Photosynthetic rates in the cyanobacterium Trichodesmium erythraeum appear to be a function of CO2, although numerical simulations of the carbon-concentrating mechanism show carboxylation to be mediated as a function of pH and HCO3–.
Keywords: Carbon acquisition, carbon concentrating mechanism (CCM), CO2, Cyanobacteria, gross photosynthesis, net photosynthesis, ocean acidification, Trichodesmium
Abstract
Increasing atmospheric CO2 concentrations are leading to increases in dissolved CO2 and HCO3– concentrations and decreases in pH and CO32– in the world’s oceans. There remain many uncertainties as to the magnitude of biological responses of key organisms to these chemical changes. In this study, we established the relationship between photosynthetic carbon fixation rates and pH, CO2, and HCO3– concentrations in the diazotroph, Trichodesmium erythraeum IMS101. Inorganic 14C-assimilation was measured in TRIS-buffered artificial seawater medium where the absolute and relative concentrations of CO2, pH, and HCO3– were manipulated. First, we varied the total dissolved inorganic carbon concentration (TIC) (<0 to ~5 mM) at constant pH, so that ratios of CO2 and HCO3– remained relatively constant. Second, we varied pH (~8.54 to 7.52) at constant TIC, so that CO2 increased whilst HCO3– declined. We found that 14C-assimilation could be described by the same function of CO2 for both approaches, but it showed different dependencies on HCO3– when pH was varied at constant TIC than when TIC was varied at constant pH. A numerical model of the carbon-concentrating mechanism (CCM) of Trichodesmium showed that carboxylation rates are modulated by HCO3– and pH. The decrease in assimilation of inorganic carbon (Ci) at low CO2, when TIC was varied, was due to HCO3– uptake limitation of the carboxylation rate. Conversely, when pH was varied, Ci assimilation declined due to a high-pH mediated increase in HCO3– and CO2 leakage rates, potentially coupled to other processes (uncharacterised within the CCM model) that restrict Ci assimilation rates under high-pH conditions.
Introduction
Over the past 150 years, atmospheric CO2 concentrations have increased from pre-industrial levels (i.e. 280 µmol mol–1) to a current value of about 400 µmol mol–1, and are predicted to increase further to 650 µmol mol–1 by mid-century, and to 750–1000 µmol mol–1 by the end of this century (Raven et al., 2005). Equilibration of CO2 between the atmosphere and the oceans is leading to increases in dissolved CO2 and HCO3– and to decreases in pH and CO32–. This process of ocean acidification is predicted to reduce the pH from average pre-industrial levels of 8.2 to about 7.9 by the end of the century (Zeebe et al., 1999; Zeebe and Wolf-Gladrow, 2001). To date, there are still many uncertainties as to the magnitude of biological responses of key organisms to these chemical changes.
One group of organisms of particular importance are the diazotrophic cyanobacteria (photosynthetic dinitrogen-fixers), notably because of their significant contribution to marine primary productivity by converting N2 into NH4+, thus providing ‘new’ nitrogen to the oceans. The filamentous cyanobacterium Trichodesmium is a colony-forming species that fixes nitrogen in an area corresponding to half the Earth’s surface (Davis and McGillicuddy, 2006), and is estimated to account for more than half of the new (combined) nitrogen production in many parts of the oligotrophic tropical and sub-tropical oceans (Capone et al., 2005).
Cyanobacteria, Trichodesmium species included, achieve high photosynthetic rates despite (i) the slow diffusion of CO2 in water (104 times slower than in air), (ii) a slow chemical equilibrium between HCO3– and CO2 within the 7–8.5 pH range, and (iii) a low affinity of Rubisco for CO2 relative to ambient CO2 concentrations. Cyanobacteria employ an intracellular carbon-concentrating mechanism (CCM) (Badger and Price, 2003; Badger et al., 2006; Kranz et al., 2010), where enhanced primary productivity significantly outweighs the metabolic costs of CCM activity (Price et al., 2008). The CCM benefits cyanobacteria by reducing photorespiration (Schwarz et al., 1995; Kaplan and Reinhold, 1999), aiding in the dissipation of excess light energy, and by maintaining an optimal intracellular pH (Badger et al., 1994; Kaplan and Reinhold, 1999). The general consensus is that up-regulation of CCM activity in response to a low-CO2 environment involves two components. Firstly, an increase in the transport of inorganic carbon (Ci) from the environment into the cell via a suite of Ci transporters, which could involve using ATP (BCT1 HCO3– transporter), NADPH, or reduced ferredoxin (CO2 conversion from passive diffusion) or coupling to an electrochemical Na+ gradient (SbtA or BicA HCO3– transport) to provide the energy for Ci uptake (Badger et al., 2002; Badger and Price, 2003). Secondly, an increased ability to reduce CO2 leakage from around the site of carboxylation, achieved via arrangement of the molecular components of the carboxysome structure and a CO2-uptake system located on the thylakoid layer, preventing the efflux of leaked CO2 to the outer cytosolic layer (Price et al., 2008).
Both 14C isotope disequilibrium experiments and simultaneous measurements of CO2 and O2 exchanges during sequential light–dark transitions indicate that HCO3– contributes >90% of the Ci assimilation by T. erythraeum IMS101 (Kranz et al., 2009; Eichner et al., 2015). This preference for HCO3– is consistent with the evidence that Trichodesmium lacks a plasma membrane-bound extracellular carbonic anhydrase (eCA) (Badger et al., 2006; Price et al., 2008). Furthermore, the T. erythraeum genome indicates the presence of both a plasma membrane HCO3– transporter (BicA) and an intracellular system for conversion of CO2 to HCO3– (NDH-I4) (Price et al., 2008). These two modes of the CCM result in the accumulation of HCO3– in the cytosol, which diffuses to the carboxysome. Inorganic carbon uptake by Trichodesmium involves the uptake of HCO3– by the BicA transporter. This transporter has a half-saturation constant, Km, of 40–100 µM HCO3–, which is well below the typical concentration of HCO3– in seawater (~2000 µM) (Badger et al., 2006). Following transport into the cell, C-fixation in Trichodesmium, like other cyanobacteria species, occurs within carboxysomes where HCO3– is converted to CO2 via a carbonic anhydrase, followed by fixation of CO2 by Rubisco. Carboxysomes provide micro-environments where CO2 is elevated to compensate for the low affinity of cyanobacterial Rubiscos for CO2 (KmCO2>150 mM) (Badger and Andrews, 1987). In Trichodesmium, CO2 that leaks from carboxysomes can be converted to HCO3– by the plasma membrane-bound NDH-I4 protein, thus reducing the efflux of CO2 from the cell, but at a cost of consuming reducing equivalents (NADPH or reduced Fd) (Price et al., 2008). Despite having a mechanism for intracellular recycling of CO2, efflux is reported to account for the loss of up to 50% of HCO3– uptake in Trichodesmium (Kranz et al., 2010; Eichner et al., 2015).
As reviewed in Boatman et al. (2017), the majority of previous studies have shown an increase (albeit not all statistically significant) in T. erythraeum IMS101 growth under predicted future CO2 concentrations (~750–1000 µmol mol–1), although the magnitudes of these responses differ between studies (see Supplementary Table S1 at JXB online). The increased growth and productivity of T. erythraeum IMS101 with increased CO2 is probably attributable to a decrease in the energy required for operation of the CCM, allowing more energy (ATP) and reductant (NADPH) to be reallocated to N2 fixation, CO2 fixation, and biosynthesis (Kranz et al., 2011).
Given the significant contribution of Trichodesmium to carbon and nitrogen biogeochemical cycles, and the predicted changes to Ci speciation over the coming decades due to ocean acidification, we performed a systematic study to assess how the kinetics of Ci assimilation of T. erythraeum IMS101 were affected by acclimation to varying CO2 concentrations. We ensured that the Ci chemistry and all other growth conditions were well defined, with cultures fully acclimated over long time periods to achieve balanced growth. We assessed how the rate of Ci assimilation was related to CO2 or HCO3– concentrations in experiments where Ci speciation was modulated by varying pH and total dissolved inorganic carbon concentration (TIC). These assays of photosynthetic performance showed that Trichodesmium productivity was influenced by high pH when TIC was held at a saturating concentration, indirectly making the rate of Ci assimilation a saturating function of CO2 concentration, and that maximum rates of CO2 fixation declined and affinity for CO2 increased when Trichodesmium was acclimated to a low-CO2 concentration. We discuss how these responses can be attributed to decreases in the cost of operating a CCM under future CO2 conditions.
Materials and methods
Trichodesmium erythraeum IMS101 was semi-continuously cultured to achieve fully acclimated balanced growth at three target CO2 concentrations (180, 380, and 720 µmol mol–1) under saturating light intensity (400 µmol photons m–2 s–1), a 12/12 h light/dark (L/D) cycle, and an optimum growth temperature (26 ± 0.7 °C) for ~5 months (~80 generations).
Experimental set-up
Cultures of T. erythraeum IMS101 were grown using YBCII medium (Chen et al., 1996) at 1.5-l volumes in 2-l Pyrex bottles that had been acid-washed and autoclaved prior to culturing. Daily growth rates were quantified from changes in baseline fluorescence (Fo) measured between 09.00 to 10.30 h on dark-adapted cultures (20 min) using a FRRfII FastAct Fluorometer System (Chelsea Technologies Group Ltd, UK). Cultures were kept at the upper section of the exponential growth phase through periodic dilution with new growth media at 3–5 d intervals. They were deemed fully acclimated and in balanced growth when both the slope of the linear regression of ln(Fo) and the ratio of live-cell to acetone-extracted (method detailed below) baseline fluorescence were constant following every dilution with fresh YBCII medium. Illumination was provided side-on by fluorescent tubes (Sylvania Luxline Plus FHQ49/T5/840). Cultures were constantly mixed using magnetic PTFE stirrer bars and aerated with a filtered (0.2-µm pore) air mixture at a rate of ~200 ml s–1. The CO2 concentration was regulated (±2 µmol mol–1) by mass-flow controllers (Bronkhorst, Newmarket, UK) and CO2-free air was supplied by an oil-free compressor (Bambi Air, UK) via a soda-lime gas-tight column that was mixed with a 10% CO2-in-air mixture from a gas cylinder (BOC Industrial Gases, UK). The CO2 concentration in the gas phase was continuously monitored and recorded by an infra-red gas analyser (Li-Cor Li-820, Nebraska USA), calibrated weekly against a standard gas (BOC Industrial Gases).
The Ci chemistry was measured prior to the dilution of each culture with fresh media; pH and TIC were measured directly, while HCO3–, CO32–, and CO2 concentrations were calculated using CO2SYS with the same constants as described in Boatman et al. (2017) (see Supplementary Information SI).
Elemental stoichiometry
Samples for elemental composition and CO2-response curves were collected at the same time of day between 4 and 6 h into the light period of the L/D cycle. Samples for determination of particulate organic carbon (POC), particulate nitrogen (PN), and particulate phosphorus (PP) were collected together with each CO2-response curve, where each sample was a biological replicate culture. Three 100-ml aliquots from each culture were vacuum-filtered onto pre-combusted 25-mm (0.45-µm pore) glass-fibre filters for measurements of POC, PN, and PP. The POC and PN filters were placed in 1.8-ml cryovials (lids off) and dried at 60 °C. The PP filters were rinsed with 2 ml of sodium sulphate (0.1 M), placed in a 20-ml glass scintillation vial, 2 ml of magnesium sulphate (0.017 M) added, and then dried at 60 °C. POC was quantified using a TC analyser (Shimadzu TOC-V Analyser & SSM-5000A Solid Sample Combustion Unit), PN by the method of Bronk and Ward (2000), and PP by the method of Solorzano and Sharp (1980).
Inorganic carbon fixation-response curves
The dependencies of CO2 fixation on CO2 and HCO3– were determined from experiments that involved varied TIC with fixed pH and varied pH with fixed TIC (see Supplementary Information SII, SIII) in TRIS-buffered YBCII medium using the 14C uptake technique (Steemann Nielsen and Jensen, 1957).
Prior to each experiment, 1 l of bicarbonate-free YBCII medium was aerated overnight with CO2-free air (soda-lime column). A 200-ml sample from each culture was gravity-filtered onto a 47-mm cyclopore filter (1-µm pore; Whatman 60750) and gently re-suspended into 50 ml of the CO2-free YBCII medium. Exactly 5 ml of concentrated culture was pipetted into each tube of the TIC or pH gradients (35 ml total volume per tube) and gently inverted to evenly distribute the trichomes. The remaining culture was used for measurement of initial activity, T0. Three replicate cultures were used per treatment. During sample preparation, test-tubes were maintained at growth temperature (26 °C) and a low light intensity (<10 µmol photons m–2 s–1).
To characterise the Ci chemistry, exactly 20 ml of culture from each treatment was filtered through a Swinnex filter (25 mm, 0.45-µm pore, glass-fibre filter): 15 ml into a plastic centrifuge tube (no headspace) for TIC analysis (Shimadzu TOC-V Analyser & ASI-V Autosampler), and 5 ml into a plastic cryogenic vial (Sigma-Aldrich V5257-250EA; no headspace) for pH analysis.
To measure chlorophyll a concentrations, a 1-ml sample from each treatment was pipetted into 9 ml of 100% acetone and left in a freezer (–20 °C) overnight (Welschmeyer, 1994). The sample was vortex-mixed and left in the dark (~30 min) to allow cell debris to precipitate and the solution to equilibrate to room temperature. A 2-ml aliquot was used to measure Fo using a FRRfII FastAct Fluorometer System (Chelsea Technologies Group Ltd, UK) with the same parameters as used for live cultures. Chlorophyll a concentrations were calculated from a calibration curve derived from a dilution series measured using a chlorophyll a standard (Sigma-Aldrich C5753).
To assess whether cells had been affected by concentration via filtration and re-suspension and exposure to the range of TIC and pH gradients over the course of the 14C incubations, 2-ml aliquots of culture from each treatment were dark-acclimated (~20 min) and the photosynthetic efficiency of PSII (Fv/Fm) was determined using a FRRfII FastAct Fluorometer System (Chelsea Technologies Group Ltd, UK) (see Supplementary Fig. S1).
Finally, 10 ml of culture from each treatment was pipetted into 12-ml glass (PTFE-capped) test-tubes and used for 14C incubations. A 14C spike solution was prepared by pipetting 45 µl of a 14C-labelled sodium bicarbonate solution (NaH14CO3) with a specific activity of 52 mCi mmol–1 (Perkin Elmer, USA) into 8 ml of bicarbonate-free YBCII media. Exactly 250 µl of the spike was added to each tube culture. The T0 tubes were immediately filtered through Swinnex filters containing 25-mm diameter (0.45-µm pore) glass-fibre filters, placed in scintillation vials, and acidified (500 µl of 3 M HCl). To determine the total activity (TC), 20 µl of the spike was added into three scintillation vials already containing 4.5 ml of scintillation cocktail (Gold LLT) and 200 µl of phenylethylamine. The TC vial caps were screwed tight immediately. The spiked test-tubes were placed within a custom-made water-jacketed incubator and maintained at 26 °C under saturating light intensity (400 ± 6 µmol photons m–2 s–1) (The Optoelectronic Manufacturing Corporation Ltd. 1ft T5 Daylight, UK). The incubations lasted between 60 and 90 min and took place between 4 to 6 h into the light period of the L/D cycle. The 14C incubations were repeated in the dark, using black-coated (Plasti-Kote paint) test-tubes. Dark 14C uptake rates were 8.25% (±0.46) and 7.05% (±0.25) of the maximum light-saturated 14C uptake rates for the TIC and pH response curves, respectively. Dark 14C uptake rates exhibited no response to varying TIC or pH and were used to correct the light-dependent rates of photosynthesis (Li and Dickie, 1991).
To terminate 14C uptake, samples were filtered through 25-mm (0.45-µm pore) glass-fibre filters (Fisherbrand FB59451, UK) using a bespoke 30-funnel filtration manifold. Test-tubes and filters were rinsed twice with 5 ml of YBCII media, before the filters were placed into scintillation vials. The vials were acidified (500 µl of 3 M HCl) overnight along with the T0 samples. Exactly 4.5 ml of scintillation cocktail (Gold LLT) was added to the acidified vials and the caps tightened. Ensuring that the scintillation cocktail and filtered samples were well mixed, the vials were placed within a scintillation counter and the disintegrations per minute (DPM) of each vial were measured (20 min per vial). The CO2 fixation rates were calculated using the following equation:
| (1) |
where DPMS, DPMT0, and DPMTC are the measurements for the sample, initial activity, and total activity vials, respectively; TIC (mmol l–1) is the mean concentration of total dissolved inorganic carbon within the sample over the course of the incubation (inclusive of the NaH14CO3 spike); VolTC and VolS are the volumes of the sample and TC vials, respectively; t is the experimental incubation time (h); and 1.05 is the radioisotope discrimination factor (12C:14C). Note that mean T0 and TC values were used when calculating the C-fixation rates (n=3).
Inorganic carbon fixation rates were normalised to a POC basis and the CO2 response curves were fitted to a Michaelis–Menten function:
| (2) |
where VC is the organic C-specific rate of CO2 fixation, VC,max is the maximum rate of CO2 fixation, and Km is the half-saturation constant. Curve-fitting was performed on individual replicates to calculate mean (±SE) curve-fit parameters (Sigmaplot 11.0), as well on the combined data where all replicates of the varied TIC (fixed pH) and varied pH (fixed TIC) data were combined per CO2 treatment.
Spectrophotometric chlorophyll a analysis
Samples for spectrophotometric determination of chlorophyll a were collected together with each CO2-response curve and were used to normalise productivity rates as well as to calculate the ratio of Chl a:C (i.e. total C). A 100-ml sample from each culture was vacuum-filtered onto a 25-mm (0.45-µm pore) glass-fibre filter (Fisherbrand FB59451, UK) and extracted in 5 ml of 100% methanol. The filters were homogenised and extracted overnight at –20 °C before being centrifuged at 12 000 g for 10 min and a 3-ml aliquot of the supernatant was transferred to a quartz cuvette. The absorption spectrum (400–800 nm) was measured using a spectrophotometer (Hitachi U-3000, Japan) and the Chl a concentration (µg l–1) was calculated using the following equation (Ritchie, 2008);
| (3) |
where Abs665 and Abs750 are the baseline-corrected optical densities of the methanol extracted sample at 665 and 750 nm, respectively; VolE is the volume of the solvent used for extraction (i.e. 5 ml); VolF is the volume of culture that was filtered (i.e. 100 ml); and 12.9447 is a cyanobacteria-specific Chl a coefficient for 100% methanol extraction.
Modelling the CCM
The CO2 and HCO3– fluxes and concentrations in an idealised Trichodesmium cell were calculated using the numerical model from Mangan et al. (2016) and Mangan and Brenner (2014). The aim was to provide a qualitatively informative view of the CCM system, without attempting to match carboxylation rates or fluxes to the experimental system or to rescale the results from the idealised cell to what would be expected from the experimental data. With the exception of a few key parameter values (Table 1), the model used was equivalent to that reported in Mangan et al. (2016). The main changes between the idealised Trichodesmium cell and previous models were an increase in cell and carboxysome size to be consistent with reported values for T. erythraeum, changes to the Rubisco kinetic constants, use of pH and external CO2 and HCO3– concentrations similar to those in the 14C incubations, updating the pKaeff for HCO3– to CO2 to match that used in the CO2SYS calculation, and re-calculating the HCO3– uptake rate to support internal inorganic carbon concentrations of ~30 mM. We scaled the Rubisco concentration by the carboxysome volume, so that the activity per volume remained the same. Similarly, we scaled the amount of carbonic anhydrase by the carboxysome surface area, so that the activity per area remained the same. The carbonic anhydrase activity was sufficient to equilibrate CO2 and HCO3– to . We set the carbonic anhydrase Kca value to preserve the correct equilibrium value for the internal pH.
Table 1.
Key parameter values used in the numerical simulation of the CCM in Trichodesmium
| Variable | Units | Model value |
|---|---|---|
| Cell radius, Rb | µm | 3 |
| Carboxysome radius, Rc | µm | 0.15 |
| Rubisco reaction rate, kRub | s–1 per active site | 1.92 |
| Rubisco KCO2 | µM | 145 |
| Rubisco KO2 | µM | 600 |
| Rubisco specificity, S | – | 45 |
| Number of Rubisco active sites | – | 54000 |
| Number of carbonic anhydrase active sites | – | 900 |
| Carbonic anhydrase half-maximum constant for CO2, Kca | µM | 104.7 |
| Internal pH | – | 8.3 |
| pKaeff for HCO3–:CO2 | – | 5.84 |
| Carboxysome permeability | cm s–1 | 3 × 10–5 |
| HCO3– uptake velocity, jc | cm s–1 | 2.4 × 10–7 |
| CO2 to HCO3– conversion at membrane | cm s–1 | 0.6 × 10–7 |
The cell radius was measured from a bioimage collected using fluorescence microscopy (Supplementary Fig. S12). Kinetic constants of Rubisco carboxylation (KCO2), oxygenation (KO2), and the specificity factor (S) for a form 1B cyanobacteria were taken from Badger et al. (1998).
Results
Inorganic carbon chemistry, growth rate, and cell composition
Overall, the CO2 drawdown in the cultures ranged between 57–78 µmol mol–1 for all CO2 treatments (Table 2) and exhibited a negligible CO2 drift over a diurnal cycle (see Supplementary Fig. S2). Dissolved inorganic NH4+ concentrations in the growth medium were ~1 µM, while NO3– concentrations were ~0.3 µM, which was below the 1 µM detection limit.
Table 2.
The growth conditions (±SE) achieved for T. erythraeum IMS101 when cultured at three target gas-phase CO2 concentrations (Low=180 µmol mol–1, Mid=380 µmol mol–1, and High=720 µmol mol–1), saturating light intensity (400 µmol photons m–2 s–1), and optimal temperature (26 °C)
| Variable | Units | Low CO2 | Mid CO2 | High CO2 |
|---|---|---|---|---|
| pH | – | 8.458 | 8.174 | 7.906 |
| H+ | nM | 3.5 (0.20) | 6.7 (0.13) | 12.4 (0.28) |
| AT | µM | 2431 (70) | 2447 (54) | 2442 (56) |
| TIC | µM | 1800 (69) | 2039 (46) | 2201 (50) |
| HCO3– | µM | 1362 (67) | 1743 (39) | 2005 (44) |
| CO32– | µM | 435 (16) | 289 (9) | 179 (6) |
| CO2 | µM | 3.3 (0.3) | 8.1 (0.2) | 17.3 (0.5) |
| NH4+ | µM | 1.03 (0.14) | 1.00 (0.08) | 1.08 (0.06) |
| NO3– | µM | 0.34 (0.05) | 0.32 (0.03) | 0.30 (0.02) |
| n | 89 | 67 | 39 |
Individual pH values were converted to a H+ concentration, allowing a mean pH value (Total scale) to be calculated. Dissolved inorganic NH4+ was determined using the phenol-hypochlorite method as described by Solorzano (1969), while dissolved inorganic NO3– was determined using the spectrophotometric method as described by Collos et al. (1999).
Balanced growth rates increased from ~0.2 d–1 at low CO2 to ~0.34 d–1 at mid-CO2 and ~0.36 d–1 at high CO2 (Table 3). The dark-adapted photochemical efficiencies of PSII (Fv/Fm) were proportionate to the CO2 treatment, increasing from 0.27 at low CO2 to ~0.31 at mid-CO2 and ~0.34 at high CO2 (Table 3). The particulate C:N ratio was independent of CO2, while the C:P and N:P ratios increased with increasing CO2 (Table 3). Both Chl a:C and Chl a:N ratios were about 30–40% higher at mid-CO2 than at low or high CO2.
Table 3.
The mean (±SE) balanced growth rate, dark-adapted photochemical efficiency of PSII (Fv/Fm), elemental stoichiometry, and chlorophyll a to C and N ratios for T. erythraeum IMS101 when acclimated to three target CO2 concentrations (Low=180 µmol mol–1, Mid=380 µmol mol–1, and High=720 µmol mol–1), saturating light intensity (400 µmol photons m–2 s–1), and optimal temperature (26 °C)
| Variable | Units | Low CO2 | Mid CO2 | High CO2 |
|---|---|---|---|---|
| Growth rate | d–1 | 0.198 (0.027)A | 0.336 (0.026)B | 0.361 (0.020)B |
| F v/Fm | dimensionless | 0.274 (0.025)A | 0.305 (0.020)B | 0.342 (0.037)C |
| Elemental stoichiometry | ||||
| C:N | mol:mol | 7.9 (0.8) | 7.8 (0.3) | 7.3 (0.8) |
| C:P | mol:mol | 91.9 (6.3)A | 143.6 (6.3)B | 155.5 (13.5)B |
| N:P | mol:mol | 11.9 (0.6)A | 18.4 (0.7)B | 21.8 (1.7)B |
| Chl a:C | g:mol | 0.052 (0.003)A | 0.089 (0.003)C | 0.066 (0.003)B |
| Chl a:N | g:mol | 0.401 (0.037)A | 0.693 (0.035)B | 0.474 (0.043)A |
Replicates comprised n=9 at low CO2, n=6 at mid- and high CO2. Letters indicate significant differences between CO2 treatments (one-way ANOVA, Tukey post hoc test; P<0.05); where B is significantly greater than A, and C is significantly greater than B and A.
CO2-response curves
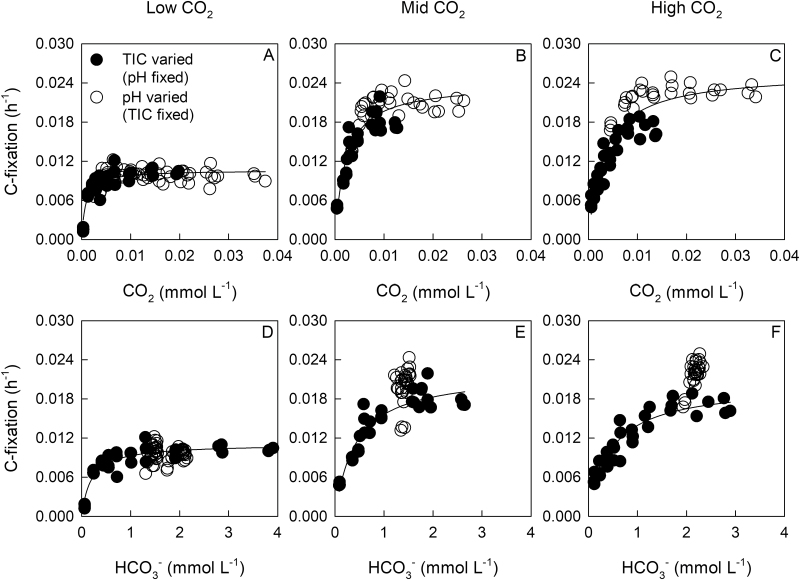
Based on the shape of the response curves, the inorganic carbon (14C) fixation rate was fitted to a saturating function of the dissolved CO2 concentration in both the pH gradient and TIC gradient experiments (Fig. 1). Although a saturating function of HCO3– concentration was observed when TIC was varied at constant pH (Fig. 1A–C), Ci assimilation could not be described by the same kinetic constants when pH was varied at constant TIC (Fig. 1D–F).
Fig. 1.
(A–C) CO2- and (D–F) HCO3–-response curves for inorganic C-fixation by T. erythraeum IMS101. C-fixation rates are normalised to a carbon h–1 basis. Filled circles indicate data obtained by varying TIC and HCO3– at a fixed pH of ~8.15. Open circles indicate data obtained by varying pH (~7.52–8.54) at a fixed TIC. Differences in the range of HCO3– and CO2 gradients between CO2 treatments were due to variability in pipetting and not from instability in the Ci chemistry. For the CO2 response, curve-fitting was performed using all replicates from both the TIC and pH gradients. For the HCO3– response, curve-fitting was performed using data from the TIC gradient only. The CO2- and HCO3–- response curves for individual experiments are shown in Supplementary Figs S6–S11.
The Km for photosynthetic C-fixation increased from 0.8 µM in cultures acclimated to low CO2 to 2.2 µM and 3.2 µM in cultures acclimated to mid- and high CO2, respectively, and were approximately 4- to 5-fold lower than the ambient CO2 concentrations in the cultures. The maximum organic carbon-specific rate of C-fixation (VC,max) was also higher in cells grown at mid-CO2 than at low CO2, although the rates at mid- and high CO2 did not differ significantly (Table 4). The affinity for CO2 (VC,max/Km) declined by about 40% between the low- and high-CO2 treatments (Table 4).
Table 4.
The physiological parameters (±SE) of the C-specific C-fixation versus CO2 concentration response curves for T. erythraeum IMS101, fitted using the Michaelis–Menten model to obtain estimates using the combined data from all replicates from both experiments employing varied TIC at fixed pH and varied pH at fixed TIC for each CO2 treatment
| Parameter | Units | Low CO2 | Mid CO2 | High CO2 |
|---|---|---|---|---|
| V C,max | h–1 | 0.011 (0.0002) | 0.024 (0.0007) | 0.026 (0.0008) |
| K m | µM CO2 | 0.8 (0.1) | 2.2 (0.3) | 3.2 (0.4) |
| Affinity | mM (CO2)–1 h–1 | 13.3 (1.7) | 10.9 (1.5) | 8.0 (1.0) |
V C,max, the C-specific maximum C-fixation rate; Km, the half saturation constant; Affinity, the C-specific initial slope of the VC,max versus CO2-response curve.
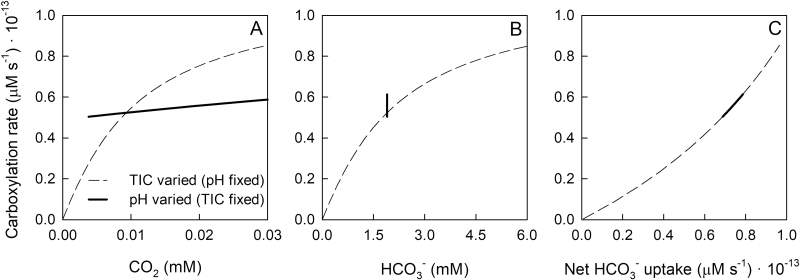
Modelled response curves
Without parameter-fitting, the CCM model of Trichodesmium produced behaviors consistent with the experimental data when either external TIC (i.e HCO3–) was varied at a fixed pH or when pH was varied at a fixed TIC (Fig. 2A, B). Assuming HCO3– is the dominant form of inorganic carbon taken up by the cell (Kranz et al., 2009; Eichner et al., 2015), Trichodesmium exhibited a significant response to changes in external pH and CO2 concentrations. The decrease in carboxylation rate with decreasing external CO2 was due to a decrease in HCO3– uptake (when TIC was varied) or an increase in HCO3– and CO2 leakage out of the cell (when pH was varied) (Supplementary Fig. S3). Modelled carboxylation rates from both numerical simulations exhibited a smooth function of HCO3– uptake, HCO3– leakage, and CO2 leakage (Fig. 2C).
Fig. 2.
Calculated carboxylation rates obtained from model simulations for T. erythraeum IMS101 as a function of external CO2 (A) and HCO3– (B) concentrations, with TIC (i.e. HCO3–) varied at a fixed pH=8.15 (dashed lines) and pH varied at a fixed HCO3–=1.9 mM (solid lines). Carboxylation rates are also plotted against the net HCO3– uptake rate (C), where HCO3– and CO2 leakage rates were subtracted from the rate of gross HCO3– transport.
The VC,max of the pH gradient and TIC gradient experiments were not significantly different (Supplementary Table S3). However, the maximum carboxylation rates from the simulations were significantly different (Fig. 2); principally because the external HCO3– concentration used in the pH-dependent simulation (chosen to be the same as the experiment) was not sufficient to saturate Rubisco. It is possible that the Km value assumed for Rubisco was set too high, or the internal pH, geometry, or HCO3– uptake values were substantially different. Note that we were simulating values beyond the range of those in the experiments, so such a discrepancy is magnified.
Discussion
The key findings of our study were as follows. The acclimated growth rate increased from low- to mid-CO2 treatments but did not increase significantly between mid- and high-CO2, suggesting that the positive effect of elevated CO2 on Trichodesmium carbon assimilation over the coming decades may only be slight. The maximum rate (VC,max) and the half-saturation constant (Km) for C-fixation increased with increasing CO2 treatment, but the affinity for CO2 (VC,max/Km) declined, which is probably attributable to the activity of the CCM in Trichodesmium. The measured inorganic C-fixation rate in Trichodesmium could be described as a saturating function of CO2, both when CO2 was manipulated by varying pH at constant TIC and when CO2 was manipulated by varying TIC at constant pH. A mechanistic model of the CCM in Trichodesmium indicated that the former was due to HCO3– uptake limitation of carboxylation rate, whereas the latter was due to a high-pH-mediated increases in HCO3– and CO2 leakage, potentially coupled to other unknown processes operating outside of the paramaterised model that were restricting Ci assimilation rates at high pH. Such processes may involve the direct effect of pH on membrane conformation, membrane transport processes, or metabolic functions.
Effect of acclimation to variations in inorganic chemistry on growth rates and elemental stoichiometry
The increased growth rates that we observed from low- (180 µmol mol–1) to mid- (380 µmol mol–1) and high-CO2 treatments (720 µmol mol–1) were similar to previous findings (Barcelos e Ramos et al., 2007; Boatman et al., 2017, 2018b). The growth rate at high CO2 was 8% greater than at mid-CO2, but this difference was not statistically significant. The magnitude of this increase at high CO2 was comparable to several recent studies, which report growth rate increases of 7–26% with increases of CO2 beyond 400 µmol mol–1 (Barcelos e Ramos et al., 2007; Hutchins et al., 2007; Levitan et al., 2007; Kranz et al., 2010; Garcia et al., 2011; Boatman et al., 2017).
The observed increases in C:P and N:P were consistent with previous findings (Barcelos e Ramos et al., 2007; Kranz et al., 2010; Levitan et al., 2010), with changes that can be ascribed to increases in cellular N and C incorporation, with P content relatively unaffected by CO2 (Hutchins et al., 2007; Kranz et al., 2010). In contrast, the C:N ratio and thus the balance between CO2 fixation and N2 fixation was not significantly affected by the CO2 treatment. Similarly, Levitan et al. (2007) found that C:N varied only slightly (from 6.5 to 7.0) across growth CO2 concentrations ranging from 250 to 900 µmol mol–1.
We report C-specific rates here as these are most directly related to changes in specific growth rate because both rates can be expressed in equivalent units of inverse time (e.g. h–1 or d–1). However, we note that due to differences in the Chl a:C ratio, chlorophyll a-specific rates showed a different pattern, increasing progressively from low through mid- to high CO2 (see Supplementary Table S2, Supplementary Fig. S4). A reduction in Chl a:C decreases the energy demands associated with synthesis of the photosynthetic apparatus and is dictated by the total demands for reductant (NADPH) and high-energy phosphate bonds (ATP) (Geider et al., 2009), the minimum turnover times for PSII (τPIIʹ) and PSI (τPIʹ), and the minimum pigment content required for effective light absorption and energy transfer (aminʹ) (Behrenfeld et al., 2008). We suggest that the reduced Chl a:C at low CO2 relative to mid-CO2 was probably due to the cost of up-regulating the CCM, whereas the reduced Chl a:C at high CO2 may have been due to an increase in carbohydrate storage granules relative to the mid-CO2 treatment (Table 3).
CO2-response curves
The growth rates reported here were comparable to the 2-µM EDTA, iron-replete (unchelated) treatments in Boatman et al. (2017), as well as 20-µM EDTA, iron-replete (chelated) cultures (Boatman et al., 2018b), which suggests that our cultures were not exposed to toxic concentrations of certain trace metals (e.g. copper) caused from low trace metal buffering capacity, as reported by Hong et al. (2017). Furthermore, dissolved inorganic NH4+ concentrations were consistently around 1.0 µM (Table 2). We are therefore confident that the observed positive effect of ocean acidification on growth and primary productivity is driven by the increased CO2 concentration, rather than being a consequence of a pH-induced shift of the NH3/NH4+ equilibrium. We determined CO2-response curves at one time of day (4–6 h into the photoperiod of a 12/12 h L/D cycle) and as such cannot extrapolate to a diel response given the reports of temporal separation of photosynthesis and N2 fixation in Trichodesmium (Berman-Frank et al., 2001).
The mechanistic model of Mangan et al. (2016) indicates that the CO2 response we observed when the TIC was varied (pH fixed) was caused by HCO3– limitation, where HCO3– uptake limits the rate of carboxylation. Conversely, the CO2 response we observed when pH was varied (TIC fixed) was a function of the pH dependency of HCO3– and CO2 leakage, which in turn could lead to CO2 limitation of C-fixation and/or diversion of reducing equivalents from powering CO2 fixation via the Calvin cycle to powering the conversion of CO2 to HCO3– by the NDH-I4 complex. The model of the CCM in Trichodesmium showed the relative importance of leakage, which is notably sensitive to certain parameters in the system such as internal pH, Rubisco activity, cell size, and carboxysome size.
Previous studies have shown a notable response in CCM activity to changes in CO2; for example, a two-fold lower dissolved inorganic carbon half-saturation concentration in cells acclimated to 150 µmol mol–1 (pH 8.56) compared with 370 µmol mol–1 (pH 8.26) (Kranz et al., 2009). Our experimental observations indicated that Ci assimilation (VC) was well described by a CO2-response curve, but not by a single HCO3–-response curve (Fig. 1). We now offer an explanation as to the response of VC to HCO3– concentration in the experiments where we varied pH from 7.65 to 8.5 at constant TIC.
Based on the numerical simulations, carboxylation rates across an external pH gradient ranging from 7.5 to 8.5 exhibited a clear linear response, which could not be ascribed to a Michaelis–Menten function (see Supplementary Fig. S3). Conversely, our experimental data showed a clear and significant decrease in Ci assimilation rates at low external CO2/high pH (Fig. 1). In addition, the Ci assimilation rates for the pH-gradient and TIC-gradient experiments, for all replicates of all three CO2 treatments, exhibited similar inflection points to external CO2 (Supplementary Fig. S5). In order for the simulated system to exhibit a rate-saturating response to external CO2, CO2 would have to be the dominant source of inorganic carbon. This would contradict all previous research showing that HCO3– accounts for >90% of inorganic carbon uptake (Kranz et al., 2009, 2010) and the currently accepted mechanism of Ci assimilation in T. erythraeum IMS101 (Badger and Price, 2003).
Given how well the numerical simulations modelled carboxylation rates as a smooth function of HCO3– uptake, HCO3– leakage, and CO2 leakage (Fig. 2C), we propose that the linear pH-dependency of carboxylation rate predicted by the model is mechanistically correct, but that processes not captured by the model are contributing to the decrease in Ci assimilation rate at high pH. Such factors could include a direct effect of high pH on cell membrane properties and alteration in membrane conformation (Myklestad and Swift, 1998), or the influence of pH on membrane transport processes and metabolic functions involved in cellular pH regulation (Raven, 1981).
Interestingly, for the mid- and high-CO2 treatments, a Michaelis–Menten function provided a better fit for the pH-varied (TIC fixed) data than a linear regression. However, there was no significant difference between a linear or Michaelis–Menten function for the low-CO2 data, which suggests that full acclimation to a high-pH environment prior to the 14C incubations lessened the negative effect that high pH had on Ci assimilation.
Based on our simulation, the actual carboxylation rate of Trichodesmium should be modelled as a function of HCO3– and pH. This is because the CO2 concentration in a saturated HCO3–/high-pH environment (i.e. 3.8 mM HCO3–, pH=8.4) could be equivalent to a limited HCO3–/present-day pH environment (i.e. 1.9 mM HCO3–, pH=8.1); which for the aforementioned reasons will impose different constraints on leakage/uptake rates. That said, our experimental data clearly suggested that high-pH-induced processes operating outside of the CCM were contributing to decrease Ci assimilation. Overall, this may allow the Ci assimilation rates of Trichodesmium to be ascribed as a function of CO2 (Fig. 1, see Supplementary Fig. S4), which would be considerably simpler to implement in biogeochemical models of Trichodesmium growth and photosynthesis (Hutchins et al., 2013) than a HCO3–-response curve in which the kinetic constants (Km and Vm) are pH-dependent. Further experimental work is needed to assess whether a CO2 parameterisation is consistent across a more extended range of pH and HCO3– conditions than those used in our experiments.
Conclusions
Climate change is driving ocean acidification, which results in higher CO2 and HCO3– concentrations and a decrease in pH. We observed systematic changes in the kinetics of inorganic carbon assimilation of T. erythraeum IMS101 in response to acclimation to increasing CO2 concentrations ranging from low CO2 (levels at the last glacial maximum) through mid-CO2 (levels at the end of the 20th century), to high CO2 (levels predicted for 2050–2100). Extrapolating these responses to future scenarios of the natural environment should take into account the fact that our findings were obtained using acclimation experiments whereas Trichodesmium may adapt to future conditions (Hutchins et al., 2015), that variability may exist between strains and clades (Hutchins et al., 2013), and that there will be additional effects of integrated abiotic variables (e.g. light and temperature) and nutrients (e.g. P and Fe) on Trichodesmium productivity (Walworth et al., 2016; Boatman et al., 2018a, 2018b).
In the context of the open oceans, our results indicate that nutrient-replete net photosynthesis and growth rates of T. erythraeum IMS101 would have been severely CO2-limited at the last glacial maximum relative to current conditions. However, future increases in CO2 (i.e. 720 µmol mol–1) may not significantly increase its growth and productivity, although we note that other studies have reported a stimulation of growth and photosynthesis by increasing CO2 beyond current ambient concentrations (Hutchins et al., 2007; Levitan et al., 2007, 2010). On the other hand, we did observe that growth under high CO2 will increase key stoichiometric ratios (N:P and C:P). Increases of N:P and C:P in Trichodesmium-dominated oceanic regimes may affect bacterial and zooplankton metabolism, the pool of bioavailable nitrogen, the depth at which sinking organic matter is remineralised, and consequently carbon sequestration via the biological carbon pump (Mulholland et al., 2004; McGillicuddy, 2014). These responses could serve as a negative feedback to climate change by increasing new N and C production and thereby increasing the organic carbon sinking to the deep ocean.
Supplementary data
Supplementary data are available at JXB online.
Information SI. Calculation of inorganic carbon speciation.
Information SII. Preparation of medium for CO2-response curves where TIC was varied at fixed pH.
Information SIII. Preparation of medium for CO2-response curves where pH was varied at fixed TIC.
Table S1. Recent literature on the C- and N2-fixation rates and elemental stoichiometry of T. erythraeum IMS101 in response to CO2, temperature, and light.
Table S2. The Chl a-specific curve-fitting parameter values of the carbon assimilation–CO2-response curves when the ‘TIC varied/pH fixed’ and ‘pH varied/TIC fixed’ data were modelled separately.
Table S3. The carbon-specific curve-fitting parameter values of the carbon assimilation–CO2-response curves when the ‘TIC varied/pH fixed’ and ‘pH varied/TIC fixed’ data were modelled separately.
Fig. S1. The effect of filtration/re-suspension and incubation on photosynthetic efficiency.
Fig. S2. The inorganic carbon chemistry of the culture vessels over a diurnal period.
Fig. S3. The modelled rates of carboxylation, CO2 leakage, HCO3–, uptake and HCO3– leakage for a Trichodesmium cell.
Fig. S4. The Chl a-specific curve fits of the carbon assimilation–CO2-response curves when the ‘TIC varied/pH fixed’ and ‘pH varied/TIC fixed’ data were modelled together.
Fig. S5. The Chl a- and carbon-specific curve fits of the carbon assimilation–CO2-response curves when the ‘TIC varied/pH fixed’ and ‘pH varied/TIC fixed’ data were modelled seperately.
Fig. S6. The Chl a- and carbon-specific curve fits of the carbon assimilation–CO2-response curves of the low-CO2 treatment for the ‘TIC varied/pH fixed’ data.
Fig. S7. The Chl a- and carbon-specific curve fits of the carbon assimilation–CO2-response curves of the low-CO2 treatment for the ‘pH varied/TIC fixed’ data.
Fig. S8. The Chl a- and carbon-specific curve fits of the carbon assimilation–CO2-response curves of the mid-CO2 treatment for the ‘TIC varied/pH fixed’ data.
Fig. S9. The Chl a- and carbon-specific curve fits of the carbon assimilation–CO2-response curves of the mid-CO2 treatment for the ‘pH varied/TIC fixed’ data.
Fig. S10. The Chl a- and carbon-specific curve fits of the carbon assimilation–CO2-response curves of the high-CO2 treatment for the ‘TIC varied/pH fixed’ data.
Fig. S11. The Chl a- and carbon-specific curve fits of the carbon assimilation–CO2-response curves of the high-CO2 treatment for the ‘pH varied/TIC fixed’ data.
Fig. S12. A bioimage of T. erythraeum IMS101 filaments cultured at mid-CO2, saturating light, and optimal temperature.
Acknowledgements
Tobias G. Boatman was supported by a UK Natural Environment Research Council PhD studentship (NE/J500379/1 DTB).
References
- Badger MR, Andrews TJ. 1987. Co-evolution of Rubisco and CO2 concentrating mechanisms. In: Biggins J, ed. Progress in photosynthesis research. Dordrecht: Springer, 601–609. [Google Scholar]
- Badger MR, Andrews TJ, Whitney S, Ludwig M, Yellowlees DC, Leggat W, Price GD. 1998. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Canadian Journal of Botany 76, 1052–1071. [Google Scholar]
- Badger MR, Hanson D, Price GD. 2002. Evolution and diversity of CO2 concentrating mechanisms in cyanobacteria. Functional Plant Biology 29, 161–173. [DOI] [PubMed] [Google Scholar]
- Badger MR, Palmqvist K, Yu JW. 1994. Measurement of CO2 and HCO3− fluxes in cyanobacteria and microalgae during steady-state photosynthesis. Physiologia Plantarum 90, 529–536. [Google Scholar]
- Badger MR, Price GD. 2003. CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. Journal of Experimental Botany 54, 609–622. [DOI] [PubMed] [Google Scholar]
- Badger MR, Price GD, Long BM, Woodger FJ. 2006. The environmental plasticity and ecological genomics of the cyanobacterial CO2 concentrating mechanism. Journal of Experimental Botany 57, 249–265. [DOI] [PubMed] [Google Scholar]
- Barcelos e Ramos J, Biswas H, Schulz KG, LaRoche J, Riebesell U. 2007. Effect of rising atmospheric carbon dioxide on the marine nitrogen fixer Trichodesmium. Global Biogeochemical Cycles 21, GB2028. [Google Scholar]
- Behrenfeld MJ, Halsey KH, Milligan AJ. 2008. Evolved physiological responses of phytoplankton to their integrated growth environment. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences 363, 2687–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman-Frank I, Lundgren P, Chen YB, Küpper H, Kolber Z, Bergman B, Falkowski P. 2001. Segregation of nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium. Science 294, 1534–1537. [DOI] [PubMed] [Google Scholar]
- Boatman TG, Lawson T, Geider RJ. 2017. A key marine diazotroph in a changing ocean: the interacting effects of temperature, CO2 and light on the growth of Trichodesmium erythraeum IMS101. PLoS ONE 12, e0168796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatman TG, Davey PA,Lawson T, Geider RJ. 2018a. The physiological cost of diazotrophy for Trichodesmium erythraeum IMS101. PLoS ONE 13, e0195638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatman TG, Oxborough K,Gledhill M,Lawson T, Geider RJ. 2018b. An integrated response of Trichodesmium erythraeum IMS101 growth and photo-physiology to iron, CO2, and light intensity. Frontiers in Microbiology 9, 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronk DA, Ward BB. 2000. Magnitude of dissolved organic nitrogen release relative to gross nitrogen uptake in marine systems. Limnology and Oceanography 45, 1879–1883. [Google Scholar]
- Capone DG, Burns JA, Montoya JP, Subramaniam A, Mahaffey C, Gunderson T, Michaels AF, Carpenter EJ. 2005. Nitrogen fixation by Trichodesmium spp.: an important source of new nitrogen to the tropical and subtropical North Atlantic Ocean. Global Biogeochemical Cycles 19, GB2024. [Google Scholar]
- Chen YB, Zehr JP, Mellon M. 1996. Growth and nitrogen fixation of the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp. IMS 101 in defined media: evidence for a circadian rhythm. Journal of Phycology 32, 916–923. [Google Scholar]
- Collos Y, Mornet F, Sciandra A, Waser N, Larson A, Harrison P. 1999. An optical method for the rapid measurement of micromolar concentrations of nitrate in marine phytoplankton cultures. Journal of Applied Phycology 11, 179–184. [Google Scholar]
- Davis CS, McGillicuddy DJ Jr. 2006. Transatlantic abundance of the N2-fixing colonial cyanobacterium Trichodesmium. Science 312, 1517–1520. [DOI] [PubMed] [Google Scholar]
- Eichner M, Thoms S, Kranz SA, Rost B. 2015. Cellular inorganic carbon fluxes in Trichodesmium: a combined approach using measurements and modelling. Journal of Experimental Botany 66, 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia NS, Fu FX, Breene CL, Bernhardt PW, Mulholland MR, Sohm JA, Hutchins DA. 2011. Interactive effects of irradiance and CO2 on CO2 fixation and N2 fixation in the diazotroph Trichodesmium erythraeum (Cyanobacteria). Journal of Phycology 47, 1292–1303. [DOI] [PubMed] [Google Scholar]
- Geider RJ, Moore CM, Ross ON. 2009. The role of cost–benefit analysis in models of phytoplankton growth and acclimation. Plant Ecology and Diversity 2, 165–178. [Google Scholar]
- Hong H, Shen R, Zhang F, et al. . 2017. The complex effects of ocean acidification on the prominent N2-fixing cyanobacterium Trichodesmium. Science 356, 527–531. [DOI] [PubMed] [Google Scholar]
- Hutchins DA, Fu F-X, Webb EA, Walworth N, Tagliabue A. 2013. Taxon-specific response of marine nitrogen fixers to elevated carbon dioxide concentrations. Nature Geoscience 6, 790–795. [Google Scholar]
- Hutchins DA, Fu F-X, Zhang Y, Warner M, Feng Y, Portune K, Bernhardt P, Mulholland M. 2007. CO2 control of Trichodesmium N2 fixation, photosynthesis, growth rates, and elemental ratios: implications for past, present, and future ocean biogeochemistry. Limnology and Oceanography 52, 1293–1304. [Google Scholar]
- Hutchins DA, Walworth NG, Webb EA, Saito MA, Moran D, McIlvin MR, Gale J, Fu F-X. 2015. Irreversibly increased nitrogen fixation in Trichodesmium experimentally adapted to elevated carbon dioxide. Nature Communications 6, 8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A, Reinhold L. 1999. CO2 concentrating mechanisms in photosynthetic microorganisms. Annual Review of Plant Physiology and Plant Molecular Biology 50, 539–570. [DOI] [PubMed] [Google Scholar]
- Kranz SA, Eichner M, Rost B. 2011. Interactions between CCM and N2 fixation in Trichodesmium. Photosynthesis Research 109, 73–84. [DOI] [PubMed] [Google Scholar]
- Kranz SA, Levitan O, Richter KU, Prásil O, Berman-Frank I, Rost B. 2010. Combined effects of CO2 and light on the N2-fixing cyanobacterium Trichodesmium IMS101: physiological responses. Plant Physiology 154, 334–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz SA, Sültemeyer D, Richter KU, Rost B. 2009. Carbon acquisition in Trichodesmium: the effect of pCO2 and diurnal changes. Limnology and Oceanography 54, 548–559. [Google Scholar]
- Levitan O, Brown CM, Sudhaus S, Campbell D, LaRoche J, Berman-Frank I. 2010. Regulation of nitrogen metabolism in the marine diazotroph Trichodesmium IMS101 under varying temperatures and atmospheric CO2 concentrations. Environmental Microbiology 12, 1899–1912. [DOI] [PubMed] [Google Scholar]
- Levitan O, Rosenberg G, Setlik I, Setlikova E, Grigel J, Klepetar J, Prasil O, Berman-Frank I. 2007. Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Global Change Biology 13, 531–538. [Google Scholar]
- Li W, Dickie P. 1991. Light and dark 14C uptake in dimly-lit oligotrophic waters: relation to bacterial activity. Journal of Plankton Research 13, 29–44. [Google Scholar]
- Mangan NM, Brenner MP. 2014. Systems analysis of the CO2 concentrating mechanism in cyanobacteria. eLIFE 3, e02043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan NM, Flamholz A, Hood RD, Milo R, Savage DF. 2016. pH determines the energetic efficiency of the cyanobacterial CO2 concentrating mechanism. Proceedings of the National Academy of Sciences, USA 113, E5354–E5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillicuddy DJ. 2014. Do Trichodesmium spp. populations in the North Atlantic export most of the nitrogen they fix?Global Biogeochemical Cycles 28, 103–114. [Google Scholar]
- Mulholland MR, Bronk DA, Capone DG. 2004. Dinitrogen fixation and release of ammonium and dissolved organic nitrogen by Trichodesmium IMS101. Aquatic Microbial Ecology 37, 85–94. [Google Scholar]
- Myklestad S, Swift E. 1998. A new method for measuring soluble cellular organic content and a membrane property, Tm, of planktonic algae. European Journal of Phycology 33, 333–336. [Google Scholar]
- Price GD, Badger MR, Woodger FJ, Long BM. 2008. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. Journal of Experimental Botany 59, 1441–1461. [DOI] [PubMed] [Google Scholar]
- Raven JA. 1981. Nutrient transport in microalgae. Advances in Microbial Physiology 21, 47–226. [DOI] [PubMed] [Google Scholar]
- Raven J, Caldeira K, Elderfield H, Hoegh-Guldberg O, Liss P, Riebesell U, Shepherd J, Turley C, Watson A. 2005. Ocean acidification due to increasing atmospheric carbon dioxide. Policy Document 12/05. London: The Royal Society. [Google Scholar]
- Ritchie R. 2008. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 46, 115–126. [Google Scholar]
- Schwarz R, Reinhold L, Kaplan A. 1995. Low activation state of ribulose-1,5-bisphosphate carboxylase/oxygenase in carboxysome-defective Synechococcus mutants. Plant Physiology 108, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solorzano L. 1969. Determination of ammonia in natural waters by the phenol hypochlorite method. Limnology and Oceanography 14, 799–801. [Google Scholar]
- Solorzano L, Sharp JH. 1980. Determination of total dissolved phosphorus and particulate phosphorus in natural waters. Limnology and Oceanography 25, 754–758. [Google Scholar]
- Steemann Nielsen E, Jensen EA. 1957. Primary oceanic production. The autotrophic production of organic matter in the oceans. In: Bruun AF, Greve SV, Spärck R, eds. Galathea Report, vol. 1. Copenhagen: The Galathea Committee, 49–135.
- Walworth NG, Fu FX, Webb EA, Saito MA, Moran D, Mcllvin MR, Lee MD, Hutchins DA. 2016. Mechanisms of increased Trichodesmium fitness under iron and phosphorus co-limitation in the present and future ocean. Nature Communications 7, 12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welschmeyer NA. 1994. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnology and Oceanography 39, 1985–1992. [Google Scholar]
- Zeebe RE, Wolf-Gladrow D, Jansen H. 1999. On the time required to establish chemical and isotopic equilibrium in the carbon dioxide system in seawater. Marine Chemistry 65, 135–153. [Google Scholar]
- Zeebe RE, Wolf-Gladrow DA. 2001. CO2 in seawater: equilibrium, kinetics, isotopes. Elsevier Oceanography Series 65. Elsevier. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.