See Gomes and Grace (doi:10.1093/brain/awy156) for a scientific commentary on this article.
Schifani et al. investigate the relationship between prefrontocortical dopamine release and stress response in schizophrenia. Associations between dopamine release and cortisol response to stress observed in controls and those at clinical high risk for psychosis are absent in schizophrenia, providing direct evidence of disrupted prefrontocortical dopamine-stress regulation in schizophrenia.
Keywords: stress, dopamine, positron emission tomography, psychosis, clinical high risk
Abstract
While alterations in striatal dopamine in psychosis and stress have been well studied, the role of dopamine in prefrontal cortex is poorly understood. To date, no study has investigated the prefrontocortical dopamine response to stress in the psychosis spectrum, even though the dorsolateral and medial prefrontal cortices are key regions in cognitive and emotional regulation, respectively. The present study uses the high-affinity dopamine D2/3 receptor radiotracer 11C-FLB457 and PET together with a validated psychosocial stress challenge to investigate the dorsolateral and medial prefrontocortical dopamine response to stress in schizophrenia and clinical high risk for psychosis. Forty participants completed two 11C-FLB457 PET scans (14 antipsychotic-free schizophrenia, 14 clinical high risk for psychosis and 12 matched healthy volunteers), one while performing a Sensory Motor Control Task (control) and another while performing the Montreal Imaging Stress Task (stress). Binding potential (BPND) was estimated using Simplified Reference Tissue Model with cerebellar cortex as reference region. Dopamine release was defined as per cent change in BPND between control and stress scans (ΔBPND) using a novel correction for injected mass. Salivary cortisol response (ΔAUCI) was assessed throughout the tasks and its relationship with dopamine release examined. 11C-FLB457 binding at control conditions was significantly different between groups in medial [F(2,37) = 7.98, P = 0.0013] and dorsolateral [F(2,37) = 6.97, P = 0.0027] prefrontal cortex with schizophrenia patients having lower BPND than participants at clinical high risk for psychosis and healthy volunteers, but there was no difference in ΔBPND among groups [dorsolateral prefrontal cortex: F(2,37) = 1.07, P = 0.35; medial prefrontal cortex: F(2,37) = 0.54, P = 0.59]. We report a positive relationship between ΔAUCI and 11C-FLB457 ΔBPND in dorsolateral and medial prefrontal cortex in healthy volunteers (r = 0.72, P = 0.026; r = 0.76, P = 0.014, respectively) and in participants at clinical high risk for psychosis (r = 0.76, P = 0.0075; r = 0.72, P = 0.018, respectively), which was absent in schizophrenia (r = 0.46, P = 1.00; r = 0.19, P = 1.00, respectively). Furthermore, exploratory associations between ΔBPND or ΔAUCI and stress or anxiety measures observed in clinical high risk for psychosis were absent in schizophrenia. These findings provide first direct evidence of a disrupted prefrontocortical dopamine-stress regulation in schizophrenia.
Introduction
Schizophrenia is a debilitating mental disease with a complex aetiology. It is believed to be caused by both genetic predisposition and environmental factors. Environmental risk factors for schizophrenia include psychosocial stress such as developmental trauma, growing up in an urban environment, and social defeat, among others (van Os et al., 2010). The impact of psychosocial stress on schizophrenia can be explained by the vulnerability-stress hypothesis. This model proposes that an endogenous diathesis/vulnerability interacts with internal or external stressors in the development of psychotic disorders (Walker and Diforio, 1997). While the importance of striatal dopamine in psychosis and stress has been well studied (Laruelle, 2000; Mizrahi et al., 2012), the role of dopamine in the prefrontal cortex (PFC) is still poorly understood.
The PFC (including its subregions the dorsolateral and medial PFC, dlPFC and mPFC, respectively) is well known for its crucial role in planning, controlling and directing behaviour in response to changing environmental demands (Miller and Cohen, 2001). The dlPFC is extensively connected with sensory and motor cortices and key region to regulate cognitive demand (Goldman-Rakic, 1995). The mPFC is extensively connected to subcortical regions that generate emotional responses such as amygdala and hypothalamus (Öngür and Price, 2000). There is compelling evidence that cognitive and emotional regulation is impaired in schizophrenia (Green et al., 2004; Holt et al., 2009), hence the importance of these two brain regions.
In contrast to the well-described striatal hyperdopaminergic state in schizophrenia, the PFC has been proposed to exhibit a hypodopaminergic response (Weinstein et al., 2017). This theory is supported primarily by preclinical schizophrenia models displaying attenuated mPFC dopamine signalling (Watt et al., 2009; Burke et al., 2010) and higher stress sensitivity (Gomes et al., 2016). However, direct evidence about a PFC hypodopaminergic state in living humans is still limited due, in part, to the lack of suitable PET tracers sensitive enough for the low density of dopamine D2/3 receptors in cortex. The high-affinity radiotracer 11C-FLB457 is a validated tool for cortical dopamine release quantification, reported to have ≤15% test-retest variability (Narendran et al., 2011b). Furthermore, 11C-FLB457 is superior to other cortical D2 receptor radioligands such as 11C-fallypride as it displays a higher signal-to-noise ratio in cortical areas (Narendran et al., 2009). Using 11C-FLB457, Slifstein et al. (2015) showed generally blunted dopamine release following an amphetamine challenge in various extrastriatal regions including a significant reduction in the dlPFC in first-episode psychosis. Furthermore, reduced dopamine release was associated with reduced working memory-related activation of the dlPFC in this population. While Hernaus et al. (2015) did investigate dopamine response to stress in mPFC previously using 18F-fallypride in a single-scan paradigm and reported no difference between individuals with non-affective psychotic disorder and healthy volunteers, so far, no data are available on PFC stress regulation in schizophrenia or its putative prodrome.
The current study aimed to examine stress-induced PFC dopamine response in patients with schizophrenia and those at clinical high risk (CHR) for psychosis using a validated two-scan paradigm with 11C-FLB457 PET. We hypothesized a reduction in mPFC and dlPFC dopamine release in response to the stress challenge given the previous study by Slifstein et al. (2015) and supporting preclinical literature on cortical dopamine response to stress (Watt et al., 2009; Burke et al., 2010). Furthermore, we examined the relationship between stress-induced PFC dopamine release and salivary cortisol response, for the first time in CHR and schizophrenia, and explored associations with stress-related behaviours.
Materials and methods
Subjects
Forty-two participants (84 scans) were included in this study, comprising 14 patients with schizophrenia, 14 individuals at CHR and 14 matched healthy volunteers. However, two healthy volunteers had to be excluded from the analysis because of excessive head motion that could not be corrected. All patients were antipsychotic-free, corroborated by clean urine drug screens, with eight of them also being antipsychotic-naïve.
To be eligible, CHR individuals had to meet the following criteria: fulfilment of diagnostic criteria for prodromal syndrome as per the Criteria of Prodromal Syndromes (COPS) (Miller et al., 2003) with no current Axis I disorder, as determined with the Structured Clinical Interview for DSM-IV (SCID) (First et al., 2002) and no history of or current treatment with antipsychotic medication. Patients with schizophrenia had to have a diagnosis of schizophrenia, schizoaffective, delusional, or schizophreniform disorder as assessed with the SCID, with no current treatment with antipsychotic medication, and no concurrent Axis I disorder. Healthy volunteers did not meet criteria for any prodromal syndrome, had any history of psychiatric illness or psychoactive drug use, and had no first-degree relative with a major mental disorder. Participants were excluded for any of the following: current diagnosis of substance abuse or positive urine drug screen; pregnancy or currently breastfeeding; clinically significant medical illness; and the presence of metal implants precluding a MRI scan.
The clinical status and severity of symptoms were assessed with the Structured Interview for Psychosis-risk Syndromes (SIPS) and the Scale of Psychosis-risk Symptoms (SOPS) (Miller et al., 2003) (CHR group), the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) (schizophrenia group), the Global Assessment of Functioning Scale (GAF), the Zung Self-rating Anxiety Scale (SAS), the Social Interaction Anxiety Scale (SIAS), the Recent Life Events questionnaire (RLE), and the Trier Inventory of the Assessment of Chronic Stress (TICS). Assessments are referenced in the online Supplementary material. This study was approved by the Research Ethics Board at the Centre for Addiction and Mental Health in accordance with the Declaration of Helsinki. All subjects provided written informed consent after being informed of all study procedures.
Montreal Imaging Stress Task
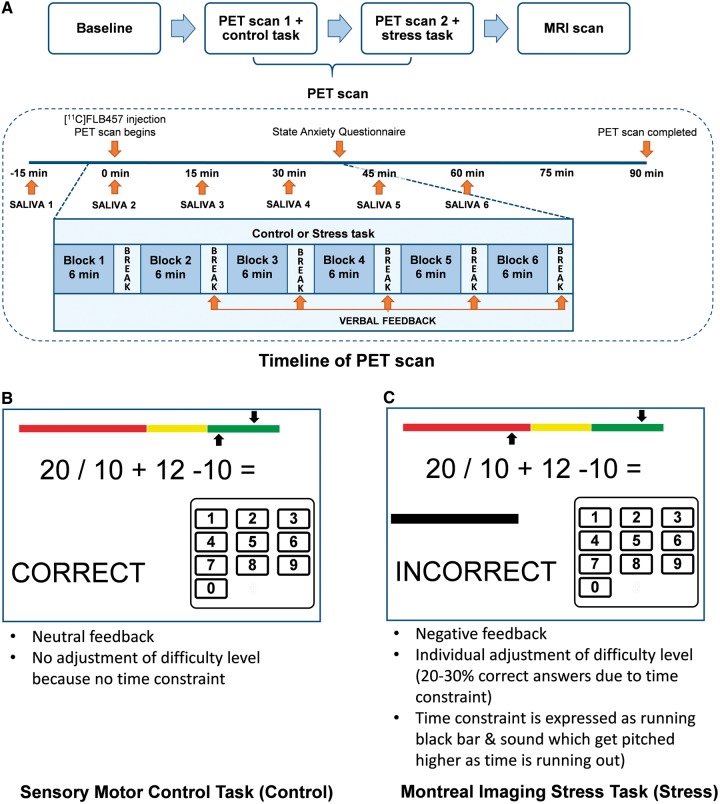
Psychological stress was induced using the Montreal Imaging Stress Task (referred to as ‘stress’) (described in detail in Dedovic et al., 2005), which has been used and validated in various functional MRI and PET studies (Lederbogen et al., 2011; Mizrahi et al., 2012). In brief, subjects perform mental arithmetic presented on a computer screen, which also displays information about the total number of errors, expected average number of errors, time spent on the current problem, and performance feedback for each problem (correct, incorrect, time out). All subjects completed six blocks of arithmetic, each approximately 6 min in length, while lying in the scanner. The time constraint was adjusted individually to be slightly beyond each subject’s abilities by adjusting each block dependent on the performance in the previous block. Because of this manipulation of the difficulty level, the average performance was set at 20–30% correct answers. Additionally, participants were given negative verbal feedback between each block, telling them that they need to improve their performance in order to reach minimum performance requirements. On a separate day before the stress session, participants were scanned while performing a Sensory Motor Control Task (referred as ‘control’), using similar arithmetic but without any time constraints or negative verbal feedback. The control scan was always performed first, to avoid any residual effects of the stress task.
In all experiments, the control or stress task was started about 6–8 min before tracer injection (see Fig. 1 for task overview). The control task was also administered as a practice trial on a separate day before the PET experiments, to reduce novelty effects.
Figure 1.
Overview of study procedures including timeline of scans (A) and the explanation of control (B) and stress task (C)..
After each PET scan session, participants’ subjective perception of stress was assessed by a short version of the State Anxiety Questionnaire (SAQ) (Spielberger et al., 1977). Further, patients’ psychotic (schizophrenia group) or attenuated psychotic (CHR group) symptoms were evaluated before and after each scan session using the PANSS or SOPS positive subscale, respectively.
Physiological measures
Saliva samples were collected every 15 min throughout the PET scanning session (six samples total) to evaluate the physiological response to the stress paradigm, starting 15 min before tracer injection and 9 min before the arithmetic task started. Saliva-derived cortisol was analysed using a time-resolved fluorescence immunoassay and the normalized area under the curve (AUCI) (g/dl/min) was calculated for each subject and each PET scan session as described elsewhere (Pruessner et al., 2003). Normalization to time point 1 was chosen due to differences in the scan time (between 9.00 am and 4.30 pm) as cortisol levels fluctuate over the course of the day (Castro et al., 2000). Cortisol data from six participants (one CHR and five schizophrenia) were not available for analysis. Change in AUCI (ΔAUCI) between control and stress task was defined as: ΔAUCI = AUCI Stress − AUCI Control.
Image acquisition and reconstruction
Every subject underwent an MRI scan to acquire a proton density-weighted image, used for delineation of individual regions of interest after co-registering with the PET image. All PET scans were performed for 90 min following intravenous bolus injection of ∼9–11 mCi 11C-FLB457 using a high resolution PET-CT scanner, Siemens-Biograph HiRez XVI (Siemens Molecular Imaging). Images were reconstructed using a 2D filtered back projection algorithm with a ramp filter at Nyquist cut-off frequency. Details of the image acquisition are summarized in the Supplementary material.
PET data analyses
Time–activity curves were extracted for the dlPFC and mPFC including both hemispheres (for detailed region of interest location, see Supplementary Fig. 5), and cerebellum using our validated in-house imaging software ROMI (Rusjan et al., 2006). All regions of interest were delineated using proton density-weighted image for each participant (Mizrahi et al., 2012). A quantitative estimate of binding was obtained from each time–activity curve with the Simplified Reference Tissue Model (SRTM) (Lammertsma and Hume, 1996) using the in-house software fMOD. The SRTM uses a within-brain reference region (cerebellar cortex in this case) instead of the arterial input function and provides an estimate of the binding potential (BPND) of the radiotracer, which is proportional to the more fundamental parameters of receptor number (Bmax) and affinity (Kd) [BPND Bmax/Kd]. It is a validated method and commonly used with 11C-FLB457 (Olsson et al., 1999; Ito et al., 2001; Narendran et al., 2009). Although few studies suggest small specific binding of 11C-FLB457 in cerebellum (Vandehey et al., 2010; Narendran et al., 2011a), no change in cerebellar distribution volume was observed following challenges with amphetamine and methylphenidate (Montgomery et al., 2007; Narendran et al., 2009). Previous studies with 11C-FLB457 have successfully used SRTM with cerebellum as reference region (Mizrahi et al., 2007; Ko et al., 2009; MacDonald et al., 2009) and a recent study showed that SRTM is a valid modelling approach to measure the percentage change in BPND () with 11C-FLB457 (Sandiego et al., 2015). Right and left regions of interest were pooled together to create a single time–activity curve used to derive BPND. As quantifying 11C-FLB457 is challenging, in part due to potential mass effects, a novel correction was applied in the current study (Gallezot et al., 2017). The correction takes competition between radioligand and dopamine in the stress condition into account while assuming negligible levels of occupied receptors in the control condition. These assumptions are supported experimentally as changes in cortical 11C-FLB457 binding has not been observed in a dopamine depletion study (Frankle et al., 2010), while ∼1000% increase of dopamine has been measured with microdialysis following 0.3 mg/kg amphetamine in non-human primates (Narendran et al., 2014). The corrected change in BPND was calculated as (Gallezot et al., 2017):
| (1) |
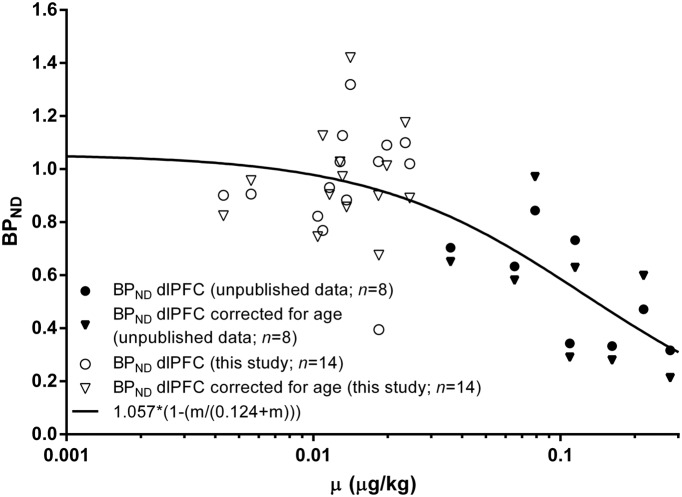
where is the ratio mass of radioligand injected to body weight and the mass injected that would reduce by 50%. The was estimated as follows: The BPND of the dlPFC of the scans from 12 healthy volunteers and two CHR gathered under control condition of this study (age 18 to 38 years, ranging from 0.004 to 0.025 μg/kg) were pooled together with the BPND of the dlPFC of eight scans from healthy subjects with very high mass injected gathered under similar control conditions (age 20 to 29 years, ranging from 0.035 to 0.28 μg/kg) from a yet unpublished study, adjusted by age (Narendran et al., 2009) and plotted in Fig. 3. = 0.124 μg/kg was estimated adjusting to the data (Logan et al., 2012).
Figure 3.
Effect of injected mass of 11C-FLB457 on the binding potential (BPND) in dlPFC. Graph shows original 11C-FLB457 BPND values of the dlPFC (circles) or corrected for age (triangles) plotted against every subject’s injected mass gathered under control condition in this study (open symbols) or control conditions of an unpublished study (filled symbols), and the non-linear fitted curve (black line). The estimated ED50 is 0.124 μg/kg.
Statistical analysis
Group differences in dopamine release were assessed using general linear models with ΔBPND value per region of interest (dlPFC or mPFC) as dependent variable and group (CHR, schizophrenia and healthy volunteers) as independent variable. BPND of the control scan was added as covariate to explore its effect on dopamine release. All analyses were two-tailed with the conventional α = 0.05. If significantly different, post hoc ANOVAs followed, using Bonferroni correction for multiple comparisons.
Group differences in the relationship between stress-induced dopamine release (ΔBPND) and salivary cortisol response (ΔAUCI) were determined using two separate general linear models to examine the group by ΔAUCI interaction, with ΔBPND value per region of interest (dlPFC or mPFC) as the dependent variable. The main analysis was followed by Pearson’s linear correlations, using Bonferroni correction for multiple comparisons.
Further, associations between ΔBPND or ΔAUCI and scores in behavioural scales (stress and anxiety) were explored using Pearson’s linear correlation analysis. As these analyses were considered exploratory, P-values were not corrected for multiple comparisons. Descriptions of further statistics can be found in the Supplementary material.
We considered results to be significant at P ≤ 0.05 and at trend levels at P ≤ 0.1.
Results
Demographics, clinical characteristics and PET scan parameters
Our analysis comprised 14 CHR, 14 patients with schizophrenia, and 12 matched healthy volunteers (80 PET scans in total). Details of demographics, clinical characteristics and scan parameters are summarized in Table 1. There were no differences between groups in sex, but a difference present in age, with schizophrenia patients being older than CHR subjects (Bonferroni-corrected P = 0.016). As age affects the BPND of 11C-FLB457 (Narendran et al., 2009) the ΔBPND was corrected for age (see ‘Materials and methods’ section and Fig. 3).
Table 1.
Participants’ demographics, clinical characteristics and radioligand injection parameters in a PET study of dopamine release in CHR and schizophrenia
| Healthy volunteers n = 12 | CHR n = 14 | Schizophrenia n = 14 | Comparisons | |
|---|---|---|---|---|
| Demographics | ||||
| Gender, male/female | 7/5 | 6/8 | 8/6 | χ2 = 0.81, P = 0.67 |
| Age, years (SD) | 26.00 (6.49) | 22.07 (3.38) | 28.29 (6.09)b | F(2,37) = 4.66, P = 0.016 |
| Clinical characteristics | ||||
| SOPS, mean (SD) | ||||
| Positive | – | 10.71 (3.45) | − | − |
| Negative | – | 9.43 (6.22) | − | − |
| Disorganized | – | 3.86 (1.75) | − | − |
| General | – | 6.71 (3.67) | − | − |
| PANSS, mean (SD) | ||||
| Positive | – | − | 17.57 (3.30) | − |
| Negative | – | − | 16.14 (7.16) | − |
| General | – | − | 35.64 (8.37) | − |
| Medication | ||||
| Antidepressants | – | 3 | 1 | − |
| Anxiolytics | – | 0 | 1 | − |
| Low dose antipsychotics | – | 0 | 1c | − |
| PET measures (11C-FLB457) | ||||
| Amount injected, mCi (SD) | ||||
| Control task | 9.83 (0.78) | 9.92 (0.58) | 10.18 (0.45) | F(2,37) = 1.21, P = 0.31 |
| Stress task | 10.28 (0.52) | 10.06 (0.58) | 10.11 (0.80) | F(2,37) = 0.40, P = 0.68 |
| Specific activity, mCi/µmol (SD) | ||||
| Control task | 3536.85 (1172.20) | 4011.15 (1977.21) | 2738.32 (1107.81) | F(2,37) = 2.61, P = 0.087 |
| Stress task | 3934.05 (1659.07) | 3264.46 (1642.78) | 3503.29 (1599.33) | F(2,37) = 0.55, P = 0.58 |
| Mass injected, µg (SD) | ||||
| Control task | 1.12 (0.29) | 1.11 (0.47) | 1.57 (0.55)a,b | F(2,37) = 4.59, P = 0.017 |
| Stress task | 1.15 (0.54) | 1.42 (0.65) | 1.30 (0.62) | F(2,37) = 0.65, P = 0.53 |
aSignificantly different to healthy volunteers (P ≤ 0.05).
bSignificantly different to CHR (P ≤ 0.05).
cQuetiapine (100 mg) taken only after the PET scan session.
PANSS = Positive and Negative Syndrome Scale; SD = standard deviation; SOPS = Scale of Psychosis-risk Symptoms.
Control and stress scans were performed on average 9.36 ± 10.22 days apart. All subjects performed the tasks during the scans successfully. There was no significant group difference in any of the PET scan parameters except for higher injected mass in patients with schizophrenia only on the control PET scan.
Furthermore, there was no difference in (attenuated) psychotic symptoms measured before the control and stress scan (Supplementary Fig. 4) or medication status between scan sessions neither in CHR nor schizophrenia. Details on current medication status per participant can be found in Table 1.
Scan paradigm effects
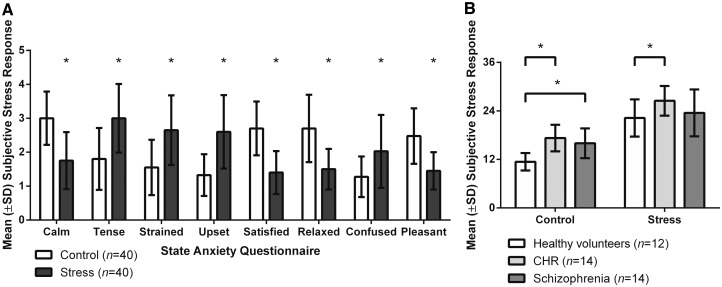
As expected, the SAQ revealed that all subjects were less calm, satisfied, relaxed and pleasant, but more tense, strained, upset and confused following the stress task than following the control task (Fig. 2A; all P < 0.0006), suggesting that the stress paradigm was effective. Total SAQ scores (Fig. 2B; positive items reversed scored) were significantly elevated in all groups following the stress as compared with the control task [effect of task: F(1,37) = 155.30, P < 0.0001, Bonferroni-corrected P < 0.0001 for all groups]. Furthermore, a group difference between SAQ scores was observed [effect of group: F(2,37) = 7.25, P = 0.0022; for post hoc results see Fig. 2 legend] with no interaction between task and group [F(2,37) = 1.47, P = 0.24].
Figure 2.
Subjective stress response following the control and stress task in healthy volunteers, CHR and schizophrenia. Stress response was assessed with the state anxiety questionnaire in individual categories for all subjects (A) and as total scores per group (B). *P ≤ 0.05 (post hoc, after Bonferroni correction).
Patients with schizophrenia showed an increase in psychotic-like experiences following the stress task (PANSS positive subscore; t = 2.60, df = 13, P = 0.022; Supplementary Fig. 1). No significant increase in the SOPS positive subscore was found in CHR (t = 1.48, df = 12, P = 0.17).
All subjects performed significantly worse in the stress task [number of errors: 34.89 ± 11.61 (healthy volunteers), 36.30 ± 9.81 (CHR) and 35.39 ± 9.99 (schizophrenia)] than in the control task [number of errors: 4.54 ± 2.13 (healthy volunteers), 5.20 ± 3.35 (CHR) and 5.20 ± 3.56 (schizophrenia); effect of task: F(1,37) = 429.56, P < 0.0001], showing that the stress task was able to adapt to the level of performance of each person and produce a tailored programmed failure within each group.
Stress-induced dopamine response in prefrontal cortex across the schizophrenia spectrum
BPND at control conditions was significantly different between groups in dlPFC [F(2,37) = 6.97, P = 0.0027] and mPFC [F(2,37) = 7.98, P = 0.0013], with patients with schizophrenia exhibiting lower BPND compared to CHR (dlPFC: Bonferroni-corrected P = 0.0019; mPFC: Bonferroni-corrected P = 0.00089) and marginally lower compared to healthy volunteers (dlPFC: Bonferroni-corrected P = 0.17; mPFC: Bonferroni-corrected P = 0.16).
11C-FLB457 ΔBPND was not different among groups in any of the PFC regions of interest investigated [dlPFC: F(2,37) = 1.07, P = 0.35; mPFC: F(2,37) = 0.54, P = 0.59]. Even if BPND in control scan had a significant effect in the model [dlPFC: F(1,36) = 5.48; P = 0.025; mPFC: F(1,36) = 5.57; P = 0.024], including it as a covariate in the analysis did not change the results [dlPFC: F(2,36) = 0.82; P = 0.45; mPFC: F(2,36) = 1.06; P = 0.36]. It is worth mentioning that ΔBPND did not differ between groups either when using the conventional calculation (Sandiego et al., 2015) (without applying any correction for injected mass 11C-FLB457).
Stress-induced dopamine response in the prefrontal cortex and salivary cortisol across the schizophrenia spectrum
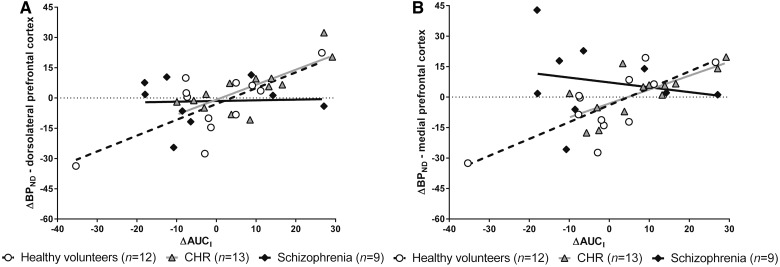
No differences in salivary levels of cortisol between groups were found, neither in AUCI during the stress scan [F(2,33) = 0.51, P = 0.60] nor in ΔAUCI [F(2,33) = 1.91, P = 0.17]. However, the relationship between ΔAUCI and 11C-FLB457 ΔBPND differed significantly between groups [omnibus test in dlPFC: F(5,28) = 5.17, P = 0.0017; mPFC F(5,28) = 3.83, P = 0.0091; interaction between group and ΔAUCI in dlPFC: F(2,28) = 3.22, P = 0.055; mPFC: F(2,28) = 3.91, P = 0.032]. The effect on ΔAUCI on 11C-FLB457 ΔBPND was significant in healthy volunteers (dlPFC: slope = 0.78, t = 3.60, Bonferroni corrected P = 0.0036; mPFC: slope = 0.83, t = 3.05, Bonferroni corrected P = 0.015) and partially in CHR (dlPFC: slope = 0.74, t = 2.91, Bonferroni corrected P = 0.021; mPFC: slope = 0.68, t = 2.13, Bonferroni corrected P = 0.13), but not in schizophrenia (dlPFC: slope = 0.030, t = 0.13, Bonferroni corrected P = 1.00; mPFC: slope = −0.24, t = −0.81, Bonferroni corrected P = 1.00) (Fig. 4), suggesting a direct relationship between dopamine release and salivary cortisol response due to the stress challenge in healthy volunteers and CHR, but not in schizophrenia.
Figure 4.
Associations between ΔBPND and ΔAUCI in healthy volunteers, CHR and schizophrenia. Lines represent the best linear model fit of the data per group (healthy volunteers: dashed line; CHR = grey line; schizophrenia = black line). The correlations were significant in healthy volunteers (A: r = 0.72, P = 0.026; B: r = 0.76, P = 0.014) and CHR (A: r = 0.76, P = 0.0075; B: r = 0.72, P = 0.018), but not in schizophrenia (A: r = 0.46, P = 1.00; B: r = 0.19, P = 1.00). P-values were Bonferroni corrected for multiple comparisons. AUCI = area under the curve; BPND = binding potential.
Associations of stress-induced dopamine and salivary cortisol response with stress/anxiety across the schizophrenia spectrum
CHR and schizophrenia subjects reported a stronger impact of stressful life events than healthy volunteers [total RLE score: F(2,37) = 6.08, P = 0.0052; CHR: Bonferroni-corrected P = 0.068, schizophrenia: Bonferroni-corrected P = 0.0045] with a higher number of stressful life events [F(2,37) = 6.24, P = 0.0046; CHR: Bonferroni-corrected P = 0.058, schizophrenia: Bonferroni-corrected P = 0.0041]. Furthermore, CHR and schizophrenia groups reported more chronic stress [TICS: F(2,37) = 13.43, P < 0.0001; CHR: Bonferroni-corrected P < 0.0001, schizophrenia: Bonferroni-corrected P = 0.00073], and displayed higher anxiety [SAS: F(2,37) = 15.22, P < 0.0001; CHR: Bonferroni-corrected P < 0.0001, schizophrenia: Bonferroni-corrected P = 0.00040; SIAS: F(2,37) = 9.65, P = 0.00042, CHR: Bonferroni-corrected P = 0.00060, schizophrenia: Bonferroni-corrected P = 0.0038] than healthy volunteers.
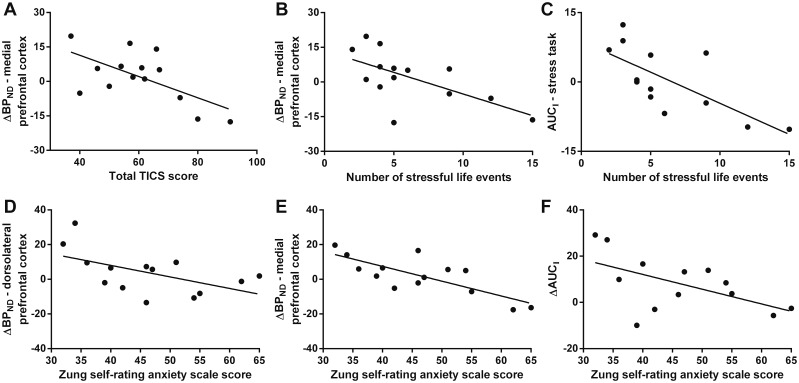
In CHR, but not in healthy volunteers or schizophrenia, chronic stress (total TICS score) was significantly negatively associated with ΔBPND specifically in mPFC (Fig. 5A; r = −0.62, P = 0.018). Furthermore, in CHR the number of stressful life events (RLE) was negatively associated with the ΔBPND in mPFC (Fig. 5B; r = −0.63, P = 0.015) as well as with cortisol levels during the stress scan (Fig. 5C; AUCI stress; r = −0.72, P = 0.0060) and marginally with ΔAUCI (r = −0.48, P = 0.093). No associations at all were observed in healthy volunteers and schizophrenia or in CHR ΔBPND in dlPFC (P > 0.05). In CHR, but not in healthy volunteers or schizophrenia, anxiety was associated with ΔBPND and ΔAUCI. In detail, SAS score was negatively associated with ΔBPND in dlPFC (Fig. 5D; r = −0.54, P = 0.045) and mPFC (Fig. 5E; r = −0.78, P = 0.0011) as well as with ΔAUCI (Fig. 5F; r = −0.56, P = 0.047), and SIAS score with ΔBPND in mPFC (r = −0.57, P = 0.034).
Figure 5.
Associations between the Trier inventory of the assessment of chronic stress (TICS), number of stressful life events or Zung SAS score and ΔBPND or ΔAUCI in CHR (n = 13–14). The line represents the best linear model fit of the data. All correlations were significant (A: r = −0.62, P = 0.018; B: r = −0.63, P = 0.015; C: r = −0.72, P = 0.0060; D: r = −0.54, P = 0.045; E: r = −0.78, P = 0.0011; F: r = −0.56, P = 0.047). P-values were not corrected for multiple comparisons. AUCI = area under the curve; BPND = binding potential.
Discussion
Our results suggest that PFC dopamine release in response to stress in healthy volunteers and CHR, but not in schizophrenia, is associated with salivary cortisol response, implying abnormal PFC stress regulation in schizophrenia. In addition, individuals at CHR with higher distress and anxiety had lower dopamine release in mPFC and salivary cortisol response following the stress challenge, associations that were absent in the schizophrenia group. A similar association between stress-induced dopamine release and cortisol response was reported in striatum for CHR (Mizrahi et al., 2014) and healthy volunteers (Pruessner et al., 2004; Mizrahi et al., 2013). This suggests an overall disrupted stress response in schizophrenia.
So far, only two studies investigated dopamine release in PFC in schizophrenia. Slifstein et al. (2015) reported lower dopamine release in dlPFC in schizophrenia following an amphetamine challenge, with no significant changes in mPFC. Similar to our study, Hernaus et al. (2015) found no change in dopamine release in mPFC in response to a similar psychosocial stress challenge in individuals with a psychotic disorder (brief psychotic episode, schizophrenia or psychosis not otherwise specified) using 18F-fallypride and a one-scan paradigm. The present study is also consistent with previous investigations reporting no association (or rather a lack of association) between dopamine release in PFC and clinical symptoms in schizophrenia (Hernaus et al., 2015; Slifstein et al., 2015). Differences between studies include the challenge conditions (amphetamine versus psychosocial stress), tracers (18F-fallypride versus 11C-FLB457) and clinical populations.
Stress-induced extrastriatal dopamine response has not been studied in CHR, but Lataster et al. (2014) reported comparable dopamine release in first-degree relatives of patients with schizophrenia and control subjects in ventromedial PFC.
A model proposed by Grace and others can explain our results. Acute stress induces an increased population activity of the ventral tegmental area (VTA) dopamine neurons (Valenti et al., 2011) leading to an increased striatal dopamine release (Rougé-Pont et al., 1993). One major regulator of the mesolimbic dopaminergic system is the mPFC, which makes direct and indirect connections to the hippocampus and amygdala (Belujon and Grace, 2015), as well as directly to the VTA (Sesack and Carr, 2002; Gabbott et al., 2005). Inhibition of the infralimbic subdivision of the mPFC was sufficient to increase the VTA dopamine neuron activity and this effect was modulated by the hippocampus (ventral subiculum) (Patton et al., 2013). Chronic stress, however, has been shown to lead to loss in dendritic material in the mPFC (Holmes and Wellman, 2009) and hippocampus (McEwen et al., 2016). This suggests that chronic stress weakens the structures that provide negative feedback for the stress response (Arnsten, 2009). This is also in line with structural changes reported in schizophrenia (Glausier and Lewis, 2013; Haijma et al., 2013). Recently Gomes and Grace (2016) observed that a mPFC lesion with ibotenic acid combined with stress during adolescence led to a long-lasting increase of VTA dopamine neuron activity accompanied by higher striatal dopamine release (measured by increased amphetamine-induced locomotor activity) and anxiety. Interestingly, we could not statistically observe an overall decreased PFC dopamine release due to the stress challenge in schizophrenia, even though the patients reported increased chronic stress and high number of past stressful events. As there is strong evidence that hippocampal hyperactivity underlies the dopamine hyperfunction in striatal regions (Lodge and Grace, 2011), it is possible that a deficient regulation of the hippocampus, rather than PFC, leads to the stress-induced increased dopamine release seen in striatal areas in schizophrenia and CHR (Mizrahi et al., 2012) and in substantia nigra only in schizophrenia (Tseng et al., 2017). Another explanation for the lack of significant difference in stress-induced PFC dopamine release among groups in our study might be a potential compensatory mechanism by recruiting more cortical dopamine, since the PFC has direct and indirect connections to the dopamine cell bodies in substantia nigra and VTA, in order to regulate its dopamine output (Arnsten, 2009). Chronic stress further compromises the plasticity in the hippocampus–PFC pathway (Rocher et al., 2004; Cerqueira et al., 2007; Garcia et al., 2008) and previous data suggest that the hippocampus-PFC pathway is compromised in patients with schizophrenia (Godsil et al., 2013). This could explain why we observed dissociation between endocrine and PFC dopamine-stress response in schizophrenia but not in CHR (or healthy volunteers). Interestingly, preclinical data using the neonatal ventral hippocampal lesion model, a validated schizophrenia model (Tseng et al., 2009), support our findings. While acute stress increased the nucleus accumbens dopamine release stronger in neonatal ventral hippocampal lesioned rats than sham-lesioned rats, there was no difference in stress-induced dopamine increase in frontal cortex between both groups. Furthermore, although the corticosterone response to the acute stressor was associated with frontal cortex dopamine release in sham-lesioned rats, this association was absent in neonatal ventral hippocampal lesion rats (Chrapusta et al., 2003), similar to the present study. We acknowledge that the present study is only a starting point in understanding how the complex dopamine-stress regulation is compromised in schizophrenia and its putative prodrome.
Accurate quantification of 11C-FLB457 is challenging as the BPND and ΔBPND values are rather small [i.e. smaller than those obtained using 11C-(+)-PHNO in striatal regions]. Although some studies argued against the use of the SRTM for quantification of 11C-FLB457 binding in view of the presence of specific binding in cerebellum and change in cerebellum distribution volume (VT) by the D2 partial agonist aripiprazole (Narendran et al., 2011a), others supported its suitability with cerebellum as reference tissue (Olsson et al., 1999; Ito et al., 2001; Olsson and Farde, 2001). Cerebellum VT and VT/fp were not observed to change pre- and post-amphetamine challenge, supporting the use of cerebellum as reference tissue in challenge-based experiments only (Sandiego et al., 2015). Moreover, SRTM was more reliable than the two-tissue compartment model in detecting ΔBPND following amphetamine challenge and had lower relative standard error. Additionally, while SRTM may lead to underestimation of BPND compared to arterial input-based models (Innis et al., 2007), this underestimation applies to both scan sessions, control and stress, such that the potential bias cancels out when calculating the ΔBPND.
The current study has limitations, many of which are inherent to neurochemical PET studies, particularly when investigating cortical dopamine. First, the resolution of the PET scanner does not allow differentiation of histological subdivisions of mPFC and dlPFC (i.e. ventromedial and dorsomedial PFC) and nearby structures. Second, the mass of 11C-FLB457 may not be at tracer dose (Narendran et al., 2011b), and hence we present our results using a novel approach to account for this issue. Third, the potential effect of specific binding of 11C-FLB457 in cerebellum may not be negligible, so we compared the cerebellar tracer uptake between scans, and showed nearly complete overlap in tracer uptake between both scans (Supplementary Fig. 2). Fourth, since our control condition may be expected to recruit dopamine activity (Egerton et al., 2009), it does not permit estimation of a true baseline D2/3 receptor availability but serves as an excellent control for the cognitive part of the stress protocol. Fifth, although our sample size provides sufficient power to detect a group effect in stress-induced dopamine release (n = 40) and its interaction with salivary cortisol response, the number of participants within each diagnostic group is small and we cannot correct for the number of exploratory correlations with questionnaires. This, however, does not change our general conclusion. Sixth, although all patients were antipsychotic-free, only eight were antipsychotic-naïve. Exploratory comparisons of dopamine release in antipsychotic-free and -naïve patients revealed no significant difference (Supplementary Fig. 3). However, since the sample size is small, an effect of past exposure to antipsychotics cannot be completely ruled out. Overall, while we acknowledge the limitations of both task and radioligand, these would not have been possible to overcome as (i) the stress task we used is the only validated one in PET imaging studies; and (ii) arterial sampling was impossible as all participants were doing the task with their hands while lying in the scanner. Thus, to date, there is no better methodology available to examine PFC dopamine response to a stress challenge in humans.
Conclusion
In summary, our results suggest that PFC dopamine release in response to stress in healthy volunteers and CHR, but not in schizophrenia, was associated with salivary cortisol response. These findings provide first direct evidence of a disrupted cortical dopamine-stress regulation in schizophrenia.
Supplementary Material
Acknowledgements
We thank the staff of the Centre for Addiction and Mental Health (CAMH) Research Imaging Centre (Alvina Ng, Laura Nguyen and Peter Bloomfield) and Focus on Youth Psychosis Prevention (FYPP) Clinic for their technical assistance, as well as Efren Navas. The authors declare no conflict of interest in relation to this work.
Funding
This work is supported by the operating grant ‘Stress-induced dopamine release in subjects at clinical high risk for psychosis: an [11C]-FLB457 PET study’ from the Canadian Institutes of Health Research (CIHR).
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- CHR
clinical high risk for psychosis
- dl/mPFC
dorsolateral/medial prefrontal cortex
- SRTM
Simplified Reference Tissue Model
References
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 2009; 10: 410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Regulation of dopamine system responsivity and its adaptive and pathological response to stress. Proc Biol Sci 2015; 282. doi: 10.1098/rspb.2014.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Renner KJ, Forster GL, Watt MJ. Adolescent social defeat alters neural, endocrine and behavioral responses to amphetamine in adult male rats. Brain Res 2010; 1352: 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro M, Elias P, Martinelli C Jr, Antonini S, Santiago L, Moreira A. Salivary cortisol as a tool for physiological studies and diagnostic strategies. Braz J Med Biol Res 2000; 33: 1171–5. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci 2007; 27: 2781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrapusta SJ, Egan MF, Wyatt RJ, Weinberger DR, Lipska BK. Neonatal ventral hippocampal damage modifies serum corticosterone and dopamine release responses to acute footshock in adult Sprague-Dawley rats. Synapse 2003; 47: 270–7. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC. The montreal imaging stress task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J Psychiatry Neurosci 2005; 30: 319–25. [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Mehta MA, Montgomery AJ, Lappin JM, Howes OD, Reeves SJ, et al. The dopaminergic basis of human behaviors: a review of molecular imaging studies. Neurosci Biobehav Rev 2009; 33: 1109–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JB. Structured clinical interview for DSM-IV-TR Axis I disorders, research version, patient edition. (SCID-I/P). New York, NY: New York State Psychiatric Institute; 2002. [Google Scholar]
- Frankle WG, Mason NS, Rabiner EA, Ridler K, May MA, Asmonga D, et al. No effect of dopamine depletion on the binding of the high-affinity D 2/3 radiotracer [11C]FLB 457 in the human cortex. Synapse 2010; 64: 879–85. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 2005; 492: 145–77. [DOI] [PubMed] [Google Scholar]
- Gallezot JD, Planeta B, Nabulsi N, Palumbo D, Li X, Liu J, et al. Determination of receptor occupancy in the presence of mass dose: [11C]GSK189254 PET imaging of histamine H3 receptor occupancy by PF-03654746. J Cereb Blood Flow Metab 2017; 37: 1095–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R, Spennato G, Nilsson-Todd L, Moreau JL, Deschaux O. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiol Learn Mem 2008; 89: 560–6. [DOI] [PubMed] [Google Scholar]
- Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience 2013; 251: 90–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsil BP, Kiss JP, Spedding M, Jay TM. The hippocampal-prefrontal pathway: the weak link in psychiatric disorders? Eur Neuropsychopharmacol 2013; 23: 1165–81. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron 1995; 14: 477–85. [DOI] [PubMed] [Google Scholar]
- Gomes FV, Grace AA. Prefrontal cortex dysfunction increases susceptibility to schizophrenia-like changes induced by adolescent stress exposure. Schizophr Bull 2016; 43: 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Rincon-Cortes M, Grace AA. Adolescence as a period of vulnerability and intervention in schizophrenia: insights from the MAM model. Neurosci Biobehav Rev 2016; 70: 260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res 2004; 72: 41–51. [DOI] [PubMed] [Google Scholar]
- Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull 2013; 39: 1129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernaus D, Collip D, Kasanova Z, Winz O, Heinzel A, van Amelsvoort T, et al. No evidence for attenuated stress-induced extrastriatal dopamine signaling in psychotic disorder. Transl Psychiatry 2015; 5: e547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev 2009; 33: 773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, et al. Extinction memory is impaired in schizophrenia. Biol Psychiatry 2009; 65: 455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007; 27: 1533–9. [DOI] [PubMed] [Google Scholar]
- Ito H, Sudo Y, Suhara T, Okubo Y, Halldin C, Farde L. Error analysis for quantification of [(11)C]FLB 457 binding to extrastriatal D(2) dopamine receptors in the human brain. Neuroimage 2001; 13: 531–9. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13: 261–76. [DOI] [PubMed] [Google Scholar]
- Ko JH, Ptito A, Monchi O, Cho SS, Van Eimeren T, Pellecchia G, et al. Increased dopamine release in the right anterior cingulate cortex during the performance of a sorting task: a [11C]FLB 457 PET study. Neuroimage 2009; 46: 516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage 1996; 4 (3 Pt 1): 153–8. [DOI] [PubMed] [Google Scholar]
- Laruelle M. The role of endogenous sensitization in the pathophysiology of schizophrenia: implications from recent brain imaging studies. Brain Res Brain Res Rev 2000; 31: 371–84. [DOI] [PubMed] [Google Scholar]
- Lataster J, Collip D, Ceccarini J, Hernaus D, Haas D, Booij L, et al. Familial liability to psychosis is associated with attenuated dopamine stress signaling in ventromedial prefrontal cortex. Schizophr Bull 2014; 40: 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P, et al. City living and urban upbringing affect neural social stress processing in humans. Nature 2011; 474: 498–501. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci 2011; 32: 507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, Carruthers NI, Letavic MA, Sands S, Jiang X, Shea C, et al. Blockade of the brain histamine H3 receptor by JNJ-39220675: preclinical PET studies with [11C]GSK189254 in anesthetized baboon. Psychopharmacology 2012; 223: 447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SW, Cervenka S, Farde L, Nyberg L, Backman L. Extrastriatal dopamine D2 receptor binding modulates intraindividual variability in episodic recognition and executive functioning. Neuropsychologia 2009; 47: 2299–304. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 2016; 41: 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001; 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull 2003; 29: 703–15. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Addington J, Rusjan PM, Suridjan I, Ng A, Boileau I, et al. Increased stress-induced dopamine release in psychosis. Biol Psychiatry 2012; 71: 561–7. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Kenk M, Suridjan I, Boileau I, George TP, McKenzie K, et al. Stress-induced dopamine response in subjects at clinical high risk for schizophrenia with and without concurrent cannabis use. Neuropsychopharmacology 2014; 39: 1479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi R, Rusjan P, Agid O, Graff A, Mamo DC, Zipursky RB, et al. Adverse subjective experience with antipsychotics and its relationship to striatal and extrastriatal D2 receptors: a PET study in schizophrenia. Am J Psychiatry 2007; 164: 630–7. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Suridjan I, Kenk M, George TP, Wilson A, Houle S, et al. Dopamine response to psychosocial stress in chronic cannabis users: a PET study with [11C]-(+)-PHNO. Neuropsychopharmacology 2013; 38: 673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery AJ, Asselin MC, Farde L, Grasby PM. Measurement of methylphenidate-induced change in extrastriatal dopamine concentration using [11C]FLB 457 PET. J Cereb Blood Flow Metab 2007; 27: 369–77. [DOI] [PubMed] [Google Scholar]
- Narendran R, Frankle WG, Mason NS, Rabiner EA, Gunn RN, Searle GE, et al. Positron emission tomography imaging of amphetamine-induced dopamine release in the human cortex: a comparative evaluation of the high affinity dopamine D2/3 radiotracers [11C]FLB 457 and [11C]fallypride. Synapse 2009; 63: 447–61. [DOI] [PubMed] [Google Scholar]
- Narendran R, Jedema HP, Lopresti BJ, Mason NS, Gurnsey K, Ruszkiewicz J, et al. Imaging dopamine transmission in the frontal cortex: a simultaneous microdialysis and [11C]FLB 457 PET study. Mol Psychiatry 2014; 19: 302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Mason NS, Chen CM, Himes M, Keating P, May MA, et al. Evaluation of dopamine D2/3 specific binding in the cerebellum for the positron emission tomography radiotracer [11C]FLB 457: implications for measuring cortical dopamine release. Synapse 2011a; 65: 991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Mason NS, May MA, Chen CM, Kendro S, Ridler K, et al. PET imaging of dopamine D2/3 receptors in the human cortex with [11C]FLB 457: reproducibility studies. Synapse 2011b; 65: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson H, Farde L. Potentials and pitfalls using high affinity radioligands in PET and SPET determinations on regional drug induced D2 receptor occupancy–a simulation study based on experimental data. Neuroimage 2001; 14: 936–45. [DOI] [PubMed] [Google Scholar]
- Olsson H, Halldin C, Swahn CG, Farde L. Quantification of [11C]FLB 457 binding to extrastriatal dopamine receptors in the human brain. J Cereb Blood Flow Metab 1999; 19: 1164–73. [DOI] [PubMed] [Google Scholar]
- Öngür D, Price J. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 2000; 10: 206–19. [DOI] [PubMed] [Google Scholar]
- Patton MH, Bizup BT, Grace AA. The infralimbic cortex bidirectionally modulates mesolimbic dopamine neuron activity via distinct neural pathways. J Neurosci 2013; 33: 16865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci 2004; 24: 2825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003; 28: 916–31. [DOI] [PubMed] [Google Scholar]
- Rocher C, Spedding M, Munoz C, Jay TM. Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cereb Cortex 2004; 14: 224–9. [DOI] [PubMed] [Google Scholar]
- Rougé-Pont F, Piazza PV, Kharouby M, Le Moal M, Simon H. Higher and longer stress-induced increase in dopamine concentrations in the nucleus accumbens of animals predisposed to amphetamine self-administration. A microdialysis study. Brain Res 1993; 602: 169–74. [DOI] [PubMed] [Google Scholar]
- Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F, et al. An automated method for the extraction of regional data from PET images. Psychiatry Res 2006; 147: 79–89. [DOI] [PubMed] [Google Scholar]
- Sandiego CM, Gallezot JD, Lim K, Ropchan J, Lin SF, Gao H, et al. Reference region modeling approaches for amphetamine challenge studies with [11C]FLB 457 and PET. J Cereb Blood Flow Metab 2015; 35: 623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Carr DB. Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia. Physiol Behav 2002; 77: 513–17. [DOI] [PubMed] [Google Scholar]
- Slifstein M, van de Giessen E, Van Snellenberg J, Thompson JL, Narendran R, Gil R, et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry 2015; 72: 316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorusch RL, Lushene RE, Vagg PR, Jacobs GA. State and trait anxiety inventory for adults. Redwood City, CA: Mind Garden; 1977. [Google Scholar]
- Tseng HH, Watts JJ, Kiang M, Suridjan I, Wilson AA, Houle S, et al. Nigral stress-induced dopamine release in clinical high risk and antipsychotic-naïve schizophrenia. Schizophr Bull 2017; 44: 542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res 2009; 204: 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti O, Lodge DJ, Grace AA. Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J Neurosci 2011; 31: 4280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature 2010; 468: 203–12. [DOI] [PubMed] [Google Scholar]
- Vandehey NT, Moirano JM, Converse AK, Holden JE, Mukherjee J, Murali D, et al. High-affinity dopamine D2/D3 PET radioligands [18F]-fallypride and [11C]-FLB457: a comparison of kinetics in extrastriatal regions using a multiple-injection protocol. J Cereb Blood Flow Metab 2010; 30: 994–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev 1997; 104: 667–85. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Burke AR, Renner KJ, Forster GL. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behav Neurosci 2009; 123: 564–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JJ, Chohan MO, Slifstein M, Kegeles LS, Moore H, Abi-Dargham A. Pathway-specific dopamine abnormalities in schizophrenia. Biol Psychiatry 2017; 81: 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.