Abstract
Background
Transcranial Alternating Current Stimulation (tACS) consists in delivering electric current to the brain using an oscillatory pattern that may entrain the rhythmic activity of cortical neurons. When delivered at gamma frequency, tACS modulates motor performance and GABA-A-ergic interneuron activity.
Objective
Since interneuronal discharges play a crucial role in brain plasticity phenomena, here we co-stimulated the primary motor cortex (M1) in healthy subjects by means of tACS during intermittent theta-burst stimulation (iTBS), a transcranial magnetic stimulation paradigm known to induce long-term potentiation (LTP)-like plasticity.
Methods
We measured and compared motor evoked potentials before and after gamma, beta and sham tACS-iTBS. While we delivered gamma-tACS, we also measured short-interval intracortical inhibition (SICI) to detect any changes in GABA-A-ergic neurotransmission.
Results
Gamma, but not beta and sham tACS, significantly boosted and prolonged the iTBS-induced after-effects. Interestingly, the extent of the gamma tACS-iTBS after-effects correlated directly with SICI changes.
Conclusions
Overall, our findings point to a link between gamma oscillations, interneuronal GABA-A-ergic activity and LTP-like plasticity in the human M1. Gamma tACS-iTBS co-stimulation might represent a new strategy to enhance and prolong responses to plasticity-inducing protocols, thereby lending itself to future applications in the neurorehabilitation setting.
Keywords: tACS, Gamma, TMS, Plasticity, Interneurons, GABA-A
Introduction
Over the last decade, a growing number of studies have demonstrated that the most important limitation of transcranial magnetic stimulation (TMS) techniques such as theta-burst stimulation (TBS), which have been designed to assess plasticity in the human primary motor cortex (M1), is the variability and the relatively short duration of their effect [1–4]. These factors have limited the application of TMS protocols in clinical and neurorehabilitation settings. New protocols that enhance and prolong TMS-induced plasticity in M1 would be useful.
Transcranial Alternating Current Stimulation (tACS), a novel non-invasive neurophysiological technique, can deliver electric current to the brain using an oscillatory pattern [5]. tACS modulates the firing rate and timing discharge of cortical neurons, and ‘entrains’ susceptible neuronal populations, synchronizing them to the stimulation frequency or harmonically related frequencies [6,7]. The effects of tACS depend on the frequency used and the cortical area being stimulated. Hence, the ability of tACS to entrain brain rhythms is higher when the externally superimposed oscillation matches, or is close to, the natural frequency of the cortical area being stimulated (resonance principle) [8,9]. tACS delivered within the range of beta (13–30 Hz) or high-gamma (60–90 Hz) frequencies modulates M1 activity, as shown by motor evoked potential (MEP) recordings or behavioral measurements [10–14]. For instance, tACS facilitates motor performances by increasing the synchronization of M1 circuitries in the gamma frequency band [14,15]. Recent TMS studies have also demonstrated that 70 Hz tACS modulates the activity of interneuronal networks in M1 by inducing an early reduction in GABA-A-ergic inhibitory neurotransmission, as measured by short-interval intracortical inhibition (SICI), and by reversing this effect after a longer stimulation period [16]. Interneurons are key physiological elements of M1 circuitries essential for several motor functions, including motor control and learning [17–19]. Importantly, modulation of the interneuronal discharge is also known to be involved in brain plasticity phenomena [20–23].
Although two studies have tested the interaction between tACS and TMS protocols driving long-term depression-like plasticity [24,25], none have yet explored whether tACS can strengthen and prolong paradigms inducing long-term potentiation (LTP)-like plasticity in M1. One approach that may shed light on this issue consists in delivering tACS over M1 during intermittent TBS (iTBS), a patterned protocol that is considered more powerful, more reliable and more easily applicable than other TMS protocols [26]. Since gamma oscillations in M1 have been found to be related to the induction of LTP in animal models [27–30], we hypothesized that the after-effects of iTBS may be modulated by applying tACS at gamma frequencies over M1. In addition, in view of evidence of GABA-A neurotransmission involvement in brain plasticity mechanisms and of SICI modulation during gamma tACS, we wished to verify whether the effects of gamma tACS on iTBS-induced plasticity are related to changes in GABA-A interneuronal activity.
To achieve these aims, we designed a protocol based on gamma tACS-iTBS co-stimulation over M1 of the dominant hemisphere in healthy subjects and examined post-intervention effects by monitoring MEP amplitudes over time. To verify whether any after-effects induced by tACS-iTBS are frequency-dependent, we also tested the effect of beta tACS-iTBS and applied sham tACS-iTBS co-stimulation. Lastly, we measured the level of SICI before and during gamma tACS.
Material and methods
Participants
Fourteen right-handed healthy young subjects (10 males; mean age ± 1 SD: 27.6 ± 3.34, age range: 24–33 years) were enrolled in the study. None of them had history of neurological/psychiatric disorders, nor were taking medications acting on brain excitability. Also, no participant had contraindications to non-invasive brain stimulation [31]. The study was approved by the local ethics committee and conducted in accordance with Declaration of Helsinki.
TMS
Single-pulse TMS was carried out by using MAGSTIM 200 and a standard figure-of-eight 70 mm coil delivering monophasic magnetic pulses (Magstim Company Limited). The optimal scalp position to elicit MEPs (i.e. ‘hotspot’) in the right first dorsal interosseous (FDI) muscle was determined with the TMS coil held at 45° to the midsagittal line with the handle pointing posteriorly. This procedure was conducted in order to center the tACS stimulating electrode and was then repeated after the electrodes had been positioned on the participant's head. At this stage, this site was marked over the sponge to ensure reproducible coil positioning. The MEPs were recorded through a pair of surface electrodes placed on the FDI muscle of the right hand in a belly/tendon montage. Electromyographic (EMG) signals were amplified (Digitimer D360 amplifier; Digitimer Ltd), digitized at 5 kHz (CED 1401 interface; Cambridge Electronic Design), and stored on a computer for off-line analysis (Signal software version 5.08; Cambridge Electronic Design). Resting motor threshold (RMT) (i.e. the output required to elicit MEPs of ≥50 μV peak-to-peak amplitude in at least 5 of 10 consecutive stimuli) was determined, as well as the minimum intensity needed to reliably produce MEPs of ≈1 mV in size (MT1mV).
Intermittent theta-burst stimulation (iTBS)
iTBS was delivered by using a high-frequency biphasic magnetic stimulator (Magstim SuperRapid; Magstim Company Limited) connected to a figure-of-eight coil placed over the right FDI hotspot. The stimulating protocol consisted of ten bursts of three pulses at 50 Hz, repeated at 200-ms intervals, delivered in short trains lasting 2 s, with an 8-s pause between consecutive trains (20 trains, 600 pulses in total). Stimulation intensity for TBS was set at 80% of the active motor threshold (AMT) [32]. AMT was defined as the minimum stimulus intensity required to evoke a 200 μV peak-to-peak MEP in at least 5 of 10 consecutive trials while subjects maintained ≈30% of the FDI maximum contraction [31].
Short-interval intracortical inhibition (SICI)
SICI was tested according to a standardized protocol [33,34]. Paired-pulses were delivered with an inter-stimulus interval (ISI) of 2 ms and at the following intensities: the conditioning stimulus (CS) was set at 70% of the RMT while the test stimulus (TS) was set at the MT1mV. We used these ISI and CS intensities to avoid any possible contamination by short-interval intracortical facilitation [35,36].
tACS
tACS was delivered through conductive rubber electrodes enclosed in saline-soaked sponges by means of a BrainSTIM stimulator (EMS, Italy). The stimulating electrode (5 × 5 cm) was centered over the FDI ‘hotspot’ whereas the reference electrode (5 × 5 cm) was positioned over Pz, according to the International 10–20 EEG system [13]. The electrodes were secured in place using rubber strips around the head. Impedance was kept at < 10 kΩ tACS was delivered at two different frequencies: 20 Hz (β) and 70 Hz (γ). A sham tACS stimulation, consisting of ramping-up and ramping-down periods, and 1 s of stimulation, was used as a control. Sinewave stimulation was delivered with no direct current offset and a peak-to-peak amplitude of 1 mA with a 3-s ramping-up and ramping-down. If this intensity induced obvious phosphenes or uncomfortable skin sensations, the stimulation amplitude was gradually lowered until they were no more present. This resulted in a stimulation intensity of 0.58 ± 0.30 mA (mean ± SD) for β tACS. The intensity did not need to be adjusted for γ tACS in any participant. Accordingly, the mean current density was 23.2 μA/cm2 for β tACS and 40 μA/cm2 for γ tACS.
Experimental design
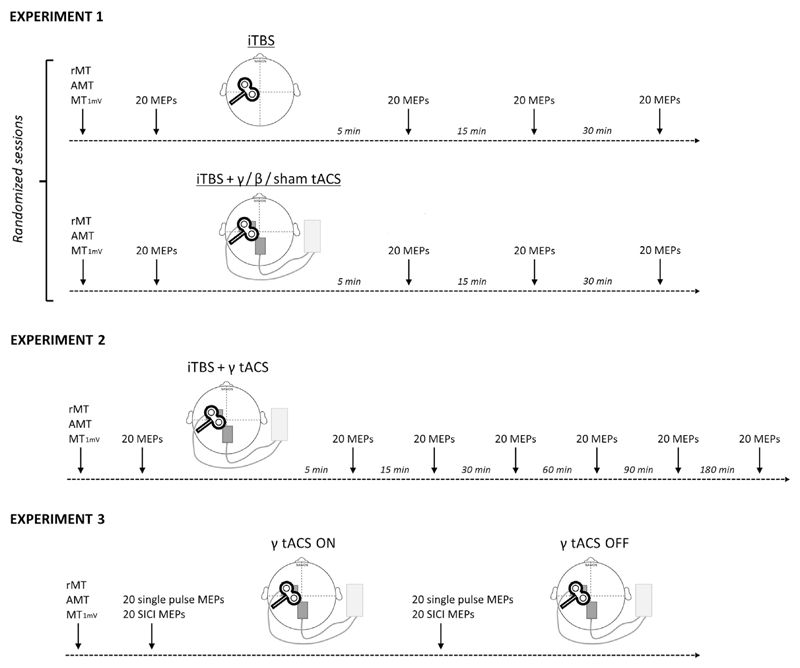
Three different experimental paradigms were used (Fig. 1). Throughout the experimental sessions, the subjects were seated comfortably in a chair with their arms fully relaxed in a natural position and their hands resting on a pillow.
Fig. 1. Experimental design.
The early part of every experiment consisted in identifying the hotspot of the first dorsal interosseous muscle and estimating the resting motor threshold (RMT), active motor threshold (AMT) and intensity that induced a motor evoked potential of about 1 mV in amplitude (MT1mV). Experiment 1: effect of iTBS-tACS co-stimulation. Twenty single TMS pulses were delivered, at rest, before and 5, 15 and 30 min after the intermittent theta burst (iTBS) protocol. The four different sessions were conducted in a random order at least one week apart. Experiment 2: time course of iTBS-γ tACS co-stimulation. Twenty single pulse MEPs were recorded before and 5, 15, 30, 60, 90 and 180 min after the iTBS-γ tACS protocol. Experiment 3: effect of γ tACS on corticospinal excitability and GABA-A interneurons. Twenty single TMS pulses and twenty paired (SICI protocol) TMS pulses were delivered in a random order, at rest, before tACS was activated and during γ tACS stimulation on M1.
Experiment 1: effect of iTBS-tACS co-stimulation
Fourteen subjects underwent four separate sessions in a randomized order, which were performed at least 1 week apart: i) iTBS without tACS (iTBS); ii) iTBS combined with 20 Hz tACS (iTBS-β tACS); iii) iTBS combined with 70 Hz tACS (iTBS-γ tACS) and iv) iTBS during sham tACS (iTBS-sh tACS). When stimulation was combined (magnetic-electric), tACS started about 7 s before the iTBS protocol started and ended immediately after the iTBS protocol ended. The duration of the tACS stimulation was thus equal to that of the iTBS protocol (i.e. 3 min and 20 s). Note that iTBS bursts were not phase-locked to the tACS sinewave (phase-independent co-stimulation). Twenty single-pulse TMS stimuli were delivered at rest before (T0) and 5 min (T1), 15 min (T2) and 30 min (T3) after iTBS. The TMS inter-trial stimulus interval was set at 4.5–5.5 s so as to avoid habituation [33,35,37].
Experiment 2: time course of iTBS-γ tACS co-stimulation
The experiment was performed in a subgroup of eight subjects. The stimulation method was exactly the same as that used in Experiment 1, the only difference being that we followed the MEP amplitude changes for up to 180 min after iTBS-γ tACS by adding three additional time points (60 min - T4; 90 min -T5; 180 min - T6).
Experiment 3: effect of γ-tACS on corticospinal excitability and GABA-A inhibitory interneurons
This experiment was performed on all the fourteen subjects who participated in Experiment 1. We recorded twenty single-pulse MEPs at the MT1mV intensity as well as twenty MEPs for SICI in a randomized order in all the participants, in both the baseline condition (γ-tACS OFF) and during 70 Hz tACS (γ-tACS ON). We started testing the effects of γ-tACS about 7 s after the electric stimulator was switched on.
Control experiment: after-effects of γ-tACS
A subgroup of eight subjects underwent an additional experiment. We recorded twenty single-pulse MEPs at MT1mV intensity before (T0) and 5 (T1), 15 (T2) and 30 min (T3) after delivering γ-tACS on M1. The electrical stimulation lasted 3 min and 20 s, i.e. the exact duration of the iTBS protocol we used, and the intensity was 1 mA as before.
Data analysis
Peak-to-peak MEP amplitudes were measured by means of a customized script on Signal software. Each trial was visually inspected and those displaying EMG activity greater than 0.1 mV in a 200 ms time-window preceding TMS were rejected (≤2 trials per condition). We then averaged peak-to-peak MEP amplitudes for each condition. SICI was expressed as the ratio between the mean conditioned MEP amplitude and the mean unconditioned MEP amplitude. A synthetic measure that reflected the overall extent of iTBS-induced potentiation was also created by averaging the percentage of MEP facilitation at the original three post-stimulation time points (T1-3).
Statistical analysis
Three separate within-group repeated measures (rm) ANOVAs were used to compare RMTs, AMTs and MT1mV in the different sessions. A rmANOVA with factors ‘session’ (4 levels: iTBS, iTBS-β tACS, iTBS-γ tACS, iTBS-sh tACS) and ‘time point’ (3 levels: T1, T2, T3) was used to test iTBS-induced changes in MEPs in each session in Experiment 1. To test the time course of iTBS-γ tACS (Experiment 2), we used a rmANOVA with ‘time point’ (7 levels: T0, T1, T2, T3, T4, T5, T6) as factor. A rmANOVA with factors ‘γ tACS’ (2 levels: γ-tACS OFF, γ-tACS ON) and ‘TMS paradigm’ (2 levels: unconditioned and conditioned MEP) was used to test the effect of γ-tACS on corticospinal excitability and intracortical inhibition, as evaluated by SICI in Experiment 3. Pearson's correlation test was used to assess any correlation between the SICI γ-tACS ON/SICI γ-tACS OFF ratio and the extent of MEP potentiation post-iTBS-γ tACS. To test possible changes in MEPs after the application of γ-tACS (Control experiment), we used a rmANOVA with ‘time point’ (4 levels: T0, T1, T2, T3) as factor. Greenhouse-Geisser corrections were applied whenever we found a violation of sphericity in Mauchly's tests. In the presence of significant interactions, post-hoc comparisons were performed by means of paired t-tests. The level of significance was initially set at P < 0.05, with Bonferroni's correction subsequently being applied to multiple comparisons. Unless otherwise stated, all the values are presented as mean ± standard error of means (SEM). Statistical analyses were performed using SPSS Statistics for Windows (version 20.0.0; IBM).
Results
Motor thresholds and pre-TBS baseline comparisons
The analysis revealed that motor thresholds (RMT, AMT, MT1mV) were comparable across the tACS sessions (Table 1), as shown by the lack of any significant effect of the main factor ‘session’ in three separate rmANOVAs (RMT: F(2,26) = 0.23, p = 0.79; AMT: F(2,26) = 0.36, p = 0.7; MT1mV: F(2,26) = 0.05, p = 0.89). No differences emerged in the baseline MEP amplitudes, i.e. pre-iTBS (T = 0), as shown by the lack of any significant effect of the main factor ‘session’ (4 levels: iTBS, iTBS-β tACS, iTBS-γ tACS, iTBS-sh tACS) in the rmANOVA (F(3,39) = 2.64, p = 0.08).
Table 1.
Transcranial alternating current stimulation (tACS) intensity, resting motor threshold (RMT), active motor threshold (AMT) and intensity used to evoke a motor evoked potential of about 1 mV in amplitude (MT1mV) for each session in Experiment 1 (mean and standard deviation - SD - values).
| tACS intensity (mA) | RMT (%) | AMT (%) | MT1mV (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β tACS | γ tACS | baseline | sh tACS | β tACS | γ tACS | baseline | sh tACS | β tACS | γ tACS | baseline | sh tACS | β tACS | γ tACS | |
| mean | 0.58 | 1 | 47.1 | 52.3 | 52.1 | 53.1 | 40.7 | 45.1 | 44.5 | 45.8 | 56.3 | 64.7 | 65.1 | 65.0 |
| SD | 0.3 | – | 6.8 | 6.9 | 6.5 | 8.0 | 6.5 | 7.0 | 5.1 | 7.0 | 8.2 | 9.7 | 9.3 | 10.2 |
Experiment 1: effect of iTBS-tACS co-stimulation
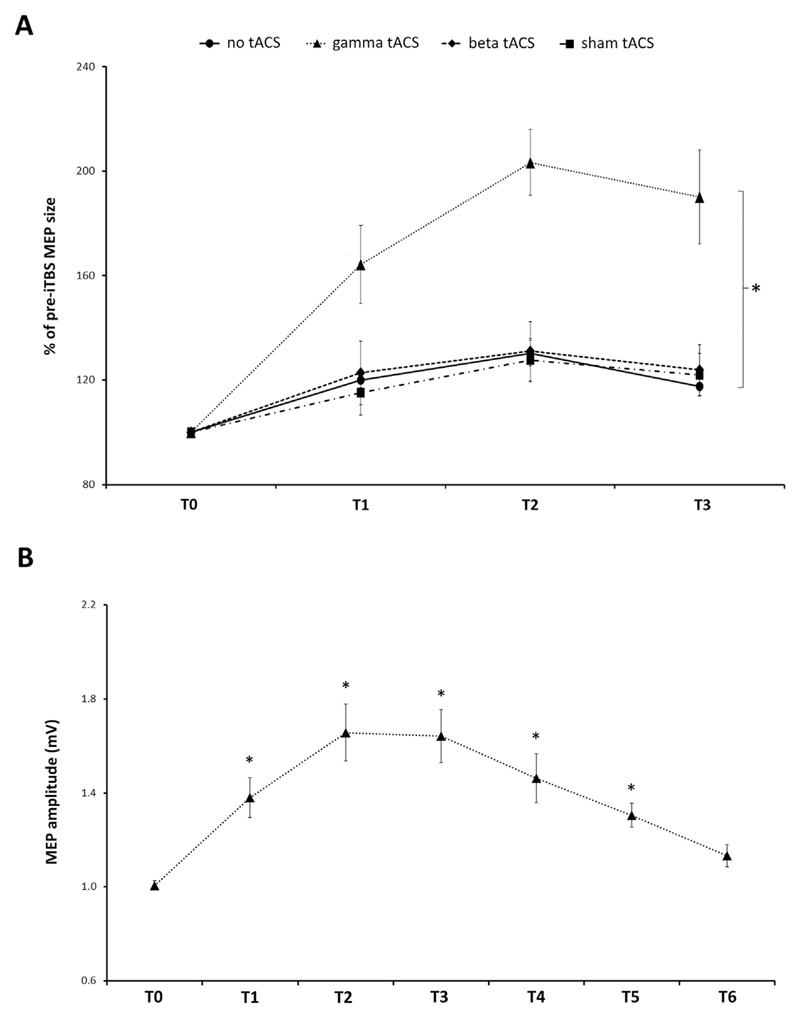
Having confirmed that the MEP size did not differ between sessions at baseline, we normalized the post-iTBS MEP amplitudes to their corresponding baseline and expressed the values as a percentage. A two-way rmANOVA identified a significant main effect of both ‘session’ (F(3,39) = 13.56, p < 0.001) and ‘time point’ (F(2,26) = 7.05, p = 0.004), whereas no interaction was detected between the two factors (F(6,78) = 1.82, p = 0.11). The post-hoc analysis on ‘session’ revealed greater MEP facilitation in the iTBS-γ tACS condition than in the other conditions (iTBS-γ tACS vs iTBS: p = 0.001; iTBS-γ tACS vs iTBS-β tACS: p = 0.005; iTBS-γ tACS vs iTBS-sh tACS: p = 0.01) (Fig. 2A). No differences were observed when the other conditions were compared with one another (all p values > 0.05). The post-hoc analysis on ‘time point’ revealed that iTBS modulated MEP facilitation over time (T1 vs T2: p = 0.01; T2 vs T3: p = 0.16; T1 vs T3: p = 0.29), regardless of session.
Fig. 2.
A, Effect of tACS-iTBS co-stimulation. When γ-tACS was delivered during iTBS, a significant MEP facilitation was observed compared with the other sessions (rm ANOVA with the factors ‘session’ and ‘time point’ identified a significant main effect of ‘session’ (p < 0.001); * = significant post-hoc t-tests for the factor ‘session’, p < 0.01). No differences were detected between iTBS (no tACS), iTBS-sham tACS and iTBS-β tACS. MEP amplitudes (average ± SEM) for each time point (i.e. after 5 min - T1, 15 min - T2, and 30 min - T3) after-iTBS are compared with pre-iTBS values (set as 100% - T0). B, Time course of iTBS-γ tACS. MEPs were recorded at six different time points after iTBS and compared with pre-iTBS (T0) MEP amplitude values: after 5 min (T1), 15 min (T2), 30 min (T3), 60 min (T4), 90 min (T5) and 180 min (T6). Average MEP amplitude ± SEM is shown. * = time points that were significantly different from T0 after post-hoc comparisons (p < 0.05). P values are presented after Bonferroni's correction for multiple comparisons.
We also assessed whether the lack of effect of iTBS-β tACS might be due to the fact that the stimulation intensity applied during iTBS-β tACS was lower than that applied during iTBS-γ tACS. We used two different approaches to test this hypothesis. First, using a median split procedure [16], we divided the participants in two groups according to the intensity of the stimulation used for β-tACS: low-β intensity (7 subjects, 0.31 ± 0.07 mA) and high-β intensity (7 subjects, 0.84 ± 0.17 mA). We then conducted a rmANOVA with the within-group factor ‘time point’ and the between-group factor ‘stimulation intensity’. This rmANOVA did not detect any significant effect of ‘stimulation intensity’ (F(1,12) = 0.48; p = 0.5) nor any ‘stimulation intensity’ x ‘time point’ interaction (F(2,24) = 0.76; p = 0.48). Secondly, we investigated a possible correlation between the stimulation intensity and the overall level of MEP potentiation after iTBS-β tACS. This analysis did not yield any significant results either (r = 0.06; p = 0.84). Accordingly, although we did not use modelling to estimate whether sufficient current density reached the target cortical area in all subjects, our statistical analyses on MEPs suggested a lack of any effect of iTBS-β tACS at any of the intensities achieved in the current study.
To sum up, the results demonstrate that when γ-tACS is continuously delivered during iTBS, it yields a greater MEP amplitude facilitation, which may reflect an enhancement of the iTBS-related LTP-like effects.
Experiment 2: time course of iTBS-γ tACS co-stimulation
Experiment 2 was performed in order to determine the duration of MEP facilitation observed in the iTBS-γ tACS condition. A rmANOVA conducted on MEP amplitudes revealed a significant effect of the factor ‘time point’ (F(2.66,18.65) = 18.38; p < 0.001). The post-hoc analysis showed that the MEP size at T0 was smaller than those recorded at all the other time points but T6, i.e. 180 min after iTBS (T0 vs T1: p = 0.04; T0 vs T2: p = 0.01; T0 vs T3: p = 0.01; T0 vs T4: p = 0.048; T0 vs T5: p = 0.01; T0 vs T6: p = 0.35) (Fig. 2B). To sum up, iTBS-γ tACS co-stimulation of M1 induced a marked LTP-like effect that peaked at 15 min and remained stable at 30 min, but gradually decreased thereafter. The potentiation in MEP amplitude was still present 90 min after iTBS-γ tACS but gradually returned toward baseline values at 180 min.
We also verified whether the extent of MEP potentiation differed from that induced by iTBS-γ tACS in Experiment 1. A two-way rmANOVA with factors ‘session’ (2 levels: Experiment 1 and Experiment 2) and ‘time point’ (3 levels: T1, T2, T3) did not detect an effect for the main factor ‘session’ (F(1,7) = 1.74; p = 0.23) or any ‘session’ x ‘time point’ interaction (F(2,14) = 0.97; p = 0.4). In addition, the data collected in Experiment 2 allowed us to test the consistency of the effect of iTBS-γ tACS. For this purpose, we calculated the overall extent of potentiation and the peak of potentiation induced by iTBS-γ tACS in Experiments 1 and 2 and then tested a possible correlation between the sessions. Despite the relatively low number of subjects, a strong trend toward significance emerged for both measures (overall extent of potentiation: r = 0.68, p = 0.06; peak of potentiation: r = 0.64, p = 0.08).
To sum up, these results demonstrate that iTBS-γ tACS co-stimulation significantly extends the duration of the after-effects on M1 excitability compared with the original iTBS protocol [26]. Moreover, the resulting effect may be relatively stable and reliable over repeated sessions.
Experiment 3: effect of γ-tACS on corticospinal excitability and GABA-A inhibitory interneurons
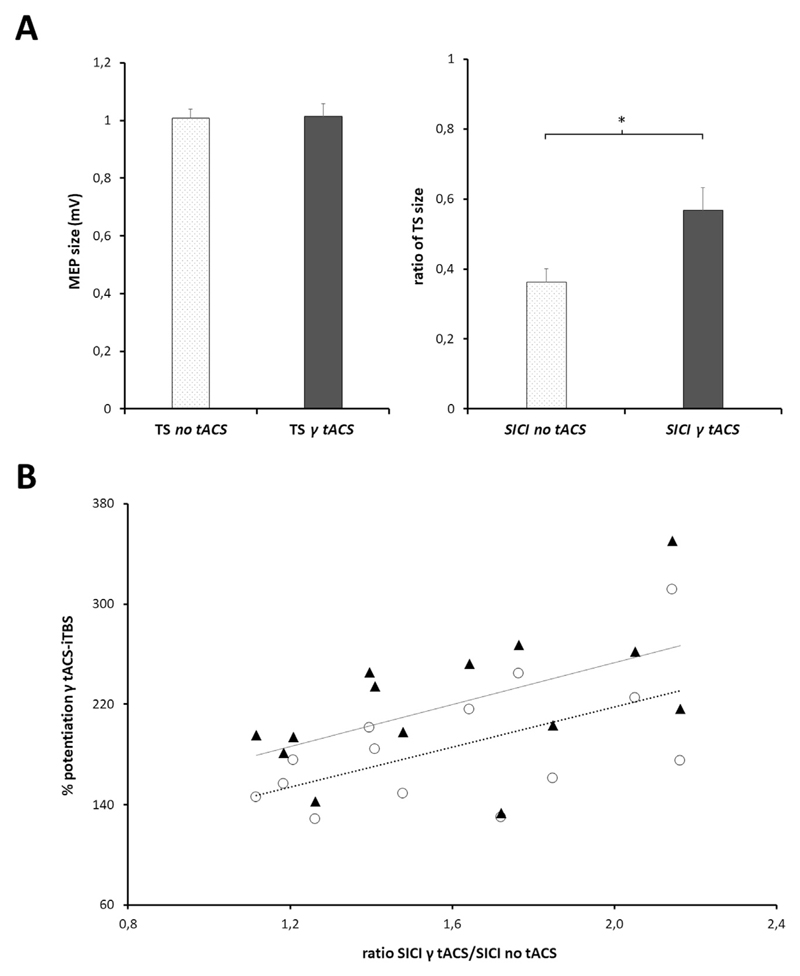
In this experiment, we first explored whether γ-tACS per se modifies corticospinal excitability and/or GABA-A neurotransmission. A rmANOVA with factors ‘γ-tACS’ and ‘TMS paradigm’ revealed a significant interaction between the two factors (F(1,13) = 13.98; p = 0.002). The post-hoc analysis showed that the conditioned MEP, i.e. SICI, was modified by tACS (p < 0.001) whereas the unconditioned MEP was not (p = 0.89) (Fig. 3A).
Fig. 3.
A, Effect of γ tACS on single pulse TMS (left panel) and short-interval intracortical inhibition (SICI - right panel). Average MEP size (and SEM) is displayed for single pulse TMS data. SICI values are expressed as the ratio of the test stimulus (TS); TS inhibition was significantly reduced when SICI was tested during γ-tACS (*p < 0.001). B, Correlation between modulation of SICI induced by γ tACS and increased plasticity during iTBS-γ tACS. SICI modulation was calculated as the ratio between the SICI obtained during γ-tACS and that obtained at baseline (no tACS condition), i.e. the higher the ratio, the greater the reduction in SICI due to γ-tACS (x axis). The level of plasticity induced by iTBS-γ tACS co-stimulation was evaluated both as a percentage of overall potentiation (mean percentage of MEP facilitation obtained at T1, T2 and T3 – empty circles) and as a percentage of the peak of potentiation (percentage of MEP facilitation obtained at the individual time peak of potentiation after iTBS – full triangles). Both correlations were significant at Pearson's correlation test (SICI ratio and overall potentiation: r = 0.57, p = 0.03; SICI ratio and peak of potentiation: r = 0.54, p = 0.04). The dotted line shows the trend for the global potentiation values; the continuous line shows the trend for the peak of potentiation values.
We also tested whether the modulation of the SICI induced by γ-tACS was correlated, at the individual level, with the increase in MEP amplitude after iTBS-γ tACS co-stimulation. To quantify any changes in SICI modulation due to γ-tACS, we calculated the ratio between the SICI during γ-tACS and the SICI in the tACS OFF condition (i.e. SICI γ-tACS ON/SICI γ-tACS OFF). We then investigated any correlations between these values and both the overall extent of potentiation (see data analysis) and the peak of potentiation (i.e. percentage of MEP facilitation obtained at the individual time peak of potentiation after iTBS) induced by iTBS-γ tACS. The analysis revealed a positive correlation between these two measures (SICI ratio and overall potentiation: r = 0.57, p = 0.03; SICI ratio and peak of potentiation: r = 0.54, p = 0.04; Fig. 3B).
To sum up, our results confirm that γ-tACS delivered over M1 induces a short-term reduction in GABA-A inhibitory intracortical neurotransmission, though without any marked change in the overall level of corticospinal excitability [16]. Moreover, our results suggest that the increased LTP-like effect induced by iTBS-γ tACS co-stimulation is likely due to the modulation of GABA-A interneurons.
Control experiment: after-effects of γ-tACS
This additional experiment was designed to evaluate possible after-effects of γ-tACS on MEPs. A rmANOVA conducted on MEP amplitudes revealed no significant effect of the factor ‘time point’ (T0: 0.99 ± 0.09 mV, T1: 0.96 ± 0.09 mV, T2: 1.03 ± 0.11 mV, T3: 1.02 ± 0.09 mV; F(3,21) = 0.74; p = 0.54), thus suggesting no relevant after-effects of γ-tACS on M1 excitability.
Discussion
Here, we provide the first evidence suggesting that tACS delivered at a gamma frequency can significantly boost and prolong iTBS-induced LTP-like plasticity in human M1. By contrast, tACS delivered at a beta frequency leaves the iTBS-related LTP-like plasticity unchanged. Lastly, γ-tACS boosts iTBS by modulating GABA-A inhibitory intracortical interneurons: the more marked the tACS-related reduction in GABA-A neurotransmission, the greater the iTBS-induced LTP-like plasticity.
The first finding that emerges from the present study is that γ-tACS boosts and prolongs the iTBS-induced after-effects in human M1. tACS may act to entrain susceptible neuronal populations where susceptibility is determined at least in part by resonance [6,7]. Beta constitutes the main intrinsic oscillatory activity in M1 [38], and tACS at 20 Hz is able to synchronize some GABA-A-ergic neurons in M1, as evinced by the tACS phase-dependent modulation of GABA-A-ergic SICI [13]. However, β-tACS did not promote the after-effects induced by iTBS. Therefore, we can exclude the possibility that the simple M1 ‘resonance’ of any externally delivered rhythm can increase the extent of LTP-like plasticity. Instead tACS in the gamma band promoted the after-effects induced by iTBS, raising the possibility of entrainment of a different gamma-resonant subpopulation of GABA-A-ergic neurons in M1. Synchronized oscillations in the gamma frequency are also a feature of the motor cortex, typically occurring before and during movement [39–45]. We suggest that tACS at 70 Hz may have promoted the after-effects of iTBS by interaction with a gamma-resonant subpopulation of GABA-A-ergic neurons in M1. It should however be noted that we have not directly demonstrated resonance, although, as will be discussed later, there is considerable evidence linking GABA-A-ergic neurons to gamma band resonant activity. In contrast, the ability of γ-tACS to boost and prolong the LTP-like effect of iTBS cannot be explained by simple modifications in M1 excitability or by a hypothetical additive effect of γ-tACS on brain plasticity (i.e. electric stimulation after-effects + iTBS after-effects). In keeping with previous experimental observations [11,16,46,47], we confirmed that tACS delivered at a gamma frequency does not change the overall level of corticospinal excitability as evaluated by single pulse TMS. Moreover, we tested possible MEPs changes up to 30 min post γ-tACS and, in line with previous studies [16,46,47], we demonstrated that this does not induce any significant after-effects.
γ-tACS potentiates iTBS by modulating GABA-A interneurons within M1
When delivered at gamma frequency, tACS modulates interneuronal GABA-A neurotransmission. We recently demonstrated that SICI, a TMS measure that reflects the activity of GABA-A inhibitory interneurons within M1 [19,33,35], is reduced when tested early during γ-tACS stimulation [16]. Here we confirm and extend these results: reduced SICI can be considered an immediate (on-line) effect as it is observed a few seconds after tACS is activated. More importantly, we now demonstrate that the increase in M1 plasticity due to γ-tACS co-stimulation is correlated with the ability of γ-tACS to modulate GABA-A inhibitory intracortical interneurons, i.e. the more marked the tACS-related reduction in GABA-A neurotransmission, the greater the iTBS-induced brain plasticity.
Physiological mechanisms responsible for γ-tACS effect on iTBS in M1
There is a body of evidence that points to a link between gamma oscillations and interneurons at the M1 level. Studies based on animals [48–50], humans [51] and biophysical or theoretical models [52–54] have demonstrated that GABA-ergic interneuronal activity contributes to the generation of gamma oscillatory activity. Moreover, gamma oscillations probably originate from superficial layers 2/3 (L2/3) of the neocortex, which are mainly populated by interneurons [55]. Evidence also exists of specific interneuronal subpopulations with an intrinsic responsivity to gamma-band synchrony, which suggests resonance in this frequency band [56,57]. GABA-A interneuronal activity also plays a crucial role in driving LTP plasticity. In animal models, the induction of LTP-like plasticity significantly reduces the amount of proteins expressed in inhibitory interneurons and related to GABA-ergic neurotransmission [21,58,59]. In humans, epidural recordings have shown that iTBS modulates the late I-waves [22], which are produced by cortical circuits selectively targeted by inhibitory projections activated in the SICI protocol [19,60,61]. Lastly, studies conducted on slice preparations and at the macroscopic level have revealed a significant involvement of oscillatory activity at the gamma frequency in LTP-induced plasticity [27–29,62–64]. A recent TMS-electrocorticography study on monkeys demonstrated that the LTP-like after-effects induced by iTBS are paralleled by an increase in high-gamma band activity in sensorimotor cortices [30]. To date, a unifying demonstration of a causal link between gamma oscillations, GABA-A interneuronal activity and LTP-like induced plasticity in M1 has been lacking. We posit that we could strongly enhance the LTP-like after-effects of iTBS by synchronizing selected neural elements of M1 in the gamma frequency band by means of tACS at this frequency. Thus the effect correlated with the ability of γ-tACS to suppress the activity of GABA-A inhibitory interneurons. Our data raise the possibility that gamma activity, intracortical inhibitory interneurons and LTP-like plasticity may be mechanistically interconnected in human M1. We speculate that specific subset of neocortical interneurons are resonant to gamma oscillations in addition to being implicated in GABA-A neurotransmission and directly involved in LTP-like plasticity induction. Candidate interneurons include those in L2/3 and layer 1 (L1) neurogliaform cells. Involvement of the latter might be made more likely by the low intensity of stimulation, so that tACS may preferentially suppress intracortical inhibitory activity by modulating more superficial cortical layers. Indeed, recent studies have described a complex inhibitory circuit composed of L1 neurogliaform cells with reciprocal connections with L2/3 interneurons, which in turn inhibit pyramidal cells [65]. Owing to their superficial location, the axons of L1 neurons might be activated by low intensity magnetic stimulation, leading to the suppression of corticospinal cell excitability in the SICI protocol [19], whereas the same population of interneurons might be modulated by γ-tACS. Gamma-tACS may enhance the activity of superficial inhibitory interneurons, which would in turn inhibit the function of GABA-Aergic interneurons in deeper layers.
Lastly, whether γ-tACS enhances cortical plasticity-modulating GABA-A-ergic interneurons by acting through homeostatic or non-homeostatic metaplasticity deserves comment [66–68]. We can reasonably exclude the involvement of homeostatic meta-plasticity since it is generally based on priming approaches that alter the level of postsynaptic activity in the corticospinal neurons to trigger homeostatic responses [67,69,70]. Our observations instead fit well with the involvement of non-homeostatic meta-plasticity, particularly with the phenomenon of ‘gating’, whereby LTP-like plasticity in M1 is boosted by reducing the excitability of GABA-A-ergic inhibitory circuits in plasticity-inducing protocols [71–74].
Conclusions
In this study, we demonstrate that γ-tACS boosts and prolongs iTBS-induced LTP-like plasticity in human M1. We also provide evidence showing that this effect is directly related to changes in GABA-A-ergic interneuronal activity. Our findings are compatible with a link between gamma oscillations, GABA-A interneuronal activity and LTP-like plasticity in human M1. Also, they may have important translational implications. Indeed, traditional neuromodulation techniques are characterized by a relatively short duration and occasionally weak effects [2,4,68]. By contrast, the tACS-TMS co-stimulation protocol we propose both enhances and prolongs M1 plasticity. However, our findings cannot be necessarily extended to all the LTP-like plasticity-inducing protocols, owing to the different physiological processes induced by these paradigms. For example, Paired Associative Stimulation (PAS) [70] depends on plasticity arising from sensori-motor integration, whereas iTBS reflects intrinsic M1 plasticity. Future studies should be performed with other plasticity-inducing protocols, including PAS, to clarify the generalisability or otherwise of the effect of co-stimulation [2,3], and test the possible phase-dependency of after-effects. It remains to be seen whether the current paradigm might provide a means of promoting motor recovery after acute brain damage in a neurorehabilitation setting [75] or even in chronic neurodegenerative disorders affecting the motor system [76].
Abbreviations
- M1
primary motor cortex
- RMT
resting motor threshold
- tACS
Transcranial Alternating Current Stimulation
- TMS
Transcranial Magnetic Stimulation
- SICI
Short-interval intracortical inhibition
- iTBS
intermittent theta-burst stimulation
- γ
gamma
- β
beta
Footnotes
Conflicts of interest
A.G., A.S., M.B., V.D.O., E.B., P.B., V.D.L., A.B. declare no competing financial interest.
References
- [1].Hinder MR, Goss EL, Fujiyama H, Canty AJ, Garry MI, Rodger J, Summers JJ. Inter- and intra-individual variability following intermittent theta burst stimulation: implications for rehabilitation and recovery. Brain Stimul. 2014;7:365–71. doi: 10.1016/j.brs.2014.01.004. [DOI] [PubMed] [Google Scholar]
- [2].Guerra A, López-Alonso V, Cheeran B, Suppa A. Variability in non-invasive brain stimulation studies: reasons and results. Neurosci Lett. 2017 Dec 30; doi: 10.1016/j.neulet.2017.12.058. pii: S0304-3940(17) 31048–0. [DOI] [PubMed] [Google Scholar]
- [3].Guerra A, López-Alonso V, Cheeran B, Suppa A. Solutions for managing variability in non-invasive brain stimulation studies. Neurosci Lett. 2017 Dec 30; doi: 10.1016/j.neulet.2017.12.060. pii: S0304-3940(17) 31051–0. [DOI] [PubMed] [Google Scholar]
- [4].Ziemann U, Siebner HR. Inter-subject and inter-session variability of plasticity induction by non-invasive brain stimulation: boon or bane? Brain Stimul. 2015;8:662–3. doi: 10.1016/j.brs.2015.01.409. [DOI] [PubMed] [Google Scholar]
- [5].Antal A, Paulus W. Transcranial alternating current stimulation (tACS) Front Hum Neurosci. 2013;7:317. doi: 10.3389/fnhum.2013.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ozen S, Sirota A, Belluscio MA, Anastassiou CA, Stark E, Koch C, Buzsáki G. Transcranial electric stimulation entrains cortical neuronal populations in rats. J Neurosci. 2010;30:11476–85. doi: 10.1523/JNEUROSCI.5252-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Reato D, Rahman A, Bikson M, Parra LC. Effects of weak transcranial alternating current stimulation on brain activity-a review of known mechanisms from animal studies. Front Hum Neurosci. 2013;7:687. doi: 10.3389/fnhum.2013.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fröhlich F, McCormick DA. Endogenous electric fields may guide neocortical network activity. Neuron. 2010;67:129–43. doi: 10.1016/j.neuron.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schutter DJLG, Hortensius R. Brain oscillations and frequency-dependent modulation of cortical excitability. Brain Stimul. 2011;4:97–103. doi: 10.1016/j.brs.2010.07.002. [DOI] [PubMed] [Google Scholar]
- [10].Pogosyan A, Gaynor LD, Eusebio A, Brown P. Boosting cortical activity at Beta-band frequencies slows movement in humans. Curr Biol. 2009;19:1637–41. doi: 10.1016/j.cub.2009.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Feurra M, Bianco G, Santarnecchi E, Del Testa M, Rossi A, Rossi S. Frequency-dependent tuning of the human motor system induced by transcranial oscillatory potentials. J Neurosci. 2011;31:12165–70. doi: 10.1523/JNEUROSCI.0978-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cappon D, D'Ostilio K, Garraux G, Rothwell J, Bisiacchi P. Effects of 10 Hz and 20 Hz transcranial alternating current stimulation on automatic motor control. Brain Stimul. 2016;9:518–24. doi: 10.1016/j.brs.2016.01.001. [DOI] [PubMed] [Google Scholar]
- [13].Guerra A, Pogosyan A, Nowak M, Tan H, Ferreri F, Di Lazzaro V, Brown P. Phase dependency of the human primary motor cortex and cholinergic inhibition cancelation during beta tACS. Cereb Cortex. 2016;26:3977–90. doi: 10.1093/cercor/bhw245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Moisa M, Polania R, Grueschow M, Ruff CC. Brain network mechanisms underlying motor enhancement by transcranial entrainment of gamma oscillations. J Neurosci. 2016;36:12053–65. doi: 10.1523/JNEUROSCI.2044-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Joundi RA, Jenkinson N, Brittain J-S, Aziz TZ, Brown P. Driving oscillatory activity in the human cortex enhances motor performance. Curr Biol. 2012;22:403–7. doi: 10.1016/j.cub.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nowak M, Hinson E, van Ede F, Pogosyan A, Guerra A, Quinn A, Brown P, Stagg CJ. Driving human motor cortical oscillations leads to behaviorally relevant changes in local GABAA inhibition: a tACS-TMS study. J Neurosci. 2017;37:4481–92. doi: 10.1523/JNEUROSCI.0098-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Di Lazzaro V, Ziemann U. The contribution of transcranial magnetic stimulation in the functional evaluation of microcircuits in human motor cortex. Front Neural Circ. 2013;7:18. doi: 10.3389/fncir.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hamada M, Galea JM, Di Lazzaro V, Mazzone P, Ziemann U, Rothwell JC. Two distinct interneuron circuits in human motor cortex are linked to different subsets of physiological and behavioral plasticity. J Neurosci. 2014;34:12837–49. doi: 10.1523/JNEUROSCI.1960-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Di Lazzaro V, Rothwell J, Capogna M. Noninvasive stimulation of the human brain: activation of multiple cortical circuits. Neuroscientist. 2017 doi: 10.1177/1073858417717660. [DOI] [PubMed] [Google Scholar]
- [20].McDonnell MN, Orekhov Y, Ziemann U. Suppression of LTP-like plasticity in human motor cortex by the GABAB receptor agonist baclofen. Exp Brain Res. 2007;180:181–6. doi: 10.1007/s00221-006-0849-0. [DOI] [PubMed] [Google Scholar]
- [21].Benali A, Trippe J, Weiler E, Mix A, Petrasch-Parwez E, Girzalsky W, Eysel UT, Erdmann R, Funke K. Theta-burst transcranial magnetic stimulation alters cortical inhibition. J Neurosci. 2011;31:1193–203. doi: 10.1523/JNEUROSCI.1379-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Di Lazzaro V, Pilato F, Dileone M, Profice P, Oliviero A, Mazzone P, Insola A, Ranieri F, Meglio M, Tonali PA, Rothwell JC. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J Physiol. 2008;586:3871–9. doi: 10.1113/jphysiol.2008.152736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Di Lazzaro V, Dileone M, Pilato F, Profice P, Oliviero A, Mazzone P, Insola A, Capone F, Ranieri F, Tonali PA. Associative motor cortex plasticity: direct evidence in humans. Cereb Cortex. 2009;19:2326–30. doi: 10.1093/cercor/bhn255. [DOI] [PubMed] [Google Scholar]
- [24].Goldsworthy MR, Vallence AM, Yang R, Pitcher JB, Ridding MC. Combined transcranial alternating current stimulation and continuous theta burst stimulation: a novel approach for neuroplasticity induction. Eur J Neurosci. 2016;43:572–9. doi: 10.1111/ejn.13142. [DOI] [PubMed] [Google Scholar]
- [25].Doeltgen SH, McAllister SM, Ridding MC. Simultaneous application of slow-oscillation transcranial direct current stimulation and theta burst stimulation prolongs continuous theta burst stimulation-induced suppression of corticomotor excitability in humans. Eur J Neurosci. 2012;36:2661–8. doi: 10.1111/j.1460-9568.2012.08181.x. [DOI] [PubMed] [Google Scholar]
- [26].Suppa A, Huang Y-Z, Funke K, Ridding MC, Cheeran B, Di Lazzaro V, Ziemann U, Rothwell JC. Ten years of theta burst stimulation in humans: established knowledge, unknowns and prospects. Brain Stimul. 2016;9:323–35. doi: 10.1016/j.brs.2016.01.006. [DOI] [PubMed] [Google Scholar]
- [27].Diba K, Buzsáki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10:1241–2. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Izaki Y, Akema T. Gamma-band power elevation of prefrontal local field potential after posterior dorsal hippocampus-prefrontal long-term potentiation induction in anesthetized rats. Exp Brain Res. 2008;184:249–53. doi: 10.1007/s00221-007-1098-6. [DOI] [PubMed] [Google Scholar]
- [29].Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–3. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- [30].Papazachariadis O, Dante V, Verschure PFMJ, Del Giudice P, Ferraina S. iTBS-induced LTP-like plasticity parallels oscillatory activity changes in the primary sensory and motor areas of macaque monkeys. PLoS One. 2014;9:e112504. doi: 10.1371/journal.pone.0112504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126:1071–107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–6. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- [33].Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol (Lond) 1993;471:501–19. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Berardelli A, Abbruzzese G, Chen R, Orth M, Ridding MC, Stinear C, Suppa A, Trompetto C, Thompson PD. Consensus paper on short-interval intracortical inhibition and other transcranial magnetic stimulation intracortical paradigms in movement disorders. Brain Stimul. 2008;1:183–91. doi: 10.1016/j.brs.2008.06.005. [DOI] [PubMed] [Google Scholar]
- [35].Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol (Lond) 1996;496:873–81. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Peurala SH, Müller-Dahlhaus JFM, Arai N, Ziemann U. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF) Clin Neurophysiol. 2008;119:2291–7. doi: 10.1016/j.clinph.2008.05.031. [DOI] [PubMed] [Google Scholar]
- [37].Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol (Lond) 2001;530:307–17. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Niedermeyer E, Lopes da Silva F. Electroencephalography: basic principles, clinical applications, and related fields. fourth ed. Lippincott Williams & Wilkins; 1999. [Google Scholar]
- [39].Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121:2301–15. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- [40].Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–57. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- [41].Pfurtscheller G, Graimann B, Huggins JE, Levine SP, Schuh LA. Spatiotemporal patterns of beta desynchronization and gamma synchronization in corticographic data during self-paced movement. Clin Neurophysiol. 2003;114:1226–36. doi: 10.1016/s1388-2457(03)00067-1. [DOI] [PubMed] [Google Scholar]
- [42].Cheyne D, Bells S, Ferrari P, Gaetz W, Bostan AC. Self-paced movements induce high-frequency gamma oscillations in primary motor cortex. Neuroimage. 2008;42:332–42. doi: 10.1016/j.neuroimage.2008.04.178. [DOI] [PubMed] [Google Scholar]
- [43].Muthukumaraswamy SD. Functional properties of human primary motor cortex gamma oscillations. J Neurophysiol. 2010;104:2873–85. doi: 10.1152/jn.00607.2010. [DOI] [PubMed] [Google Scholar]
- [44].Cheyne DO. MEG studies of sensorimotor rhythms: a review. Exp Neurol. 2013;245:27–39. doi: 10.1016/j.expneurol.2012.08.030. [DOI] [PubMed] [Google Scholar]
- [45].Gaetz W, Liu C, Zhu H, Bloy L, Roberts TPL. Evidence for a motor gamma-band network governing response interference. Neuroimage. 2013;74:245–53. doi: 10.1016/j.neuroimage.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Antal A, Boros K, Poreisz C, Chaieb L, Terney D, Paulus W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. 2008;1:97–105. doi: 10.1016/j.brs.2007.10.001. [DOI] [PubMed] [Google Scholar]
- [47].Moliadze V, Antal A, Paulus W. Boosting brain excitability by transcranial high frequency stimulation in the ripple range. J Physiol (Lond) 2010;588:4891–904. doi: 10.1113/jphysiol.2010.196998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Traub RD, Whittington MA, Colling SB, Buzsáki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol (Lond) 1996;493:471–84. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Towers SK, Gloveli T, Traub RD, Driver JE, Engel D, Fradley R, Rosahl TW, Maubach K, Buhl EH, Whittington MA. Alpha 5 subunit-containing GABAA receptors affect the dynamic range of mouse hippocampal kainate-induced gamma frequency oscillations in vitro. J Physiol (Lond) 2004;559:721–8. doi: 10.1113/jphysiol.2004.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ahmed OJ, Mehta MR. Running speed alters the frequency of hippocampal gamma oscillations. J Neurosci. 2012;32:7373–83. doi: 10.1523/JNEUROSCI.5110-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gaetz W, Edgar JC, Wang DJ, Roberts TPL. Relating MEG measured motor cortical oscillations to resting γ-aminobutyric acid (GABA) concentration. Neuroimage. 2011;55:616–21. doi: 10.1016/j.neuroimage.2010.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wang XJ, Buzsáki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci. 1996;16:6402–13. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci. 2007;30:343–9. doi: 10.1016/j.tins.2007.05.003. [DOI] [PubMed] [Google Scholar]
- [54].Buia CI, Tiesinga PH. Role of interneuron diversity in the cortical microcircuit for attention. J Neurophysiol. 2008;99:2158–82. doi: 10.1152/jn.01004.2007. [DOI] [PubMed] [Google Scholar]
- [55].Arnal LH, Giraud A-L. Cortical oscillations and sensory predictions. Trends cogn sci (regul ed) 2012;16:390–8. doi: 10.1016/j.tics.2012.05.003. [DOI] [PubMed] [Google Scholar]
- [56].Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai L-H, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–7. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Otte S, Hasenstaub A, Callaway EM. Cell type-specific control of neuronal responsiveness by gamma-band oscillatory inhibition. J Neurosci. 2010;30:2150–9. doi: 10.1523/JNEUROSCI.4818-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Aydin-Abidin S, Trippe J, Funke K, Eysel UT, Benali A. High- and low-frequency repetitive transcranial magnetic stimulation differentially activates c-Fos and zif268 protein expression in the rat brain. Exp Brain Res. 2008;188:249–61. doi: 10.1007/s00221-008-1356-2. [DOI] [PubMed] [Google Scholar]
- [59].Trippe J, Mix A, Aydin-Abidin S, Funke K, Benali A. θ burst and conventional low-frequency rTMS differentially affect GABAergic neurotransmission in the rat cortex. Exp Brain Res. 2009;199:411–21. doi: 10.1007/s00221-009-1961-8. [DOI] [PubMed] [Google Scholar]
- [60].Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. 2000;111:794–9. doi: 10.1016/s1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- [61].Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Dileone M, Insola A, Tonali PA, Rothwell JC. Short-term reduction of intracortical inhibition in the human motor cortex induced by repetitive transcranial magnetic stimulation. Exp Brain Res. 2002;147:108–13. doi: 10.1007/s00221-002-1223-5. [DOI] [PubMed] [Google Scholar]
- [62].Buzsaki G. The hippocampo-neocortical dialogue. Cereb Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- [63].Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–9. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- [64].Buzsaki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron. 2010;68:362–85. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ. The organization of two new cortical interneuronal circuits. Nat Neurosci. 2013;16:210–8. doi: 10.1038/nn.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Karabanov A, Ziemann U, Hamada M, George MS, Quartarone A, Classen J, Massimini M, Rothwell J, Siebner HR. Consensus paper: probing homeostatic plasticity of human cortex with non-invasive transcranial brain stimulation. Brain Stimul. 2015;8:993–1006. doi: 10.1016/j.brs.2015.06.017. [DOI] [PubMed] [Google Scholar]
- [67].Müller-Dahlhaus F, Ziemann U. Metaplasticity in human cortex. Neuroscientist. 2015;21:185–202. doi: 10.1177/1073858414526645. [DOI] [PubMed] [Google Scholar]
- [68].Huang Y-Z, Lu M-K, Antal A, Classen J, Nitsche M, Ziemann U, Ridding M, Hamada M, Ugawa Y, Jaberzadeh S, Suppa A, et al. Plasticity induced by non-invasive transcranial brain stimulation: a position paper. Clin Neurophysiol. 2017;128:2318–29. doi: 10.1016/j.clinph.2017.09.007. [DOI] [PubMed] [Google Scholar]
- [69].Ziemann U, Siebner HR. Modifying motor learning through gating and homeostatic metaplasticity. Brain Stimul. 2008;1:60–6. doi: 10.1016/j.brs.2007.08.003. [DOI] [PubMed] [Google Scholar]
- [70].Suppa A, Quartarone A, Siebner H, Chen R, Di Lazzaro V, Del Giudice P, Paulus W, Rothwell JC, Ziemann U, Classen J. The associative brain at work: evidence from paired associative stimulation studies in humans. Clin Neurophysiol. 2017;128:2140–64. doi: 10.1016/j.clinph.2017.08.003. [DOI] [PubMed] [Google Scholar]
- [71].Hess G, Aizenman CD, Donoghue JP. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophysiol. 1996;75:1765–78. doi: 10.1152/jn.1996.75.5.1765. [DOI] [PubMed] [Google Scholar]
- [72].Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124:1171–81. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]
- [73].Quartarone A, Rizzo V, Bagnato S, Morgante F, Sant'Angelo A, Girlanda P, Siebner HR. Rapid-rate paired associative stimulation of the median nerve and motor cortex can produce long-lasting changes in motor cortical excitability in humans. J Physiol (Lond) 2006;575:657–70. doi: 10.1113/jphysiol.2006.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Siebner HR. A primer on priming the human motor cortex. Clin Neurophysiol. 2010;121:461–3. doi: 10.1016/j.clinph.2009.12.009. [DOI] [PubMed] [Google Scholar]
- [75].Guerra A, Costantini EM, Maatta S, Ponzo D, Ferreri F. Disorders of consciousness and electrophysiological treatment strategies: a review of the literature and new perspectives. Curr Pharmaceut Des. 2014;20:4248–67. [PubMed] [Google Scholar]
- [76].Giovanni A, Capone F, di Biase L, Ferreri F, Florio L, Guerra A, Marano M, Paolucci M, Ranieri F, Salomone G, Tombini M, et al. Oscillatory activities in neurological disorders of elderly: biomarkers to target for neuromodulation. Front Aging Neurosci. 2017;9(189) doi: 10.3389/fnagi.2017.00189. Erratum in: Front Aging Neurosci. 2017;9:252. [DOI] [PMC free article] [PubMed] [Google Scholar]