sustained entrainment. However, our findings showed that there is a bimodal response, and appear to support the theory by Dittman et al. (2000) that there may be interplay between facilitation and depression. In many synapses (especially glutamatergic, due to their lower probabilities of neurotransmitter release) there is a ‘short-lived’ synaptic facilitation that occurs at the onset of repeated stimulation, believed to occur by increased presynaptic calcium (Katz and Miledi, 1968). The facilitation is followed in short order by synaptic depression (Katz, 1966; Malenka and Siegelbaum, 2001; Fioravante and Regehr, 2011), believed to occur by vesicle depletion and/or decreased presynaptic calcium (Zucker and Regehr, 2002; Fioravante and Regehr, 2011). When a rapid stimulus results in release of a readily releasable pool of neurotransmitter vesicles, subsequent stimuli delivered before replenishment will release fewer vesicles, eventually depleting the pool (Zucker, 1989; Rosenmund and Stevens, 1996). Modelling studies have shown that synaptic depression increases when the initial release probability and/or frequency of activation are increased (Dittman and Regehr, 1998; Zucker and Regehr, 2002; Rizzoli and Betz, 2005; Fioravante and Regehr, 2011). Indeed, these findings have been found to hold true in glutamatergic cortico-thalamic synapses in a rat brain slices (Ran et al., 2009).
With lower stimulation frequencies, which would allow sufficient time for vesicle replenishment, the driving response should be sustained (Supplementary Fig. 1). Although we were not able to measure synaptic field potentials, previous studies from our group (Liu et al., 2012; Milosevic et al., 2017) have shown that the rate of attenuation of extracellular inhibitory postsynaptic potentials in SNr and GPi increases as stimulation frequency is increased, indicative of frequency-dependent neurotransmitter depletion/synaptic depression as a mechanism of HFS.
An intracellular sensorimotor thalamic rat brain slice study by Anderson et al. (2004) has indeed shown that HFS leads to an initial transient depolarization, characterized by a burst of action potentials. Following the initial burst, the neurons were either quickly repolarized and returned to a quiescent baseline, or maintained some level of membrane depolarization, with or without spike firing. Reduction in the initial depolarization was achieved with application of kynurenate, a non-specific antagonist of ionotropic glutamate receptors, as well as with application of NMDA receptor blocker, and sodium channel blocker. This suggests that the HFS-induced depolarization was primarily mediated by glutamate. Furthermore, blockade of voltage-dependent calcium channels, which reversibly inhibited the depolarization, suggested that the depolarization was mediated primarily though pre-synaptic calcium channels (Anderson et al., 2004), which are known to facilitate transmitter release (Zucker and Regehr, 2002). Thus, Anderson et al. (2004) hypothesize that HFS in the ventral thalamus disrupts local synaptic function and neuronal firing thereby leading to a ‘functional deafferentation’.
Alternatively, other postsynaptic mechanisms may underlie the stimulation-induced burst at the onset of HFS. When thalamic neurons are hyperpolarized for 50–100 ms, incoming excitatory synaptic potentials trigger activation of T-type Ca2+ currents (Jahnsen and Llinas, 1984a), which causes the cell to fire a burst of action potentials. This leads to further calcium channel openings, which eventually trigger calcium-activated potassium currents, which quickly hyperpolarize the cell and reset it for another cycle of bursting. While these mechanisms may explain the generation of rhythmic bursts (i.e. tremor cells), they are less likely to explain the lack of continued bursting (/sustained inhibition) that we have shown here occurs during HFS. The more likely involvement of the T-current is that the initial excitatory response (via glutamate release) leads to inactivation of T-type Ca2+ channels, thereby preventing bursting activity. Beurrier et al. (2001) have shown that in the STN of rat brain slices, there is an inhibition of neuronal activity that outlasts a 1-min train of HFS. They found that (L- and) T-type Ca2+ currents were indeed transiently depressed during the HFS-induced silence. Additionally, they found that the HFS-induced inhibition was persistent in the presence of blockers of ionotropic GABA and glutamate receptors, and suggest that the inhibition was non-synaptic. However, they did not study the synaptic function during HFS. Thus, neurotransmitter blockers would not affect the persistent inhibition if synaptic function was already depressed due to the HFS.
Furthermore, thalamic inhibition has been linked to the activity of neuromodulators. Bekar et al. (2008) found that in rodent thalamic slices, DBS caused increased levels of adenosine, which they hypothesized led to neuronal inhibition that was necessary for suppression of tremor. Additionally, Dirkx et al. (2017) showed that the treatment of Parkinson’s tremor with levopoda was associated with increased thalamic self-inhibition, which may be a physiological mechanism that protects the thalamus from a permanent oscillatory state.
Thalamic gating
This study offers mechanistic insight on the gating properties of the Vim and its thalamo-cortical projection. The Vim sends excitatory glutamatergic projections to cortical motor regions in order to modulate movements (Rouiller et al., 1994). In this study, we have identified five different types of Vim firing patterns that corresponded to different motor states. First, there were three described previously in the literature that occurred in the absence of electrical stimulation, exemplified in Fig. 7. When the patient was at rest with no tremor, the neurons exhibited (i) tonic irregular firing. Both passive and voluntary manipulations of the limb led to (ii) kinaesthetic movement-related responses (Ohye and Narabayashi, 1979; Lenz et al., 1990). When the patient had tremor, the neuron exhibited (iii) tremor-related (4–6 Hz) bursting (Albe-Fessard et al., 1963). The significance of these classifications is the potential to use this real-time information in an application of closed-loop DBS (Priori et al., 2013; Arlotti et al., 2016) for the control of tremor. A novel finding of this study was the stimulation-induced (iv) transient driving of Vim neurons that reset the regular periodic rhythmicity of the tremor (Fig. 6). The most likely explanation of this is that the transient neuronal driving response leads to an activation of thalamo-cortical motor neurons either in the primary or supplementary motor cortical areas (Rouiller et al., 1994) via collaterals that give rise to the transcortical reflex that then quickly activate the forearm muscles. Our EMG results showed a fast latency muscle activation that is consistent with thalamo-cortical activation of the transcortical reflex. In many simple laboratory models of central pattern generators, such as the locust thoracic ganglion motor neuron recordings, a very similar phenomenon of rhythmic reset is observed with short train out-of-phase stimulation of the isolated proprio-sensory input from the wing to the central pattern generator (Pearson, 1991; Marder and Bucher, 2001). In fictive locomotion induced by mesencephalic locomotor region stimulation in the decerebrate paralysed cat, a prominent reset of the step cycle is produced by brief out-of-phase 100 Hz stimulation of the Group I muscle spindle afferents (Guertin et al., 1995; Hiebert et al., 1996). This would suggest that tremor reset and tremor reduction is due to interruption of the pacing of proprioceptive input in human thalamus, which is found near the Vim-Vc border that receives input from deep muscles (Tasker et al., 1987; Vitek et al., 1994). Indeed, our results show that more efficacious tremor reduction was at stimulation sites closest to the Vim-Vc border.
While the phase reset demonstrates that a transient excitatory neuronal response in Vim would facilitate a brief movement, the subsequent (v) inhibition of neuronal activity was associated with a reduction of tremor. This finding supports the hypothesis (Anderson et al., 2004) that DBS at a high frequency may in effect function as a reversible lesion, which disrupts the pathological tremor-genic rhythmicity of Vim (Fig. 2B). Indeed, we have found that at a lower stimulation frequency (100 Hz) that is less effective at inhibiting the firing of Vim neurons, the tremor and tremor-related bursting persists (Fig. 2A). These findings support recent functional MRI findings by Dirkx et al. (2017), which suggest that efficacious treatment of tremor with levodopa may act by increasing thalamic self-inhibition. However, it is unlikely that the stimulation-induced inhibition of Vim only effects tremor, but may also be associated with a more widespread inhibition of movements. The continuous inhibition of neuronal activity in this area may explain the commonly reported adverse effects on other motor functions such as gait disturbances and ataxia (Cury et al., 2017), or less commonly weakness/uncertainty of the treated limbs (Takahashi et al., 1998). With respect to the gating function of Vim, it supports the notion that inhibition of neuronal activity has a role in downregulation of movements, including perhaps non-pathological (Strafella et al., 1997). This would further justify the need for a closed-loop system to selectively control tremor, in order to offset the chronic adverse effects of unnecessary continuous stimulation.
Taken together, these observations support the theory that the Vim acts as a gate for incoming information required to trigger movements. Depending on the input it receives (inhibitory, excitatory, rhythmic, etc.), its thalamo-cortical projection gives rise to an appropriate motor action. It also shows that the Vim can be selectively modulated by external stimuli. This likely explains why HFS relieves tremor, low frequency stimulation has been shown to induce or worsen tremor (Hassler et al., 1960; Barnikol et al., 2008; Pedrosa et al., 2013) likely due to persistent driving/entrainment of neuronal activity (Supplementary Fig. 1), and also why additional incoming proprioceptive information may desynchronize tremor-related activity (Naros et al., 2018). It may also explain why anti-phasic rhythmic stimulation has been reported to be efficacious for suppressing tremor (Cagnan et al., 2013), which likely works by regularizing the overall neuronal firing in Vim by producing short excitations between tremor bursts, rather than by overall inhibition which we have shown here appears to be the mechanism of continuous HFS.
Clinical utility
We found that the degree of cell inhibition was correlated to the degree of tremor reduction, suggesting that suppression of neuronal firing in the Vim is likely an important mechanism of DBS for the control of tremor. Our finding of better tremor suppression with 200 Hz supports clinical studies (Blomstedt et al., 2007; Earhart et al., 2007; Kuncel et al., 2012), which suggest that Vim-DBS produces better tremor benefit with higher programmed stimulation frequencies than typically used for STN (∼185 Hz versus ∼130 Hz). Single and multicentre studies have reported an average tremor reduction of ∼80% with Vim-DBS in essential tremor patients (Ondo et al., 1998; Koller et al., 1999; Rehncrona et al., 2003). We found a reduction of 53 ± 5% with microstimulation at 200 Hz, which is likely due to stimulating a much smaller population of neurons as well as testing less effective sites dorso-anterior to the tentative target site. The most effective sites for tremor reduction were in close proximity to the Vim-Vc border. At stimulation sites within 1 mm of the Vim-Vc border, 200 Hz microstimulation led to a tremor reduction of 70 ± 4%, comparable to that of the reported benefit of DBS macro-stimulation. This finding is important in informing surgical electrode placement, which can be accounted for intraoperatively with micro-recording and stimulation. It also supports neurosurgical observations that the ideal location for a Vim thalamotomy is the small section of Vim near Vc that receives proprioceptive input (Tasker et al., 1987). A recent study identified that more posterior DBS electrode placements were associated with failure of benefit, and more anterior placements were optimal (Sandoe et al., 2018). Our study shows that micro-stimulation of the ventroposterior region of Vim (i.e. as close to Vc as possible, without inducing paraesthesia) yielded the best tremor reduction, within the standard Vim-DBS trajectory. This is likely due to the larger size of DBS electrodes and the contacts being too close to Vc, producing paraesthesias that limit the current density required for tremor reduction. In the advent of novel ‘current-steering’ electrodes, this finding may be able to inform stimulation delivery, i.e. placement of the DBS electrode near the Vim/Vc border, but directing the current away from Vc.
Functional implications
Additionally, suboptimal electrode placement can be clinically compensated for by increasing the volume of tissue activation. However, this increases the risk of stimulating different neuro-circuits that lack relevance to the patient pathology, which likely gives rise to side-effects such as paraesthesia and dysarthria (Cury et al., 2017). Our study confirms the existence of an optimal site within the standard Vim trajectory, just anterior to the Vim-Vc border. At sites within 1 mm of the Vim-Vc border, 200 Hz microstimulation led to comparable long-term benefit of previously reported DBS macro-stimulation, despite stimulating a smaller population of neurons. This demonstrates the potential for improving therapeutic window by (i) minimizing the volume of tissue activation (reduces risk of side-effects); and (ii) minimizing the size of the stimulating electrode to have a more focal target (reduces risk of oedema, haemorrhage, micro-lesion, etc.).
Additionally, having an embedded electrode with a significantly smaller effective contact size can allow for the possibility to chronically record single neurons (DBS macroelectrodes are limited to local field potentials). Although ambitious, DBS technologies are evolving more rapidly than ever (Arlotti et al., 2016). This would allow for measurement of tremor-related neuronal activity to be used as a control parameter for adaptive DBS systems. Since tremor amplitude and prevalence can fluctuate over time, within seconds or minutes (Beuter and Vasilakos, 1995a, b), continuous open-loop strategies present an inefficient solution. Closed-loop DBS has been explored in Parkinson’s disease using beta (12–35 Hz) oscillations (Little and Brown, 2012; Little et al., 2013), but tremor-related activity in Vim may be a more robust and promising symptomatic correlate (Fig. 7).
Finally, we have shown that HFS can downregulate activity, which is important in essential tremor (where Vim receives pathophysiological input from cerebellum) and Parkinson’s disease (where the STN is believed to be overactive; Delong, 1990). However, we propose that stimulation at lower frequencies (conducive to excitation, but insufficient for neuronal inhibition) may be able to persistently drive/entrain neuronal firing in a target structure with predominantly glutamatergic inputs (Supplementary Fig. 1). This could have implications for upregulating activity in pathologies where structures may be underactive.
Limitations
One limitation of human intraoperative studies is the inability to use pharmacological agents to elucidate specific synaptic mechanisms. In contrast, these studies have the advantage over animal studies in that it is not known how well animal models correspond to human conditions, or anatomy. Furthermore, DBS is delivered chronically over a long period of time, while the time course of our intraoperative stimulation is limited. DBS macroelectrodes also stimulate a much larger population of neurons, with a current density that is capable of spreading up to 2 mm from the centre of a contact (Wu et al., 2001; Erez et al., 2009). Despite the short durations of stimulation and smaller volume of tissue activation with a microelectrode, we were still able to produce marked therapeutic symptomatic benefit, especially when delivered to the optimal location. Thus, our findings should be applicable to understanding the mechanisms that might be involved in Vim-DBS. A future study to validate our findings within the Vim would be the demonstration of tremor reduction in response to direct activation of the afferent dentatothalamic tracts (Coenen et al., 2014). While our results suggest that HFS of the Vim, and in particular ventroposterior Vim/Vc border region, does lead to marked tremor reduction, it would also be of interest to compare our results to other targets implicated in tremor suppression, such as caudal zona incerta, prelemniscal radiations, or subthalamic nucleus, as outlined in Elble and Deuschl (2011), which may have stronger effects. Another interesting prospective study would be the investigation of the effect of low frequency stimulation on Vim neuronal activity, and the potential relationship with the purported worsening of tremor.
Conclusions
Our study shows that the degree of neuronal inhibition in the Vim is associated with the degree of tremor suppression. The predominance of glutamatergic boutons located on somas of Vim neurons may explain why Vim was more resistant to neuronal inhibition than structures such as STN, SNr and GPi, which have predominantly GABAergic inputs. Hence, the mechanism of action of electrical stimulation is dependent on the underlying anatomical and physiological properties of the stimulated target structures. The transient excitatory responses at the onset of stimulation likely reflect those glutamatergic inputs, whereas the subsequent inhibition may be due to synaptic fatigue. Furthermore, we have shown that the location for maximal tremor suppression within the Vim is the ventroposterior region proximal to the Vim-Vc border. Finally, some of the response properties described in this study can help guide advancement of DBS therapy. First, the potential for using Vim tremor-related spike bursting as a robust, real time predictor of tremor onset and occurrence, and second, the potential for using electrical stimulation to upregulate neuronal activity.
Supplementary Material
Acknowledgements
We would like to thank Dr Rick Helmich for his insightful comments on the manuscript. Additionally, we thank the functional neurosurgical fellows (Robert Dallapiazza, Darren Lee, and Philippe De Vloo) who assisted in the operations, and the patients who participated in this study.
Glossary
Abbreviations
- DBS
deep brain stimulation
- GPi
globus pallidus internus
- HFS
high frequency stimulation
- SNr
substantia nigra pars reticulata
- STN
subthalamic nucleus
- Vc
ventral caudal nucleus
- Vim
ventral intermediate nucleus
- Voa
ventral oral anterior nucleus
- Vop
ventral oral posterior nucleus
Funding
This work was supported in part by the Natural Sciences and Engineering Research Council: Discovery Grant RGPIN-2016–06358 (M.R.P), and the Dystonia Medical Research Foundation (W.D.H).
Conflict of interest
S.K.K., M.H., A.M.L., and W.D.H. have received honoraria, travel funds, and/or grant support from Medtronic. M.R.P. is a shareholder in MyndTec Inc. and an advisor to Myant Inc. A.M.L. is a co-founder of Functional Neuromodulation Ltd.
Supplementary material
Supplementary material is available at Brain online.
References
- Albe-Fessard D, Guiot G, Hardy J. Electrophysiological localization and identification of subcortical structures in man by recording spontaneous and evoked activities. EEG Clin Neurophysiol 1963; 15: 1052–3. [Google Scholar]
- Ambardekar AV, Ilinsky IA, Froestl W, Bowery NG, Kultas-Ilinsky K. Distribution and properties of GABAB antagonist [3H] CGP 62349 binding in the rhesus monkey thalamus and basal ganglia and the influence of lesions in the reticular thalamic nucleus. Neuroscience 1999; 93: 1339–47. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Turner RS. Activity of neurons in cerebellar-receiving and pallidal-receiving areas of the thalamus of the behaving monkey. J Neurophysiol 1991; 66: 879–93. [DOI] [PubMed] [Google Scholar]
- Anderson T, Hu B, Pittman Q, Kiss ZH. Mechanisms of deep brain stimulation: an intracellular study in rat thalamus. J Physiol 2004; 559: 301–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlotti M, Rosa M, Marceglia S, Barbieri S, Priori A. The adaptive deep brain stimulation challenge. Parkinsonism Relat Disord 2016; 28: 12–17. [DOI] [PubMed] [Google Scholar]
- Asanuma C, Thach WT, Jones EG. Distribution of cerebellar terminations and their relation to other afferent terminations in the ventral lateral thalamic region of the monkey. Brain Res Rev 1983; 5: 237–65. [DOI] [PubMed] [Google Scholar]
- Atkinson JD, Collins DL, Bertrand G, Peters TM, Pike GB, Sadikot AF. Optimal location of thalamotomy lesions for tremor associated with Parkinson disease: a probabilistic analysis based on postoperative magnetic resonance imaging and an integrated digital atlas. J Neurosurg 2002; 96: 854–66. [DOI] [PubMed] [Google Scholar]
- Axelrad JE, Louis ED, Honig LS, Flores I, Ross GW, Pahwa R, et al. Reduced Purkinje cell number in essential tremor: a postmortem study. Arch Neurol 2008; 65: 101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnikol UB, Popovych OV, Hauptmann C, Sturm V, Freund HJ, Tass PA. Tremor entrainment by patterned low-frequency stimulation. Philos Trans A Math Phys Eng Sci 2008; 366: 3545–73. [DOI] [PubMed] [Google Scholar]
- Basha D, Dostrovsky JO, Rios AL, Hodaie M, Lozano AM, Hutchison WD. Beta oscillatory neurons in the motor thalamus of movement disorder and pain patients. Exp Neurol 2014; 261: 782–90. [DOI] [PubMed] [Google Scholar]
- Bekar L, Libionka W, Tian GF, Xu Q, Torres A, Wang X, et al. Adenosine is crucial for deep brain stimulation–mediated attenuation of tremor. Nat Med 2008; 14: 75. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gao D, Hoffmann D, Limousin P, Gay E, et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg 1996; 84: 203–14. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Hoffmann D, Gervason C, Hommel M, Perret JE, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 1991; 337: 403–6. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Seigneuret E, Hoffmann D, Gay E, Perret J. Chronic VIM thalamic stimulation in Parkinson’s disease, essential tremor and extra-pyramidal dyskinesias. In: Advances in stereotactic and functional neurosurgery. 10th edn Springer, Vienna; 1993. p. 39–44. [DOI] [PubMed] [Google Scholar]
- Beurrier C, Bioulac B, Audin J, Hammond C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol 2001; 85: 1351–6. [DOI] [PubMed] [Google Scholar]
- Beuter A, Vasilakos K. Fluctuations in tremor and respiration in patients with Parkinson’s disease. Parkinsonism Relat Disord 1995; 1: 103–11. [DOI] [PubMed] [Google Scholar]
- Beuter A, Vasilakos K. Tremor: is Parkinson’s disease a dynamical disease? Chaos 1995; 5: 35–42. [DOI] [PubMed] [Google Scholar]
- Birdno MJ, Tang W, Dostrovsky JO, Hutchison WD, Grill WM. Response of human thalamic neurons to high-frequency stimulation. PLoS One 2014; 9: e96026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomstedt P, Hariz GM, Hariz MI, Koskinen LO. Thalamic deep brain stimulation in the treatment of essential tremor: a long-term follow-up. Br J Neurosurg 2007; 21: 504–9. [DOI] [PubMed] [Google Scholar]
- Bromberg MB, Penne JB Jr, Stephenson BS, Young AB. Evidence for glutamate as the neurotransmitter of corticothalamic and corticorubral pathways. Brain Res 1981; 215: 369–74. [DOI] [PubMed] [Google Scholar]
- Cagnan H, Brittain JS, Little S, Foltynie T, Limousin P, Zrinzo L, et al. Phase dependent modulation of tremor amplitude in essential tremor through thalamic stimulation. Brain 2013; 136: 3062–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen VA, Allert N, Paus S, Kronenbürger M, Urbach H, Mädler B. Modulation of the cerebello-thalamo-cortical network in thalamic deep brain stimulation for tremor: a diffusion tensor imaging study. Neurosurgery 2014; 75: 657–70. [DOI] [PubMed] [Google Scholar]
- Cury RG, Fraix V, Castrioto A, Fernández MA, Krack P, Chabardes S, et al. Thalamic deep brain stimulation for tremor in Parkinson disease, essential tremor, and dystonia. Neurology 2017; 89: 1416–23. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Pollak P, Passingham R, Landais P, Gervason C, Cinotti L, et al. Thalamic stimulation and suppression of parkinsonian tremor: evidence of a cerebellar deactivation using positron emission tomography. Brain 1993; 116: 267–79. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 1990; 13: 281–5. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Bain P, Brin M. Consensus statement of the movement disorder society on tremor. Mov Disord 1998; 13(S3): 2–3. [DOI] [PubMed] [Google Scholar]
- Dirkx MF, den Ouden HE, Aarts E, Timmer MH, Bloem BR, Toni I, et al. Dopamine controls Parkinson’s tremor by inhibiting the cerebellar thalamus. Brain 2017; 140: 721–34. [DOI] [PubMed] [Google Scholar]
- Dittman JS, Kreitzer AC, Regehr WG. Interplay between facilitation, depression, and residual calcium at three presynaptic terminals. J Neurosci 2000; 20: 1374–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman JS, Regehr WG. Calcium dependence and recovery kinetics of presynaptic depression at the climbing fiber to Purkinje cell synapse. J Neurosci 1998; 18: 6147–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval C, Daneault J F, Hutchison W D, Sadikot A F. A brain network model explaining tremor in Parkinson’s disease. Neurobiol Dis 2016; 85; 49–59. [DOI] [PubMed] [Google Scholar]
- Earhart GM, Hong M, Tabbal SD, Perlmutter JS. Effects of thalamic stimulation frequency on intention and postural tremor. Exp Neurol 2007; 208: 257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elble R, Deuschl G. Milestones in tremor research. Mov Disord 2011; 26: 1096–105. [DOI] [PubMed] [Google Scholar]
- Elble RJ, Deuschl G. An update on essential tremor. Curr Neurol Neurosci Rep 2009; 9: 273–7. [DOI] [PubMed] [Google Scholar]
- Erez Y, Czitron H, McCairn K, Belelovsky K, Bar-Gad I. Short-term depression of synaptic transmission during stimulation in the globus pallidus of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-treated primates. J Neurosci 2009; 29: 7797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravante D, Regehr WG. Short-term forms of presynaptic plasticity. Curr Opin Neurobiol 2011; 21: 269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman PS. Paradoxical aspects of parkinsonian tremor. Mov Disord 2008; 23: 168–73. [DOI] [PubMed] [Google Scholar]
- Goldman MS, Ahlskog JE, Kelly PJ. The symptomatic and functional outcome of stereotactic thalamotomy for medically intractable essential tremor. J Neurosurg 1992; 76: 924–8. [DOI] [PubMed] [Google Scholar]
- Guertin P, Angel MJ, Perreault MC, McCrea DA. Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. J Physiol 1995; 487: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido WI, Weyand T. Burst responses in thalamic relay cells of the awake behaving cat. J Neurophysiol 1995; 74: 1782–6. [DOI] [PubMed] [Google Scholar]
- Hassler R, Riechert T, Mundinger F, Umbach W, Ganglberger JA. Physiological observations in stereotaxic operations in extrapyramidal motor disturbances. Brain 1960; 83: 337–50. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Toni I. Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol 2011; 69: 269–81. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Toni I, Deuschl G, Bloem BR. The pathophysiology of essential tremor and Parkinson’s tremor. Curr Neurol Neurosci Rep 2013; 13: 378. [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Whelan PJ, Prochazka AR, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol 1996; 75: 1126–37. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson’s disease. Arch Neurol 1993; 50: 140–8. [DOI] [PubMed] [Google Scholar]
- Hutchison W D, Lozano A M, Tasker R R, Lang A E, Dostrovsky J O. Identification and characterization of neurons with tremor-frequency activity in human globus pallidus. Exp Brain Res 1997; 113: 557–63. [DOI] [PubMed] [Google Scholar]
- Ilinsky IA, Ambardekar AV, Kultas‐Ilinsky K. Organization of projections from the anterior pole of the nucleus reticularis thalami (NRT) to subdivisions of the motor thalamus: light and electron microscopic studies in the rhesus monkey. J Comp Neurol 1999; 409: 369–84. [PubMed] [Google Scholar]
- Jahnsen H, Llinas R. Electrophysiological properties of guinea‐pig thalamic neurones: an in vitro study. J Physiol 1984a; 349: 205–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R. Ionic basis for the electro‐responsiveness and oscillatory properties of guinea‐pig thalamic neurones in vitro. J Physiol 1984b; 349: 227–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. Never, muscle and synapse. New York, NY: McGraw-Hill; 1966. [Google Scholar]
- Katz B, Miledi R. The role of calcium in neuromuscular facilitation. J Physiol 1968; 195: 481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller WC, Busenbark K, Miner K. The relationship of essential tremor to other movement disorders: report on 678 patients. Ann Neurol 1994; 35: 717–23. [DOI] [PubMed] [Google Scholar]
- Koller WC, Lyons KE, Wilkinson SB, Pahwa R. Efficacy of unilateral deep brain stimulation of the VIM nucleus of the thalamus for essential head tremor. Move Disord 1999; 14: 847–50. [DOI] [PubMed] [Google Scholar]
- Kultas-Ilinsky K, Ilinsky IA. Fine structure of the ventral lateral nucleus (VL) of the Macaca mulatta thalamus: cell types and synaptology. J Comp Neurol 1991; 314: 319–49. [DOI] [PubMed] [Google Scholar]
- Kuncel AM, Birdno MJ, Swan BD, Grill WM. Tremor reduction and modeled neural activity during cycling thalamic deep brain stimulation. Clin Neurophysiol 2012; 123: 1044–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramoto E, Fujiyama F, Nakamura KC, Tanaka Y, Hioki H, Kaneko T. Complementary distribution of glutamatergic cerebellar and GABAergic basal ganglia afferents to the rat motor thalamic nuclei. Eur J Neurosci 2011; 33: 95–109. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Kwan HC, Dostrovsky JO, Tasker RR, Murphy JT, Lenz YE. Single unit analysis of the human ventral thalamic nuclear group: activity correlated with movement. Brain 1990; 113: 1795–821. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Tasker RR, Kwan HC, Schnider S, Kwong R, Murayama Y, et al. Single unit analysis of the human ventral thalamic nuclear group: correlation of thalamic” tremor cells” with the 3–6 Hz component of parkinsonian tremor. J Neurosci 1988; 8: 754–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S, Brown P. What brain signals are suitable for feedback control of deep brain stimulation in Parkinson’s disease? Ann N Y Acad Sci 2012; 1265: 9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M, et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol 2013; 74: 449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LD, Prescott IA, Dostrovsky JO, Hodaie M, Lozano AM, Hutchison WD. Frequency-dependent effects of electrical stimulation in the globus pallidus of dystonia patients. J Neurophysiol 2012; 108: 5–17. [DOI] [PubMed] [Google Scholar]
- Llinás R, Paré D. Role of intrinsic neuronal oscillations and network ensembles in the genesis of normal and pathological tremors. In: Findley LJ, Koller WC, editors. Handbook of Tremor Disorders. New York: Marcel Dekker; 1995. p. 7–36. [Google Scholar]
- Llinás R, Urbano FJ, Leznik E, Ramírez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci 2005; 28: 325–33. [DOI] [PubMed] [Google Scholar]
- Llinás RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science 1988; 242: 1654–64. [DOI] [PubMed] [Google Scholar]
- Louis ED, Faust PL, Ma KJ, Yu M, Cortes E, Vonsattel JP. Torpedoes in the cerebellar vermis in essential tremor cases vs. controls. Cerebellum 2011; 10: 812–19. [DOI] [PubMed] [Google Scholar]
- Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 2007; 130: 3297–307. [DOI] [PubMed] [Google Scholar]
- Louis ED, Ottman R, Allen Hauser W. How common is the most common adult movement disorder? Estimates of the prevalence of essential tremor throughout the world. Mov Disord 1998; 13: 5–10. [DOI] [PubMed] [Google Scholar]
- Magnin M, Morel A, Jeanmonod D. Single-unit analysis of the pallidum, thalamus and subthalamic nucleus in parkinsonian patients. Neuroscience 2000; 96: 549–64. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Siegelbaum SA. Chapter 9: Synaptic plasticity: diverse targets and mechanisms for regulating synaptic efficacy. In: Cowan WM, Südhof TC, Stevens CF, editors. Synapses. Baltimore, MD: John Hopkins University Press; 2001. p. 393–413. [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Curr Biol 2001; 11: R986–96. [DOI] [PubMed] [Google Scholar]
- Mathai A, Smith Y. The corticostriatal and corticosubthalamic pathways: two entries, one target. So what? Front Syst Neurosci 2011; 5: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer HG, Krupa M, Cagnan H, Lourens MA, Heida T, Martens HC, et al. From Parkinsonian thalamic activity to restoring thalamic relay using deep brain stimulation: new insights from computational modeling. J Neural Eng 2011; 8: 066005. [DOI] [PubMed] [Google Scholar]
- Milosevic L, Kalia SK, Hodaie M, Lozano AM, Fasano A, Popovic MR, et al. Neuronal inhibition and synaptic plasticity of basal ganglia neurons in Parkinson’s disease. Brain 2017; 141: 177–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar GF, Pilliar A, Lozano AM, Dostrovsky JO. Differences in neuronal firing rates in pallidal and cerebellar receiving areas of thalamus in patients with Parkinson’s disease, essential tremor, and pain. J Neurophysiol 2005; 93: 3094–101. [DOI] [PubMed] [Google Scholar]
- Monakow KH, Akert K, Künzle H. Projections of the precentral motor cortex and other cortical areas of the frontal lobe to the subthalamic nucleus in the monkey. Exp Brain Res 1978; 33: 395–403. [DOI] [PubMed] [Google Scholar]
- Nambu A, Takada M, Inase M, Tokuno H. Dual somatotopical representations in the primate subthalamic nucleus: evidence for ordered but reversed body-map transformations from the primary motor cortex and the supplementary motor area. J Neurosci 1996; 16: 2671–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naros G, Grimm F, Weiss D, Gharabaghi A. Directional communication during movement execution interferes with tremor in Parkinson’s disease. Mov Disord 2018; 33: 251–61. [DOI] [PubMed] [Google Scholar]
- Nguyen JP, Degos JD. Thalamic stimulation and proximal tremor: a specific target in the nucleus ventrointermedius thalami. Arch Neurol 1993; 50: 498–500. [DOI] [PubMed] [Google Scholar]
- Ohye C, Narabayashi H. Physiological study of presumed ventralis intermedius neurons in the human thalamus. J Neurosurg 1979; 50: 290–7. [DOI] [PubMed] [Google Scholar]
- Ohye CH, Shibazaki TO, Hirai TA, Wada HI, Hirato M, Kawashima YA. Further physiological observations on the ventralis intermedius neurons in the human thalamus. J Neurophysiol 1989; 61: 488–500. [DOI] [PubMed] [Google Scholar]
- Ondo W, Jankovic J, Schwartz K, Almaguer M, Simpson RK. Unilateral thalamic deep brain stimulation for refractory essential tremor and Parkinson’s disease tremor. Neurology 1998; 51: 1063–9. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev 1995a; 20: 91–127. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidium in basal ganglia circuitry. Brain Res Rev 1995b; 20: 128–54. [DOI] [PubMed] [Google Scholar]
- Paris-Robidas S, Brochu E, Sintes M, Emond V, Bousquet M, Vandal M, et al. Defective dentate nucleus GABA receptors in essential tremor. Brain 2012; 135: 105–16. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Sensory elements in pattern-generating networks. In Making them move. Morgan Kaufmann Publishers Inc; 1991. p. 111–27. [Google Scholar]
- Pedrosa DJ, Auth M, Eggers C, Timmermann L. Effects of low-frequency thalamic deep brain stimulation in essential tremor patients. Exp Neurol 2013; 248: 205–12. [DOI] [PubMed] [Google Scholar]
- Pedrosa DJ, Reck C, Florin E, Pauls KA, Maarouf M, Wojtecki L, et al. Essential tremor and tremor in Parkinson’s disease are associated with distinct ‘tremor clusters’ in the ventral thalamus. Exp Neurol 2012; 237: 435–43. [DOI] [PubMed] [Google Scholar]
- Priori A, Foffani G, Rossi L, Marceglia S. Adaptive deep brain stimulation (aDBS) controlled by local field potential oscillations. Exp Neurol 2013; 245: 77–86. [DOI] [PubMed] [Google Scholar]
- Rajput AH, Rozdilsky B, Rajput A. Accuracy of clinical diagnosis in parkinsonism—a prospective study. Can J Neurol Sci 1991; 18: 275–8. [DOI] [PubMed] [Google Scholar]
- Ran I, Quastel DM, Mathers DA, Puil E. Fluctuation analysis of tetanic rundown (short-term depression) at a corticothalamic synapse. Biophys J 2009; 96: 2505–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehncrona S, Johnels B, Widner H, Törnqvist AL, Hariz M, Sydow O. Long‐term efficacy of thalamic deep brain stimulation for tremor: double‐blind assessments. Mov Disord 2003; 18: 163–70. [DOI] [PubMed] [Google Scholar]
- Rinvik E, Ottersen OP. Terminals of subthalamonigral fibres are enriched with glutamate-like immunoreactivity: an electron microscopic, immunogold analysis in the cat. J Chem Neuroanat 1993; 6: 19–30. [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci 2005; 6: 57–69. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron 1996; 16: 1197–207. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Liang F, Babalian A, Moret V, Wiesendanger M. Cerebellothalamocortical and pallidothalamocortical projections to the primary and supplementary motor cortical areas: a multiple tracing study in macaque monkeys. J Comp Neurol 1994; 345: 185–213. [DOI] [PubMed] [Google Scholar]
- Sandoe C, Krishna V, Basha D, Sammartino F, Tatsch J, Picillo M, et al. Predictors of deep brain stimulation outcome in tremor patients. Brain Stimul 2018; 11: 592–9. [DOI] [PubMed] [Google Scholar]
- Schaltenbrand G, Wahren W. Atlas for stereotaxy of the human brain. Stuttgart: Thieme-Verlag; 1977. [Google Scholar]
- Sherman SM, Guillery RW. Functional organization of thalamocortical relays. J Neurophysiol 1996; 76: 1367–95. [DOI] [PubMed] [Google Scholar]
- Shill HA, Adler CH, Sabbagh MN, Connor DJ, Caviness JN, Hentz JG, et al. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology 2008; 70(16 Part 2): 1452–5. [DOI] [PubMed] [Google Scholar]
- Strafella A, Ashby P, Munz M, Dostrovsky JO, Lozano AM, Lang AE. Inhibition of voluntary activity by thalamic stimulation in humans: relevance for the control of tremor. Mov Disord 1997; 12: 727–37. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Watanabe K, Satake K, Hirato M, Ohye C. Effect of electrical stimulation of the thalamic Vim nucleus on hand tremor during stereotactic thalamotomy. Electroencephalogr Clin Neurophysiol 1998; 109: 376–84. [DOI] [PubMed] [Google Scholar]
- Tasker RR, Lenz FA, Dostrovksy JO, Yamashiro K, Chodakiewitz J, Albe-Fessard DG. The physiological basis of Vim thalamotomy for involuntary movement disorders. In: Clinical aspects of sensory motor integration. Springer, Berlin; 1987. p. 265–76. [Google Scholar]
- Vitek JL, Ashe J, DeLong MR, Alexander GE. Physiologic properties and somatotopic organization of the primate motor thalamus. J Neurophysiol 1994; 71: 1498–513. [DOI] [PubMed] [Google Scholar]
- Volkmann J, Joliot M, Mogilner A, Ioannides AA, Lado F, Fazzini E, et al. Central motor loop oscillations in parkinsonian resting tremor revealed magnetoencephalography. Neurology 1996; 46: 1359. [DOI] [PubMed] [Google Scholar]
- Wu YR, Levy R, Ashby P, Tasker RR, Dostrovsky JO. Does stimulation of the GPi control dyskinesia by activating inhibitory axons? Mov Disord 2001; 16: 208–16. [DOI] [PubMed] [Google Scholar]
- Yu M, Ma K, Faust PL, Honig LS, Cortés E, Vonsattel JP, et al. Increased number of Purkinje cell dendritic swellings in essential tremor. Eur J Neurol 2012; 19: 625–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci 1989; 12: 13–31. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol 2002; 64: 355–405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.