Abstract
Glucocorticoids (GC), produced and released by the adrenal glands, regulate numerous physiological processes in a wide range of tissues. Because of their profound immunosuppressive and anti-inflammatory actions, GC are extensively used for the treatment of immune and inflammatory conditions, the management of organ transplantation, and as a component of chemotherapy regimens for cancers. However, both pathologic endogenous elevation and long-term use of exogenous GC are associated with severe adverse effects. In particular, excess GC has devastating effects on the musculoskeletal system. GC increase bone resorption and decrease formation leading to bone loss, microarchitectural deterioration and fracture. GC also induce loss of muscle mass and strength leading to an increased incidence of falls. The combined effects on bone and muscle account for the increased fracture risk with GC. This review summarizes the advance in knowledge in the last two decades about the mechanisms of action of GC in bone and muscle and the attempts to interfere with the damaging actions of GC in these tissues with the goal of developing more effective therapeutic strategies.
Keywords: Glucocorticoids, osteoporosis, fracture, osteonecrosis, myopathy, muscle atrophy, osteocyte, osteoblast, osteoclast, apoptosis
1. CLINICAL EFFECTS OF GLUCOCORTICOIDS ON THE MUSCULOSKELETAL SYSTEM
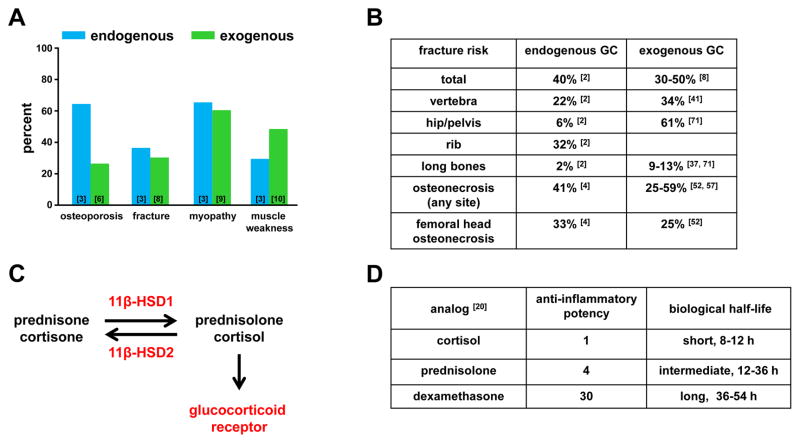
Excess of glucocorticoids (GC) in humans produces detrimental effects in a wide range of tissues. Fat tissue increases and redistributes to abdomen, shoulders, and face, a diabetic metabolic syndrome evolves, blood pressure increases causing hypertensive disease, skin thins and ecchymosis and pink striae appear, acne and hirsutism and irregular menstruation develop, kidney stones form, and there is a failure of growth in children [1–3]. However, among the most common and serious effects occur in the musculoskeletal system [1–3]. In bone, excess GC causes osteoporosis and osteonecrosis, whereas in skeletal muscle, GC causes proximal myopathy and muscle atrophy [3,4] (Figure 1A). The combined detrimental effects of GC on bone mass and muscle strength causes falls and results in increased bone fracture risk, which is a major clinical feature of GC excess. In children GC also markedly retard skeletal growth and maturation [5]. Both endogenous elevation of GC, caused by cortisol over-secretion from the adrenal cortex due to adrenal disease or to ACTH stimulation by pituitary disease or by ectopic tissue, or exogenous GC administered for the treatment of a serious disorder, lead to the same phenotype, commonly known as Cushing syndrome. Exogenous GC produce similar adverse effects on bone and muscle to those in endogenous GC excess, regardless of the underlying chronic disease being treated with exogenous GC. The majority of patients with endogenous GC elevations exhibit osteoporosis (64%) and myopathy (65%) and approximately half of these individuals experience fractures (Figure 1A [3,6–10]). The fractures can occur at any skeletal site but commonly affect vertebrae and ribs (Figure 1B) One study reported that 26% of patients receiving chronic oral corticosteroid developed osteoporosis as defined by bone mineral density ( BMD) t-scores equal to or lower than −2.5 [11]. However, some studies indicate that BMD alone underestimates the fracture risk for patients on GC treatment [11,12]. The myopathy induced by GC increases the incidence of falls and leads to a further increase in fracture risk.
Figure 1. Adverse effects of GC in the musculoskeletal system.
A. Percent occurrence of adverse effects in bone and muscle with endogenous or exogenous GC excess. B. Comparison of fracture and osteonecrosis occurrence in specific bones induced by endogenous and exogenous GC excess. C. GC metabolism: Inactive GC metabolites are converted to active metabolites and vice versa by the enzymatic activity of 11β-HSD 1 and 2, respectively. Active GC metabolites bind to the glucocorticoid receptor to initiate the GC response. D. Relative potency of active GC analogs. References in the graph and tables correspond to data sources. See abbreviations in text.
The biological effect of GC and the pathophysiology of excess GC result from activation of the nuclear GC receptor (GR) (Figure 1C), which regulates a large number and wide spectrum of genes controlling cell metabolism [13]. Mutations in the GR gene, prevent the Cushingoid phenotype from developing [14]. Absence of the phenotype also occurs if the 11β-hydroxysteroid dehydrogenase type1 (11β-HSD1), which converts inactive to active GC metabolites, is defective [13,15,16], or if there is a defect in a coenzyme, H6PDH [17,18]. 11β-HSD1 regulates the level of cortisol and its inactive precursor, cortisone, in target tissues (Figure 1C) [19]. Hydroxylation of the 11-carbon position in GC is essential for activity, and 11β-HSD1 regulates the conversion of inactive cortisol analogues such as prednisone to prednisolone, the active form. The clinical features of GC on bone and muscle varies in severity depending on the endogenous activity of 11β-HSD1.
In most patients with disease caused by endogenous hormonal activity, the high secretion rate of GC relates to the severity of the complications, whereas the side effects in disease caused by exogenous GC excess relate to the dose of hormonal analogue, its potency, and the length of treatment. A number of analogues are currently in clinical use in doses equivalent to the anti-inflammatory activity of cortisol (Figure 1D). However, largely because of higher affinities for GR, their pharmacokinetics differ and biological half-lives are longer than cortisol, thus increasing their potency [20]. All GC are strong anti-inflammatory drugs, and patients chronically treated for over one month with high dose of GC of over 5mg prednisone or its equivalent often have severe musculoskeletal side effects. However, even relatively small doses of GC in the order of 1.5mg/day, such as those used for treatment of asthma, cause adrenal suppression and may lead to adverse effects on the skeletal system [21]. GC cause adverse effects on bone and muscle mainly by direct action on the GR expressed in bone and muscle cells. However, GC also indirectly affect bone and muscle adversely by suppressing sex steroid secretion [22,23], lowering serum 1,25 dihydroxy-Vitamin D levels and calcium absorption [24], decreasing collagen synthesis [25], and inducing diabetes mellitus [26].
Whether GC in the normal range affect the musculoskeletal system is an unsettled, but central question [27]. There is some evidence in children that cortisol in the normal range negatively effects bone geometry and density [28]. In bone, 11β-HSD1 increases with age and increased cortisol production locally may account in part for age-related bone loss [29]. The report of a negative relationship between circulating cortisone and bone formation markers and BMD supports such a finding [30]. Further, cortisol is higher in women with lower BMD at the hip, which in turn appears to relate to GC receptor polymorphisms [31]. A better understanding of the role of normal GC levels in determining bone mass and muscle function awaits much more extensive studies. However, there is little disagreement that states of increased cortisol secretion due to chronic stress such as burns [32–34] and alcoholism [35] increase the incidence of osteoporosis and muscle atrophy. The clinical effects of GC excess are dose and time dependent and many of the features are reversible with removal of the excess. Both BMD and myopathy improve by discontinuing the treatment. On the other hand, fracture and osteonecrosis are adverse events that require prevention. Thus, a comprehensive understanding of the mechanism of action of GC on bone and muscle cells is essential both for understanding the pathophysiology of the clinical presentation of disease and for the development of drug treatments. Currently, replacement of GC with drugs that do not have serious effects on the musculoskeletal system is not always possible.
1. Bone: Osteoporosis and Osteonecrosis
Skeletal fractures are a common and well-recognized clinical manifestation of both endogenous and exogenous chronic GC. Fractures of the ribs and vertebrae occur frequently (Figure 1B). Surprisingly, femoral fracture is not a feature documented in published cases of endogenous Cushing syndrome [1–3], whereas it is a prominent feature of exogenous GC use [36–41]. This difference is probably because exogenous GC increase hip fracture risk by exacerbating a reduction in age-related bone mass and an increase in frequency of age-related falls.
Studies suggest that fracture occurs with GC excess at a higher BMD than in age-related osteoporosis indicating that decrease in bone quality with chronic GC play an important role in fracture risk [11,42]. However, not all studies agree [43]. What is a consistent finding is that the low BMD occurs in the presence of obesity induced by GC, whereas simple obesity is associated with normal to high BMD [44,45]. Acute GC administration decreases bone formation and increases bone resorption [46,47]. However, the increased bone resorption phase does not persist and the osteoporosis of chronic GC use has low to normal bone turnover. Histologically, there is a decrease in bone volume, trabecular width, osteoid surface, and mineralization rate [48]. In GC induced osteoporosis, both bisphosphonates, perhaps paradoxically in view of the low bone turnover, and intermittent PTH increase BMD and reduce fracture [49].
Osteonecrosis (also called avascular necrosis or aseptic necrosis) is a rare disease [50,51], but it occurs commonly with chronic GC use [4,52]. Pathogenesis, although not proven, may to be due to interruption of the blood supply to bone with death of osteocytes and destruction of normal bone structure [53,54]. Multiple etiologies are involved [55]. In osteonecrosis induced by GC, the precise pathogenesis is unclear but may relate to GC effects on fat in the bone marrow. In general, its occurrence relates to the length of exposure and dose of GC although even relatively small doses of GC given over short time periods may precipitate the disease. In patients with inflammatory disease prescribed 4mg methylprednisone 21 tablet taper-pack over 6 days, the relative risk of osteonecrosis increases with one pack and rises further with multiple packs [56]. Common skeletal sites are in bones contiguous with large joints, including head of the femur and humerus, femur and tibia at the knee joint, and bones of the wrist and foot [55]. Radiological imaging establishes the diagnosis, with magnetic resonance imaging being most sensitive and standard radiography most commonly used. In the vertebrae, it may be difficult to distinguish a crush fracture due to osteonecrosis from that due to osteoporosis. Treatment is unsatisfactory particularly in end-stage disease, and bisphosphonates, although often advocated, showed no benefit in clinical trial [57].
2. Muscle: Myopathy and Muscle Atrophy
Proximal myopathy can be a striking clinical feature of Cushing syndrome [10,58,59]. The pathogenesis is obscure. It is similar clinically to the proximal myopathy of vitamin D deficiency and other endocrine myopathies and may have a common mechanism. It is most noticeable at the hip girdle muscles and rising from a sitting position and climbing stairs are difficult. It regresses with the removal of the GC excess. In asthmatics, the myopathy may affect the diaphragm and worsen respiratory distress [60]. The terms myopathy and muscle atrophy are not interchangeable. The former indicates the muscle fibers are normal in size and number but are dysfunctional, whereas the latter indicates the muscle fibers are smaller, fewer, and wasted. Muscle atrophy with GC excess is associated with abnormalities in transcription factors, nuclear cofactors, hyperacetylation, cell-calcium metabolism and insulin signaling [61,62]. GC excess also has major effects on muscle protein synthesis and degradation, and myoblast proliferation [63]. Atrophy affects all voluntary muscles and may be difficult to recognize because of the concomitant increase in fat. Clinically it manifests as both generalized peripheral and central weakness.
2. GLUCOCORTICOID EFFECTS ON BONE CELLS
1. Effects of GC excess on the bone remodeling rate and osteonecrosis
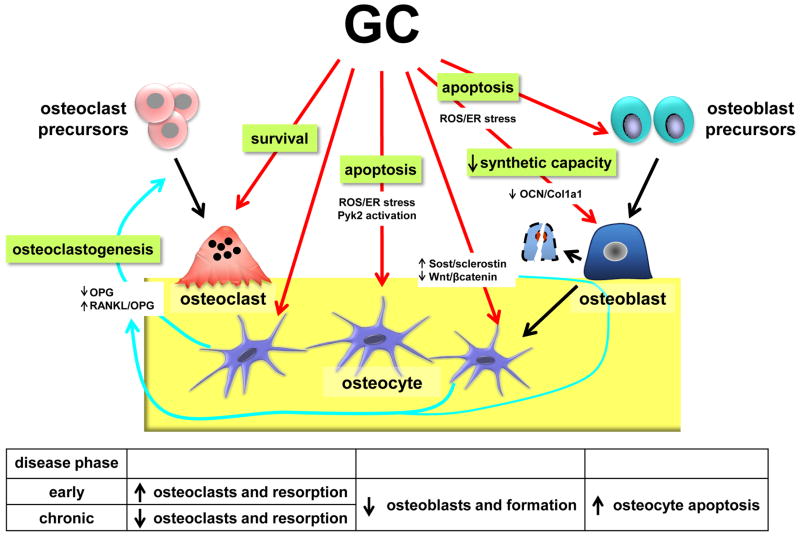
GC induce osteoporosis by increasing bone resorption, decreasing bone formation, and increasing apoptosis of osteocytes and osteoblasts, resulting in decreased bone strength causing elevated fracture risk in humans and animal models of GC excess [12,41,64–71] (Figure 2). The initial phase of GC-induced bone loss occurs rapidly with reports of BMD loss as early as 3–5 months after initiation of immunosuppressant therapy in patients [72–74] and with losses of 6–12% after the first year of GC treatment [75]. This early bone-loss phase is characterized by robust resorption activity; however, inhibition of osteoid production and osteoblast and osteocyte apoptosis also contribute to the rapid bone loss exhibited in this phase. The increased resorption is attributed to GC effects on both prolonging the lifespan of pre-existing osteoclasts as well as a transient, initial increase of osteoclastogenesis [64,76,77]. Bone formation is also readily suppressed by GC in short-term intervention models [78,79], which is attributed to the downregulation of osteocalcin (OCN) [80,81] and collagen 1 (Col1A1) [82,83] resulting in decreased production of osteoid. GC also increase the prevalence of apoptotic osteoblasts and osteocytes, which occurs as early as 10 days after GC initiation in mouse models and accumulates with continued administration [65,70,78,84].
Figure 2. Mechanisms of action of GC on bone cells.
GC induce an early increase in osteoclasts and a decrease in osteoblasts and osteoid production. The early increase in osteoclasts is due to prolongation of survival of preexisting osteoclasts and stimulation of osteoclastogenesis via increased RANKL/OPG ratio, through combined downregulation of OPG and upregulation of RANKL expression in osteoblasts and osteocytes. GC also induce apoptosis of osteoblasts and osteocytes through activation of the Pyk2 kinase, and accumulation of ROS and the resulting endoplasmic reticulum (ER) stress. GC inhibit the synthetic capacity of osteoblasts through suppression of OCN and Col 1a1 transcription. Osteocytic Sost/sclerostin expression is increased by GC, which reduces Wnt/β-catenin signaling in the bone microenvironment, with the consequent decreased in OPG expression in osteocytes and osteoblasts. Table summarizes GC effects at the tissue and cellular levels. The early phase of GC-induced bone disease is due to increased osteoclasts and bone resorption and decreased osteoblasts and bone formation. The late, chronic disease phase is associated with decreased osteoclasts and osteoblasts, leading to a low bone remodeling state. Increased osteocyte apoptosis in both phases of GC-induced bone disease contributes to decreased bone strength by disrupting the function of the osteocytic lacunar-canalicular network.
Long-term treatment of GC induces bones losses after the first year of up to 3–5% per continued year of GC therapy [75]. However, it should be noted that the bone changes in humans is confounded by the activity and etiology of the underlying disease for which exogenous GC are being used to treat. In contrast to the initial phase of GC-induced bone loss, the chronic phase is characterized by reductions in resorption activity and osteoclast number, which is attributed to the loss of receptor activator of NFKB ligand (RANKL)- expressing osteoblastic cells required for osteoclast development [70]. The reduced bone formation and apoptosis of osteoblasts and osteocytes exhibited in the early phase continues throughout long-term GC exposure, corresponding to the strong correlations between the cumulative GC dose and the percentage of BMD loss exhibited in patients [72]. Thus, imbalance between formation and resorption causing negative bone balance might be responsible for the continuous bone loss in chronic GC excess states.
A recent study in a murine model of GC excess examined the temporal sequence of pathogenic events leading to GC-induced osteonecrosis and provided evidence that the femoral head is more sensitive to the adverse effects of GC excess compared to other anatomic bone sites [85]. The femoral head of mice given GC exhibited decreased expression of the hypoxia-inducible factor (Hif-1α) and vascular endothelial growth factor (VEGF), a reduction in the number of osteoblasts, bone formation rate, and strength and an increase in number of osteoclasts, before changes were detected in either the femoral midshaft cortex or the cancellous bone of the distal femur. These molecular and cellular effects in the femoral head were accompanied by conversion of the normal vasculature to areas of edema as assessed by magnetic resonance imaging providing diagnostic evidence of osteonecrosis. These effects of GC preceded any detectable changes in bone density, cortical or cancellous bone architecture, bone volume in the cortical midshaft or distal cancellous bone, or accumulation of empty osteocyte lacunae (a late sign of osteocyte apoptosis). These findings strongly suggest that alterations in bone vascularity leading to osteonecrosis precede bone loss and deterioration of bone microarchitecture, and explain the vulnerability of the femoral head to collapse in GC excess.
A separate study showed that GC-induced osteonecrosis in a murine model as well as in human bone is associated with decreased remodeling of the osteocyte lacunar space and with decreased expression of metalloproteinase 13 (MMP13) by these cells [86]. Suppression of MMP13 expression occurs in parallel, but independently of GC-induced osteocyte apoptosis; and the changes in matrix hypermineralization characteristic of osteonecrosis occur before any detectable reduction in bone mass induced by GC. These findings suggest that suppression of osteocytic perilacunar remodeling is an early event associated with osteonecrosis induced by GC [86].
2. Direct effects of GC on cells of the osteoblastic lineage
Endogenous GC activity is regulated by two enzymes: 11β-HSD type 1 and type 2 (Figure 1C). 11β-HSD1 is a nicotinamide adenine dinucleotide phosphate (NADPH)-dependent reductase that converts inert 11-ketometabolites into biologically active GC; whereas 11β-HSD2 is a nicotinamide adenine dinucleotide (NAD+)-dependent dehydrogenase that converts active GC into inactive metabolites. Several transgenic overexpression mouse models have taken advantage of this enzymatic system to investigate the direct effects of GC on osteoblastic cells. By overexpressing 11β-HSD2 under the control of promoters active at different stages of differentiation of the lineage, GC action can be blocked in a cell specific manner. Blocking GC action by overexpressing 11β-HSD2 in osteoblast precursors, immature as well as mature osteoblasts, using the 3.6-kb or 2.3-kb fragments of the rat Col1a1 promoter, decreases bone mass accrual in growing mice, highlighting the importance of GC signaling for physiological bone development and growth [87–89]. Similarly, mice lacking the GR in cells expressing the osteoblast specific transcription factor Runt Related Transcription Factor 2 (Runx2) also exhibit reductions in cancellous bone accompanied by reduced expression of osteoblast-related genes (Runx2, Col1a1, and Bglap2) [83]. The impact of endogenous GC signaling on early osteoblastic linage cells decreases with age, as no differences in bone volume or cancellous bone parameters were found in skeletally mature 24-week old Col1a1-2.3kb-11β-HSD2 compared to control mice [90]. In addition, transgenic expression of 11β-HSD2 in mature osteoblasts and osteocytes under the control of the osteocalcin promoter does not negatively affect skeletal development or peak bone mass accrual [66]. Overall, the evidence indicates that GC signaling in early osteoblastic differentiation stages, but not in late osteoblastic or osteocytic stages, is required for optimal bone mass acquisition during bone growth.
In contrast, mice overexpressing 11β-HSD2 under the control of the murine osteocalcin gene 2 (OG2) promoter, which is active only in mature osteoblasts and osteocytes [91,92] were protected from GC-induced apoptosis of these cells [66]. Prevention of osteoblast/osteocyte apoptosis preserved cancellous osteoblast function and osteoid production, thus preventing the decrease in bone formation. Importantly, bone strength was preserved in the transgenic mice despite loss of bone mass, suggesting a potential effect of osteocyte viability in preserving bone strength. In addition, the initial rapid bone loss induced by GC was not prevented by blocking GC action in osteoblasts and osteocytes, strongly suggesting that the early phase of bone loss is due to GC action on osteoclasts [64] (see below Effects of GC excess on osteoclasts).
A major effect of GC on cells of the osteoblastic lineage is the inhibition of osteoblast differentiation and reduction of the synthetic activity of osteoblasts. This action is dependent upon the duration of the exposure and the dose of GC, and the stage of differentiation of the osteoblasts. Low GC doses or short durations of in vitro administration of embryonic or adult mesenchymal stem cells (MSC), as well as immature osteoblast progenitor cells, promote early osteoblastic differentiation with increases in the mRNA expression of osteoblastic genes [1,93]. In contrast, higher GC doses and longer exposure of cells committed to the osteoblastic lineage suppress differentiation and inhibit the expression of osteoblast markers [94,95]. At least part of the inhibitory effect of high dose GC on osteoblast maturation and matrix production is due to direct downregulation of osteoblastic genes, including collagen 1 and osteocalcin [80–83].
3. GC and osteoblast and osteocyte apoptosis: inside-out kinase signaling, reactive oxygen species (ROS), and endoplasmic reticulum (ER) stress
A hallmark of GC excess on cells of the osteoblastic lineage is the promotion of apoptosis [12,70] (Figure 2). The increase in the prevalence of osteoblast apoptosis partially explains the reduced osteoblast number and decreased bone formation induced by GC. Further, accumulation of apoptotic osteocytes contributes to osteoporosis of GC excess. As discussed earlier, GC induce apoptosis by direct actions on osteoblasts and osteocytes as in vivo blockade of GC signaling in these cells preserved viability [66]. Accordingly, the apoptotic effect of GC observed in vivo are readily reproduced in vitro in cultured osteoblasts and osteocytes and depend on the expression of the glucocorticoid receptor (GR) [96,97].
Binding of GC to the GR is followed by cis- or trans-interactions between the ligand-bound receptor with DNA and induction or repression of gene transcription [98,99]. However, GC also exert actions mediated by the GR independently of direct changes in gene transcription, including modulation of the activity of intracellular kinases such as the extracellular signal-regulated kinases (ERKs), the c-Jun N-terminal kinase (JNK) and the proline-rich tyrosine kinase 2 (Pyk2) [100–105]. Pyk2 also known as related adhesion focal tyrosine kinase (RAFTK), cellular adhesion kinase β (CAKβ), and calcium-dependent tyrosine kinase (CADTK) [106,107] is a member of the focal adhesion kinase (FAK) family of non-receptor tyrosine kinases. Although Pyk2 and FAK are highly homologous, they exhibit opposite effects on cell fate. Whereas FAK activation leads to cell spreading and survival, Pyk2 induces cell detachment and apoptosis [106,108]. In particular, the survival of osteoblasts and osteocytes and their interaction with the extracellular matrix are controlled by focal adhesions, sites at the plasma membrane in which integrins connect extracellular matrix proteins with intracellular structural and catalytic molecules [109–111]. Signaling mediated by integrins is bidirectional. Extracellular matrix proteins induce integrin engagement and activate intracellular signaling (referred to as outside-in signaling). Conversely, activation of intracellular signaling or changes in the composition of the focal adhesions regulate the interaction of integrins with extracellular matrix proteins (referred to as inside-out signaling) [112,113]. Whereas association of integrins with the extracellular matrix leads to survival, loss of this interaction causes detachment-induced apoptosis or anoikis [111]. For osteocytes, integrin engagement mediated by FAK, and potentiated by mechanical signals, maintains osteocyte survival [114]. Conversely, the pro-apoptotic effect of GC in osteocytes is preceded by cell detachment due to interference with FAK-mediated survival signaling generated by integrins [97]. GC oppose this integrin/FAK-dependent anti-apoptotic signaling by activating Pyk2, which in turn activates pro-apoptotic JNK signaling. This rapid kinase activation is followed by inside-out signaling that causes osteocyte detachment and leads to anoikis. Remarkably, although this action of GC is exerted via a receptor-mediated mechanism, it is independent of new gene transcription [97]. This evidence highlights the importance of alterations in rapid kinase signaling independent of nuclear actions of the GR and open new avenues for the design of GC analogs with the ability to activate transcription-mediated versus kinase-mediated actions of the GR.
Changes in FAK and Pyk2 kinase signaling induced by GC, combined with down-regulation of genes that prolong survival, such as interleukin-6, insulin growth factors, transforming growth factor β, collagenase type I, and integrin β1 [98,115–118], could result in the increase in osteocyte and osteoblast apoptosis observed in vivo.
GC also increase reactive oxygen species (ROS) production in bone in vivo and in osteoblasts in vitro [119], which could contribute to their in vivo effects. Endoplasmic reticulum (ER) stress is associated with increased ROS, resulting from accumulation of misfolded/unfolded proteins, and can trigger apoptosis. ER stress is alleviated by phosphorylation of eukaryotic translation initiation factor 2α (eIF2α), which slows the global rate of protein translation to provide time for the ER to recover from the excessive protein load, thus allowing the cell to escape from apoptosis [120,121]. Consistent with a role for ROS/ER stress, GC effects are prevented by the compounds, salubrinal and guanabenz [122], eIF2α dephosphorylation inhibitors that block ROS-induced ER stress [123,124]. Salubrinal and guanabenz prevented the pro-apoptotic effect of GC on osteoblasts and osteocytes in vitro as well as the decrease in differentiation induced by GC in osteoblastic cell cultures. Further, salubrinal prevented apoptosis of osteoblasts and osteocytes in vivo and blunted the decrease in bone mass and bone formation induced by GC. Salubrinal increased the number of alkaline phosphatase positive colonies in bone marrow cell cultures [125] and osteocalcin expression in osteoblastic MC3T3-E1 cells [126]. Conversely in vitro exposure to thapsigargin or tunicamycin induces elevated ER stress and increased apoptosis of osteoblasts and changes in osteoblast differentiation [127,128]. Increased ER stress appears to have a time-dependent biphasic effect inducing rapid increase in osteoblast markers Runx2 and osterix, followed by a reduction in the expression of these transcription factors as well as osteocalcin [127].
4. Effects of GC excess on osteoclasts
One of the features of GC-induced bone loss is a rapid, early increase in bone resorption, which is associated with increased osteoclasts on bone surfaces (Figure 2). The effect of GC on osteoclasts results from two different mechanisms: GC stimulate osteoclast generation and also prolong the life span of preexisting osteoclasts. GC increase the RANKL/osteoprotegerin (OPG) ratio in bone, mainly by downregulating the expression of OPG, a Wnt/β catenin target gene, resulting in increased osteoclast generation [129,130]. Consistent with this notion, activation of Wnt/β catenin signaling prevents osteoclast increase and halts bone resorption induced by GC [129]. In addition, GC promote survival of osteoclasts [76], and oppose the pro-apoptotic effects of bisphosphonates [64]. The effect of GC delaying osteoclast apoptosis explains why mice receiving GC exhibit increased number of osteoclasts on bone surfaces even when osteoclast progenitors in the bone marrow are reduced. GC exert an anti-apoptotic effect on osteoclasts by direct actions on osteoclasts demonstrated by the evidence that GC do not increase osteoclasts in mice expressing 11β-HSD2 specifically in these cells [76]. Consistent with a causative role of delayed osteoclast apoptosis in the early bone loss induced by GC, the loss of bone observed in WT mice was prevented in transgenic mice expressing 11β-HSD2 specifically in osteoclasts. Remarkably, these mice exhibit the expected decrease in osteoblast number and bone formation rate and increased osteoblast apoptosis [76]. Thus, GC act on cells of the osteoclastic and osteoblastic lineage independently and by different mechanisms.
In contrast to the early, acute increased osteoclast and decreased osteoblast activity, late effects of GC decrease both osteoclasts and osteoblasts [131]. Whereas osteoblast number is reduced and osteoblast and osteocyte apoptosis is increased at all stages of GC-induced bone disease, at the late stage inhibition of osteoclastogenesis prevails over delayed osteoclast apoptosis. The latter effect is attributed to a reduction in osteoblastic lineage cells that support osteoclastogenesis and leads to the low bone remodeling rate that characterizes chronic GC-induced bone disease.
5. GC and Wnt/β-catenin signaling
Wnt/β-catenin signaling and GC action lead to opposing effects on bone. The Wnt/β-catenin pathway has a critical role in the control of bone acquisition and maintenance. This pathway is activated by ligands of the Wnt family that bind to frizzled receptors and co-receptors, and also by downregulation of antagonists, including Dkk1 and Sost/sclerostin [132]. Human mutations responsible for high bone mass diseases, including gain-of-function of the LRP5 Wnt co-receptor, loss of expression of the Sost/sclerostin inhibitor in Van Buchem disease and sclerosteosis type 1, and loss-of-function of the sclerostin chaperone LRP4 in sclerosteosis type 2, demonstrate that activation of the Wnt/β-catenin pathway is linked to increased bone formation and bone gain [133–135]. In addition however, genetic manipulation of β-catenin, the mediator of the canonical Wnt pathway, affects bone resorption. This is due to up-regulation of OPG, the decoy receptor for RANKL [136,137]. Therefore, the Wnt/β-catenin signaling cascade regulates bone mass by both bone anabolic and anti-catabolic mechanisms. Further, activation of Wnt/β-catenin signaling promotes osteoblast and osteocyte survival [138]. This collective evidence demonstrates that activation of the Wnt/β-catenin signaling pathway and GC action lead to opposing cellular and tissue level effects on bone. Based on this findings, we recently investigated the effects of GC excess in mice exhibiting activated Wnt/β-catenin signaling by virtue of genetic deletion of the Wnt antagonist Sost/sclerostin (Sost−/− mice) [129]. Sost−/− mice were protected from the decrease in bone mass, deterioration in microarchitecture, and reduced structural and material strength induced by GC. Although the high bone mass exhibited by Sost−/− mice is due to increased bone formation with unchanged resorption, protection from bone loss in Sost−/− mice was due to prevention of GC-induced bone resorption and not to restoration of bone formation. In WT mice, GC increased the expression of Sost and the number of sclerostin positive osteocytes. GC also altered the molecular signature of the Wnt/β-catenin pathway by decreasing expression of genes associated with both anti-catabolism, including OPG, as well as anabolism/survival. In contrast, GC did not decrease OPG or other anti-catabolic markers in Sost−/− mice, but did reduce genes associated with anabolism and survival. Thus, in the context of GC excess, activation of Wnt/β-catenin signaling induced by Sost/sclerostin deficiency sustained bone integrity by opposing bone catabolism despite markedly reduced bone formation and increased apoptosis. These results indicate that the Wnt/β-catenin pathway, which is predominantly anabolic for bone, is switched to anti-catabolic in the frame of GC excess.
These findings suggest that therapeutic interventions activating Wnt/β-catenin signaling could halt the high bone resorption responsible for the early rapid bone loss induced by GC, which in humans ranges 6–12% during the first year of treatment [12]. Consistent with this notion, inhibition of sclerostin with a neutralizing antibody in growing mice opposed the lack of bone gain and the loss of strength induced by GC [139,140]. It was proposed that these effects were due to preservation of osteoblast activity [140]. However, compared to control mice treated with GC alone, mice treated with GC and the anti-sclerostin antibody exhibited lower circulating tartrate-resistant acid phosphatase (TRAP5b), a marker of osteoclast number, [139] and CTX-1 [140], but still markedly reduced bone formation markers osteocalcin and amino-terminal propeptide (PINP) [139]. Similarly, GC are unable to decrease OPG and increase the RANKL/OPG ratio ex vivo in bones from Sost−/− mice or from WT mice treated with an anti-sclerostin antibody [129]. These findings demonstrate that Sost/sclerostin deficiency, either genetically or pharmacologically achieved, maintains bone mass and strength in conditions of GC excess by inhibiting bone resorption through sustained anti-catabolic signaling driven by OPG.
This crosstalk between GC and Wnt/β-catenin signaling could be exploited therapeutically not only to halt bone resorption and bone loss induced by GC, but also to inhibit the exaggerated bone formation and bone mass in diseases due to hyperactivation of Wnt/β-catenin signaling. Indeed, it has been shown that GC stopped the bone gain and reduced high P1NP in a patient with Van Buchem disease, a genetic disease that results from lack of sclerostin expression and in which continuous bone anabolism causes life-threatening increased intracranial pressure [141]. Prior to GC intervention, the patient exhibited annual BMD gains of 4 to 9% in the lumbar spine and of 4 to 24% in the hip. Treatment with prednisone blunted the anabolic effect of Sost deficiency as evidenced by no gain in BMD over two years (−0.7% in lumbar spine and 0.4% in the hip). The demonstration that bone formation and Wnt/β-catenin anabolic signaling is decreased in Sost/sclerostin deficient mice treated with GC [129] provides a mechanistic explanation for these clinical findings. Thus, GC oppose the effects of Sost/sclerostin deficiency on bone formation in both humans and mice [129,141].
Inhibition of resorption with bisphosphonates is current standard of care for GC-induced osteoporosis [12,142], as these drugs protect from the loss of bone mass in animal models and patients. However, bone formation is decreased even further by bisphosphonates compared to GC alone [49,78,122,143]. In patients receiving GC, treatment with anti-RANKL antibody induced more pronounced reductions in bone formation compared to bisphosphonate [144]. Profound reduction in bone turnover is undesirable since it increases the potential for developing osteonecrosis of the jaw and atypical femoral fracture [145–148]. In contrast to bisphosphonates, Sost deficiency confers high bone formation, and Sost deficient mice treated with GC exhibit bone formation levels comparable to WT mice treated with placebo [129]. Even when the reduction in P1NP induced by GC in Sost−/− mice is more severe than in WT mice, the reduced P1NP is similar to that of placebo-treated WT mice. Similarly, the reduced bone formation exhibited by Sost−/− mice treated with GC is equivalent to bone formation in placebo-treated WT mice. Therefore, Sost/sclerostin deficiency maintains tissue-level toughness by preserving modest amounts of bone formation while preventing GC-induced increases in resorption. This finding points to a potential benefit of neutralizing sclerostin strategies compared to the current therapeutic approach to treat GC-induced bone fragility.
In summary, the deleterious effects of GC on the skeleton are linked to increased expression of the osteocyte-derived Wnt/β-catenin antagonist Sost/sclerostin and to downregulation of Wnt/β-catenin target genes; and Sost/sclerostin deficiency prevents GC-induced osteoporosis by anti-catabolic, not anabolic, actions.
3. GLUCOCORTICOID EXCESS IN MUSCLE AND ATROPHY-RELATED GENES IN BONE AND MUSCLE
1. Effects of GC excess in muscle
GC induce loss of skeletal muscle mass and strength particularly at the hip and shoulder girdle, which in turn increase the risk of falls. These effects on muscle are of rapid onset and detected as early as 7 days after initiation of GC administration in humans [149,150]. GC reduce sarcolemma excitability, decrease serum levels of creatine kinase and myoglobin, decrease cross sectional area of type 1, 2A and 2B myofibers, and reduce the specific force (strength) of muscle fibers. Muscle atrophy induced by GC is accompanied by suppression of protein synthesis with simultaneous increase in protein catabolism, leading to reduced myotube diameter [61,151]. The formation of new myotubes is also impaired, as GC inhibit myogenesis by down-regulating myogenin gene expression. GC-induced protein catabolism in skeletal muscle is associated with enhanced Forkhead Box O (FoxO)-dependent transcription of members of the protein degradation machinery, including the ubiquitin-proteasome system of E3 ubiquitin ligases, the lysosomal system of cathepsins, and the calcium-dependent system of calpains [61,152]. E3 ubiquitin ligases muscle atrophy F-box (MAFbx, also known as atrogin1) and muscle RING finger 1 (MuRF1, also known as TRIM63) are known regulators of GC-induced muscle atrophy both in vivo and in vitro, as well as a number of other sarcopenia-inducing conditions including denervation, immobilization, disuse, diabetes, and renal failure [153–155]. Mice lacking MuRF1 are partially protected from muscle loss induced by GC, and muscle-specific deletion of the GR prevents the increased atrophy gene expression and muscle loss induced by GC [156–158]. The ubiquitin ligase MUSA1 (muscle ubiquitin ligase of the SCF complex in atrophy-1, also known as Fbxo30) has also been associated with increased protein catabolism and reductions in total protein content in muscle models of denervation injury/disuse [159].
2. Regulation of atrophy related genes in muscle and bone
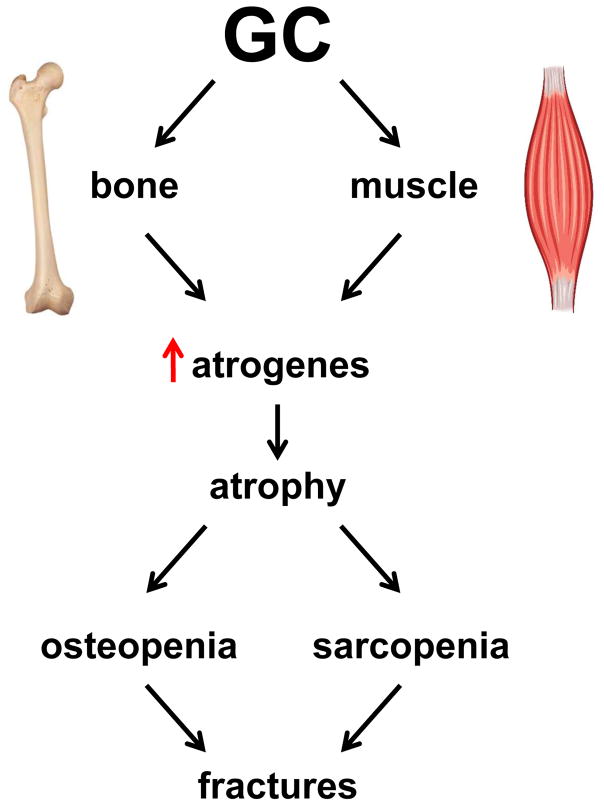
A recent study in a mouse model of GC excess provided information about the mechanisms by which GC upregulate the expression of atrophy-related genes in muscle and demonstrated that GC also increase atrophy-related genes in bone [79]. GC increase the expression in bone in vivo and in osteoblasts and osteocytes in vitro of the traditionally considered muscle-specific E3 ligases atrogin1, MuRF1, and MUSA1 (Figure 3). GC also increase the expression of Notch ligands, receptors, and target genes in muscle, but not in bone. GC-induced expression of atrophy-related genes and muscle cell atrophy were prevented by pharmacologic inhibition of the Notch pathway. These findings demonstrate that GC-induced loss of bone and muscle mass are accompanied by increased expression of atrophy-related genes, although the upstream mechanisms are tissue-specific. Further, they identify the Notch signaling pathway as a potential therapeutic intervention to prevent skeletal muscle atrophy and weakness induced by GC, and provide the mechanistic basis for combining therapies that target each tissue to treat GC-induced osteopenia and sarcopenia.
Figure 3. Regulation of atrophy-related genes in muscle and bone by GC.
GC activate distinct pathways in muscle and bone that converge in the upregulation of atrophy-related E3 ubiquitin ligase genes or atrogens (atrogin1, MuRF1, and MUSA1); these in turn lead to atrophy and loss of mass in both tissues (sarcopenia and osteopenia). The combined adverse effects of GC in bone and muscle contribute to GC-induced myopathy and the increased occurrence of bone fractures.
This evidence notwithstanding, the relevance of atrophy gene expression for the action of GC in bone remains unclear. Earlier studies have shown that genetic global deletion in mice of MuRF1 protects from the loss of bone induced by hind limb unloading [160]. MuRF1 KO mice were also protected from the decrease in bone formation and the increase in osteoclasts [160]. This evidence, together with the findings in our study [79], raises the possibility that upregulation of MuRF1, and possibly other atrophy-related genes, contributes to the reduced bone formation and increased bone resorption induced by GC, and suggests that targeting the atrophy pathway may block GC action in bone.
The decrease in muscle mass, quantified by lean body mass, mass of individual muscles rich in fast-twitch fibers like the extensor digitorium longus (EDL) induced by GC in our murine model, is detected as early as after 14 days of GC administration [79]. Importantly, muscles from GC-treated mice exhibited decreased strength (force), quantified ex vivo by contractility tests of individual muscles, and in vivo, by measuring isometric plantarflexion torque generated by muscles of the posterior compartment of the leg. Remarkably, we detected decreased strength (specific force) in the slow-twitch soleus muscle in the absence of reduction of soleus mass, suggesting that GC might alter muscle function in the absence of detectable tissue loss, at least in muscles traditionally considered resistant to GC [161,162]. In contrast, the reduction in strength in the EDL muscle can be explained by loss of muscle mass, because it is corrected by normalizing for tissue mass. The lower rate of fatigue detected in the fast-twitch EDL muscles treated with GC compared to placebo-treated muscles may be explained by the fact that EDL muscles are mainly composed of the highly fatigable myosin-rich type 2 fibers, which are preferential targets of GC-induced muscle atrophy [163,164]. Thus, GC might induce loss of highly fatigable fibers and the remaining fibers fatigue more slowly in EDL muscles. In contrast, slow-twitch soleus muscles fatigue at the same rate in GC- and placebo-treated mice despite reductions in overall strength. This effect may in part explain the proximal myopathy seen in patients with Cushing syndrome. The current findings with the C57BL/6 mouse model of GC excess are consistent with the previously reported reduction in muscle fiber specific force in humans treated with GC [150]. Importantly, the reduction in muscle mass correlated with increased expression of the atrophy genes. Future studies are warranted to establish the role of E3 ubiquitin ligases in muscle weakness induced by GC.
GC decrease C2C12 myotube diameter by 20%, comparable with earlier in vitro and in vivo studies [150,157,158,162,165], as well as studies with starvation [162,166] and denervation [158,166]. Decreases of 10–17% myotube diameter translates into reductions of 25–35% in strength (specific force) with GC administration in humans, suggesting that modest reductions in myotube diameter result in a notable impairment of muscle function [150].
Activation of the Notch signaling pathway is required for expansion of a satellite cell population, a known critical event for skeletal muscle repair [167,168]. Notch signaling also inhibits myogenic differentiation of progenitor cells by decreasing myoblast determination protein 1 (MyoD) and myogenin expression and by reducing MyoD activity [167,169,170]. These findings support the notion that Notch signaling is crucial for maintaining the self-renewal capacity of muscle satellite cells. The recent findings showing that the expression of components of the Notch pathway was upregulated by GC in skeletal muscle reveal a novel role of this pathway. [79]. Moreover, the fact that inhibition of Notch signaling with the gamma secretase inhibitor, GSI XX, blocked the upregulation of atrophy-related genes and prevented the reduction in C2C12 myotube diameter induced by GC, suggest a potential therapeutic benefit of blocking Notch signaling to maintain muscle strength.
In contrast to the effects on muscle, GC did not increase the expression of components of the Notch pathway in vivo, ex vivo, or in vitro models of bone and osteoblastic/osteocytic cells [79]. This finding is consistent with previous studies demonstrating that the expression Dll1 and Jag1 Notch ligands was not altered by GC administered to osteoblastic cells [171]. Thus, GC activate Notch signaling in muscle, but not in bone, to induce atrophy. Although the mechanism underlying the upregulation of atrophy genes by GC in bone remains unknown, it is possible that Forkhead box O (FoxO) transcription factors are involved. In muscle, FoxO family members (FoxO1, 3, and 4) regulate atrogen-related gene expression and are required for the full atrophic response by several inducers of skeletal muscle wasting, including starvation, denervation, and chronic kidney disease [165,166,172]. In bone, FoxOs are activated by and are critical for the defense against ROS [173]. In addition, at least part of the effects of GC in bone are due to accumulation of ROS as well as to ER stress, and are abolished by ROS or ER stress inhibitors [122]. Activation of FoxO in osteoblasts by GC is blocked by ROS inhibition [119]. FoxO-mediated transcription is favored over Wnt/β-catenin transcription [119], and activation of Wnt/β-catenin signaling protects from GC-induced osteoporosis [129]. Future studies will be required to reveal the role of FoxOs in the upregulation of atrophy-related genes by GC in bone.
4. CONCLUSIONS AND FUTURE STUDIES
Research in the last two decades have enormously increased our understanding of the mechanisms of action of GC excess in the musculoskeletal system. It is now clear that GC act directly on individual bone cell types (osteoclasts, osteoblasts and osteocytes) (Figure 2), and that they also affect muscle cells. GC stimulate the production of osteoclasts and delay apoptosis, thus increasing number and resorption activity, primarily in the early phase of GC bone disease. GC induce the premature death of osteoblasts and markedly decrease their matrix synthesizing activity, resulting in marked decreased bone formation rate throughout GC treatment. GC increase apoptosis of osteocytes, contributing to deterioration of the osteocytic canalicular network, which is crucial for the regulation of bone metabolism and function. GC increase the expression of the osteocyte-derived bone formation antagonist Sost/sclerostin, which in turn decreases Wnt/β-catenin signaling and reduces OPG expression contributing to the increase in bone resorption. GC induce in both muscle and bone the expression of atrophy-related genes (Figure 3), which are involved in muscle and bone atrophy. GC are important therapeutic agents for many chronic diseases, but they produce serious adverse effects on the musculoskeletal system. Future research is urgently required to develop therapeutic interventions that simultaneously prevent the loss of bone and muscle mass and preserve the functionality of musculoskeletal tissues. In addition, clinical studies on early osteonecrosis and cell biology studies to clarify the mechanisms that lead to osteonecrosis are needed to develop preventative and curative treatment. Further, studies to clarify differences between myopathy and muscle atrophy and the development of therapeutic interventions for prevention and treating myopathy induced by GC are warranted.
Acknowledgments
Funding: This research was supported by the National Institutes of Health (grants AR059357, AT008754, AT008754-O2S1, and CA209882 to TB) and the United States Department of Veterans Affairs (I01BX002104-01 to TB). AYS was partially supported by T32-AR065971.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: Authors declare no conflict of interest
Research Involving Human Participants: This article does not contain any studies with human participants performed by any of the authors.
Research Involving Animals: This article does not contain any studies with animals performed by any of the authors.
Informed Consent: Not applicable.
References
- 1.Plotz CM, Knowlton AI, Ragan C. The natural history of Cushing’s syndrome. Am J Med. 1952;13:597–614. doi: 10.1016/0002-9343(52)90027-2. [DOI] [PubMed] [Google Scholar]
- 2.Soffer LJ, Iannacconone A, Gabrilove JL. Cushing’s syndrome. American Journal of Medicine. 1961;30:129–146. [Google Scholar]
- 3.Ross EJ, Linch DC. Cushing’s syndrome--killing disease: discriminatory value of signs and symptoms aiding early diagnosis. Lancet. 1982;2:646–649. doi: 10.1016/s0140-6736(82)92749-0. [DOI] [PubMed] [Google Scholar]
- 4.Littooij AS, Kwee TC, Enriquez G, et al. Whole-body MRI reveals high incidence of osteonecrosis in children treated for Hodgkin lymphoma. Br J Haematol. 2017;176:637–642. doi: 10.1111/bjh.14452. [DOI] [PubMed] [Google Scholar]
- 5.Aljebab F, Choonara I, Conroy S. Systematic Review of the Toxicity of Long-Course Oral Corticosteroids in Children. PLoS ONE. 2017;12:e0170259. doi: 10.1371/journal.pone.0170259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gudbjornsson B, Juliusson UI, Gudjonsson FV. Prevalence of long term steroid treatment and the frequency of decision making to prevent steroid induced osteoporosis in daily clinical practice. Ann Rheum Dis. 2002;61:32–36. doi: 10.1136/ard.61.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Overman RA, Gourlay ML, Deal CL, et al. Fracture rate associated with quality metric-based anti-osteoporosis treatment in glucocorticoid-induced osteoporosis. Osteoporos Int. 2015;26:1515–1524. doi: 10.1007/s00198-014-3022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canalis E, Mazziotti G, Giustina A, et al. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319–1328. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 9.Batchelor TT, Taylor LP, Thaler HT, et al. Steroid myopathy in cancer patients. Neurology. 1997;48:1234–1238. doi: 10.1212/wnl.48.5.1234. [DOI] [PubMed] [Google Scholar]
- 10.Bowyer SL, LaMothe MP, Hollister JR. Steroid myopathy: incidence and detection in a population with asthma. J Allergy Clin Immunol. 1985;76:234–242. doi: 10.1016/0091-6749(85)90708-0. [DOI] [PubMed] [Google Scholar]
- 11.Van Staa TP, Laan RF, Barton IP, et al. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum. 2003;48:3224–3229. doi: 10.1002/art.11283. [DOI] [PubMed] [Google Scholar]
- 12.Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62–70. doi: 10.1056/NEJMcp1012926. [DOI] [PubMed] [Google Scholar]
- 13.Chapman K, Holmes M, Seckl J. 11beta-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev. 2013;93:1139–1206. doi: 10.1152/physrev.00020.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charmandari E, Kino T, Chrousos GP. Primary generalized familial and sporadic glucocorticoid resistance (Chrousos syndrome) and hypersensitivity. Endocr Dev. 2013;24:67–85. doi: 10.1159/000342505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomlinson JW, Draper N, Mackie J, et al. Absence of Cushingoid phenotype in a patient with Cushing’s disease due to defective cortisone to cortisol conversion. J Clin Endocrinol Metab. 2002;87:57–62. doi: 10.1210/jcem.87.1.8189. [DOI] [PubMed] [Google Scholar]
- 16.Arai H, Kobayashi N, Nakatsuru Y, et al. A case of cortisol producing adrenal adenoma without phenotype of Cushing’s syndrome due to impaired 11beta-hydroxysteroid dehydrogenase 1 activity. Endocr J. 2008;55:709–715. doi: 10.1507/endocrj.k08e-008. [DOI] [PubMed] [Google Scholar]
- 17.Draper N, Walker EA, Bujalska IJ, et al. Mutations in the genes encoding 11beta-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase interact to cause cortisone reductase deficiency. Nat Genet. 2003;34:434–439. doi: 10.1038/ng1214. [DOI] [PubMed] [Google Scholar]
- 18.Lavery GG, Idkowiak J, Sherlock M, et al. Novel H6PDH mutations in two girls with premature adrenarche: ‘apparent’ and ‘true’ CRD can be differentiated by urinary steroid profiling. Eur J Endocrinol. 2013;168:K19–K26. doi: 10.1530/EJE-12-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan SA, Hassan-Smith ZK, Lavery GG. Mechanisms in endocrinology: Tissue-specific activation of cortisol in Cushing’s syndrome. Eur J Endocrinol. 2016;175:R83–R89. doi: 10.1530/EJE-15-1237. [DOI] [PubMed] [Google Scholar]
- 20.Swartz SL, Dluhy RG. Corticosteroids: clinical pharmacology and therapeutic use. Drugs. 1978;16:238–255. doi: 10.2165/00003495-197816030-00006. [DOI] [PubMed] [Google Scholar]
- 21.Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: A systematic review and meta-analysis. Arch Intern Med. 1999;159:941–955. doi: 10.1001/archinte.159.9.941. [DOI] [PubMed] [Google Scholar]
- 22.Crilly RG, Marshall DH, Nordin BE. Metabolic effects of corticosteroid therapy in post-menopausal women. J Steroid Biochem. 1979;11:429–433. doi: 10.1016/0022-4731(79)90063-3. [DOI] [PubMed] [Google Scholar]
- 23.Lukert BP, Johnson BE, Robinson RG. Estrogen and progesterone replacement therapy reduces glucocorticoid-induced bone loss. J Bone Min Res. 1992;7:1063–1069. doi: 10.1002/jbmr.5650070909. [DOI] [PubMed] [Google Scholar]
- 24.Crilly RG, Marshall DH, Horsman A, Nordin BEC, Peacock M. Corticosteroid Osteoporosis. In: Dixon ASJ, Russell RGG, Stamp TCB, editors. Osteoporosis, A Multi-Disciplinary Problem. Academic Press Inc and Royal Society of Medicine; London: 1983. pp. 153–159. [Google Scholar]
- 25.Oikarinen A, Haapasaari KM, Sutinen M, et al. The molecular basis of glucocorticoid-induced skin atrophy: topical glucocorticoid apparently decreases both collagen synthesis and the corresponding collagen mRNA level in human skin in vivo. Br J Dermatol. 1998;139:1106–1110. doi: 10.1046/j.1365-2133.1998.02646.x. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz AV. Diabetes Mellitus: Does it Affect Bone? Calcif. Tissue Int. 2003;73:515–519. doi: 10.1007/s00223-003-0023-7. [DOI] [PubMed] [Google Scholar]
- 27.Cooper MS. Glucocorticoids in bone and joint disease: the good, the bad and the uncertain. Clin Med (Lond) 2012;12:261–265. doi: 10.7861/clinmedicine.12-3-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi L, Sanchez-Guijo A, Hartmann MF, et al. Higher glucocorticoid secretion in the physiological range is associated with lower bone strength at the proximal radius in healthy children: importance of protein intake adjustment. J Bone Miner Res. 2015;30:240–248. doi: 10.1002/jbmr.2347. [DOI] [PubMed] [Google Scholar]
- 29.Cooper MS, Rabbitt EH, Goddard PE, et al. Osteoblastic 11beta-hydroxysteroid dehydrogenase type 1 activity increases with age and glucocorticoid exposure. J Bone Miner Res. 2002;17:979–986. doi: 10.1359/jbmr.2002.17.6.979. [DOI] [PubMed] [Google Scholar]
- 30.Cooper MS, Syddall HE, Fall CH, et al. Circulating cortisone levels are associated with biochemical markers of bone formation and lumbar spine BMD: the Hertfordshire Cohort Study. Clin Endocrinol (Oxf) 2005;62:692–697. doi: 10.1111/j.1365-2265.2005.02281.x. [DOI] [PubMed] [Google Scholar]
- 31.van Schoor NM, Dennison E, Lips P, et al. Serum fasting cortisol in relation to bone, and the role of genetic variations in the glucocorticoid receptor. Clin Endocrinol (Oxf) 2007;67:871–878. doi: 10.1111/j.1365-2265.2007.02978.x. [DOI] [PubMed] [Google Scholar]
- 32.Suman OE, Spies RJ, Celis MM, et al. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol (1985 ) 2001;91:1168–1175. doi: 10.1152/jappl.2001.91.3.1168. [DOI] [PubMed] [Google Scholar]
- 33.Przkora R, Herndon DN, Sherrard DJ, et al. Pamidronate preserves bone mass for at least 2 years following acute administration for pediatric burn injury. Bone. 2007;41:297–302. doi: 10.1016/j.bone.2007.04.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norbury WB, Herndon DN, Branski LK, et al. Urinary cortisol and catecholamine excretion after burn injury in children. J Clin Endocrinol Metab. 2008;93:1270–1275. doi: 10.1210/jc.2006-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Besemer F, Pereira AM, Smit JW. Alcohol-induced Cushing syndrome. Hypercortisolism caused by alcohol abuse. Neth J Med. 2011;69:318–323. [PubMed] [Google Scholar]
- 36.Van Staa TP, Leufkens HG, Cooper C. Use of inhaled corticosteroids and risk of fractures. J Bone Miner Res. 2001;16:581–588. doi: 10.1359/jbmr.2001.16.3.581. [DOI] [PubMed] [Google Scholar]
- 37.Van Staa TP, Abenhaim L, Cooper C, et al. Public health impact of adverse bone effects of oral corticosteroids. Br J Clin Pharmacol. 2001;51:601–607. doi: 10.1046/j.0306-5251.2001.1385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Staa TP, Leufkens HGM, Abenhaim L, et al. Use of oral corticoisteroids and risk of fractures. J Bone Min Res. 2000;15:993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 39.Walsh LJ, Wong CA, Oborne J, et al. Adverse effects of oral corticosteroids in relation to dose in patients with lung disease. Thorax. 2001;56:279–284. doi: 10.1136/thorax.56.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hubbard RB, Smith CJ, Smeeth L, et al. Inhaled corticosteroids and hip fracture: a population-based case-control study. Am J Respir Crit Care Med. 2002;166:1563–1566. doi: 10.1164/rccm.200206-606OC. [DOI] [PubMed] [Google Scholar]
- 41.Van Staa TP. The pathogenesis, epidemiology and management of glucocorticoid-induced osteoporosis. Calcif Tissue Int. 2006;79:129–137. doi: 10.1007/s00223-006-0019-1. [DOI] [PubMed] [Google Scholar]
- 42.Luengo M, Picado C, Del Rio L, et al. Vertebral fractures in steroid dependent asthma and involutional osteoporosis: a comparative study. Thorax. 1991;46:803–806. doi: 10.1136/thx.46.11.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selby PL, Halsey JP, Adams KR, et al. Corticosteroids do not alter the threshold for vertebral fracture. J Bone Min Res. 2000;15:952–956. doi: 10.1359/jbmr.2000.15.5.952. [DOI] [PubMed] [Google Scholar]
- 44.Yang S, Shen X. Association and relative importance of multiple obesity measures with bone mineral density: the National Health and Nutrition Examination Survey 2005–2006. Arch Osteoporos. 2015;10:14. doi: 10.1007/s11657-015-0219-2. [DOI] [PubMed] [Google Scholar]
- 45.Zhu K, Hunter M, James A, et al. Associations between body mass index, lean and fat body mass and bone mineral density in middle-aged Australians: The Busselton Healthy Ageing Study. Bone. 2015;74:146–152. doi: 10.1016/j.bone.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 46.Cosman F, Nieves J, Herbert J, et al. High-dose glucocorticoids in multiple sclerosis patients exert direct effects on the kidney and skeleton. J Bone Miner Res. 1994;9:1097–1105. doi: 10.1002/jbmr.5650090718. [DOI] [PubMed] [Google Scholar]
- 47.Dovio A, Perazzolo L, Osella G, et al. Immediate fall of bone formation and transient increase of bone resorption in the course of high-dose, short-term glucocorticoid therapy in young patients with multiple sclerosis. J Clin Endocrinol Metab. 2004;89:4923–4928. doi: 10.1210/jc.2004-0164. [DOI] [PubMed] [Google Scholar]
- 48.Aaron JE, Francis RM, Peacock M, et al. Contrasting microanatomy of idiopathic and corticosteroid-induced osteoporosis. Clin Orthop Relat Res. 1989:294–305. [PubMed] [Google Scholar]
- 49.Saag KG, Shane E, Boonen S, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357:2028–2039. doi: 10.1056/NEJMoa071408. [DOI] [PubMed] [Google Scholar]
- 50.Cooper C, Steinbuch M, Stevenson R, et al. The epidemiology of osteonecrosis: findings from the GPRD and THIN databases in the UK. Osteoporos Int. 2010;21:569–577. doi: 10.1007/s00198-009-1003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikeuchi K, Hasegawa Y, Seki T, et al. Epidemiology of nontraumatic osteonecrosis of the femoral head in Japan. Mod Rheumatol. 2015;25:278–281. doi: 10.3109/14397595.2014.932038. [DOI] [PubMed] [Google Scholar]
- 52.Kubo T, Ueshima K, Saito M, et al. Clinical and basic research on steroid-induced osteonecrosis of the femoral head in Japan. J Orthop Sci. 2016;21:407–413. doi: 10.1016/j.jos.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 53.Assouline-Dayan Y, Chang C, Greenspan A, et al. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum. 2002;32:94–124. [PubMed] [Google Scholar]
- 54.Weinstein RS. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol Metab Clin North Am. 2012;41:595–611. doi: 10.1016/j.ecl.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphey MD, Foreman KL, Klassen-Fischer MK, et al. From the radiologic pathology archives imaging of osteonecrosis: radiologic-pathologic correlation. Radiographics. 2014;34:1003–1028. doi: 10.1148/rg.344140019. [DOI] [PubMed] [Google Scholar]
- 56.Dilisio MF. Osteonecrosis following short-term, low-dose oral corticosteroids: a population-based study of 24 million patients. Orthopedics. 2014;37:e631–e636. doi: 10.3928/01477447-20140626-54. [DOI] [PubMed] [Google Scholar]
- 57.Chen CH, Chang JK, Lai KA, et al. Alendronate in the prevention of collapse of the femoral head in nontraumatic osteonecrosis: a two-year multicenter, prospective, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2012;64:1572–1578. doi: 10.1002/art.33498. [DOI] [PubMed] [Google Scholar]
- 58.Khaleeli AA, Edwards RH, Gohil K, et al. Corticosteroid myopathy: a clinical and pathological study. Clin Endocrinol (Oxf) 1983;18:155–166. doi: 10.1111/j.1365-2265.1983.tb03198.x. [DOI] [PubMed] [Google Scholar]
- 59.Gupta A, Gupta Y. Glucocorticoid-induced myopathy: Pathophysiology, diagnosis, and treatment. Indian J Endocrinol Metab. 2013;17:913–916. doi: 10.4103/2230-8210.117215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Janssens S, Decramer M. Corticosteroid-induced myopathy and the respiratory muscles. Report of two cases. Chest. 1989;95:1160–1162. doi: 10.1378/chest.95.5.1160. [DOI] [PubMed] [Google Scholar]
- 61.Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol. 2008;197:1–10. doi: 10.1677/JOE-07-0606. [DOI] [PubMed] [Google Scholar]
- 62.Hasselgren PO, Alamdari N, Aversa Z, et al. Corticosteroids and muscle wasting: role of transcription factors, nuclear cofactors, and hyperacetylation. Curr Opin Clin Nutr Metab Care. 2010;13:423–428. doi: 10.1097/MCO.0b013e32833a5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morgan SA, Hassan-Smith ZK, Doig CL, et al. Glucocorticoids and 11beta-HSD1 are major regulators of intramyocellular protein metabolism. J Endocrinol. 2016;229:277–286. doi: 10.1530/JOE-16-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weinstein RS, Chen JR, Powers CC, et al. Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J Clin Invest. 2002;109:1041–1048. doi: 10.1172/JCI14538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinstein RS, Nicholas RW, Manolagas SC. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab. 2000;85:2907–2912. doi: 10.1210/jcem.85.8.6714. [DOI] [PubMed] [Google Scholar]
- 66.O’Brien CA, Jia D, Plotkin LI, et al. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145:1835–1841. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- 67.Reid IR. Glucocorticoid osteoporosis--mechanisms and management. Eur J Endocrinol. 1997;137:209–217. doi: 10.1530/eje.0.1370209. [DOI] [PubMed] [Google Scholar]
- 68.Plotkin LI, Weinstein RS, Parfitt AM, et al. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104:1363–1374. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Newell-Price J, Bertagna X, Grossman AB, et al. Cushing’s syndrome. Lancet. 2006;367:1605–1617. doi: 10.1016/S0140-6736(06)68699-6. [DOI] [PubMed] [Google Scholar]
- 70.Weinstein RS. Glucocorticoid-induced osteoporosis. Rev Endocr Metab Disord. 2001;2:65–73. doi: 10.1023/a:1010007108155. [DOI] [PubMed] [Google Scholar]
- 71.Mazziotti G, Angeli A, Bilezikian JP, et al. Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab. 2006;17:144–149. doi: 10.1016/j.tem.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 72.Van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777–787. doi: 10.1007/s001980200108. [DOI] [PubMed] [Google Scholar]
- 73.Laan RFJM, Van Riel PLCM, Van de Putte LBA, et al. Low-dose prednisone induces rapid reversible axial bone loss in patients with rheumatoid arthritis: A randomized, controlled study. Ann Intern Med. 1993;119:963–968. doi: 10.7326/0003-4819-119-10-199311150-00001. [DOI] [PubMed] [Google Scholar]
- 74.Devogelaer JP, Adler RA, Recknor C, et al. Baseline glucocorticoid dose and bone mineral density response with teriparatide or alendronate therapy in patients with glucocorticoid-induced osteoporosis. J Rheumatol. 2010;37:141–148. doi: 10.3899/jrheum.090411. [DOI] [PubMed] [Google Scholar]
- 75.LoCascio V, Bonucci E, Imbimbo B, et al. Bone loss in response to long-term glucocorticoid therapy. Bone Miner. 1990;8:39–51. doi: 10.1016/0169-6009(91)90139-q. [DOI] [PubMed] [Google Scholar]
- 76.Jia D, O’Brien CA, Stewart SA, et al. Glucocorticoids act directly on osteoclasts to increase their lifespan and reduce bone density. Endocrinology. 2006;147:5592–5599. doi: 10.1210/en.2006-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hofbauer LC, Zeitz U, Schoppet M, et al. Prevention of glucocorticoid-induced bone loss in mice by inhibition of RANKL. Arthritis Rheum. 2009;60:1427–1437. doi: 10.1002/art.24445. [DOI] [PubMed] [Google Scholar]
- 78.Plotkin LI, Bivi N, Bellido T. A bisphosphonate that does not affect osteoclasts prevents osteoblast and osteocyte apoptosis and the loss of bone strength induced by glucocorticoids in mice. Bone. 2011;49:122–127. doi: 10.1016/j.bone.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sato AY, Richardson D, Cregor M, et al. Glucocorticoids induce bone and muscle atrophy by tissue-specific mechanisms upstream of E3 ubiquitin ligases. Endocrinology. 2017;158:664–677. doi: 10.1210/en.2016-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leclerc N, Noh T, Cogan J, et al. Opposing effects of glucocorticoids and Wnt signaling on Krox20 and mineral deposition in osteoblast cultures. J Cell Biochem. 2008;103:1938–1951. doi: 10.1002/jcb.21587. [DOI] [PubMed] [Google Scholar]
- 81.Mortensen RF, Shapiro J, Lin BF, et al. Interaction of recombinant IL-1 and recombinant tumor necrosis factor in the induction of mouse acute phase proteins. J Immunol. 1988;140:2260–2266. [PubMed] [Google Scholar]
- 82.Advani S, LaFrancis D, Bogdanovic E, et al. Dexamethasone suppresses in vivo levels of bone collagen synthesis in neonatal mice. Bone. 1997;20:41–46. doi: 10.1016/s8756-3282(96)00314-6. [DOI] [PubMed] [Google Scholar]
- 83.Rauch A, Seitz S, Baschant U, et al. Glucocorticoids Suppress Bone Formation by Attenuating Osteoblast Differentiation via the Monomeric Glucocorticoid Receptor. Cell Metab. 2010;11:517–531. doi: 10.1016/j.cmet.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 84.Weinstein RS, Jilka RL, Parfitt AM, et al. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weinstein RS, Hogan EA, Borrelli MJ, et al. The Pathophysiological Sequence of Glucocorticoid-induced Osteonecrosis of the Femoral Head in Male Mice. Endocrinology. 2017 doi: 10.1210/en.2017-00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fowler TW, Acevedo C, Mazur CM, et al. Glucocorticoid suppression of osteocyte perilacunar remodeling is associated with subchondral bone degeneration in osteonecrosis. Sci Rep. 2017;7:44618. doi: 10.1038/srep44618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang M, Trettel LB, Adams DJ, et al. Col3.6-HSD2 transgenic mice: A glucocorticoid loss-of-function model Spanning early and late osteoblast differentiation. Bone. 2010;47:573–582. doi: 10.1016/j.bone.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sher LB, Harrison JR, Adams DJ, et al. Impaired cortical bone acquisition and osteoblast differentiation in mice with osteoblast-targeted disruption of glucocorticoid signaling. Calcif Tissue Int. 2006;79:118–125. doi: 10.1007/s00223-005-0297-z. [DOI] [PubMed] [Google Scholar]
- 89.Kalak R, Zhou H, Street J, et al. Endogenous glucocorticoid signalling in osteoblasts is necessary to maintain normal bone structure in mice. Bone. 2009;45:61–67. doi: 10.1016/j.bone.2009.03.673. [DOI] [PubMed] [Google Scholar]
- 90.Sher LB, Woitge HW, Adams DJ, et al. Transgenic expression of 11beta-hydroxysteroid dehydrogenase type 2 in osteoblasts reveals an anabolic role for endogenous glucocorticoids in bone. Endocrinology. 2004;145:922–929. doi: 10.1210/en.2003-0655. [DOI] [PubMed] [Google Scholar]
- 91.Aarden EM, Wassenaar AM, Alblas MJ, et al. Immunocytochemical demonstration of extracellular matrix proteins in isolated osteocytes. Histochem Cell Biol. 1996;106:495–501. doi: 10.1007/BF02473312. [DOI] [PubMed] [Google Scholar]
- 92.Frendo JL, Xiao G, Fuchs S, et al. Functional hierarchy between two OSE2 elements in the control of osteocalcin gene expression in vivo. J Biol Chem. 1998;273:30509–30516. doi: 10.1074/jbc.273.46.30509. [DOI] [PubMed] [Google Scholar]
- 93.Cheng SL, Yang JW, Rifas L, et al. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134:277–286. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- 94.Ishida Y, Heersche JN. Glucocorticoid-induced osteoporosis: both in vivo and in vitro concentrations of glucocorticoids higher than physiological levels attenuate osteoblast differentiation. J Bone Miner Res. 1998;13:1822–1826. doi: 10.1359/jbmr.1998.13.12.1822. [DOI] [PubMed] [Google Scholar]
- 95.Ito S, Suzuki N, Kato S, et al. Glucocorticoids induce the differentiation of a mesenchymal progenitor cell line, ROB-C26 into adipocytes and osteoblasts, but fail to induce terminal osteoblast differentiation. Bone. 2007;40:84–92. doi: 10.1016/j.bone.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 96.Gohel A, McCarthy MB, Gronowicz G. Estrogen prevents glucocorticoid-induced apoptosis in osteoblasts in vivo and in vitro. Endocrinology. 1999;140:5339–5347. doi: 10.1210/endo.140.11.7135. [DOI] [PubMed] [Google Scholar]
- 97.Plotkin LI, Manolagas SC, Bellido T. Glucocorticoids induce osteocyte apoptosis by blocking focal adhesion kinase-mediated survival: evidence for inside-out signaling leading to anoikis. J Biol Chem. 2007;282:24120–24130. doi: 10.1074/jbc.M611435200. [DOI] [PubMed] [Google Scholar]
- 98.Necela BM, Cidlowski JA. Mechanisms of glucocorticoid receptor action in noninflammatory and inflammatory cells. Proc Am Thorac Soc. 2004;1:239–246. doi: 10.1513/pats.200402-005MS. [DOI] [PubMed] [Google Scholar]
- 99.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 100.Druilhe A, Letuve S, Pretolani M. Glucocorticoid-induced apoptosis in human eosinophils: mechanisms of action. Apoptosis. 2003;8:481–495. doi: 10.1023/a:1025590308147. [DOI] [PubMed] [Google Scholar]
- 101.Limbourg FP, Liao JK. Nontranscriptional actions of the glucocorticoid receptor. J Mol Med. 2003;81:168–174. doi: 10.1007/s00109-003-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chauhan D, Pandey P, Ogata A, et al. Dexamethasone induces apoptosis of multiple myeloma cells in a JNK/SAP kinase independent mechanism. Oncogene. 1997;15:837–843. doi: 10.1038/sj.onc.1201253. [DOI] [PubMed] [Google Scholar]
- 103.Blaukat A, Ivankovic-Dikic I, Gronroos E, et al. Adaptor proteins Grb2 and Crk couple Pyk2 with activation of specific mitogen-activated protein kinase cascades. J Biol Chem. 1999;274:14893–14901. doi: 10.1074/jbc.274.21.14893. [DOI] [PubMed] [Google Scholar]
- 104.Tokiwa G, Dikic I, Lev S, et al. Activation of Pyk2 by stress signals and coupling with JNK signaling pathway. Science. 1996;273:792–794. doi: 10.1126/science.273.5276.792. [DOI] [PubMed] [Google Scholar]
- 105.Chauhan D, Hideshima T, Pandey P, et al. RAFTK/PYK2-dependent and -independent apoptosis in multiple myeloma cells. Oncogene. 1999;18:6733–6740. doi: 10.1038/sj.onc.1203082. [DOI] [PubMed] [Google Scholar]
- 106.Xiong W, Parsons JT. Induction of apoptosis after expression of PYK2, a tyrosine kinase structurally related to focal adhesion kinase. J Cell Biol. 1997;139:529–539. doi: 10.1083/jcb.139.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sasaki H, Nagura K, Ishino M, et al. Cloning and characterization of cell adhesion kinase beta, a novel protein-tyrosine kinase of the focal adhesion kinase subfamily. J Biol Chem. 1995;270:21206–21219. doi: 10.1074/jbc.270.36.21206. [DOI] [PubMed] [Google Scholar]
- 108.Avraham H, Park S, Schinkmann K, et al. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12:123–133. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 109.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 110.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 111.Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 112.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005;17:509–516. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 113.Hynes R. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 114.Plotkin LI, Mathov I, Aguirre JI, et al. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases and ERKs. Am J Physiol Cell Physiol. 2005;289:C633–C643. doi: 10.1152/ajpcell.00278.2004. [DOI] [PubMed] [Google Scholar]
- 115.Vanden Berghe W, Francesconi E, De Bosscher K, et al. Dissociated glucocorticoids with anti-inflammatory potential repress interleukin-6 gene expression by a nuclear factor-kappaB-dependent mechanism. Mol Pharmacol. 1999;56:797–806. [PubMed] [Google Scholar]
- 116.Cheng SL, Zhang SF, Mohan S, et al. Regulation of insulin-like growth factors I and II and their binding proteins in human bone marrow stromal cells by dexamethasone. J Cell Biochem. 1998;71:449–458. doi: 10.1002/(sici)1097-4644(19981201)71:3<449::aid-jcb13>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 117.Chang DJ, Ji C, Kim KK, et al. Reduction in transforming growth factor beta receptor I expression and transcription factor CBFa1 on bone cells by glucocorticoid. J Biol Chem. 1998;273:4892–4896. doi: 10.1074/jbc.273.9.4892. [DOI] [PubMed] [Google Scholar]
- 118.Doherty WJ, Derome ME, McCarthy MB, et al. The effect of glucocorticoids on osteoblast function. The effect of corticosterone on osteoblast expression of beta 1 integrins. J Bone Joint Surg Am. 1995;77:396–404. doi: 10.2106/00004623-199503000-00009. [DOI] [PubMed] [Google Scholar]
- 119.Almeida M, Han L, Ambrogini E, et al. Glucocorticoids and tumor necrosis factor (TNF) alpha increase oxidative stress and suppress WNT signaling in osteoblasts. J Biol Chem. 2011;286:44326–44335. doi: 10.1074/jbc.M111.283481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Harding HP, Zhang Y, Zeng H, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 121.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 122.Sato AY, Tu X, McAndrews KA, et al. Prevention of glucocorticoid induced-apoptosis of osteoblasts and osteocytes by protecting against endoplasmic reticulum (ER) stress in vitro and in vivo in female mice. Bone. 2015;73:60–68. doi: 10.1016/j.bone.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boyce M, Bryant KF, Jousse C, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 124.Tsaytler P, Harding HP, Ron D, et al. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332:91–94. doi: 10.1126/science.1201396. [DOI] [PubMed] [Google Scholar]
- 125.Yokota H, Hamamura K, Chen A, et al. Effects of salubrinal on development of osteoclasts and osteoblasts from bone marrow-derived cells. BMC Musculoskelet Disord. 2013;14:197. doi: 10.1186/1471-2474-14-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hamamura K, Tanjung N, Yokota H. Suppression of osteoclastogenesis through phosphorylation of eukaryotic translation initiation factor 2 alpha. J Bone Miner Metab. 2013;31:618–628. doi: 10.1007/s00774-013-0450-0. [DOI] [PubMed] [Google Scholar]
- 127.Hamamura K, Yokota H. Stress to endoplasmic reticulum of mouse osteoblasts induces apoptosis and transcriptional activation for bone remodeling. FEBS Lett. 2007;581:1769–1774. doi: 10.1016/j.febslet.2007.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saito A, Ochiai K, Kondo S, et al. Endoplasmic reticulum stress response mediated by the PERK-eIF2(alpha)-ATF4 pathway is involved in osteoblast differentiation induced by BMP2. J Biol Chem. 2011;286:4809–4818. doi: 10.1074/jbc.M110.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sato AY, Cregor M, Delgado-Calle J, et al. Protection from glucocorticoid-induced osteoporosis by anti-catabolic signaling in the absence of Sost/sclerostin. J Bone Miner Res. 2016;31:1791–1802. doi: 10.1002/jbmr.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Piemontese M, Xiong J, Fujiwara Y, et al. Cortical bone loss caused by glucocorticoid excess requires RANKL production by osteocytes and is associated with reduced OPG expression in mice. Am J Physiol Endocrinol Metab. 2016;311:E587–E593. doi: 10.1152/ajpendo.00219.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med. 2009;360:53–62. doi: 10.1056/NEJMoa0802633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 133.Balemans W, Ebeling M, Patel N, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 134.Dixon JM, Cull RE, Gamble P. Two cases of Van Buchem’s disease. J Neurol Neurosurg Psychiatry. 1982;45:913–918. doi: 10.1136/jnnp.45.10.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Leupin O, Piters E, Halleux C, et al. Bone overgrowth-associated mutations in the LRP4 gene impair sclerostin facilitator function. J Biol Chem. 2011;286:19489–19500. doi: 10.1074/jbc.M110.190330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Glass DA, Bialek P, Ahn JD, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 137.Holmen SL, Zylstra CR, Mukherjee A, et al. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem. 2005;280:21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- 138.Jilka RL, Bellido T, Almeida M, Plotkin LI, O’Brien CA, Weinstein RS, Manolagas SC. Apoptosis in bone cells. In: Bilezikian JP, Raisz LG, Martin TJ, editors. Principles of Bone Biology. Academic Press; San Diego, San Francisco, New York, London, Sydney, Tokyo: 2008. pp. 237–261. [Google Scholar]
- 139.Marenzana M, Greenslade K, Eddleston A, et al. Sclerostin antibody treatment enhances bone strength but does not prevent growth retardation in young mice treated with dexamethasone. Arthritis Rheum. 2011;63:2385–2395. doi: 10.1002/art.30385. [DOI] [PubMed] [Google Scholar]
- 140.Yao W, Dai W, Jiang L, et al. Sclerostin-antibody treatment of glucocorticoid-induced osteoporosis maintained bone mass and strength. Osteoporos Int. 2016;27:283–294. doi: 10.1007/s00198-015-3308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van Lierop AH, Hamdy NA, Papapoulos SE. Glucocorticoids are not always deleterious for bone. J Bone Miner Res. 2010;25:2796–2800. doi: 10.1002/jbmr.151. [DOI] [PubMed] [Google Scholar]
- 142.Rizzoli R, Adachi JD, Cooper C, et al. Management of glucocorticoid-induced osteoporosis. Calcif Tissue Int. 2012;91:225–243. doi: 10.1007/s00223-012-9630-5. [DOI] [PubMed] [Google Scholar]
- 143.Saag KG, Emkey R, Schnitzer TJ, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med. 1998;339:292–299. doi: 10.1056/NEJM199807303390502. [DOI] [PubMed] [Google Scholar]
- 144.Mok CC, Ho LY, Ma KM. Switching of oral bisphosphonates to denosumab in chronic glucocorticoid users: a 12-month randomized controlled trial. Bone. 2015;75:222–228. doi: 10.1016/j.bone.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 145.Allen MR, Iwata K, Phipps R, et al. Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone. 2006;39:872–879. doi: 10.1016/j.bone.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 146.Mashiba T, Turner CH, Hirano T, et al. Effects of suppressed bone turnover by bisphosphonates on microdamage accumulation and biomechanical properties in clinically relevant skeletal sites in beagles. Bone. 2001;28:524–531. doi: 10.1016/s8756-3282(01)00414-8. [DOI] [PubMed] [Google Scholar]
- 147.O’Ryan FS, Khoury S, Liao W, et al. Intravenous bisphosphonate-related osteonecrosis of the jaw: bone scintigraphy as an early indicator. J Oral Maxillofac Surg. 2009;67:1363–1372. doi: 10.1016/j.joms.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 148.Allen MR, Burr DB. Mineralization, Microdamage, and Matrix: How Bisphosphonates Influence Material Properties of Bone. BoneKEy-osteovision. 2007;4:49–60. [Google Scholar]
- 149.Minetto MA, Botter A, Lanfranco F, et al. Muscle fiber conduction slowing and decreased levels of circulating muscle proteins after short-term dexamethasone administration in healthy subjects. J Clin Endocrinol Metab. 2010;95:1663–1671. doi: 10.1210/jc.2009-2161. [DOI] [PubMed] [Google Scholar]
- 150.Minetto MA, Qaisar R, Agoni V, et al. Quantitative and qualitative adaptations of muscle fibers to glucocorticoids. Muscle Nerve. 2015;52:631–639. doi: 10.1002/mus.24572. [DOI] [PubMed] [Google Scholar]
- 151.Schakman O, Kalista S, Barbe C, et al. Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol. 2013;45:2163–2172. doi: 10.1016/j.biocel.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 152.Sandri M, Lin J, Handschin C, et al. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci U S A. 2006;103:16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bodine SC, Latres E, Baumhueter S, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 154.Gomes MD, Lecker SH, Jagoe RT, et al. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Menconi M, Gonnella P, Petkova V, et al. Dexamethasone and corticosterone induce similar, but not identical, muscle wasting responses in cultured L6 and C2C12 myotubes. J Cell Biochem. 2008;105:353–364. doi: 10.1002/jcb.21833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Furlow JD, Watson ML, Waddell DS, et al. Altered gene expression patterns in muscle ring finger 1 null mice during denervation- and dexamethasone-induced muscle atrophy. Physiol Genomics. 2013;45:1168–1185. doi: 10.1152/physiolgenomics.00022.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Baehr LM, Furlow JD, Bodine SC. Muscle sparing in muscle RING finger 1 null mice: response to synthetic glucocorticoids. J Physiol. 2011;589:4759–4776. doi: 10.1113/jphysiol.2011.212845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Watson ML, Baehr LM, Reichardt HM, et al. A cell-autonomous role for the glucocorticoid receptor in skeletal muscle atrophy induced by systemic glucocorticoid exposure. Am J Physiol Endocrinol Metab. 2012;302:E1210–E1220. doi: 10.1152/ajpendo.00512.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sartori R, Schirwis E, Blaauw B, et al. BMP signaling controls muscle mass. Nat Genet. 2013;45:1309–1318. doi: 10.1038/ng.2772. [DOI] [PubMed] [Google Scholar]
- 160.Kondo H, Ezura Y, Nakamoto T, et al. MURF1 deficiency suppresses unloading-induced effects on osteoblasts and osteoclasts to lead to bone loss. J Cell Biochem. 2011;112:3525–3530. doi: 10.1002/jcb.23327. [DOI] [PubMed] [Google Scholar]
- 161.Fournier M, Huang ZS, Li H, et al. Insulin-like growth factor I prevents corticosteroid-induced diaphragm muscle atrophy in emphysematous hamsters. Am J Physiol Regul Integr Comp Physiol. 2003;285:R34–R43. doi: 10.1152/ajpregu.00177.2002. [DOI] [PubMed] [Google Scholar]
- 162.Dekhuijzen PN, Gayan-Ramirez G, Bisschop A, et al. Corticosteroid treatment and nutritional deprivation cause a different pattern of atrophy in rat diaphragm. J Appl Physiol. 1995;78:629–637. doi: 10.1152/jappl.1995.78.2.629. [DOI] [PubMed] [Google Scholar]
- 163.Rosenblatt JD, Parry DJ. Gamma irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J Appl Physiol (1985) 1992;73:2538–2543. doi: 10.1152/jappl.1992.73.6.2538. [DOI] [PubMed] [Google Scholar]
- 164.Falduto MT, Czerwinski SM, Hickson RC. Glucocorticoid-induced muscle atrophy prevention by exercise in fast-twitch fibers. J Appl Physiol. 1990;69:1058–1062. doi: 10.1152/jappl.1990.69.3.1058. [DOI] [PubMed] [Google Scholar]
- 165.Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Milan G, Romanello V, Pescatore F, et al. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat Commun. 2015;6:6670. doi: 10.1038/ncomms7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mourikis P, Sambasivan R, Castel D, et al. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells. 2012;30:243–252. doi: 10.1002/stem.775. [DOI] [PubMed] [Google Scholar]
- 168.Wen Y, Bi P, Liu W, et al. Constitutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol Cell Biol. 2012;32:2300–2311. doi: 10.1128/MCB.06753-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Buas MF, Kabak S, Kadesch T. The Notch effector Hey1 associates with myogenic target genes to repress myogenesis. J Biol Chem. 2010;285:1249–1258. doi: 10.1074/jbc.M109.046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kopan R, Nye JS, Weintraub H. The intracellular domain of mouse Notch: a constitutively activated repressor of myogenesis directed at the basic helix-loop-helix region of MyoD. Development. 1994;120:2385–2396. doi: 10.1242/dev.120.9.2385. [DOI] [PubMed] [Google Scholar]
- 171.Pereira RM, Delany AM, Durant D, et al. Cortisol regulates the expression of Notch in osteoblasts. J Cell Biochem. 2002;85:252–258. doi: 10.1002/jcb.10125. [DOI] [PubMed] [Google Scholar]
- 172.Xu J, Li R, Workeneh B, et al. Transcription factor FoxO1, the dominant mediator of muscle wasting in chronic kidney disease, is inhibited by microRNA-486. Kidney Int. 2012;82:401–411. doi: 10.1038/ki.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ambrogini E, Almeida M, Martin-Millan M, et al. FoxO-Mediated Defense against Oxidative Stress in Osteoblasts Is Indispensable for Skeletal Homeostasis in Mice. Cell Metab. 2010;11:136–146. doi: 10.1016/j.cmet.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]