Abstract
Objective
Several distinctive clinical features of patients with familial dysautonomia (FD) including dysarthria and dysphagia suggest a developmental defect in brainstem reflexes. Our aim was to characterize the neurophysiological profile of brainstem reflexes in these patients.
Methods
We studied the function of sensory and motor trigeminal tracts in 28 patients with FD. All were homozygous for the common mutation in the IKAP gene. Each underwent a battery of electrophysiological tests including; blink reflexes, jaw jerk reflex, masseter silent periods and direct stimulation of the facial nerve. Responses were compared with 25 age-matched healthy controls.
Results
All patients had significantly prolonged latencies and decreased amplitudes of all examined brainstem reflexes. Similar abnormalities were seen in the early and late components. In contrast, direct stimulation of the facial nerve revealed relative preservation of motor responses.
Conclusions
The brainstem reflex abnormalities in FD are best explained by impairment of the afferent and central pathways. A reduction in the number and/or excitability of trigeminal sensory axons is likely the main problem.
Significance
These findings add further evidence to the concept that congenital mutations of the elongator-1 protein (or IKAP) affect the development of afferent neurons including those carrying information for the brainstem reflex pathways.
Keywords: Familial dysautonomia, blink reflex, jaw jerk reflex, afferent disorder, hereditary sensory neuropathy, hereditary sensory and autonomic neuropathy type III
INTRODUCTION
Familial dysautonomia (FD) is a congenital autosomal recessive neuropathy caused by mutations in the elongator-1/IKAP gene (Anderson et al., 2001, Slaugenhaupt et al., 2001). The resulting protein deficiency selectively impairs the development of particular sensory (afferent) neurons (Mezey et al., 2003, Close et al., 2006). In addition to relative indifference to pain and insensitivity to temperature (Riley et al., 1949), affected patients are born with signs of cranial nerve dysfunction including afferent baroreflex failure (Norcliffe-Kaufmann et al., 2010), blunted hypoxic ventilatory drive (Filler et al., 1965) and absent corneal reflexes (Mahloudji et al., 1970).
Several lines of evidence suggest abnormalities in the trigeminal nerve (cranial nerve V) and medullary pathways. Post-mortem examinations showed a marked reduction in sensory neuron counts in the trigeminal ganglia (Brown et al., 1964, Aguayo et al., 1971, Pearson et al., 1971, Pearson et al., 1978a, Pearson et al., 1978c) and gross atrophy of the medulla (Brown et al., 1964, Pearson et al., 1971, Pearson et al., 1978a, Pearson et al., 1978c). Patients are born with feeding difficulties, likely due to the inability to coordinate the repetitive sucking and swallowing motor pattern (Geltzer et al., 1964), a primitive mechanism that involves sensory inputs carried by the trigeminal nerve (Barlow, 2009). Poor control of jaw, tongue, cheeks and lip movements persist throughout life, manifesting as chewing difficulties (Mass et al., 1992), dysphagia (Margulies et al., 1968) and dysarthria (Halpern et al., 1967), processes that rely on sensory feedback from trigeminal nerve afferents (Barlow, 2009). Patients appear to have diminished facial sensation and may self-mutilate (Mass et al., 1994). They also develop a peculiar craniofacial appearance with abnormal mandibular growth (Mass, 2012), as seen in other congenital hereditary neuropathies (Varon et al., 2003).
Recent studies from our laboratory support the concept of FD as a disorder of afferent nerve function, with relative sparing of the efferent motor neurons (Norcliffe-Kaufmann et al., 2010, Macefield et al., 2011, Norcliffe-Kaufmann et al., 2012). Surprisingly, brainstem reflexes involving the trigeminal nerves have never been assessed systematically in patients with FD. We expected that patients with FD would have impaired brainstem reflexes, due to either abnormal trigeminal sensory fibers, failure of the interneuronal networks within the brainstem or abnormalities in the efferent motor neurons controlling the craniofacial muscles. Here we used electrophysiological techniques to examine afferent, central and efferent pathways of the trigeminal nerve by evaluating trigeminal-facial and trigeminal-trigeminal brainstem reflexes.
METHODS
Participants
From September to November 2012, we studied 28 patients with FD (age 26±12 years; 10 male, 18 female). All had typical clinical histories and confirmation of the gene mutation (Anderson et al., 2001, Slaugenhaupt et al., 2001). Seven patients were taking benzodiazepines (diazepam4.5 mg/day, clonazepam1 mg/day, mean dosages). Twenty-five age matched healthy controls were also studied (age 32±17 years; 10 male, 18 female). The procedures were approved by the institutional review board of NYU and informed consent was obtained from all participants.
Preparation for the study
Subjects were seated in a semi-supine position. Room temperature was maintained constant at 25°C. All measurements were made using a Nicolet Viking IV EMG machine (VIASYS Healthcare, Madison, Wisconsin). All tests were performed in compliance with standards recommended by the International Federation of Clinical Neurophysiology (Deuschl et al., 1999). Participants were instrumented with Ag/AgCl surface electrodes. The order of the tests was randomized. All responses were measured by the same evaluator that was blinded to whether the tracings were from patients or controls.
Electrophysiological recordings
Electrical thresholds
The individual thresholds for the detection of electrical stimulation, defined as the minimum intensity the subject could perceive, were determined on the right side only by delivering a series of square pulses of 0.1 ms duration and increasing stimulus intensity in steps of 0.3 mA over the right supraorbital nerve.
Blink reflex
Participants were instructed to remain immobile and keep their eyes directed towards their knees. Muscular responses were recorded bilaterally with electrodes placed over the orbicularis oculi muscles. The active recording electrode was at the mid-lower lid and the reference electrode was 1 cm lateral to the eye cantus (i.e., 30 mm apart). The ground electrode was placed at the middle of the forehead. The supraorbital nerves on the right and left sides were stimulated percutaneously at the supraorbital foramen using square pulses of 0.2 ms.
For standard recordings, stimulus intensity was set at five to eight times the individual perception threshold (Rossi et al., 1989, Rossi et al., 1993, Meincke et al., 1999). To develop the intensity versus response (amplitude and latency) curves, stimuli of increasing intensity (5, 10, 15, 20, 25, 30 and 35 mA) were delivered to the right supraorbital nerve at random intervals between 45 and 60 seconds. Stimulation was repeated until 3 reproducible recordings were obtained at each level. Only reproducible blink reflex responses were measured and used for analysis. If after 5 stimuli responses were not reproducibly and clearly elicited, stimulation was stopped and responses were designated as absent.
Raw blink reflex responses were superimposed. Onset latencies were identified within the following time windows: R1 (9–24 ms), R2 (27–70 ms), and R3: (70–100 ms) according to published standards (Esteban, 1999, Blumenthal et al., 2005). Computer-generated peak-to-peak amplitude cursors were placed at the highest and lowest points of the superimposed raw responses. To avoid amplitude overestimation, care was taking to exclude peaks exceeding the 95th percentile, as these outlier values were likely artifacts in the measurement of the peak-to-peak amplitude. Manual corrections of automatic cursor positioning were used when necessary. Duration was measured from the onset to the end of the responses. Scale magnification was used to help clearly identify reflex components in the patient recordings.
Direct stimulation of the facial nerve and EMG of orbicularis oculi muscle
Facial nerve compound muscle action potentials (CMAP) were evaluated on the right side, using direct stimulation of the facial nerve, as previously described (Kimura, 1982). In brief, percutaneous supramaximal electrical stimuli (0.2 ms pulses) were applied to the facial nerve, just anterior to the tragus. Onset latency and peak-to-peak amplitude were measured on the CMAP recorded from the orbicularis oculi muscle. Measurements taken from the mastoid process to the nasion point were used to estimate the length of the facial nerve segments evaluated in both groups. Surface EMG of the right orbicularis oculi muscle was evaluated during light and maximal voluntary contraction.
Jaw jerk reflex (JJR) and EMG of masseter muscle
EMG responses (bandpass 30 Hz to 3 kHz) were recorded bilaterally with electrodes on the belly of each masseter muscle and reference electrodes at the angle of the mandible. Subjects were instructed to hold their mouths open with the incisor teeth 1 cm apart. An electronic hammer was applied to the chin to trigger the electrophysiological recordings. Two series of four tapping stimuli were applied. Minimal onset latency, maximal peak-to-peak amplitude, and negativephase duration parameters were evaluated. Surface EMG of the right masseter muscle, was evaluated during light and maximal voluntary contraction.
Masseter silent period (MSP)
Subjects were asked to voluntarily maximally contract their masseter muscles. A pre-stimulus period of 20 ms was used to monitor the level of muscular contraction before two series of four single pulses (0.2 ms) were applied to the right mental nerve. Stimulus intensity was increased to six-times the sensory threshold in the perioral skin (Maillou et al., 2007). Auditory feedback was provided. Mean onset latency and total duration of the two masseter silent periods (SP 1 and 2) were measured.
Statistical analysis
Comparison of mean of the brainstem responses were performed using unpaired t-tests (for latencies and durations) and the Mann-Whitney U test (for amplitudes). Comparison of mean electrically evoked blink reflex responses between groups at each level of stimulus intensity was performed with the Kruskal-Wallis test. The severity of the abnormality was estimated from the percentage difference between responses observed in FD patients and normal controls. The percentage of abnormal responses between groups was evaluated using the Z test. Significance was set at p<0.05. All statistical analyses were performed on the STATISTICA data analysis software system, version 8.0 (StatSoft, Inc. (2007).
RESULTS
Blink reflexes
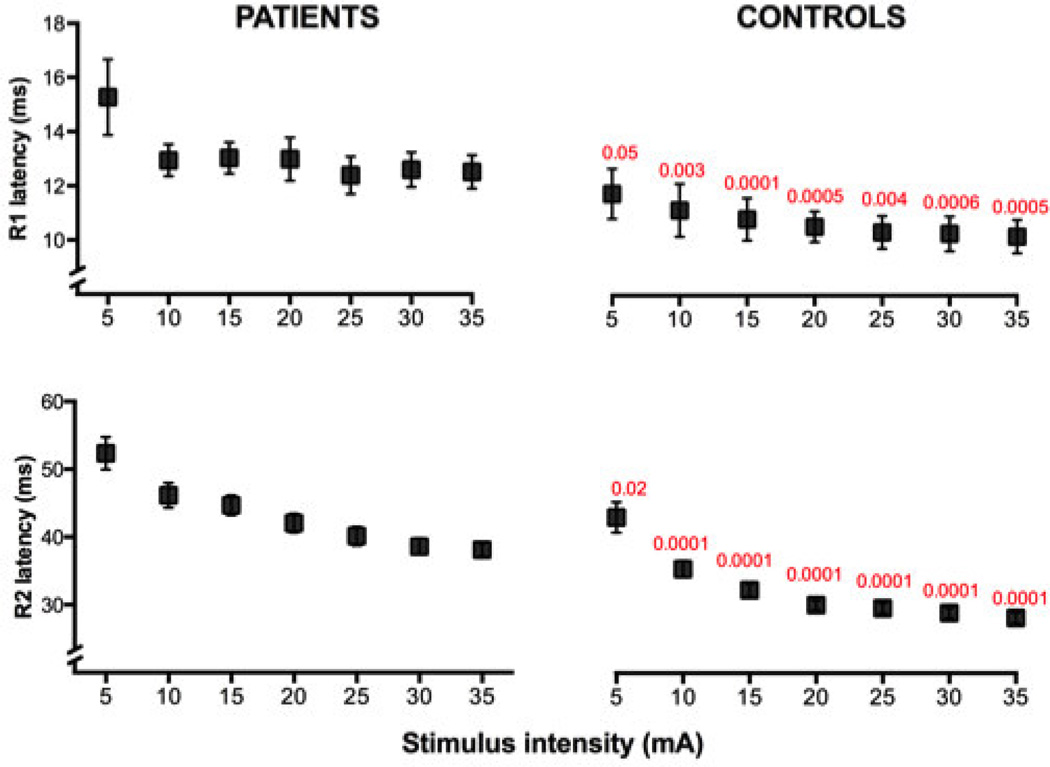
Latency
Latencies of all three components of the blink reflex were significantly prolonged in patients with FD. Experimental records from one patient and a control subject are shown in Fig. 1. The latencies of late and early components of the blink reflex showed a similar proportion of delay, as compared to normal subjects. There were no significant differences between right and left sides for R1, R2 or R2-contralateral. Mean data for the patients and controls are provided in Table 1.
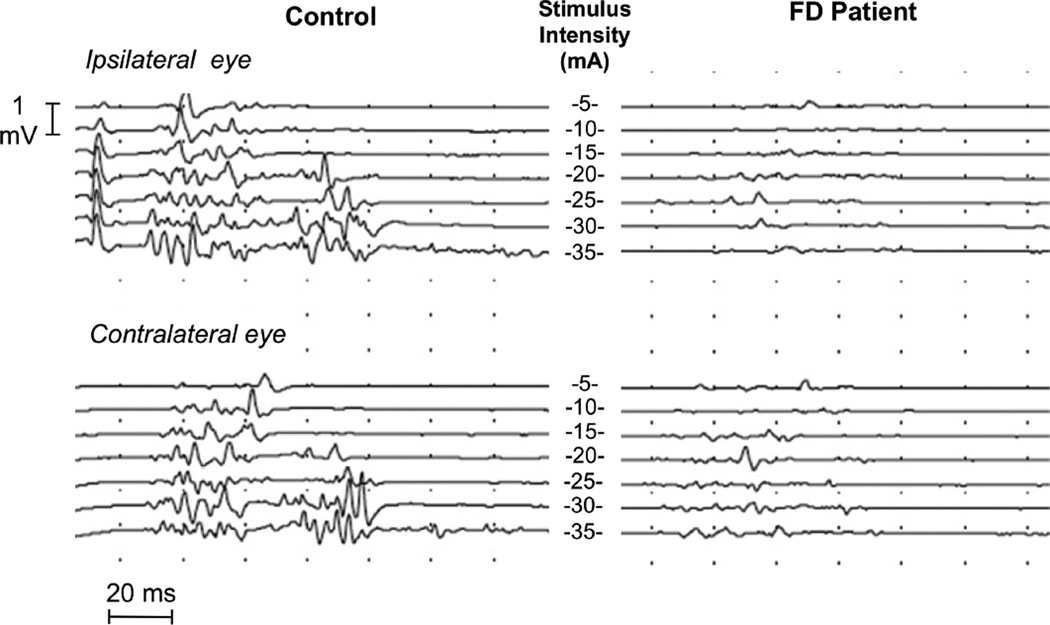
Figure 1.
Electrically elicited blink reflex recordings in a normal control and a patient with FD, during stepwise incremental stimulation of the supraorbital nerve from 5 to 35 mA (center). Stimuli were delivered to the right supraorbital nerve at random intervals between 45 and 60 seconds. Note, the reduced amplitude and increased latencies of R1, R2, and R3 responses at all intensities of stimulation in the patient.
Table 1.
Latencies of blink reflex components.
| BR parameters |
Latencies (ms) | % | p value | |
|---|---|---|---|---|
| Patients, n=28 | Controls, n=25 | |||
| R1: Right | 13.0±2.8 | 10.7±0.6 | 18 | 0.00 |
| R1: Left | 12.9±3.0 | 10.7±0.5 | 17 | 0.00 |
| R2: Right | 42.7±5.5 | 33.0±4.9 | 23 | 0.00 |
| R2: Left | 42.3±6.8 | 33.2±3.6 | 22 | 0.00 |
| R2C: Right | 45.09±7.4 | 34.6±5.9 | 23 | 0.00 |
| R2C: Left | 47.29±9.6 | 36.0±5.5 | 24 | 0.00 |
| R3: Right | 91.8±8.4 | 77.2±6.6 | 16 | 0.00 |
| R3: Left | 87.7±10.6 | 79.3±5.8 | 10 | 0.02 |
| R3C: Right | 91.1±8.4 | 77.6±7.2 | 15 | 0.00 |
| R3C: Left | 84.9±16.7 | 78.0±5.9 | 8 | 0.03 |
n: number of subjects, %: Percent difference, patients vs. controls. p: significance of unpaired t-tests. All data are mean±SD.
In the seven patient staking benzodiazepines, latencies for all the components of the blink reflex were slightly prolonged compared with patients not taking benzodiazepines, particularly for the R1 component. This, however, did not reach significance (R1: 13.2±2.8 vs. 12.7±1.4 ms; R2: 42.8±4.9 ms vs. 42.5±6.9 ms). Removing the patients taking benzodiazepines from the FD group did not change the statistically significant difference in latencies between patients and controls (table 1)
The blink reflex latencies of the patients with FD were significantly prolonged and more dispersed than the latencies of the controls for all intensities of stimulation (Fig. 2) Increasing stimulus intensity tended to decrease the latency of the R1 and R2 components (Figures 1 and 2).
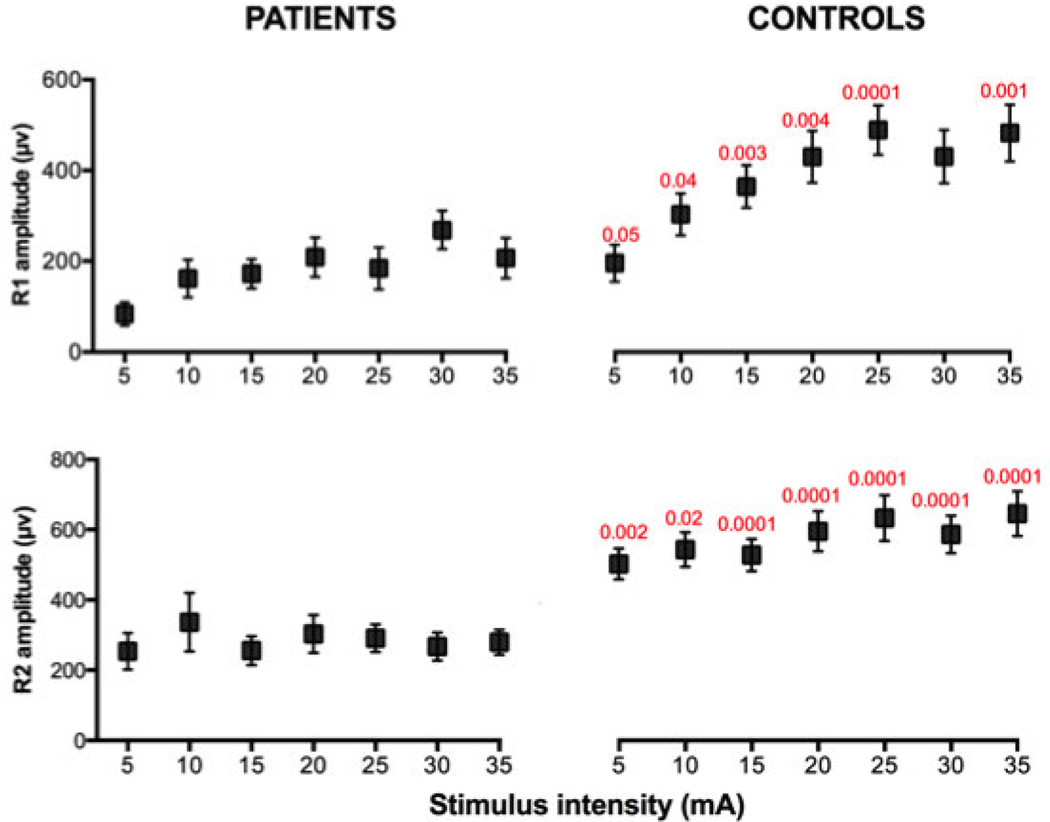
Figure 2.
Latency vs. intensity of stimulation (mean±SEM) of R1 and R2 responses in patients with FD and in control subjects. R1 and R2 latencies were significantly increased in the patients at all intensities of stimulation. Normal controls, but not patients, showed a significant negative correlation between intensity and latency of the R1 response. Significant differences are listed in red (Kruskal-Wallis test).
Amplitude
R1, R2 and R3 amplitudes were significantly depressed in patients with FD. (Table 2, Fig. 1). The amplitudes of late and early components of the blink reflex showed a similar proportion of decrement. There were no significant differences between right and left sides for the amplitudes of R1, R2 or R2-contralateral. Mean data for the amplitudes of patients and controls are provided in Table 2.
Table 2.
Amplitudes of blink reflex components.
| BR parameters | Amplitudes (mv) | % | p value | |
|---|---|---|---|---|
| Patients, n=28 | Controls, n=25 | |||
| R1: Right | 187±141 | 372±236 | −50 | 0.00 |
| R1: Left | 132±114 | 388±228 | −66 | 0.00 |
| R2: Right | 287±141 | 565±221 | −49 | 0.00 |
| R2: Left | 223±183 | 491±178 | −55 | 0.00 |
| R2C: Right | 227±169 | 467±180 | −51 | 0.00 |
| R2C: Left | 172±143 | 505±214 | −66 | 0.00 |
| R3: Right | 275±148 | 405±152 | −32 | 0.00 |
| R3: Left | 246±111 | 438±160 | −44 | 0.02 |
| R3C: Right | 315±133 | 396±155 | −20 | 0.00 |
| R3C: Left | 213±182 | 379±107 | −44 | 0.03 |
n: number of subjects, %: Percent difference, patients vs. controls (i.e., ((Patients × 100)/controls)-100). p: significance of Mann–Whitney U-test. Right/Left indicate side of stimulation. R2C: contralateral R2. R3C: contralateral R3. All data are mean±SD.
Patients taking benzodiazepines showed further decreased amplitudes compared with patients not taking these drugs (R1: 158±143 vs. 207±192uv; R2: 240±240 vs. 295±200 uv). Again, these differences did not reach significance when comparing the patients with and without benzodiazepines. The statistical difference between the patients and controls remained when the patients taking benzodiazepines were removed from the analysis (table 2)
Increasing stimulus intensities tended to increase blink reflex amplitudes (Fig. 3). Both R1 and R2 amplitudes were depressed in the patients at each level of stimulus intensity (Fig. 3 and 1).
Figure 3.
Amplitude vs. intensity of stimulation (means ± SD) of R1 and R2 responses in patients with FD and controls. Note, R1 and R2 amplitudes are severely decreased in the patients at all intensities of stimulation. Significant differences between patients and controls are listed in red (Kruskal-Wallis test).
Responses to facial nerve stimulation and EMG of orbicularis oculi
In patients with FD, there was a trend for lower amplitude and longer latency CMAPs evoked by direct stimulation of the facial nerve (table 3). Surface EMG of the orbicularis oculi muscle showed a normal pattern of recruitment and normal motor units potentials.
Table 3.
Threshold of sensory perception, latency and amplitude of facial CMAP and amplitude of masseter muscle EMG recordings.
| Patients n=28 |
Controls n=25 |
% | p value | |
|---|---|---|---|---|
| Sensory perception (mv) | 2.6±0.6 | 1.8±0.3 | 17 | 0.01 |
| Facial CMAP Latency (ms) | 3.1±0.6 | 2.8±0.4 | 10 | 0.08 |
| Facial CMAP Amplitude (uv) | 1650±886 | 2079±832 | −21 | 0.09 |
| Mastoid-Nose Distance (cm) | 15.4±1.3 | 15.1±1.2 | 2 | 0.33 |
| Amplitude SEMG Masseter (uv) | 709±402 | 1045±332 | −32 | 0.02 |
n: number of subjects, %: Percentage of change in patients vs. controls. p: significance of t-tests and Mann–Whitney U-test. SEMG: Surface EMG, CMAP: Compound muscle action potential. All data are mean±SD.
Threshold of sensory perception
Patients with FD reported a significantly higher mean threshold of perception of the electrical stimulus delivered on the face than normal subjects (table 3).
Jaw Jerk Reflex
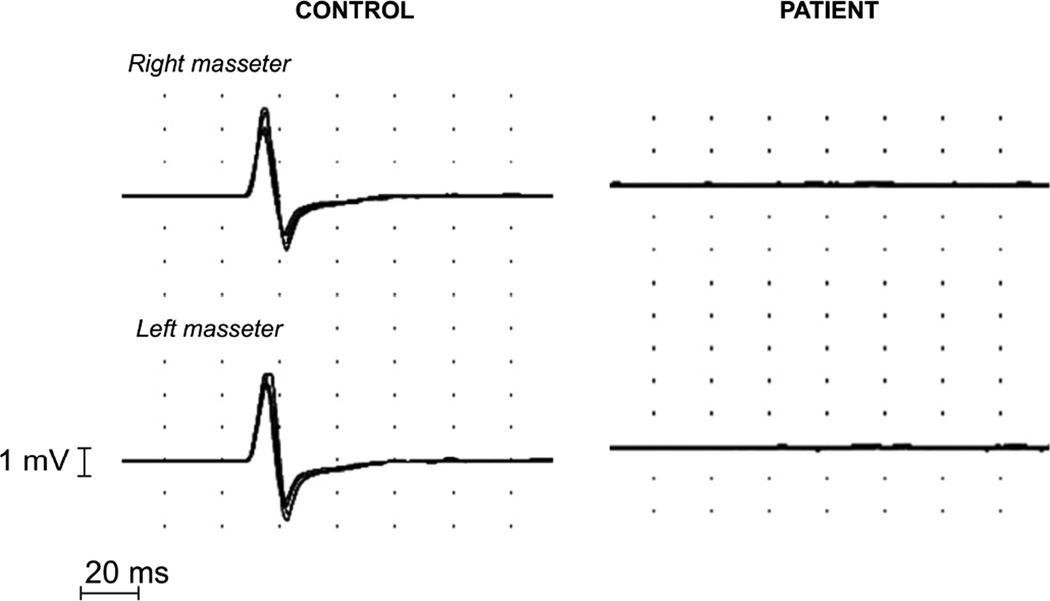
Clinical jaw jerk (i.e., overt masseter muscle contraction to chin percussion) was absent in all patients. EMG jaw jerk-like responses were evoked in all controls, but in contrast could only be provoked in two patients. In these two patients, the responses were not reproducible and had very long latencies (>14 ms), casting doubt as to whether they were truly elicited jaw jerk potentials. In the remaining patients, mechanical stimulation failed to evoke an identifiable jaw jerk response (fig. 4). The amplitude of masseter EMG recordings during maximal voluntary contraction was significantly reduced in patients with FD (Table 3). The surface EMG of the masseter muscle showed a normal pattern of recruitment and normal motor units potentials.
Figure 4.
Superimposed recordings of the jaw jerk reflex in a normal control and a patient with FD. The patient showed bilateral absence of JJR responses. Recordings have a delay of −20 ms.
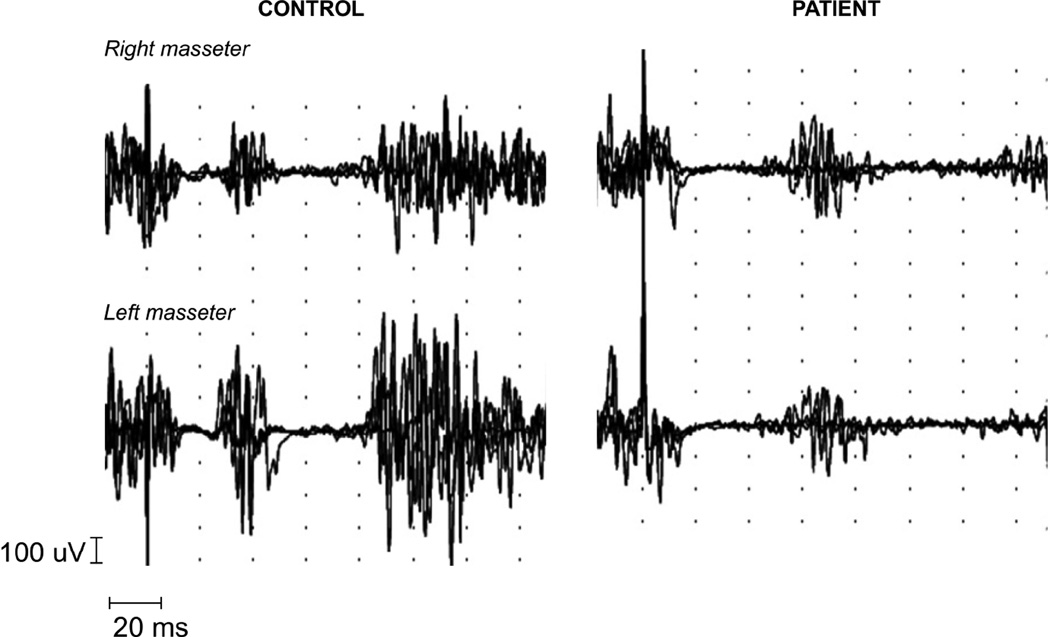
Latency and duration of masseter silent periods
SP1 and SP2 responses were recorded in all normal controls and patients with FD. In patients, latencies of the first and second silent periods (SP1 and SP2) were significantly prolonged (Table 4, Fig. 5). Delays were similar in the early and late inhibitory responses (Table 4). The duration of SP1 and SP2 was similar for both groups (Table 4, Fig. 5) and there were no right/left sided differences (Table 4).
Table 4.
Latency and duration of masseter silent period (MSP) responses
| Patients n=28 |
Controls n=25 |
% | p value | |
|---|---|---|---|---|
| SP1 Latency: Left (ms) | 16.2±4.3 | 12.9±1.7 | 26 | 0.00 |
| SP1 Latency: Right (ms) | 17.0±4.1 | 13.4±1.7 | 27 | 0.00 |
| SP1 Duration: Left (ms) | 18.8±10.8 | 17.5±3.3 | 7 | 0.55 |
| SP1 Duration: Right (ms) | 18.9±12.0 | 16.7±3.4 | 14 | 0.36 |
| SP2 Latency: Left (ms) | 63.2±17.1 | 49.1±6.2 | 28 | 0.00 |
| SP2 Latency: Right (ms) | 65.0±15.6 | 49.6±7.5 | 31 | 0.00 |
| SP2 Duration: Left (ms) | 39.0±14.9 | 33.8±12.1 | 15 | 0.20 |
| SP2 Duration: Right (ms) | 40.3±14.9 | 34.7±10.4 | 16 | 0.15 |
n: number of subjects. %: Percentage of change in patients vs. controls. p: significance of t-tests.
Figure 5.
Masseteric silent period recordings in a normal control and a patient with FD. SP1 and SP2 are clearly defined in both subjects, with almost complete inhibition of voluntary contraction of masseter muscles. Note, mildly reduced amplitude of the EMG signal in the patient with increased latency but preserved duration of SP1 and SP2. Recordings have a delay of −20 ms.
DISCUSSION
The main finding of our study was that patients with FD have abnormal brainstem reflexes. All tested trigeminal reflex latencies were delayed and amplitudes reduced. Motor responses provoked by direct stimulation and voluntary contraction were, by comparison, preserved, suggesting relative sparing of the motor efferent neurons.
Whether the impairment is located at the peripheral neurons or the central circuits of these reflexes is an intriguing question. Early components (R1, JJR and SP1) are believed to be mainly dependent on the peripheral neurons (i.e. trigeminal and facial nerve fibers (Cruccu et al., 2005) while late components (R2, R2-contralateral, R3, R3-contralateral, and SP2) involve both peripheral and central neurons (i.e., within the brainstem). Since both early and late components were affected in a similar proportion, it is likely that both peripheral and central neurons are affected similarly in patients with FD. In support of this, brainstem auditory evoked potentials in children with FD were reported to have increased latencies of waves III and V and durations of I–III and III–V intervals, which can only be explained by concomitant peripheral and central lesions throughout the auditory neuronal pathway (Lahat et al., 1992). Furthermore, post-mortem neuropathology studies in patients with FD showed involvement of cranial nerves (including the trigeminal peripheral fibers) and brainstem reticular formation (Cohen et al., 1955, Brown et al., 1964).
The particular kind of damage (axonal vs. demyelinating) and the type of fibers that are affected are also interesting questions. Severe demyelination of brainstem pathways is unlikely to account for the observed abnormalities, since the degree of slowing in patients with FD is considerably milder than that seen in demyelinating neuropathies (Kimura, 1982, Cruccu et al., 1998, Ishpekova et al., 2005, Kokubun et al., 2007).While scattered irregular sheaths in the mesencephalic tract of the trigeminal nerve were reported in one case, (Brown et al., 1964) significant demyelination was not reported in other post mortem samples (Brown et al., 1964, Yatsu et al., 1964, Aguayo et al., 1971, Pearson et al., 1975). However, the finding that both early and late components showed a similar proportion of delay (around 20%), suggest that patients with FD have the same severity of slowing, probably due to demyelination or hypo-myelination, of both peripheral and central neurons.
Since the different reflexes tested are mediated by a variety of sensory fiber types, (Shahani, 1970, Cruccu et al., 1987, Ellrich et al., 1997, Esteban, 1999) and all were affected, it is likely that multiple afferent fiber types, both small and large diameter, are abnormal in FD. In contrast, there was relative sparing of the efferent motor neurons, a finding supported by the study of Dyck et al., who described normal number and morphology of the a motor neuron cell bodies in an autopsy sample of a patient with FD (Dyck et al., 1978).
The reduction of amplitudes of brainstem responses could be caused by several factors: decreased population of axons, de-synchronization, phase cancellation and muscle atrophy (Cruccu et al., 2000). The similar proportion of amplitude decrement for the early and late components, approximately 50%, indicates that both peripheral trigeminal afferents and central pathways within the brainstem are affected in a similar proportion, as occurred with the latencies of these responses. This is in line with pathology samples in FD showing involvement of brainstem reticular formation (Cohen et al., 1955, Brown et al., 1964) and marked reduction in the number of sensory neurons in the trigeminal ganglia (Brown et al., 1964, Aguayo et al., 1971, Pearson et al., 1971, Pearson et al., 1975, Pearson et al., 1978b), as well as the decreased sensory nerve action potentials in the limbs (Hilz et al., 2000). Impairment of the efferent neurons, as suggested by the slightly decreased orbicularis oculi CMAP and masseter EMG amplitudes, is unlikely to fully explain the marked decrease in reflex amplitudes.
The lack of stepwise change in blink reflex latencies and amplitudes with increasing stimulus intensity was a surprising finding. The observed saturation of the responses at lower intensities could be explained by the inability to progressively recruit sensory trigeminal fibers (Sanes et al., 1982). This particular profile can be due to the reduction in the absolute number of sensory trigeminal fibers, as shown in post mortem studies (Brown et al., 1964, Pearson et al., 1971, Pearson et al., 1975) or decreased excitability of trigeminal afferents, as suggested by the increased threshold of sensory perception recorded in the patients.
A decreased number of trigeminal afferent fibers would generate a low response for all the reflexes mediated by the trigeminal nerve, including both the early and late components of the response as both depend on the same reduced input, and would explain the relative preservation of direct motor responses, which are not dependent on trigeminal inputs. Therefore, this could be the main pathological mechanism leading to a reduction in the amplitude of brainstem reflexes in patients with FD.
The absence of the jaw jerk reflex in almost all patients is consistent with the absence of other myotatic reflexes (Axelrod et al., 1984). EMG recordings of the masseter muscle did show a moderate decrement in amplitude, which could account for a minor decrement of jaw jerk reflex amplitude, but not for the complete lack of JJR response observed in the patients. This, again, suggests that the impairment lies mainly within the afferent limb or the central portion of this reflex arc, which in this case are Ia proprioceptive fibers from the muscle spindles of the masseter and temporalis muscles (Finan et al., 2005). Absence of functional muscle spindle afferents in the lower limbs has been shown recently in these patients (Macefield et al., 2011) and likely accounts for the characteristic gait ataxia. Impairment of the Ia afferent fibers from the masseter muscles could contribute to the poor motor coordination during chewing (Tartaglia et al., 2008), speech (Franz et al., 1992, Smith, 1992),and feeding (Finan et al., 2005), and subsequent dental trauma (Mass et al., 1992), which are all typical features of the disorder.
Silent period 1 and 2 latencies were also increased in the patients with FD, with a similar proportion of slowing found in both components. Previous studies in patients with demyelinating and diabetic neuropathies have demonstrated that these silent periods are the brainstem responses most frequently affected (Cruccu et al., 1998). Since both SP 1 and 2 afferents are the intermediately myelinated Ab group (Cruccu et al., 1987), this suggests possible impairment of small afferent cutaneous sensory fibers from the lower face. These fibers are essential for the feedback regarding the jaw muscle contraction, and their absence is also associated with dental trauma (Mass et al., 1992).
Our study has limitations. The intensity of the mechanical stimulus used for eliciting the jaw jerk reflex was not standardized. Nevertheless, we were able to demonstrate differences between patients and controls. Due to technical limitations, we were unable to perform a selective evaluation of the conduction through the peripheral fibers of the trigeminal nerve, which would have permitted a more definitive assessment of the functional state of these axons. Peak-to-peak amplitude measurements were useful to demonstrate amplitude differences between groups; but rectifying and integrating the responses would have allowed better quantification of the amplitudes. Sub-group analysis indicates that the use of benzodiazepines, may have somewhat influenced our results. The presence of abnormal reflex responses in the patients not taking benzodiazepines suggest that the use of these medications does not fully account for the reduction in the amplitudes.
The role of trigeminal nerve involvement in the clinical manifestations of familial dysautonomia warrants further investigation. In contrast to acquired disorders of the trigeminal nerve, comparatively little is known about the clinical features in congenital disorders affecting trigeminal nerve pathways.
In conclusion, our findings show that brainstem reflexes are delayed and diminished in patients with FD. Neurophysiology and pathological evidence supports involvement of the afferent trigeminal nerve fibers. These findings fit with the clinical phenotype of FD and add further support to the concept that FD is a developmental disorder that affects primarily sensory afferents with functional preservation of motor efferent fibers.
Highlights.
Patients with familial dysautonomia (FD) have severe and widespread disorders of brainstem reflexes.
Sensory afferent fibers are more affected than motor efferent fibers.
These findings could explain some clinical features of patients with FD, such as slurred speech, dental trauma, chewing difficulties, dysphagia and dysarthria.
Acknowledgments
We thank all staff and fellows of the Dysautonomia Center for their help with this study. This research was supported by grants from the Dysautonomia Foundation, Inc. and National Institutes for Health (U54NS065736 to LJNK and HK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: There are no conflicts of interest.
References
- Aguayo AJ, Nair CP, Bray GM. Peripheral nerve abnormalities in the Riley-Day syndrome. Findings in a sural nerve biopsy. Arch Neurol. 1971;24:106–116. doi: 10.1001/archneur.1971.00480320034003. [DOI] [PubMed] [Google Scholar]
- Anderson SL, Coli R, Daly IW, Kichula EA, Rork MJ, Volpi SA, et al. Familial dysautonomia is caused by mutations of the IKAP gene. Am J Hum Genet. 2001;68:753–758. doi: 10.1086/318808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod FB, Pearson J. Congenital sensory neuropathies. Diagnostic distinction from familial dysautonomia. Am J Dis Child. 1984;138:947–954. [PubMed] [Google Scholar]
- Barlow SM. Central pattern generation involved in oral and respiratory control for feeding in the term infant. Curr Opin Otolaryngol Head Neck Surg. 2009;17:187–193. doi: 10.1097/MOO.0b013e32832b312a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Brown WJ, Beauchemin JA, Linde LM. A Neuropathological Study of Familial Dysautonomia (Riley-Day Syndrome) in Siblings. J Neurol Neurosurg Psychiatry. 1964;27:131–139. doi: 10.1136/jnnp.27.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close P, Hawkes N, Cornez I, Creppe C, Lambert CA, Rogister B, et al. Transcription impairment and cell migration defects in elongator-depleted cells: implication for familial dysautonomia. Mol Cell. 2006;22:521–531. doi: 10.1016/j.molcel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Cohen P, Solomon NH. Familial dysautonomia; case report with autopsy. J Pediatr. 1955;46:663–670. doi: 10.1016/s0022-3476(55)80171-0. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Agostino R, Inghilleri M, Innocenti P, Romaniello A, Manfredi M. Mandibular nerve involvement in diabetic polyneuropathy and chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 1998;21:1673–1679. doi: 10.1002/(sici)1097-4598(199812)21:12<1673::aid-mus8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Deuschl G. The clinical use of brainstem reflexes and hand-muscle reflexes. Neurophysiol Clin. 2000;111:371–387. doi: 10.1016/s1388-2457(99)00291-6. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Iannetti GD, Marx JJ, Thoemke F, Truini A, Fitzek S, et al. Brainstem reflex circuits revisited. Brain. 2005;128:386–394. doi: 10.1093/brain/awh366. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Inghilleri M, Fraioli B, Guidetti B, Manfredi M. Neurophysiologic assessment of trigeminal function after surgery for trigeminal neuralgia. Neurology. 1987;37:631–638. doi: 10.1212/wnl.37.4.631. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Eisen A. Long-latency reflexes following electrical nerve stimulation. The Inter national Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:263–268. [PubMed] [Google Scholar]
- Dyck PJ, Kawamura Y, Low PA, Shimono M, Solovy JS. The number and sizes of reconstructed peripheral autonomic, sensory and motor neurons in a case or dysautonomia. J Neuropathol Exp Neurol. 1978;37:741–755. doi: 10.1097/00005072-197811000-00003. [DOI] [PubMed] [Google Scholar]
- Ellrich J, Bromm B, Hopf HC. Pain-evoked blink reflex. Muscle Nerve. 1997;20:265–270. doi: 10.1002/(SICI)1097-4598(199703)20:3<265::AID-MUS1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Esteban A. A neurophysiological approach to brainstem reflexes. Blink reflex. Neurophysiol Clin. 1999;29:7–38. doi: 10.1016/S0987-7053(99)80039-2. [DOI] [PubMed] [Google Scholar]
- Filler J, Smith AA, Stone S, Dancis J. Respiratory Control in Familial Dysautonomia. J Pediatr. 1965;66:509–516. doi: 10.1016/s0022-3476(65)80115-9. [DOI] [PubMed] [Google Scholar]
- Finan DS, Smith A. Jaw stretch reflexes in children. Exp Brain Res. 2005;164:58–66. doi: 10.1007/s00221-005-2217-x. [DOI] [PubMed] [Google Scholar]
- Franz EA, Zelaznik HN, Smith A. Evidence of Common Timing Processes in the Control of Manual, Orofacial, and Speech Movements. J Mot Behav. 1992;24:281–287. doi: 10.1080/00222895.1992.9941623. [DOI] [PubMed] [Google Scholar]
- Geltzer AI, Gluck L, Talner NS, Polesky HF. Familial Dysautonomia; Studies in a Newborn Infant. N Engl J Med. 1964;271:436–440. doi: 10.1056/NEJM196408272710903. [DOI] [PubMed] [Google Scholar]
- Halpern H, Hochberg I, Rees N. Speech and hearing characteristics in familial dysautonomia. J Speech Hear Res. 1967;10:361–366. doi: 10.1044/jshr.1002.361. [DOI] [PubMed] [Google Scholar]
- Hilz MJ, Axelrod FB. Quantitative sensory testing of thermal and vibratory perception in familial dysautonomia. Clin Auton Res. 2000;10:177–183. doi: 10.1007/BF02291353. [DOI] [PubMed] [Google Scholar]
- Ishpekova BA, Christova LG, Alexandrov AS, Thomas PK. The electrophysiological profile of hereditary motor and sensory neuropathy-Lom. J Neurol Neurosurg Psychiatry. 2005;76:875–878. doi: 10.1136/jnnp.2004.040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J. Conduction abnormalities of the facial and trigeminal nerves in polyneuropathy. Muscle Nerve. 1982;5:S139–S144. [PubMed] [Google Scholar]
- Kokubun N, Hirata K. Neurophysiological evaluation of trigeminal and facial nerves in patients with chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2007;35:203–207. doi: 10.1002/mus.20679. [DOI] [PubMed] [Google Scholar]
- Lahat E, Aladjem M, Mor A, Azizi E, Arlazarof A. Brainstem auditory evoked potentials in familial dysautonomia. Dev Med Child Neurol. 1992;34:690–693. doi: 10.1111/j.1469-8749.1992.tb11504.x. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Norcliffe-Kaufmann L, Gutierrez J, Axelrod FB, Kaufmann H. Can loss of muscle spindle afferents explain the ataxic gait in Riley-Day syndrome? Brain. 2011;134:3198–3208. doi: 10.1093/brain/awr168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahloudji M, Brunt PW, McKusick VA. Clinical neurological aspects of familial dysautonomia. J Neurol Sci. 1970;11:383–395. doi: 10.1016/0022-510x(70)90083-3. [DOI] [PubMed] [Google Scholar]
- Maillou P, Cadden SW. Characteristics of a jaw reflex in humans with temporomandibular disorders: a preliminary report. J Oral Rehabil. 2007;34:329–335. doi: 10.1111/j.1365-2842.2007.01723.x. [DOI] [PubMed] [Google Scholar]
- Margulies SI, Brunt PW, Donner MW, Silbiger ML. Familial dysautonomia. A cineradiographic study of the swallowing mechanism. Radiology. 1968;90:107–112. doi: 10.1148/90.1.107. [DOI] [PubMed] [Google Scholar]
- Mass E. A review of the oro-dento-facial characteristics of hereditary sensory and autonomic neuropathy type III (familial dysautonomia) Spec Care Dentist. 2012;32:15–20. doi: 10.1111/j.1754-4505.2011.00225.x. [DOI] [PubMed] [Google Scholar]
- Mass E, Gadoth N. Oro-dental self-mutilation in familial dysautonomia. J Oral Pathol. 1994;23:273–276. doi: 10.1111/j.1600-0714.1994.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Mass E, Sarnat H, Ram D, Gadoth N. Dental and oral findings in patients with familial dysautonomia. Oral Surg Oral Med Oral Pathol. 1992;74:305–311. doi: 10.1016/0030-4220(92)90064-w. [DOI] [PubMed] [Google Scholar]
- Meincke U, Topper R, Hoff P. The electrically elicited startle blink reflex in patients with schizophrenia: A threshold study of different reflex components. Neuropsychobiology. 1999;39:76–80. doi: 10.1159/000026564. [DOI] [PubMed] [Google Scholar]
- Mezey E, Parmalee A, Szalayova I, Gill SP, Cuajungco MP, Leyne M, et al. Of splice and men: what does the distribution of IKAP mRNA in the rat tell us about the pathogenesis of familial dysautonomia? Brain Res. 2003;983:209–214. doi: 10.1016/s0006-8993(03)03090-7. [DOI] [PubMed] [Google Scholar]
- Norcliffe-Kaufmann L, Axelrod F, Kaufmann H. Afferent baroreflex failure in familial dysautonomia. Neurology. 2010;75:1904–1911. doi: 10.1212/WNL.0b013e3181feb283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcliffe-Kaufmann LJ, Axelrod FB, Kaufmann H. Cyclic Vomiting Associated With Excessive Dopamine in Riley-day Syndrome. J Clin Gastroenterol. 2012;47:136–138. doi: 10.1097/MCG.0b013e3182582cbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J, Budzilovich G, Finegold MJ. Sensory, motor, and autonomic dysfunction: the nervous system in familial dysautonomia. Neurology. 1971;21:486–493. doi: 10.1212/wnl.21.5.486. [DOI] [PubMed] [Google Scholar]
- Pearson J, Dancis J, Axelrod F, Grover N. The sural nerve in familial dysautonomia. J Neuropathol Exp Neurol. 1975;34:413–424. doi: 10.1097/00005072-197509000-00004. [DOI] [PubMed] [Google Scholar]
- Pearson J, Pytel B. Quantitative studies of ciliary and sphenopalatine ganglia in familial dysautonomia. J Neurol Sci. 1978a;39:123–130. doi: 10.1016/0022-510x(78)90193-4. [DOI] [PubMed] [Google Scholar]
- Pearson J, Pytel BA. Quantitative studies of sympathetic ganglia and spinal cord intermediolateral gray columns in familial dysautonomia. J Neurol Sci. 1978b;39:47–59. doi: 10.1016/0022-510x(78)90187-9. [DOI] [PubMed] [Google Scholar]
- Pearson J, Pytel BA, Grover-Johnson N, Axelrod F, Dancis J. Quantitative studies of dorsal root ganglia and neuropathologic observations on spinal cords in familial dysautonomia. J Neurol Sci. 1978c;35:77–92. doi: 10.1016/0022-510x(78)90103-x. [DOI] [PubMed] [Google Scholar]
- Riley CM, Day RA, Greeley DM, Landford WS. Central autonomic dysfunction with defective lacrimation: I. Report of five cases. Pediatrics. 1949;3:468–478. [PubMed] [Google Scholar]
- Rossi B, Risaliti R, Rossi A. The R3 component of the blink reflex in man: a reflex response induced by activation of high threshold cutaneous afferents. Electroencephalogr Clin Neurophysiol. 1989;73:334–340. doi: 10.1016/0013-4694(89)90111-9. [DOI] [PubMed] [Google Scholar]
- Rossi B, Vignocchi MG. Methodological considerations on the use of the blink reflex R3 component in the assessment of pain in man. Ital J Neurol Sci. 1993;14:217–224. doi: 10.1007/BF02335662. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Foss JA, Ison JR. Conditions that affect the thresholds of the components of the eyeblink reflex in humans. J Neurol Neurosurg Psychiatry. 1982;45:543–549. doi: 10.1136/jnnp.45.6.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahani B. The human blink reflex. J Neurol Neurosurg Psychiatry. 1970;33:792–800. doi: 10.1136/jnnp.33.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaugenhaupt SA, Blumenfeld A, Gill SP, Leyne M, Mull J, Cuajungco MP, et al. Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. Am J Hum Genet. 2001;68:598–605. doi: 10.1086/318810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. The control of orofacial movements in speech. Crit Rev Oral Biol Med. 1992;3:233–267. doi: 10.1177/10454411920030030401. [DOI] [PubMed] [Google Scholar]
- Tartaglia GM, Testori T, Pallavera A, Marelli B, Sforza C. Electromyographic analysis of masticatory and neck muscles in subjects with natural dentition, teeth-supported and implant-supported prostheses. Clin Oral Implants Res. 2008;19:1081–1088. doi: 10.1111/j.1600-0501.2008.01574.x. [DOI] [PubMed] [Google Scholar]
- Varon R, Gooding R, Steglich C, Marns L, Tang H, Angelicheva D, et al. Partial deficiency of the C-terminal-domain phosphatase of RNA polymerase II is associated with congenital cataracts facial dysmorphism neuropathy syndrome. Nat Genet. 2003;35:185–189. doi: 10.1038/ng1243. [DOI] [PubMed] [Google Scholar]
- Yatsu F, Zussman W. Familial Dysautonomia (Riley-Day Syndrome). Case Report with Postmortem Findings of a Patient at Age 31. Arch Neurol. 1964;10:459–463. doi: 10.1001/archneur.1964.00460170029004. [DOI] [PubMed] [Google Scholar]