Abstract
Background
Painful intervertebral disc (IVD) degeneration has tremendous societal costs and few effective therapies. Intradiscal tumor necrosis factor‐alpha (TNFα) is commonly associated with low back pain, but the direct relationship remains unclear.
Purpose
Treatment strategies for low back pain require improved understanding of the complex relationships between pain, intradiscal pro‐inflammatory cytokines, and structural IVD degeneration. A rat in vivo lumbar IVD puncture model was used to 1) determine the role of TNFα in initiating painful IVD degeneration, and 2) identify statistical relationships between painful behavior, IVD degeneration, and intradiscal pro‐inflammatory cytokine expression.
Methods
Lumbar IVDs were punctured anteriorly and injected with TNFα, anti‐TNFα, or saline and compared with sham and naive controls. Hindpaw mechanical hyperalgesia was assayed weekly to determine pain over time. 6‐weeks post‐surgery, animals were sacrificed, and IVD degeneration, IVD height, and intradiscal TNFα and interleukin‐1 beta (IL‐1β) expressions were assayed.
Results
Intradiscal TNFα injection increased pain and IVD degeneration whereas anti‐TNFα alleviated pain to sham level. Multivariate step‐wise linear regression identified pain threshold was predicted by IVD degeneration and intradiscal TNFα expression. Pain threshold was also linearly associated with IVD height loss and IL‐1β.
Discussion
The significant associations between IVD degeneration, height loss, inflammation, and painful behavior highlight the multifactorial nature of painful IVD degeneration and the challenges to diagnose and treat a specific underlying factor. We concluded that TNFα is an initiator of painful IVD degeneration and its early inhibition can mitigate pain and degeneration. Intradiscal TNFα inhibition following IVD injury may warrant investigation for its potential to alter downstream painful IVD degeneration processes.
Keywords: axial back pain, discogenic pain, disc height, infliximab, intervertebral disc degeneration, low back pain, mechanical hyperalgesia, tumor necrosis factor‐alpha
1. INTRODUCTION
Low back pain has a lifetime prevalence of up to 85% and is the leading cause of disability worldwide.1, 2, 3 Intervertebral disc (IVD) degeneration is highly associated with back pain.4, 5, 6, 7, 8 However, many patients with magnetic resonance imaging evidence for IVD degeneration do not report back pain,9, 10 highlighting challenges in diagnosis and treatment. Consequently, nonsurgical interventions are most commonly recommended, and often of limited efficacy.11, 12 Surgery for disc degeneration has also yielded mixed clinical results. Specific phenotypes of painful IVD degeneration have been introduced to help distinguish aging from painful IVD degeneration conditions, and these phenotypes often involve structural defects and pro‐inflammatory conditions.4, 7, 13, 14, 15, 16 IVD injuries are known to precipitate a strong pro‐inflammatory responses with macrophage infiltration that can induce permanent alterations to IVD structure and function.17, 18, 19 Together, these findings demonstrate that IVD degeneration and pro‐inflammatory cytokines play important roles in back pain, but the relationships are complex and nonlinear. A better understanding of the relationship between IVD degeneration and back pain is an important societal research priority, and a clearer understanding may provide insights to identifying therapeutic targets and to developing preventative treatment strategies for discogenic pain.
IVD degeneration‐related pain is multifactorial and associated with intradiscal inflammation, neurovascular ingrowth into the IVD, sensitization of nervous system (ie, upregulation of pain‐related neuropeptides in the dorsal root ganglia), impingement of adjacent nerve roots, biomechanical instability, and increased demand on the surrounding spinal muscles.20, 21, 22, 23, 24 In particular, intradiscal pro‐inflammatory cytokines tumor necrosis factor‐alpha (TNFα) and interleukin‐1 beta (IL‐1β) are highly implicated in the development and progression of IVD degeneration and discogenic pain, and these cytokines can be produced by native nucleus pulposus (NP) and annulus fibrosus (AF) cells as well as infiltrating inflammatory cells.18, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 Upon IVD injury, macrophages and mast cells are recruited and release TNFα and IL‐1β in the IVD and also induce native IVD cells to further produce pro‐inflammatory cytokines, including TNFα, IL‐1β, IL‐6, and IL‐8.35, 36, 37 TNFα is highly associated with IVD degeneration because it has been demonstrated to promote extracellular matrix degradation via upregulation of catabolic mediators, including matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS).38, 39, 40 TNFα can also upregulate substance P, nerve growth factor (NGF), and vascular endothelial growth factor production, which may induce painful conditions through sensitization of the nervous system and neurovascular ingrowth into the IVD.41, 42, 43, 44 The importance of TNFα in discogenic back pain led to multiple human clinical trials treating chronic discogenic pain with TNFα inhibitors, yet results were mixed highlighting a need for further research.45, 46, 47 There is a high research priority for investigating a direct relationship between TNFα and discogenic back pain that can further assess contribution of specific pain generators, including intradiscal pro‐inflammatory cytokines and structural IVD degeneration, and such a controlled investigation requires an animal study.
Animal models are commonly used for studying IVD degeneration, with pain often used as motivation for studying IVD degeneration. In vivo rodent models play an important role in determining the underlying pathophysiology of painful IVD degeneration because known quantitative methods are available that can characterize specific painful responses.24, 48 However, few studies have directly assessed the pain associated with IVD degeneration, and no study to date has investigated the direct associations between pain, structural degeneration, and intradiscal pro‐inflammatory cytokines in order to characterize the most important contributors to the painful process. Furthermore, rats offer advantages over mice because the larger size enables more precise control of induced injuries. Annular injury models (using puncture or stab injury) are commonly adopted to induce IVD degeneration in rat models because the severity of injury can be controlled. Our group previously developed an in vivo painful IVD degeneration model in rat and showed that AF puncture with intradiscal injection of phosphate buffered saline (PBS) produced painful behavior and degeneration in the injured IVDs, and intradiscal injection of TNFα into the injured IVD further increased painful behavior up to 6 weeks post‐injury.48, 49 The findings suggested that TNFα plays an important role in the development of IVD degeneration‐related pain; however, it is unclear whether inhibiting TNFα at the time of IVD injury can prevent or mitigate IVD degeneration and degeneration‐related pain. Furthermore, the relationships between pain, intradiscal pro‐inflammatory cytokines, and IVD structural degeneration in this model are unknown.
This study applied an in vivo rat model of painful IVD degeneration to determine (1) a role of TNFα in causing painful IVD degeneration following an IVD puncture injury; and (2) the relationships between pain, IVD structural degeneration, and intradiscal pro‐inflammatory cytokines in this model. We hypothesized that annular injury with intradiscal administration of TNFα would increase painful IVD degeneration, whereas blocking TNFα at the time of injury with intradiscal injection of anti‐TNFα would prevent this painful IVD degeneration cascade. We also hypothesized that the painful behavior would be multifactorial but be predicted by intradiscal pro‐inflammatory cytokines expression and IVD structural degeneration.
2. MATERIALS AND METHODS
2.1. Experimental design
A total of 36 skeletally mature50 male Sprague‐Dawley rats (4‐5 months old) were used and randomly assigned into one of five experimental groups: naive (n = 8), sham (n = 8), PBS (n = 8), TNFα (n = 6), or anti‐TNFα (n = 6). In the PBS, TNFα, and anti‐TNFα groups, rat lumbar IVDs were exposed, punctured, and intradiscally injected with PBS, TNFα, or anti‐TNFα, respectively. The injection of TNFα aimed to further increase the content of intradiscal TNFα in addition to the expected inflammatory response resulting from IVD injury; while anti‐TNFα was injected to downregulate the intradiscal TNFα response induced by IVD injury. The sham group involved surgery to expose lumbar IVDs only without puncture or injection, and naive group involved no surgical procedures at all. Pain behavior and IVD height were measured before surgery and then weekly after surgery using hindpaw mechanical hyperalgesia test and lateral radiographs, respectively. After pain behavior and radiographic measurements were taken at the 6‐week time point, the rats were euthanized, and the lumbar spines were isolated for morphological and biochemical analyses. All experimental procedures were approved by the Institutional Animal Care and Use Committee.
2.2. Lumbar IVD puncture and injection injury
Surgery was performed under sterile conditions and general anesthesia (2% isoflurane in oxygen). The lumbar spine was accessed via an anterior approach with an abdominal midline incision. The lumbar spine was visualized, and the IVD levels were identified using the pelvic rim and aortic bifurcation as anatomical landmarks. L3/4, L4/5, and L5/6 IVDs were then punctured along the midline with a 26‐gauge needle at a depth of 3.0 mm guided by a needle stopper followed by intradiscal injection of PBS (2.5 μL), TNFα (0.25 ng in 2.5 μL; 80045RNAE50; Sino Biological Inc., Beijing, China),49 or anti‐TNFɑ (2.5 μL, 0.5 mg/kg; Remicade; Janssen Biotech Inc., Horsham, Pennsylvania),51 for PBS, TNFα, and anti‐TNFα groups, respectively, with all three IVDs receiving the same injectate (Figure 1). The anti‐TNFα agent, infliximab, is a monoclonal antibody that binds with high specificity to soluble and transmembrane TNFα and prevents TNFα from binding to its receptor thereby blocking its pro‐inflammatory effects. The peritoneum was then closed with 3‐0 silk sutures, and the skin was closed with 4‐0 nylon sutures. Rats were allowed ad libitum access to food and water. Rats were closely monitored for complications. Volume and method of intradiscal injection were determined from preliminary in vitro and in vivo studies demonstrating no visible fluid leakage or nerve irritation.
Figure 1.
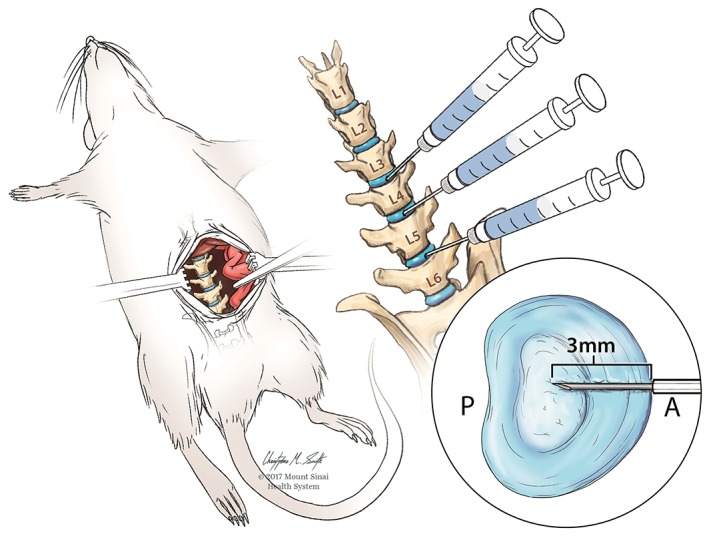
Induced intervertebral disc (IVD) injury illustration. Image created from Mount Sinai Health System. Used with permission
2.3. Pain behavior measurement
Severity of pain was measured using hindpaw mechanical hyperalgesia test, shown to be a sensitive measurement technique to quantify the pain associated with IVD degeneration in rats.48 A decrease in paw withdrawal threshold indicated an increase in pain sensitivity. The test was carried out in wire mesh‐floored cages, and the rats were placed in the cages at least 20 min prior to measurement for acclimation. Calibrated von Frey filaments, ranging from 0.4 to 26.0 g (Stoelting, Wood Dale, Illinois), were then applied to the plantar surface of the hindpaw with sufficient force to cause buckling of the filament. The 50% paw withdrawal thresholds were determined for both left and right hindpaws using the up‐down method with three trials for each side.52 The withdrawal thresholds from left and right hindpaws were then averaged for analysis and normalized to preoperative values.
2.4. Radiographic IVD height measurement
IVD heights of noninjured IVD (ie, L2/3 IVD) and injured IVDs (i.e. L4/5 and L5/6 IVDs) were measured via lateral radiographs with the animals under general anesthesia. Animals were anesthetized for approximately 10 min before taking the radiographs to minimize the effect of anesthesia on IVD height resulting from muscle relaxation and IVD swelling.53 After the radiographic film was digitized using a flatbed scanner with backlighting, the image was magnified and analyzed in ImageJ. Both upper and lower vertebral boundaries of the motion segment were manually identified, and coordinates of the vertebral boundaries were obtained. The coordinates were then transferred to the MATLAB (Mathworks, Inc., Natick, Massachusetts),49 which measured the averaged distance between the vertebral boundaries. A step wedge was used as a scale reference. Each disc was measured thrice and averaged for analysis. The IVD heights from the injured IVDs were also averaged for analysis and normalized by dividing by the presurgical heights of each IVD. Interrater and intrarater reliability tests were used to determine the consistency and repeatability for measuring IVD height using this technique. A total of 30 IVDs were randomly picked and measured by three researchers on two separate days with 7 days apart. The interrater reliability was excellent with intraclass correlation coefficient, ICC(3,3), of 0.983; and the interrater reliability was also excellent with ICC(3,3) for the three researchers of 0.992, 0.993, and 0.977 for the three researchers, respectively.
2.5. Tissue harvesting
Rats were euthanized at 6 weeks after surgery via carbon dioxide inhalation. Immediately after sacrifice, the lumbar spine (L1/2‐L3/4) was harvested, fixed in formalin, decalcified, embedded, and sectioned sagittally at 5 μm intervals.
2.6. IVD morphology and semi‐quantitative degeneration grading
Mid‐sagittal sections were stained with safranin‐O/light green/hematoxylin to assess IVD morphology and evaluated using bright‐field microscopy. The severity of IVD degeneration of each section was assessed using a semi‐quantitative IVD degeneration grading scale adapted from the grading scales proposed by Masuda et al54 and Rutges et al.55 The combined degeneration scale consisted of five categories, including (1) AF, (2) border between AF and NP, (3) cellularity of NP, (4) matrix of NP, and (5) endplate (Table 1). Each category received a score of 0‐2 with score 0 for normal morphology and score 2 for characteristic severe degeneration; therefore, the overall degenerative score for each section was between 0 and 10. All sections were assessed by two researchers blinded to the experimental groups. Intra‐ and inter‐rater reliability scores were obtained for error analysis purposes. Interrater reliability was excellent with intraclass correlation coefficient, ICC(1,3), for the two researchers of 0.961 and 0.968; and interrater reliability was excellent with intraclass correlation coefficient, ICC(1,3), of 0.887. Each section was graded twice at least 2 weeks apart by each researcher, and the four grades were then averaged for analysis.
Table 1.
Intervertebral disc (IVD) degeneration grading scalea
| Annulus fibrosus | ||
| Grade | 0 | Normal, pattern of fibrocartilage lamellae (U‐shaped in the posterior aspect and slightly convex in the anterior aspect) without ruptured fibers and without a serpentine appearance anywhere within the annulus |
| 1 | Ruptured or serpentine pattern fibers in less than 30% of the annulus | |
| 2 | Ruptured or serpentine pattern fibers in greater than 30% of the annulus | |
| Border between annulus fibrosus and nucleus pulposus | ||
| Grade | 0 | Normal |
| 1 | Minimally interrupted | |
| 2 | Moderate/severe interruption | |
| Cellularity of nucleus pulposus | ||
| Grade | 0 | Normal cellularity with large vacuoles in the gelatinous structure of the matrix |
| 1 | Slight decrease in the number of cells and fewer vacuoles | |
| 2 | Moderate/severe decrease (>50%) in the number of cells and no vacuoles | |
| Matrix of nucleus pulposus | ||
| Grade | 0 | Normal gelatinous appearance |
| 1 | Slight condensation of the extracellular matrix | |
| 2 | Moderate/severe condensation of the extracellular matrix | |
| Endplate | ||
| Grade | 0 | Homogenous structure; regular thickness |
| 1 | Slight irregularity with limited number of microfractures; locally decreased thickness | |
| 2 | Severe irregularity with multiple microfractures of endplate; generalized decrease of thickness | |
2.7. Intradiscal pro‐inflammatory cytokine expressions
Expression of intradiscal pro‐inflammatory cytokines TNFα and IL‐1β was determined using immunohistochemistry. Mid‐sagittal IVD sections were selected from each rat. Sections were rehydrated, treated with protein block reagent, and incubated overnight with either rabbit polyclonal primary antibody against rat TNFα (NB600‐587; Novus, Littleton, Colorado), rabbit polyclonal primary antibody against rat IL‐1β (bs‐6319R; Bioss, Woburn, Massachusetts), or normal rabbit serum (Biocare Medical, Concord, California) as negative control. Sections were incubated with a horseradish peroxidase‐conjugated anti‐rabbit secondary antibody (MP‐7451, ImmPRESS VR Reagent; Vector Laboratories, Burlingame, California) for 30 min and 3% hydrogen peroxide for 10 min, then treated with diaminobenzidine‐based horseradish‐peroxidase substrate (ImmPACT DAB; Vector Laboratories) to visualize the immunoreactivity. Sections were counterstained with toluidine blue, dehydrated, mounted, and evaluated using bright‐field microscopy.
Nine area‐of‐interest regions were identified from each IVD section, including outer anterior AF (three), inner anterior AF (two), NP (two), and posterior AF (two). The percentage of immunopositive cells from each area‐of‐interest region was determined via manual cell counting and then averaged for analysis.
2.8. Statistical analysis
The results of hindpaw mechanical hyperalgesia and IVD height obtained at different time points were normalized to those obtained before surgery to minimize interanimal variability, and the changes of the normalized results with time within and across experimental groups were analyzed using two‐way repeated measures ANOVA with Tukey's test as post hoc comparison. The morphological degeneration grades as well as percentages of intradiscal TNFα and IL‐1β immunopositive cells across experimental groups were compared using one‐way ANOVA with Tukey's test as post hoc comparison. The correlation between paw withdrawal threshold, IVD height, IVD degeneration grade, and percentage intradiscal TNFα and IL‐1β immunopositivities were analyzed using Pearson's correlation. A multivariate stepwise linear regression analysis was used to determine the predictive factors for paw withdrawal threshold, where all other outcome measures were defined as the independent variables. All the statistical analyses were conducted using Prism version 7 (GraphPad Software, Inc., La Jolla, California) and SPSS Statistics version 22 (IBM Corporation, Armonk, New York), and the level of significance was set to 0.05. D'Agostino and Pearson test demonstrated all data were normally distributed, so parametric tests were performed for data analyses.
3. RESULTS
3.1. Surgery did not affect rat general health
Both sham surgery and spinal injury procedures were well‐tolerated by the rats. The rat mean ±SD body weight of 495 ±48 g, 504 ±52 g, 522 ±51 g, 533 ±52 g, 544 ±53 g, 555 ±53 g, and 565 ±55 g, for presurgery and postsurgery weeks 1, 2, 3, 4, 5, and 6, respectively. There were no significant differences between groups at any time point. No intraoperative complications or obvious stress or discomfort were observed from the general physical examination.
3.2. TNFα inhibition at time of IVD injury prevents development of acute and long‐term painful behavior
The continuous changes of normalized hindpaw withdrawal threshold within each group, as well as the comparison of withdrawal threshold across groups at acute (ie, 1‐week postsurgery) and long‐term (ie, 6‐week postsurgery) were documented (Figure 2). Paw withdrawal thresholds were normalized to pre‐op levels for each animal. There were no significant changes in hindpaw withdrawal thresholds among naive and sham animals across the 6‐week duration of the study (Figure 2A). The paw withdrawal thresholds were significantly decreased following TNFα and PBS injections, and maintained throughout the duration of the 6‐week study (P < .05 at all time points compared to presurgery, Figure 2A). Their withdrawal thresholds were lower than both naive and sham groups for both 1‐week and 6‐week time points (P < .05, Figure 2B,C, respectively). However, intradiscal injection of anti‐TNFα prevented the decrease in paw withdrawal threshold induced by IVD injury and did not show significant change across the 6‐week duration of the study (P > .05 compared to presurgery for all time points). Paw withdrawal thresholds following anti‐TNFα injection were significantly higher than following both PBS and TNFα injections at both 1‐week and 6‐week time points (P < .05 with both groups at both time points).
Figure 2.
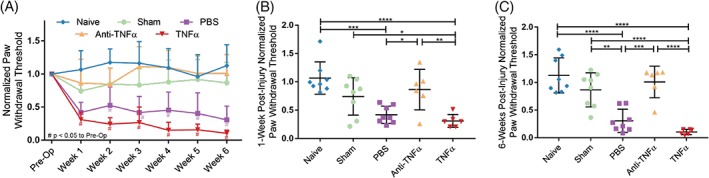
Tumor necrosis factor‐alpha (TNFα) inhibition at time of intervertebral disc (IVD) injury prevents development of acute and long‐term painful behavior. A, Normalized paw withdrawal threshold by experimental group over time. Scatter plots presents the comparison of normalized withdrawal threshold across groups at B (acute [1‐week postsurgery]) and C (long‐term [6 weeks postsurgery]) time points. Data presented as mean ± SD. # indicates P < .05 compared to presurgery baseline value. *P < .05, **P < .01, ***P < .001, ****P < .0001 by a one‐way ANOVA with Tukey's post hoc
3.3. TNFα inhibition at time of IVD injury prevents IVD structural degeneration but not height loss
Normal IVD morphology was observed in both naive and sham animals. AF puncture with intradiscal injections of PBS, TNFα, or anti‐TNFα induced observable degenerative changes, including smaller and more fibrous NP, decreased number of NP cells, less distinct NP‐AF boundary, and disorganized AF lamellae (Figure 3A). There was no evidence of NP herniation in any IVD upon histological analysis.
Figure 3.
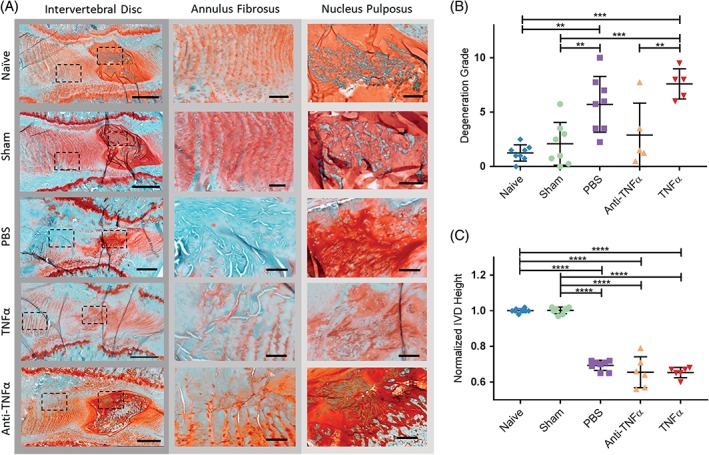
Tumor necrosis factor‐alpha (TNFα) inhibition at time of intervertebral disc (IVD) injury prevents IVD structural degeneration but not IVD height loss. A, Mid‐sagittal sections stained with hematoxylin/safranin‐O/light green. Boxed regions shown under higher magnification. B, IVD degeneration by group. C, Radiographic IVD height by group normalized to presurgery levels. Scale bars (whole IVD) = 500 μm. Scale bars (annulus fibrosus [AF] and nucleus pulposus [NP]) = 100 μm. Data presented as mean ± SD. *P < .05, **P < .01, ***P < .001, ****P < .0001 by a one‐way ANOVA with Tukey's post hoc
The semi‐quantitative degeneration grading scale showed that both naive and sham IVDs had minimal degeneration with mean ±SD degeneration grade of 1.25 ±0.74 and 2.09 ±1.97, respectively (Figure 3B). PBS‐injected (5.72 ±2.57) and TNFα‐injected (7.60 ±1.39) IVDs had significantly increased degeneration than both naive and sham IVDs and some evidence of increased glycosaminoglycan loss. Anti‐TNFα‐injected (2.90 ±2.93) IVDs had significantly lower degeneration than TNFα‐injected IVDs but did not show significant difference from naive‐, sham‐, or PBS‐injected IVDs (P = .615. P = .955, and P = .133, respectively).
The IVD heights obtained at 6‐week postsurgery were normalized to presurgery baseline. The normalized heights of all injured IVDs (including PBS, TNFα, and anti‐TNFα) were significantly smaller than those of naive and sham IVDs (Figure 3B). There was no significant difference between naive and sham groups, or among the three injury groups (Figure 3C).
3.4. TNFα inhibition at time of IVD injury prevents long‐term upregulation of intradiscal TNFα
At 6‐week postsurgery, compared to naive and sham IVDs, the percentage of intradiscal TNFα immunopositive cells was increased following PBS or TNFα injections. However, intradiscal injection of anti‐TNFα alleviated the changes in intradiscal TNFα immunopositivity induced by IVD injury and showed no significant difference from both naive or sham IVDs (Figure 4E).
Figure 4.
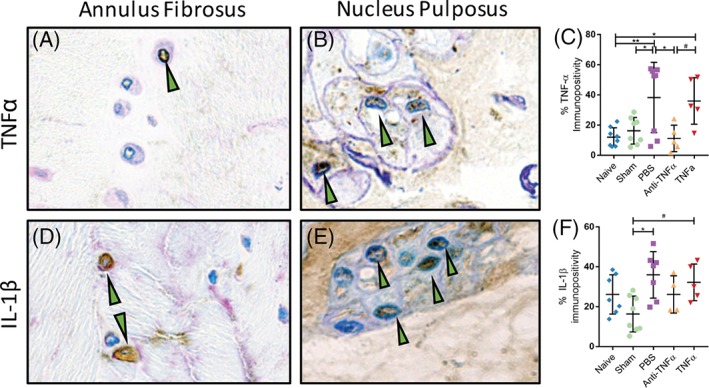
Tumor necrosis factor‐alpha (TNFα) inhibition at time of intervertebral disc (IVD) injury prevents intradiscal TNFα upregulation at 6 weeks postsurgery. Representative anti‐TNFα immunostained sections for A (annulus fibrosus) and B (nucleus pulposus). C, Quantified TNFα by group. Representative anti‐interleukin (IL)‐1β immunostained sections for D (annulus fibrosus) and E (nucleus pulposus). F, Quantified IL‐1β by group. Green arrows denote immunopositive cells. Scatter plots presents the percentage of immunopositive cells for E (TNFα) and F (IL‐1β). Data presented as mean ± SD. *P < .05, **P < .01, #P < .10 by a one‐way ANOVA with Tukey's post hoc
In contrast, the percentage of intradiscal IL‐1β immunopositive cells was only increased following PBS injection relative to sham at 6‐week postsurgery. IL‐1β trends to increase in TNFα‐injected IVDs relative to sham. The anti‐TNFα‐injected IVDs did not show significant difference in intradiscal IL‐1β immunopositivity from both naive or sham IVDs (Figure 4F).
3.5. Painful behavior predicted by IVD degeneration using stepwise linear regression analysis
Multivariate stepwise linear regression analysis showed that the normalized paw withdrawal threshold could be significantly predicted by IVD degeneration grade (β = −0.533, P = .001) and intradiscal TNFα immunopositivity (β = −0.355, P = .016) (Table 2). Normalized paw withdrawal threshold was also linearly correlated to IVD degeneration grade and intradiscal TNFα and IL‐1β immunopositivity with R 2 of 0.481, 0.315, and 0.204, respectively (Figure 5A‐C). IVD degeneration grade correlated with intradiscal TNFα and IL‐1β immunopositivity with R 2 of 0.226 and 0.123, respectively (Figure 5D,E). Intradiscal TNFα and IL‐1β immunopositivity with R 2 of 0.453 (Figure 5F).
Table 2.
Painful behavior predicted by intervertebral disc (IVD) degeneration and intradiscal tumor necrosis factor‐alpha (TNFα)a
| Multivariate | ||
|---|---|---|
| β | P‐value | |
| IVD degeneration | −0.533 | 0.001 |
| Intradiscal % TNFα positivity | −0.355 | 0.016 |
| Intradiscal % interleukin‐1 beta positivity | −0.048 | 0.777 |
| Normalized IVD height | −0.026 | 0.876 |
β, standardized coefficient of variation. Bold indicates statistical significance.
Relationship of paw withdrawal threshold to structural IVD degeneration, IVD height, and intradiscal pro‐inflammatory cytokines using multivariate stepwise linear regression.
Figure 5.
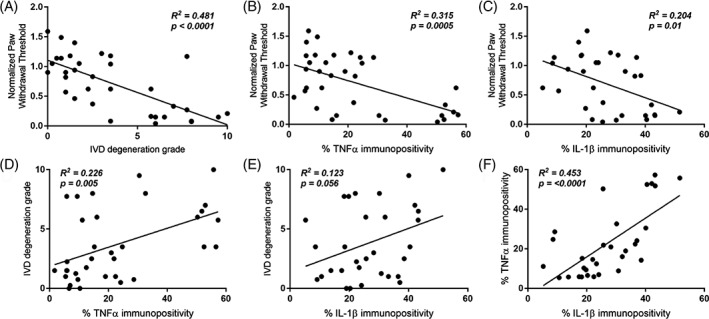
Pain, structural parameters, and intradiscal pro‐inflammatory cytokines correlate. A, Normalized paw withdrawal threshold vs intervertebral disc (IVD) degeneration. B, Normalized paw withdrawal threshold vs intradiscal tumor necrosis factor‐alpha (TNFα) immunopositivity. C, Normalized paw withdrawal threshold vs intradiscal interleukin (IL)‐1β immunopositivity. D, IVD degeneration vs intradiscal TNFα immunopositivity. E, IVD degeneration vs intradiscal IL‐1β immunopositivity. F, Intradiscal TNFα immunopositivity vs intradiscal IL‐1β immunopositivity
3.6. Pain threshold, structural degeneration and intradiscal pro‐inflammatory cytokines correlate with each other
Pearson's correlation analysis showed that the normalized paw withdrawal threshold, IVD degeneration grade, normalized IVD height, as well as intradiscal TNFα and IL‐1β immunopositivity were significantly correlated with each other (P < .05), except for the association between IVD degeneration and intradiscal IL‐1β expression, which showed a trend of correlation with R 2 of 0.351 (P = .053) (Table 3).
Table 3.
Pain, structural intervertebral disc (IVD) degeneration, and intradiscal pro‐inflammatory cytokines correlate
| IVD degeneration | Normalized IVD height | % Intradiscal TNFα positivity | % IntradiscalI L‐1β positivity | ||
|---|---|---|---|---|---|
| Normalized paw withdrawal threshold | r (P) | −0.694 (<0.001) | 0.508 (0.002) | −0.562 (0.001) | −0.452 (0.011) |
| IVD degeneration | r (P) | 1 | −0.630 (<0.001) | 0.476 (0.004) | 0.351 (0.05) |
| NormalizedIVD height | r (P) | 1 | −0.407 (0.017) | −0.466 (0.008) | |
| % Intradiscal TNFα positivity | r (P) | 1 | 0.674 (<0.001) | ||
Abbreviations: IL‐1β, interleukin‐1 beta; TNFα, tumor necrosis factor‐alpha. Bold indicates significant correlations.
4. DISCUSSION
This study identified multifactorial causes of painful IVD degeneration and determined a causal role for TNFα on the initiation of painful IVD degeneration in an in vivo rat IVD puncture and injection model. Intradiscal TNFα was directly and causally involved in the initiation of a painful IVD degeneration cascade because anti‐TNFα injection into lumbar IVDs at the time of IVD puncture injury reduced pain and IVD degeneration grade to sham levels. IVD degeneration grade and intradiscal TNFα expression were identified as predictive factors for IVD degeneration‐related pain using stepwise multivariate regression, while univariate correlation analysis further identified associations of pain with IVD height loss and intradiscal IL‐1β expression.
This study injected anti‐TNFα intradiscally at the time of injury and identified TNFα as an essential initiator of a painful IVD degeneration cascade. Relatively few animal models exist that identify causative factors underlying painful IVD degeneration without direct herniation. Annular puncture with intradiscal injection of TNFα previously demonstrated significantly increased IVD degeneration and increased painful behavior, similar to the current study which showed enhanced pain sensitivity behavior with either TNFα or PBS injection.49 Intradiscal injection of complete Freund's adjuvant into the rat lumbar IVD also significantly increased painful behavior in rats in vivo as measured by the hindpaw mechanical hyperalgesia test.56 Both of these studies implicate TNFα and intradiscal pro‐inflammatory cytokines more generally as sufficient to initiate a painful IVD degeneration cascade, yet neither determined if TNFα was an essential initiator of the painful IVD degeneration. Taken together with the literature, the results from this study suggest local TNFα inhibition at early stages after IVD injury may offer potential benefit for preventing painful IVD degeneration progression, although immediate intradiscal injection of anti‐TNFα would be difficult to directly translate to the human clinical condition.
The moderate to severe IVD degeneration of the current study is comparable to other injury models in the literature. Previous puncture injury studies demonstrated that IVD degeneration occurs with needle puncture, and IVD degeneration severity increased with needle diameter and needle puncture depth.49, 54, 57, 58, 59 Needle puncture injuries with complete AF puncture (ie, into the NP) with larger needle diameters (~50% IVD height) induced moderate to severe IVD degeneration, similar to the current study. Multiple studies also inject pro‐inflammatory factors or matrix degrading enzymes to induce matrix changes more representative of chronic degeneration conditions in acute puncture injury models. Specifically, annular puncture with chondroitinase ABC, complete Freund's adjuvant, and TNFα have all been used to induce moderate to severe IVD degeneration with significantly reduced IVD height, diminished proteoglycan content, loss of NP cells, and altered IVD biomechanics.49, 56, 60, 61 While these studies contextualize the current model of painful IVD degeneration as a model of moderate to severe IVD degeneration, several comprehensive reviews of other animal models of IVD degeneration exist.24, 62, 63, 64
TNFα inhibition has been studied clinically for treating low back pain; however, the effects are inconclusive. Patient‐reported levels of pain and disability were decreased up to 8 weeks after intradiscal administration of the TNFα inhibitor etanercept (2 mL bupivacaine with 10 mg etanercept) in a prospective, randomized clinical trial.45 Similar findings were observed in a retrospective analysis, which showed perispinal delivery of etanercept (25 mg) significantly reduced pain and weakness in patients with severe, chronic discogenic pain.46 However, another study found no reduction in patient‐reported pain 1‐month after intradiscal etanercept administration in a double‐blind placebo‐controlled study.47 The current findings in the context of the more mixed human clinical literature suggest that TNFα inhibition immediately after acute, traumatic IVD injury, or possibly early in the IVD degenerative process offers promise for mitigating a painful degenerative cascade. We speculate the limited effectiveness of TNFα inhibition in clinical studies may be the result of administration long after the onset of discogenic pain, so the timing of anti‐TNFα administration is of utmost importance. Future studies using the current animal model are warranted to determine the importance of TNFα inhibition timing, specifically whether intradiscal anti‐TNFα injection can reduce pain after the onset of painful IVD degeneration. However, we also provided local treatment at the source of pain in this model and it is possible that some of the limited efficacy of the clinical trials is due to ineffective dosing at the source of the pain. Further investigations are also warranted to assess dosing effects and administration routes, and to investigate TNFα inhibition in chronic painful IVD degeneration conditions clinically.
In this in vivo rat study, the pain behavior was significantly associated with IVD degeneration and intradiscal TNFα expression as predictive factors using multivariate stepwise linear regression, and univariate correlation analysis also showed that the pain was significantly associated with loss of IVD height and intradiscal IL‐1β expression. These results are consistent with another study that used an annular puncture model with NP removal that found a correlation between IVD degeneration and mechanical hyperalgesia, which was assayed directly on the low back.65 The multiple associations of pain with IVD structural and biochemical degenerative changes in this in vivo rat model have many similarities with painful IVD degeneration in humans, which supports the feasibility of using this rat model to understand the pathophysiology of discogenic pain.4, 6, 13, 66, 67, 68 The lack of significant herniation or endplate damage in the observed IVD degeneration in this study is suggestive of intradiscal pro‐inflammatory cytokines as a cause for pain. It should be noted that anti‐TNFα prevented IVD degeneration but not a loss of IVD height. We believe this is because anti‐TNFα mitigated some of the inflammation‐induced matrix catabolism, as observed by decreased IVD degeneration, but did not restore the IVD height loss caused by the puncture.
We chose to study the role of TNFα in initiating discogenic pain and the effect of its inhibition because it is thought to initiate an inflammatory cascade, which is then propagated by other pro‐inflammatory cytokines. TNFα stimulates IL‐1β expression, which plays a significant role in upregulating catabolic MMPs and ADAMTS enzymes.38, 69, 70, 71 Bovine IVDs cultured in the presence of TNFα had increased gene expression of multiple pro‐inflammatory and catabolic factors after 7 days, which persisted to 21 days without recovery after TNFα was removed from culture.70 Similarly, human NP cells cultured with TNFα increased gene expression of IL‐1β, IL‐6, and IL‐8, which was inhibited by early anti‐TNFα administration.72 Interestingly, human NP cells cultured with TNFα significantly upregulated IL‐1β gene expression, and NP cells treated with IL‐1β significantly upregulated MMPs to a much greater extent than when treated with TNFα,69 further highlighting the role of TNFα in inflammation initiation and of IL‐1β in enhancing catabolism. Accordingly, we believed inhibiting specifically TNFα at the time of annular injury would have the greatest effect on the inflammatory response, which the current data support. This study adds to the literature by showing that TNFα plays a key role in initiating a painful inflammatory cascade, which early inhibition can prevent. A previous clinical study found the serum half‐life of infliximab, the TNFα inhibitor used in this study, is 7 to 12 days when given intravenously.73 While the IVD is avascular and infliximab may have remained in the IVD for a longer period of time, it is highly unlikely that the original infliximab remained present at the 6‐week end point. Thus, the significant decrease in intradiscal TNFα after intradiscal anti‐TNFα injection suggested that intradiscal anti‐TNFα administration was effective in inhibiting the inflammatory cascade in response to IVD puncture injury.
Furthermore, TNFα and intradiscal pro‐inflammatory cytokines more generally are known to be associated with multiple pain pathways.16, 27, 33, 34, 74, 75 TNFα can induce inflammatory pain as it directly sensitizes sensory neurons through a reduction in the firing thresholds, which contributes to maintenance of pain.76, 77, 78, 79, 80, 81 An electrophysiology study demonstrated TNFα application to rat nociceptive neurons decreased response latency and increased spontaneous firing frequency, supporting the role of TNFα sensitizing peripheral nerves on the cellular level.76 TNFα has also been shown to induce pain on the behavioral level. TNFα injection directly into rat hindpaws resulted in increased hyperalgesia demonstrating a role of TNFα in the initiation of inflammatory pain, and this painful behavior was inhibited by local TNFα inhibition, demonstrating the potential of local TNFα inhibition to alleviate the inflammatory pain in the periphery.82 In an investigation of the inflammatory response and effect of anti‐TNFα on pain in a surgically induced IVD herniation model with autologous IVD autograft and spinal nerve ligation, local application of anti‐TNFα at the time of herniation surgery significantly improved paw withdrawal threshold in a rat model.37 These finding further support the role of TNFα in peripheral inflammatory pain and the concept of locally inhibiting TNFα at early stages after injury to prevent the development of long‐term pain. However, it should be noted that the pain pathophysiology is different between the two models. The pain pathophysiology is much clearer in IVD herniation models with inflamed IVD tissue directly impinging a nerve but more difficult to identify in discogenic pain models, which highlights the importance of the validation in the current model. TNFα upregulates NGF in IVD cells, and nociceptive nerves, usually restricted to the outer AF in normal healthy IVDs, can be stimulated with NGF to grow into the inner AF and NP as was found in the painful human IVDs.23, 41, 83, 84 In this model, anti‐TNFα may have worked in several different ways: mitigating intradiscal inflammation, thereby preventing innervated nerve stimulation, decreasing the inflammatory environment of adjacent nerve roots, or inhibiting neovascular innervation. Likely, the mitigation of pain behavior following annular injury is a combination of the aforementioned factors, and the specific mechanism requires future investigations. However, histological assessments with this model did not identify obvious nerves or vessels, suggesting neovascularization is not likely to be a dominant cause of observed pain.
The current study focused on the changes in painful behavior and the structural and biochemical changes in the IVD in response to IVD injury and was limited because we did not investigate changes in the central nervous system, which plays important roles in both acute and chronic pain and warrants future investigation. Previous work has shown TNFα upregulates NGF in NP and AF cells in vitro,41 so annular puncture with intradiscal injections of TNFα or PBS may facilitate nerve ingrowth and upregulation of neurotropic factors including NGF and VEGF, while anti‐TNFα injection mitigates nerve ingrowth and expression of these neurotropic factors. Similarly, local anti‐NGF treatment attenuated the development of mechanical hyperalgesia in a rat facet joint distraction model,85 so there is some evidence to support TNFα and NGF play important roles on pain initiation. Understanding the relationship of other inflammation‐related proteins and NGF to pain and the broader inflammatory changes after TNFα injection or inhibition is an interesting area of future investigation. Additionally, only male rats were used in this study to minimize variability in behavioral and biochemical measurements, and it should be noted that there is evidence that pain pathways differ between sexes.86, 87 While it is not expected that the conclusions of the current findings would differ across sexes, male and female rats likely have sex differences in the neural pathophysiology of painful IVD degeneration that warrant further investigation. Lastly, rodent IVDs contain different cell types to those during human degeneration, and thus the responses may vary. Nonetheless, rodents are widely used to study IVD pathologies and treatment strategies.88
In conclusion, the results demonstrate that intradiscal TNFα plays a critical role initiating an inflammatory cascade that results in painful IVD degeneration and suggest that inhibiting TNFα at early stages after IVD injury may offer potential benefit for preventing pain symptoms and structural IVD degeneration. The pain behavior following IVD injury was significantly associated with IVD degeneration and intradiscal pro‐inflammatory cytokine expressions, which supports that this in vivo rat model has a phenotype similar to the human condition of painful IVD degeneration and could be a useful tool to study the underlying pathophysiology of painful IVD degeneration and for screening therapeutic strategies for discogenic pain.
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers R01AR064157 & R01AR057397 (Iatridis, PI) and in part by the Botanical Center for Promotion of Cognitive and Psychological Resilience as supported by the National Center for Complementary and Integrative Health of the National Institutes of Health under Award Number P50AT008661 (Pasinetti, PD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. S.K.C. receives grant funding from Zimmer Biomet on projects unrelated to the current study and is a consultant for Zimmer Biomet, Stryker, Medtronic, and DePuy Synthes on projects unrelated to the current study. A.C.H. is a consultant for Zimmer Spine and Medtronic on projects unrelated to the current study.
Conflict of interest
The authors declare no potential conflict of interests.
Author contributions
T.W.E.R. and A.L. were involved in the study conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, and critical revisions. H.W. was involved in the study conception and design, acquisition of data, analysis and interpretation of data, and critical revisions. J.M.S. was responsible for the acquisition of data, analysis and interpretation of data, and critical revisions. B.A.W. and S.K.C. were responsible for the analysis and interpretation of data and critical revisions. A.C.H. was involved in the study conception and design, analysis and interpretation of data, and critical revisions. J.C.I. was involved in the study conception and design, analysis and interpretation of data, drafting of manuscript, and critical revisions.
Evashwick‐Rogler TW, Lai A, Watanabe H, et al. Inhibiting tumor necrosis factor‐alpha at time of induced intervertebral disc injury limits long‐term pain and degeneration in a rat model. JOR Spine. 2018;1:e1014. 10.1002/jsp2.1014
Thomas W. Evashwick‐Rogler and Alon Lai contributed equally to this work.
Funding information National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health, Grant/Award Number: R01AR064157; NIAMS, Grant/Award Number: R01AR057397; in part by the Botanical Center for Promotion of Cognitive and Psychological Resilience as supported by the National Center for Complementary and Integrative Health of the National Institutes of Health, Grant/Award Number: P50AT008661
REFERENCES
- 1. Walker BF. The prevalence of low back pain: a systematic review of the literature from 1966 to 1998. J Spinal Disord. 2000;13(3):205‐217. [DOI] [PubMed] [Google Scholar]
- 2. Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(6):968‐974. [DOI] [PubMed] [Google Scholar]
- 3. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163‐2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheung KMC, Karppinen J, Chan D, et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty‐three individuals. Spine. 2009;34(9):934‐940. [DOI] [PubMed] [Google Scholar]
- 5. De Schepper EIT, Damen J, van Meurs JBJ, et al. The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine. 2010;35(5):531‐536. [DOI] [PubMed] [Google Scholar]
- 6. Livshits G, Popham M, Malkin I, et al. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann Rheum Dis. 2011;70(10):1740‐1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adams MA, Dolan P. Intervertebral disc degeneration: evidence for two distinct phenotypes. J Anat. 2012;221(6):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ito K, Creemers L. Mechanisms of intervertebral disk degeneration/injury and pain: a review. Global Spine J. 2013;3(3):145‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic‐resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(3):403‐408. [PubMed] [Google Scholar]
- 10. Savage RA, Whitehouse GH, Roberts N. The relationship between the magnetic resonance imaging appearance of the lumbar spine and low back pain, age and occupation in males. Eur Spine J. 1997;6(2):106‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166(7):514‐530. [DOI] [PubMed] [Google Scholar]
- 12. Lu Y, Guzman JZ, Purmessur D, et al. Nonoperative management of discogenic back pain: a systematic review. Spine. 2014;39(16):1314‐1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheung KMC, Samartzis D, Karppinen J, Luk KDK. Are “patterns” of lumbar disc degeneration associated with low back pain?: new insights based on skipped level disc pathology. Spine. 2012;37(7):E430‐E438. [DOI] [PubMed] [Google Scholar]
- 14. Iatridis JC, Nicoll SB, Michalek AJ, Walter BA, Gupta MS. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013;13(3):243‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weber KT, Alipui DO, Sison CP, et al. Serum levels of the proinflammatory cytokine interleukin‐6 vary based on diagnoses in individuals with lumbar intervertebral disc diseases. Arthritis Res Ther. 2016;18:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakazawa KR, Walter BA, Laudier DM, et al. Accumulation and localization of macrophage phenotypes with human intervertebral disc degeneration. Spine J. 2018;18(2):343‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ulrich JA, Liebenberg EC, Thuillier DU, Lotz JC. ISSLS prize winner: repeated disc injury causes persistent inflammation. Spine. 2007;32(25):2812‐2819. [DOI] [PubMed] [Google Scholar]
- 19. Walter BA, Likhitpanichkul M, Illien‐Junger S, Roughley PJ, Hecht AC, Iatridis JC. TNFα transport induced by dynamic loading alters biomechanics of intact intervertebral discs. PLoS One. 2015;10(3):e0118358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brisby H. Pathology and possible mechanisms of nervous system response to disc degeneration. J Bone Joint Surg Am. 2006;88(suppl 2):68‐71. [DOI] [PubMed] [Google Scholar]
- 21. Faustmann PM. Neuroanatomic basis for discogenic pain. Z Orthop Ihre Grenzgeb. 2004;142(6):706‐708. [DOI] [PubMed] [Google Scholar]
- 22. Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O'Brien J, Jayson MIV. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350(9072):178‐181. [DOI] [PubMed] [Google Scholar]
- 23. Freemont AJ, Watkins A, Le Maitre C, et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197(3):286‐292. [DOI] [PubMed] [Google Scholar]
- 24. Mosley GE, Evashwick‐Rogler TW, Lai A, Iatridis JC. Looking beyond the intervertebral disc: the need for behavioral assays in models of discogenic pain. Ann N Y Acad Sci. 2017;1409(1):51‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoshida M, Nakamura T, Sei A, Kikuchi T, Takagi K, Matsukawa A. Intervertebral disc cells produce tumor necrosis factor alpha, interleukin‐1 beta, and monocyte chemoattractant protein‐1 immediately after herniation: an experimental study using a new hernia model. Spine. 2005;30(1):55‐61. [DOI] [PubMed] [Google Scholar]
- 26. Miyagi M, Ishikawa T, Orita S, et al. Disk injury in rats produces persistent increases in pain‐related neuropeptides in dorsal root ganglia and spinal cord glia but only transient increases in inflammatory mediators: pathomechanism of chronic diskogenic low back pain. Spine. 2011;36(26):2260‐2266. [DOI] [PubMed] [Google Scholar]
- 27. Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL‐1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9(4):R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ahn S‐H, Cho Y‐W, Ahn M‐W, Jang SH, Sohn YK, Kim HS. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine. 2002;27(9):911‐917. [DOI] [PubMed] [Google Scholar]
- 29. Akyol S, Eraslan BS, Etyemez H, et al. Catabolic cytokine expressions in patients with degenerative disc disease. Turk Neurosurg. 2010;20(4):492‐499. [DOI] [PubMed] [Google Scholar]
- 30. Bachmeier BE, Nerlich AG, Weiler C, et al. Analysis of tissue distribution of TNF‐alpha, TNF‐alpha‐receptors, and the activating TNF‐alpha‐converting enzyme suggests activation of the TNF‐alpha system in the aging intervertebral disc. Ann N Y Acad Sci. 2007;1096:44‐54. [DOI] [PubMed] [Google Scholar]
- 31. Dongfeng R, Hou S, Wu W, et al. The expression of tumor necrosis factor‐α and CD68 in high‐intensity zone of lumbar intervertebral disc on magnetic resonance image in the patients with low back pain. Spine. 2011;36(6):E429‐E433. [DOI] [PubMed] [Google Scholar]
- 32. Lee S, Moon CS, Sul D, et al. Comparison of growth factor and cytokine expression in patients with degenerated disc disease and herniated nucleus pulposus. Clin Biochem. 2009;42(15):1504‐1511. [DOI] [PubMed] [Google Scholar]
- 33. Park JY, Kuh SU, Park HS, Kim KS. Comparative expression of matrix‐associated genes and inflammatory cytokines‐associated genes according to disc degeneration: analysis of living human nucleus pulposus. J Spinal Disord Tech. 2011;24(6):352‐357. [DOI] [PubMed] [Google Scholar]
- 34. Weiler C, Nerlich AG, Bachmeier BE, Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine. 2005;30(1):44‐53; discussion 54. [DOI] [PubMed] [Google Scholar]
- 35. Peng B, Hao J, Hou S, et al. Possible pathogenesis of painful intervertebral disc degeneration. Spine. 2006;31(5):560‐566. [DOI] [PubMed] [Google Scholar]
- 36. Kim JH, Studer RK, Sowa GA, Vo NV, Kang JD. Activated macrophage‐like THP‐1 cells modulate anulus fibrosus cell production of inflammatory mediators in response to cytokines. Spine. 2008;33(21):2253‐2259. [DOI] [PubMed] [Google Scholar]
- 37. Takada T, Nishida K, Maeno K, et al. Intervertebral disc and macrophage interaction induces mechanical hyperalgesia and cytokine production in a herniated disc model in rats. Arthritis Rheum. 2012;64(8):2601‐2610. [DOI] [PubMed] [Google Scholar]
- 38. Séguin CA, Pilliar RM, Roughley PJ, Kandel RA. Tumor necrosis factor‐alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine. 2005;30(17):1940‐1948. [DOI] [PubMed] [Google Scholar]
- 39. Wang J, Markova D, Anderson DG, Zheng Z, Shapiro IM, Risbud MV. TNF‐α and IL‐1β promote a disintegrin‐like and metalloprotease with thrombospondin type I motif‐5‐mediated aggrecan degradation through syndecan‐4 in intervertebral disc. J Biol Chem. 2011;286(46):39738‐39749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tian Y, Yuan W, Fujita N, et al. Inflammatory cytokines associated with degenerative disc disease control aggrecanase‐1 (ADAMTS‐4) expression in nucleus pulposus cells through MAPK and NF‐κB. Am J Pathol. 2013;182(6):2310‐2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abe Y, Akeda K, An HS, et al. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine. 2007;32(6):635‐642. [DOI] [PubMed] [Google Scholar]
- 42. Ohba T, Haro H, Ando T, et al. TNF‐alpha‐induced NF‐kappaB signaling reverses age‐related declines in VEGF induction and angiogenic activity in intervertebral disc tissues. J Orthop Res. 2009;27(2):229‐235. [DOI] [PubMed] [Google Scholar]
- 43. Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res Ther. 2008;10(4):R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kemp SWP, Webb AA, Dhaliwal S, Syed S, Walsh SK, Midha R. Dose and duration of nerve growth factor (NGF) administration determine the extent of behavioral recovery following peripheral nerve injury in the rat. Exp Neurol. 2011;229(2):460‐470. [DOI] [PubMed] [Google Scholar]
- 45. Sainoh T, Orita S, Miyagi M, et al. Single intradiscal administration of the tumor necrosis factor‐alpha inhibitor, etanercept, for patients with discogenic low back pain. Pain Med. 2016;17(1):40‐45. [DOI] [PubMed] [Google Scholar]
- 46. Tobinick E, Davoodifar S. Efficacy of etanercept delivered by perispinal administration for chronic back and/or neck disc‐related pain: a study of clinical observations in 143 patients. Curr Med Res Opin. 2004;20(7):1075‐1085. [DOI] [PubMed] [Google Scholar]
- 47. Cohen SP, Wenzell D, Hurley RW, et al. A double‐blind, placebo‐controlled, dose‐response pilot study evaluating intradiscal etanercept in patients with chronic discogenic low back pain or lumbosacral radiculopathy. Anesthesiology. 2007;107(1):99‐105. [DOI] [PubMed] [Google Scholar]
- 48. Lai A, Moon A, Purmessur D, et al. Assessment of functional and behavioral changes sensitive to painful disc degeneration. J Orthop Res. 2015;33(5):755‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lai A, Moon A, Purmessur D, et al. Annular puncture with tumor necrosis factor‐alpha injection enhances painful behavior with disc degeneration in vivo. Spine J. 2016;16(3):420‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hughes PC, Tanner JM. The assessment of skeletal maturity in the growing rat. J Anat. 1970;106(pt 2):371‐402. [PMC free article] [PubMed] [Google Scholar]
- 51. Nakamae T, Ochi M, Olmarker K. Pharmacological inhibition of tumor necrosis factor may reduce pain behavior changes induced by experimental disc puncture in the rat: an experimental study in rats. Spine. 2011;36(4):E232‐E236. [DOI] [PubMed] [Google Scholar]
- 52. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55‐63. [DOI] [PubMed] [Google Scholar]
- 53. Lai A, Chow DHK, Siu WS, Holmes AD, Tang FH. Reliability of radiographic intervertebral disc height measurement for in vivo rat‐tail model. Med Eng Phys. 2007;29(7):814‐819. [DOI] [PubMed] [Google Scholar]
- 54. Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2005;30(1):5‐14. [DOI] [PubMed] [Google Scholar]
- 55. Rutges JPHJ, Duit RA, Kummer JA, et al. A validated new histological classification for intervertebral disc degeneration. Osteoarthritis Cartilage. 2013;21(12):2039‐2047. [DOI] [PubMed] [Google Scholar]
- 56. Lee M, Kim B‐J, Lim EJ, et al. Complete Freund's adjuvant‐induced intervertebral discitis as an animal model for discogenic low back pain. Anesth Analg. 2009;109(4):1287‐1296. [DOI] [PubMed] [Google Scholar]
- 57. Sobajima S, Kompel JF, Kim JS, et al. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X‐ray, and histology. Spine. 2005;30(1):15‐24. [DOI] [PubMed] [Google Scholar]
- 58. Elliott DM, Yerramalli CS, Beckstein JC, Boxberger JI, Johannessen W, Vresilovic EJ. The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine. 2008;33(6):588‐596. [DOI] [PubMed] [Google Scholar]
- 59. Martin JT, Gorth DJ, Beattie EE, Harfe BD, Smith LJ, Elliott DM. Needle puncture injury causes acute and long‐term mechanical deficiency in a mouse model of intervertebral disc degeneration. J Orthop Res. 2013;31(8):1276‐1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Norcross JP, Lester GE, Weinhold P, Dahners LE. An in vivo model of degenerative disc disease. J Orthop Res. 2003;21(1):183‐188. [DOI] [PubMed] [Google Scholar]
- 61. Boxberger JI, Auerbach JD, Sen S, Elliott DM. An in vivo model of reduced nucleus pulposus glycosaminoglycan content in the rat lumbar intervertebral disc. Spine. 2008;33(2):146‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Daly C, Ghosh P, Jenkin G, Oehme D, Goldschlager T. A review of animal models of intervertebral disc degeneration: pathophysiology, regeneration, and translation to the clinic. Biomed Res Int. 2016;2016:5952165, 1‐5952114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Singh K, Masuda K, An HS. Animal models for human disc degeneration. Spine J. 2005;5(6 suppl):267S‐279S. [DOI] [PubMed] [Google Scholar]
- 64. Lotz JC. Animal models of intervertebral disc degeneration: lessons learned. Spine. 2004;29(23):2742‐2750. [DOI] [PubMed] [Google Scholar]
- 65. Kim J‐S, Kroin JS, Li X, et al. The rat intervertebral disk degeneration pain model: relationships between biological and structural alterations and pain. Arthritis Res Ther. 2011;13(5):R165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Borenstein DG, O'Mara JW, Boden SD, et al. The value of magnetic resonance imaging of the lumbar spine to predict low‐back pain in asymptomatic subjects: a seven‐year follow‐up study. J Bone Joint Surg Am. 2001;83‐A(9):1306‐1311. [DOI] [PubMed] [Google Scholar]
- 67. MacGregor AJ, Andrew T, Sambrook PN, Spector TD. Structural, psychological, and genetic influences on low back and neck pain: a study of adult female twins. Arthritis Rheum. 2004;51(2):160‐167. [DOI] [PubMed] [Google Scholar]
- 68. Samartzis D, Karppinen J, Luk K, Cheung K. Baseline MRI characteristics in asymptomatic subjects as predictors for future first‐time LBP episode. Global Spine J. 2012;2(1 suppl):s‐0032‐1319911‐s‐0032‐1319911. [Google Scholar]
- 69. Millward‐Sadler SJ, Costello PW, Freemont AJ, Hoyland JA. Regulation of catabolic gene expression in normal and degenerate human intervertebral disc cells: implications for the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2009;11(3):R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Purmessur D, Walter BA, Roughley PJ, Laudier DM, Hecht AC, Iatridis J. A role for TNFα in intervertebral disc degeneration: a non‐recoverable catabolic shift. Biochem Biophys Res Commun. 2013;433(1):151‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL‐1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxford). 2008;47(6):809‐814. [DOI] [PubMed] [Google Scholar]
- 72. Walter BA, Purmessur D, Likhitpanichkul M, et al. Inflammatory kinetics and efficacy of anti‐inflammatory treatments on human nucleus pulposus cells. Spine. 2015;40(13):955‐963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Klotz U, Teml A, Schwab M. Clinical pharmacokinetics and use of infliximab. Clin Pharmacokinet. 2007;46(8):645‐660. [DOI] [PubMed] [Google Scholar]
- 74. Zhang J‐M, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45(2):27‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin‐1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7(4):R732‐R745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu B, Li H, Brull SJ, Zhang J‐M. Increased sensitivity of sensory neurons to tumor necrosis factor alpha in rats with chronic compression of the lumbar ganglia. J Neurophysiol. 2002;88(3):1393‐1399. [DOI] [PubMed] [Google Scholar]
- 77. Cheng J‐K, Ji R‐R. Intracellular signaling in primary sensory neurons and persistent pain. Neurochem Res. 2008;33(10):1970‐1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Andrade P, Visser‐Vandewalle V, Hoffmann C, Steinbusch HWM, Daemen MA, Hoogland G. Role of TNF‐alpha during central sensitization in preclinical studies. Neurol Sci. 2011;32(5):757‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Junger H, Sorkin LS. Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain. 2000;85(1–2):145‐151. [DOI] [PubMed] [Google Scholar]
- 80. Ozaktay AC, Kallakuri S, Takebayashi T, et al. Effects of interleukin‐1 beta, interleukin‐6, and tumor necrosis factor on sensitivity of dorsal root ganglion and peripheral receptive fields in rats. Eur Spine J. 2006;15(10):1529‐1537. [DOI] [PubMed] [Google Scholar]
- 81. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 suppl):S2‐S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107(3):660‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hayashi S, Taira A, Inoue G, et al. TNF‐alpha in nucleus pulposus induces sensory nerve growth: a study of the mechanism of discogenic low back pain using TNF‐alpha‐deficient mice. Spine. 2008;33(14):1542‐1546. [DOI] [PubMed] [Google Scholar]
- 84. Binch ALA, Cole AA, Breakwell LM, et al. Nerves are more abundant than blood vessels in the degenerate human intervertebral disc. Arthritis Res Ther. 2015;17:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kras JV, Kartha S, Winkelstein BA. Intra‐articular nerve growth factor regulates development, but not maintenance, of injury‐induced facet joint pain & spinal neuronal hypersensitivity. Osteoarthritis Cartilage. 2015;23(11):1999‐2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sorge RE, Mapplebeck JCS, Rosen S, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18(8):1081‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mogil JS, Wilson SG, Chesler EJ, et al. The melanocortin‐1 receptor gene mediates female‐specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci U S A. 2003;100(8):4867‐4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17(1):2‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]


