Abstract
Purpose of Review
Dementia detection in the community is challenging. The purpose of this paper is to review methods of dementia screening and provide a useable algorithm for screening for dementia a variety of clinical settings.
Recent Findings
In recent years, a number of brief performance and informant-based assessments have been developed and validated in research, clinical, and community samples. These assessments are now complemented by patient self-reports that afford the ability to detect subjective cognitive impairment.
Summary
An optimal approach to dementia screening is to combine performance, informant, and self-reports, many of which can be completed in the waiting room or by non-physician staff prior to the start of the office visit. This diverse information may help inform the provider as to the presence or absence of a cognitive disorder, assist in staging the extent of the disorder, and help to develop a differential diagnosis and management plan.
Keywords: Screening, mild cognitive impairment, dementia, Alzheimer’s disease, AD8, quick dementia rating system, Montreal Cognitive Assessment
INTRODUCTION
Alzheimer’s disease and related dementias (ADRD) current affects over 5.5 million Americans and over 35 million people worldwide. It is expected that the number of ADRD cases will continue to increase as the number of people over age 65 continues to increase [1]. More than one in eight adults over age 65 has dementia, and current projections indicate a three-fold increase by 2050. In addition to ADRD, many older adults suffer from multiple co-morbid medical conditions (e.g., heart disease, stroke, depression) that can affect cognitive abilities, behavior, and daily functioning [2]. Primary care providers, neurologists, psychiatrists, and geriatricians are often responsible for the detection, diagnosis, and treatment of ADRD as the number of dementia specialty centers is not sufficient to meet the growing demands [3]. Over the next 2 decades, the number of people over age 65 is expected to grow by 62% and the number over age 85 is expected to grow by 84%, [1,3]. Thus, the prevalence, incidence, morbidity and mortality for ADRD will increase dramatically and the societal financial burden of illness and dependency will expand exponentially.
Despite these obvious demands for early detection, ADRD is often under-recognized in community practice, with many individuals obtaining a diagnosis in the mild-to-moderate stages of dementia. Screening for ADRD would likely increase case identification, however there are questions as to whether increased screening and case identification has value in the absence of more effective medications and interventions that can patient outcomes [3,4]. Effective public health efforts aimed at secondary prevention (i.e. screening) permits early detection of core elements of disease, hopefully to be coupled with treatment or preventive actions to reduce the burden of disease to patients, families, and society [4].
Currently, there are many dementia-screening measures available for use, each capturing different aspects of impairment. Some rely on patient performance, while others rely on interviews with collateral sources such as family members who have witnessed change in the patients from their premorbid abilities. More recently, screening measures have been developed that rely on self-report from the patient himself or herself.. Some batteries are extensive but time-consuming, making them impractical for use in the context of a busy office setting. Other screening measures are brief, but lack sensitivity and specificity required for accurate capture of those at risk for ADRD [3,4]. Here we will discuss the pros and cons of various screening methods and propose an algorithm that can be applied across a variety of clinical and research settings.
Potential Benefits of Dementia Screening
It is worth considering what benefits exist for dementia detection. First and foremost, screening may identify individuals who are experiencing mild symptoms but have not yet brought these symptoms to medical attention [4]. The objective in this case would be to detect the disease earlier than current usual care and to begin interventions and treatments when they might be most effective. Additional benefits would provide patients and providers to address modification of risk factors through change in diet, exercise and life-style [5,6].
Dementia screening would likely be most beneficial at the earliest detectable signs of disease, particularly if the detection measures reflect pathology and biomarker changes associated with the earliest stages of ADRD [7,8]. In this way, treatments (both current and future) can be initiated to potentially alter the pathologic cascade [5,6]. Furthermore, early dementia recognition would afford clinicians the opportunity to enhance patient adherence to medical recommendations by providing information, educational materials, and support to patients and their family caregivers.
Performance-Based Assessment
The essential elements of a comprehensive cognitive evaluation include assessment of diverse cognitive domains, patient functionality, and behavior. Even at the initial contact with the patient, the clinician can assess whether cognitive (including memory, attention, language, executive function), affective, or behavioral problems are present, and then plan a methodical evaluation. This evaluation should include the major domains of neuropsychological function, not only memory but also attention, language, visuospatial skills, and executive function. Using performance-based assessments, the clinician can compare how the patient performs in comparison to published normative values, corrected for age and education. If the patient was previously assessed, the clinician can compare current performance to previous abilities to determine the extent of cognitive decline and stage the patient. Outside the office setting, referral to a neuropsychologist for formal neuropsychological testing provides a more detailed evaluation of cognitive abilities, however outside of major metropolitan areas the availability of a neuropsychologist may be limited.
Brief performance tests while providing a “snap shot” of abilities at the time of evaluation, cannot provide information regarding the change from previous cognitive function unless the patient was previously tested. Additionally, the tests themselves offer little information as to how the scores related to interference with the patients’ activities of daily living. A large number of brief cognitive tests are available each with their pros and cons. Examples include the Mini-Mental State Exam [9], the Mini-Cog [10], and the Montreal Cognitive Assessment [11]. If sufficient time is available, a selection of individual cognitive domains can be assessed either on their own, or in addition to brief screening tests (Table 1). If office staff is available, many of these tests can be performed by medical assistants, physician assistants or nurses.
TABLE 1.
Example of a Brief, In-Office Neurocognitive Battery
| Domains | Example tests |
|---|---|
|
| |
| Language | Category (Animal, Vegetable) naming |
| Letter (F,A,S) fluency | |
| 15-item Boston naming | |
|
| |
| Working memory | Digit span forward |
| Digit span backward | |
|
| |
| Episodic memory | Word list recall (Hopkins, California Verbal Learning Test) |
| Paragraph recall | |
|
| |
| Visual construction | Clock drawing |
|
| |
| Psychomotor speed | Trailmaking A |
|
| |
| Executive function | Trailmaking B |
| Digit symbol substitution | |
|
| |
| Abstraction | Similarities and differences |
| Proverb interpretation | |
|
| |
| Concentration | Months in reverse order |
| Counting backward from 20 | |
|
| |
| Global measurement (Choose one) | Mini-mental State Exam |
| Montreal Cognitive Assessment | |
|
| |
| Mood (Choose one) | Geriatric Depression Scale |
| Patient Health Questionnaire (9, 4, or 2 item) | |
| Hospital anxiety and depression scale | |
|
| |
| Memory Self-Report (Choose one) | Cognitive Change Index |
| Cognitive Function Instrument | |
|
| |
| Informant Global Rating (Choose one) | Quick Dementia Rating Scale |
| AD8 | |
Attention, working memory and concentration are very important in order to process other cognitive abilities and are important to assess when considering whether a cognitive impairment is present [12]. Examples of attention and concentration tasks include counting backwards from 20 to 1, reciting the months in reverse order, performing serial subtraction, or determining digit span forwards and backwards [13]. Orientation to person, place, time and situation can be easily elicited from most patients and informs the clinician as to whether delirium is present. Episodic memory is most readily assessed by list learning, recall, and recognition. As patients with milder stages of ADRD can still often remember short lists (3 items), longer lists (5–12 items) are preferred. It is also useful to see if cues can improve recall supporting relative preservation of primary hippocampal circuitry. Verbal and semantic memory can be easily assessed by asking the patient to generate a list of words (i.e., animals) and name objects presented to them [14]. Construction skills can be assessed by having the patient copy figures such as a cube or intersecting pentagons, or drawing a clock. Executive function is critical to assessing cognition but is more challenging in a brief evaluation. Trailmaking A and B tests can be used to give an overall impression of ability.
Though the idea of creating a unique psychometric battery for use in the office might initially seem appealing, even a brief battery can take 30 minutes to administer to account for delayed recall. Alternatively, there are a variety of brief, cognitive tests developed and validated to provide global assessment of cognitive states. Each test has limitations, but may provide the quickest way to get a global assessment in a busy office practice. The most commonly used instrument is the MMSE, (approximately 10 minutes to complete), is frequently used in general clinical practice and in hospital settings. The MMSE score can be tracked longitudinally to account for cognitive decline [9]. Although among the easiest to administer and score, the MMSE may not capture early stages of ADRD, particularly because of its greater emphasis on orientation (10 of 30 points) and lack of emphasis on memory (3 of 30 points). The MMSE is copyrighted and use is associated with a fee. Issues associated with bias according to age, race, education, and socioeconomic status have also been reported [3,4]. The Mini Cognitive Assessment Instrument (Mini-Cog) combines 3-item learning, clock-drawing as distractor, and then 3-item recall. Quick and easy to interpret (approximately 3 minutes), the Mini-Cog requires no special equipment or specialized training [10], but does not test many cognitive domains. The Saint Louis University Mental Status (SLUMS) is a 30-point, 11-item, clinician-administered test of orientation, memory, attention, and executive function [15]. The SLUMS, although similar in format and scoring to the MMSE adds tasks of attention, calculation, immediate and delayed recall, animal naming, digit span, clock drawing, and figure copying. The Montreal Cognitive Assessment (MoCA) is a 30-point test (approximately 10 minutes) originally developed to improve detection of mild cognitive impairment (MCI) [11]. The MoCA addresses additional frontal-executive function domains not commonly found in other brief performance tests but is more complex to administer and score. Because the language and executive function tests require higher educational attainment, adjustments for education and culture may be necessary. The MoCA attempts for educational correction by the addition 1 point if the patient has less than a 12th grade education.
There is increasing use of computerized batteries for the assessment of patients with cognitive complaints, particularly in clinical trials and longitudinal research projects. Many of these batteries take 30 minutes or more to complete and have licensing and equipment costs associated with them that may limit their use in general practice. One example is the National Institutes of Health Toolbox for the Assessment of Neurological and behavioral Function [16]. The NIH Toolbox contains a range of measures to evaluate cognitive, motor, emotional and sensory function with normative values across the spectrum of aging (published ranges 3–85 years). While used predominantly in research studies, some clinicians may find the NIH Toolbox useful for diagnosis. Access to the cognitive measures requires obtaining permission from NIH after demonstrating knowledge of how to administer and interpret neuropsychological tests. Other examples of commercial companies that market computerized assessments include Cogstate (Cogstate Healthcare), CANTAB (Cambridge Cognition), and NeuroTrax (NeuroTrax Corporation).
Therefore, although incredibly useful in the office, brief instruments may present challenges to detect very early impairment in a short period of time vs. more detailed assessment that takes too much time from the rest of the office visit. To summarize, brief performance-based testing may be: (1) unable to detect or quantify change from previous levels of function; (2) insensitive to subtle changes in high functioning individuals (i.e., ceiling effects); (3) may overestimate deficits in low functioning individuals (i.e. floor effects); and (4) culturally insensitive. To overcome these challenges, clinicians may consider adding informant-based assessments.
Informant-Based Assessment
The diagnosis of dementia is a clinical one that requires intra-individual decline in cognitive function that interferes with everyday functioning. The limitations to all the brief performance measures described above is that they may (1) fail to capture the “change” and “interference” on an individual basis, and (2) have biases due to age, gender, race, education, and culture. If available, questioning of an informant (spouse, adult child, paid caregiver) may highlight changes in cognition, function and behavior that the patient either is not aware of or denies. Informant-based instruments rely on an observant collateral source to assess whether (1) there are changes in cognition, and (2) that change interferes with everyday activities of daily living [17]. Informant assessments are relatively unaffected by education and premorbid ability or by proficiency in the culture’s dominant language as each person serves as their own control. A potentially significant limitation of informant assessments is that they are highly dependent on the reliability of the individual providing the information. Lack of sufficient exposure may prevent an informant from being able to judge the patient’s memory, problem-solving abilities, or activities of daily living. Informants may also minimize deficits either to protect the patient or because they attribute cognitive decline solely to age [17]. Three examples of informant assessments are discussed.
The AD8 [16] is a brief screening (2–3 minute) interview that can differentiate between individuals with and without cognitive impairment. The AD8 has eight yes/no questions about memory, orientation, judgment, and everyday function where the informant rates changes. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) –short form is a 16-item questionnaire that measures cognitive decline from a premorbid level [18]. Each item is rated on a 5-point scale from 1 (“much better”) to 5 (“much worse”) and ratings are averaged to give a 1–5 score, with three representing no change on any item. However, the IQCODE may not be able to detect mild cognitive impairment and other prodromal forms of ADRD [19]. More recently, the Quick Dementia Rating System (QDRS) has been introduced to provide rapid but comprehensive assessment for both baseline evaluations and longitudinal follow-up [20]. The QDRS is a 10-item questionnaire (3–5 minutes) completed by an informant, without the need of a trained clinician or rater. The QDRS reliably discriminates individuals with and without dementia and can provide an accurate staging of individuals compared with longer Gold Standard evaluations such as the Clinical Dementia Rating [20]. The simple format provides ease of use in clinical practice, clinical research, and epidemiological projects [20,21]. The QDRS consists of ten domains with 5 choices per domain: (1) memory and recall, (2) orientation, (3) decision-making and problem-solving abilities, (4) activities outside the home, (5) function at home and hobbies, (6) toileting and personal hygiene, (7) behavior and personality changes, (8) language and communication abilities, (9) mood, and (10) attention and concentration. The QDRS total score (range 0–30) is derived by summing up the 10 domains with higher scores representing greater cognitive impairment. Cognitively normal individuals score 0–1; MCI scores between 2–5; Mild dementia scores between 6–12; Moderate dementia scores between 13–20; and Severe dementia scores between 20–30. The QDRS has the potential to provide a clearer, more accurate staging for those patients who are unable to receive an evaluation by a dementia specialist, can assist in case ascertainment, and may help improve clinical trial eligibility [20].
Self-Report Of Cognitive Impairment
It is common to think that cognitively impaired individuals are not reliable reporters of their cognitive symptoms due to lack of awareness or denial of deficits (cognitive anosognosia) [22]. However, awareness of deficits varies greatly between individuals, with some patients offering reliable accounts of cognitive change, and others failing to appreciate symptoms. Denial of cognitive deficits does not correlate with age of dementia onset, duration of illness or education but does correlate with dementia severity [23,24]. However, ADRD patients can self-rate a number of physical and psychological symptoms (e.g., depression) [25] and whether they believe they are experiencing cognitive decline using patient versions of informant interviews such as the AD8 [26].
The concept of subjective cognitive impairment has recently gained traction as a sensitive marker of decline and a possible early warning sign of those at risk for future ADRD. The Cognitive Function Instrument (CFI) [23] is a 14-item Yes/No checklist with reference to performance 1 year prior. The CFI is sensitive to decline from a non-dementia status but not if the patient already exhibited objective cognitive impairment. The Cognitive Change Index [23] is a 20-item question that uses a Likert scale to detect the level of change from previous abilities. In the absence of an informant, informant tests such as the QDRS and AD8 can be directly administered to the patient as a self-rating tool [20,26]. Alternatively, complaints of subjective impairment can be extracted from other standardized scales such as the Geriatric Depression Scale [27].
The Self-Administered Gerocognitive Examination (SAGE) was developed as a 10–15 minute self-administered cognitive assessment tool that does not require any staff time to conduct [28]. The SAGE may identify those individuals with MCI and early-stage ADRD by testing orientation, language, cognition, visuospatial-construction, executive, and memory domains. Of particular interest, is a prospective memory task requiring the patient to remember to write “I am done” (a task given to them at the beginning of the assessment) after completion of the SAGE. The SAGE can be administered to the patient outside the office setting with acceptable sensitivity (79%) and specificity (95%) and then provided to the clinician [28]. Limitations include low educational attainment, visual impairment, and the inability to monitor “cheating”, particularly for the prospective memory task.
Mood Assessment
Depression is common in older adults, either as a separate condition, or as a precursor to ADRD. The most common cognitive complaint in individuals with depression revolves around memory. Interesting, if the cognitive symptoms wax and wane in relation to mood symptoms, then depression is more likely to be the root cause of the cognitive decline. If depression improves, but memory doesn’t, then ADRD is more likely to be the underlying culprit. Because of this complex relationship, early detection and appropriate management of depression are important. Several brief scales are available that are commonly used for detecting depression in older adults. The Geriatric Depression Scale (GDS) comes as a 15-item or a 30-item questionnaire that takes 5–10 minutes [25]. The Patient Health Questionnaire (PHQ-9) is a 9-item questionnaire widely used primary care settings that is also available in briefer 2- and 4-item versions [29]. The Hospital Anxiety and Depression Scale (HADS), is a 14-item questionnaire (7-item depression subscale and 7-item anxiety subscale) for use in general medical outpatient clinic settings and has the advantage of distinguishing between anxiety and depression [30].
CONCLUSIONS
Cognitive disorders are increasingly common in older adults who present to any clinical setting. The elements of a comprehensive cognitive evaluation include observational, cognitive, and neuropsychiatric assessments. However, for a variety of reasons (anosagnosia, lack of insight, fear, lack of knowledge, confusion about what is normal for age) cognitive complaints may not be readily offered by patients. A coexist factor is that clinicians may have limited time during the office visit to elicit cognitive symptoms. In the absence of a time and ability to perform a comprehensive evaluation, it is unlikely that a clinician will detect impairment at the mildest stages when intervention may offer the greatest potential for benefit. In addition, cognitive impairments leads to poorer adherence, higher medical costs, and worse outcomes for other medical conditions compared with age-matched older adults without cognitive impairment [1]. On possibility to alleviate this is to conduct a staged screening approach, taking advantage of observations from patients and families, completing some assessments in the waiting room, and training non-physician staff to administer brief tests of mental status. However the clinician chooses to conduct a cognitive evaluation (design their own battery, use some of the commercially available computer programs, or use one or more of the standardized assessments described here), the failure to include a cognitive evaluation in the assessment of older adults represents a missed opportunity for early detection and early intervention.
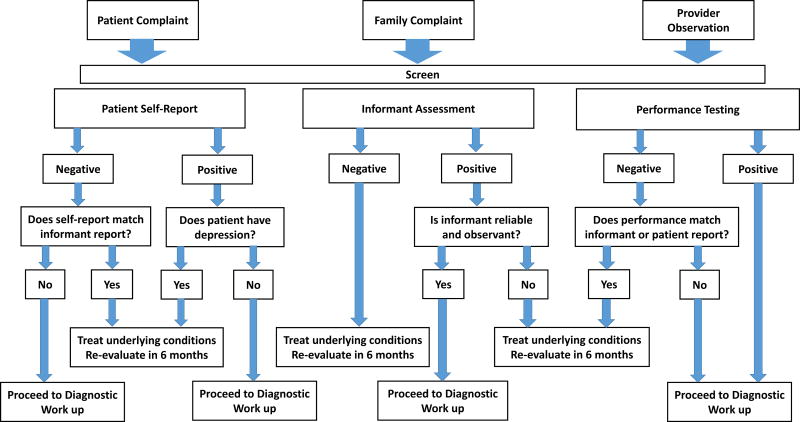
Figure 1. Screening Algorithm for Dementia Screening in the Office Setting.
Screening for cognitive impairment can be triggered by any one of three possible events: patient complaints, family complaints, or a provider’s suspicion. Three categories of assessments should be considered depending on situation and resources: patient self-reports, informant reports, and patient performance. Patient self-reports document subjective cognitive concerns. If the patient endorses symptoms, the next step should be to screen for depression. If the patient denies symptoms, does this match what the family is observing? Informant reports provide a measure of change from baseline and extent of interference with activities of daily living. If the informant reports change, does this match the patient’s performance? Is the informant observant and reliable? Performance testing compares the patient to age- and education-matched normative data. If the patient performs within normal range, does this match was the family is noticing in real-world situations? These assessments can then inform the clinician to either proceed with a more extensive diagnostic work-up or to treat underlying conditions (e.g., depression) and re-evaluate at a later time.
TABLE 2.
Useful brief dementia screening test for the office setting
| Screening test |
Numbers of items |
Scoring system | Validity | Limitations |
|---|---|---|---|---|
|
| ||||
| MMSE | 30 items | Cutoff 23–24 | Sensitivity 85–100% Specificity 66–100% | Score influenced by education, ethnicity, social class. Not ideal to identify mild impairment. Copyright fees |
|
| ||||
| Mini-Cog | 3-item recall with clock drawing | Recall 2/3, use clock to determine presence of dementia | Sensitivity and specificity comparable to MMSE | Test focus on immediate and delay recall, and construction. |
| Does not consider other cognitive domains | ||||
|
| ||||
| SLUMS | 11 items | Cutoff of 21–26: mild cognitive impairment (MCI), 20 and below: dementia | Sensitivity 96–98% Specificity 61–100% | Limited validation on different groups of patients from original study. |
| Tests are complicated and take time to use in an office setting. | ||||
|
| ||||
| MoCA | 12 items | Less than 26 detect MCI or dementia | Sensitivity of 90 for MCI and 100 for dementia | Takes 10 minutes or more for patients with more severe impairment. |
| Not as extensively studied as MMSE. | ||||
|
| ||||
| AD8 | 8 items | Scores greater than 2 signify impairment | Sensitivity 90% Specificity 68% | Depends on observer? informant. |
| In the absence of informant, the AD8 can be administered to the patient. | ||||
|
| ||||
| QDRS | 10 items | Scores of 2 or greater signify impairment | Sensitivity 84% Specificity 75% | Depends on observant informant. |
| In the absence of informant, the QDRS can be administered to the patient. | ||||
|
| ||||
| IQCODE | 16 items | Scores greater than 3.44 signify impairment | Sensitivity 76–100% Specificity 65–86% | Depends on observant informant. |
| May not be sensitive to mild cognitive | ||||
Key: MMSE=Mini Mental State Exam; SLUMS=Saint Louis University Mental Status; MoCA=Montreal Cognitive Assessment; QDRS=Quick Dementia Rating System; IQCODE=Informant Questionnaire on Cognitive Decline in the Elderly
Acknowledgments
James Galvin is the primary copyright holder of the AD8 (with Washington University) and the QDRS (with New York University). His research is supported by grants from NIH (R01 AG040211, U01 NS100610, R01 AG054425, and R01 AG056610), the Florida Department of Health, and the Harry T. Mangurian Foundation. He serves as a consultant for Biogen, Axovant, Eli Lilly, and Eisai. He directs clinical trials for Novartis, Amgen, Genentech, Biogen, Janssen, and Axovant. None the sponsors are involved in the development or publication of this manuscript.
Footnotes
Compliance with Ethical Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
* Of Importance
- 1*.Alzheimer's Association. 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. An annually updated paper covering all aspects of Alzheimer’s disease. [DOI] [PubMed] [Google Scholar]
- 2*.Haaksma ML, Vilela LR, Marengoni A, Calderón-Larrañaga A, Leoutsakos JS, Olde Rikkert MGM, Melis RJF. Comorbidity and progression of late onset Alzheimer's disease: A systematic review. PLoS One. 2017;12:e0177044. doi: 10.1371/journal.pone.0177044. A well-done paper discussing the role that multiple co-morbidities may plan in onset and progression of Alzheimer’s disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tolea MI, Galvin JE. Current guidelines for dementia screening: shortcomings and recommended changes. Neurodegener Dis Manag. 2013;3:565–573. doi: 10.2217/nmt.13.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galvin JE. Dementia screening, biomarkers and protein misfolding: Implications for public health and diagnosis. Prion. 2011;5:16–21. doi: 10.4161/pri.5.1.14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galvin JE. Optimizing diagnosis and management in mild-to-moderate Alzheimer’s disease. Neurodegener Dis Manag. 2012;2:291–304. doi: 10.2217/nmt.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galvin JE, Sadowsky CH. Practical guidelines for the recognition and diagnosis of dementia. J Am Board Fam Med. 2012;25:367–382. doi: 10.3122/jabfm.2012.03.100181. [DOI] [PubMed] [Google Scholar]
- 7.Biagioni MC, Galvin JE. Using biomarkers to improve detection of Alzheimer's disease. Neurodegener Dis Manag. 2011;1:127–139. doi: 10.2217/NMT.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galvin JE, Fagan AM, Holtzman DM, Mintun MA, Morris JC. Relationship of dementia screening tests with biomarkers of Alzheimer's disease. Brain. 2010;133:3290–3300. doi: 10.1093/brain/awq204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical methods for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 10.Borson S, Scanlan JM, Watanabe J, Tu SP, Lessig M. Simplifying detection of cognitive impairment: comparison of the Mini-Cog and Mini-Mental State Examination in a multiethnic sample. J Am Geriatr Soc. 2005;53:871–874. doi: 10.1111/j.1532-5415.2005.53269.x. [DOI] [PubMed] [Google Scholar]
- 11.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 12.Karantzoulis S, Galvin JE. Distinguishing Alzheimer’s disease from other major forms of dementia. Expert Rev Neurother. 2011;11:1579–1591. doi: 10.1586/ern.11.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karzmark P. Validity of serial seven procedure. Int J Geriatr Psychiatry. 2000;15:677–679. doi: 10.1002/1099-1166(200008)15:8<677::aid-gps177>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Brandt J, Manning KJ. Patterns of world list generation in Mild Cognitive Impairment and Alzheimer disease. Clin Neuropsychol. 2009;23:870–879. doi: 10.1080/13854040802585063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tariq SH, Tumosa N, Chibnall JT, Perry MH, 3rd, Morley JE. Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14:900–910. doi: 10.1097/01.JGP.0000221510.33817.86. [DOI] [PubMed] [Google Scholar]
- 16*.NIH Toolbox. [accessed Dec 12, 2017]; http://www.healthmeasures.net/explore-measurement-systems/nih-toolbox Information and permission inquiries for the NIH Toolbox.
- 17.Galvin JE, Roe CM, Xiong C, Morris JC. The validity and reliability of the AD8 informant interview for dementia. Neurology. 2006;67:1942–1948. doi: 10.1212/01.wnl.0000247042.15547.eb. [DOI] [PubMed] [Google Scholar]
- 18.Jorm AF. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): a review. Int Psychogeriatr. 2004;16:275–293. doi: 10.1017/s1041610204000390. [DOI] [PubMed] [Google Scholar]
- 19.Razavi M, Tolea MI, Margrett J, Martin P, Oakland A, Tscholl DW, Ghods S, Mina M, Galvin JE. Comparison of 2 informant questionnaire screening tools for dementia and mild cognitive impairment: AD8 and IQCODE. Alzheimer Dis Assoc Disord. 2014;28:156–161. doi: 10.1097/WAD.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Galvin JE. The Quick Dementia Rating System (QDRS): A rapid dementia staging tool. Alzheimers Dement (Amst) 2015;1:249–259. doi: 10.1016/j.dadm.2015.03.003. A new instrument that permits the rapid staging of cognitive impairment with high correlation to gold standard measurements such as the Clinical Dementia Rating and neuropsychological testing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Berman SE, Koscik RL, Clark LR, Mueller KD, Bluder L, Galvin JE, Johnson SC. Use of the Quick Dementia Rating System (QDRS) as an Initial Screening Measure in a Longitudinal Cohort at Risk for Alzheimer's Disease. JAD Rep. 2017;1:9–13. doi: 10.3233/ADR-170004. A demonstration of the QDRS in a community-based longitudinal Alzheimer Prevention study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelleher M, Tolea MI, Galvin JE. Anosognosia increases caregiver burden in mild cognitive impairment. Int J Geriatr Psychiatry. 2016;31:799–808. doi: 10.1002/gps.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Li C, Neugroschl J, Luo X, Zhu C, Aisen P, Ferris S, Sano M. The Utility of the Cognitive Function Instrument (CFI) to Detect Cognitive Decline in Non-Demented Older Adults. J Alzheimers Dis. 2017;60:427–437. doi: 10.3233/JAD-161294. The CFI is a useful tool to quantify subjective memory complaints and predict whether patient is likely to go to develop cognitive impairment and dementia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Rattanabannakit C, Risacher SL, Gao S, Lane KA, Brown SA, McDonald BC, Unverzagt FW, Apostolova LG, Saykin AJ, Farlow MR. The Cognitive Change Index as a Measure of Self and Informant Perception of Cognitive Decline: Relation to Neuropsychological Tests. J Alzheimers Dis. 2016;51:1145–1155. doi: 10.3233/JAD-150729. Another recently developed instrument, the CCI helps to quantify subjective memory complaints and predict whether patient is likely to go to develop cognitive impairment and dementia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leier VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 26.Galvin JE, Roe CM, Coats MA, Morris JC. Patient’s rating of cognitive ability: using the AD8, a brief informant interview, as a self-rating tool to detect dementia. Arch Neurol. 2007;64(5):725–730. doi: 10.1001/archneur.64.5.725. [DOI] [PubMed] [Google Scholar]
- 27*.Vogel JW, Varga Doležalová M, La Joie R, Marks SM, Schwimmer HD, Landau SM, Jagust WJ. Subjective cognitive decline and β-amyloid burden predict cognitive change in healthy elderly. Neurology. 2017;89:2002–2009. doi: 10.1212/WNL.0000000000004627. A recent study examining the relationship between subjective cognitive decline and amyloid burden, but is limited by the capture of data extracted from the Geriatric Depression Scale. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scharre DW, Chang SI, Murden RA, Lamb J, Beversdorf DQ, Kataki M, Nagaraja HN, Bornstein RA. Self-administered Gerocognitive Examination (SAGE): a brief cognitive assessment Instrument for mild cognitive impairment (MCI) and early dementia. Alzheimer Dis Assoc Disord. 2010;24:64–71. doi: 10.1097/WAD.0b013e3181b03277. [DOI] [PubMed] [Google Scholar]
- 29.Park SC, Lee HY, Lee DW, Hahn SW, Park SH, Kim YJ, Choi JS, Lee HS, Lee SI, Na KS, Jung SW, Shim SH, Kim KW, Paik JW, Kwon YJ. Screening for Depressive Disorder in Elderly Patients with Chronic Physical Diseases Using the Patient Health Questionnaire-9. Psychiatry Investig. 2017;14:306–313. doi: 10.4306/pi.2017.14.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olssøn I, Mykletun A, Dahl AA. The Hospital Anxiety and Depression Rating Scale: a cross sectional study of psychometrics and case-finding abilities in general practice. BMC Psychiatry. 2005;5:46. doi: 10.1186/1471-244X-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]