Abstract
Links between individual differences in risk processing and high-risk behaviors such as binge-drinking have long been the focus of active research. However, investigations in this area almost exclusively utilize decision-making focused paradigms. This emphasis makes it difficult to assess links between risk behaviors and raw risk reactivity independent of decision and feedback processes. A deeper understanding of this association has the potential to shed light on the role of risk reactivity in high-risk behavior susceptibility. To contribute toward this aim, this study utilizes a popular risk-taking game, the crocodile dentist, to assess links between individual differences in decision-free risk-reactivity and reported binge-drinking frequency levels. In this task, participants engage in a series of decision-free escalating risk responses. Risk-reactivity was assessed by measuring late positive potential responses toward risk-taking action initiation cues using high-density 256-Channel EEG. The results indicate that, after controlling for overall alcohol consumption frequency, higher rates of reported binge-drinking are associated with both increased general risk-taking responsivity and increased risk-reactivity escalation as a function of risk level. These findings highlight intriguing links between risk reactivity and binge-drinking frequency, making key contributions in the areas of risk-taking and affective science.
Keywords: risk-taking, risk-reactivity, alcohol, binge-drinking, EEG, ERP
Introduction
Binge-drinking is a public health issue associated with a wide array of health, economic and social consequences. Frequently defined as the number of occasions in the past month involving the consumption of five or more alcoholic drinks on a single occasion (Kann et al., 2014), binge-drinking among college students in the United States has declined relative to peak levels in the 1990s (Johnston et al., 2017). Nonetheless, binge-drinking rates continue to be of concern with 32.3% of US college students in 2016 reporting binge-drinking at least once in the past month, and 12% reporting consumption of 10 or more drinks on those occasions (Johnston et al., 2017).
Individual differences in binge-drinking frequency are often associated with elevated activity in neural regions and functional networks associated with affective and reward reactivity (Xiao et al., 2013; Whelan et al., 2014). Given these findings, it is unsurprising that binge-drinking is linked with heightened social anxiety (Biolcati et al., 2016; Goodman et al., 2018) and sensation-seeking behavior (Adan et al., 2017; Doumas et al., 2017).
With regard to risk-taking responses, sensation-seeking, in particular, is positively associated with increased risk-taking (Adan et al., 2017), elevated approach responses toward intense stimuli (Zuckerman, 2005; Joseph et al., 2009), as well as blunted responses to stress (Roberti, 2004) and threat-related cues (Lissek et al., 2005). Conversely, social anxiety is linked with elevated stress reactivity (Zorn et al., 2017) while, nonetheless, still being associated with increased risk-taking (Reynolds et al., 2013) and reward-sensitivity (Richards et al., 2015) under high-stress conditions.
Given the links stress and risk reactivity have with sensation-seeking and social anxiety, it is significant to note that prior work assessing risk reactivity among binge-drinkers (Johnson et al., 2008; Xiao et al., 2009, 2013; Carbia et al., 2017) largely utilize relatively unstressful decision-making focused paradigms. This focus on paradigms with with strong decision-making elements has arguably led to a strong emphasis on the role of executive processes in binge-drinking behavior (Johnson et al., 2008; Xiao et al., 2009, 2013; Carbia et al., 2017). As highlighted by Lannoy et al. (2014), this paradigmatic focus has left the role of less reflective and more automatic affective risk reactivity in binge drinkers relatively underexplored.
Some of the most widely used paradigms in this area include the Columbia Card Task (Figner et al., 2009), the Iowa Gambling Task (Bechara et al., 1994) and the Balloon Analogue Risk Task (Lejuez et al., 2002). In the Columbia Card Task (Figner et al., 2009) participants draw cards from four decks, half with advantageous and half with disadvantageous odds, with the difference between the number of overall disadvantageous and advantageous selections being the primary measure of interest. In the Iowa Gambling Task (Bechara et al., 1994), participants turn over cards placed face down in a grid either one-at-a-time or in predeclared numbers, earning a number of points equal to the number of turned-up gain cards so long as no loss cards are also turned-up in the process. Finally, in the Balloon Analogue Risk Task (Lejuez et al., 2002) participants are presented with a series of virtual balloons which they proceed to inflate, with each inflation earning a number of points. This continues until the participant either decides to cash out their winnings on that trial or the balloon explodes, resulting in a loss of all earnings on that trial.
Critically, all of these tasks involve decision-making by the participant to varying degrees. While some of these tasks have varients designed to tap into affective risk-related processes more strongly, such as the ‘hot’ version of the Columbia Card Task (Figner et al., 2009), these varients are nonetheless intended to focus on affective decision-making as opposed to individual differences in reactivity toward escalating risk, independent of higher-level decision-making processes. Thus building on the focus of these classic paradigms, prior work in this area has primarily shed light on links between executive-functioning and binge-drinking, typically finding that a deficit in various aspects of executive functioning is associated with increased binge-drinking susceptibility (Johnson et al., 2008; Xiao et al., 2009; though see also Lannoy et al., 2017).
While these findings provide valuable insights into the dynamics of risk decision-making among binge-drinkers, the simultaneous engagement of evaluative, response-initiation and outcome-anticipation related processes in the paradigms utilized in prior investigations make it difficult to isolate independent links between these processes and binge-drinking susceptibility. In addition to this, given the tendency toward higher levels of boredom proneness among binge drinkers (Biolcati et al., 2016), the use of paradigms lacking intrinsic engagement value may also interfere with the simulation of risk-taking dynamics involved in real-world binge-drinking, which often occurs in relatively stimulating social environments. Finally, the frequent use of financial incentives in prior work also potentially introduces monetary risk-related influences that may not always be associated with the differences in risk-taking behavior linked with binge-drinking.
Of particular interest to the present study, interactions between the abovementioned factors make it difficult to assess the role of risk-related reactivity in binge-drinking behavior. A useful framework to build on in assessing this issue is the “hot-cold” dual-system view of risk-taking. Models drawing on this framework posit risk-taking to be the product of two systems, a phylogenetically older, more affective socioemotional system and a phylogenetically younger, more deliberate one focused on controlled executive processes (Steinberg, 2008). While not without limitations (Gladwin and Figner, 2015), there is evidence to suggest that dual-systems models continue to have significant value for understanding risk-taking behavior (Shulman et al., 2016). With this in mind, several current alcohol use models extend the hot/cold framework to binge-drinking, proposing that the behavior may be driven by imbalances arising from a hyperactive “hot” affective response system combined with dysfunctional “cold” executive control processes (Lannoy et al., 2014). Drawing on these frameworks and psychophysiological models of sensation-seeking behavior (Zuckerman, 2005), we propose that elevated responsivity to risk-taking under high arousal conditions plays a key role in driving binge-drinking behavior. However, the absence of prior work focused on assessing reactivity to risk-taking among binge drinkers independent of decision-making makes it difficult to draw firm conclusions regarding the link between individual differences in this type of responsivity and binge-drinking behavior.
To address this gap in the literature regarding the role of raw risk-taking reactivity in binge-drinking, this investigation utilizes a simple aversive risk-taking game to assess differences in risk reactivity as a function of binge-drinking frequency. Specifically, this investigation assesses differences in anticipatory risk-taking reactivity using the popular crocodile dentist game (see ‘Method’ section for details) created by Robert Fuhrer (Fetherston, 1994). By administering the game in a manner which removes its decision-making elements, the crocodile task becomes a means by which raw risk-taking reactivity can be potentially isolated from decision-making processes. Furthermore, given the game’s structure, the risk of experiencing an adverse outcome escalates as participants proceed through the game. With these characteristics, the crocodile task provides an intrinsically engaging task with minimal levels of higher-level decision-making and a structure inherently suited for assessing individual differences in risk reactivity as a function of escalating situational risk.
To decompose and isolate the neural responses associated with risk-reactivity as a function of risk level, this study utilizes high-density EEG. The high temporal-resolution of this imaging modality makes it particularly suited for decomposing and distinguishing between responses within temporally contiguous risk processing stages (Kiat et al., 2016). By leveraging this capability, this study aims to test the hypothesis that binge-drinkers will exhibit increased anticipatory reactivity toward risk-taking in the absence of decision-making relative to non-binge drinkers. The target index component of interest is the late positive potential (LPP), a posterior ERP component that typically emerges 300–400 ms post-stimulus onset and often lasts for the duration of the stimulus presentation (Cuthbert et al., 2000). Activity in this range has frequently been associated with elevated levels of reactivity toward motivationally relevant stimuli both directly (Cuthbert et al., 2000; Schupp et al., 2004) and from an anticipatory perspective (Howsley and Levita, 2017), making it an ideal target response for this investigation.
Materials and methods
Power estimation
The target sample size was estimated using empirical power simulation in SAS 9.3 with Late Positivity component variability estimates (SD = 3.05 μV) from a prior investigation conducted by the researchers (Kiat et al., 2017, 2018). With these parameters, 10 000 datasets were simulated for seven potential target sample sizes (10–40 in increments of 5) with a main effect of risk level (i.e. high vs low risk) effect size of +1.5 μV (Effect SE = 1.5 μV) and an interaction between binge-drinking and risk level (i.e. relative increase in the observed main effect among binge drinkers effect size) of the the same magnitude, both reasonable target effect sizes for event-related potential work. The results of this analysis indicated that based on these parameters, a sample size of 25 participants was sufficient to provide 95% power to detect an overall main effect of risk level on this task and 75% power to detect an interaction between risk and binge-drinking frequency levels.
Participants
Twenty-six subjects (20 females, 6 males, mean age = 20.00, SD =1.74, range 18–24) were recruited from the research subject pool at a large Midwestern University for this study. All experimental procedures were approved by the university institutional review board (IRB#20121212948EP) with all subjects providing informed consent and receiving course credit for their participation. None of the participants indicated having had prior experience with the task used in this study.
Materials and tasks
Alcoholic consumption frequency self-report measures
Individual differences in binge-drinking susceptibility was assessed using two items from the 2013 Centers for Disease Control and Prevention (CDC) Youth Risk Behavior Survey (Kann et al., 2014), a national school-based survey administered yearly by the CDC. The first item, (General Drinking Frequency) asked participants to indicate the number of days in the past month in which they had at least one alcoholic drink. The second (binge-drinking frequency) asked participants to indicate the number of days in the past month in which they had more than five alcoholic drinks in a row within a few hours. Both items were multiple choice responses with seven response options (0, 1–2, 3–5, 6–9, 10–19, 20–29 and 30 days). Each item was scored on an integer scale ranging from 1 to 7, corresponding to the selected response, with higher scores indicating higher drinking frequencies.
Crocodile dentist game
This task (shown in Figure 1) consists of a plastic prop in the shape of a crocodile with ten teeth that lock into place upon being pressed down by the player. One of the teeth (randomly selected by a hidden gear mechanism on each trial) would, upon being depressed, cause the jaws to snap shut, stopping just short of making contact with the player’s finger. Participants were instructed to engage with this prop in the following fashion. At the start of the experiment, they were shown the prop and told that the teeth of the prop were numbered 1 through 10 which to half of the participants started on the right and the other half on the left. At the start of each trial, they were then asked to select the lowest numbered undepressed tooth upon hearing a 500 Hz tone, placing their right index finger on the identified tooth and verbally indicating they had done so by stating the phrase “Ready.” Upon receiving this acknowledgment, the researcher triggered the experimental program that played a second 500 Hz tone 2000 ms after the researcher’s response. Participants’ were instructed to minimize their motion as well as blinking upon hearing this tone and prepare to press down on the tooth. Two thousand milliseconds after the second tone, a third 1000 Hz tone was played to which participants were instructed to press down the tooth upon hearing. This procedure was repeated until the participant triggered the prop to close at which point the prop was reopened for the start of the next round. Participants engaged in as many trials of the task as could be completed in the 30-min task runtime (mean number of rounds = 24.15, SD = 3.63, mean number of trials = 94.04, SD = 11.38).
Fig. 1.
Crocodile dentist task prop.
Data collection procedure
Participants completed the Edinburgh Handedness Inventory (Oldfield, 1971), with 24 participants indicating right, and two left, handed dominance. All participants then completed the aversive risk-taking task with the experimenter monitoring the real-time EEG waveforms for excessive data artifacts. After completing the task, each participant completed the alcoholic consumption frequency self-report measures.
Risk response classification
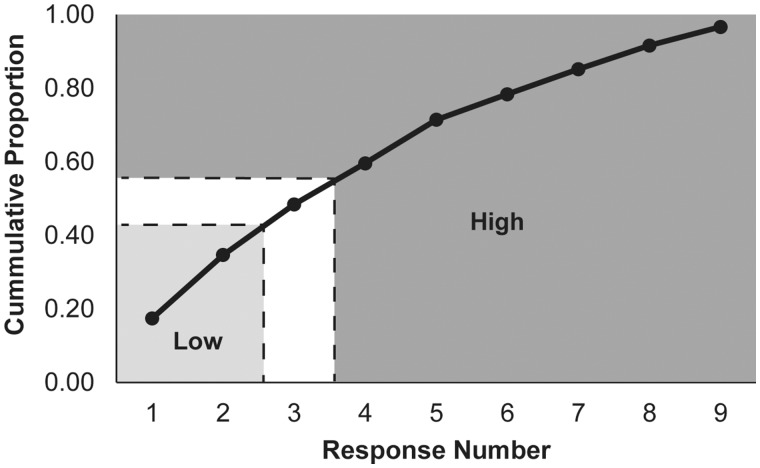
Risk-taking responses on the crocodile task were first categorized by response number (i.e. first tooth press, second tooth press, etc.), with trials resulting in the jaws of the crocodile closing being discarded. The categorized trials were then classified into lower and higher risk trials by determining the median response number on the task. This point was determined by plotting the frequency curve of the scored responses to identify the 50% cumulative proportion mark. As shown in Figure 2, that point was at the third response. With this response as the dividing point, the first two responses (i.e. responses one and two) were classified as lower risk trials whereas responses four through nine were classified as higher risk trials. An overall total of 849 low (participant mean = 32.65, SD = 4.82) and 968 higher (participant mean = 25.85, SD = 6.77) risk responses were recorded.
Fig. 2.
Cumulative proportion of crocodile trial response numbers.
EEG data acquisition
Data were recorded using a 256 high-density AgCl electrode Hydrocel Geodesic Sensor Net connected to a high-input impedance NetAmps 300 amplifier running Netstation version 4.4.2. Participants were seated with the task prop in front of them on a table at approximately mid-chest height. Recordings were collected using a vertex sensor (Cz), later referenced to an average reference. Electrode impedances were kept below 45 kΩ, a level appropriate for the system that had been designed to accommodate high impedances. The EEG data were digitized at 1000 Hz from the DC to 500 Hz range using a 24-bit analog-to-digital converter.
EEG pre-processing
The ongoing EEG data were digitally filtered using a 0.1 Hz first order high-pass and a 30 Hz (−40 dB stop-band attenuation, 2.00 Hz roll-off) finite impulse response low-pass filter. The continuous data were then downsampled to 250 Hz and segmented to onset of the 1000 Hz tone which signaled participants to initiate their response, beginning 200 ms before onset and continuous for 1000 ms onwards.
All segments were then baseline corrected using the 200 ms prestimulus average. Ocular artifacts were reduced via decomposing the data into ICA components and removing components which correlated highly (>0.80) with a blink template created via averaging 200 blinks from open eye resting state data recorded from 40 subjects from a separate study (each subject contributing five blinks) using an identical system setup.
After the artifact reduction process, bad channels were identified and interpolated in the ERP PCA Toolkit version 2.54 (Dien, 2010). Bad channels were identified across the entire session via poor overall correlations (r <0.50) between neighboring channels, and within each segment via either unusually high differences between an electrode’s average voltage and that of their neighbors (>30 µv) or extreme voltage differences within the electrode (>100 µv min to max) within an 80 ms moving average window. A channel was also marked as bad for the entire session if more than 20% of its segments were classified as bad. All identified bad channels were replaced using whole head spline interpolation. After bad channels were identified and interpolated, trials with more than 10% of their channels interpolated were removed from the analysis set. Retained trials had an average of 6.02 (SD = 2.77) interpolated channels, 2.4% out of the total 256.
At the end of this process, each subject retained an average of 31.92 low (SD = 5.03) and 25.62 high (SD = 6.48) risk trials. These trial numbers have been shown to be more than sufficient for reliable measurement of the LPP component (Moran et al., 2013).
ERP component extraction processing
ERP components were quantified using temporal-spatial PCA in the ERP PCA Toolkit. First, a temporal PCA was conducted using all time points from each participant’s averaged ERP as variables, and condition and recording sites as observations. Promax rotation was used to extract temporal components based on a 95% variance-accounted-for criterion. The spatial distribution of these factors was then reduced via spatial ICA using the Infomax algorithm with a parallel analysis criterion, using all recording sites as variables and considering participants, conditions, and temporal component scores as observations. The covariance matrix and Kaiser normalization were used for both the temporal PCA and spatial ICA steps. To facilitate interpretation, the waveforms for each temporal-spatial component were converted to microvolts by multiplying the factor pattern matrix with the standard deviations.
Using this procedure, the temporal-spatial variance in the response ERPs was reduced to 60 temporal and 6 spatial components. Temporal-spatial components that accounted for at least 50 ms of the full ERP variance with non-artifactual topographies were then identified. Only one temporal-spatial component, the predicted LPP component spanning (temporal loadings > 0.6) 592–736 ms (peak latency = 668 ms), met the inclusion criterion (see Figure 3).
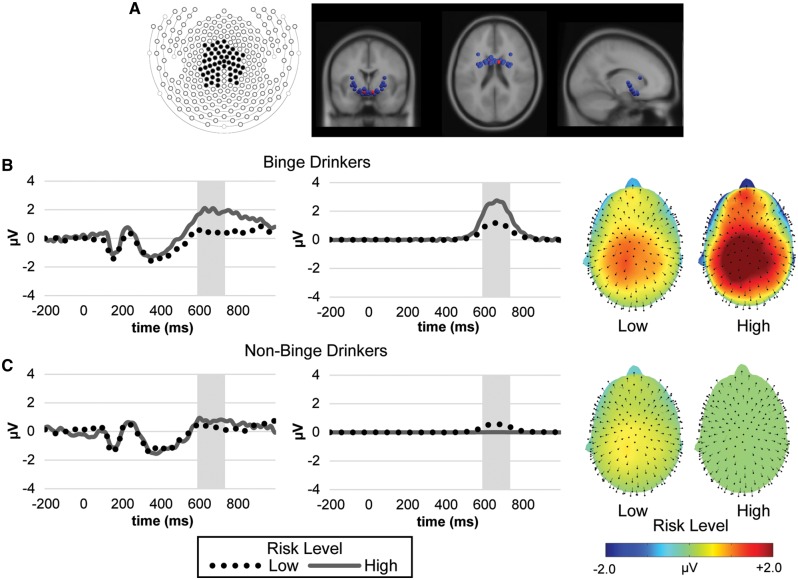
Fig. 3.
(A) High-loading (>0.60 shaded in black) electrode map and jack-knifed dipole solution for the LPP component. (B to C: from left to right) Grand average ERP waveforms for high LPP loading electrodes, LPP component waveforms (high loading [>0.60] time points shaded in gray) and LPP scalp topographies by risk-taking level for (B) binge and (C) non-binge drinkers.
Source localization of the neural sources of this component was conducted by specifying a pair of hemispheric dipoles (mirrored in position but not orientation) using a four-shell standard Boundary Element Method volume conduction model. Stability of the solution was then assessed with a jack-knife technique where the spatial PCA solution was recomputed 24 times, each with one of the participants left out. The spatial factor best corresponding to that of the original in scalp topography was then identified and source localized for each jack-knifed solution. As indicated by the red marker in Figure 3A, the source localization solution identified the subcallosal gyrus (Talairach coordinates: X = −7, Y = 3, Z = −11) as the most likely neural generator source for this component with the solution exhibiting a good level of fit (residual variance = 7.91%) with the localization solution being relatively stable across subsamples. A jackknife-based assessment of the relative strength of the hemispheric dipoles also showed that the relative amplitude of dipoles across the two hemispheres was relatively equivalent t(9) = 0.227, P = 0.826.
Analytic approach
All analysis models were estimated using maximum likelihood estimated general linear mixed models in SAS version 9.3 with between-within degrees of freedom. Unstructured covariance matrixes were used to model the covariances of risk-level, a within-subjects’ factor.
Results
Behavioral measure associations
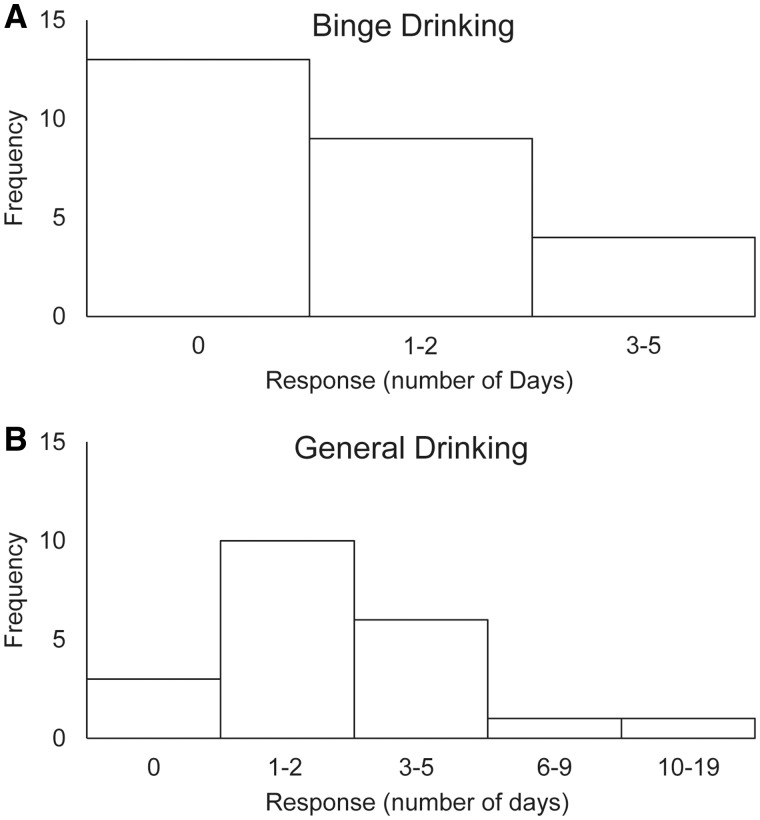
As shown in Figure 4, 50% (N = 13) of the participants reporting no prior binge-drinking instances in the past 30 days with 35% (N = 9) indicating 1–2 days and 15% (N = 4) 3–5 days involving at least one binge-drinking session. General drinking frequency responses had 11, 38, 23, 3 and 3% of participants reporting 0, 1–2, 3–5, 6–9 and 10–19 days, respectively, in the past 30 days in which they had at least one alcoholic drink.
Fig. 4.
Frequency distributions for (A) binge and (B) general drinking report measures.
The correlations between binge and general drinking frequencies with participant age were non-significantly negative in the study sample [r(26) = −0.277, P = 0.171 and r(26) = −0.148, P = 0.471, respectively]. Binge and general drinking frequencies also did not significantly vary as a function of participant gender [t(24) = 0.665, P = 0.512 and t(24) = 0.784, P = 0.440, respectively]. Binge and general drinking frequency reports were however highly correlated (r = 0.703, P <.001).
Primary LPP analyses
An unconditional contrast between lower (M = 0.789 μV, SE = 0.447) and higher (M = 1.389 μV, SE = 0.650) risk responses showed no significant difference in LPP amplitude between them, MD = 0.600, SE = 0.439, 95% confidence interval (CI) = [−0.305, 1.505], F(1, 25) = 1.87, P = 0.184, R2 = 0.011%. Higher and lower risk LPP levels were however significantly correlated within-participants, r(26) = 0.739, P < 0.0001.
LPP and drinking behavior analyses
The relationship between LPP response levels and binge-drinking as well as general drinking frequency was first estimated independently. The results of this analysis showed the main effect of risk level was not statistically significant after controlling for overall drinking (MD = 0.600 μV, SE = 0.405, 95% CI = [−0.235, 1.435]), F(1, 24) = 2.20 μV, P = 0.151) or binge-drinking frequency [MD = 0.600 μV, SE = 0.370, 95% CI = (−0.163, 1.363), F(1, 24) = 2.63, P = 0.118)] in isolation. Overall drinking and binge-drinking frequencies also did not have significant main effects on observed LPP amplitude levels [−0.086 μV, SE = 0.420, 95% CI = (−0.952, 0.780), F(1, 24) = 0.04, P = 0.890, and +0.758 μV, SE = 0.594, 95% CI = (−0.468, 1.984), F(1, 24) = 1.63, P = 0.214, respectively]. Both variables did, however, significantly moderate the main effect of risk level. Specifically, a significantly greater increase in the LPP response as participants transitioned from low to high risk levels was observed as a function of higher levels of binge [+1.656 μV, SE = 0.506, 95% CI = (0.612, 2.700), F(1, 24) = 10.71, P = 0.003] and general drinking frequency [+0.820 μV, SE = 0.380, 95% CI = (0.037, 1.603), F(1, 24) = 4.67, P = 0.041]. Independently, binge-drinking and overall drinking frequency accounted for 21.98 and 4.89% of the variance in LPP responses, respectively.
The relationship between LPP amplitude levels and general drinking as well as binge-drinking frequencies was then estimated simultaneously in a joint model. The results of this model showed that the link between the LPP response and drinking measures was primarily driven by binge-drinking frequency. After controlling for binge-drinking frequency levels, overall drinking frequency did not have a significant main effect [−0.891 μV, SE = 0.545, 95% CI = (−2.018, 0.237), F(1, 23) = 2.67, P = 0.116] or moderating influence on observed LPP levels as a function of risk level [−0.044 μV, SE = 0.488, 95% CI = (−0.965, 1.052), F(1, 23) = 0.01, P = 0.930].
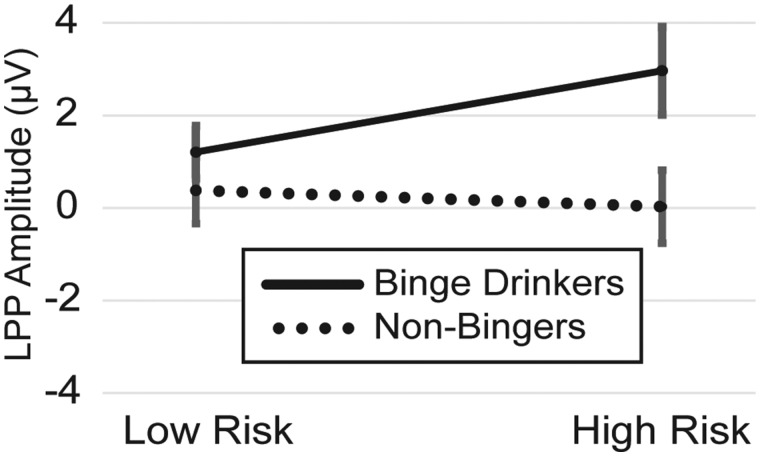
However, as shown in Figure 3, after controlling for overall drinking frequency, binge-drinking frequency had both a significant positive main effect on overall LPP amplitude levels [+1.671 μV, SE = 0.795, 95% CI = (0.026, 3.316), F(1, 23) = 4.42, P = 0.047] as well as a significant moderating influence on the rate of increase in the LPP response transitioning from low to high risk levels [+1.611 μV, SE = 0.711, 95% CI = (0.141, 3.083), F(1, 23) = 5.14, P = 0.033]. Jointly, binge-drinking and overall drinking frequency accounted for 27.29% of the LPP component variance. While binge-drinking frequencies were modeled in their original form, purely for a visual representation purposes a median split was used to divide the sample into binge (N = 13) and non-binge (N = 13) drinkers to form the ERP and component waveforms in Figure 3B and C. A plot of LPP component levels as a function of both this median split and risk level is also presented in Figure 5.
Fig. 5.
Plot of LPP component amplitudes by binge drinking status and risk level.
Discussion
The results of this investigation show binge-drinking frequency to be linked with significantly increased levels of anticipatory risk-taking reactivity on a simple decision-free aversive risk-taking task. After controlling for general drinking frequency, binge-drinking frequency was uniquely associated with elevated reactivity as indexed by the LPP ERP component. The LPP component was elevated both in general and as a function of higher levels of situational risk during the aversive risk-taking task. By largely eliminating the decision-making aspect of the task, these results arguably represent a relatively pure measure of “raw” risk-taking reactivity with minimal levels of decision-making associated with higher level executive processing.
These findings provide the first example of individual differences in raw risk reactivity as a function of reported binge-drinking frequency. These results indicate that individual differences in binge-drinking rates are associated with increased levels of anticipatory risk-taking reactivity even in the absence of a decision-making element. Furthermore, after controlling for general drinking frequency, these differences were found to be present not only in general but also intensified at higher levels of situational risk. These results also lend support to prior work showing evidence of hyperreactivity in affect-related neural responses in general affective responding (Garfield et al., 2015) and affective risk-taking (Xiao et al., 2013) as a function of binge-drinking susceptibility. While the premorbid status of the effects observed in this study has yet to be determined, these findings are congruent with recent research on binge-drinking which suggest that increased affective responsiveness is the true precursor of this health risk behavior (Garfield et al., 2015).
Of particular interest, the results of this study lend support to the idea that exaggerated risk-taking and affective reactivity play key roles in driving binge-drinking behavior (Garfield et al., 2015). Placed in the context of dual-systems models of risk-taking (Steinberg, 2008), the observed differences in the LPP response to signals of risk-taking initiation are in line with the idea that binge-drinking is associated with hyperactive reactive systems (Lannoy et al., 2014). While acknowledging the limitations inherent in source localizing scalp level EEG, this point gains additional support from the localization of LPP response to the subcallosal gyrus, an area of the limbic system. Projections from the subcallosal gyrus to other affective areas such as the amygdala have been proposed to play a key role in suppressing the responsiveness of those regions (Vermetten and Lanius, 2012), with prior research showing that stronger connectivity between the subcallosal gyrus and the amygdala are associated with increased impulsivity and reduced impulsive control in heroin addicts (Xie et al., 2011). Activation in the subcallosal gyrus has also been associated with the reward anticipation (Gloria et al., 2009; Jia et al., 2011) and responsivity to craving cues (Li et al., 2012; Hong et al., 2017).
While this study makes important contributions to our understanding of the potential links between raw risk reactivity and high risk behaviors, it is not without limitations. Of primary importance, replication of the observed effects with larger, more diverse, samples would be an important next step. Such larger scale investigations would also gain significant value from administering other behavioral inventories such as measures of depression, social anxiety or impulsivity as well as measures of health related outcomes such as blood pressure or sleep quality. Understanding the potentially rich interplay between these factors and risk reactivity would almost certainly shed valuable light on the cognitive-affective framework underlying real-world risk taking behavior. In addition to measures of drinking frequency, it would also be important to consider administering additional measures of alcohol consumption level to control for the potential role of raw alcohol consumption level with regard to all observed effects.
Another potential limitation in this study arises with regard to differentiating the observed LPP response from other motor- and pre-motor-related components. There are several factors that lend support to the argument that the observed component is not driven by motor activity, most notably the absence of lateralization in the component at both the scalp and neural level and the virtual absence of the component in non-binge drinkers. Nonetheless, the potential contribution of motor activity should be taken into consideration and potentially tested for in future work, perhaps through the use of a task prop with built-in pressure sensors. It would also be interesting to utilize a prop wired in this manner to assess feedback-related processing via ERP segmentation to the onset of participants’ responses. Such an approach would allow for a more complete modelling of the full risk response process as well as the integration of this approach with prior work on cue and feedback reactivity modulations as a function of alcohol consumption (Bailey et al., 2014; Fleming and Bartholow, 2014).
In conclusion, by removing the decision-making component of a popular risk-taking game, this study highlights intriguing individual differences in decision-free raw risk-taking reactivity as a function of binge-drinking frequency. These results expand on prior work on affective decision making in binge drinkers (Johnson et al., 2008; Xiao et al., 2009, 2013; Carbia et al., 2017; Lannoy et al., 2017) by demonstrating the elevated risk reactivity in this target population is independent of actual choice, or deliberative decision-making processing. The characteristics of the task in this study (i.e. its inherently high engagement value, accessibility and ease of administration) also suggest that it has significant value with regard to future work involving neuroimaging data collection from populations from whom the collection of high-quality data can be challenging (e.g. young children). While further work is needed to assess the precursor vs consequence status of the effects observed here, these findings highlight effects which have important theoretical and practical implications for understanding the links between fundamental aspects of psychological processing and high-risk health behaviors.
Conflict of interest. None declared.
References
- Adan A., Forero D.A., Navarro J.F. (2017). Personality traits related to binge drinking: a systematic review. Frontiers in Psychiatry, 8, 134.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey K., Bartholow B.D., Saults J.S., Lust S.A. (2014). Give me just a little more time: effects of alcohol on the failure and recovery of cognitive control. Journal of Abnormal Psychology, 123(1), 152–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A., Damasio A.R., Damasio H., Anderson S.W. (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition, 50(1), 7–15. [DOI] [PubMed] [Google Scholar]
- Biolcati R., Passini S., Mancini G. (2016). “I cannot stand the boredom.” Binge drinking expectancies in adolescence. Addictive Behaviors Reports, 3(Suppl C), 70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbia C., Cadaveira F., Caamaño-Isorna F., Rodríguez Holguín S., Corral M. (2017). Binge drinking trajectory and decision-making during late adolescence: gender and developmental differences. Frontiers in Psychology, 8, 783.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert B.N., Schupp H.T., Bradley M.M., Birbaumer N., Lang P.J. (2000). Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology, 52(2), 95–111. [DOI] [PubMed] [Google Scholar]
- Dien J. (2010). The ERP PCA Toolkit: an open source program for advanced statistical analysis of event-related potential data. Journal of Neuroscience Methods, 187(1), 138–45. [DOI] [PubMed] [Google Scholar]
- Doumas D.M., Miller R., Esp S. (2017). Impulsive sensation seeking, binge drinking, and alcohol-related consequences: do protective behavioral strategies help high risk adolescents? Addictive Behaviors, 64(Suppl C), 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetherston D. (1994). Playing With Toys Is Serious Work: Robert B. Fuhrer's life revolves around games. Newsday, 12 May 1994.
- Figner B., Mackinlay R.J., Wilkening F., Weber E.U. (2009). Affective and deliberative processes in risky choice: age differences in risk taking in the Columbia Card Task. Journal of Experimental Psychology: Learning, Memory, and Cognition, 35(3), 709–30. [DOI] [PubMed] [Google Scholar]
- Fleming K.A., Bartholow B.D. (2014). Alcohol cues, approach bias, and inhibitory control: applying a dual process model of addiction to alcohol sensitivity. Psychology of Addictive Behaviors: journal of the Society of Psychologists in Addictive Behaviors, 28(1), 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield J.B.B., Allen N.B., Cheetham A., Simmons J.G., Lubman D.I. (2015). Attention to pleasant stimuli in early adolescence predicts alcohol-related problems in mid-adolescence. Biological Psychology, 108(Suppl C), 43–50. [DOI] [PubMed] [Google Scholar]
- Gladwin T., Figner B. (2015). “Hot” Cognition and Dual Systems: Introduction, Criticisms, and Ways Forward. New York: Psychological Press. [Google Scholar]
- Gloria R., Angelos L., Schaefer H.S., et al. (2009). An fMRI investigation of the impact of withdrawal on regional brain activity during nicotine anticipation. Psychophysiology, 46(4), 681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman F.R., Stiksma M.C., Kashdan T.B. (2018). Social Anxiety and the Quality of Everyday Social Interactions: the Moderating Influence of Alcohol Consumption. Behavior Therapy, 49(3), 373–87. [DOI] [PubMed] [Google Scholar]
- Hong J.S., Kim S.M., Jung H.Y., Kang K.D., Min K.J., Han D.H. (2017). Cognitive avoidance and aversive cues related to tobacco in male smokers. Addictive Behaviors, 73, 158–64. [DOI] [PubMed] [Google Scholar]
- Howsley P., Levita L. (2017). Anticipatory representations of reward and threat in perceptual areas from preadolescence to late adolescence. Developmental Cognitive Neuroscience, 25(Suppl C), 246–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z., Worhunsky P.D., Carroll K.M., et al. (2011). An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biological Psychiatry, 70(6), 553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.A., Xiao L., Palmer P., et al. (2008). Affective decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in 10th grade Chinese adolescent binge drinkers. Neuropsychologia, 46(2), 714–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L.D., O’Malley P.M., Miech R.A., Bachman J.G., Schulenberg J.E. (2017). Monitoring the Future National Survey Results on Drug Use, 1975–2016: Overview, Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research, The University of Michigan. [Google Scholar]
- Joseph J.E., Liu X., Jiang Y., Lynam D., Kelly T.H. (2009). Neural correlates of emotional reactivity in sensation seeking. Psychological Science, 20(2), 215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann L., Kinchen S., Shanklin S.L., et al. (2014). Youth risk behavior surveillance—United States, 2013. Morbidity and Mortality Weekly Report Supplements, 63(4), 1–168. [PubMed] [Google Scholar]
- Kiat J., Straley B., Cheadle J.E. (2016). The moderating effect of resistance to peer influence on the P200 and feedback related negativity. Social Cognitive and Affective Neuroscience, 11(3), 377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiat J.E., Goosby B.J., Cheadle J.E. (2018). The impact of social exclusion on anticipatory attentional processes. International Journal of Psychophysiology, 123, 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiat J.E., Straley E., Cheadle J.E. (2017). Why won’t they sit with me? An exploratory investigation of stereotyped cues, social exclusion, and the P3b. Social Neuroscience, 12(5), 612–25. [DOI] [PubMed] [Google Scholar]
- Lannoy S., Billieux J., Maurage P. (2014). Beyond inhibition: a dual-process perspective to renew the exploration of binge drinking. Frontiers in Human Neuroscience, 8, 405.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannoy S., D'Hondt F., Dormal V., Billieux J., Maurage P. (2017). Electrophysiological correlates of performance monitoring in binge drinking: impaired error-related but preserved feedback processing. Clinical Neurophysiology, 128(11), 2110–21. [DOI] [PubMed] [Google Scholar]
- Lejuez C.W., Read J.P., Kahler C.W., et al. (2002). Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART). Journal of Experimental Psychology: Applied, 8(2), 75–84. [DOI] [PubMed] [Google Scholar]
- Li Q., Wang Y., Zhang Y., et al. (2012). Craving correlates with mesolimbic responses to heroin-related cues in short-term abstinence from heroin: an event-related fMRI study. Brain Research, 1469, 63–72. [DOI] [PubMed] [Google Scholar]
- Lissek S., Baas J.M., Pine D.S., et al. (2005). Sensation seeking and the aversive motivational system. Emotion, 5(4), 396–407. [DOI] [PubMed] [Google Scholar]
- Moran T.P., Jendrusina A.A., Moser J.S. (2013). The psychometric properties of the late positive potential during emotion processing and regulation. Brain Research, 1516, 66–75. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. (1971). The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Reynolds E.K., Schreiber W.M., Geisel K., MacPherson L., Ernst M., Lejuez C.W. (2013). Influence of social stress on risk-taking behavior in adolescents. Journal of Anxiety Disorders, 27(3), 272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J.M., Patel N., Daniele-Zegarelli T., MacPherson L., Lejuez C.W., Ernst M. (2015). Social anxiety, acute social stress, and reward parameters interact to predict risky decision-making among adolescents. Journal of Anxiety Disorders, 29(Suppl C), 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberti J.W. (2004). A review of behavioral and biological correlates of sensation seeking. Journal of Research in Personality, 38(3), 256–79. [Google Scholar]
- Schupp H., Cuthbert B., Bradley M., Hillman C., Hamm A., Lang P. (2004). Brain processes in emotional perception: motivated attention. Cognition and Emotion, 18(5), 593–611. [Google Scholar]
- Shulman E.P., Smith A.R., Silva K., et al. (2016). The dual systems model: review, reappraisal, and reaffirmation. Developmental Cognitive Neuroscience, 17(Suppl C), 103–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. (2008). A social neuroscience perspective on adolescent risk-taking. Developmental Review, 28(1), 78–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermetten E., Lanius R.A. (2012). Chapter 18 - Biological and clinical framework for posttraumatic stress disorder In: Michael F.B., Aminoff J., Dick F.S., editors. Handbook of Clinical Neurology, Vol. 106 New York: Elsevier, 291−342. [DOI] [PubMed] [Google Scholar]
- Whelan R., Watts R., Orr C.A., et al. (2014). Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature, 512(7513), 185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Bechara A., Gong Q., et al. (2013). Abnormal affective decision making revealed in adolescent binge drinkers using a functional magnetic resonance imaging study. Psychology of Addictive Behaviors, 27(2), 443–54. [DOI] [PubMed] [Google Scholar]
- Xiao L., Bechara A., Grenard L.J., et al. (2009). Affective decision-making predictive of Chinese adolescent drinking behaviors. Journal of the International Neuropsychological Society, 15(4), 547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Shao Y., Fu L., et al. (2011). Identification of hyperactive intrinsic amygdala network connectivity associated with impulsivity in abstinent heroin addicts. Behavioural Brain Research, 216(2), 639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn J.V., Schür R.R., Boks M.P., Kahn R.S., Joëls M., Vinkers C.H. (2017). Cortisol stress reactivity across psychiatric disorders: a systematic review and meta-analysis. Psychoneuroendocrinology, 77(Suppl C), 25–36. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. (2005). Psychobiology of Personality, 2nd edn New York, NY: Cambridge University Press. [Google Scholar]