Abstract
The local anesthetics lidocaine and articaine are among the most widely used drugs in the dentist's arsenal, relieving pain by blocking voltage-dependent Na+ channels and thus preventing transmission of the pain signal. Given reports of infrequent but prolonged paresthesias with 4% articaine, we compared its neurotoxicity and functional impairment by screening cultured neural SH-SY5Y cells with formulations used in patients (2% lidocaine + 1:100,000 epinephrine or 4% articaine + 1:100,000 epinephrine) and with pure formulations of the drugs. Voltage-dependent sodium channels Na(v)1.2 and Na(v)1.7 were expressed in SH-SY5Y cells. To test the effects on viability, cells were exposed to drugs for 5 minutes, and after washing, cells were treated with the ratiometric Live/Dead assay. Articaine had no effect on the survival of SH-SY5Y cells, while lidocaine produced a significant reduction only when used as pure powder. To determine reversibility of blockage, wells were exposed to drugs for 5 minutes and returned for medium for 30 minutes, and the calcium elevation induced by depolarizing cells with a high-potassium solution was measured using the calcium indicator Fura-2. High potassium raised calcium in control SH-SY5Y cells and those treated with articaine, but lidocaine treatment significantly reduced the response. In conclusion, articaine does not damage neural cells more than lidocaine in this in vitro model. While this does not question the safety of lidocaine used clinically, it does suggest that articaine is no more neurotoxic, at least in the in vitro setting.
Key Words: Lidocaine, Articaine, Paresthesia, Sodium channel, Neurotoxicity, Delayed responsiveness, Calcium, Neurons
The development of safe and effective local anesthetic agents is possibly the most important advance in providing pain control in dental practice.1 The agents currently available in dentistry are extremely safe and fulfill most of the characteristics of an ideal local anesthetic. These agents can be administered with minimal tissue irritation or likelihood of inducing allergic reactions. A variety of agents are available that provide rapid onset and adequate duration of surgical anesthesia that is completely reversible, and systemic toxicity is rarely reported; these events are invariably the result of overdoses in young children.2,3
Two percent lidocaine with 1:100,000 epinephrine is the most widely used local anesthetic in the United States,1 while in Canada and several European countries, articaine with 1:100,000 epinephrine has supplanted lidocaine as the most frequently employed local anesthetic agent.4 Four percent articaine with 1:100,000 epinephrine provides more profound infiltration anesthesia than does 2% lidocaine with 1:100,000 epinephrine.5 While it is not as clear if 4% articaine with 1:100,000 epinephrine is superior in anesthetic efficacy compared with 2% lidocaine with 1:100,000 epinephrine with regard to mandibular (inferior alveolar and lingual nerve) block anesthesia, a recent meta-analysis revealed the superiority of the former when this injection technique is employed.6 A large prospective safety study of 1325 individuals revealed no difference in systemic or local toxicity between 4% articaine with 1:100,000 epinephrine and 2% lidocaine with 1:100,000 epinephrine.7 However, retrospective studies and case reports have associated the use of 4% articaine with 1:100,000 epinephrine with a higher, albeit relatively rare, incidence of paresthesia, following inferior alveolar nerve block than 2% lidocaine with 1:100,000 epinephrine.4,8 An additional study reported that articaine was shown to contribute to more than a 20-fold increase in reported paresthesia compared with all other local anesthetics combined.9 Nonsurgical cases of paresthesia in dentistry are almost exclusively related to inferior alveolar nerve block injection and appear to affect the lingual nerve more frequently than the inferior alveolar nerve.4,9 Available data indicate that 85–94% of such cases resolve spontaneously within 8 weeks; however, about two-thirds of those that do not recover quickly may never fully recover.10
This study asked whether in vitro applied articaine was more neurotoxic than lidocaine at levels used clinically and whether the channel block was more sustained with articaine than lidocaine. In contrast to our predictions, we found lidocaine both more toxic and with a greater residual block to cellular responsiveness.
METHODS
Drugs
Clinically relevant formulations of lidocaine (2% Xylocaine, Dentsply Pharmaceutical) and articaine (4% Septocaine, Septadont) were used (both with 1:100,000 epinephrine). Drugs were delivered from the cartridges at full strength or diluted 1:3 and 1:9 with DMEM/Ham F12 medium (1:1). According to the accompanying literature, each milliliter of the solution in the Xylocaine cartridge contained lidocaine hydrochloride (20 mg), epinephrine bitartrate (as base, 0.01 mg), NaCl (6.5 mg), potassium metabisulfite (1.2 mg), and edetate disodium (EDTA) (0.25 mg). Each milliliter of the solution in the Septocaine cartridge contained articaine hydrochloride (40 mg), epinephrine tartrate (as base, 0.01 mg), NaCl (1.6 mg), and sodium metabisulfite (0.5 mg). Additional experiments were performed with pure powdered lidocaine (RBI, No. L-102) and articaine (obtained from Septodont) to test for the effects of these additional constituents, particularly EDTA. Drugs were dissolved in DMEM/Ham F12 medium, and concentrations were chosen so that the maximum levels were approximately the same for drugs in powder and cartridge formulations.
Cell Culture
The SH-SY5Y neuroblastoma cell line was used to examine the effects of the 2 drugs on cell survival. Cells were maintained in DMEM/Ham F12 medium (1:1), 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% amphotericin B (Fungizone). In some experiments, wells were coated with poly-L-lysine (Peptides International, UKK-0356). Cells were grown for a minimum of 5 days. Initial experiments were performed on differentiated SH-SY5Y cells induced by reducing fetal bovine serum to 1%, adding 10 μM retinoic acid 1 and 3 days after plating, and then adding brain-derived neurotrophic factor (25 ng/mL) in a serum-free medium 4–5 days later. While this led to a more neural phenotype and increased expression of sodium channels described below, the reduced cell attachment complicated their use in assays. Although the use of ratiometric assays enabled measurement of viability and calcium levels independent of cell number, experiments reported here were performed on undifferentiated cells to maximize accurate evaluation.
Polymerase Chain Reaction
Confluent plates of SH-SY5Y cells were homogenized in 1 mL TRIzol (Invitrogen Corp), and total RNA was purified using RNeasy mini kit (No. 79254, Qiagen, Inc, Gaithersburg, Md). cDNA was synthesized from 500 ng of total RNA per reaction using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems No. 4368814). Quantitative polymerase chain reaction was performed using SYBR Green and the 7300 RealTimePCR system (Invitrogen Corp) as described.11 Primers used were Na(v)1.2: F: TGATGGTGATGTGTTTGTG, R: TCTCTGTCTTGTTATAGGCACTG, 109 base pairs; Na(v)1.7: F:AGACCTCTCTTTCCATGTAGATTAC, R: TGTAACTGCCTTTCTGTATTGTTG, 129 base pairs. Primers were designed from published sequences.12
Live/Dead Assay
The Live/Dead assay (ThermoFisher No. L3224) was used to determine cell viability. Cells grown in 96-well plates were washed and incubated with 10 μL ethidium homodimer-1 (EthD-1) and 5 μL calcein-AM in 5 mL medium for 30 minutes at 25°C. A positive control was applied by adding 70% methanol for 5 minutes. After washing, cells were imaged with a microplate fluorometer (Fluoroskan Ascent, Labsystems, Franklin, Mass). Calcein (Live) was excited at 488 nm and emitted at 560 nm to indicate healthy cells with functioning esterases to cleave the AM bond and render the dye fluorescent. EthD-1 was excited at 544 nm (em 590 nm) to quantify cells with compromised plasma membranes. The ratio of light excited at 488 nm to 544 nm provides an index of cell viability independent of cell number. For images, cells were exposed to full-strength lidocaine or articaine for 5 minutes, washed with medium, then to 3 μl of 1 mM Calcein AM (ThermoFisher Scientific No. C31100MP) mixed with 2 μL ethidium homodimer (ThermoFisher Scientific No. L7013) for 20 minutes at room temperature before washing then imaged on a Nikon Eclipse E600 (Nikon USA, Melville, NY) at ex 460–500 Chroma filter for live cells and ex 530–550 Chroma filter for dead cells. Images were captured with a Nikon DS-Fi1 camera and processed with ImageJ software,13 with parallel modifications performed for all images.
Cytoplasmic Ca2+ Measurement
Neuronal activity was determined by examining the influx of Ca2+ upon depolarization. The Goldman/Hodgkin/Katz equation predicts a shift in membrane potential from −71 mV to −20 mV when cells are exposed to the high-K solution; this is raised above the threshold for activation of the voltage-dependent Na+ channels, and thus an action potential is expected. This is predicted to induce an influx of Ca2+ through voltage-dependent channels, which was measured with the dye Fura-2. Calcium levels were measured from SH-SY5Y cells grown in 96-well plates with the dye Fura-2 as described.14 Basically, Fura-2 AM (ThermoFisher Scientific, No. F1201) was loaded by incubating wells for 30 minutes with 5 μM Fura-2 AM and 0.02% pluronic acid. After washing, 90 μL of isotonic control solution containing 5 mM KCl and 120 mM NaCl was added, and the ratio of light excited at 340/380 nm emitted >510 nm was determined in the microplate fluorometer. A baseline reading was obtained for 5 minutes, after which a high K+ solution was injected through the fluorimeter to give a final concentration of 50 mM KCl and 75 mM NaCl.
Statistical Analysis
Data are reported as mean ± SEM. Analysis was performed in a masked fashion whenever possible. Statistical analysis on the Live/Dead assay was performed using a Kruskal-Wallis 1-way analysis on ranks with Dunn's post hoc test versus control. Analysis of the calcium response was performed using a Student's t test. Both sets of analysis were performed using Systat Software Inc (San Jose, Calif). Results with p < 0.05 were considered significant.
RESULTS
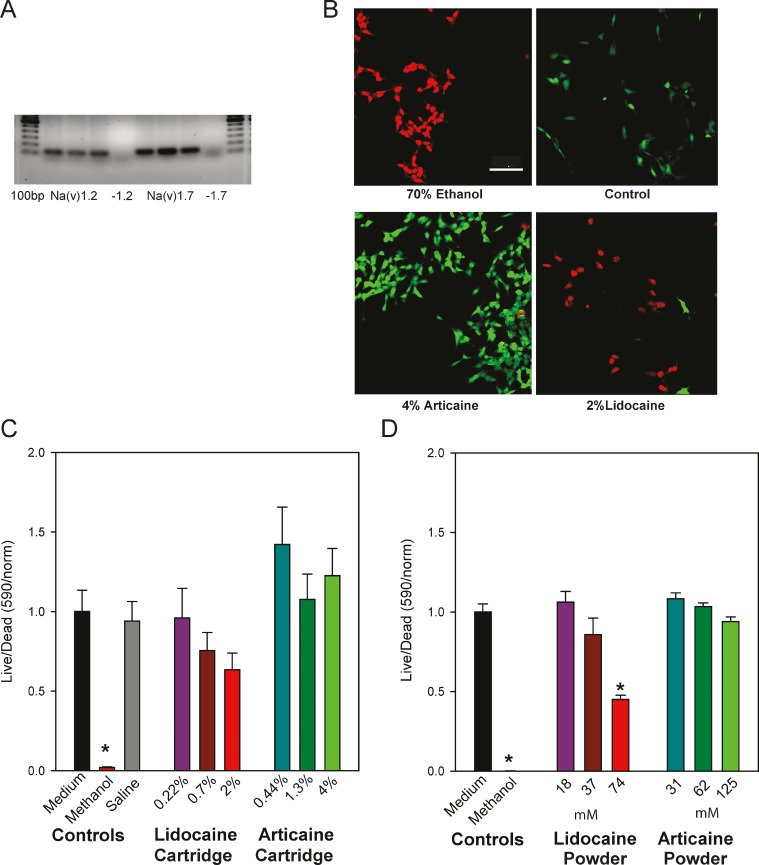
The SH-SY5Y cells used in this study expressed the voltage-dependent sodium channels Na(v)1.2 and Na(v)1.7, predicted to be targeted by the drugs (Figure 1A). To determine whether lidocaine or articaine were neurotoxic, SH-SY5Y cells were exposed to drugs for 5 minutes. Each drug was obtained directly from the cartridge used to treat patients and employed at full strength or diluted in cell medium 1:3 or 1:9. After washing, cells were exposed to the Live/Dead assay for 30 minutes, and the relative levels of green and red fluorescence were quantified (Figure 1B). Neither articaine nor lidocaine had a significant effect on cell viability at any concentration when obtained from the cartridge (Figure 1C), although 2% lidocaine increased the ratio of dead to live cells substantially.
Figure 1.
Effects of lidocaine and articaine on viability of SH-SY5Y cells. (A) Expression of Na(V) in SH-SY5Y cells. Polymerase chain reaction gel showing cells expressed mRNA for both Na(V)1.2 and Na(V)1.7. Gels show bands of expected size from 3 cell preparations. “-1.2” and “-1.7” indicate lanes where reverse transcriptase was omitted from the mix for Na(V)1.2 and Na(V)1.7, respectively. Bars are 100 base pairs. (B) Example of images of SH-SY5Y cells loaded with the Live/Dead assay in response to various conditions. Cells treated for 5 minutes with 4% articaine or 2% lidocaine (both from the cartridge), washed, then loaded with the Live/Dead dye. Positive control of cells treated with 70% ethanol are shown on the top left, while untreated cells are shown on the right. Green, calcein indicating healthy cells; red, ethidium homodimer indicating compromised cells. Bar = 100 μM. (C) Quantification of Live/Dead levels from SH-SY5Y cells treated with lidocaine + 1 : 100,000 epinephrine or articaine + 1 : 100000 epinephrine from the cartridges used clinically. The reduced viability observed using lidocaine at full strength was not significant (Kruskal-Wallis 1-way analysis on ranks with Dunn's post hoc test). Articaine did not lead to cell death at any strength. Numbers along the abscissa axis indicate the percentage of drug, with 2% lidocaine and 4% articaine the full strength from the cartridge. Numbers along the ordinate represent the ratio of light excited at 488 nm versus 544 nm, normalized to the mean control for each set. *p < .001 methanol versus saline; n = 10. (D) Quantification of the Live/Dead levels from SH-SY5Y cells treated with pure lidocaine or articaine. Lidocaine increased the number of dead cells when used in pure powdered form at the highest concentration, while pure articaine did not alter cell survival. Numbers along the abscissa indicate the concentration in mM, with the highest levels of both drugs equal to the maximum level with the cartridge. Numbers along the ordinate represent the Live/Dead ratio normalized as in C. *p < .001 (methanol and 74 mM lidocaine), n = 18.
As cells displayed some signs of detachment in preliminary trials, several steps were taken to minimize this effect. The use of ratiometric assays meant that cell survival measures were independent of cell numbers, and undifferentiated cells survived manipulations more robustly, with poly-L-lysine coating the substrate increasing this further. Of note was the presence of 0.7 mM of calcium/magnesium chelator EDTA. While this level of EDTA was less than that used experimentally to detach cells (1–10 mM), there was some concern that the presence of EDTA in the lidocaine but not articaine formulations could influence the outcome. To control for this, the effect of adding 0.7 mM EDTA on cell adherence was examined directly; there was no increase in cell detachment with EDTA, however.
The cell viability experiments were confirmed with pure lidocaine and articaine from powder to avoid influence from secondary components such as EDTA or epinephrine. Concentrations were chosen so that the highest level of lidocaine and articaine from powder equaled the full-strength drug from the cartridge. While there was no effect of purified articaine at any concentration, 74 mM of purified lidocaine significantly increased the percentage of dead cells to 55% (Figure 1D).
Delayed Neuronal Recovery
Given reports of delayed local anesthetic recovery with articaine, experiments were designed to determine whether treatment with either anesthetic led to a delayed recovery from nerve block by examining the response to depolarization 30 minutes after treatment with drugs. Specifically, the Ca2+ influx to depolarizing cells with high (50 mM) K+ solution was measured. Raising the level of extracellular K+ from 5 mM to 50 mM was calculated to raise the membrane potential from −71 mV to −20 mV, above the threshold to activation of the voltage-dependent Na(v)1.2 and Na(v)1.7 channels.15 Cellular Ca2+ was used as a proxy for membrane potential as the ratiometric output of Ca2+-sensor Fura-2 was very sensitive and provided reading independent of cell number.
Baseline levels of cytoplasmic Ca2+ were not affected by exposure to 2% lidocaine or 4% articaine 30 minutes previously (Figure 2A). However, cells treated with lidocaine displayed a significantly reduced responsiveness to depolarization as compared with controls (Figure 2B), while the effect of articaine was not significant.
Figure 2.
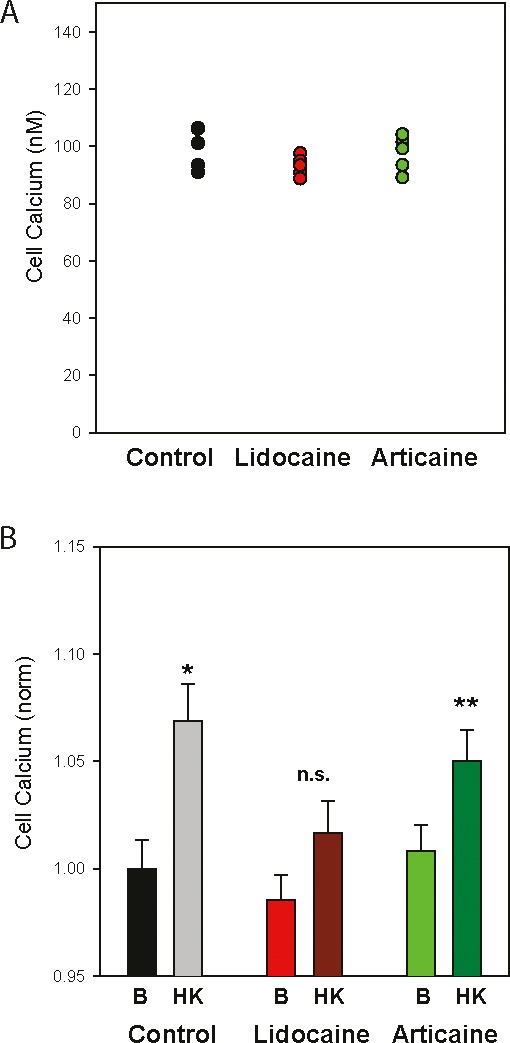
Neuronal responsiveness impaired by previous lidocaine treatment. (A) Typical baseline cytoplasmic Ca2+ levels in SH-SY5Y cells. (B) Mean levels of Ca2+ under baseline conditions (B, 5 mM K+) and after exposure to 50 mM K+ (HK) in cells exposed to 2% lidocaine, 4% articaine or control solution 30 minutes before measurements were made. Baseline Ca2+ levels show no significant difference between the 3 treatment groups. While depolarization with the high K+ solution significantly raised cellular Ca2+ levels in the control cells (*p =0.004) and those previously exposed to articaine (**p = .031), the response in cells previously exposed to 2% lidocaine was not significant, Student's t test, n = 15.
DISCUSSION
In the current study, the use of the ratiometric Live/Dead assay to determine cell viability suggests that articaine was no more likely to kill cultured neuronal SY-SY5Y cells than lidocaine. However, solutions made with pure lidocaine from powder led to a small but significant increase in neuronal death. In addition, results obtained with the ratiometric Ca2+ indicator Fura-2 imply that articaine does not produce a more sustained blockage of neural response in vitro than lidocaine. Lidocaine treatment led to a reduced cell responsiveness 30 minutes after the drugs were washed off. While these findings are the opposite of what was predicted based on the proposed enhancement of paresthesia by articaine in the clinical setting,4 data from the 2 different assays in the present study support the conclusion that articaine does not directly lead to neuronal damage in vitro. The findings agree with a report suggesting lidocaine had a lower LD50 than articaine.16 These published data were based on the production of glycolytic production of NADPH and are thus a simplistic measure of total metabolic activity. The combined use of the Live/Dead and Fura-2 assays in the present study provides a more accurate measure of cell stress that is independent of the number of cells.
While the use of drugs directly from the injection cartridges provides relevance to the clinical condition, we felt it was important to verify the results using the purified forms of the drugs. This was particularly true of the lidocaine, as inclusion of EDTA may have interacted with cells and complicated interpretation. While there was a trend toward decreased cell viability in experiments where lidocaine from the cartridge was used at full strength, cell death was significant only with the powder form. Given that lidocaine is considered a very safe drug clinically, it is likely that this level of significance in the powder form of the drug does not translate to the clinic.
While both lidocaine and articaine are thought to produce a reversible block of the voltage-dependent Na+ channels associated with the transmission of dental pain, several reports link articaine with sustained paresthesia.4,9,17 However, the current study found that neural responsiveness was reduced in SH-SY5Y cells 30 minutes after cells were exposed to lidocaine, but not articaine. Of course, the measure of cellular responsiveness used here, based on Ca2+ rise, provided an indirect measure of Na+ channel activity and may reflect effects of lidocaine downstream from the Na+ channel. The similar baseline levels in cells pretreated with lidocaine, articaine, and control solution suggest there is not an overall change in Ca2+ regulation in the cells. As exposure to high levels of KCl is expected to depolarize the cells similarly, the reduced rise in intracellular Ca2+ is predicted to reflect a difference in the activation of the voltage-dependent Na+ channels needed to depolarize the cells into the range of Ca2+ channel activation. While the precise site of action cannot be determined without direct inspection of the ionic currents, the parallel findings with the 2 assays imply articaine is not affecting the cells.
Although the results of the current study suggest articaine is no more disruptive than lidocaine to neural cells, there are several other mechanisms that could underlie differential rates of paresthesia reported in the literature. For example, the low overall occurrence suggests that genetic polymorphisms in the ionic channels may contribute, as polymorphisms in Na(v)1.7 result in a range of pain phenotypes.18 A cell culture model such as the one employed in these experiments does not account for rare genetic differences in sodium channel sensitivity to potential neurotoxic agents. The actual magnitude of the problem with articaine is also somewhat unclear, because, unfortunately, clinicians typically file FDA Medwatch reports only when there is a fear of litigation. In addition to genetic polymorphisms, local anesthetic neurotoxicity may also be concentration related. As neither articaine in the pure form nor articaine from the clinical cartridge led to issues in the present study, it is unlikely that additives increase neurotoxicity.
In summary, articaine did not produce a prolonged block of neuronal responsiveness or an increased toxicity as compared with lidocaine in SH-SY5Y cells. The use of ratiometric assays to determine viability and Ca2+ levels strengthens the conclusions. It should be stressed that numerous studies have found lidocaine to be remarkably safe in a clinical setting.1 The findings of the present study refer specifically to SH-SY5Y cells in an in vitro setting and should not be taken to imply there is any additional concern with the use of lidocaine in patients. The corollary that articaine does not produce a prolonged loss of responsiveness or cell death as compared with lidocaine under these reductionist conditions is perhaps the most relevant conclusion.
ACKNOWLEDGMENTS
This work was supported by a grant from the Rabinowitz Foundation to Drs Mitchell and Hersh. Dr Albalawi is supported by King Saud bin Abdulaziz University for Health Sciences. Portions of this work were previously presented in abstract form.19
DISCLOSURES
Dr Hersh, representing the Trustees of the University of Pennsylvania, received research grants from Septodont (the maker of articaine plus 1:100,000 and 1:200,000 epinephrine) for research he performed on the clinical development of articaine formulations between 2003 and 2005. He has also received funding from Septodont for research performed on phentolamine mesylate (Oraverse) in pediatric dental patients from 2011–2014.
REFERENCES
- 1. Moore PA, Hersh EV, . Local anesthetics: pharmacology and toxicity. Dent Clin North Am. 2010; 54: 587– 599. [DOI] [PubMed] [Google Scholar]
- 2. Goodson JM, Moore PA, . Life-threatening reactions after pedodontic sedation: an assessment of narcotic, local anesthetic, and antiemetic drug interaction. J Am Dent Assoc. 1983; 107: 239– 245. [DOI] [PubMed] [Google Scholar]
- 3. Hersh EV, Helpin ML, Evans OB, . Local anesthetic mortality: report of case. ASDC J Dent Child. 1991; 58: 489– 491. [PubMed] [Google Scholar]
- 4. Haas DA, Lennon D. A, . 21 year retrospective study of reports of paresthesia following local anesthetic administration. J Can Dent Assoc. 1995; 61: 323– 316, 319–320, 329– 330. [PubMed] [Google Scholar]
- 5. Snoeck M, . Articaine: a review of its use for local and regional anesthesia. Local Reg Anasthes. 2012; 5: 23– 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brandt RG, Anderson PF, McDonald NJ, Sohn W, Peters, MC, . The pulpal anesthetic efficacy of articaine versus lidocaine in dentistry: a meta-analysis. J Am Dent Assoc. 2011; 142: 493– 504. [DOI] [PubMed] [Google Scholar]
- 7. Malamed SF, Gagnon S, Leblanc D, . Articaine hydrochloride: a study of the safety of a new amide local anesthetic. J Am Dent Assoc. 2001; 132: 177– 185. [DOI] [PubMed] [Google Scholar]
- 8. Garisto GA, Gaffen AS, Lawrence HP, Tenenbaum HC, Haas DA, . Occurrence of paresthesia after dental local anesthetic administration in the United States. J Am Dent Assoc. 2010; 141: 836– 844. [DOI] [PubMed] [Google Scholar]
- 9. Hillerup S, Jensen R, . Nerve injury caused by mandibular block analgesia. Int J Oral Maxillofac Surg. 2006; 35: 437– 443. [DOI] [PubMed] [Google Scholar]
- 10. Pogrel MA, . Permanent nerve damage from inferior alveolar nerve blocks—an update to include articaine. J Calif Dent Assoc. 2007; 35: 271– 273. [PubMed] [Google Scholar]
- 11. Guha S, Baltazar GC, Coffey EE,et al. Lysosomal alkalinization, lipid oxidation, and reduced phagosome clearance triggered by activation of the P2X7 receptor. FASEB J. 2013; 27: 4500– 4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vetter I, Mozar CA, Durek T,et al. Characterisation of Na(v) types endogenously expressed in human SH-SY5Y neuroblastoma cells. Biochem Pharmacol. 2012; 83: 1562– 1571. [DOI] [PubMed] [Google Scholar]
- 13. Schindelin J, Rueden CT, Hiner MC, Eliceiri KW, . The ImageJ ecosystem: an open platform for biomedical image analysis. Mol Reprod Dev. 2015; 82: 518– 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reigada D, Lu W, Zhang X,et al. Degradation of extracellular ATP by the retinal pigment epithelium. Am J Physiol Cell Physiol. 2005; 289: C617– C624. [DOI] [PubMed] [Google Scholar]
- 15. Catterall WA, Goldin AL, Waxman SG, . International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005; 57: 397– 409. [DOI] [PubMed] [Google Scholar]
- 16. Malet A, Faure MO, Deletage N, Pereira B, Haas J, Lambert G, . The comparative cytotoxic effects of different local anesthetics on a human neuroblastoma cell line. Anesth Analg. 2015; 120: 589– 596. [DOI] [PubMed] [Google Scholar]
- 17. Pedlar J, . Prolonged paraesthesia. Br Dent J. 2003; 195: 119. [DOI] [PubMed] [Google Scholar]
- 18. Drenth JP, Waxman SG, . Mutations in sodium-channel gene SCN9A cause a spectrum of human genetic pain disorders. J Clin Invest. 2007; 117: 3603– 3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DiRenzo KV, Lim JL, Albalawi F, Hersh EV, Mitchell CH, . Differential effects of sodium channel blockers on neural survival and responsiveness; 2% lidocaine kills more SHSY-5Y cells and reduces cellular activity as compared to 4% articaine. J Dent Res. 2016; 95special issue A:0790. [Google Scholar]