Abstract
Goals
To analyze the neurochemical profile during the recurrent attacks of nausea and vomiting in patients with Riley Day syndrome.
Background
One of the most disabling features of patients with Riley Day syndrome are recurrent attacks of severe nausea/retching/vomiting accompanied by hypertension, tachycardia and skin flushing, usually triggered by emotional or other stresses.
Study
We monitored blood pressure and heart rate and measured plasma catecholamines during typical dysautonomic crises triggered by emotionally charged situations. For comparison, measurements were repeated at follow-up after the symptoms had resolved and the patients were feeling calm and well.
Results
During a typical attack, patients were hypertensive and tachycardic. In all patients, circulating levels of norepinephrine (p<0.002) and dopamine (p<0.007) increased significantly.
Conclusions
Activation of dopamine receptors in the chemoreceptor trigger zone may explain the cyclic nausea/retching/vomiting of patients with Riley-Day syndrome.
Keywords: familial dyautonomia, afferent baroreflex failure, vomiting, dopamine
INTRODUCTION
The Riley Day syndrome, commonly referred to as familial dysautonomia (FD) is a type of hereditary sensory and autonomic neuropathy. FD is caused by a deficiency of the protein IKAP that affects development of specific sensory and autonomic neurons. Patients have decreased/absent myotatic reflexes, impaired pain and temperature perception, abnormal swallowing and gait ataxia. We have recently shown that patients with FD have a selective defect in the afferent neurons of the baroreflex, 1 which explains their extremely labile blood pressure and recurrent attacks of hypertension and tachycardia, because similar episodes occur in patients with acquired baroreflex failure due to surgical lesions. 2
However, a puzzling feature unique to patients with FD is that during these crises, in addition to hypertension and tachycardia, patients also experience severe nausea and vomiting that can last for hours or even days 3. These disabling vomiting attacks, described by Riley et al as a diagnostic feature of the disease, 3 result in frequent aspiration pneumonia leading to chronic pulmonary damage, but their mechanism remains unknown. Here we report marked increases in circulating levels of dopamine during typical vomiting crises suggesting that activation of the dopamine receptors in the chemoreceptor trigger zone of the brainstem may be the likely mechanism of vomiting. 4
PATIENTS AND METHODS
Participants
Seven patients with typical clinical feature of FD confirmed by genetic testing participated in the study (table 1). The Institutional Review Board of New York University approved all procedures and all subjects signed informed consent.
Table 1.
Demographics, crisis frequency and medications.
| Patient No. |
Age/ Sex |
Crisis Frequency |
Standing medications (mg/day) |
|---|---|---|---|
| 1 | 14/F | 4/week | 0.1 mg* clonidine, 2mg clonazepam, 2.5 mg diazepam, 150 mg pregabalin |
| 2 | 20/M | Daily | 0.25 mg clonidine, 2.5 mg clonazepam, 250 mg pregabalin, 40 mg fluoxetine |
| 3 | 2/M | Daily | 0.1 mg* clonidine, 5 mg diazepam |
| 4 | 17/F | 3/week | 0.1 mg* clonidine, 1 mg clonazepam, 5 mg diazepam, 75 mg pregabalin, |
| 5 | 28/F | Daily | 0.3 mg* clonidine, 1.5 mg alprazolam, 1.5 mg lorazepam, 100 mg pregabalin |
| 6 | 20/M | Daily | 0.1 mg* clonidine, 3.5 mg clonazepam, 10 mg diazepam |
| 7 | 17/F | Daily | 0.1 mg* clondine, 1 mg clonazepam, 40 mg paroxetine |
indicates transdermal patch with continuous systemic delivery.
Measurements
We monitored blood pressure and heart rate and measured plasma catecholamines during typical dysautonomic crises triggered by emotionally charged situations. Measurements were repeated at follow-up after the symptoms had resolved and the patients were feeling calm and well. Blood pressure recordings were taken in the left arm with an automatic sphygmomanometer (Colin Press-Mate 7800, San Antonio, TX). Heart rate was derived from RR intervals recorded from 3 precordial electrodes. Blood samples were obtained in the supine position through an indwelling venous catheter, which was placed in the arm at least 15 minutes prior. Samples were drawn into a heparin Vacutainer tube (BD, Franklin Lakes, NJ). Blood was centrifuged, the plasma extracted and frozen at −70°C until assayed. Plasma norepinephrine, epinephrine and dopamine were measured using high-performance liquid chromatography with electrochemical detection. Average plasma catecholamine concentrations, blood pressure and heart rate values during crisis were compared with values obtained during follow-up testing when the symptoms had resolved using paired t-tests. All values are expressed as mean±SEM; p<0.05 was considered statistically significant.
RESULTS
Patient characteristics are shown in table 1. All patients had a history of emotional lability, anxiety, orthostatic hypotension and episodic hypertension. All were being treated with benzodiazepines and clonidine in an attempt to control the crises. Other medications intended for crisis prevention included pregabalin (cases #1, #3, #4 and #5) and selective serotonin reuptake inhibitors (case # 2 and #7, see table 1). All patients had had fundoplication surgery to control vomiting. Six patients underwent the procedure as infants (mean age, 6 months) and the remaining patient (#2) at age 19 years. The most severe dysautonomic crises tended to occur early in the day. Anxiety or emotional upset was noted as a provocative factor. All patients had a history hospitalization for uncontrollable retching. Because of the frequency and severity of their crises (table 1), the children missed a significant number of school days per week and the adult patients were unable to work.
BASELINE
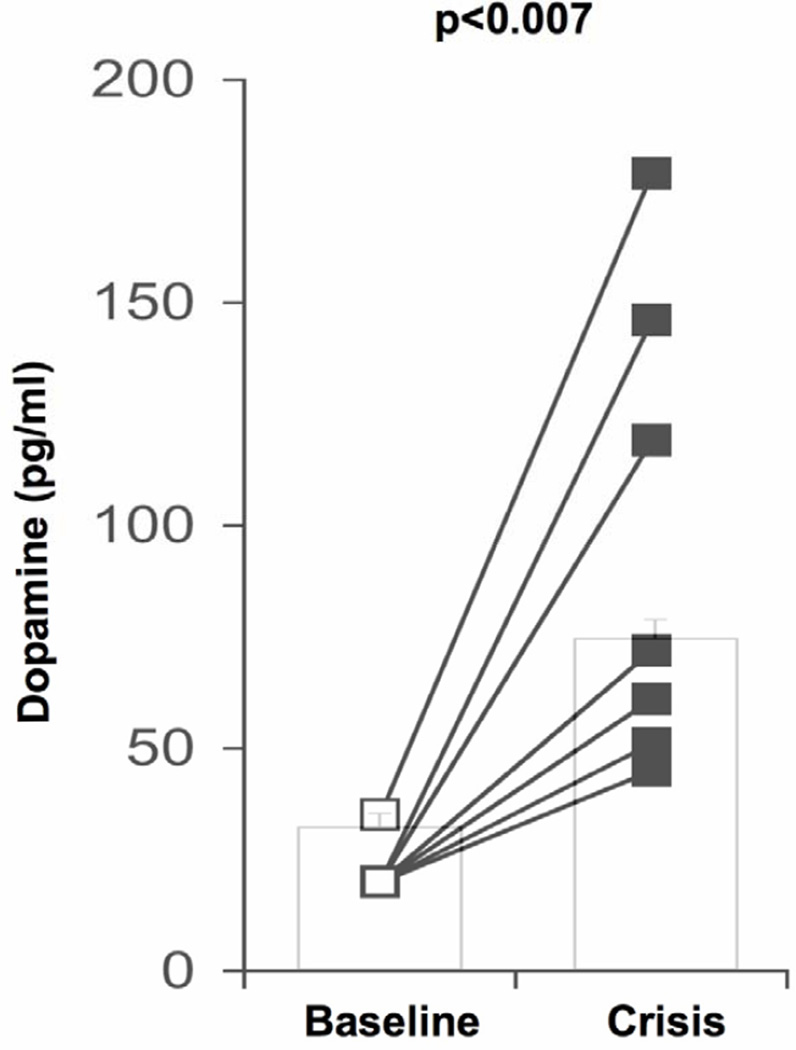
When the patients were feeling well, average supine blood pressure was 124±7/64±5 mmHg with a heart rate of 81±3 beats/min. Four out of the 7 patients were hypertensive for their age. Resting tachycardia was noted in three cases (table 2). In the supine position, average plasma norepinephrine concentration at that time was 130± 45 pg/ml (range 38 to 369 pg/ml), plasma epinephrine concentration was 20±5 pg/ml (range 10 to 40 pg/ml) and dopamine levels were 22±2 pg/ml (figure).
Table 2.
Blood pressure and heart rate at baseline and during typical crisis.
| Patient No. |
BASELINE | CRISIS | ||
|---|---|---|---|---|
| BP mm Hg |
HR bpm |
BP mm Hg |
HR bpm |
|
| 1 | 133/52* | 71 | 222/134 | 135 |
| 2 | 120/59 | 90 | 190/115 | 120 |
| 3 | 146/89* | 90 | 174/114 | 130 |
| 4 | 141/66* | 75 | 177/109 | 138 |
| 5 | 94/57 | 82 | 182/124 | 105 |
| 6 | 113/60 | 60 | 146/96 | 125 |
| 7 | 125/68* | 75 | 169/117 | 117 |
denotes hypertensive value for age
Figure 1.
DYSAUTONOMIC CRISIS
All patients experienced typical crises when they complained of severe nausea and were retching loudly but vomiting was prevented by the fundoplication. In all cases, the face and torso appeared flushed, there was excessive drooling and sweating. Patients were uncommunicative and withdrawn. Akathisia was apparent with repetitive rocking, rubbing of the legs, thrashing and teeth grinding. There were no other overt signs of physical illness and blood counts were normal making infection unlikely. All patients were hypertensive and tachycardic (table 2) but denied feeling palpitations. Plasma norepinephrine levels in the supine position were elevated compared to baseline (772±151 pg/ml, range 426 to 1558 pg/ml, p<0.004), plasma epinephrine concentration was also higher than at baseline, with average plasma concentrations reaching 63±20 pg/ml (range 13 to 169 pg/ml, p=0.07). Plasma dopamine levels were markedly increased in all patients (figure), average plasma dopamine concentration was 96±20 pg/ml (range 45 to 175 pg/ml, p<0.007).
DISCUSSION
The finding of high levels of circulating dopamine in patients with FD during typical attacks of nausea and vomiting provides a plausible neurochemical explanation for these symptoms. The most likely target site triggering vomiting are dopamine receptors, primarily D2 but also D3 in the chemoreceptor trigger zone in the area postrema, outside of the blood-brain barrier. 4
That high circulating levels of dopamine triggers nausea and vomiting was strongly suggested by the induction of nausea and vomiting in patients with Parkinson disease given levodopa, and its prevention with coadministration of carbidopa which blocks the conversion of levodopa to dopamine in the systemic circulation. 5
Contrary to its prominence in the FD dysautonomic crisis, nausea is not a feature of the hyperadrenergic hypertensive crises that occur in patients with acquired afferent baroreflex failure as a result of injury to the glossopharyngeal or vagus nerves. In these patients, resting plasma dopamine levels are normal 6, 7 and during hypertensive crises are either undetectable 6 or only slightly elevated.7
Episodes of hypertension, diaphoresis and flushing also occur frequently in patients with high spinal cord lesions when sympathetic neurons are activated by afferent stimulation at the spinal cord level. Symptoms are similar to those experienced by patients with FD, again with the exception of nausea, however, which is seldom reported in patients with spinal cord lesions. The lack of nausea in patients with spinal cord lesions, may also be explained by lack of increase in plasma dopamine levels during these episodes. Dopamine levels while resting are reportedly low 8 or normal 9 and do not increase in parallel with norepinephrine levels during exercise 9 or food intake. 8 During episodes of hypertension, however, plasma dopamine-beta-hydroxylase spills over into the circulation and urinary excretion of dopamine metabolites is increased. 10 The increased conversion of dopamine to norepinephrine likely prevents plasma levels from rising excessively and causing nausea. It should also be noted that during hypertensive episodes patients with spinal cord injury have bradycardia rather than tachycardia.
Patients with dopamine beta hydoxylase deficiency have high circulating levels of dopamine, but do not experience nausea or cyclic vomiting, perhaps because the dopamine levels are persistently elevated in this disorder. In contrast, although plasma dopamine levels are usually higher than normal in patients with FD, during the vomiting episodes, there is a paroxysmal increase and the levels of dopamine rise even further. While the concentration of circulating dopamine during crisis in patients with FD is lower than the circulating levels of dopamine known to produce nausea and vomiting in patients with Parkinson disease receiving levodopa, it is likely that the concentration locally at the receptor site in the area postrema is much higher than levels measured in blood and this can account for the nausea and vomiting 11.
Recently, nausea and vomiting during exercise has been reported in patients with pheochromocytoma and paraganglioma, likely as a result of increased circulating catecholamines 12.
It is likely that patients with FD have an abnormality in the catecholamine pathway that explains their peculiar neurochemical profile. At rest, patients have increased plasma levels of 3,4-dihydroxyphenylalanine (DOPA) and dopamine while norepinephrine levels are normal and dopamine metabolites are high in plasma and urine 13, 14. This particular pattern could be explained by a number of enzymatic abnormalities. Tyrosine hydroxylase, a rate limiting enzyme in the catecholamine pathway, is upregulated in sympathetic terminals of patients with FD 15, which may explain the increased levels of DOPA. Moreover, because DOPA-decaboxylase is widely expressed and is not a rate-limiting enzyme, DOPA can be extensively converted to dopamine, which may explain the high levels of dopamine. In contrast, dopamine beta hydoxylase (DBH) is a rate-limiting enzyme, and is not upregulated 13 therefore at times of increased dopamine production, not all of it can be hydroxylated to yield norepinephrine. In addition, a possible deficiency in monoamine oxidase (MAO) has also been proposed 16. We postulate that during stress-induced sympathetic activation, when catecholamine production is increased, dopamine formation exceeds the rate of its conversion and metabolism, causing dopamine to spill over from the nerves resulting in high circulating levels. This finding has potential therapeutic implications.
Acknowledgments
Dr. Norcliffe-Kaufmann receives research support from the NIH and the FDA. Dr. Kaufmann serves on a scientific advisory board for Chelsea Therapeutics; serves as Editor-in-Chief of Clinical Autonomic Research; and receives research support from the NIH, the FDA, and the Dysautonomia Foundation, Inc.
Financial support: This work was supported by a grant from the Dysautonomia Foundation, Inc., the National Institutes of Health (U54-NS065736-01) and the Food and Drug Administration Office of Rare Disorders (R01-FD003731-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no conflict of interest to declare.
Disclosures: Dr. Axelrod has nothing to disclose.
REFERENCES
- 1.Norcliffe-Kaufmann L, Axelrod F, Kaufmann H. Afferent baroreflex failure in familial dysautonomia. Neurology. 2010;75:1904–1911. doi: 10.1212/WNL.0b013e3181feb283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson D, Hollister AS, Biaggioni I, et al. The diagnosis and treatment of baroreflex failure [see comments] N Engl J Med. 1993;329:1449–1455. doi: 10.1056/NEJM199311113292003. [DOI] [PubMed] [Google Scholar]
- 3.Riley CM, Day RA, Greeley DM, Landford WS. Central autonomic dysfunction with defective lacrimation: I. Report of five cases. Pediatrics. 1949;3:468–478. [PubMed] [Google Scholar]
- 4.Miller AD, Leslie RA. The area postrema and vomiting. Front Neuroendocrinol. 1994;15:301–320. doi: 10.1006/frne.1994.1012. [DOI] [PubMed] [Google Scholar]
- 5.Markham C, Diamond SG, Treciokas LJ. Carbidopa in Parkinson disease and in nausea and vomiting of levodopa. Arch Neurol. 1974;31:128–133. doi: 10.1001/archneur.1974.00490380076010. [DOI] [PubMed] [Google Scholar]
- 6.Jordan J, Shannon JR, Black BK, et al. Malignant vagotonia due to selective baroreflex failure. Hypertension. 1997;30:1072–1077. doi: 10.1161/01.hyp.30.5.1072. [DOI] [PubMed] [Google Scholar]
- 7.Aksamit TR, Floras JS, Victor RG, Aylward PE. Paroxysmal hypertension due to sinoaortic baroreceptor denervation in humans. Hypertension. 1987;9:309–314. doi: 10.1161/01.hyp.9.3.309. [DOI] [PubMed] [Google Scholar]
- 8.Christensen NJ, Mathias CJ, Frankel HL. Plasma and urinary dopamine: studies during fasting and exercise and in tetraplegic man. Eur J Clin Invest. 1976;6:403–409. doi: 10.1111/j.1365-2362.1976.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 9.Schmid A, Huonker M, Stahl F, et al. Free plasma catecholamines in spinal cord injured persons with different injury levels at rest and during exercise. J Auton Nerv Syst. 1998;68:96–100. doi: 10.1016/s0165-1838(97)00127-6. [DOI] [PubMed] [Google Scholar]
- 10.Sell GH, Naftchi NE, Lowman EW, Rusk HA. Autonomic hyperreflexia and catecholamine metabolites in spinal cord injury. Arch Phys Med Rehabil. 1972;53:415–417. [PubMed] [Google Scholar]
- 11.Jovanovic-Micic D, Samardzic R, Beleslin DB. The role of alpha-adrenergic mechanisms within the area postrema in dopamine-induced emesis. Eur J Pharmacol. 1995;272:21–30. doi: 10.1016/0014-2999(94)00622-e. [DOI] [PubMed] [Google Scholar]
- 12.King KS, Darmani NA, Hughes MS, et al. Exercise-induced nausea and vomiting: another sign and symptom of pheochromocytoma and paraganglioma. Endocrine. 2010;37:403–407. doi: 10.1007/s12020-010-9319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein DS, Holmes C, Axelrod FB. Plasma catechols in familial dysautonomia: a long-term follow-up study. Neurochem Res. 2008;33:1889–1893. doi: 10.1007/s11064-008-9662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Axelrod FB, Goldstein DS, Holmes C, et al. Pattern of plasma levels of catecholamines in familial dysautonomia. Clin Auton Res. 1996;6:205–209. doi: 10.1007/BF02291135. [DOI] [PubMed] [Google Scholar]
- 15.Pearson J, Brandeis L, Goldstein M. Tyrosine hydroxylase immunoreactivity in familial dysautonomia. Science. 1979;206:71–72. doi: 10.1126/science.39339. [DOI] [PubMed] [Google Scholar]
- 16.Rubin BY, Anderson SL. The molecular basis of familial dysautonomia: overview, new discoveries and implications for directed therapies. Neuromolecular Med. 2008;10:148–156. doi: 10.1007/s12017-007-8019-5. [DOI] [PubMed] [Google Scholar]