Abstract
Biosensors have been developed to measure transdermal alcohol concentration (TAC), but converting TAC into interpretable indices of blood/breath alcohol concentration (BAC/BrAC) is difficult because of variations that occur in TAC across individuals, drinking episodes, and devices. We have developed mathematical models and the BrAC Estimator software for calibrating and inverting TAC into quantifiable BrAC estimates (eBrAC). The calibration protocol to determine the individualized parameters for a specific individual wearing a specific device requires a drinking session in which BrAC and TAC measurements are obtained simultaneously. This calibration protocol was originally conducted in the laboratory with breath analyzers used to produce the BrAC data. Here we develop and test an alternative calibration protocol using drinking diary data collected in the field with the smartphone app Intellidrink to produce the BrAC calibration data. We compared BrAC Estimator software results for 11 drinking episodes collected by an expert user when using Intellidrink versus breath analyzer measurements as BrAC calibration data. Inversion phase results indicated the Intellidrink calibration protocol produced similar eBrAC curves and captured peak eBrAC to within .0003%, time of peak eBrAC to within 18 minutes, and area under the eBrAC curve to within .025% alcohol-hours as the breath analyzer calibration protocol. This study provides evidence that drinking diary data can be used in place of breath analyzer data in the BrAC Estimator software calibration procedure, which can reduce participant and researcher burden and expand the potential software user pool beyond researchers studying participants who can drink in the laboratory.
Keywords: estimated breath alcohol concentration, transdermal alcohol concentration, alcohol biosensor, drinking diary, smartphone app, ecological momentary assessment
1. Introduction
1.1 Background
The alcohol research and treatment communities have long recognized the potential value of being able to passively obtain continuous quantitative measures of alcohol levels of people drinking in naturalistic settings. This is viewed as an improvement from methods that rely on breath analyzers and drink diaries, which have either heavy subject burden or large time gaps between assessment points, hinder naturalistic drinking behavior, and may not be accurate even when individuals are trying to be compliant (e.g., breath analyzer readings being too high due to mouth alcohol or too low due to not taking deep lung breaths; drink diaries being inaccurate due to not knowing the alcohol content of a drink or the amount consumed). In a recent series of Challenges in 2016 and 2017 on Wearable Alcohol Biosensors, NIAAA (2016, 2017) requested the development of biosensor technology to give researchers additional tools for studying the effects of alcohol and to give consumers valuable personal data. They emphasized the importance of these biosensors being quantitative, well-calibrated devices if they are to be used effectively to objectively measure of alcohol consumption.
To date, the alcohol biosensors with the most promise for doing this have been those measuring transdermal alcohol concentration (TAC), the amount of alcohol diffusing through the skin. The measurement of TAC has been shown to be relatively easy to measure via electrochemical sensors placed on the skin (Swift & Swette, 1992). Several TAC devices have been available to the legal system and to alcohol researchers for decades (e.g., AMS SCRAM®, Giner WrisTASTM) and others are under development with the intent to be commercially available to the public (e.g., BACtrack Skyn®, Milo PROOFTM). An often overlooked, yet critical issue for these devices, is that the raw TAC data they record do not consistently relate to breath and blood alcohol concentrations (BrAC/BAC) across individuals, devices, and environmental conditions (see Figure 1; Swift, 2000; 2003; Swift & Swette, 1992). Unlike a breath analyzer, which relies on a relatively simple model that is reasonably robust across people (Dominick, 1990; Labianca, 1990), the transport and filtering of alcohol by the skin is physiologically more complex and based on a number of factors that differ across individuals (e.g., skin layer thickness, tortuosity) and drinking episodes within individuals (e.g., temperature, skin hydration), as well as across devices (e.g. hardware variations; Dumett et al., 2008; Leffingwell et al., 2013). Addressing this variability in the TAC-BrAC/BAC relationship is difficult, but necessary to produce interpretable estimates of alcohol in the body (Webster & Hampton, 2007a, 2007b). Thus, the development of a reliable and valid processing system for converting TAC into quantitatively and temporally accurate estimates of BrAC (eBrAC) is needed to allow for the passive, accurate, and precise monitoring of alcohol consumption patterns and levels in naturalistic settings (Barnett, 2014; Dougherty et al., 2012; Karns-Wright et al., 2017; Swift & Swette, 1992).
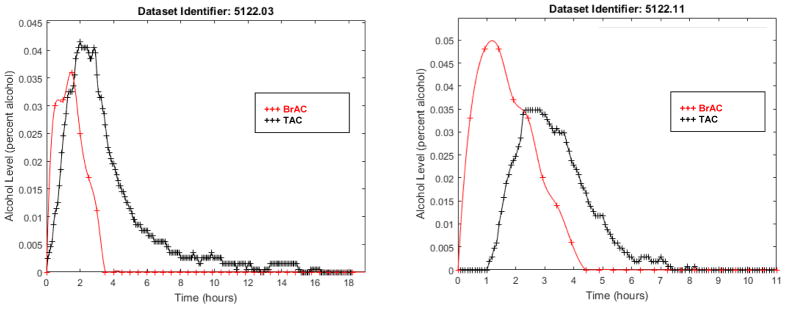
Figure 1. Corresponding BrAC (red crosses) and TAC (black crosses) data for two drinking episodes.
TAC is delayed compared with BrAC in both episodes, but TAC reaches a higher percent alcohol than BrAC in the left panel and a lower percent alcohol in the right panel, indicating the lack of a consistent relationship between BrAC and TAC data.
With the development and dissemination of such a quantitative processing system, alcohol researchers would be able to obtain eBrAC from TAC data at the required resolution for advancing a great variety of experimental and analytic studies on alcohol-related phenomena, including studies aimed at understanding differences between social and problem drinkers, acute and long-term consumption and consequences, progression of drinking (e.g. initiation, harmful use, dependence), symptomatology of dependence (e.g., sensitization, craving, tolerance, withdrawal), evaluation of clinical interventions, and development of prevention programs. This system also could help clinicians and physicians monitor patients in personalized prevention and treatment efforts and be an important component of precision medicine. Finally, being able to quantitatively monitor real-time alcohol levels is now recognized as broadly applicable to lay individuals monitoring their own personal health and for public safety.
1.2 Prior development of a TAC-BrAC physics/physiological-based model and BrAC Estimator conversion and consolidation software
Over the past decade, our research team has been developing a data analysis system to accompany TAC devices that produces quantitative eBrAC from TAC data (Dai, Rosen, Wang, Barnett, & Luczak, 2016; Dumett et al., 2008; Rosen, Luczak, Hu, & Hankin, 2013; Rosen, Luczak, & Weiss, 2014). We developed a two-step mathematical modeling process (calibration, inversion) based upon a first principles forward model consistent with human physiology for the transport and filtering of alcohol from the blood through the skin to the TAC device, and its inversion back to the blood/breath (Banks & Ito, 1997; Curtain & Salamon, 1986; Gibson & Rosen, 1988; Pritchard & Salamon, 1987). In the calibration phase of the modeling process, simultaneously-collected TAC and BrAC data from a single drinking episode are used to determine the individualized parameter values of the forward model for a particular person wearing a particular TAC device (i.e., the “person-device pair”). Then, in the inversion step of the modeling process, the individualized parameters determined in the calibration phase are used to invert the model and produce eBrAC from the TAC data of all subsequent drinking episodes without requiring any additional BrAC data.
These models have been compiled in our BrAC Estimator software program (Luczak & Rosen, 2014). The software uses simultaneously-collected TAC and BrAC data from a calibration drinking episode to choose optimal values for the model parameters for a person-device pair (calibration phase), and then uses these individualized parameter values to invert TAC into eBrAC for all subsequent drinking episodes during which the TAC device is worn (inversion phase). The software produces as output an eBrAC data point for every TAC reading (placed in the original data file as a new column), plots of the TAC and eBrAC curves, and summary scores for each drinking episode, including peak eBrAC, time of peak eBrAC, and area under the drinking curve, AUC. We refer to this system as a BrAC estimator, but the system will produce eBAC if the models are calibrated with BAC data instead of with BrAC data.
1.3 Prior data collection protocols for obtaining the calibration data
In our original calibration data collection protocol, the simultaneously-collected BrAC (via a breath analyzer) and TAC (via the biosensor device) data are obtained during a laboratory alcohol administration session (Fairbairn et al., submitted; Luczak & Rosen, 2014; Luczak, Rosen, & Wall, 2015). We showed this laboratory calibration protocol produced well-fitting curves in the calibration phase, and in the inversion phase produced peak eBrACs that were on average within .01mg% alcohol of peak BrACs, within 30 minutes for time of peak, and within .01–.02mg% alcohol-hours for AUC of breath analyzer readings taken by an expert user at 30-minute intervals during 11 drinking episodes (Luczak & Rosen, 2014, Rosen et al., 2014). This calibration procedure, however, requires a full day in the laboratory and thus has high researcher and subject burden. In addition, this procedure would not be appropriate in some circumstances (e.g., with individuals trying to abstain) and would not feasible for lay users and clinicians/physicians who do not have access to an alcohol administration laboratory and medical-grade breath analyzers.
In a recent study (Dai et al., 2016), we investigated if we could use alternative methods to obtain the BrAC data required to calibrate the model for the person-device pair that would be less burdensome than our original calibration data collection protocol. We replaced the breath analyzer BrAC data obtained in the laboratory with an estimated BrAC calculated from drinking diary data of a single controlled drinking episode (note that this is also an “estimated BrAC”, but to distinguish such methods from the eBrAC derived from the software we will refer to this as “calculated BrAC”). This was done by using six well-established relatively simple linear models (Carey & Hustad, 2002; Forrest, 1986; Lewis, 1986; Matthews & Miller, 1979; NHTSA, 1994; Watson, Watson, & Batt, 1981) and a dynamic nonlinear Michaelis-Menten model for the enzyme catalyzed metabolism of ethanol in the liver (Goudar, Sonnad, & Duggleby, 1999) to convert the diary-recorded number of standard drinks into calculated BrAC using the same dataset as in Luczak and Rosen (2014). We then tested how using these seven sets of calculated BrAC in place of the breath analyzer data altered the calibration and inversion phase results. Our results indicated that using the non-linear physiological-based models to convert the drinking diary data into calculated BrAC most closely approximated the breath analyzer BrAC curve and resulted in only minimal degradation of the parameter estimates in the calibration phase. When these drinking diary-produced calibration parameters were used to invert 10 subsequent drinking sessions in the inversion phase, they provided values close to those obtained using the breath analyzer-produced calibration parameters—capturing on average peak BrAC to within 0.003% alcohol, time of the peak to within 17 minutes, and AUC to within 0.01% alcohol–hours.
1.4 Current study
In the current study, we tested whether we could further reduce the burden of the calibration data collection protocol by using drinking diary data obtained and converted into a calculated BrAC curve via a smart phone app Intellidrink. This would provide an alternative calibration data collection procedure that would make it possible for a lay user to enter drinking data in real-time during a drinking session of his/her choice. Furthermore, if the phone app were automatically synchronized with the output from the TAC device and directly entered as the calibration data into the BrAC Estimator software, this would also reduce researcher/administrator burden. Here we report on our efforts to create such an integrated data collection and processing system. To test how well this alternative calibration data collection protocol worked, we compared the calibration and inversion output results of the BrAC Estimator software when using this new data collection protocol compared with when using the laboratory breath analyzer data collection protocol that we used in Luczak and Rosen (2014).
2. Materials and Methods
2.1 Creating an integrated data collection system
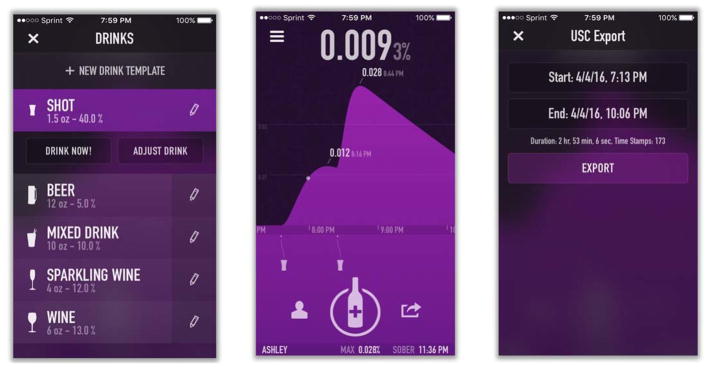
The Intellidrink smart phone app uses Widmark equations (Forrest, 1986; Watson et al., 1981) to model alcohol absorption, metabolism, and elimination and calculate BrAC. One author (RW) modified the Intellidrink app to have the wireless transmission of the data go directly to the BrAC Estimator software. The app requires the user to enter basic demographic data once and then for each drinking episode to enter the type of alcohol, number of standard drinks consumed and times at which they were consumed, and estimated fullness of stomach. Figure 2 shows three screen shots of the app to provide examples of how data are entered into the app, displayed by the app, and exported for this study.
Figure 2. Screenshots of the Intellidrink app.
Drinking diary is entered by drink type or actual brand of alcohol (left panel) and the program calculates a BrAC curve (center panel). The app was modified to export the calculated BrAC curve directly into the BrAC Estimator software (right panel) to be used with simultaneously-collected TAC data to calibrate the model and produce the individualized parameter values for the person-device pair (i.e., the eBrACapp method).
The BrAC Estimator software has previously been described in detail (Luczak & Rosen, 2014). Three authors (ZD, IGR, and CW) modified the software to be compatible with Intellidrink. The software also was modified to automatically use the episode with the breath analyzer or Intellidrink data as the calibration episode and to automatically identify all drinking episodes. For each episode identified, as in prior versions, the software output includes comprehensive reports and plots of eBrAC signal and summary measures including peak, time of peak, and AUC. The software also writes back to the TAC data file a new column of eBrAC data for every raw TAC data point.
2.2 Data
We used the same dataset we previously showed produced well-fitting models when calibrated with breath analyzer data (Luczak & Rosen, 2014, Rosen et al., 2014) and when calibrated with a paper drinking diary data entered into a non-linear model (Dai et al., 2016). This dataset was obtained from a single expert subject, which enabled us to test the model fit and software output using highly accurate breath analyzer and drinking diary data without individual- and device-level variation; this means differences in the software output were only due to BrAC calibration data and episode-level variations.
We briefly describe the dataset (see Luczak & Rosen, 2014 for additional details). One of the authors (SEL) wore a WrisTAS™ 7 sensor set to take readings every 5 minutes over an 18-day period. The first drinking episode was a standard laboratory calibration session with alcohol consumed evenly over the first 15 minutes of the session designed to reach a peak of approximately .050%, and the next 10 episodes were naturalistic drinking episodes in the field. During drinking episodes, she maintained a real-time drinking diary and collected breath measurements with an Alco-sensor IV (Intoximeters, Inc., St. Louis, MO) at 30-minute intervals (15-minute intervals in the first two episodes). Table 1 shows the alcohol types, consumption levels and durations, and data obtained for the 11 drinking episodes.
Table 1. Summary of drinking episodes.
For each of the 11 drinking episodes, data obtained included the time interval for recording breath analyzer (BrAC) and drinking diary data, types of alcohol consumed, total number of standard drinks consumed, number of drinking diary readings obtained until drinking commenced, number of BrAC readings obtained until BrAC returned to .000%, and total duration of BrAC readings from start of drinking until BrAC returned to .000%.
| Drinking episode | BrAC interval (minutes) | Alcohol type | Standard drinks | Number of diary recordings | Duration of drinking (minutes) | Number of BrAC readings | Duration of BrAC readings (minutes) |
|---|---|---|---|---|---|---|---|
| 1 | 15 | 95% ethanol in 1:4 mixer | 2 | 2 | 15 | 15 | 225 |
| 2 | 15 | Beer | 1 | 4 | 45 | 7 | 90 |
| 3 | 30 | Beer | 2 | 4 | 75 | 8 | 150 |
| 4 | 30 | Beer | 3.5 | 6 | 150 | 9 | 225 |
| 5 | 30 | Mixed drink, beer, wine | 3 | 11 | 300 | 14 | 420 |
| 6 | 30 | Mixed drink | 1 | 2 | 30 | 5 | 120 |
| 7 | 30 | Wine | 1.5 | 4 | 90 | 6 | 150 |
| 8 | 30 | Beer | 1 | 2 | 30 | 5 | 105 |
| 9 | 30 | Beer, wine | 2.5 | 5 | 120 | 9 | 250 |
| 10 | 30 | Wine | 1 | 3 | 60 | 5 | 120 |
| 11 | 30 | Mixed drink, wine | 3 | 5 | 120 | 10 | 270 |
Note. In Episodes 1, 5, and 8, a single BrAC reading was omitted. In Episode 4, BrAC was recorded only down to .036, whereas in all other episodes BrAC was recorded down to .000.
Another author (AH) entered the drinking diary data into the Intellidrink app for all drinking episodes. In the actual protocol of the new calibration method, this information would be entered by the drinker in real time for a single drinking episode of his/her choice (and no breath analyzer data would be required). Because we were testing the accuracy of this protocol in this study, we entered diary data for all 11 episodes to examine how each episode performed as the calibration episode. Person-level data entered once into the app included age, height, weight, gender, and drinking frequency (rare, occasional, frequent). Episode-level data included the type of alcohol (wine, beer, liquor) and percent alcohol (if known), stomach content (empty, half, full), drinking start time, and cumulative standard drinks consumed at each time point recorded in the diary. The data were then synthesized in the BrAC Estimator software by importing the downloaded TAC excel file and Intellidrink .csv file and running the software program in automatic mode.
2.3 Data analysis
We denote the output from the BrAC Estimator software calibrated with raw breath analyzer data as the “eBrACraw” method and the output calibrated with Intellidrink app as the “eBrACapp” method. To compare these two calibration methods, we examined the BrAC Estimator software outputs of the eBrAC plots and the absolute differences in the summary scores of Peak (% alcohol), Time of Peak (hours), and AUC (% alcohol-hours) between the two methods (eBrACraw - eBrACapp). We also report the differences between the output of these two calibration methods and the raw breath analyzer data (“BrAC”); to calculate the AUC of the BrAC, we fit the data points to a smooth piecewise polynomial curve (Schultz, 1973). For additional reference, we include the raw TAC data from the biosensor (“TAC”) in the tables and figures. We calculated pair-wise t-tests and correlations among the three sets of values (from BrAC, eBrACraw, eBrACapp), recognizing that any statistics for only 10–11 scores should be interpreted with caution.
3. Results
3.1 Calibration phase
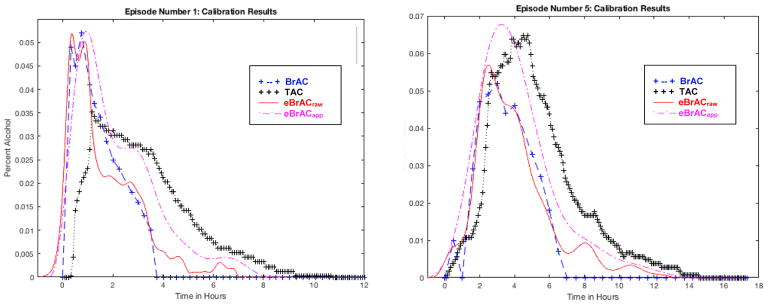
Table 2 shows the Peak, Time of Peak, and AUC values for BrAC, eBrACraw, eBrACapp, and their mean differences from one another when each of the 11 episodes was used as the calibration session. Example plots of the curves are shown in Figure 3 for Episode 1 where eBrACraw and eBrACapp Peak matched closely and for Episode 5 where the summary scores were the most discrepant.
Table 2. Calibration phase.
Results using each of the 11 drinking episodes as the calibration session with the model calibrated either with breath analyzer data (eBrACraw) or Intellidrink-calculated data (eBrACapp)
| Drinking episode | Mdiff (SD) | r | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |||
| Peak (% alcohol) | |||||||||||||
| TAC | .035 | .009 | .042 | .048 | .065 | .012 | .010 | .011 | .030 | .014 | .035 | ||
| BrAC | .052 | .023 | .036 | .057 | .052 | .023 | .018 | .017 | .026 | .013 | .048 | ||
| eBrACraw | .052 | .016 | .034 | .063 | .057 | .043 | .018 | .020 | .024 | .015 | .047 | ||
| eBrACapp | .052 | .022 | .035 | .061 | .068 | .039 | .020 | .022 | .030 | .015 | .051 | ||
| Differences | |||||||||||||
| (BrAC - eBrACraw) | .000 | .007 | .002 | −.006 | −.005 | −.020 | .000 | −.003 | .002 | −.002 | .001 | −.002 (.007) | .92*** |
| (BrAC - eBrACapp) | .000 | .001 | .001 | −.004 | −.016 | −.016 | −.002 | −.005 | −.004 | −.002 | −.003 | −.005 (006)* | .94*** |
| (eBrACraw - eBrACapp) | −.001 | −.005 | −.001 | .002 | −.011 | .004 | −.002 | −.003 | −.006 | .000 | −.004 | −.002 (.004) | .97*** |
| Time of peak (hours) | |||||||||||||
| TAC | 1.17 | 1.67 | 2.00 | 3.92 | 4.50 | 1.92 | 2.75 | 1.33 | 3.42 | 1.25 | 2.33 | ||
| BrAC | 0.75 | 0.50 | 1.50 | 2.00 | 3.00 | 0.58 | 1.58 | 0.83 | 2.00 | 1.08 | 0.92 | ||
| eBrACraw | 0.37 | 1.25 | 1.15 | 2.58 | 2.50 | 1.10 | 1.48 | 0.70 | 2.27 | 0.92 | 1.75 | ||
| eBrACapp | 0.95 | 1.00 | 1.58 | 3.07 | 3.27 | 1.25 | 1.82 | 1.03 | 2.42 | 1.02 | 2.22 | ||
| Differences | |||||||||||||
| (BrAC - eBrACraw) | 0.38 | −0.75 | 0.35 | −0.58 | 0.50 | −0.52 | 0.10 | 0.13 | −0.27 | 0.16 | −0.83 | −.121 (.484) | .79** |
| (BrAC - eBrACapp) | −0.20 | −0.50 | −0.08 | −1.07 | −0.27 | −0.67 | −0.24 | −0.20 | −0.42 | 0.06 | −1.30 | −.445 (.419)** | .87*** |
| (eBrACraw - eBrACapp) | −0.58 | 0.25 | −0.43 | −0.48 | −0.77 | −0.15 | −0.33 | −0.33 | −0.15 | −0.10 | −0.47 | −.324 (.277)** | .95*** |
| Area under curve (% alcohol-hours) | |||||||||||||
| TAC | .137 | .016 | .157 | .179 | .326 | .021 | .018 | .031 | .085 | .029 | .100 | ||
| BrAC | .102 | .016 | .075 | .167 | .199 | .027 | .027 | .015 | .055 | .018 | .118 | ||
| eBrACraw | .112 | .016 | .089 | .170 | .227 | .029 | .027 | .021 | .059 | .018 | .114 | ||
| eBrACapp | .149 | .039 | .109 | .205 | .321 | .030 | .038 | .039 | .080 | .020 | .147 | ||
| Differences | |||||||||||||
| (BrAC - eBrACraw) | −.010 | .000 | −.014 | −.003 | −.028 | −.002 | .000 | −.006 | −.004 | .000 | .004 | −.006 (.009) | 1.00*** |
| (BrAC - eBrACapp) | −.047 | −.023 | −.034 | −.038 | −.122 | −.003 | −.011 | −.024 | −.025 | −.002 | −.029 | −.033 (.033)** | .98*** |
| (eBrACraw - eBrACapp) | −.038 | −.023 | −.020 | −.035 | −.095 | −.001 | −.011 | −.018 | −.021 | −.002 | −.033 | −.027 (.025)** | .99*** |
Note. TAC = transdermal alcohol concentration, BrAC = breath analyzer BrAC measurements, eBrACraw = estimated BrAC using breath analyzer BrAC as the calibration data in the BrAC Estimator software, eBrACapp = estimated BrAC using Intellidrink app-calculated BrAC as the calibration data in the BrAC Estimator software, Mdiff = mean difference over all episodes.
p < .05.
p < .01.
p < .001. (two-tailed tests)
Figure 3. Calibration results.
Plots shown for Episode 1 in the left panel, where the two calibration data-collection methods (eBrACraw from breath analyzer data shown in red solid line, and eBrACapp from Intellidrink app drinking diary data shown in purple dashed line) capture Peak within .001 of one another, and for Episode 5 in the right panel, which had the largest Peak discrepancy between the two calibration methods at .011.
Peak eBrAC
Peak eBrACapp was on average within .002 (+/−.004% alcohol) of Peak eBrACraw and the two were correlated at .97 (p < .001). The two peak values differed by more than .005, the reliability level of the breath analyzer, only in Episodes 5 (.011%) and 11 (.006%). The mean difference from Peak BrAC was significant for eBrACapp (.005%, p<.05), but not for eBrACraw (.002%).
Time of Peak eBrAC
Time of Peak eBrACapp on average was within 19 minutes (.32 hours +/− .28 hours, p<.01) of Time of Peak eBrACraw and the two were correlated at .95 (p<.001). The difference in Time of Peak was under 30 minutes, the time between BrAC readings, for all but Episodes 1 (35 minutes) and 5 (46 minutes). The mean difference from Time of Peak BrAC was significant for eBrACapp (26 minutes, p<.01), but not for eBrACraw (7 minutes).
Area Under eBrAC Curve
The AUC eBrACapp on average was within .027 (+/− .025% alcohol–hours, p<.01) of AUC eBrACraw and the two were correlated at .99 (p<.001). The AUC difference was over .050 only in Episode 5 (.095). The mean difference from AUC BrAC was significant for eBrACapp (.033% alcohol–hours, p<.01), but not for eBrACraw (.006% alcohol–hours).
3.2 Inversion phase
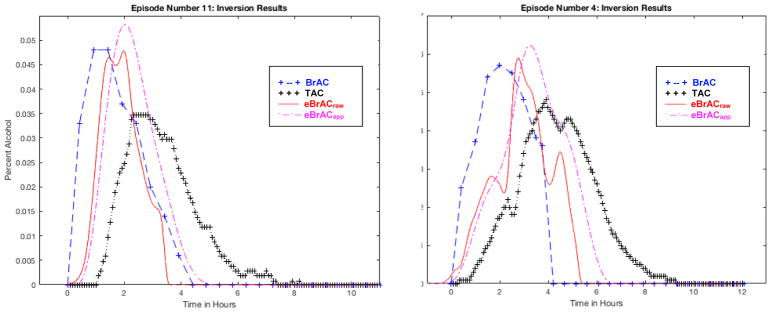
We then used the model calibrated with Episode 1, the laboratory drinking session that was the calibration episode in the original protocol, to invert the TAC data for each of the subsequent 10 drinking episodes (see Table 3). Example plots of the curves are shown in Figure 4 for Episode 11 where eBrACraw and eBrACapp Time of Peak matched closely and for Episode 4 where they were more discrepant.
Table 3. Inversion phase.
Inversion results for the 10 drinking episodes with model calibrated on Episode 1 using breath analyzer BrAC (eBrACraw) or Intellidrink (eBrACapp) calibration protocols
| Drinking episode | Mdiff (SD) | r | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |||
| Peak (% alcohol) | ||||||||||||
| TAC | .009 | .042 | .048 | .065 | .012 | .010 | .011 | .030 | .014 | .035 | ||
| BrAC | .023 | .036 | .057 | .052 | .023 | .018 | .017 | .026 | .013 | .048 | ||
| eBrACraw | .015 | .051 | .059 | .090 | .021 | .012 | .019 | .037 | .028 | .048 | ||
| eBrACapp | .013 | .056 | .062 | .084 | .020 | .014 | .018 | .040 | .023 | .053 | ||
| Differences | ||||||||||||
| (BrAC - eBrACraw) | .008 | −.015 | −.002 | −.038 | .002 | .006 | −.002 | −.011 | −.015 | .000 | −.007 (.014) | .85** |
| (BrAC - eBrACapp) | .010 | −.020 | −.005 | −.032 | .003 | .004 | −.001 | −.014 | −.010 | −.005 | −.007 (.012) | .89*** |
| (eBrACraw - eBrACapp) | .002 | −.005 | −.003 | .006 | .001 | −.002 | .001 | −.004 | .005 | −.005 | −.000 (.004) | .99*** |
| Time of peak (hours) | ||||||||||||
| TAC | 1.67 | 2.00 | 3.92 | 4.50 | 1.92 | 2.75 | 1.33 | 3.42 | 1.25 | 2.33 | ||
| BrAC | 0.50 | 1.50 | 2.00 | 3.00 | 0.58 | 1.58 | 0.83 | 2.00 | 1.08 | 0.92 | ||
| eBrACraw | 1.00 | 0.90 | 2.77 | 2.23 | 1.25 | 1.83 | 0.80 | 2.53 | 0.77 | 1.97 | ||
| eBrACapp | 1.23 | 1.50 | 3.25 | 2.60 | 1.58 | 1.88 | 1.13 | 2.72 | 1.10 | 2.02 | ||
| Differences | ||||||||||||
| (BrAC - eBrACraw) | −0.50 | 0.60 | −0.77 | 0.77 | −0.67 | −0.25 | 0.03 | −0.53 | 0.31 | −1.05 | −.206 (.611) | .68* |
| (BrAC - eBrACapp) | −0.73 | 0.00 | −1.25 | 0.40 | −1.00 | −0.30 | −0.30 | −0.72 | −0.02 | −1.10 | −.502 (.542)* | .75** |
| (eBrACraw - eBrACapp) | −0.23 | −0.60 | −0.48 | −0.37 | −0.33 | −0.05 | −0.33 | −0.18 | −0.33 | −0.05 | −.296 (.174)*** | .97*** |
| Area under curve (% alcohol - hours) | ||||||||||||
| TAC | .016 | .157 | .179 | .326 | .021 | .018 | .031 | .085 | .029 | .100 | ||
| BrAC | .016 | .075 | .167 | .199 | .027 | .027 | .015 | .055 | .018 | .118 | ||
| eBrACraw | .014 | .127 | .150 | .267 | .020 | .018 | .026 | .073 | .026 | .085 | ||
| eBrAC-app | .018 | .170 | .194 | .357 | .026 | .022 | .035 | .096 | .032 | .109 | ||
| Differences | ||||||||||||
| (BrAC - eBrACraw) | .002 | −.052 | .017 | −.068 | .007 | .009 | −.011 | −.018 | −.008 | .033 | −.009 (.031) | .93*** |
| (BrAC - eBrACapp) | −.002 | −.095 | −.027 | −.158 | .001 | .005 | −.020 | −.041 | −.014 | .009 | −.034 (.053) | .93*** |
| (eBrACraw - eBrACapp) | −.004 | −.043 | −.044 | −.089 | −.005 | −.004 | −.009 | −.023 | −.007 | −.024 | −.025 (.027)* | 1.00*** |
Note. TAC = transdermal alcohol concentration, BrAC = breath analyzer BrAC measurements, eBrACraw = estimated BrAC using breath analyzer BrAC as the calibration data in the BrAC Estimator software, eBrACapp = estimated BrAC using Intellidrink app-calculated BrAC as the calibration data in the BrAC Estimator software, Mdiff = mean difference over all episodes.
p < .05.
p < .01.
p < .001. (two-tailed tests)
Figure 4. Inversion results using Episode 1 as the calibration session.
Differences in Peak eBrACs for the two calibration methods (eBrACraw from breath analyzer data shown in red solid line, and eBrACapp from Intellidrink app drinking diary data shown in purple dashed line) only differed by .001–.006 in all episodes, whereas the difference in Time of Peak eBrAC for the two calibration methods was short in Episode 11 at 3 minutes shown in the left panel and longer in Episode 4 at 29 minutes shown in the right panel.
Peak eBrAC
Peak eBrACapp was on average within .0003 (+/−.004% alcohol) of Peak eBrACraw and the two were correlated at .99 (p < .001). Peak values differed by more than .005 only in Episode 5 (.006%). The mean difference from Peak BrAC was not significant for either eBrACapp (.007%) or eBrACraw (.007%).
Time of Peak eBrAC
Time of Peak eBrACapp was on average within 18 minutes (.30 hours +/− .17 hours, p<.001) of Time of Peak eBrACraw and the two were correlated at .97 (p<.001). The difference in Time of Peak was less than 30 minutes in all episodes. The mean difference from Time of Peak BrAC was significant for eBrACapp (30 minutes, p<.05), but not for eBrACraw (12 minutes).
Area Under eBrAC Curve
The AUC eBrACapp on average was within .025 (+/− .027% alcohol–hours, p<.05) of AUC eBrACraw and the two were correlated at 1.00 (p<.001). The AUC difference was over .050 only in Episode 5 (.089). The mean difference from AUC BrAC was not significant for either eBrACapp (.034% alcohol–hours) or eBrACraw (.009% alcohol–hours).
4. Discussion
4.1 Main findings
Results of this study indicate that using a real-time calibration protocol that only requires the user to enter drinking diary data into a commercially available smartphone app for a single drinking episode produces relatively similar results in the BrAC Estimator software as using a highly regimented laboratory alcohol administration with breath analyzer data obtained by trained research staff. The accuracy of the eBrAC output, however, did show some degradation when using the smart phone calibration method compared with the eBrAC laboratory method, moreso for time of peak than the actual peak value. The amount of quantitative precision required for each research question will vary, so researchers will need to weigh the costs and benefits of obtaining detailed laboratory calibration data to provide more accurate output versus a simpler calibration procedure that produces some additional variability but has lower burden and risk. For lay users and clinicians for whom laboratory calibration with medical-grade devices is not an option, this new protocol integrated into a hybrid data analysis system makes it possible to use the BrAC Estimator software.
We previously showed that 32 participants were willing and able to record drinking diary data on smart phones as part of a larger real-time assessment battery over a 2-week period of naturalistic drinking (Luczak et al., 2015). We were not able to obtain accurate field breath analyzer BrAC data to compare with the real-time drinking diary data so we could not determine the accuracy of the diary data, but the results of the diary compliance were very promising. In the Intellidrink app calibration protocol examined in this study, the actual protocol would allow the user to choose the episode to record a drinking diary, which should help maximize the likelihood of obtaining accurate self-report data (e.g., when drinking known quantities of alcohol such as bottles of beer, in a private setting, with people who are tolerant of interruptions to socializing). Testing how accurate the diaries are from typical device wearers will be done in future studies.
4.2 Study limitations and future directions
The results of this study should be viewed within its limitations, including utilizing a single expert user to collect the data, low-to-moderate alcohol consumption that resulted in low-to-moderate BrACs, and relatively short periods of drinking. This study does not prove that the models or the drinking diary calibration procedure work across individuals. How these results from a single expert subject fit with additional subjects, higher alcohol quantities, and more varied patterns of drinking (i.e., fast/slow and short/long temporal patterns of consumption) needs to be determined. Future research will test the BrAC Estimator calibration methods using more varied consumption patterns and peak BrACs, as well as participants who vary on factors such as skin thickness, height, weight, gender, ethnicity, and age. Obtaining these data would allow us to conduct sensitivity analyses and examine within- and between-subject covariates that affect model fit.
Several modifications to the models and protocols also may enhance the system. It is possible the field drinking diary method could be improved by incorporating non-linear models (e.g., Dai et al, 2016). In addition, the BrAC Estimator calibration scheme could be adapted such that if drinking diary were available from multiple drinking episodes, the software could incorporate all available data to produce more accurate estimates. There also is a need to establish a baseline model (i.e., population-based parameters) when no individual-level calibration data are available. Finally, the software currently inverts an episode once it has the TAC data, but we plan to test models that use machine learning and auto-regressive moving average models to generate real-time eBrAC, which could be displayed to individuals wishing to view their eBrAC during drinking episodes.
This study used a TAC device and currently these are the most well-established alcohol biosensor devices, but the conversion models and software system in the BrAC Estimator software are applicable to any biosensor device, with some adjustment, that measures alcohol content in the body using optical, thermal, infrared, chemical, or electronic technology. As bioengineering companies develop biosensor devices that are more reliable, precise, durable, inexpensive, and appealing to the wearer, this need to adapt various forms of alcohol biosensor data into meaningful eBrAC becomes even more critical. Our end goal for this research program is to produce a single smart phone app that automatically integrates alcohol biosensor data, drinking diary calibration data, and the BrAC Estimator software to produce eBrAC signals and summary scores, which would be a powerful tool for researchers, clinicians, and lay users who wish to unobtrusively obtain quantified alcohol consumption levels in naturalistic settings. Producing this system as a stand-alone program as well as a plug-in for other software (e.g., health monitors, intervention smart phone apps) would maximize the versatility of this technology.
4.3. Conclusions
The development of an integrated system that converts TAC into continuous measures of eBrAC and produces drinking episode summary scores makes it possible for researchers to obtain reliable, quantitative eBrACs in naturalistic settings. Here we showed that calibrating the BrAC Estimator software models using drinking diary data entered into the Intellidrink app produced relatively similar results as using breath analyzer data obtained in the laboratory. Thus, using this type of hybrid data collection system becomes a potential alternative method for calibrating the software model to the individual when it is not possible to do so in a laboratory setting. To be able to obtain eBrAC with this simplified calibration method would greatly reduce participant and researcher burden, expand the utility of TAC biosensors to non-researchers, and improve the ease of using the BrAC Estimator software. This important step in the development of the BrAC Estimator software expands its use as a tool for passively monitoring naturalistic alcohol consumption.
Acknowledgments
This work was funded by the National Institutes of Health grant R21AA17711 to SEL and IGR and a Women in Science and Engineering undergraduate summer fellowship to AH.
Footnotes
Conflict of interest
Wichmann receives royalties for sales of the smart phone app Intellidrink. All other authors declare no conflict of interest.
References
- Banks HT, Ito K. Approximation in LQR problems for infinite dimensional systems with unbounded input operators. Journal of Mathematical Systems, Estimation and Control. 1997;7:1–34. [Google Scholar]
- Barnett NP. Alcohol sensors and their potential for improving clinical care. Addiction. 2014;110:1–3. doi: 10.1111/add.12764. [DOI] [PubMed] [Google Scholar]
- Carey KB, Hustad JTP. Are retrospectively reconstructed blood alcohol concentrations accurate? Preliminary results from a field study. Journal of Studies on Alcohol. 2002;63:762–766. doi: 10.15288/jsa.2002.63.762. [DOI] [PubMed] [Google Scholar]
- Chen C-T. Linear system theory and design. New York: Holt Rinehart and Winston; 1970. [Google Scholar]
- Curtain RF, Salamon D. Finite dimensional compensators and infinite dimensional systems with unbounded input operators. SIAM Journal on Control and Optimization. 1986;24:797–816. [Google Scholar]
- Dai Z, Rosen IG, Wang C, Barnett NJ, Luczak SE. Identifying drinking diary based pharamacokinetic models to calibrate transdermal alcohol biosensor data analysis software. Mathematical Biosciences and Engineering. 2016;13:911–934. doi: 10.3934/mbe.2016023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominick AL. The chemical basis of the breathalyzer, a critical analysis. Journal of Chemical Education. 1990;67:259–261. [Google Scholar]
- Dougherty DM, Charles NE, Acheson A, John S, Furr RM, Hill-Kapturczak N. Comparing the detection of transdermal and breath alcohol concentrations during periods of alcohol consumption ranging from moderate drinking to binge drinking. Experimental and Clinical Psychopharmocology. 2012;20:373–381. doi: 10.1037/a0029021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumett M, Rosen IG, Sabat J, Shaman A, Tempelman LA, Wang C, Swift RM. Deconvolving an estimate of breath measured blood alcohol concentration from biosensor collected transdermal ethanol data. Applied Mathematics and Computation. 2008;196:724–743. doi: 10.1016/j.amc.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn CE, Bresin K, Kang D, Rosen IG, Ariss T, Barnett NP, Luczak SE, Eckland NS. A multimodal investigation of contextual effects on alcohol’s emotional rewards. doi: 10.1037/abn0000346. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest ARW. The estimation of Widmark’s factor. Journal of the Forensic Science Society. 1986;26:249–252. doi: 10.1016/s0015-7368(86)72491-2. [DOI] [PubMed] [Google Scholar]
- Gibson JS, Rosen IG. Approximation of discrete time LQG compensators for distributed systems with boundary input and unbounded measurement. Automatica. 1988;24:517–529. [Google Scholar]
- Goudar CT, Sonnad JR, Duggleby RG. Parameter estimation using a direct solution of the integrated Michaelis-Menten equation. Biochimica et Biophysica Acta – Protein Structure and Molecular Enzymology. 1999;1429:377–383. doi: 10.1016/s0167-4838(98)00247-7. [DOI] [PubMed] [Google Scholar]
- Karns-Wright TE, Roache JD, Hill-Kapturczak NH, Liang Y, Mullen J, Dougherty DM. Time delays in transdermal alcohol concentrations relative to breath alcohol concentrations. Alcohol and Alcoholism. 2016;52:35–41. doi: 10.1093/alcalc/agw058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labianca DA. The chemical basis of the breathalyzer, a critical analysis. Journal of Chemical Education. 1990;67:259–261. [Google Scholar]
- Leffingwell TR, Cooney NJ, Murphy JG, Luczak SE, Rosen IG, Dougherty DM, Barnett NP. Transdermal alcohol monitoring: 21st century measurement for an age-old problem. Alcoholism: Clinical and Experimental Research. 2013;37:16–22. doi: 10.1111/j.1530-0277.2012.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MJ. The individual and the estimation of his blood alcohol concentration from intake, with particular reference to the “hip-flask” drink. Journal of the Forensic Science Society. 1986;26:19–27. doi: 10.1016/s0015-7368(86)72442-0. [DOI] [PubMed] [Google Scholar]
- Luczak SE, Rosen IG. Estimating BrAC from transdermal alcohol concentration data using the BrAC Estimator software program. Alcoholism: Clinical and Experimental Research. 2014;38:2243–2252. doi: 10.1111/acer.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak SE, Rosen IG, Wall TL. Development of a real-time repeated-measures assessment protocol to capture change over the course of drinking episodes. Alcohol and Alcoholism. 2015;50:1–8. doi: 10.1093/alcalc/agu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DB, Miller WR. Estimating blood alcohol concentration: Two computer programs and their applications in therapy and research. Addictive Behaviors. 1979;4:55–60. doi: 10.1016/0306-4603(79)90021-2. [DOI] [PubMed] [Google Scholar]
- National Highway Traffic Safety Administration. Computing a BAC estimate. Washington, D.C: Department of Transportation; 1994. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. A wearable alcohol biosensor – A second challenge. 2017 Available at: https://www.niaaa.nih.gov/challenge-prize and https://www.challenge.gov/challenge/wearable-alcohol-biosensor/
- National Institute on Alcohol Abuse and Alcoholism. NIH competition seeks wearable device to detect alcohol levels in real-time. 2016 Available at: https://www.niaaa.nih.gov/news-events/news-releases/nih-competition-seeks-wearable-device-detect-alcohol-levels-real-time.
- National Institutes of Health. All of Us Research Program. 2017 Available at: https://www.nih.gov/research-training/allofus-research-program.
- National Institutes of Health. New NIH Strategic Plan launched. 2015 Available at: https://www.niaaa.nih.gov/news-events/news-noteworthy/new-nih-strategic-plan-launched.
- Pritchard AJ, Salamon D. The linear quadratic control problem for infinite dimensional systems with unbounded input and output operators. SIAM Journal on Control and Optimization. 1987;25:121–144. [Google Scholar]
- Rosen IG, Luczak SE, Weiss J. Blind deconvolution for distributed parameter systems with unbounded input and output and determining blood alcohol concentration from transdermal biosensor data. Applied Math and Computation. 2014;213:357–376. doi: 10.1016/j.amc.2013.12.099. http://www.ncbi.nlm.nih.gov/pubmed/24707065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen IG, Luczak SE, Hu W, Hankin M. Discrete-time blind deconvolution for distributed parameter systems with dirichlet boundary input and unbounded output with application to a transdermal alcohol biosensor. Proceedings of 2013 SIAM Conference on Control and its Applications; 2013. pp. 160–167. [Google Scholar]
- Schultz M. Spline analysis. Englewood Cliffs, NJ: Prentice Hall; 1973. [Google Scholar]
- Swift RM. Transdermal alcohol measurement for estimation of blood alcohol concentration. Alcoholism: Clinical and Experimental Research. 2000;24:422–423. [PubMed] [Google Scholar]
- Swift RM. Direct measurement of alcohol and its metabolites. Addiction. 2003;98S:73–80. doi: 10.1046/j.1359-6357.2003.00605.x. [DOI] [PubMed] [Google Scholar]
- Swift RM, Swette LL. Assessment of ethanol consumption with a wearable, electronic ethanol sensor/recorder. In: Litten R, Allen J, editors. Measuring alcohol consumption: Psychosocial and biological methods. Totowa, NJ: Humana Press; 1992. [Google Scholar]
- Watson PE, Watson ID, Batt RD. Prediction of blood alcohol concentrations in human subjects: Updating the Widmark equation. Journal of Studies on Alcohol. 1981;42:547–556. doi: 10.15288/jsa.1981.42.547. [DOI] [PubMed] [Google Scholar]
- Webster GD, Gabler HC. Feasibility of transdermal ethanol sensing for the detection of intoxicated drivers. Annual Proceedings for the Advancement of Medicine. 2007a;51:449–464. [PMC free article] [PubMed] [Google Scholar]
- Webster GD, Gabler HC. Modeling of transdermal transport of alcohol: effect of body mass and gender. Biomedical Sciences Instrumentation. 2007b;44:361–366. [PubMed] [Google Scholar]