We are conducting a nationwide multicenter Phase II/III trial in children and adolescents with newly diagnosed B-cell precursor acute lymphoblastic Leukemia in Japan.
Keywords: acute lymphoid leukemia, randomization, trial protocol
Abstract
B-cell precursor acute lymphoblastic leukemia is the most common pediatric malignancy, but its treatment needs to be modified to cause low acute toxicity and few late complications with a high cure rate. In this trial, we will stratify patients with B-cell precursor acute lymphoblastic leukemia into standard, intermediate and high risk groups according to prognostic factors. In addition, we will establish an evaluation system for minimal residual disease that will enable us to stratify patients based on minimal residual disease in subsequent clinical trials. We will clarify the impact of dexamethasone/vincristine pulse therapy during maintenance therapy in the standard risk group, and intensive l-asparaginase therapy in the intermediate risk group. In the high risk group, usefulness of vincristine intensification will be assessed. This trial has been registered in the UMIN Clinical Trials Registry as UMIN000009339 [http://www.umin.ac.jp/ctr/].
Introduction
Acute lymphoblastic leukemia (ALL) is the most common pediatric malignant neoplasm, and there are expected to be 450–500 patients per year in Japan with newly diagnosed ALL based on the total number registered in the Japan Children’s Cancer Group clinical trial. Among them, 85–90% will have B-cell precursor acute lymphoblastic leukemia (BCP-ALL). The outcome of Pediatric ALL has been dramatically improved by the progress of research over the past 50 years (1), with stratification of treatment based on risk of relapse and the biological features of leukemic cells, as well as better supportive care. However, the increase of long-term survivors has led to problems with late complications (2,3). Recently, risk classification has become more detailed, and the categories range from a group with the best prognosis and an expected to cure rate of 90% or more (4,5) to a group that is difficult to cure with chemotherapy (6,7). Accordingly, it is necessary to establish optimum treatments for each group with low acute/long-term toxicity, high cure rates and as few late complications as possible.
A study performed at St. Jude Children’s Research Hospital in the USA (8) first demonstrated the breakthrough outcome of long-term remission for pediatric ALL by combining prophylactic cranial irradiation with multi-agent combination chemotherapy. After that, the Berlin-Frankfurt-Munster (BFM) group established a ‘remission induction/early consolidation therapy (Protocol I)’ that achieved a 5-year event-free survival (EFS) rate of 55% ± 6% (9). Protocol I consists of ‘Protocol IA’, in which adds daunorubicin is added to vincristine (VCR), prednisolone and l-asparaginase (L-ASP), or ‘Protocol IB’, in which cyclophosphamide, 6-mercaptopurine and cytarabine are administered immediately after Protocol IA. By incorporating ‘re-induction therapy (Protocol II)’ in the BFM 76/79 study, they achieved a 5-year EFS rate of about 70% in patients with high risk (HR) ALL(high leukocyte count at diagnosis) (10). The children’s Cancer Group (CCG) subsequently studied the efficacy of ‘remission induction/early consolidation therapy’ and ‘re-induction therapy,’ and established these regimens as an essential part of chemotherapy for pediatric ALL (11). Today, this ‘BFM-backbone treatment’ is used worldwide, from advanced countries such as the United States (Children’s Oncology Group: COG), United Kingdom, France, the Netherlands and Scandinavia to semi-developed countries such as those in South America and eastern Europe. It is considered to be the ‘standard’ treatment for pediatric ALL from the viewpoint of versatility.
Many clinical trials performed in Japan differ greatly in detail but use BFM-backbone, including induction therapy, consolidation therapy with high-dose methotrexate, and maintenance therapy (12). Thus, we adopted the BFM backbone for the first nationwide clinical trial of pediatric ALL in Japan, aiming to confirm the feasibility of this therapy based on stratification by age, initial leukocyte count and early response to prednisolone. Furthermore, based on the results of the BFM95 clinical trial (13), we planned to perform a randomized clinical trial in each risk group to obtain clinical evidence for improving the outcome of pediatric ALL.
In the standard risk (SR) group, we planned a randomized trial to verify the effectiveness of VCR/dexamethasone (DEX) pulse therapy during maintenance therapy. VCR/steroid pulse therapy was found to improve treatment outcomes by a meta-analysis of studies conducted in the 1980s (14). However, its usefulness was regarded as inconsistent in the late 1990s. The international BFM group failed to detect any advantage of pulse therapy during maintenance for the intermediate risk group (15), while the European Organisation for Research and Treatment of Cancer (EORTC) 58591 study demonstrated better disease free survival with additional pulse therapy during maintenance (16,17). These results suggested the possibility that intensive maintenance therapy is only useful if treatment before maintenance therapy has been less intensive, while the advantage of intensified maintenance therapy is attenuated when consolidation therapy was intensive. Therefore, we hypothesized that pulse intensification of maintenance therapy might improve the outcome for the SR group who received less intensive induction/consolidation therapy.
In the IR group, we will perform a randomized clinical trial to verify the effectiveness of L-ASP intensification throughout consolidation therapy. In this study, intensification of L-ASP will be performed with IB, IIIB and Protocol M. The feasibility of intensification in each phase has been confirmed by randomized clinical trials, including the EORTC 58951 study (16) and the BFM 90 study (18). The total dose of L-ASP in the study group (intensive L-ASP group) will be 290 000 U/m2, which will be significantly higher than in the control arm (120 000 U/m2). However, there is no marked increase in the study dose compared to 36 600 U/m2 in the augmented BFM study (19).
The BFM-2000 HR regimen was reported to improve 5-year EFS for prednisolone poor responders (PPR) with no other HR factors from 65% to 73% (20), by restoring IB and intensifying L-ASP in Block treatment compared with the regimen used in the 95HR study. This also exceeded the results obtained in the Associazione Italiana di Ematologia Oncologia Pediatrica (AIEOP)-95 HR study (8) by performing Protocol II twice, which is currently the best treatment for PPR among the BFM-backbone regimens. However, the usefulness of Block treatment, which has been adopted for patients HR since the BFM-90 study, has not been verified. Meanwhile, intensification of VCR/L-ASP that causes comparatively mild myelosuppression is one of the important components of augmented BFM therapy, with which the CCG obtained excellent results in patients showing a poor response to initial treatment. BFM backbone therapy incorporates a relatively low dose of VCR, so there is a possibility of improving treatment outcomes. Since standard therapy has not been established for HR patients, we will perform a comparison between BFM-HR Block treatment and standard BFM therapy with intensified VCR/L-ASP.
Allogeneic stem cell transplantation is indicated for patients who have high minimal residual disease (MRD) at the end of consolidation therapy, as well as for those with failure of induction therapy or poor cytogenetic features such as hypodiploidy and TCF3-HLF.
Treatment outcomes will not only be assessed by EFS/overall survival (OS), but also on the basis of quality of life.
Eligibility criteria
Inclusion criteria
Diagnosis of BCP-ALL.
Aged 1–19 years at the time of diagnosis.
Eastern Cooperative Oncology Group performance status (PS) score of 0–2. However, a PS of up to 3 is allowed when deterioration of PS is caused by leukemia.
No history of receiving anticancer drugs or radiotherapy.
- Fully maintained organ functions that simultaneously satisfy the following two conditions. (Laboratory data shall be obtained within 7 days of the registration date.)
- Total bilirubin: less than 3 times the upper limit of the reference value adjusted for age.
- Creatinine: less than 3 times the upper limit of the reference value adjusted for age.
Written consent to participation in this study provided by the patient/legal representatives.
Exclusion criteria
Diagnosis of mature B cell ALL.
Diagnosis of Ph+ ALL.
Diagnosis of true mixed-lineage leukemia.
Symptoms of central nervous system bleeding of Grade 3 or more according to the Common Terminology Criteria for Adverse Events v4.0.
Infection that is difficult to control, including patients with active tuberculosis and those positive for human immunodeficiency virus antibody.
Females who are pregnant, breastfeeding or possibly pregnant.
A history of congenital or acquired immunodeficiency syndrome.
QTfc (with Fridericia’s correction) of 0.45 s or more (QTfc = QT/RR*1/3)
Patients otherwise deemed unsuitable for this study by the investigator.
Methods
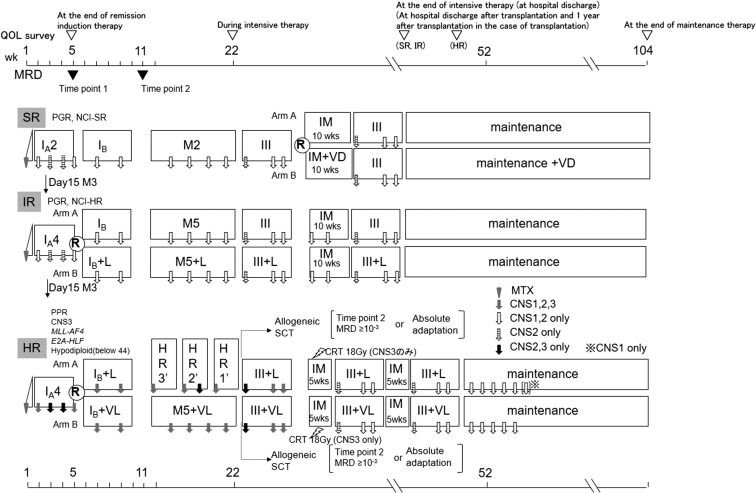
Randomization will be performed in all risk groups. The design of this trial is shown in Fig. 1 (details in Table 1).
Figure 1.
Treatment outline ALL-B12.
Table 1.
Treatment protocol ALL-B12
| Treatment element/drug | Single or daily dose | Days of application per element |
|---|---|---|
| Remission–induction therapy | ||
| IP | ||
| Prednisolone (PO or IV) | 15/30/45/60 mg/m2 | 1–7 |
| Methotrexate (IT) | 1 | |
| IA2(SR) | ||
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 8,15,22,29 |
| Prednisolone (PO or IV) | 60 mg/m2 | 8–28 |
| Prednisolone (PO or IV) | 30/15/7.5 mg/m2 | 29–37 |
| Daunorubicin (IV 1 h) | 30 mg/m2 | 8,15 |
| l-asparaginase (IM or IV 1 h) | 5000 U/m2 | 12,15,18,21,24,27,30,33 |
| Methotrexate/cytarabine/prednisolone (IT) | 12,33b | |
| IA4(IR, HR) | ||
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 8,15,22,29 |
| Prednisolone (PO or IV) | 60 mg/m2 | 8–28 |
| Prednisolone (PO or IV) | 30/15/7.5 mg/m2 | 29–37 |
| Daunorubicin (IV 1 h) | 30 mg/m2 | 8,15,22,29 |
| l-asparaginase (IM or IV 1 h) | 5000 U/m2 | 12,15,18,21,24,27,30,33 |
| Methotrexate/cytarabine/prednisolone (IT) | 12,33c | |
| Early consolidation therapy | ||
| IB(SR, HR Arm A) | ||
| Cyclophosphamide (IV 1 h) | 1000 mg/m2 | 36,64 |
| Cytarabine (IV push or ≤15 min) | 75 mg/m2 | 38–41,45–48,52–55,59–62 |
| 6-mercaptopurine (PO) | 60 mg/m2 | 36–63 |
| Methotrexate/cytarabine/prednisolone (IT) | 45,59 | |
| IB + L(IR ArmB, HR Arm A) | ||
| Cyclophosphamide (IV 1 h) | 1000 mg/m2 | 36,64 |
| l-asparaginase (IM) | 5000 U/m2 | 38,41,45,48,52,55,59,62 |
| Cytarabine (IV push or ≤15 min) | 75 mg/m2 | 38–41,45–48,52–55,59–62 |
| 6-mercaptopurine (PO) | 60 mg/m2 | 36–63 |
| Methotrexate/cytarabine/prednisolone (IT) | 45,59 | |
| IB + VL(HR-VCR Arm B) | ||
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 50,57 |
| Cyclophosphamide (IV 1 h) | 1000 mg/m2 | 36,64 |
| l-asparaginase (IM) | 5000 U/m2 | 38,41,45,48,52,55,59,62 |
| Cytarabine (IV push or ≤15 min) | 75 mg/m2 | 38–41,45–48,52–55,59–62 |
| 6-mercaptopurine (PO) | 60 mg/m2 | 36–63 |
| Methotrexate/cytarabine/prednisolone (IT) | 45,59 | |
| Consolidation therapy | ||
| M2(SR) | ||
| HD-Methotrexate (IV 24 h)a | 2 g/m2 | 8,22,36,50 |
| Leucovorin rescue (IV) | 15 mg/m2 | at 42,48 and 54 h |
| 6-mercaptopurine (PO) | 25 mg/m2 | 1–56 |
| Methotrexate/cytarabine/prednisolone (IT) | 8,22,36,50 | |
| M5(IR Arm A) | ||
| HD-Methotrexate (IV 24 h) | 5 g/m2 | 8,22,36,50 |
| Leucovorin rescue (IV) | 15 mg/m2 | at 42,48 and 54 h |
| 6-mercaptopurine (PO) | 25 mg/m2 | 1–56 |
| Methotrexate/cytarabine/prednisolone (IT) | 8,22,36,50 | |
| M5+L(IR Arm B) | ||
| HD-Methotrexate (IV 24 h)c | 5 g/m2 | 8,22,36,50 |
| Leucovorin rescue (IV) | 15 mg/m2 | at 42,48 and 54 h |
| 6-mercaptopurine (PO) | 25 mg/m2 | 1–56 |
| l-asparaginase (IM) | 12 500 U/m2 | 10,24,38,52 |
| Methotrexate/cytarabine/prednisolone (IT) | 8,22,36,50 | |
| M5+VL(HR-VCL Arm B) | ||
| HD-Methotrexate (IV 24 h)a | 5 g/m2 | 8,22,36,50 |
| Leucovorin rescue (IV) | 15 mg/m2 | at 42,48 and 54 h |
| 6-mercaptopurine (PO) | 25 mg/m2 | 1–56 |
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 8,22,36,50 |
| l-asparaginase (IM) | 12 500 U/m2 | 10,24,38,52 |
| Methotrexate/cytarabine/prednisolone (IT) | 8,22,36,50 | |
| Consolidation therapy(HR3 -> HR2 -> HR1) | ||
| HR3 | ||
| Dexamethasone (PO or IV) | 20 mg/m2 | 1–5 |
| HD-Cytarabine (IV 3 h) | 2000 mg/m2 | 1–2 (4 doses, 12 h intervals) |
| Etoposide (IV 1 h) | 100 mg/m2 | 3–5 (5 doses, 12 h intervals) |
| l-asparaginase (IM) | 25 000 U/m2 | 6,11 |
| Methotrexate/cytarabine/prednisolone (IT) | 5 | |
| HR2 | ||
| Dexamethasone (PO or IV) | 20 mg/m2 | 1–5 |
| Vindesine (IV) | 3 mg/m2 (max 5 mg) | 1, 6 |
| HD-Methotrexate (IV 24 h)a | 5 g/m2 | 1 |
| Leucovorin rescue (IV) | 15 mg/m2 | at 42, 48 and 54 h |
| Ifosfamide (IV 1 h) | 800 mg/m2 | 2–4 (5 doses, 12 h intervals) |
| Daunorubicin (IV 24 h) | 30 mg/m2 | 5 |
| l-asparaginase (IM) | 25 000 U/m2 | 6,11 |
| Methotrexate/cytarabine/prednisolone (IT) | 1d | |
| HR1 | ||
| Dexamethasone (PO or IV) | 20 mg/m2 | 1–5 |
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 1,6 |
| HD-Methotrexate (PI 24 h)a | 5 g/m2 | 1 |
| Leucovorin rescue(IV) | 15 mg/m2 | at 42,48 and 54 h |
| HD-Cytarabine (IV 3 h) | 2 000 mg/m2 | 5 (2 doses, 12 h interval) |
| Cyclophosphamide (IV 1 h) | 200 mg/m2 | 2–4 |
| l-asparaginase (IM) | 25 000 U/m2 | 6,11 |
| Methotrexate/cytarabine/prednisolone (IT) | 1 | |
| Reinduction therapy | ||
| III(SR,SR Arm A,SR Arm B,SR Arm B, IR Arm A) | ||
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 1,8 |
| Dexamethasone (PO or IV) | 10 mg/m2 | 1–14 |
| Dexamethasone (PO or IV) | 5 –>2.5 –>1.25 mg/m2 | 15–23 |
| Pirarubicin hydrochloride (IV 1 h) | 25 mg/m2 | 1,8 |
| l-asparaginase (IM) | 10 000 U/m2 | 1,4,8,11 |
| Cyclophosphamide (IV 1 h) | 500 mg/m2 | 15 |
| Cytarabine (IV push or ≤15 min) | 75 mg/m2 | 17–20,24–27 |
| 6-mercaptopurine (PO) | 60 mg/m2 | 15–28 |
| Methotrexate/cytarabine/prednisolone (IT) | 17,24e | |
| III + L(IR Arm B, HR Arm A) | ||
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 1,8 |
| Dexamethasone (PO or IV) | 10 mg/m2 | 1–14 |
| Dexamethasone (PO or IV) | 5 –> 2.5 –> 1.25 mg/m2 | 15–23 |
| Dexamethasone (PO or IV) | 10 mg/m2 | 1–7,15–21 |
| Pirarubicin hydrochloride (IV 1 h) | 25 mg/m2 | 1,8 |
| l-asparaginase (IM) | 10 000 U/m2 | 1,4,8,11,15,18,22,25 |
| Cyclophosphamide (IV 1 h) | 500 mg/m2 | 15 |
| Cytarabine (IV push or ≤15 min) | 75 mg/m2 | 17–20,24–27 |
| 6-mercaptopurine (PO) | 60 mg/m2 | 15–28 |
| Methotrexate/cytarabine/prednisolone (IT) | 17,24f,g,h | |
| III + VL(HR-VCR Arm B) | ||
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 1,8,15,22 |
| Dexamethasone (PO or IV) | 10 mg/m2 | 1–14 |
| Dexamethasone (PO or IV) | 5 –> 2.5 –> 1.25 mg/m2 | 15–23 |
| Dexamethasone (PO or IV) | 10 mg/m2 | 1–7,15–21 |
| Pirarubicin hydrochloride (IV 1 h) | 25 mg/m2 | 1,8 |
| l-asparaginase (IM) | 10 000 U/m2 | 1,4,8,11,15,18,22,25 |
| Cyclophosphamide (IV 1 h) | 500 mg/m2 | 15 |
| Cytarabine (IV push or ≤15 min) | 75 mg/m2 | 17–20,24–27 |
| 6-mercaptopurine (PO) | 60 mg/m2 | 15–28 |
| Methotrexate/cytarabine/prednisolone (IT) | 17,24i,j | |
| Interim maintenance therapy | ||
| IM(SR Arm A) | ||
| Methotrexate (PO) | 20 mg/m2/week | 1,8,15,22,29,36,43,50 |
| 6-mercaptopurine (PO) | 50 mg/m2 | 1–56 |
| IM(IR Arm A, IR Arm B) | ||
| Methotrexate (PO) | 20 mg/m2/week | 1,8,15,22,29,36,43,50 |
| 6-mercaptopurine (PO) | 50 mg/m2 | 1–56 |
| Methotrexate/cytarabine/prednisolone (IT) | 1,29 | |
| IM(HR Arm A-1, HR-VCR Arm B-1) | ||
| Methotrexate (PO) | 20 mg/m2/week | 1,8,15,22 |
| 6-mercaptopurine (PO) | 50 mg/m2 | 1–28 |
| CRT (only CNS3) | 18 Gy/12fr | |
| IM(HR Arm A-2, HR-VCR Arm B-2) | ||
| Methotrexate (PO) | 20 mg/m2/week | 1,8,15,22 |
| 6-mercaptopurine (PO) | 50 mg/m2 | 1–28 |
| IM + VD(SR Arm B) | ||
| Methotrexate (PO) | 20 mg/m2/week | 1,8,15,22,29,36,43,50 |
| 6-mercaptopurine (PO) | 50 mg/m2 | 1–56 |
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 1,29 |
| Dexamethasone (PO or IV) | 6 mg/m2 | 1–55,29–33 |
| Maintenance therapy | ||
| Maintenance (SR Arm A, IR Arm A, IR Arm B) | ||
| Methotrexate (PO) | 20 mg/m2/week | 1,8,15,22,29,36,43,50 |
| 6-mercaptopurine (PO) | 50 mg/m2 | 1–56 |
| Maintenance (HR Arm A) | ||
| Methotrexate (PO) | 20 mg/m2/week | 1,8,15,22,29,36,43,50 |
| 6-mercaptopurine (PO) | 50 mg/m2 | 1–56 |
| Methotrexate/cytarabine/prednisolone (IT) | 1(CNS1:cycle #1–6,CNS2: cycle #1–5) | |
| Maintenance (HR Arm B) | ||
| Methotrexate (PO) | 20 mg/m2/week | 1,8,15,22,29,36,43,50 |
| 6-mercaptopurine (PO) | 50 mg/m2 | 1–56 |
| Methotrexate/cytarabine/prednisolone (IT) | 1(CNS1,2: cycle #1–5) | |
| Maintenance + VD(SR Arm B) | ||
| Methotrexate (PO) | 20 mg/m2/week | 1,8,15,22,29,36,43,50 |
| 6-mercaptopurine (PO) | 50 mg/m2 | 1–56 |
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 1,29 |
| Dexamethasone (PO) | 6 mg/m2 | 1–55,29–33 |
PO indicates orally; IV, intravenous push; PI, intravenous infusion; IT, intrathecally; CRT, cranial radiotherapy
aA loading dose of 10% is infused over 30 min, the remaining 90% over 23.5 h.
bPatients with CNS status CNS 2 receive additional IT on Days 18 and 27.
cPatients with CNS status CNS 2 or 3 receive additional IT on Days 18 and 27.
dPatients with CNS status CNS 2 or 3 receive additional IT on Day 5.
ePatients with CNS status CNS 2 receive additional IT on Day 1.
fIn IR, patients with CNS status CNS 2 receive additional IT on Day 1.
gIn HR, patients with CNS status CNS 2 or 3 receive additional IT on Day 1 in the first course.
hIn HR, patients with CNS status CNS 2 or 3 receive additional IT on Day 1 in the second and third course.
iPatients with CNS status CNS 2 or 3 receive additional IT on Day 1 in the first course.
jPatients with CNS status CNS 2 receive additional IT on Day 1 in the second and third course.
SR:
Criteria: National Cancer Institute (NCI)-SR (age <10 years and white blood cell [WBC] <50 000/mm3) and prednisolone good responder (PGR; peripheral blood blast count of less than 1 000/μl on Induction Day 8) and bone marrow (BM) on Induction Day 15 = M1 (leukemic blasts <5%)/2 (leukemic blasts >5%, <25%) and time point (TP) 1 (TP 1; after Induction Ia) BM = M1 without HR features.
Treatment (experimental arm): Reduced intensity BFM-backbone treatment with VCR/DEX pulse therapy during the maintenance phase.
Treatment (standard arm): Reduced intensity BFM-backbone treatment without VCR/DEX pulse therapy during the maintenance phase.
IR:
Criteria:
• NCI-SR and PGR and BM on Induction Day 15 = M3 and TP1 BM = M1 without HR features.
• NCI-HR (age >10 years or WBC >50 000/mm3) and PGR and BM on Day 15 = M1/2 and TP1 BM = M1 without HR features.
Treatment (experimental arm): Standard BFM-backbone treatment with.
Treatment (standard arm): Standard BFM-backbone treatment without intensive L-ASP during the consolidation phase.
HR:
Criteria: at least one of the following features:
- NCI-HR and PGR and BM on Induction Day 15 = M3
- Central nervous system (CNS)-3
- PPR; peripheral blood blast count of at least 1000/μl on Induction Day 8
- MLL-AF4
- E2A-HLF
- Hypodiploid (<44)
Treatment (experimental arm A: HR-VCR): BFM-backbone treatment with L-ASP and intensive intrathecal therapy combined with intensive VCR.
Treatment (experimental arm B: HR-Block): BFM-backbone treatment with L-ASP and intensive intrathecal therapy combined with BFM-backbone Block treatment.
Outcomes
Primary endpoint
Five-year EFS in the SR group, IR group and HR group.
The events included in EFS are induction failure, relapse, secondary-cancer (including myelodysplastic syndromes), and all cause of death.
Secondary endpoints
- Five-year EFS, 5-year OS, and 5-year CNS recurrence for pooled risk groups.
- Five-year OS and 5-year CNS recurrence in each risk group.
- Remission induction rate after remission induction therapy (IA) and early consolidation therapy (IB) in each risk group and pooled risk groups.
- Incidence of adverse events.
- The percentage of patients in which MRD can be evaluated at TP1 and TP2 (after consolidation IB), and the correlation between MRD at these times and 5-year EFS/5-year OS.
- Evaluation of quality of life by the patients and their families (evaluation by representatives).
- Exploratory evaluation of the relationship between molecular genetic abnormality and the prognosis
Sample size
The planned number of patients for registration is 1560 patients (SR group: 800, IR group: 490, and HR group: 270).
-
- SR group
The expected treatment outcome in the SR standard treatment group is assumed to be about 85%, based on the following: the 6-year EFS rate of the NCI-SR group with BCP-ALL was 86.5% in the BFM 95 study (13); this study will be performed based on the BFM 95 study (excluding patients with PPR, 25% or more myeloblasts (M3) on Day 15 of remission induction therapy, CNS infiltration, and poor prognostic chromosomal abnormalities); and there is a possibility that therapeutic results will decline somewhat by treatment alleviation. VCR/DEX pulse therapy can be judged to be useful in the SR group if the 5-year EFS rate of patients receiving VCR/DEX pulse therapy is at least 6% higher than that of those without VCR/DEX pulse therapy (assumed to be 85%). Accordingly, the required number of patients per group (both groups) is calculated as 325 (650) for a detection power of 80%, 372 (744) for 85%, and 435 (870) for 90%, with a registration period of 5 years, follow-up period of 5 years, and two-tailed significance level of 5% by the log rank test. The registration rate of Down syndrome patients without randomization is estimated to be about 1%. From these considerations, and allowing for a few ineligible cases, we set the required number of patients (including Down syndrome patients without randomization) as 800 in both groups.
-
- IR group
Intensive L-ASP therapy can be judged to be useful in the SR group if the 5-year EFS rate of the patients receiving intensive L-ASP therapy is at least 10% higher than that of those without intensive L-ASP therapy (assumed to be 70%). Accordingly, the required number of patients per group (both groups) for each are calculated as 213 (426) for a detection power of 80%, 244 (488) for 85%, and 285 (570) for 90%, with a registration period of 5 years, follow-up period of 5 years, and two-tailed significance level of 5% by the log rank test. The registration rate of Down syndrome patients without randomization is estimated to be about 1%. From these considerations, and allowing for a few ineligible cases, we set the required number of patients (including Down syndrome patients without randomization) as 490 in both groups.
-
- HR group
There are currently few data on the outcome of HR, as classified in this study, in Japan or other countries. We predicted the survival rate of each treatment arm based on the following data: the 6-year EFS rate was 58.8% for HR patients in the AIEOP-BFM 2000 study (20) and the 5-year EFS rate was about 70% for patients with 25% or more myeloblasts (M3) on Day 7 of CCG/COG induction therapy (21). Taking into consideration that the HR group in this study will mainly contain patients with a poor initial response and some patients with poor prognostic chromosomes/genes, the outcome is estimated to be similar to those of the AIEOP-BFM and CCG/COG studies. Thus, the 5-year EFS rate is assumed to be 60% in both groups. We plan to identify promising treatments from the results of this study by setting the detection power within a certain range for the situation in which the hazard ratio for one group is 1.5–1.7 worse than that for the other group. Under this condition, we calculated the required number of patients with a registration period of 5 years, follow-up period of 5 years, two-tailed significance level of 10%, and detection power of 70–90%.
We found that the detection power is 80% with a hazard ratio of 1.6 when the number of patients is 254. From these considerations, and allowing for a few ineligible cases, we set the required number of HR patients as 270 in both groups.
Statistical analysis
Analysis sets
A total of three analysis sets are defined for this study, including two sets for efficacy evaluation [‘full analysis set (FAS)’ and ‘per protocol set (PPS)’] and one set for safety evaluation [‘safety analysis set (SAF)’].
The FAS is defined as the patients eligible for registration in this study, excluding those found to be ineligible after registration, duplicate registrations and misregistrations. The PPS is defined as the patients among the FAS who are judged to be valid for evaluation, excluding patients in whom protocol treatment was not initiated and patients excluded from analysis by the judgment of the ALL Committee due to severe protocol violations that could have a significant effect on the primary endpoints. In view of the characteristics of this study, the FAS is the main analysis set for efficacy evaluation. Results from analysis PPS will also be calculated as reference values for the main evaluation items. Moreover, a breakdown of all registered examples will also be shown and taken into account in interpretation of the results of analysis.
The SAF is defined as all eligible patients, except for those in whom protocol treatment is not initiated.
Parallel-group comparison will be performed, limited to randomized patients.
Main analysis for each group
Kaplan–Meier method is used for estimation of survival curve. The log rank test is used for comparison of survival curve. Hazard ratio is estimated by Cox proportional hazard model.
Interim analysis
For each risk group, interim analysis is planned when around two-thirds of the patients have been registered. The primary endpoint and adverse events will be evaluated. The primary endpoint is analyzed by the log rank test using an O’Brien-Fleming type alpha spending function method based on the information time of events.
Treatment will be discontinued in the following cases: (1) non-remission at the time of BMA 4, (2) recurrence of the primary disease, (3) if treatment cannot be completed/resumed within the prescribed period, (4) if protocol treatment is discontinued or substituted by the patient, (5) when the attending physician judges that it is necessary to withdraw treatment, (6) if ineligibility is found after registration, (7) if death occurs during protocol treatment, or (8) if discontinuation is ordered by the operations committee due to marked deviation from the protocol.
Discussion
Establishment of a standard treatment framework for BCP-ALL will make it easier to evaluate new therapeutic options, including new drugs, in the future. In addition, establishing an evaluation system for MRD will allow us to stratify patients based on MRD in subsequent clinical trials. The significance of performing DEX/VCR pulse therapy during maintenance therapy for SR patients, and that of intensive L-ASP therapy for IR patients will be clarified. In the HR group, comparison between BFM-HR-type Block treatment and standard BFM therapy with intensified VCR/L-ASP will be performed.
Abbreviations
AIEOP, Associazione Italiana di Ematologia Oncologia Pediatrica; ALL, acute lymphoblastic leukemia; BCP-ALL, B cell precursor acute lymphoblastic leukemia; BFM, Berlin-Frankfurt-Munster; BM, bone marrow; CCG, Children’s Cancer Group; CNS, central nervous system; COG, Children’s Oncology Group; DEX, dexamethasone; EORTC, European Organisation for Research and Treatment of Cancer; EFS, event-free survival; FAS, full analysis set; HR, high risk; IR, intermediate risk; L-ASP, l-asparaginase; MRD, minimal residual disease; NCI, National Cancer Institute; OS, overall survival; PGR, prednisolone good responder; PPR, prednisolone poor responder; PPS, Per Protocol Set; PS, performance status; SAF, Safety Analysis Set; SR, standard risk; TP, time point; VCR, vincristine; WBC, white blood cell.
Funding
This study is supported by a Grant for Establishment of Standard Treatment for Acute Lymphoblastic Leukemia in Children from the Japan Agency for Medical Research and Development (AMED) (17ck0106334h0001).
Conflict of interest statement
The authors declare that they have no competing interests.
Authors’ contributions
K.K., M.K., H.K., Y.O., T.I. and A.M.S. developed the clinical protocol. K.K. approved the final protocol and is managing the entire study. A.K. is performing the statistical analysis. K.H. and A.M. contributed to conception of the study.
References
- 1. Pui CH, Yang JJ, Hunger SP, et al. . Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol 2015;33:2938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Essig S, Li Q, Chen Y, et al. . Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol 2014;15:841–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vora A, Andreano A, Pui CH, et al. . Influence of cranial radiotherapy on outcome in children with acute lymphoblastic leukemia treated with contemporary therapy. J Clin Oncol 2016;34:919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhojwani D, Pei D, Sandlund JT, et al. . ETV6-RUNX1-positive childhood acute lymphoblastic leukemia: improved outcome with contemporary therapy. Leukemia 2012;26:265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kato M, Imamura T, Manabe A, et al. . Prognostic impact of gained chromosomes in high-hyperdiploid childhood acute lymphoblastic leukaemia: a collaborative retrospective study of the Tokyo Children’s Cancer Study Group and Japan Association of Childhood Leukaemia Study. Br J Haematol 2014;166:295–8. [DOI] [PubMed] [Google Scholar]
- 6. Nachman JB, Heerema NA, Sather H, et al. . Outcome of treatment in children with hypodiploid acute lymphoblastic leukemia. Blood 2007;110:1112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schrappe M, Hunger SP, Pui CH, et al. . Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med 2012;366:1371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aur RJ, Simone J, Hustu HO, et al. . Central nervous system therapy and combination chemotherapy of childhood lymphocytic leukemia. Blood 1971;37:272–81. [PubMed] [Google Scholar]
- 9. Schrappe M, Reiter A, Zimmermann M, et al. . Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995. Berlin-Frankfurt-Munster. Leukemia 2000;14:2205–22. [DOI] [PubMed] [Google Scholar]
- 10. Moricke A, Zimmermann M, Reiter A, et al. . Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia 2010;24:265–84. [DOI] [PubMed] [Google Scholar]
- 11. Tubergen DG, Gilchrist GS, O’Brien RT, et al. . Improved outcome with delayed intensification for children with acute lymphoblastic leukemia and intermediate presenting features: a Children’s Cancer Group phase III trial. J Clin Oncol 1993;11:527–37. [DOI] [PubMed] [Google Scholar]
- 12. Manabe A, Ohara A, Hasegawa D, et al. . Significance of the complete clearance of peripheral blasts after 7 days of prednisolone treatment in children with acute lymphoblastic leukemia: the Tokyo Children’s Cancer Study Group Study L99-15. Haematologica 2008;93:1155–60. [DOI] [PubMed] [Google Scholar]
- 13. Möricke A, Reiter A, Zimmermann M, et al. . Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood 2008;111:4477–89. [DOI] [PubMed] [Google Scholar]
- 14. Group CAC. Duration and intensity of maintenance chemotherapy in acute lymphoblastic leukaemia: overview of 42 trials involving 12 000 randomised children. Childhood ALL Collaborative Group. Lancet 1996;347:1783–8. [DOI] [PubMed] [Google Scholar]
- 15. Conter V, Valsecchi MG, Silvestri D, et al. . Pulses of vincristine and dexamethasone in addition to intensive chemotherapy for children with intermediate-risk acute lymphoblastic leukaemia: a multicentre randomised trial. Lancet 2007;369:123–31. [DOI] [PubMed] [Google Scholar]
- 16. Moerloose B, Suciu S, Bertrand Y, et al. . Improved outcome with pulses of vincristine and corticosteroids in continuation therapy of children with average risk acute lymphoblastic leukemia (ALL) and lymphoblastic non-Hodgkin lymphoma (NHL): report of the EORTC randomized phase 3 trial 58951. Blood 2010;116:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sirvent N, Suciu S, Benoit Y, et al. . Prognostic Significance of Central Nervous System (CNS) status of children with Acute Lymphoblastic Leukemia (ALL) treated without cranial irradiation: results of European Organization for Research and Treatment of Cancer (EORTC) Children Leukemia Group Study 58951. Blood 2008;112:303. [Google Scholar]
- 18. Schrappe M, Reiter A, Ludwig WD, et al. . Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: results of trial ALL-BFM 90. German-Austrian-Swiss ALL-BFM Study Group. Blood 2000;95:3310–22. [PubMed] [Google Scholar]
- 19. Nachman JB, Sather HN, Sensel MG, et al. . Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med 1998;338:1663–71. [DOI] [PubMed] [Google Scholar]
- 20. Conter V, Bartram CR, Valsecchi MG, et al. . Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood 2010;115:3206–14. [DOI] [PubMed] [Google Scholar]
- 21. Gaynon PS, Angiolillo AL, Carroll WL, et al. . Long-term results of the children’s cancer group studies for childhood acute lymphoblastic leukemia 1983–2002: a Children’s Oncology Group Report. Leukemia 2010;24:285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]