Abstract
The β1-adrenergic receptor (β1-AR) is a major cardiac G protein-coupled receptor, which mediates cardiac actions of catecholamines and is involved in genesis and treatment of numerous cardiovascular disorders. In mammalian cells, catecholamines induce the internalization of the β1-AR into endosomes and their removal promotes the recycling of the endosomal β1-AR back to the plasma membrane; however, whether these redistributive processes occur in terminally differentiated cells is unknown. Compartmentalization of the β1-AR in response to β-agonists and antagonists was determined by confocal microscopy in primary adult rat ventricular myocytes (ARVMs), which are terminally differentiated myocytes with unique structures such as transverse tubules (T-tubules) and contractile sarcomeres. In unstimulated ARVMs, the fluorescently labeled β1-AR was expressed on the external membrane (the sarcolemma) of cardiomyocytes. Exposing ARVMs to isoproterenol redistributed surface β1-ARs into small (∼225–250 nm) regularly spaced internal punctate structures that overlapped with puncta stained by Di-8 ANEPPS, a membrane-impermeant T-tubule-specific dye. Replacing the β-agonist with the β-blocker alprenolol, induced the translocation of the wild-type β1-AR from these punctate structures back to the plasma membrane. This step was dependent on two barcodes, namely, the type-1 PDZ binding motif and serine at position 312 of the β1-AR, which is phosphorylated by a pool of cAMP-dependent protein kinases anchored at the type-1 PDZ of the β1-AR. These data show that redistribution of the β1-AR in ARVMs from internal structures back to the plasma membrane was mediated by a novel sorting mechanism, which might explain unique aspects of cardiac β1-AR signaling under normal or pathologic conditions.
Introduction
Activation of cardiac β1-adrenergic receptor (β1-AR) and β2-adrenergic receptor (β2-AR) by synaptic or circulating catecholamines increases cardiac cAMP that in turn activates cAMP-dependent protein kinase (PKA), which mediates catecholamine-dependent changes in rate, force, and speed of myocardial contractions (Lefkowitz et al., 2000). The activity of cAMP is vectored to specific cellular regions by compartmentalized signaling scaffolds that target phosphodiesterases and A-kinase anchoring proteins (AKAPs) to specific end locations within the cell (Baillie, 2009; Ellisdon and Halls, 2016). Moreover, spatial distribution of β1-AR and β2-AR in cardiomyocytes might provide an additional regulatory mechanism for controlling cAMP production and action (Valentine and Haggie, 2011). For example, spatial redistribution of cardiac β2-AR in heart failure was associated with altered compartmentalization of cAMP and its downstream signaling in the heart (Nikolaev et al., 2010).
Given the prominence of β1-ARs in regulating physiologic and pathologic signaling of catecholamine in the heart, it is important to know their distribution within the various cellular compartments of quiescent and catecholamine-activated adult rat ventricular cardiomyocytes. The distribution of many G protein-coupled receptors (GPCRs) in mammalian cells is altered by exposing these cells to chronic high concentrations of a GPCR agonist, which promotes the sequestration of many GPCRs, including the β1-AR from the membrane to intracellular endosomes (Ferguson et al., 1996; Lefkowitz, 1998; Li et al., 2013). The fate of GPCRs in endosomes is regulated by barcodes that mediate either their retention and subsequent degradation in endosomes or their sorting out of endosomes and redistribution back to the plasma membrane (Hanyaloglu and von Zastrow, 2008).
A major barcode that governs the fate of the agonist-internalized GPCRs in endosomes is a C-terminal type-1 PDZ binding motif (PBM) found in many recycling GPCRs, including the β1-ARs (Romero et al., 2011). In some cases, post-translational modifications such as ubiquitination assist the PBM in regulating the fate of a given GPCR or override the effect of the PBM on redistribution (Shenoy et al., 2001; Marchese and Trejo, 2013). Unlike other GPCRs, translocation of the agonist-internalized β1-AR from endosomes to the plasma membrane was dependent on two barcodes, the PBM and a PKA-substrate serine at position 312 (Ser312) in the third intracellular loop of the β1-AR. Inactivation of these barcodes did not affect β-agonist-mediated translocation of the β1-AR to endosomes, but inhibited β1-AR redistribution from endosomes to the plasma membrane of mammalian cells, including cardiomyocytes prepared from neonatal rodents (Gardner et al., 2004; Li et al., 2013; Nooh and Bahouth, 2017).
In addition to barcodes, several GPCR accessory proteins were implicated in the regulatory processes that governed the compartmentalization of the β1-ARs and other GPCRs. These proteins are divided into two types. The first are accessory compartmentalizing proteins such as SAP97 and AKAP5, which are involved in regulating the localization of β-adrenergic receptors (β-ARs) in the cell and in compartmentalization of cAMP, respectively (Gardner et al., 2006, 2007; Valentine and Haggie, 2011; Nooh et al., 2013). Endosomal proteins such as the PDZ protein sorting nexin-27 (SNX27) represent the second class, which are involved in the redistribution of the agonist-internalized GPCR from endosomes to the plasma membrane (Seaman et al., 2013).
These paradigms were identified in mammalian cells, but their general validity to β1-AR compartmentalization in complex cells such as adult rat ventricular myocytes (ARVMs) is unknown. ARVMs contain an extensive network of transverse tubules (T-tubules) and their juxtaposed sarcoplasmic reticula, as well as an extensive set of sarcomeres, which alone represent 45%–60% of the cardiomyocyte volume (Bers, 2001). While ARVMs express both β1-AR and β2-AR, the β1-AR complement was involved in PKA-mediated effects on contractility and in inducing hypertrophy and cardiomyocyte apoptosis (Morisco et al., 2001; Xiao, 2001; Zhu et al., 2001). A better characterization of the role of barcodes on spatiotemporal localization and redistribution of β1-AR in ARVMs would provide crucial new information on the factors that regulate compartmentalization and subsequent signaling of cardiac β1-AR.
Material and Methods
Adult Rat Ventricular Myocytes Cultures.
Animal experiments were performed according to protocols that were approved by the University of Tennessee–HSC Institutional Animal Care and Use Committee. Studies conformed to the Guide for Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (Publication No. 85-23, revised 1996, https://grants.nih.gov/grants/.../guide-for-the-care-and-use-of-laboratory-animal). Sprague-Dawley rats (200–250 g) were heparinized and anesthetized with 4% isoflurane. The hearts were excised, cannulated on a Langendorff apparatus, and then perfused for 5 minutes with oxygenated modified perfusion buffer composed of 133 mM NaCl, 4 mM KCl, 11 mM glucose, 1.2 mM NaH2PO4, 1.2 mM MgCl2, and 10 mM HEPES (pH 7.4 with NaOH), and supplemented with 0.1% butanedione monoxime until the blood cleared (Louch et al., 2011). Thereafter, the heart was perfused with perfusion buffer supplemented with 0.1% collagenase type II (CLS2; Worthington, Lakewood, NJ) for 20 minutes to break down the extracellular matrix. The enzymatically digested ventricles were minced and gently shaken in perfusion buffer supplemented with 0.2% bovine serum albumin. Undigested ventricular tissue was removed using a 250-μm-mesh sieve. Then, the concentration of Ca2+ was increased stepwise to 0.5 mM in perfusion buffer containing 0.2% bovine serum albumin. The cells were concentrated by centrifugation and resuspended in culture media composed of (per liter) 9.3 g of Medium-199 Powder (Sigma-Aldrich, St. Louis, MO), 5 mM creatine, 2 mM carnitine, 5 mM taurine, 10 mM NaHCO3, 10 mM HEPES (pH 7.4), and 100 U/ml penicillin plus 100 μg/ml streptomycin. Rod-shaped ventricular myocytes were counted with a hemocytometer, and ∼40,000 cells were plated onto laminin-coated (10 μg/ml) coverslips in tissue culture dishes. After 12 hours, the medium was aspirated and the myocytes were infected with 50 multiplicities of infection with adenoviruses harboring the desired construct for 6 hours. All confocal imaging experiments were conducted on cells that were fixed within 72 hours from preparation to minimize ongoing morphologic changes in cardiomyocytes during culture (Banyasz et al., 2008; Pavlović et al., 2010).
Antibodies, siRNA, and Additional Reagents.
Cy3-labeled anti-FLAG M2 monoclonal antibody was obtained from Sigma. Polyclonal rabbit anti-FLAG IgG and CF-350, CF-543, and CF-633; CF-770-conjugated anti-rabbit IgG and anti-mouse IgG; and Di-8-ANEPPS were obtained from Biotium (Fremont, CA). Monoclonal anti-SAP97 was obtained from Enzo Life Sciences (Farmingdale, NY), while H-89 and myristoylated PKA-inhibitor peptide (mPKI) 14-22 amide were obtained from EMD-Millipore (Billerica, MA). st-Ht31 and st-Ht31-pro were obtained from Promega (Fitchburg, WI). Anti-SNX27(366−415) (ab178388) and anti-β-actin (ab8226 and ab8227) were obtained from Abcam (Cambridge, MA). The adenovirus (Ad) containing short hairpin RNA (shRNA) for rat SAP97 (shSAP97) sense 5′-GATATCCAGGAGCATAAAT-3′ was kindly provided by M. B. Anumowno, SUNY Upstate Medical University, Syracuse, NY (Vaidyanathan et al., 2010). The sequence between 861 and 881 in AKAP150 (AAGAAGACAAAATCCAAACTT) or its inactive control (GTCTCCACGCGCAGTACATTT) was used to generate the AKAP150-specific shRNA (shAKAP5) in adenovirus. Adenoviruses pAd5CMV-NpA harboring the wild-type (WT) β1-AR, β1-AR deleted PBM (∆PDZ), mutation of Ser312 to alanine (S312A) β1-AR, were previously described (Li et al., 2013). These viruses were generated, purified, and tittered at the Vector Core of the University of Tennessee Health Sciences Center.
Acid Strip Confocal Microscopy and Dual Microscopy Protocols.
Cardiomyocytes in serum-free culture medium were incubated with either Cy3-conjugated to monoclonal anti-FLAG M2 IgG or with unconjugated rabbit anti-FLAG IgG (4 μg/ml) for 1 hour at 37°C. In some experiments diluents, 0.3 μM H-89, mPKI, or 50 µM st-Ht-31 peptides was added in the middle of the antibody incubation step. ARVMs were exposed to 10 μM isoproterenol (ISO) for 30 minutes at 37°C to activate the receptor and promote the redistribution of the agonist-mediated β1-AR. Then, the slides were chilled on ice and exposed to 0.5 M NaCl and 0.2 M acetic acid (pH 3.5) for 4 minutes on ice to strip antibody bound to the FLAG-tag of extracellularly oriented FLAG-tagged β1-AR (Ehlers, 2000; Snyder et al., 2001; Delos Santos et al., 2006). Cultures were quickly rinsed in warm culture medium and incubated in culture medium supplemented with 100 µM of the β-antagonist alprenolol (ALP) at 37°C for 60 minutes to promote the redistribution of the agonist-exposed β1-AR. After each time period, cover slips were either acid stripped as described previously, or rinsed and fixed in phosphate-buffered saline containing 4% paraformaldehyde and 4% sucrose (pH 7.4) for 10 minutes at room temperature. To permeabilize the myocytes, fixed slides were incubated in HEPES-buffered saline solution containing 0.2% Triton X-100 for 10 minutes at 4°C (Li et al., 2013).
At this stage, coded slides that were incubated with Cy3-anti-FLAG IgG were examined by confocal microscopy using Cy3 (λex 550 nm, λem 570 nm) and 4′,6-diamidino-2-phenylindole (λex 358 nm, λem 461) channels and their pseudo fluorescence red for Cy3 and pseudo fluorescence violet results for 4′,6-diamidino-2-phenylindole are presented. To label the β1-AR in cells that were incubated with rabbit anti-FLAG IgG, the slides were exposed to a 1:500 dilution of fluorescent anti-rabbit IgG for 30 minutes in blocking buffer prior to their examination by confocal microscopy. Permeabilized slides for dual confocal microscopy were incubated with 1:1000 dilution of the primary antibody under study (such as anti-SAP97 or anti-SNX27) for 30 minutes at 37°C, washed and incubated with 1:500 dilution of fluorescent anti-mouse or anti-rabbit IgG, and then analyzed by confocal microscopy. Confocal fluorescence microscopy was performed at room temperature on coded slides and optical section thicknesses of 1.0 µm images were acquired by an Olympus FV1000 confocal microscope equipped with 40 or 60 Å oil immersion (numerical aperture 1.30) objective lens, using FV10-ASW 3.1 acquisition software (Olympus, Center Valley, PA). (Nooh and Bahouth, 2017). TIFF files of each image were exported and analyzed for pixel intensity and distribution by ImageJ software (https://imagej.nih.gov/ij/). Each cell was partitioned by a line, every point of which was at a distance of 400 nm from the outer periphery of the ARVMs, using the ImageJ software. This line formed the outer limit of the area used to index pixel intensity of internal β1-ARs in a given cell, while the area formed between the peripheral cell membrane and this line was used to index the distribution of pixels associated with cell surface β1-ARs (Gardner et al., 2004, 2006, 2007; Li et al., 2013; Nooh and Bahouth, 2017).
Radioligand Binding and Western Blotting Procedures.
Radioligand binding was carried out on whole cell homogenates prepared from ARVMs that were infected with either the empty adenoviral vector or the WT β1-AR expressing virus. ARVMs were scrapped, concentrated by centrifugation, and homogenized in hypotonic lysis buffer (50 mM HEPES, pH 7.4 and 10 mM MgCl2) supplemented with 1X cOmplete protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Saturation binding experiments involved mixing equal amounts of protein (5 µg) prepared from different ARVMs with 5–200 pM (125I)Iodocyanopindolol in duplicate in the absence or presence of 100 µM ISO as described previously (Delos Santos et al., 2006). The KD and the Bmax values for (125I)Iodocyanopindolol binding were estimated by parametric fitting of the data using the Prism 7 software (GraphPad Software Inc., San Diego, CA).
For western blotting, ARVMs expressing the appropriate construct were lysed in lysis buffer composed of: 150 mM NaCl, 50 mM Tris, pH 8.0, 5 mM EDTA, 0.2% Triton X-100, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml chymostatin, and 1 mM phenyl methyl sulfonyl fluoride for 1 hour at 4°C. Then, insoluble cellular debris was removed by centrifugation at 14,000g for 15 minutes at 4°C. After equalizing protein concentrations across all samples, lysates in 2X-Laemmli sample buffer were subjected to SDS-PAGE under denaturing conditions and electroblotted to nitrocellulose. Identical gels were run and transferred for separate detection of the desired protein by western blotting, as described previously (Nooh and Bahouth, 2017).
Statistics.
Data were derived from image analysis that determined specific total pixels and pixels outside versus inside the 400-nm partition that was drawn around the inner circumference of cardiomyocytes. The ratio of pixels residing outside the 400-nm partition to that of the percentage of total pixels was calculated for each image. The average ± S.E. of percentile pixel ratios from three separate experiments derived from 10 images/experiment (n = 30 images) is presented. Statistical comparison between two groups was performed by unpaired t tests and for multiple groups by analysis of variance (ANOVA) followed by Bonferroni’s test with a single pooled variance test in which the family-wise error rate was set at 0.05, using GraphPad Prism 7 software (GraphPad Software Inc.).
Results
Spatiotemporal Localization of the Human β1-AR in ARVMs.
The ARVMs used in this study were maintained in defined media without serum to minimize their transformation and all experiments were carried out within 72 hours after their isolation (Banyasz et al., 2008). Under these conditions, cardiomyocytes maintained their rod-shaped appearance and parallel myofibrillar architecture for several days (Simpson et al., 1999). Our studies were carried out by adenoviral-mediated expression of an N-terminally modified WT β1-AR that expressed the FLAG epitope (FLAG-tagged WT β1-AR) because there were no antibodies we could find that would detect native β1-AR in mammalian cells. Radioligand binding studies indicated that infection of ARVMs with 50 multiplicities of infection of either control or WT β1-AR-expressing adenovirus increased the Bmax value of β-AR from 112 ± 19 to 216 ± 43 fmol/mg protein with a KD value of 19 ± 6 pM (n = 5). Cardiomyocytes infected with the WT FLAG-β1-AR adenovirus were incubated with Cy3-labeled anti-FLAG IgG to label the β1-AR, and then fixed and monitored by confocal microscopy (Fig. 1). The images were exported as TIFF files into the ImageJ software and analyzed for pixel distribution outside and inside a 400-nm partition drawn around the inner circumference of the ARVMs.
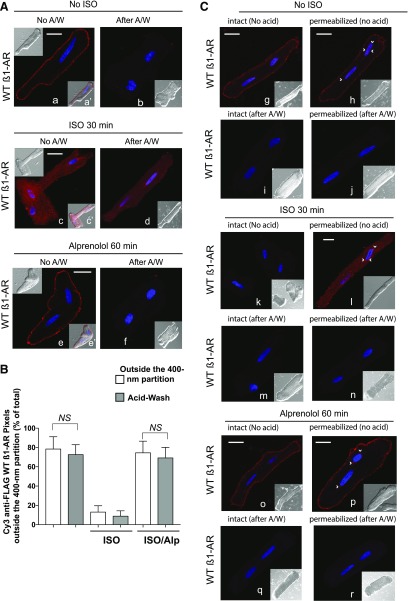
Fig. 1.
Compartmentalization of WT β1-AR in ARVMs. (A) ARVMs expressing the FLAG-tagged human WT β1-AR were prelabeled for 1 hour with Cy3-anti-FLAG antibody and fixed (image a). The rest of the slides were exposed to 10 µM ISO for 30 minutes, fixed (image c) or washed and incubated with 100 µM ALP for 1 hour, and fixed (image e). Slides corresponding to images a, c, and e were subjected to a mild acid wash prior to fixing (images b, d, and f). Cells were imaged and pseudo fluorescence of Cy3 (red) and 4′,6-diamidino-2-phenylindole (violet) is presented. Nomarski image or Cy3-superimposed to the Nomarski image are presented in the upper left or in the right lower quadrant, respectively. (B) Cy3 pixel distribution (mean ± S.E. from a total of 30 images derived from three separate experiments) in ARVMs that were untreated and exposed to ISO or ISO/ALP by the microscopic partition vs. the acid-strip procedure were analyzed by one-way ANOVA followed by Bonferroni’s test with a single pooled variance test. NS, indicates nonsignificant differences between the column pairs. (C) Intact or permeabilized ARVMs prepared as in (A), were incubated with buffer (images g–j), ISO 30′ (images k–n), or ISO/ALP (images o–r) as described in Material and Methods. These slides were incubated with Cy3-anti-FLAG IgG for 1 hour and exposed to neutral (images g, h, k, l, o, and p) or acidic buffer (images i, j, m, n, q and r), and then fixed and visualized by confocal microscopy. Each scale bar in (A) and (B) represents 15 µm. Small arrows indicate nuclear fluorescence.
In quiescent ARVMs, 79% ± 12% Cy3-conjugated anti-FLAG IgG pixel intensities (pseudo red fluorescence in Fig. 1A, images a and a′) were localized outside the 400-nm partition (P < 0.05 compared with inside the partition), indicating that WT β1-ARs were expressed at the plasma membrane of ARVMs. The orientation of Cy3 fluorescence, which indexes the orientation of the FLAG epitope, was determined by an acid wash procedure applied to intact cells prior to fixing (Fig. 1A, image b). This procedure would strip the fluorescent anti-FLAG antibody bound to the extracellular FLAG epitope. The acid wash procedure expunged 73% ± 9% of total Cy3 fluorescence, indicating that the majority of β1-ARs were confined at the surface sarcolemma membrane of ARVMs.
Exposing ARVMs to a high concentration of the β-agonist ISO resulted in redistribution of membranous Cy3 fluorescence into numerous small punctate structures throughout the cell (Fig. 1A, images c and c′). The fluorescence of the Cy3 puncta outside versus inside the 400-nm divider accounted for 13% ± 5% versus 86% ± 11% of the total pixel intensities, respectively, and these differences were statistically significant (P < 0.05). Treatment of these cells with a mild acid wash removed 8.7% ± 3% of the pixels, indicating that Cy3 pixels were intracellularly in an acid inaccessible compartment (Fig. 1A, image d).
Replacing ISO with the β-blocker ALP induced the translocation of the WT β1-AR from inside to outside the 400-nm partition (Fig. 1A, images e and e′). Under these conditions, fluorescence outside the 400-nm partition accounted for 74% ± 9% of total pixel intensity (P < 0.05, compared with inside the divider). The amount of Cy-3 fluorescence that was stripped by acid wash in these ARVMs amounted to 66% ± 8%, indicating that ALP induced the redistribution of punctal WT β1-AR to the surface membrane (Fig. 1A, image f). Statistical comparisons were made between the Cy3 pixel distributions by the partition method versus the acid-strip procedure in untreated ARVMs by ANOVA followed by Bonferroni’s test (Fig. 1B). These analyses showed statistically insignificant differences (P > 0.05) between the percentile distribution of pixels outside the 400-nm partition (the image a condition in Fig. 1A) compared with the percentile of fluorescence stripped by the acid wash method in untreated control ARVMs (the image b condition in Fig. 1A). Moreover, no statistically significant difference (P > 0.05) occurred when the percentile of Cy3 pixels outside the 400-nm partition in ISO/ALP ARVMs were compared with the percentile of fluorescence stripped by the acid wash method in ISO/ALP-treated ARVMs (Fig. 1B). These findings indicate that the partition method is a valid approach for compartmental analysis of GPCR distribution in ARVMs.
We conducted additional studies to determine the distribution of the WT β1-AR in intact versus permeabilized naive or ISO-treated ARVMs (Fig. 1C, images g and h, respectively). Staining permeabilized naive ARVMs with Cy-3 anti-FLAG revealed additional limited amounts of β1-AR staining around the nuclei (Fig. 1C, arrows in image h). The mild acidic wash stripped the Cy3 fluorescence from these cells, indicating that Cy3 staining was limited to the plasmalemmal membrane in intact or permeabilized naive ARVMs (Fig. 1C, images i and j). Staining ISO-exposed intact or permeabilized cardiomyocytes with Cy3-anti-FLAG IgG revealed that β1-AR staining in permeabilized ISO-exposed ARVMs, but not in intact ISO-exposed ARVMs (Fig. 1C, compare image k to image i). These findings suggest that ISO induced the redistribution of the WT β1-AR into compartments in ARVMs that were inaccessible to staining with Cy3-labeled anti-FLAG IgG. As expected, the acid wash procedure stripped Cy3 fluorescence from permeabilized ISO-treated ARVMs (Fig. 1C, images m and n). Finally, we determined the effect of ALP on the distribution of the WT β1-AR in intact ISO-exposed versus permeabilized ISO-exposed ARVMs (Fig. 1C, images o–r). Cy-3 staining of intact or permeabilized ISO/ALP-treated myocytes was limited to the surface cell membrane (Fig. 1C, images o and p, respectively), and was stripped by mild acid treatment (Fig. 1C, images q and r). Therefore, it appears that WT β1-AR redistributed from an acid-inaccessible compartment to an extracellularly exposed compartment in response to ALP.
Characterization of the Compartment Harboring the WT β1-AR in Agonist-Exposed ARVMs.
Membranous GPCR translocate from their membranous compartment to intracellular early endosomes in response to agonist via two main redistribution pathways, namely, clathrin coat–mediated endocytosis and caveolar mechanisms (Baig et al., 2002; Pelkmans et al., 2004; Wolfe and Trejo, 2007) (Fig. 2A). The average diameter of the puncta harboring the ISO-activated WT β1-AR in ARVMs was ∼230 ± 50 nm (n = 30, Fig. 1). This diameter is smaller than the diameter of early endosomal vesicles, which varies from 250 to 400 nm for small diameter smooth vesicles to ∼600–900 nm for large endocytic vesicles with tubular extensions (Wileman et al., 1985; Jean-Alphonse et al., 2014).
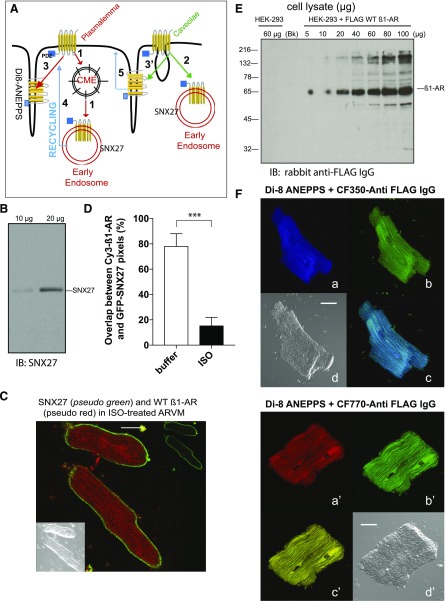
Fig. 2.
Fluorescence colocalization microscopy between WT β1-AR and intracellular markers in ARVMs. (A) Cartoon representing modes of agonist-mediated GPCR internalization. The majority of GPCRs are endocytosed from the surface membrane to early endosomes by clathrin-mediated endocytosis into early endosomes (number 1), or from caveolae into early endosomes (number 2). The cartoon also indicates that GPCRs may translocate to T-tubules, which are continuous with the plasma membrane in agonist-exposed ARVMs (number 3). Specific markers for early endosomes and T-tubules, such as SNX27 and Di-8 ANEPPS, respectively, may provide clues about the intracellular compartment harboring the β1-AR in ISO-exposed ARVMs. Also depicted are possible mechanisms for GPCR recycling from their intracellular compartment back to the surface membrane (number 4) or caveolae (number 5). (B) Western blotting of 10 or 20 µg of ARVM cell lysates with anti-SNX27. (C) Colocalization between CF-770 labeled WT β1-AR (pseudo red) and GFP-labeled SNX27 (pseudo green) in ISO-exposed ARVMs. The scale bar represents 20 µm. (D) Statistical comparison of CF-770 vs. GFP pixel overlap (mean ± S.E. from 30 images derived from three separate experiments) in ARVMs that were untreated (buffer) or exposed to ISO for 30 minutes. Statistical comparisons in the percentile of overlapping pixels between the two groups were carried out by student’s t test. P values are expressed as nonsignificant to indicate no significant difference or *, **, and *** to indicate P < 0.05, P < 0.01, and P< 0.001, respectively. (E) Western blotting of increasing amounts of cell lysates prepared from HEK-293 stably expressing FLAG-β1-AR that were probed with rabbit polyclonal anti-FLAG IgG. (F) ARVMs expressing the WT FLAG β1-AR were incubated with 5 µg/ml of rabbit anti-FLAG IgG for 1 hour and then exposed to buffer or ISO for 30 minutes as described in Fig. 1A. These slides were incubated with 10 µM Di-8 ANEPPS, washed, and then fixed. Slides were permeabilized and incubated with CF350- (a–d) or CF770-goat anti-rabbit IgG (images a′–d′) for 30 minutes and visualized by confocal microscopy. Di-8 ANEPPS staining (pseudo green in images b and b′) and Nomarski images (images d and d′) are shown. Distribution of fluorescent pixels for CF-350 (pseudo violet in image a) or CF-770 (pseudo red in image a′) were superimposed on Di-8 ANEPPS staining (pseudo cerulean or yellow in images c and c′, respectively). Each scale bar in (A) and (B) represents 10 µm.
We conducted colocalization studies between the WT β1-AR and the PDZ protein SNX27, which is an early endosome resident protein expressed in ARVMs (Fig. 2B). SNX27 is universally involved in recycling of PBM-containing cargoes, such as β1-AR, β2-AR, and many other GPCRs from early endosomes to the plasma membrane (Lauffer et al., 2010; Steinberg et al., 2013). Distribution of SNX27 and the WT-β1-AR in control versus ISO-treated and permeabilized ARVMs revealed that while SNX27 was colocalized with the WT β1-AR in naive ARVMs, its distribution was significantly different from that of the WT β1-AR in ISO-exposed ARVMs (Fig. 2, C and D). In ISO-exposed ARVMs, SNX27 was confined to the cytosolic side of the cardiac plasmalemmal membrane (pseudo green in Fig. 2C), while the WT β1-AR was distributed in puncta throughout the ARVMs (pseudo red in Fig. 2C).
The diameter of β1-AR puncta, however, was close to the diameter of T-tubules, which are plasma membrane tubular invaginations that rapidly propagate excitatory signals to inside cardiomyocytes through tubules with diameters between 180 and 280 nm (Soeller and Cannell, 1999). To find out if these structures were colocalized, we performed dual-fluorescence microscopy on ARVMs that expressed the WT β1-AR and the T-tubule-specific fluorescent dye Di-8 ANEPPS (Lyon et al., 2009). A problem encountered in using Di-8 ANEPPS was its broad excitation/emission spectra (λex = 498 nm and λem = 713 nm), which overlapped with the wavelength maxima of Cy3 (λex = 550 nm and λem = 570 nm) and many other fluorescent dyes. Therefore, we used fluorescent dyes with nonoverlapping excitation and emission spectra, such as CF-350 (absorbance/emission maxima: 347/448 nm) or CF-770 (absorbance/emission maxima: 770/797 nm) that were conjugated to goat anti-rabbit IgG. We verified by western blotting that the polyclonal rabbit anti-FLAG IgG selectively and quantitatively recognized the Mr = 66–70 kDa mature form of the FLAG-tagged WT β1-AR in cell lysates prepared from HEK-293 cells stably expressing the FLAG WT β1-AR (Fig. 2E).
To detect the β1-AR and Di-8 ANEPPS by dual-fluorescence confocal microscopy in ARVMs, cell expressing FLAG-β1-AR were exposed to ISO, fixed, and incubated with Di-8 ANEPPS for 10 minutes. The slides were washed, permeabilized, and incubated with rabbit anti-FLAG IgG to label the β1-AR, followed by CF-350 or CF-770-conjugated to goat anti-rabbit IgG. In ISO-treated ARVMs, CF-350-labeled WT β1-AR (pseudo violet in Fig. 2F, image a) and Di-8 ANNEPS (pseudo green in Fig. 2F, image b) were largely colocalized by >87% ± 12% (P > 0.05 from 30 images derived from n = 3 experiments). Similar findings were obtained with the CF-770 conjugated anti-FLAG IgG as well (Fig. 2F, images a′–d′).
Barcoding of β1-AR Trafficking in ARVMs.
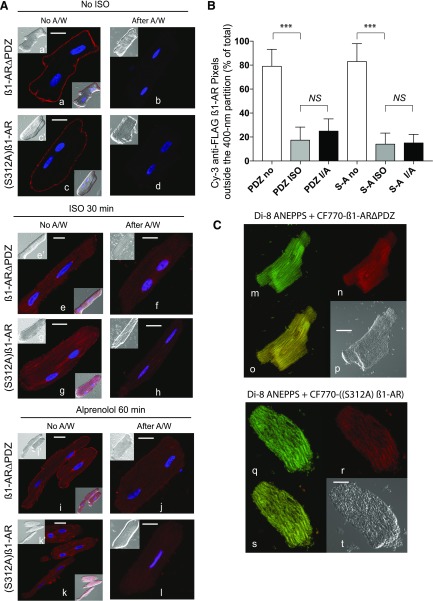
In mammalian cells, specific barcodes, such as the PBM and PKA-substrate Ser312 are involved in translocating endosomal WT β1-AR back to plasma membrane, but whether these barcodes are also involved in the redistribution of β1-AR in ARVMs is unknown (Nooh and Bahouth, 2017). ARVMs were infected with adenovirus harboring either the PBM inactivated β1-AR (β1-AR∆PDZ) or a β1-AR construct with a Ser312 to Ala312 [(S312A) β1-AR] inactivating point mutation (Gardner et al., 2004, 2006; Li et al., 2013). In untreated myocytes, Cy3 labeled β1-AR∆PDZ or (S312A) β1-AR were localized by 79% ± 12% and by 83% ± 14%, respectively, at the surface membrane of naive ARVMs (Fig. 3A, images a and a′ and images c and c′, respectively; Fig. 3B) and their fluorescence was expunged after exposing these cells to a mild acid treatment (Fig. 3A, images b and d, respectively). Exposing these ARVMs to ISO, redistributed 83% ±13% of β1-AR∆PDZ and 86% ± 15% of (S312A) β1-AR into puncta inside the 400-nm partition (Fig. 3A, images e and e′ and images g and g′, respectively; Fig. 3B). The fluorescence of these cells was resistant to mild acid exposure (Fig. 3A, images f and h, respectively). The distribution of β1-AR∆PDZ or (S312A) β1-AR overlapped with the distribution of Di-8 ANEPPS in ISO-treated ARVMs (Fig. 3C, images m–o and q–s, respectively, from 30 images derived from n = 3 separate experiments). Punctal distribution of β1-AR∆PDZ or (S312A) β1-AR did not change significantly (P > 0.05) upon replacing ISO with ALP (Fig. 3A, images i and i′ and images k and k′, respectively; Fig. 3B), and their fluorescence was not affected by exposure to a mild acid solution (Fig. 3A, images j and l, respectively). Therefore, these two mutations in the β1-AR markedly inhibited its redistribution from the acid-resistant compartment back to the surface sarcolemmal membrane.
Fig. 3.
Compartmentalization of β1-AR∆PDZ and (S312A) β1-AR in ARVMs. (A) Confocal microscopy of FLAG β1-AR∆PDZ or FLAG (S312A) β1-AR in ARVMs was carried as previously described in Fig. 1A. Each scale bar in (A) represents 15 µm. (B) Pixel distribution of Cy3 β1-AR ∆PDZ (PDZ) or Cy3-[(S312A) β1-AR] (S-A) outside the 400-nm partition (mean ± S.E.) of ARVMs that were untreated and exposed to ISO or ISO/ALP by the microscopic partition procedure from 30 images/condition that were derived for three separate experiments is presented. Statistical comparisons were carried out by one-way ANOVA followed by Bonferroni’s test. NS, indicates nonsignificant differences between the column pairs or *, **, and *** to indicate P < 0.05, P < 0.01, and P < 0.001, respectively. (C) ARVMs expressing FLAG-β1-AR ∆PDZ or FLAG (S312A) β1-AR were processed as described in Fig. 2F. Slides were permeabilized and incubated with CF770-goat anti-rabbit IgG and visualized. β1-AR∆PDZ staining (pseudo red, image n) or (S312A) β1-AR (pseudo red, image r) and that of with Di-8 ANEPPS (images m and q) were superimposed (images o and s, respectively). Each scale bar in (A) and (B) represents 15 µm.
Involvement of PKA and PKA-AKAP Interactions in β1-AR Trafficking in ARVMs.
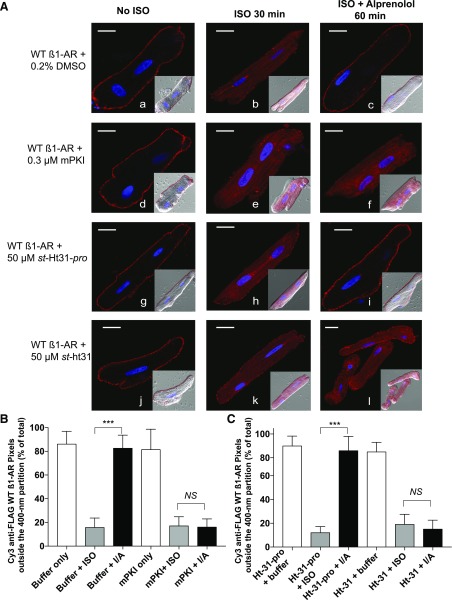
The involvement of PKA or AKAP-PKA interactions on the distribution of β1-AR in ARVMs was determined using PKA or PKA-AKAP interaction inhibitors (Fig. 4). Inhibition of PKA with the mPKI or disruption of PKA-AKAP interactions with the cell-permeable st-Ht31 peptide did not affect the localization of Cy-3 labeled WT β1-AR in naive ARVMs (84% ± 12% and 79% ± 11% of the pixels were distributed outside the partition, respectively, as illustrated in Fig. 4, B and C. These pixels redistributed toward the inside of this partition (84% ± 16% and 83% ± 15%, respectively) in response to ISO (Fig. 4A, images a and b, d and e, g and h, and j and k; Fig. 4, B and C). However, inactivation of PKA prevented the translocation of the ISO-exposed WT β1-AR from inside to outside the partition in response to ALP (Fig. 4A, compare the pixel distribution in image c to that in images f and i and the graphical data in Fig. 4B). In ARVMs that were pretreated with st-Ht31-pro, which is the inactive counterpart of st-Ht31, ALP induced the redistribution of WT β1-AR pixels from inside (88% ± 16%) into the region outside the 400-nm barrier by 84% ± 13% (Fig. 4A, image i; Fig. 4C). However, internal pixels in ARVMs that were pretreated with st-Ht31 did not redistribute to the cell surface membrane in response to ALP (compare images k–l in Fig. 4A). In buffer or st-Ht31-pro pretreated myocytes, replacing ISO with ALP induced significant redistribution of β1-AR pixels from inside to outside the 400-nm partition (Fig. 4, B and C, P < 0.001 by one-way ANOVA followed by Bonferroni’s analysis). However, ALP failed to induce the translocation of WT β1-AR from inside to outside the 400-nm partition in mPKI or st-Ht-31 pretreated myocytes (Fig. 4, B and C, P > 0.05 by one-way ANOVA followed by Bonferroni’s analysis).
Fig. 4.
Effect of mPKI and st-Ht-31/pro on compartmentalization of WT β1-AR in ARVMs. (A) ARVMs expressing the FLAG-tagged WT β1-AR were exposed to Cy3-anti-Flag antibody and pretreated for 30 minutes either with buffer (images a–c), 0.3 µM mPKI (images d–f), 50 µM st-Ht-31-pro (images g–i), or 50 µM st-Ht-31 (images j–l). The indicated slides were exposed to ISO or ISO/ALP as described in Fig 1A. The distribution of fluorescent pixels in confocal images (pseudo red) is shown alone or superimposed on the Nomarski image in the right lower quadrant. Each scale bar represents 15 µm. (B) Pixel distribution of Cy3 WT β1-AR outside the 400-nm partition (mean ± S.E.) in ARVMs that were untreated or pretreated with mPKI, or in (C) for cells pretreated with st-Ht31 or st-Ht31-pro is presented. Statistical comparisons were carried out by one-way ANOVA followed by Bonferroni’s test. NS, indicates nonsignificant differences between the column pairs or *, **, and *** to indicate P < 0.05, P < 0.01, and P < 0.001, respectively.
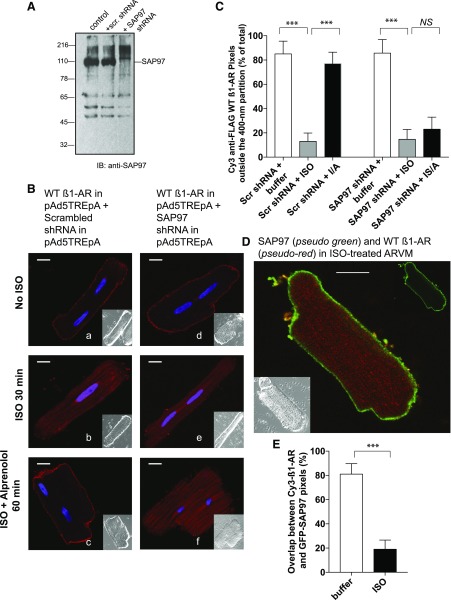
Involvement of the PDZ Protein SAP97 on β1-AR Redistribution in ISO-Exposed ARVMs.
In addition to PKA-AKAP interactions, the PDZ protein SAP97 was involved in translocation of the agonist-internalized β1-AR from endosomes to the plasma membrane of mammalian cells (Li et al., 2013; Nooh et al., 2014; Nooh and Bahouth, 2017). SAP97 is a PDZ scaffolding protein that is localized at the cytoplasmic side of the surface membrane of ARVMs (Shcherbakova et al., 2007). SAP97 is expressed in ARVMs cells as a ∼110 kDa protein (Fig. 5A), and its expression was effectively reduced by >85% after infecting these cells with an adenovirus harboring a highly effective and validated shRNA to SAP97 (Fig. 5A, compare lane 2 with lane 3). In naive ARVMs expressing either the scrambled Ad-shRNA or the SAP97 Ad-shRNA, 88% ± 15% and 80% ± 13% of WT β1-AR pixels were distributed outside the 400-nm partition, respectively (Fig. 5B, images a and d, respectively). These findings indicate that downregulation of SAP97 did not affect the compartmentalization of WT β1-AR in ARVMs. ISO induced the redistribution of the WT β1-AR from the surface plasmalemmal membrane into puncta inside the 400-nm partition by 85% ± 15% in scrambled Ad-shRNA-infected ARVMs and by 88% ± 13% in SAP97 Ad-shRNA-infected ARVMs (P > 0.05 derived from 30 images/condition similar to those in Fig. 5B, images b and e, respectively). In ARVMs infected with scrambled Ad-shRNA, 85% ± 10% of WT β1-AR puncta redistributed back to the cell membrane (outside the 400-nm partition) upon replacing ISO with ALP (Fig. 5B, image c). However, in ARVMs that were infected with SAP97 Ad-shRNA, more than 85% of the WT β1-AR fluorescence remained inside the 400-nm partition. Comparisons of pixel distribution between 30 images per condition, indicated that knockdown of SAP97 significantly inhibited the redistribution of β1-AR puncta to the cell membrane of ARVMs compared with its scrambled control Ad-shRNA (Fig. 5C).
Fig. 5.
Characterization of the role and distribution of SAP97 on ISO-mediated translocation of WT β1-AR in ARVMs. (A) Western blotting of cell lysates (50 µg) prepared from ARVMs that were infected with scrambled Ad-shRNA or Ad-SAP97 shRNA, and then probed with anti-SAP97 IgG. (B) Effect of SAP97 knockdown on WT β1-AR compartmentalization in ARVMs. ARVMs infected simultaneously with 50 multiplicities of infection (m.o.i.) of Ad-WT FLAG β1-AR and with either 50 m.o.i. scrambled Ad-shRNA (images a–c) or Ad-SAP97 shRNA (images d–f) were used. These slides were incubated with buffer (images a and d), ISO for 30 minutes (images b and e) or ISO/ALP (images c and f) and processed as described in Fig. 1A. Each scale bar in (B) represents 15 µm. (C) Effect of scrambled vs. SAP97 shRNA on the mean ± S.E. for the distribution of WT β1-AR pixels outside the 400-nm partition in ARVMs exposed to buffer, ISO, and ISO/ALP is presented. These data were derived from 10 images/condition for three separate experiments, n = 30 images). Statistical comparisons were carried out by one-way ANOVA followed by Bonferroni’s test. NS, indicates nonsignificant differences between the column pairs or *, **, and *** to indicate P < 0.05, P < 0.01, and P < 0.001, respectively. (D) Dual-fluorescence confocal microscopy between WT β1-AR (pseudo red) and SAP97 (pseudo green) in ISO-exposed ARVMs. (E) Statistical comparisons in the percentile of overlapping pixels between the two groups were carried out by student’s t test. P values are expressed as NS to indicate no significant difference or *, **, and *** to indicate P < 0.05, P < 0.01, and P < 0.001, respectively.
Dual-fluorescence microscopy indicated that in permeabilized ARVMs, SAP97 was confined to the cytosolic side of the surface membrane in buffer or in ISO-treated ARVMs (pseudo green in Fig. 5D) and colocalized with the WT β1-AR in naive ARVMs (Fig. 5E). However, in ISO-exposed ARVMs, the WT β1-AR was localized in puncta distributed throughout the ARVMs (pseudo red in Fig. 5D) and its distribution was significantly different (P < 0.05) from that of SAP97 (Fig. 5E).
Discussion
Characterization of the Compartmentalization of β1-AR in ARVMs.
Spatial localization of β-ARs and vectoring their signaling to specific intracellular compartments through compartmentalization are thought to play a major role in the development of heart diseases, such as heart failure (Head et al., 2005; Fischmeister et al., 2006; Lyon et al., 2009; Nikolaev et al., 2010). Assessment of the precise distribution of β-ARs in the heart by direct visualization is hampered by technical hurdles, the chief among them being the low densities of β-ARs in the heart and insufficient sensitivity of currently available antibodies to detect these receptors in intact tissue or ARVMs. We overcame these difficulties by expressing N-terminally FLAG-tagged β1-AR constructs at relatively low levels and minimized serum-induced cellular differentiation and β1-AR activation by culturing the ARVMs in serum-free media (Banyasz et al., 2008; Pavlović et al., 2010; Louch et al., 2011). Our results indicate that in quiescent ARVMs, β1-ARs were localized (>85%) to nontubular external membrane (surface sarcolemma) and to structures around the nucleus that were described previously (Boivin et al., 2006). Activation of the β1-AR by ISO induced the redistribution of surface β1-AR into acid and antibody inaccessible vesicles. These findings are significant on several fronts. First, they correlate findings made by other techniques that selective local stimulation of β1-ARs in the crest region or around T-tubules of ARVMs induced robust widespread activation of cAMP signals, while stimulation of β2-ARs in T-tubules generated restricted cAMP signals (Nikolaev et al., 2010). Other studies using similar techniques to those described here have shown that β1-ARs were uniformly distributed at the cell surface membrane and T-tubules, resulting in a striated fluorescence pattern (Zhou et al., 2000). We attribute differences in β1-AR distribution to the use of fetal bovine serum, which contains catecholamines that can significantly activate β-ARs in some cells lines (Dibner and Insel, 1981; Patrizio et al., 1996) and might alter the cellular distribution of the β1-AR in naive ARVMs. Finally, we point out that results similar to those described here were obtained by [3H]CGP-12177 binding to cell-surface β-ARs in mouse ventricular myocytes (Cheng et al., 2005). In this study, [3H]CGP-12177 binding was reduced by >60% upon exposing the myocytes to ISO, followed by restoring the density of cell-surface β-ARs by >80%, within 1 hour after ISO washout.
Novel Aspects of β1-AR Redistribution in ARVMs.
Distribution of β1-ARs in ARVMs under β-agonist activating conditions indicated that β1-ARs redistributed to small round structures and were no longer exposed to the extracellular milieu. These findings raise a question on the nature of the punctate vesicular structures harboring the β1-AR in agonist-exposed ARVMs. Our findings indicate that these small diameter vesicles overlapped by >90% with Di-8 ANEPPS staining, a widely used fluorescent dye that specifically labels T-tubules in muscle tissue (Lyon et al., 2009; Pérez-Treviño et al., 2015; Schobesberger et al., 2017). Since T-tubules are continuous with the plasma membrane (Soeller and Cannell, 1999), we did not refer to this process as agonist-mediated internalization of the GPCRs, rather as redistribution/translocation of the GPCRs into T-tubules. We speculate that redistribution of β1-ARs in ISO-exposed ARVMs into the t-tubular membrane might occur either by vesicular budding (pathways 1 and 2 in Fig. 2A) or translocation of outer membrane structures into T-tubules as illustrated by pathway 3 in Fig. 2A. In this regard, we determined that SNX27 was not colocalized with β1-AR-containing vesicles in ISO-treated ARVMs (Fig. 2C). This was surprising because SNX27 is an early endosomal PDZ protein that is universally involved in endosome to plasma membrane recycling of PBM-containing cargoes, such as β1-ARs, β2-ARs, and many other GPCRs (Lauffer et al., 2010; Steinberg et al., 2013). We interpret this finding as an indication that the agonist-activated β1-ARs did not migrate into classic endosomes, but might have redistributed to deep invaginations that are continuous with the plasma membrane of cardiac myocytes, such as T-tubules or caveolae (Soeller and Cannell, 1999) (Fig. 2C).
Finally, we determined that ISO washout promoted the translocation of β1-ARs from these punctate structures back into the plasma membrane, in line with observations concerning the distribution of WT β1-ARs in other mammalian cells (Gardner et al., 2004; Li et al., 2013; Nooh and Bahouth, 2017). While these results indicate significant similarities in compartmentalization of β1-ARs in terminally differentiated ARVMs to those in established cell lines (Valentine and Haggie, 2011; Bahouth and Nooh 2017; Nooh and Bahouth, 2017), it also reveals some major differences in redistribution that will be discussed in the subsequent sections.
Role of Barcodes in Setting the Trafficking Itinerary of the β1-AR in ARVMs.
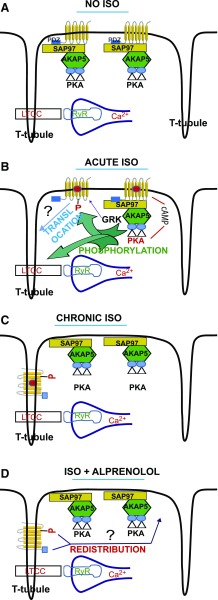
A consistent finding in GPCR trafficking studies is the paramount role of barcodes in dictating the trafficking itineraries of GPCRs. Barcodes are either short amino acid sequences found in the C-tail of the GPCR or reversibly modified amino acids (either by phosphorylation or by ubiquitination), which dictate the trafficking outcome of a given GPCR (Marchese and Trejo, 2013; Bahouth and Nooh, 2017). We concentrated on the roles of the PBM in the C-tail and Ser312 in the third intracellular loop in regulating the redistribution of the agonist-activated β1-AR from intracellular punctate vesicles to the surface membrane of ARVMs because these barcodes were also involved in β1-AR trafficking in mammalian cells (Gardner et al., 2004, 2007; Nooh et al., 2013; Nooh and Bahouth, 2017). This study confirmed that these domains mediated their effects on the distribution of the WT β1-AR in ARVMs by a crosstalk mechanism centered around the PBM of the β1-AR and its binding partner SAP97 (Figs. 4 and 5). Bipartite binding of SAP97 to the PBM of the β1-AR and to an AKAP5/PKA complex tethered a multi-protein complex to the PBM of the β1-AR in the surface membrane of quiescent ARVMs (Fig. 6A). Mechanistically, increased cAMP in response to agonist-mediated activation of the β1-AR activates the pool of PKA bound to the PBM of the β1-AR to phosphorylate Ser312 and other substrates such as L-type Ca2+ channels and others (Fig. 6B). In addition, activation of the β1-AR promotes GRK-mediated phosphorylation of the C-tail of β1-AR and binding of β-arrestin, which serves as a primary node for GPCR sequestration (Fig. 4B) (Wolfe and Trejo, 2007; Walther and Ferguson, 2013). Our findings show that chronic stimulation of ARVMs with ISO induced the redistribution of the majority of WT β1-AR into punctate structures that overlapped with the structures stained by the T-tubule-specific dye Di-8 ANEPPS (Fig. 2F; Fig. 6C). In this regard, the WT β1-AR translocated away from the SAP97/AKAP5/PKA complex, which remained in its original compartment at the inner leaflet of the plasma membrane (Fig. 6C). The data in Figs. 3–5 confirm that the type-1 PDZ and its associated receptosome, as well as phospho-Ser312, played a major role in translocating the WT β1-AR from intracellular structures to the surface plasmalemma of ARVMs (Fig. 6D). This report introduces the (S312A) β1-AR point mutant as a novel easy-to-use tool for analyzing compartmentalized β1-AR signaling in intracellular puncta versus the cell surface membrane. These studies would be best carried out by adenoviral-mediated expression of the (S312A) β1-AR in β1/β2-AR knockout mouse cardiomyocytes (Rohrer et al., 1999; Zhou et al., 2000). For example, acute activation of the (S312A) β1-AR in naive versus ISO-exposed and then rested β1/β2-AR–/–cardiomyocytes might provide clues regarding the dynamics of β1-AR signaling to cAMP from the surface membrane or from intracellular structures, respectively, in normal or experimental pathologic conditions.
Fig. 6.
Effect of ISO on WT β1-AR compartmentalization in ARVMs. (A) In naive no ISO ARVMs, WT β1-AR are confined to the surface plasmalemmal membrane. In this compartment, the β1-AR associates with the PDZ protein SAP97 via its type-1 PDZ. SAP97 in turn binds to the AKAP5/PKA (Gardner et al., 2007) complex. (B) Exposing ARVMs to ISO activated the β1-AR signaling pathway and increased cAMP, which in turn activated PKA bound to the β1-AR microdomain. The activated catalytic subunits of PKA phosphorylate many proteins including the β1-AR on Ser312, L-type calcium channels (LTCCs) and many others. In conditions associated with chronic ISO, the agonist-activated β1-AR translocated away from the SAP97/AKAP5/PKA complex on the surface membrane to punctate invaginations that appear to correspond to T-tubules by an, as yet, undetermined mechanism. (C) Our results show that under chronic ISO conditions, the β1-AR resides exclusively in these intracellular structures that overlap with the T-tubule-specific dye Di-8 ANEPPS. (D) Upon replacing ISO with the β-blocker ALP, internal β1-AR recycled in a type-1 PDZ- and phospho-Ser312-dependent manner from their intracellular compartment to the surface plasmalemmal membrane to restore the organization depicted in the no ISO condition shown in (A). RyR, Ryanodine receptors.
Summary and Significance of Our Findings in ARVMs.
Sustained elevation of circulating catecholamines is a common theme in many pathologic conditions such as cardiac hypertrophy, heart failure, and hypertension (Prichard et al., 1991; Keys and Koch, 2004). In heart failure, increased sympathetic nervous system activity is associated with selective downregulation of cardiac β1-AR and in worsening prognosis (Bristow et al., 1982; Bristow et al., 1993; Brodde, 1993; Keys and Koch, 2004). In addition, loss or remodeling of T-tubules is a common observation in several cardiovascular diseases such as myocardial infarction and heart failure (Balijepalli et al., 2003; Lyon et al., 2009; Schobesberger et al., 2017). In this report, we show that β-agonists induce the redistribution of the β1-AR to what appears to be T-tubules or caveolae, which is a novel observation with significant potential for regulating the stability of signaling outputs of cardiac β1-AR. Whether these combined structural and biochemical abnormalities work cooperatively in altering the compartmentalized signaling outputs or stability of cardiac β1-AR is not known. However, these changes could provide another investigative dimension for understanding the basis for selective downregulation and desensitization of cardiac β1-AR seen in heart failure and other pathologic conditions.
Abbreviations
- Ad
adenovirus
- AKAP
A-kinase anchoring protein
- ALP
alprenolol
- ANOVA
analysis of variance
- ARVM
adult rat ventricular myocyte
- β-AR
β-adrenergic receptor
- β1-AR
β1-adrenergic receptor
- β2-AR
β2-adrenergic receptor
- GPCR
G protein-coupled receptor
- ISO
isoproterenol
- mPKI
myristoylated cAMP-dependent protein kinase inhibitory peptide
- PBM
type-1 PDZ binding motif
- ∆PDZ
deleted type-1 PDZ binding motif
- PKA
cAMP-dependent protein kinase A
- Ser312
serine at position 312
- shRNA
short hairpin RNA
- S312A
mutation of serine at position 312 to alanine
- SNX27
sorting nexin-27
- T-tubule
transverse tubule
- WT
wild type
Authorship Contributions
Participated in research design: Nooh, Mancarella, Bahouth.
Conducted experiments: Nooh, Mancarella.
Contributed new reagents or analytic tools: Mancarella, Bahouth.
Performed data analysis: Mancarella, Bahouth.
Wrote or contributed to the writing of the manuscript: Nooh, Mancarella, Bahouth.
Footnotes
This work was supported by the National Institutes of Health [Grant HL-085848] and by Bridge Funding support from the Office of the Vice Chancellor of The University of Tennessee–Health Sciences Center [B-2015-8].
References
- Bahouth SW, Nooh MM. (2017) Barcoding of GPCR trafficking and signaling through the various trafficking roadmaps by compartmentalized signaling networks. Cell Signal 36:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig AH, Swords FM, Szaszák M, King PJ, Hunyady L, Clark AJ. (2002) Agonist activated adrenocorticotropin receptor internalizes via a clathrin-mediated G protein receptor kinase dependent mechanism. Endocr Res 28:281–289. [DOI] [PubMed] [Google Scholar]
- Baillie GS. (2009) Compartmentalized signalling: spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. FEBS J 276:1790–1799. [DOI] [PubMed] [Google Scholar]
- Balijepalli RC, Lokuta AJ, Maertz NA, Buck JM, Haworth RA, Valdivia HH, Kamp TJ. (2003) Depletion of T-tubules and specific subcellular changes in sarcolemmal proteins in tachycardia-induced heart failure. Cardiovasc Res 59:67–77. [DOI] [PubMed] [Google Scholar]
- Banyasz T, Lozinskiy I, Payne CE, Edelmann S, Norton B, Chen B, Chen-Izu Y, Izu LT, Balke CW. (2008) Transformation of adult rat cardiac myocytes in primary culture. Exp Physiol 93:370–382. [DOI] [PubMed] [Google Scholar]
- Bers DM. (2001) Excitation–Contraction Coupling and Cardiac Contractile Force, 2nd ed, Kluwer Academic, Dordrecht, The Netherlands. [Google Scholar]
- Boivin B, Lavoie C, Vaniotis G, Baragli A, Villeneuve LR, Ethier N, Trieu P, Allen BG, Hébert TE. (2006) Functional β-adrenergic receptor signalling on nuclear membranes in adult rat and mouse ventricular cardiomyocytes. Cardiovasc Res 71:69–78. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. (1982) Decreased catecholamine sensitivity and β-adrenergic-receptor density in failing human hearts. N Engl J Med 307:205–211. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Minobe WA, Raynolds MV, Port JD, Rasmussen R, Ray PE, Feldman AM. (1993) Reduced β1 receptor messenger RNA abundance in the failing human heart. J Clin Invest 92:2737–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodde OE. (1993) Beta-adrenoceptors in cardiac disease. Pharmacol Ther 60:405–430. [DOI] [PubMed] [Google Scholar]
- Cheng G, Qiao F, Gallien TN, Kuppuswamy D, Cooper G., IV (2005) Inhibition of β-adrenergic receptor trafficking in adult cardiocytes by MAP4 decoration of microtubules. Am J Physiol Heart Circ Physiol 288:H1193–H1202. [DOI] [PubMed] [Google Scholar]
- Delos Santos NM, Gardner LA, White SW, Bahouth SW. (2006) Characterization of the residues in helix 8 of the human β1-adrenergic receptor that are involved in coupling the receptor to G proteins. J Biol Chem 281:12896–12907. [DOI] [PubMed] [Google Scholar]
- Dibner MD, Insel PA. (1981) Serum catecholamines desensitize β-adrenergic receptors of cultured C6 glioma cells. J Biol Chem 256:7343–7346. [PubMed] [Google Scholar]
- Ehlers MD. (2000) Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron 28:511–525. [DOI] [PubMed] [Google Scholar]
- Ellisdon AM, Halls ML. (2016) Compartmentalization of GPCR signalling controls unique cellular responses. Biochem Soc Trans 44:562–567. [DOI] [PubMed] [Google Scholar]
- Ferguson SSG, Downey WE, III, Colapietro AM, Barak LS, Ménard L, Caron MG. (1996) Role of β-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 271:363–366. [DOI] [PubMed] [Google Scholar]
- Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J, Vandecasteele G. (2006) Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res 99:816–828. [DOI] [PubMed] [Google Scholar]
- Gardner LA, Delos Santos NM, Matta SG, Whitt MA, Bahouth SW. (2004) Role of the cyclic AMP-dependent protein kinase in homologous resensitization of the β1-adrenergic receptor. J Biol Chem 279:21135–21143. [DOI] [PubMed] [Google Scholar]
- Gardner LA, Naren AP, Bahouth SW. (2007) Assembly of an SAP97-AKAP79-cAMP-dependent protein kinase scaffold at the type 1 PSD-95/DLG/ZO1 motif of the human β1-adrenergic receptor generates a receptosome involved in receptor recycling and networking. J Biol Chem 282:5085–5099. [DOI] [PubMed] [Google Scholar]
- Gardner LA, Tavalin SJ, Goehring AS, Scott JD, Bahouth SW. (2006) AKAP79-mediated targeting of the cyclic AMP-dependent protein kinase to the β1-adrenergic receptor promotes recycling and functional resensitization of the receptor. J Biol Chem 281:33537–33553. [DOI] [PubMed] [Google Scholar]
- Hanyaloglu AC, von Zastrow M. (2008) Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol 48:537–568. [DOI] [PubMed] [Google Scholar]
- Head BP, Patel HH, Roth DM, Lai NC, Niesman IR, Farquhar MG, Insel PA. (2005) G-protein-coupled receptor signaling components localize in both sarcolemmal and intracellular caveolin-3-associated microdomains in adult cardiac myocytes. J Biol Chem 280:31036–31044. [DOI] [PubMed] [Google Scholar]
- Jean-Alphonse F, Bowersox S, Chen S, Beard G, Puthenveedu MA, Hanyaloglu AC. (2014) Spatially restricted G protein-coupled receptor activity via divergent endocytic compartments. J Biol Chem 289:3960–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys JR, Koch WJ. (2004) The adrenergic pathway and heart failure. Recent Prog Horm Res 59:13–30. [DOI] [PubMed] [Google Scholar]
- Lauffer BE, Melero C, Temkin P, Lei C, Hong W, Kortemme T, von Zastrow M. (2010) SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J Cell Biol 190:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ. (1998) G protein-coupled receptors. III. New roles for receptor kinases and β-arrestins in receptor signaling and desensitization. J Biol Chem 273:18677–18680. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Rockman HA, Koch WJ. (2000) Catecholamines, cardiac β-adrenergic receptors, and heart failure. Circulation 101:1634–1637. [DOI] [PubMed] [Google Scholar]
- Li X, Nooh MM, Bahouth SW. (2013) Role of AKAP79/150 protein in β1-adrenergic receptor trafficking and signaling in mammalian cells. J Biol Chem 288:33797–33812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louch WE, Sheehan KA, Wolska BM. (2011) Methods in cardiomyocyte isolation, culture, and gene transfer. J Mol Cell Cardiol 51:288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon AR, MacLeod KT, Zhang Y, Garcia E, Kanda GK, Lab MJ, Korchev YE, Harding SE, Gorelik J. (2009) Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc Natl Acad Sci USA 106:6854–6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese A, Trejo J. (2013) Ubiquitin-dependent regulation of G protein-coupled receptor trafficking and signaling. Cell Signal 25:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisco C, Zebrowski DC, Vatner DE, Vatner SF, Sadoshima J. (2001) β-adrenergic cardiac hypertrophy is mediated primarily by the β1-subtype in the rat heart. J Mol Cell Cardiol 33:561–573. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE, Gorelik J. (2010) β2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science 327:1653–1657. [DOI] [PubMed] [Google Scholar]
- Nooh MM, Bahouth SW. (2017) Two barcodes encoded by the type-1 PDZ and by phospho-Ser312 regulate retromer/WASH-mediated sorting of the ß1-adrenergic receptor from endosomes to the plasma membrane. Cell Signal 29:192–208. [DOI] [PubMed] [Google Scholar]
- Nooh MM, Chumpia MM, Hamilton TB, Bahouth SW. (2014) Sorting of β1-adrenergic receptors is mediated by pathways that are either dependent on or independent of type I PDZ, protein kinase A (PKA), and SAP97. J Biol Chem 289:2277–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooh MM, Naren AP, Kim SJ, Xiang YK, Bahouth SW. (2013) SAP97 controls the trafficking and resensitization of the β-1-adrenergic receptor through its PDZ2 and I3 domains. PLoS One 8:e63379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrizio M, Riitano D, Costa T, Levi G. (1996) Selective enhancement by serum factors of cyclic AMP accumulation in rat microglial cultures. Neurochem Int 29:89–96. [DOI] [PubMed] [Google Scholar]
- Pavlović D, McLatchie LM, Shattock MJ. (2010) The rate of loss of T-tubules in cultured adult ventricular myocytes is species dependent. Exp Physiol 95:518–527. [DOI] [PubMed] [Google Scholar]
- Pelkmans L, Bürli T, Zerial M, Helenius A. (2004) Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 118:767–780. [DOI] [PubMed] [Google Scholar]
- Pérez-Treviño P, Pérez-Treviño J, Borja-Villa C, García N, Altamirano J. (2015) Changes in T-tubules and sarcoplasmic reticulum in ventricular myocytes in early cardiac hypertrophy in a pressure overload rat model. Cell Physiol Biochem 37:1329–1344. [DOI] [PubMed] [Google Scholar]
- Prichard BN, Owens CW, Smith CC, Walden RJ. (1991) Heart and catecholamines. Acta Cardiol 46:309–322. [PubMed] [Google Scholar]
- Rohrer DK, Chruscinski A, Schauble EH, Bernstein D, Kobilka BK. (1999) Cardiovascular and metabolic alterations in mice lacking both β1- and β2-adrenergic receptors. J Biol Chem 274:16701–16708. [DOI] [PubMed] [Google Scholar]
- Romero G, von Zastrow M, Friedman PA. (2011) Role of PDZ proteins in regulating trafficking, signaling, and function of GPCRs: means, motif, and opportunity. Adv Pharmacol 62:279–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobesberger S, Wright P, Tokar S, Bhargava A, Mansfield C, Glukhov AV, Poulet C, Buzuk A, Monszpart A, Sikkel M, et al. (2017) T-tubule remodelling disturbs localized β2-adrenergic signalling in rat ventricular myocytes during the progression of heart failure. Cardiovasc Res 113:770–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MN, Gautreau A, Billadeau DD. (2013) Retromer-mediated endosomal protein sorting: all WASHed up! Trends Cell Biol 23:522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcherbakova OG, Hurt CM, Xiang Y, Dell’Acqua ML, Zhang Q, Tsien RW, Kobilka BK. (2007) Organization of β-adrenoceptor signaling compartments by sympathetic innervation of cardiac myocytes. J Cell Biol 176:521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. (2001) Regulation of receptor fate by ubiquitination of activated β2-adrenergic receptor and β-arrestin. Science 294:1307–1313. [DOI] [PubMed] [Google Scholar]
- Simpson DG, Majeski M, Borg TK, Terracio L. (1999) Regulation of cardiac myocyte protein turnover and myofibrillar structure in vitro by specific directions of stretch. Circ Res 85:e59–e69. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. (2001) Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci 4:1079–1085. [DOI] [PubMed] [Google Scholar]
- Soeller C, Cannell MB. (1999) Examination of the transverse tubular system in living cardiac rat myocytes by 2-photon microscopy and digital image-processing techniques. Circ Res 84:266–275. [DOI] [PubMed] [Google Scholar]
- Steinberg F, Gallon M, Winfield M, Thomas EC, Bell AJ, Heesom KJ, Tavaré JM, Cullen PJ. (2013) A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat Cell Biol 15:461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan R, Taffet SM, Vikstrom KL, Anumonwo JM. (2010) Regulation of cardiac inward rectifier potassium current (IK1) by synapse-associated protein-97. J Biol Chem 285:28000–28009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine CD, Haggie PM. (2011) Confinement of β1- and β2-adrenergic receptors in the plasma membrane of cardiomyocyte-like H9c2 cells is mediated by selective interactions with PDZ domain and A-kinase anchoring proteins but not caveolae. Mol Biol Cell 22:2970–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther C, Ferguson SS. (2013) Arrestins: role in the desensitization, sequestration, and vesicular trafficking of G protein-coupled receptors. Prog Mol Biol Transl Sci 118:93–113. [DOI] [PubMed] [Google Scholar]
- Wileman T, Harding C, Stahl P. (1985) Receptor-mediated endocytosis. Biochem J 232:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe BL, Trejo J. (2007) Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic 8:462–470. [DOI] [PubMed] [Google Scholar]
- Xiao RP. (2001) β-Adrenergic signaling in the heart: dual coupling of the β2-adrenergic receptor to Gs and Gi proteins. Sci STKE 2001:re15. [DOI] [PubMed] [Google Scholar]
- Zhou YY, Yang D, Zhu WZ, Zhang SJ, Wang DJ, Rohrer DK, Devic E, Kobilka BK, Lakatta EG, Cheng H, et al. (2000) Spontaneous activation of β2- but not β1-adrenoceptors expressed in cardiac myocytes from β1β2 double knockout mice. Mol Pharmacol 58:887–894. [DOI] [PubMed] [Google Scholar]
- Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. (2001) Dual modulation of cell survival and cell death by β2-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci USA 98:1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]