Abstract
Occult hepatitis B virus infection (OBI) is manifested by presence of HBV-DNA in the absence of detectable Hepatitis B surface antigen (HBsAg) with or without anti-HBV antibodies. Hence it is a potential threat in blood transfusion medicine. This study was carried out to determine the prevalence of OBI as well as evaluate the effectiveness of using Hepatitis B surface antigen (HBsAg) marker alone in the diagnosis of HBV infection among HBsAg negative blood donors in Ilorin, Nigeria. A purposive sampling, including samples from 206 already donated and prescreened blood units from HBsAg negative from apparently healthy volunteer blood donors at the General Hospital Blood Transfusion Centre, Ilorin, Nigeria, were collected for further laboratory analysis for this study. Five millilitres of blood was collected and plasma sample tested for the presence of HBsAg using a commercially available ELISA kit. In addition, Polymerase Chain Reaction (PCR) was used for molecular detection of HBV DNA in each of the samples. Data was analyzed using descriptive statistics, Chi square at p = 0.05. Of the 206 HBsAg Micropoint® rapid kits pre-screened seronegative samples collected from the blood transfusion centre, 8 (3.9%) samples were positive for the presence of HBsAg when retested using ELISA in the laboratory. Eighteen of the 206 samples (8.7%) were HBV-DNA positive by a semi-nested PCR technique giving an OBI rate of 8.7%. Out of the 18 HBV-DNA positive samples, 17 (4.4%) were from males and only one (5.6%) was from a female donor. Analysis of the 18 HBV DNA positive samples using genotype specific primers into genotype A and Non-A showed that 15 (83.3%) were HBV genotype A, while 2 (11.1%) were genotypes other than A (Non-A), one (5.6%) sample had mixed genotypes (A & non-A). A prevalence of 8.7% OBI found in this study indicates substantial risk of post transfusion HBV infection in the study area in Nigeria. Hence, the need to include HBV DNA detection in the routine blood screening that is, using Nucleic Acid Testing (NAT) technique for transfusion safety in the country.
Keywords: Occult hepatitis B virus, HBsAg, ELISA, PCR, NAT
INTRODUCTION
Hepatitis B virus (HBV) is a potentially known life-threatening infection of the liver. Globally, HBV is ranked among the deadliest infectious diseases with increasing public health consequences (WHO, 2015). For instance, liver cirrhosis and hepatocellular carcinoma (HCC), a major cause of high mortality in humans are complications arising from infection with hepatitis B virus (HBV) (Lok., 2000). Like other hepatitidies of viral origin, HBV manifests clinically and in an asymptomatic fashion, but also with unusual serological patterns (Ola et al., 2000, Odemuyiwa et al., 2001).
In Nigeria, detection of HBV infection is mostly performed with the estimation of hepatitis B surface antigen (HBsAg) serological marker alone (Ola et al., 2002, Ajayi et al., 2007). Therefore, many screened seronegative subjects for HBsAg which may have been said to be free of HBV infection and patients transfused with such blood have been reported to have developed post- transfusion hepatitis from HBV infection (Czaja A., 1993, Behzad-Behbahani et al., 2006).
Occult hepatitis B virus infection (OBI) has been described as the presence of HBV-DNA in the absence of detectable HBsAg with or without anti-HBV antibodies. Nucleic Acid Testing (NAT) for HBV-DNA detection has been used to confirm the presence of HBV, a phenomenon which is now increasingly recognized in several clinical settings worldwide (Raimondo et al., 2008, Brechot et al., 2001). Mandatory NAT for transfused blood units in many developed countries on a large set of blood donors has further confirmed the phenomenon of OBI (Panhotra et al., 2005, Prati et al., 2006). This transfusion safety algorithm is yet to be incorporated into many laboratories and blood safety measures in developing countries including Nigeria.
Though previous studies in Nigeria and some other parts of the world show that the prevalence of OBI in blood donors ranges from 1% to 16% depending on the endemicity of HBV infection as well as the geographic areas (Candotti et al., 2008, Candotti et al., 2012, Huang et al., 2012), the point prevalence and burden of OBI in Nigeria is unclear. A study with sample size of 28 (n = 28) in Oyo showed zero prevalence of OBI in healthy subjects while a prevalence of 5.4% was reported in blood donors from Ile Ife, both in Nigeria (Ola et al., 2009, Olotu et al., 2016). Albeit, other investigators reported prevalence of 8.0% from south-eastern part of Nigeria (Nna et al., 2014) while Opaleye et al., (2016) reported 17% prevalence of OBI from blood donors in southwestern part of the country using NAT but hitherto, there has been no report among blood donors from the north central part of the country. However, studies from other subject populations found OBI prevalence of 62% in hepatocellular carcinoma (HCC) patients, 64% in liver transplanted patients, 27% in hemodialysis patients and up to 45% in hepatitis C virus (HCV) and HIV infected patients respectively (Samal et al., 2012, Raimando et al., 2007). This study was carried out to determine the rate of HBV-DNA in blood donors in Ilorin, Nigeria using blood units that have been prescreened and reported to be negative for HBsAg.
MATERIALS AND METHODS
Ethics Statement
Ethical clearance for this study was obtained from the Health Ethical Research Committee, Kwara State Ministry of Health, Ilorin, Kwara State, Nigeria with reference number MOH/KS/EC/777/103.
Study Area
This study was carried out among volunteer blood donors in Ilorin, Kwara State, Nigeria. Kwara State is located in the northcentral region of Nigeria between coordinates 8° 30′ 0″ North, 4° 33′ 0″ East. It has a land area of about 36,825 km sq, the ninth largest state in Nigeria, with a total population of 2.37 million people based on the Nigeria 2006 census and constitute about 1.69% of the Nation’s total population (Census, 2006). The state borders Niger and Kebbi States in the north, Republic of Benin to the west, Kogi State to the east and Osun, Oyo and Ekiti States in the south (Nigeria master web).
Sampling
A total of 206 samples from blood units donated at the Ilorin General Hospital blood transfusion centre, Ilorin, Kwara State, Nigeria (male/female: 196/10) were purposively included in the present study. As a routine, the blood bank screens for HBsAg using a commercially available rapid screening test kit (Micropoint Diagnostics, USA). HBsAg negative blood donors were prescreened for HIV status by ELISA in addition, aliquot of each prescreened blood units was collected and transferred into labelled sterile anticoagulant free tubes and each tube was centrifuged at 3500rpm for 5 mins within 24hours of collection. The plasma was separated from the whole blood and stored at −80°C until analysed.
Serological analysis
The 206 samples collected were retested for presence of HBsAg using commercially available ELISA kit (WANTAI HBV Surface Antigen; Beijing Wantai Biological Pharmacy Enterprise CO., Ltd; China) following the manufacturer’s instructions.
Detection of HBV-DNA
DNA was extracted from each of the 206 blood samples using the guanidium thiocyanate extraction method (Manzin et al., 1991) and detection of HBV-DNA was done by a routine diagnostic PCR in the the department of virology, University of Ibadan HBV laboratory. A semi- nested PCR was performed using a pair of primers (Forward and Reverse) for the first round while the forward primer was changed in the second round of PCR amplification. The primers used are: SF 5′- GTGTCTTGGCCA AAATTCGCAGT-3′ (sense), MF -5′-TCGGATCCGGTA TGTTGCCCGTTTGTCC-3′ (sense), and 979,5′CAAAAG ACCCACAATTCTTTGACATACTTTCCAAT-3′ (antisense). PCR amplification was carried out in a 25μl reaction volume containing 5μl PCR mix (Jena Bioscience; Germany), 2.0μl each of primers SF and 979, 10.0μl of PCR grade water and 6.0μl of the extracted DNA as template for the synthesis. The amplified fragment of the Pol gene was then used as a template for the second run at 4.0μl volume, 5.0 μl PCR mix, 2.0μl each of primers MF and 979, and12.0μl of PCR buffer and set in a Applied Biosystem Veriti ™ 9700 Thermal Cycler. Primers MF and 979 amplified a 562 bp DNA fragment from the first round product. Thermal cycling parameters were: initial denaturation at 95°C for 5 minutes, followed by 40 cycles of 30 seconds at 95°C denaturation, 60 seconds at 62°C annealing temperature, 90 seconds at 72°C extension and 10 minutes at 72°C for the final extension of the reaction process. Thermal cycling parameters for the initial denaturation, pre and final extension of the second run remained unaltered but the annealing condition; denaturation time as well as the number of cycles was adjusted to 63°C for 20 seconds, 20 seconds and 30 cycles respectively. Furthermore, a positive control (HBV plasmid DNA) and a negative control of the master mix were integrated to each run to validate the PCR amplification products.
Genotyping of Hepatitis B Virus
Genotyping of the HBV DNA samples was carried out using specific primers PC1, rvA, rvnonA and fw1865 for amplification of the preC/C region. The primers used for genotyping (rvA and rvnonA) were designed based on the 6-nucleotide insertion detected in HBV/A. These 6-nucleotides were distinguished for the designing of the primer rvA while the primer rvnonA is specific for any HBV that is not genotype A and anneals to it (Olinger et al., 2006). The reaction is a semi-nested one. The nucleotide sequences of the primers are: PCI (5′-GGAGACCACCGTGAACGC-3′), rvA (5′-TTCTTC TTCTAGGGGACCTGCCTCAGTCC-3′) rvnonA (5′-TTCT TCTTCTAGGGGACCTGCCTCATCGT-3′) and fw1865 (5′-CAAGCCTCCAAGCTGTGCCTTGGGTGGCCTT-3′). A 25.0μl reaction containing 5.0μl of Jena PCR mix, 1.0 μl each of primers PC1 as forward and rvA/rvnonA as reverse primers, 12.0μl of PCR buffer and 6.0μl of the extracted DNA as template were used for the PCR. The reaction led to amplification of a larger fragment of the preC/C gene (768bp) which was used as a template for the second round. The forward primer PC1 was replaced with fw1865 and a 25.0 μl reaction containing 5.0 μl PCR mix, 1.0 μl each of primers fw1865 as forward and rvA/rvnonA reverse primers, 16.0μl of PCR buffer and 2.0μl of the amplified DNA template for the PCR and amplified on Applied Biosystem Veriti ™ 9700 Thermal Cycler. Primers fw1865 and rvA/rvnonA amplified a 513bp DNA fragment from the first round product. The thermal cycling parameters for the first and second run were the same except for the annealing temperature of the second run set at 70°C, other parameters remained the same with initial denaturation at 95°C for 5 minutes, followed by 30 cycles of 60 seconds at 95°C denaturation, 60 seconds at 60°C annealing temperature, 2 minutes at 72°C extension and 10 minutes at 72°C for the final extension of the reaction process. The amplified products were separated in a 1.5% agarose gel and visualized by staining with Cyber green. A positive control (HBV plasmid DNA) and a negative control of the master mix were included in each run to validate the PCR reaction.
Statistical analysis
Data were entered into an Excel software and analysed using the Statistical Package for Social Sciences (SPSS) programme version 21 (Chicago, IL, USA). Analyses were carried out using descriptive statistics for continuous variables while differences and relationships were determined using Chi‑square test where applicable and a P < 0.05 was regarded as significant.
RESULTS
Of the 206 HBsAg blood donor samples that were pre-screened and said to be HBV free, 8 (3.9%) tested positive for HBsAg when retested using another ELISA in the laboratory, while 18 (8.7%) of the 206 samples were HBV-DNA positive by a semi-nested PCR using HBV specific primer pairs (Table 1). Comparison of rates of detection of evidence of HBV infection based on the methods used for detection showed a statistically significant difference in the sensitivity of the assay (P = 0.003).
Table 1.
HBV markers detection among volunteer blood donors by gender in Ilorin, Nigeria.
| Gender | χ2 | P | ||
|---|---|---|---|---|
| Male | Female | |||
| Age (Years) | ||||
| ≤ 25 | 10 (5.1) | 0 (0.0) | 0.464 | 0.601 |
| >25 | 186 (94.9) | 10 (100.0) |
||
| HBV test Result | ||||
| Positive (%) | 22 (11.2) | 1 (10.0) | 0.014 | 0.905 |
| Negative (%) | 174 (88.8) | 9 (90.0) | ||
| ELISA test results | ||||
| Positive (%) | 7 (3.6) | 1 (10.0) | 1.053 | 0.305 |
| Negative (%) | 189 (96.4) | 9 (90.0) | ||
| PCR test Result | ||||
| Positive (%) | 17 (8.7) | 1 (10.0) | 0.021 | 0.885 |
| Negative (%) | 179 (91.3) | 9 (90.0) | ||
| Genotype Result | ||||
| rvA | 15 (83.3) | 0 (0.0) | ||
| rv non A | 1 (5.6) | 1 (5.6) | ||
| rvA/non A | 1 (5.6) | 0 (0.0) | ||
Of the 18 positive HBV-DNA samples 17 (94.4%) were males and one (5.6%) was a female donor. There was no significant association between OBI and gender. Figure 1 shows the gel picture of PCR products with the expected band size of 562 bp of fragment of the pol gene amplified.
Figure 1.
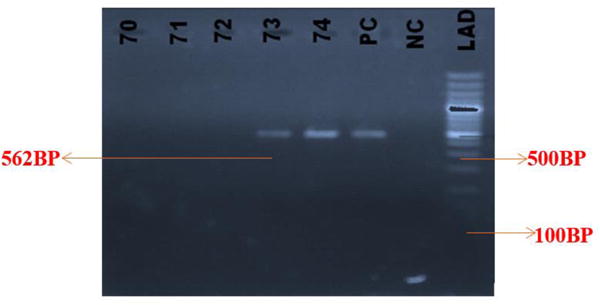
Gel picture of PCR product showing amplification of fragment HBV pol gene (Band size= 562bp).
Lanes: 70 – 72 are empty wells, 73 – 74 are samples positive for HBV pol gene, PC is Positive Control, NC is Negative Control and LAD is the Ladder.
Genotyping of the OBI samples using genotype specific primers with detectable HBV DNA showed that 15 (83.3%) of the 18 OBI positive samples were HBV genotype A, 2 (11.1%) were HBV genotypes other than A (Non-A), while one (5.6%) sample had mixed HBV genotypes (A and non A).
Figures 2 and 3 show the gel pictures of product of PCR amplification fragment of HBV DNA for the genotyping of preC/C gene of HBV/A and HBV/non A respectively. The highest rate of occurrence of the OBI was found among donors who were in the age group of 25-36 years.
Figure 2.
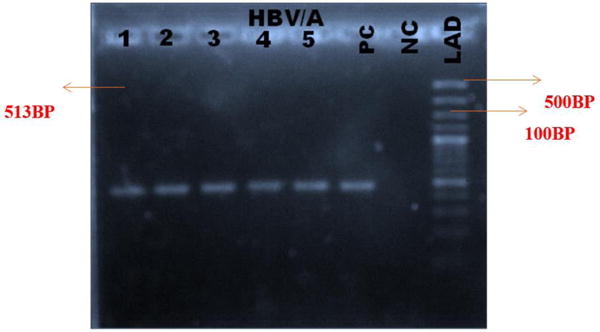
Gel image of PCR product for the genotyping of HBV (pre C/C gene of HBV/A) (Band size = 513bp).
Lanes: 1 – 5 are samples positive for HBV genotype A, PC is Positive Control, NC is Negative Control and LAD is the Ladder.
Figure 3.
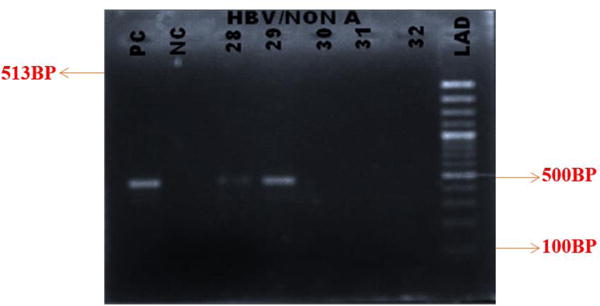
Gel image of PCR product for the genotyping of HBV (pre C/C gene of HBV/non A) (Band size = 513bp).
Lanes: 28 – 29 are positive samples for HBV genotype Non A, PC is Positive Control, NC is Negative Control and LAD is the Ladder
DISCUSSION
The emergence of mutants of HBV and the need for early diagnosis of infection with the virus requires improvement of currently available immunoassays (Erhabor et al., 2014). High prevalence of OBI in high and low endemic areas worldwide is a general burden particularly for tranfussion safety and management of infected persons. The results obtained from this study showed that the rate of HBsAg in Nigeria is high which agrees with previous reports that the virus endemic in the country (Hodges et al., 1998). The sero-positivity of HBsAg (87%) found in this study is in accordance with the findings in other studies in Nigeria and other sub-Saharan African Countries. In a similar study Nna et al., (2014), reported a prevalence of 11.5% among blood donors in southeastern Nigeria while Motayo et al. (2015) found 10% in parts of southwestern part of the country. Fashola et al. (2009) reported 13.2% in Ibadan, southwestern Nigeria. In other studies, Esumeh et al. (2003) reported 10.6% from the south-south region of Nigeria and 9% by Erhabor et al. (2014) from Sokoto, in northwestern Nigeria. On the other hand, Salihu (2013) and Olokoba et al. (2009) reported 4.7% and 2.4% from Lagos and Yola respectively. the sub-Saharan Africa, Nigeria has HBV infection rate that is similar to the situation in some neighboring countries such as Ghana (5-10%), Cameroon (8-20%), Benin (12%) and Chad (12%) (Dongdem et al., 2009; Fomulu et al., 2013; WHO, 2006). South Africa has a prevalence of about 10%, Tanzania, Burkina Faso, South Sudan, and Eritrea have 8.3%, 13.4%, 6.25% and 10% infection rate respectively (Muktar et al., 2005, Nagalo et al., 2012, Abou et al., 2009, Fessehayle et al., 2011). The disparities observed in the prevalence may be due to variation in lifestyle, behavioral changes, level of awareness about HBV as well as access to standard healthcare systems within the country (Nwokediuko, 2011; Setia et al., 2013).
Global studies on OBI have shown varied rates, depending on the population studied, demography, and sensitivity of assays used (Chemin and Trepo. 2005; Hisham et al. 2010). Ola et al. (2009) reported 7.2% amongst patients with viral hepatitis while Opaleye et al. (2014) reported 11.2% among HIV infected patients and recently a rate of 17% among blood donors in Nigeria (Opaleye et al., 2015). The high prevalence of OBI observed in this study further underscores the fact that HBV is still endemic in Nigeria.
Detection of HBV was higher when PCR was used compared to the ELISA techniques (18% versus 5%). This indicates that PCR technique is more sensitive than ELISA especially for the detection of OBI (Mekuria et al., 2003; Hairul Aini et al., 2008). Testing of blood donors for HBV infection in most blood banks in Nigeria still utilizes only rapid diagnostic kits, safety of which previous reports have questioned especially when used as a sole assay for the diagnosis of HBV for transfusion safety (Moore C., 2013; Susmita et al., 2012). The results of this study has also shown that rapid tests are inferior compared to ELISA and PCR methods and this is consistent with previous report by Busch (2004). There is therefore need to use a combination of HBsAg rapid test along with ELISA and NAT for screening of donors blood because the present situation with high possibility of transfusion of infected blood have far-reaching consequences, not only to the recipients but also their families, communities and society at large (Iqbal et al., 2005). Although the presence of HBsAg in the blood indicates acute or chronic infection, patients with detectable HBSAg in their blood but undetectable HBV DNA could be inactive HBV carriers (Odaibo et al., 2013).
The majority of volunteer blood donors in this study were young adult males; 196 (95.1%) out of 206 were less than 36 years. This pattern is in agreement with some previous reports on the rate of HBV infection among volunteer donors in Nigeria (Motayo et al., 2015 ; Musa et al., (2015). Several reasons have been offered for this pattern which include altruism, social influence and fear about giving blood among women but not men (Healy, 2000; Ferguson et al., 2008). On the other hand, the believe that blood donation is beneficial to health has been reported to be twice as high a motivation for donating blood among men compared to women (Glynn et al., 2002). Furthermore, adverse reactions and advice to female not to donate blood (during their menstrual period or pregnancy) by Doctors has also been offered as a reason for the poor attitude to donation. Untoward reactions, vasovagal reactions, perceived anxiety, nausea, arm pain, haematoma and bruises coupled with dizziness, sweating, weakness and fainting which may occur during or immediately after the donation of whole blood have been reported to be more frequent among females than their male counterparts (Newman et al.,2003; Newman et al., 2006; Trouern-Trend et al., 1999; Hinrichs et al., 2008; Nilsson et al., 2003).
Typing of the 18 samples that had detectable HBV DNA indicate occurrence of, genotypes HBV/A, HBV/non-A and HBV A/non-A at a rate of 83.3%, 11.1% and 5.6% respectively. This funding contrasts previous reports (Odemuyiwa et al., 2001; Mulders et al., 2004; Olinger, 2006; Forbi et al., 2010; Opaleye et al., 2015) in Nigeria. that showed that HBV subgenotype A2, A3 and the recombinants of genotypes A and E were observed were frequently in West Africa (Makuwa et al., 2006; Kurbanov et al., 2005). Therefore, further studies are needed on large scale to determine the circulating genotypes among volunteer blood donors in Nigeria.
Primer specific typing or molecular sequencing can be used to ascertain the specific HBV genotypes (B-J). Only one of the samples which had detectable HBV DNA was a mixed genotype HBV A/non A. Mixed genotypes are more frequently encountered in patients with chronic hepatitis compared to acute hepatitis which has been associated with increased HBV replication (Toan et al., 2006). HBV genetic variability has implications in the diagnosis, HBeAg seroconversion rate, treatment, vaccination, vaccine escape mutants, prognosis, occult infection (Guettouche and Hnatyszyn, 2005; Sunbul, 2014). The findings from a study conducted by Kew et al., (2005) indicate that the relative risk of developing hepatocellular carcinoma in HBsAg-positive individuals infected with HBV/A is 4.5 times when compared with other genotypes.
In conclusion, careful implementation of HBsAg screening as well as HBV NAT donor blood screening can result in reduction of HBV residual risk and occult infection. With endemicity of HBV in Nigeria and prevalence of OBI at 8.7% found in this study, it is imperative that screening algorithms of blood donors or blood units for transfusion be enhanced by pretesting for OBI using nucleic acid testing (NAT) and/or anti HBc prior to transfusion to minimize the HBV infection risk. The results of this study further justify the need for use of highly sensitive assays for transfusion safety in HBV endemic countries including Nigeria. It is therefore proposed that use of rapid test kits should be validated with ELISA assays in routine screening for HBV in transfusion centres in the country. Also, samples of blood units should be tested for OBI status using NATs such as nested PCR or real time PCR and/or at least by anti-HBc screening prior to transfusion thereby minimizing the risk of acquiring HBV infection by transfused individuals.
Acknowledgments
We acknowledge the Health Ethical Research Committee, Kwara State Ministry of Health, Ilorin, Kwara State, Nigeria, the entire staff of Ilorin General Hospital blood transfusion centre, Ilorin, Kwara State for their technical assistance, as well as the Laboratory staff of the Department of Virology, University College Hospital, University of Ibadan, Ibadan, Nigeria.
This study was supported by Medical Education Partnership Initiative Nigeria (MEPIN) through National Institute of Health (NIH) USA grant funded by Fogarty International Centre, the office of AIDS Research and National Human Genome Research Institute of NIH, the Health Resources and Services Administration (HRSA) and the Office of the U.S. Global AIDS Coordinator under award number R24TW008878.
References
- Abou MA, Eltahir YM, Ali AS. Seroprevalence of HBV and HCV among blood donors in Nyala, South Dar Fur, Sudan. Virol J. 2009;23(6):146. doi: 10.1186/1743-422X-6-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajayi AO, Komolafe AO, Ajumobi K. Seroprevalence of hepatitis B surface antigenaemia among health care workers in a Nigerian tertiary health institution. Nig J Clin Pract. 2007;10:287–289. [PubMed] [Google Scholar]
- Antar W, El Shokry MH, Abd EL, Hamid WA, Helmy MF. Significance of detecting anti-HBc among Egyptian male blood donors negative for HBsAg. Transfusion Medicine. 2010;20:409–413. doi: 10.1111/j.1365-3148.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Behzad-Behbehani A, Mafi-Nejad A, Tabei SZ, Lankareni KB, Torab A, Moaddeb A. Anti- HBC &HBV-DNA detection in blood donors negative for hepatitis B virus surface antigen in reducing risk of transfusion associated HBV infection. Indian J Med Res. 2006;123:37–42. [PubMed] [Google Scholar]
- Brechot C, Thiers V, Kremsdorf D, Nalpas B, Pol S, Paterlini-Brechot P. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: Clinically significant or purely “occult”? Hepatology. 2001;34:194–203. doi: 10.1053/jhep.2001.25172. [DOI] [PubMed] [Google Scholar]
- Busch MP. Should HBV DNA NAT replace HBsAg and/or anti-HBc screening of blood donors? Transfus Clin Biol. 2004;11(1):26–32. doi: 10.1016/j.tracli.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Candotti D, Grabarczyk P, Ghiazza P, Roig R, Casamitjana N, Iudicone P, et al. Characterization of occult hepatitis B virus from blood donors carrying genotype A2 or genotype D strains. Journal of Hepatology. 2008;49:537–547. doi: 10.1016/j.jhep.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Candotti D, Lin CK, Belkhiri D, Sakuldamrongpanich T, Biswas S, Lin S, et al. Occult hepatitis B infection in blood donors from South East Asia: molecular characterisation and potential mechanisms of occurrence. Gut gutjnl-2011. 2012 doi: 10.1136/gutjnl-2011-301281. 301281. [DOI] [PubMed] [Google Scholar]
- Chemin I, Trepo C. Clinical Impact of occult HBV infection. J Clinical Virol. 2005;34(Suppl 1):S15–S21. doi: 10.1016/s1386-6532(05)80005-8. [DOI] [PubMed] [Google Scholar]
- Czaja Albert J. Chronic Active Hepatitis: The Challenge for a New Nomenclature. Ann Intern Med. 1993;119:510–517. doi: 10.7326/0003-4819-119-6-199309150-00011. [DOI] [PubMed] [Google Scholar]
- Erhabor O, Kwaifa IK, Bayawa AM, Isaac ZI, Dorcas I, et al. Comparison of ELISA and Rapid screening techniques for the detection of HBsAg among blood donors in Usmanu Danfodiyo University Teaching Hospital Sokoto, North Western Nigeria. J Blood Lymph. 2014;4:124. [Google Scholar]
- Esumeh FI, Ugbomioko D, Isibor JO. Seroprevalence of HIV and HBsAg in central hospital Benin city, Nigeria. J Med Lab Sci. 2003;12(2):52–55. [Google Scholar]
- Fang Y, Shang QL, Liu JY, Li D, Xu WZ, Teng X, et al. Prevalence of occult hepatitis B virus infection among hepatopathy patients and healthy people in China. Journal of Infection. 2009;58:383–388. doi: 10.1016/j.jinf.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Fashola FA, Kotila TR, Akinyemi JO. Trends in transfusion transmissible viral infections from 2001–2009 in Ibadan, Nigeria. Intervirology. 2009;51(6):427–431. doi: 10.1159/000209671. [DOI] [PubMed] [Google Scholar]
- Ferguson E, Farrell K, Lawrence C. Blood Donation is an act of Benevolence Rather Than Altruism. Health Psychology. 2008;27:327–336. doi: 10.1037/0278-6133.27.3.327. [DOI] [PubMed] [Google Scholar]
- Fessehayle N, Naik D, Fessehaye T. Transfussion transmited infections-a retrospective analysis from the National blood transfusion service, Eritrea. Pan Afr Med J. 2011;9:40. doi: 10.4314/pamj.v9i1.71219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbi JC, Vaughan G, Purdy MA, Campo DS, Xia GL, Ganova-Raeva LM, et al. Epidemic history and evolutionary dynamics of hepatitis B virus infection in two remote communities in rural Nigeria. PLoS One. 2010;5:e11615. doi: 10.1371/journal.pone.0011615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn SA, Kleinman SH, Schreiber GB, Zuck T, McCombs S, Bethel J, Garratty G, Williams AE. Motivations to donate blood: Demographic comparisons. Transfusion. 2002;42(2):216–225. doi: 10.1046/j.1537-2995.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- Guettouche T, Hnatyszyn HJ. Chronic hepatitis B and viral genotype: the clinical significance of determining HBV genotypes. Antiviral Therapy. 2005;10:593–604. 2005. [PubMed] [Google Scholar]
- Hairul Aini H, Omar AR, Hair-Bejo M, Aini I. Comparison of Sybr Green I, ELISA and conventional agarose gel-based PCR in the detection of infectious bursal disease virus. Microbiological Research. 2008;163(5):556–563. doi: 10.1016/j.micres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Healy K. Embedded Altruism: Blood Collection Regimes and the European Union’s Donor Population. Princeton University; 2000. [Google Scholar]
- Hinrichs A1, Picker SM, Schneider A, Lefering R, Neugebauer EA, Gathof BS. Effect of blood donation on well-being of blood donors. Transfus Med. 2008;18(1):40–8. doi: 10.1111/j.1365-3148.2007.00805.x. [DOI] [PubMed] [Google Scholar]
- Ismail Hisham, Soliman Mohamed, Ismail Nahed. Occult hepatitis B virus infection in Egyptian hemodialysis patients with or without hepatitis C virus infection. Journal of Pathology and Laboratory Medicine International. 2010;2:113–120. [Google Scholar]
- Hodges M, Sanders E, Aitken C. Seroprevalence of hepatitis markers: HAV, HBV, HCV, and HEV amongst primary school children in Freetown Sierra Leone. West African J Med. 1998;17:3–7. [PubMed] [Google Scholar]
- Huang CH, Yuan Q, Chen PJ, Zhang YL, Chen CR, Zheng QB, et al. Influence of mutations in hepatitis B virus surface protein on viral antigenicity and phenotype in occult HBV strains from blood donors. J Hepatol. 2012;57:720–729. doi: 10.1016/j.jhep.2012.05.009. S0168-8278(12)00363-7. [DOI] [PubMed] [Google Scholar]
- Iqbal HS, Solomon S, Murugavel KG, Solomon SS, Balakrishnan P. Evaluation and diagnostic usefulness of domestic and imported enzyme-linked immunosorbent assay for the detection of HIV type 1 antibody in India. Clin Diagn Lab Immunol. 2005;12:1425–1428. doi: 10.1128/CDLI.12.12.1425-1428.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Yotsuyanagi H, Yatsuhashi H, Karino Y, Takikawa Y, Saito T, Arase Y, Imazeki F, Kurosaki M, Umemura T. Risk factors for long-term persistence of serum hepatitis B surface antigen following acute hepatitis B virus infection in Japanese adults. Hepatology. 2014;59:89–97. doi: 10.1002/hep.26635. [DOI] [PubMed] [Google Scholar]
- Jutavijittum P, Andernach IE, Yousukh A, Samountry B, Samountry K, Thammavong T, et al. Occult hepatitis B infections among blood donors in Lao PDR. Vox Sang. 2014;106:31–37. doi: 10.1111/vox.12073. [DOI] [PubMed] [Google Scholar]
- Kew M, Kramvis A, Francois G. Hepatitis B virus genotypes. Vaccine. 2005;23:2409–2423. doi: 10.1016/j.vaccine.2004.10.045. [DOI] [PubMed] [Google Scholar]
- Kurbanov F, Tanaka Y, Fujiwara K, Sugauchi F, Mbanya D, Zekeng L, et al. A new subtype (subgenotype) Ac (A3) of hepatitis B virus and recombination between genotypes A and E in Cameroon. J Gen Virol. 2005;86:2047–2056. doi: 10.1099/vir.0.80922-0. [DOI] [PubMed] [Google Scholar]
- Li L, Chen PJ, Chen MH, Chak KF, Lin KS, Tsai SJL. A pilot study for screening blood donors in Taiwan by nucleic acid amplification technology: detecting occult hepatitis B virus infections and closing the serologic window period for hepatitis C virus. Transfusion. 2008;48:1198–1206. doi: 10.1111/j.1537-2995.2008.01672.x. [DOI] [PubMed] [Google Scholar]
- Lok ASF. Hepatitis B infection: pathogenesis and management. Journal of Hepatology. 2000;32:89–97. doi: 10.1016/s0168-8278(00)80418-3. [DOI] [PubMed] [Google Scholar]
- Makuwa M, Souquiere S, Telfer P, Apetrei C, Vray M, Bedjabaga I, et al. Identification of hepatitis B virus subgenotype A3 in rural Gabon. J Med Virol. 2006;78:1175–1184. doi: 10.1002/jmv.20678. [DOI] [PubMed] [Google Scholar]
- Manzin A, Salvoni G, Bagnarelli P, Menzo S, Carloni G, Clementi M. A single-step DNA extraction procedure for the detection of serum hepatitis B virus sequences by the polymerase chain reaction. J Virol Methods. 1991;32:245–253. doi: 10.1016/0166-0934(91)90055-5. [DOI] [PubMed] [Google Scholar]
- Mekuria G, Ramesh SA, Alberts E, Bertozzi T, Wirthensohn M, Collins G, Sedgley M. Comparison of ELISA and RT-PCR for the detection of Prunus necrotic ring spot virus and prune dwarf virus in almond (Prunus dulcis) Journal of Virology Methods. 2003;114(1):65–69. doi: 10.1016/j.jviromet.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Moore C. Point-of-care tests for infection control: should rapid testing be in the laboratory or at the front line? Journal of Hospital Infection. 2013;85:1. doi: 10.1016/j.jhin.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Motayo BO, Faneye AO, Udo UA, Olusola BA, Ezeani I, Ogiogwa JI. Seroprevalence of transfusion transmissible infections (TTI), in first time blood donors in Abeokuta, Nigeria. Afr Health Sci. 2015;15(1):19–24. doi: 10.4314/ahs.v15i1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudawi H, Hussein W, Mukhtar M, Yousif M, Nemeri O, Glebe D, et al. Overt and occult hepatitis B virus infection in adult Sudanese HIV patients. Int J Infect Dis. 2014;29:65–70. doi: 10.1016/j.ijid.2014.07.004. S1201-9712(14)01588-4. [DOI] [PubMed] [Google Scholar]
- Muktar HM, Suleman AM, Jones M. Safety of blood transfusion: prevalence of HBsAg in blood donors in Zaria, Northern, Nigeria. Nigerian Journal of Surgical Research. 2005;7(3&4):290–292. [Google Scholar]
- Mulders MN, Venard V, Njayou M, Edorh AP, Oyefolu AOB, Kehinde MO, et al. Low genetic diversity despite hyperendemicity of hepatitis B virus genotype E throughout West Africa. Journal of Infectious Diseases. 2004;190:400–408. doi: 10.1086/421502. [DOI] [PubMed] [Google Scholar]
- Musa B, Bussell S, Borodo MM, Samaila AA, Femi OL. Prevalence of hepatitis B virus infection in Nigeria, 2000-2013: A systematic review and meta-analysis. Niger J Clin Pract. 2015;18:163–72. doi: 10.4103/1119-3077.151035. [DOI] [PubMed] [Google Scholar]
- Nagalo BM, Bisseye C, Sanou M, Kienou K, Nebie YK, et al. Seroprevalence and incidence of transmission tranmissible infectious diseases among blood donors from regional blood transfusion centers in Burkina Faso, West Africa. Trop Med Int Health. 2012;17(2):247–253. doi: 10.1111/j.1365-3156.2011.02902.x. [DOI] [PubMed] [Google Scholar]
- N’Dri-Yoman T, Anglaret X, Messou E, Attia A, Polneau S, Toni T, et al. Occult HBV infection in untreated HIV-infected adults in Cote d’Ivoire. Antivir Ther. 2010;15:1029–1034. doi: 10.3851/IMP1641. [DOI] [PubMed] [Google Scholar]
- Newman BH. Vasovagal reaction rates and body weight: findings in high- and low-risk populations. Transfusion. 2003;43:1084–1088. doi: 10.1046/j.1537-2995.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- Newman BH. Donor reactions and injuries from whole blood donation. Transfusion Med Rev. 2006;11:64–75. doi: 10.1016/s0887-7963(97)80011-9. [DOI] [PubMed] [Google Scholar]
- Nna E, Mbamalu C, Ekejindu I. Occult hepatitis B viral infection among blood donors in South-Eastern Nigeria. Pathog Glob Health. 2014;108:223–228. doi: 10.1179/2047773214Y.0000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwokediuko SC. Chronic Hepatitis B: Management Challenges in Resource- Poor Countries. Hepatology. 2011;11(10):786–763. doi: 10.5812/kowsar.1735143X.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odaibo GN, Ola SO, Olaleye DO. Hepatitis B Virus DNA in Patients with HBsAg in South Western Nigeria. Journal of Medical Virology. 2013;85:214–218. doi: 10.1002/jmv.23418. [DOI] [PubMed] [Google Scholar]
- Odemuyiwa SO, Mulders MN, Oyedele OI, Ola SO, Odaibo GN, Olaleye DO, et al. Phylogenetic analysis of new hepatitis B virus isolates from Nigeria supports endemicity of genotype e in west Africa. Journal of Medical Virology. 2001;65:463–469. [PubMed] [Google Scholar]
- Ola SO, Anomneze EE, Chukwuani CM, Ojo OS, Ndububa DA, Onyenkwe B, Nasidi A. Interferon alfa-2a (Roferon-A) in the management of Chronic Hepatitis B infection: Results of an open prospective study in Nigerian patients. West Afri Med J. 2000;19:259–264. [PubMed] [Google Scholar]
- Ola SO, Otegbayo JA, Odaibo GN, Olaleye OD, Olubuyide IO. Serum hepatitis C virus and hepatitis B surface antigenaemia in Nigerian patients with acute icteric hepatitis. West Afr J Med. 2002;21:215–217. doi: 10.4314/wajm.v21i3.28033. [DOI] [PubMed] [Google Scholar]
- Ola SO, Otegbayo JA, Odaibo GN, Olaleye DO, Olubuyide IO, Summerton CB. Occult HBV infection among a cohort of Nigerian adults. J Infect Dev Ctries. 2009;3:442–446. doi: 10.3855/jidc.415. [DOI] [PubMed] [Google Scholar]
- Christophe Olinger M, Venard Véronique, Njayou Mounjohou, Bola Oyefolu Akeeb O, Maïga Ibrahim, Kemp Alain J, Omilabu Sunday A, Faou Alain le, Muller Claude P. Phylogenetic analysis of the precore/core gene of hepatitis B virus genotypes E and A in West Africa: new subtypes, mixed infections and recombinations. Journal of General Virology. 2006;87:1163–1173. doi: 10.1099/vir.0.81614-0. [DOI] [PubMed] [Google Scholar]
- Olokoba AB, Salawu FK, Danburam A, Desalu OO, Olokoba LB, et al. Viral Hepatitides in voluntary blood donors in Yola, Nigeria. European Journal of Scientific Research. 2009;31(3):329–334. [Google Scholar]
- Amadin A Olotu A, Oyelese Adesola O, Salawu Lateef, Audu Rosemary A, Okwuraiwe Azuka P, Aboderin Aaron O. Occult Hepatitis B virus infection in previously screened, blood donors in Ile-Ife, Nigeria: implications for blood transfusion and stem cell transplantation. Virology Journal. 2016;13:76. doi: 10.1186/s12985-016-0533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opaleye OO, Oluremi AS, Atiba AB, Adewumi MO, Mabayoje OV, Donbraye E, Ojurongbe O, Olowe OA. Occult Hepatitis B Virus Infection among HIV Positive Patients in Nigeria. Journal of Tropical Medicine. 2014:796121. doi: 10.1155/2014/796121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opaleye OO, Tong HV, Bui Tien S, Fagbami AH, Adekanle O, Ojurongbe O, et al. Occult Hepatitis B Virus Infection in Nigerian Blood Donors and Hepatitis B Virus Transmission Risks. PLoS ONE. 2015;10(7):e0131912. doi: 10.1371/journal.pone.0131912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panhotra BR, Bahrani A, Joshi CS, Hassan ZU. Occult hepatitis B virus infection among anti-HBc positive blood donors: Necessitates substitution of screening by HBVNAT. Journal of Infection. 2005;51:263. doi: 10.1016/j.jinf.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Prati D, Gerosa A, Porretti L. Occult HBV infection and blood transfusion. Journal of Hepatology. 2006;44:818. doi: 10.1016/j.jhep.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Raimondo G, Pollicino T, Cacciola I, Squadrito G. Occult hepatitis B virus infection. J Hepatol. 2007;46:160–170. doi: 10.1016/j.jhep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Raimondo G, Allain JP, Brunetto MR, Buendia MA, Chen DS, Colombo M, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008;49:652–657. doi: 10.1016/j.jhep.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Salihu I. Policies, processes and procedures to ensure safe blood for transfusion. The Medical Laboratory Scientist. 2013;34(1&2):6–12. [Google Scholar]
- Samal J, Kandpal M, Vivekanandan P. Molecular mechanisms underlying occult hepatitis B virus infection. Clin Microbiol Rev. 2012;25:142–163. doi: 10.1128/CMR.00018-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setia S, Gambhir RS, Kapoor V, Jindal G, Garg S, Setia S. Attitudes and Awareness Regarding Hepatitis B and Hepatitis C Amongst Health-care Workers of a Tertiary Hospital in India. Annual Medical Health Science Research. 2013;3(4):551–558. doi: 10.4103/2141-9248.122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunbul M. Hepatitis B virus genotypes: Global distribution and clinical importance. World Journal of Gastroenterology. 2014;20(18):5427–5434. doi: 10.3748/wjg.v20.i18.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susmita M, Srijita N, Subrata B, Salil S, Malay S. Performance and diagnostic usefulness of commercially available enzyme linked immunosorbent assay and rapid kits for detection of HIV, HBV and HCV in India. Virology Journal. 2012;9:290. doi: 10.1186/1743-422X-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toan NL, Song LH, Kremsner PG, Duy DN, Binh VQ, Koeberlein B. Impact of the hepatitis B virus genotype and genotype mixtures on thecourse of liver disease in Vietnam. Hepatology. 2006;43:1375–1384. doi: 10.1002/hep.21188. [DOI] [PubMed] [Google Scholar]
- Trouern-Trend JJ, Cable RG, Badon SJ, et al. A case-controlled multicenter study of vasovagal reactions in blood donors: influence of sex, age, donation status, weight, blood pressure, and pulse. Transfusion. 1999;39:316–320. doi: 10.1046/j.1537-2995.1999.39399219291.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Hepatitis B Global Infection rate. 2006 Available from: http://www.pkids.org/files/pdf/phr/02-09globalhbv.pdf.
- WHO. Hepatitis B Fact sheet N° 204 2015 [Google Scholar]


