Abstract
Lymphadenectomy is a prerequisite for most malignancies to define the precise staging of cancer, as well as resect the possible metastases completely. While it improves prognosis, lymphadenectomy often causes postoperative edema or bleeding because of unclear surgical margins. In this study, we synthesized near-infrared (NIR) fluorescent nanoprobes with conjugating various mannose moieties on the surface to target macrophages in the lymph node. Armed with these NIR nanoprobes, we demonstrated the feasibility of intraoperative pan lymph nodes (PLN) mapping and real-time optical imaging under the NIR fluorescence imaging system. We found that even single mannose-conjugated ZW800-1 showed specific uptake in lymph nodes within 4 h, and multiple mannose-employed polyrotaxanes highlighted PLN efficiently with low background signals in major organs. This technology can help surgeons perform lymphadenectomy with ease and safety by identifying all regional lymph nodes proficiently after a single intravenous injection of NIR nanoprobes.
Keywords: Near-infrared fluorescence, targeted fluorophore, optical imaging, lymph node targeting, signal-to-background ratio
Graphical Abstract
INTRODUCTION
Lymph node metastasis is an important prognostic factor in many solid malignancies. Diagnostic, prophylactic, or curative lymphadenectomy is usually performed as a standard procedure during the cancer surgery [1–3]. Since the distribution and extent of regional lymph nodes varies in each malignancy, lymphadenectomy often requires that surgeons have advanced techniques; as a result, the quality of lymphadenectomy in each surgery is mostly dependent on a surgeon’s skill. Furthermore, this procedure sometimes causes serious postoperative complications such as postoperative edema and bleeding, lymphatic fistula, and tissue injury [4].
Previously, we reported various near-infrared (NIR) fluorophores for mapping pan lymph nodes (PLN) and sentinel lymph nodes (SLN), and performed lymphadenectomy in pigs and humans with real-time intraoperative navigation [5–7]. A small molecule NIR fluorophore ZW800-3C was recently used for PLN mapping with improved accumulation and retention in lymph nodes compared to quantum dot-based NIR fluorophores [4,8]. Unfortunately, ZW800-3C largely accumulates in the liver and often results in confusion of lymph node imaging in the abdomen [9]. ZW800-1, however, is a zwitterionic heptamethine indocyanine fluorophore with showing minimal binding on serum proteins or cell surface [10,11]. Preclinical evaluations of toxicity and scale-up chemistry were successfully finished, and clinical trials are ongoing, so ZW800-1 could be clinically available in the near future [10–12]. In addition, it shows excellent optical properties, including a high extinction coefficient, high quantum yield, and excellent optical stability [10]. Therefore, ZW800-1 has been used for synthesizing novel receptor-targeted NIR fluorophores by conjugating highly specific targeted ligands including small molecules, peptides, proteins, and antibodies [10–16].
In order to target lymph nodes, many trials have been made to use mannose receptors because they express on the surface of macrophages and dendritic cells in the lymph node [17–19]. Lymphoseek is the most broadly used radiotracer for SLN mapping, which includes dextran and multiple units of mannose and diethylene triamine pentaacetic acid [20–22]. Nevertheless, this theory has never been translated into mapping all regional lymph nodes via single intravenous administration. In this study, we designed various mannose-conjugated nanoprobes and exploited the feasibility of PLN mapping under real-time NIR fluorescence imaging. Various numbers of mannose moieties were employed on small molecules and polymeric nanoparticles to verify their targeting efficiency to lymph nodes in terms of size, lipophilicity, charge, shape, and flexibility.
RESULTS
Preparation of mannose-conjugated nanoprobes
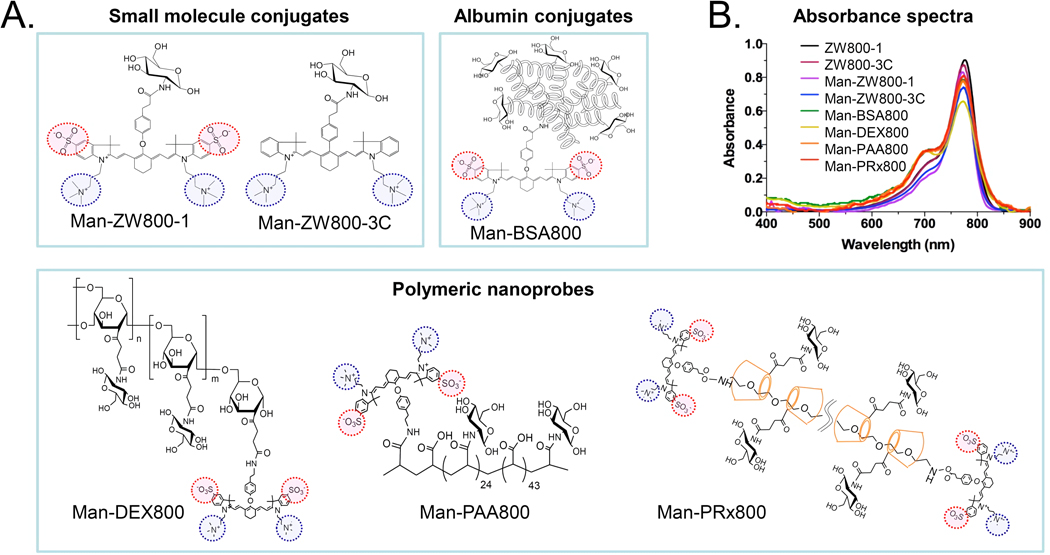
We prepared the mono- and multivalent mannose-conjugated NIR fluorophores by conjugating mannose moiety to ZW800-1, ZW800-3C, bovine serum albumin (BSA), and polymers such as dextran (DEX), polyacrylic acid (PAA) and polyrotaxane (PRx) (Fig. 1). The chemical profiles of each mannose conjugates are shown in Table 1. For single mannose nanoprobes, an amino-mannose was conjugated to the NHS ester activated NIR fluorophores such as ZW800-1 and ZW800-3C (Supplementary Methods). The conjugation yield was >92%, and the final products showed >98% purity after prep-HPLC purification (Figs. S1–S3) [11,23]. To improve PLN targeting with higher receptor binding on macrophages, we introduced multiple mannose moieties on various biomolecules: 1) BSA was used as a model globular-structured protein, 2) DEX is a clinically available semi-globular structured biomolecule and the main composition of Lymphoseek, 3) PAA is a linear polymer with high negative charges on the surface, and 4) PRx is a supramolecular structure, in which many α-cyclodextrins (α-CDs) are threaded onto the polyethylene glycol (PEG) chain. Since the overall hydrodynamic diameter (HD) of Lymphoseek is about 6 nm (mainly composed of 10 kDa dextran and about 25 mannose units), we prepared our nanoprobes based on this commercial product in terms of the number of mannose units, MW and HD. The final HD of multivalent mannose-conjugated nanoprobes are found to be 5–7 nm (Table 1, Fig. S4). All biomolecules contain ZW800-1 for tracking their behavior in the body.
Figure 1. Design of mannose-conjugated nanoprobes.
a) Chemical structures of NIR fluorophores and nanoprobes conjugated with mannose moieties. b) Absorption spectra of mannose-conjugated nanoprobes. 4 µM of each nanoprobe was measured in 100% serum, pH 7.4.
Table 1.
Composition of NIR nanoprobes in terms of molecular weight (MW), hydrodynamic diameter (HD), LogD at pH 7.4, net charges, and the density of mannose moieties and NIR fluorophores.
| Nanoprobe | MW | HD | LogD, pH 7.4 |
Net charges |
# of mannose |
# of fluorophores |
|---|---|---|---|---|---|---|
| ZW800-1 | 944 | 1.0 | −3.35 | 0 | 0 | 1.0 |
| ZW800-3C | 770 | 1.0 | −1.63 | +2 | 0 | 1.0 |
| Man-ZW800-1 | 1105 | 1.2 | −2.99 | +1 | 1 | 1.0 |
| Man-ZW800-3C | 931 | 1.2 | −5.40 | +3 | 1 | 1.0 |
| Man-BSA800 | 73k | 7.0 | ND | −18 | 10 | 2.6 |
| Man-DEX800 | 16k | 6.0 | ND | +2 | 25 | 2.0 |
| Man-PAA800 | 11k | 5.0 | ND | −42 | 25 | 2.0 |
| Man-PRx800 | 36k | 7.0 | ND | +2 | 25 | 2.0 |
ND = not determined. In silico calculations of LogD and net charges were calculated using Marvin and JChem calculator plugins (ChemAxon, Budapest, Hungary).
PLN mapping of monovalent mannose-conjugated ZW800-1 and ZW800-3C
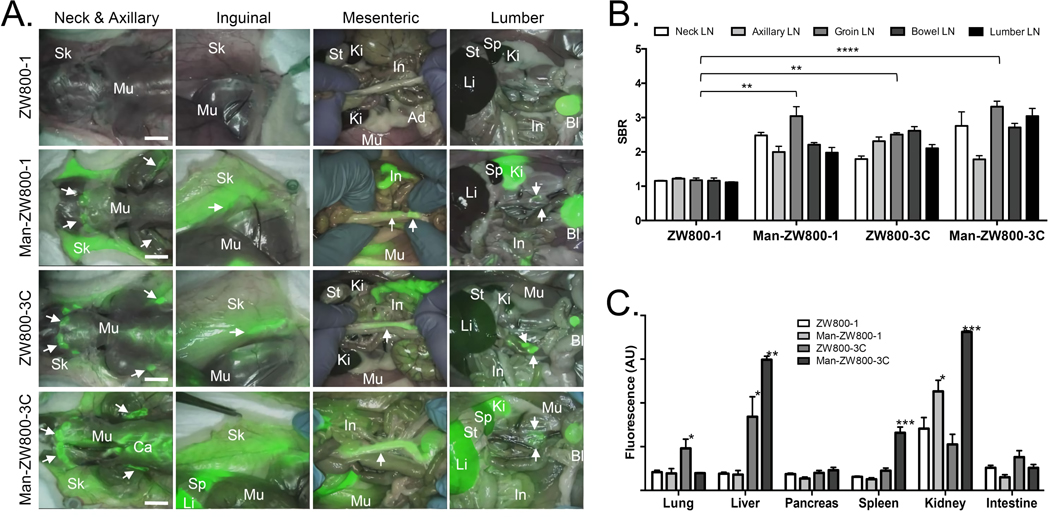
As shown in Figure 2a, hydrophilic and zwitterionic ZW800-1 (logD = −3.35, net charges = 0) shows virtually no uptake in any lymph nodes in the rat model, while lipophilic and positive charged ZW800-3C (logD = −1.63, net charges = +2) highlights all regional lymph nodes after a single intravenous injection. By introducing a single mannose moiety on nonsticky ZW800-1, Man-ZW800-1 (logD = −2.99, net charges = +1) targeted pan lymph nodes within 4 h post-intravenous injection (**P < 0.005; Fig. 2b). Mannose conjugation on lipophilic ZW800-3C resulted in decreased logD at pH 7.4 (−5.40), but increased background uptake in major organs including liver, spleen, and cartilages (Fig. 2c). In addition, the lymph node uptake was not improved significantly (P > 0.05). There is no significant differences in signal-to-background ratio (SBR) of lymph nodes against muscle among Man-ZW800-1, ZW800-3C, and Man-ZW800-3C (Fig. 2b), but the background signals in major organs of zwitterionic Man-ZW800-1 injected rats were significantly lower than those from both ZW800-3C and Man-ZW800-3C injected animals (Fig. 2c). The nonspecific uptake of Man-ZW800-3C in liver (**P < 0.005), spleen (***P < 0.0005) and kidneys (***P < 0.0005) were significantly higher compared with other tissues/organs due to the positive surface charges.
Figure 2. Intraoperative PLN mapping of ZW800-1 and ZW800-3C with or without a single mannose moiety.
a) Each NIR probe was injected intravenously into 250 g SD rats (100 nmol, 0.4 mg/kg) 4 h prior to imaging. Abbreviations used are: Ad, adipose tissue; Bl, bladder; Ca, cartilage; In, intestine; Ki, kidney; Li, liver; Mu, muscle; Sk, skin; Sp, spleen; St, stomach. Arrows indicate lymph nodes (LNs). Scale bars = 1cm. b) SBR (mean ± SD) of neck, axillary, groin, bowel, and lumbar LNs against muscle was compared. c) Fluorescence intensity (mean ± SD) of major organs resected after intraoperative imaging at 4 h post-intravenous injection, representing nonspecific uptake. *P < 0.05, **P < 0.005, ***P < 0.0005, ****P < 0.00005.
PLN mapping of multivalent mannose-conjugated nanoprobes
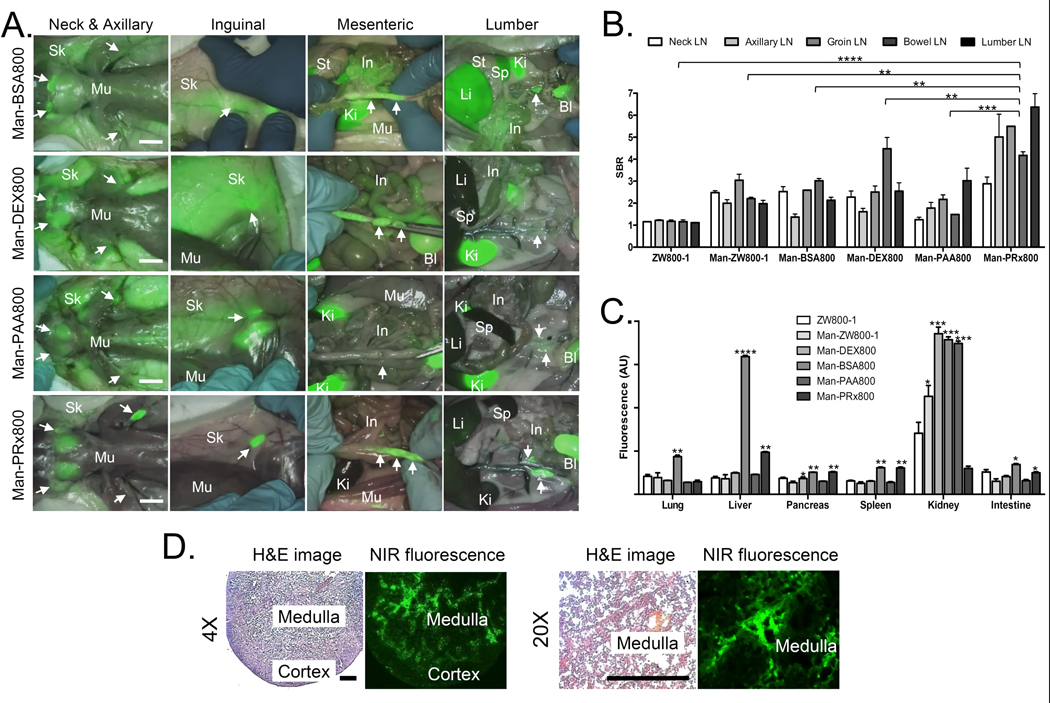
To confirm the effectiveness of mannose conjugation on different biomolecules, 25 mannose moieties were conjugated on PAA, DEX, and PRx with varying net surface charges, shape, and flexibility (Table 1, Figs. S4–S6). BSA was used as a protein control with employing only 10 mannose units due to the limited surface area, while ZW800-1 was used as a negative control. Man-BSA800, Man-DEX800, and Man-PRx800 showed high regional lymph node signals (Fig. 3a): Globular structured Man-BSA800 was found in neck and lumbar lymph nodes, and semi-globular structured Man-DEX800 was specific to mesenteric lymph nodes. Linear and rigid Man-PAA800, however, exhibited weak uptake for most lymph nodes. Flexible Man-PRx800 was found from all regional lymph nodes including axillary, inguinal, mesenteric and lumber areas compared with all other groups with significant difference (**P < 0.005; Fig. 3b).
Figure 3. Intraoperative PLN mapping of multiple mannose-conjugated nanoprobes in rats.
a) Each NIR nanoprobe was injected intravenously into 250 g SD rats (100 nmol) 4 h prior to imaging. Abbreviations used are: Ad, adipose tissue; Bl, bladder; In, intestine; Ki, kidney; Li, liver; Mu, muscle; Sk, skin; Sp, spleen; St, stomach. Scale bars = 1cm. b) SBR (mean ± SD) of neck, axillary, inguinal, mesenteric, and lumbar lymph nodes (LNs) against muscle was compared using various multiple mannose-conjugated nanoprobes. *P < 0.05, **P < 0.005, ***P < 0.0005, ****P < 0.00005. c) Fluorescence intensity (mean ± SD) of major organs resected after intraoperative imaging at 4 h post-intravenous injection, representing nonspecific uptake. d) Histopathological analysis of neck lymph nodes resected from a rat injected with Man-PRx800. Scale bars = 300 µm.
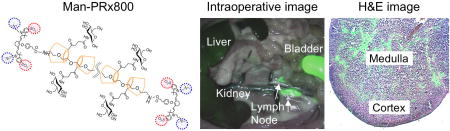
Background signals from the surrounding tissues/organs of lymph nodes are important to determine the SBR of lymph nodes. As shown in Fig. 3c, Man-BSA800, Man-DEX800, and Man-PAA800 exhibited high nonspecific uptake in the skin and muscle as well as major organs such as liver or kidneys. Man-PRx800 showed minimal to no fluorescence signals in normal tissues and organs except for nominal liver uptake, which contributed to the high SBR of most lymph nodes. The resected lymph nodes were applied for histopathological analysis combined with NIR fluorescence microscopy. As shown in Fig. 3d, the neck lymph node resected from the rat injected with 100 nmol of Man-PRx800 represented the major uptake in the medulla region, where macrophages overexpress mannose receptors [19].
DISCUSSION
SLN biopsy is the standard of care for some of malignancies such as breast cancer and malignant melanoma [1–3]. Likewise, prophylactic regional lymphadenectomy is of significant importance in most malignancies to provide precise staging and to predict the prognosis [5]. Furthermore, curative lymphadenectomy can improve the prognosis of many cancer patients with lymph node metastasis [5]. However, numerous complications accompanied by the lymphadenectomy such as postoperative edema, postoperative bleeding, lymphatic fistula, and tissue injury are significant drawbacks [4].
Under the intraoperative NIR fluorescence imaging system, mono- or multivalent mannose-targeted nanoprobes enable to highlight all lymph nodes in the whole body after a single intravenous injection. Of note, the success of imaging and surgery is largely dependent on strong signals from the lymph nodes as well as low uptake in the surrounding tissues and major organs, which together decide the relative SBR [24,25]. For example, as shown in Fig. 2, ZW800-3C, Man-ZW800-1, and Man-ZW800-3C showed similarly high uptake in PLNs, but only zwitterionic Man-ZW800-1 displayed minimum to no signals in the background tissues. ZW800-3C and Man- ZW800-3C include 3 positive charges that result in high nonspecific uptake in major organs including lung, liver, spleen, and kidneys [25].
Similarly, the physicochemical properties of nanoparticles such as size, lipophilicity, charge, shape, and flexibility determine their biodistribution and targeting efficiency to lymph nodes [26,27]. As shown in Fig. 3, multiple mannose-conjugated nanoprobes (HD = 5–7 nm) targeted almost all lymph nodes within 4 h post-intravenous injection. However, globular-shaped and highly negative charged BSA (−18) resulted in high uptake in lung, liver, pancreas, and kidneys. PAA composed of linear but less flexible backbone and −42 charges ended up in the kidneys and bladder with less targetability in the lymph nodes. 10 kDa dextran, however, is the main component of Lymphoseek along with 25 mannose units, but the PLN targetability of Man-DEX800 was not considerably higher than other nanoprobes. Indeed, Man-PRx800 showed much higher specificity in almost all regional lymph nodes (**P < 0.005). Although the targeting affinity and kinetics are not fully investigated, this could be because of the hydrophilicity and flexibility of Man-PRx800, which are the two main factors to avoid nonspecific uptake and facilitate renal clearance [26–28]. Furthermore, the α-CDs in the PRx structure can move freely along the PEG chain like a necklace, the thermodynamics for mannose receptor binding are favorable because of negative free energy.
Nevertheless, there are some limitations in the present study. First, we tested mannose-conjugated nanoprobes only in rats. We will need to optimize the pharmacokinetics and pharmacodynamics of nanoprobes in a larger species such as pigs prior to testing in humans. In addition, although Man-PRx800 showed best performance in vivo, both ZW800-1 and PRx have not received FDA approval yet. It will require the same regulatory path like any other diagnostic agents prior to human use. The good news is that all the components for PRx such as PEG and α-CD are already clinically available, which will lower the barrier for FDA approval of PRx. Lastly, further studies including binding affinity and kinetics as well as immunohistological evaluations of targeted nanoprobes need to be followed in the future.
In summary, the combination of mannose-decorated NIR nanoprobes and real-time FLARE imaging system enabled intraoperative PLN mapping. This technology could be useful for surgeons to find regional lymph nodes (PLNs) in a fast and accurate manner. This will further empower surgeons to perform such complicated surgery safely and quickly. In addition, PLN mapping allows pathologists to find all the resected lymph nodes efficiently and undoubtedly, which also contributes to precise postoperative diagnosis.
MATERIALS AND METHODS
Preparation of mono- and multivalent mannose-conjugated NIR nanoprobes
All chemicals and solvents were commercially obtained from Sigma Aldrich (Saint Louis, MO) or Fisher Scientific (Pittsburgh, PA, USA) and were of the highest purity level available. The synthetic routes of ZW800-1 and ZW800-3C were described in detail previously [8,11,12]. For preparation of single mannose conjugates, D-mannosamine (MW: 216) was conjugated to the ZW800-1 and ZW800-3C NIR fluorophores using N-hydroxysuccinimide (NHS) ester chemistry in dimethyl sulfoxide (DMSO). The NHS ester activated ZW800-1 was also used for the conjugation into BSA (the labeling ratio between ZW800-1 and BSA is 2.6), followed by the reconjugation of D-mannosamine on the purified BSA800 conjugates in the presence of 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM) in an aqueous solution. The amount of mannose moieties in the Man-BSA800 conjugate was determined by colorimetry, using phenol and sulfuric acid [29]. For the preparation of multiple mannose-polymer conjugates, D-mannosamine and amino-functionalized ZW800-1 were respectively conjugated into PAA (MW, 5 kDa) directly, and DEX (MW, 10 kDa) after carboxylation using succinic anhydride, in the presence of DMT-MM. PRx (MW of PEG, 4 kDa; the average number of threaded α-cyclodextrins (α-CDs), approximately 25) in particular was prepared by inclusion complexation between PEG and α-CDs in water and end-capped by ZW800-1 fluorophores [30]. Subsequently, D-mannosamine was randomly conjugated on the α-CDs of PRx after carboxylation of hydroxyl groups using succinic anhydride in DMSO.
Purification of mannose-conjugated nanoprobes
Mono-mannose conjugates of Man-ZW800-1 and Man-ZW800-3C were separated by preparative HPLC system (Waters, Milford, MA, USA) equipped with a PrepLC 150 mL fluid handling unit, a manual injector (Rheodyne 7725i), a 2487 dual wavelength absorbance detector (Waters) and an evaporative light scatter detection (ELSD, Richards Scientific, Novato, CA, USA). For purification of multiple mannose conjugates, gel-filtration chromatography (GFC) was performed on a P-6 cartridge (Bio-Rad, Hercules, CA) using PBS, pH 7.4 as mobile phase. The hydrodynamic diameter (HD) of all products was simultaneously measured at 280 nm (BSA and polymer) and 770 nm (ZW800-1) using an absorbance spectrophotometer.
Optical property measurements
All optical measurements were performed at 37°C in phosphate buffered saline (PBS) and 100% fetal bovine serum (FBS) buffered with 50 mM HEPES, pH 7.4. Absorbance and fluorescence emission spectra NIR fluorophores were measured using fiber optic HR2000 absorbance (200–1100 nm) and USB2000FL fluorescence (350–1000 nm) spectrometers (Ocean Optics, Dunedin, FL) were employed. NIR excitation was provided by a 770 nm NIR laser diode light source (Electro Optical Components, Santa Rosa, CA) set to 8 mW and coupled through a 300 µm core diameter, NA 0.22 fiber (Fiberguide Industries, Stirling, NJ). The optical and physicochemical properties of ZW800-1 and ZW800-3C have been previously described in detail [11,23].
Animal models
Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-certified facility and were studied under the supervision of BIDMC IACUC in accordance with approved institutional protocols (#101–2011). Sprague-Dawley (SD) rats (n = 24) averaging 253 g (Charles River Laboratories, Wilmington, MA) were anesthetized with 80 mg/kg ketamine and 8 mg/kg xylazine intraperitoneally (Webster Veterinary, Fort Devens, MA).
NIR fluorescence imaging system
The dual-NIR channel FLARE imaging system has been described in detail [31]; in this study, 11 mW/cm2 of 760 nm excitation light was used with white light (400–650 nm) at 40,000 lx. Color and NIR fluorescence images were acquired simultaneously with custom software at rates up to 15 Hz over a 15-cm diameter field of view. In the color-NIR merged image, 800 nm fluorescence was pseudo-colored green. The imaging system was positioned at a distance of 18 inches from the surgical field. For each experiment, camera exposure time and image normalization were held constant.
Intraoperative pan lymph node mapping in rats
To confirm the in vivo performance of mannose-conjugated nanoprobes, 100 nmol (0.4 mg/kg) of ZW800-1, Man-ZW800-1, ZW800-3C and Man-ZW800-3C were injected intravenously into SD rats, and their lymph node targetability and biodistribution was compared. Next, the same dose (100 nmol) of Man-DEX800, Man-BSA800, Man-PAA800 and Man-PRx800 were injected into SD rats 4 h prior to imaging to evaluate their PLN targeting with the same condition. FLARE imaging system was used to take intraoperative images, and the fluorescence signal of each organ and signal-to-background ratio (SBR) were analyzed by the FLARE software.
NIR fluorescence microscopy
For histological evaluations, lymph nodes were resected from the Man-ZW800-1 injected rats after scheduled imaging, embedded in Tissue-Tek OCT compound (Fisher Scientific, Pittsburgh, PA), and flash frozen in liquid nitrogen. Tissue was cryosectioned at 10 µm intervals and observed using a NIR fluorescence microscope. Consecutive sections were stained with hematoxylin and eosin (H&E). NIR fluorescence microscopy was performed on a 4 filter-set Nikon Eclipse TE300 epifluorescence microscope to confirm the fluorescence of lymph node as previously described [10]. The microscope was equipped with a 100 W mercury light source, NIR-compatible optics, and a NIR-compatible 4×, 10×, 20×, and 40X Plan Fluor objective lens (Nikon, Melville, NY). Custom filter sets (Chroma Technology Corporation, Brattleboro, VT) composed of 750 ± 25 nm excitation filter, 785 nm dichroic mirror and 810 ± 20 nm emission filter were used to detect the fluorescent signal in the frozen tissue samples. Images were acquired on an Orca-AG (Hamamatsu, Bridgewater, NJ) and QImaging 12-bit camera for color imaging (Surrey, BC, Canada). Image acquisition and analysis was performed using iVision software (BioVision Technologies, Exton, PA).
Quantification and statistical analysis
The fluorescent intensity (FI) of a region of interest (ROI) over the neck, axillary, inguinal, mesenteric and lumbar lymph nodes were quantified using custom FLARE software, and the signal-to-background ratio (SBR) was calculated in each experiment to compare the performance. SBR = FI of ROI / background (BG) intensity. The rectus abdominis muscles were used as BG. FI of organs such as lung, liver, pancreas, spleen, kidney and intestine was also quantified and compared. At least 3 animals were analyzed for SBR in each compound. Results were presented as mean ± standard deviation (SD). The statistical analysis was performed using a one-way ANOVA followed by Tukey’s multiple comparisons test between multiple groups. A P value of less than 0.05 was considered significant: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Supplementary Material
Research highlights.
NIR fluorescent nanoprobes to target macrophages in the lymph node.
Renal clearable nanoprobes enhance the SBR of target tissue.
Intraoperative pan lymph nodes mapping is demonstrated.
Surgeons can perform lymphadenectomy with ease and safety using NIR.
Acknowledgments
We thank Ivey Choi for manuscript editing. This study was supported by the US National Institute of Health grants: NIBIB #R01-EB017699 and NIBIB #R01-EB022230. This work was also supported by the Pioneer Research Center Program #2015M3C1A3056410 funded by the Ministry of Science, ICT and Future Planning and the Marine Biotechnology Program #PJT200979 funded by the Ministry of Oceans and Fisheries, South Korea.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: None.
Author’s Contributions: HW, HH, and HSC designed the study. HW, HH, JHL and KB performed the experiments. HW, HH, GEF, YC and HSC reviewed, analyzed, and interpreted the data. HW, HH, YC and HSC wrote and revised the paper. All authors discussed the results and commented on the manuscript. YC and HSC approved the submitted version.
References
- 1.Zhao BW, Chen YM, Jiang SS, Chen YB, Zhou ZW, Li YF. Lymph Node Metastasis, a Unique Independent Prognostic Factor in Early Gastric Cancer. PLoS One. 2015;10:e0129531. doi: 10.1371/journal.pone.0129531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiriyama M, Ebata T, Aoba T, Kaneoka Y, Arai T, Shimizu Y, Nagino M. Prognostic impact of lymph node metastasis in distal cholangiocarcinoma. Br J Surg. 2015;102:399–406. doi: 10.1002/bjs.9752. [DOI] [PubMed] [Google Scholar]
- 3.Adachi T, Eguchi S, Beppu T, Ueno S, Shiraishi M, Okuda K, Yamashita Y, Kondo K, Nanashima A, Ohta M, Takami Y, Noritomi T, Kitahara K, Fujioka H. Prognostic Impact of Preoperative Lymph Node Enlargement in Intrahepatic Cholangiocarcinoma: A Multi-Institutional Study by the Kyushu Study Group of Liver Surgery. Ann Surg Oncol. 2015;22:2269–2278. doi: 10.1245/s10434-014-4239-8. [DOI] [PubMed] [Google Scholar]
- 4.Wada H, Hyun H, Vargas C, Gravier J, Park G, Gioux S, Frangioni JV, Henary M, Choi HS. Pancreas-targeted NIR fluorophores for dual-channel image-guided abdominal surgery. Theranostics. 2015;5:1–11. doi: 10.7150/thno.10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mieog JS, Troyan SL, Hutteman M, Donohoe KJ, van der Vorst JR, Stockdale A, Liefers GJ, Choi HS, Gibbs-Strauss SL, Putter H, Gioux S, Kuppen PJ, Ashitate Y, Lowik CW, Smit VT, Oketokoun R, Ngo LH, van de Velde CJ, Frangioni JV, Vahrmeijer AL. Toward optimization of imaging system and lymphatic tracer for near-infrared fluorescent sentinel lymph node mapping in breast cancer. Ann Surg Oncol. 2011;18:2483–2491. doi: 10.1245/s10434-011-1566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka E, Choi HS, Fujii H, Bawendi MG, Frangioni JV. Image-guided oncologic surgery using invisible light: completed pre-clinical development for sentinel lymph node mapping. Ann Surg Oncol. 2006;13:1671–1681. doi: 10.1245/s10434-006-9194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutteman M, Choi HS, Mieog JS, van der Vorst JR, Ashitate Y, Kuppen PJ, van Groningen MC, Lowik CW, Smit VT, van de Velde CJ, Frangioni JV, Vahrmeijer AL. Clinical translation of ex vivo sentinel lymph node mapping for colorectal cancer using invisible near-infrared fluorescence light. Ann Surg Oncol. 2011;18:1006–1014. doi: 10.1245/s10434-010-1426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashitate Y, Hyun H, Kim SH, Lee JH, Henary M, Frangioni JV, Choi HS. Simultaneous mapping of pan and sentinel lymph nodes for real-time image-guided surgery. Theranostics. 2014;4:693–700. doi: 10.7150/thno.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wada H, Hyun H, Vargas C, Genega EM, Gravier J, Gioux S, Frangioni JV, Choi HS. Sentinel Lymph Node Mapping of Liver. Ann Surg Oncol. 2015;22(Suppl 3):S1147–1155. doi: 10.1245/s10434-015-4601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi HS, Gibbs SL, Lee JH, Kim SH, Ashitate Y, Liu F, Hyun H, Park G, Xie Y, Bae S, Henary M, Frangioni JV. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat Biotechnol. 2013;31:148–153. doi: 10.1038/nbt.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi HS, Nasr K, Alyabyev S, Feith D, Lee JH, Kim SH, Ashitate Y, Hyun H, Patonay G, Strekowski L, Henary M, Frangioni JV. Synthesis and in vivo fate of zwitterionic near-infrared fluorophores. Angew Chem Int Ed Engl. 2011;50:6258–6263. doi: 10.1002/anie.201102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyun H, Bordo MW, Nasr K, Feith D, Lee JH, Kim SH, Ashitate Y, Moffitt LA, Rosenberg M, Henary M, Choi HS, Frangioni JV. cGMP-Compatible preparative scale synthesis of near-infrared fluorophores. Contrast Media Mol Imaging. 2012;7:516–524. doi: 10.1002/cmmi.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bao K, Nasr KA, Hyun H, Lee JH, Gravier J, Gibbs SL, Choi HS. Charge and hydrophobicity effects of NIR fluorophores on bone-specific imaging. Theranostics. 2015;5:609–617. doi: 10.7150/thno.11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim KS, Hyun H, Yang JA, Lee MY, Kim H, Yun SH, Choi HS, Hahn SK. Bioimaging of Hyaluronate-Interferon alpha Conjugates Using a Non-Interfering Zwitterionic Fluorophore. Biomacromolecules. 2015;16:3054–3061. doi: 10.1021/acs.biomac.5b00933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SH, Lee JH, Hyun H, Ashitate Y, Park G, Robichaud K, Lunsford E, Lee SJ, Khang G, Choi HS. Near-infrared fluorescence imaging for noninvasive trafficking of scaffold degradation. Sci Rep. 2013;3:1198. doi: 10.1038/srep01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung JE, Tan S, Gao SJ, Yongvongsoontorn N, Kim SH, Lee JH, Choi HS, Yano H, Zhuo L, Kurisawa M, Ying JY. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nat Nanotechnol. 2014;9:907–912. doi: 10.1038/nnano.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robbins JC, Lam MH, Tripp CS, Bugianesi RL, Ponpipom MM, Shen TY. Synthetic glycopeptide substrates for receptor-mediated endocytosis by macrophages. Proc Natl Acad Sci U S A. 1981;78:7294–7298. doi: 10.1073/pnas.78.12.7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Chirino AJ, Misulovin Z, Leteux C, Feizi T, Nussenzweig MC, Bjorkman PJ. Crystal structure of the cysteine-rich domain of mannose receptor complexed with a sulfated carbohydrate ligand. J Exp Med. 2000;191:1105–1116. doi: 10.1084/jem.191.7.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marttila-Ichihara F, Turja R, Miiluniemi M, Karikoski M, Maksimow M, Niemela J, Martinez-Pomares L, Salmi M, Jalkanen S. Macrophage mannose receptor on lymphatics controls cell trafficking. Blood. 2008;112:64–72. doi: 10.1182/blood-2007-10-118984. [DOI] [PubMed] [Google Scholar]
- 20.Vera DR, Wallace AM, Hoh CK, Mattrey RF. A synthetic macromolecule for sentinel node detection: (99m)Tc-DTPA-mannosyl-dextran. J Nucl Med. 2001;42:951–959. [PubMed] [Google Scholar]
- 21.Kim HK, Kim S, Park JJ, Jeong JM, Mok YJ, Choi YH. Sentinel node identification using technetium-99m neomannosyl human serum albumin in esophageal cancer. Ann Thorac Surg. 2011;91:1517–1522. doi: 10.1016/j.athoracsur.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Pirmettis I, Arano Y, Tsotakos T, Okada K, Yamaguchi A, Uehara T, Morais M, Correia JD, Santos I, Martins M, Pereira S, Triantis C, Kyprianidou P, Pelecanou M, Papadopoulos M. New (99m)Tc(CO)(3) mannosylated dextran bearing S-derivatized cysteine chelator for sentinel lymph node detection. Mol Pharm. 2012;9:1681–1692. doi: 10.1021/mp300015s. [DOI] [PubMed] [Google Scholar]
- 23.Hyun H, Owens EA, Narayana L, Wada H, Gravier J, Bao K, Frangioni JV, Choi HS, Henary M. Central C-C Bonding Increases Optical and Chemical Stability of NIR Fluorophores. RSC Adv. 2014;4:58762–58768. doi: 10.1039/C4RA11225C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owens EA, Henary M, El Fakhri G, Choi HS. Tissue-Specific Near-Infrared Fluorescence Imaging. Acc Chem Res. 2016;49:1731–1740. doi: 10.1021/acs.accounts.6b00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owens EA, Lee S, Choi J, Henary M, Choi HS. NIR fluorescent small molecules for intraoperative imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:828–838. doi: 10.1002/wnan.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi HS, Frangioni JV. Nanoparticles for biomedical imaging: fundamentals of clinical translation. Mol Imaging. 2010;9:291–310. [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JH, Park G, Hong GH, Choi J, Choi HS. Design considerations for targeted optical contrast agents. Quant Imaging Med Surg. 2012;2:266–273. doi: 10.3978/j.issn.2223-4292.2012.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pesez M BJ. Colorimetric and fluorimetric analysis of organic compounds and drugs. Marcel Dekker; New York: 1974. [Google Scholar]
- 30.Hyun H, Yui N. Mono-, di-, or triazidated cyclodextrin-based polyrotaxanes for facile and efficient functionalization via click chemistry. Macromol Rapid Commun. 2011;32:326–331. doi: 10.1002/marc.201000631. [DOI] [PubMed] [Google Scholar]
- 31.Gioux S, Choi HS, Frangioni JV. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol Imaging. 2010;9:237–255. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.