Short abstract
Inflammatory bowel disease is a complex collection of disorders. Microbial dysbiosis as well as exposure to toxins including xenoestrogens are thought to be risk factors for inflammatory bowel disease development and relapse. Bisphenol-A has been shown to exert estrogenic activity in the colon and alter intestinal function, but the role that xenoestrogens, such as bisphenol-A , play in colonic inflammation has been previously described but with conflicting results. We investigated the ability of bisphenol-A to exacerbate colonic inflammation and alter microbiota metabolites derived from aromatic amino acids in an acute dextran sulfate sodium-induced colitis model. Female C57BL/6 mice were ovariectomized and exposed to bisphenol-A daily for 15 days. Disease activity measures include body weight, fecal consistency, and rectal bleeding. Colons were scored for inflammation, injury, and nodularity. Alterations in the levels of microbiota metabolites derived from aromatic amino acids known to reflect phenotypic changes in the gut microbiome were analyzed. Bisphenol-A exposure increased mortality and worsened disease activity as well as inflammation and nodularity scores in the middle colon region following dextran sulfate sodium exposure. Unique patterns of metabolites were associated with bisphenol-A consumption. Regardless of dextran sulfate sodium treatment, bisphenol-A reduced levels of tryptophan and several metabolites associated with decreased inflammation in the colon. This is the first study to show that bisphenol-A treatment alone can reduce microbiota metabolites derived from aromatic amino acids in the colon which may be associated with increased colonic inflammation and inflammatory bowel disease.
Impact statement
As rates of inflammatory bowel disease rise, discovery of the mechanisms related to the development of these conditions is important. Environmental exposure is hypothesized to play a role in etiology of the disease, as are alterations in the gut microbiome and the metabolites they produce. This study is the first to show that bisphenol-A alone alters tryptophan and microbiota metabolites derived from aromatic amino acids in a manner consistent with autoimmune diseases, specifically inflammatory bowel diseases, regardless of dextran sulfate sodium treatment. These findings indicate a potential mechanism by which bisphenol-A negatively affects gut physiology to exacerbate inflammation.
Keywords: Bisphenol-A, colitis, inflammatory bowel disease, microbiota metabolites, tryptophan, xenoestrogen
Introduction
Inflammatory bowel disease (IBD) is a complex collection of gastrointestinal disorders. IBD incidence is on the rise, a concerning trend, as treatment is lifelong and often requires surgery, and colitis-associated inflammation is a risk factor for developing colon cancer.1–3 Increased prevalence of these diseases has been observed in North American and European nations for decades, but as developing nations become more industrialized, IBD prevalence increases in these countries.1,2 A growing body of data suggests environmental exposures significantly influence IBD development and relapse.1–3 The two most common IBDs, Crohn’s disease (CD) and ulcerative colitis (UC), differ in their pathophysiology, patterns of incidence, and environmental risk factors, complicating the elucidation of the role of the environment in IBD.2 Proposed environmental risk factors for IBD include diet, smoking, infections and pharmaceutical usage, altered gut microbiome, estrogen-containing medication usage, and toxins or pollutants.1,2,4
Both endogenous estrogens as well as pharmaceutical estrogens in oral contraceptive pills and hormone replacement therapy are potential risk factors for IBD development and relapse.1,2,4 Therefore, it is plausible that environmental exposure to xenoestrogens (XEs) could increase the risk of IBD. One such XE, bisphenol A (BPA) is used in the production of polymers including those that compose polycarbonate plastics, epoxy resins, and thermal paper.5 A major source of human exposure to BPA is in the diet, particularly through canned foods.5,6 Epoxy resins line metal food and beverage containers, and polycarbonate plastics are also used in a variety of food-related containers.6 Worldwide, over 3.8 million tons of BPA are produced annually, and because BPA is used in a wide variety of consumer and industrial applications, it is pervasive in the environment and human tissues.6,7 For example, in the United States, BPA was detected in the urine of 92.6% of tested individuals over the age of 6.8 One review found BPA levels in human serum between 0.2 and 20 ng/mL, and these levels are above those BPA concentrations known to cause adverse effects in vitro.7 Exposure to the compound has been linked with obesity, reproductive issues, metabolic disorders, hormone-dependent tumors, and other health effects.7,9 The Environmental Protection Agency has established guidelines for acceptable levels of BPA exposure in humans.10 The no observed adverse effect level (NOAEL) is 5 mg/kg-bw/day, the lowest observed adverse effect level (LOAEL) is 50 mg/kg-bw/day, and the reference dose is 50 µg/kg-bw/day.10 This reference dose is an estimate of the daily exposure level that is unlikely to cause deleterious effects in humans over the course of the lifespan. However, several studies have shown negative effects of this or lower doses, and a lower reference dose of 16 µg/kg-bw/day has been proposed.11–13
BPA is considered an endocrine disruptor capable of binding estrogen receptor α and β (ERα and ERβ, respectively), as well as G-protein coupled receptor 30 (GPR30), and other non-classical estrogen-related receptors, which may provide a mechanistic explanation for these adverse effects.14 More specifically, BPA mimics 17β-estradiol (E2) when binding to ERα, but acts as an antagonist when binding ERβ.14 This is particularly relevant in the colon, where ERβ is the primary ER and is considered to mediate the protective effects of estrogen in inflammation-associated and sporadic colon cancer.15,16 It is has been previously shown that BPA is linked to changes in gut barrier function, inflammation, and altered gut microbiome.11,17,18 Previous studies have linked changes in gut microbiome and the levels of metabolites present in the feces with colonic inflammation and IBD development.19 Reduced levels of tryptophan (Trp) and several microbiota metabolites derived from aromatic amino acids (MDAs) including serotonin have been associated with IBD and with increased severity of symptoms in human patients and animal models.20 Therefore, compounds that alter the gut microbiome and, as a result, the metabolome of the colon, could impact IBD development and symptom severity.
The purpose of this study was to determine the effects of BPA exposure on colonic inflammation and the intestinal metabolome both in the absence of and during dextran sulfate sodium (DSS)-induced colitis. Previous studies have shown that BPA does not alter disease severity or is mildly protective against 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis.11,21 However, previous studies in our laboratory and others have demonstrated differential effects of E2 signaling on varying models of colitis, particularly a worsening of disease severity during DSS-induced colitis.22–24 In the work presented here, we hypothesized that BPA exacerbates DSS-induced colitis and reduces Trp and MDAs in the colon.
Materials and methods
Animal model
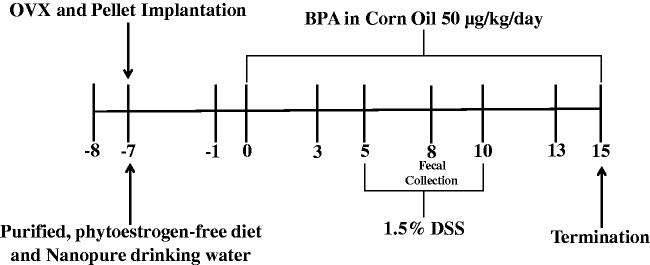
Wild type C57BL/6 mice were obtained from Charles River Laboratories. The mice were housed at the Laboratory Animal Resources and Research facility at Texas A&M University. All procedures were performed under a protocol approved by the Institutional Animal Care and Use Committee at Texas A&M University; 10-week-old female C57BL/6 mice were randomly divided into four groups that received either no treatment (n = 12), BPA alone (n = 10), DSS alone (n = 12), or BPA and DSS (n = 12). The experimental timeline is shown in Figure 1. Animals were allowed to acclimatize for one week prior to the start of the study. To limit the effects of variations in endogenous estrogen production, animals were ovariectomized as described previously.15 Mice were transferred to a pelleted, purified, phytoestrogen-free diet (Baker Amino Acid Diet 5CC7, Test Diet) at the time of surgery and allowed food and reverse osmosis and UVUF-treated water (Nanopure Diamond, Barnstead) ad libitum. Mice were housed in polyethylene cages and provided drinking water in polyethylene bottles.
Figure 1.
Experimental design.
Beginning one week after ovariectomy, animals were gavaged with 50 µg/kg-bw/day BPA (Sigma Aldrich) dissolved in corn oil or vehicle control for 15 days at the same time each morning. BPA was first dissolved in ethanol for a final ethanol concentration in treatment and controls groups of 0.01%. BPA was dissolved in corn oil at a concentration of 6.25 µg/mL. This concentration allowed gavage of 1.25 µg BPA per 200 µL corn oil for a 25 g mouse such that the maximum gavage volume of 1% body weight was not exceeded. The same calculations were used to dose vehicle controls with corn oil. Treatments were prepared fresh daily.
Induction of colitis
DSS (1.5%) (MP Biomedicals; 36–50 kDa) was provided ad libitum in drinking water from days 5 through 10 of BPA treatment. DSS was replaced every 48 h. Animals in control groups received normal drinking water. Body weight, fecal consistency score, and macroscopic fecal blood scores were obtained daily on all animals. Disease activity index (DAI), providing an average measure of body weight loss, fecal consistency score, and macroscopic rectal bleeding scores were adapted from previous work.25,26 Briefly, body weight loss percentage from start of the study was scored as 0: weight gain or 0–1% loss, 1: 1–5% loss, 2: 5–10% loss, 3: 10–15% loss, 4: >15% loss compared to study day −1. Fecal consistency was scored as 0: normal stool, 1: soft but formed pellet, 2: very soft pellet, 3: diarrhea (no pellet), or 4: dysenteric diarrhea (blood in diarrhea). Rectal bleeding was scored as 0: no bleeding, 2: presence of visible blood in stool (red/dark pellet), 4: gross macroscopic bleeding (blood around anus). Body weight as well as fecal consistency, rectal bleeding, and disease activity scores are only reported through day 12. After this point, the loss of animals influenced these data points such that they were not interpretable.
Fecal and tissue collection
Animals were singly housed for up to 2 h, and feces were collected prior to BPA treatment (day −1), and on days 3, 8, and 13 of BPA treatment, and at termination. For targeted metabolomics, day 8 samples were chosen because this time point would best allow for determining the effects of BPA on the gut metabolome during DSS-induced inflammation. Fecal pellets were flash frozen and stored at −80°C until analysis.
Animals were terminated on study day 15, 5 days following cessation of DSS. Final treatments of BPA or vehicle control were gavaged 2 h before termination. Blood was collected via cardiac puncture, and plasma was stored at −20°C. Colons were resected, flushed with PBS, and opened longitudinally. Half of each colon was Swiss rolled, fixed in 4% paraformaldehyde (JT Baker) for 4 h, and then sectioned for pathological analysis; 4 µm, non-serial sections from fixed colons were H&E stained and scored for severity of acute colonic inflammation and injury by a blinded, board-certified pathologist (B. Weeks). Degree of inflammation was scored as 0: no unexpected inflammation, 1: minimal to very mild inflammation, 2: mild to moderate inflammation, or 3: moderate to severe inflammation. Degree of tissue injury was scored as 0: no unexpected injury, 1: minimal to very mild injury, 2: mild to moderate injury, or 3: moderate to severe injury. Nodularity or aggregation of inflammation was scored as 0: diffuse inflammation, 1: minimal to very mild nodular inflammation 2: moderately nodular inflammation, or 3: very nodular inflammation.
Cytokine analysis
Cytokine analysis was performed as described previously.22 Briefly, the middle third of snap frozen colons were homogenized in 333 µL tissue protein extraction reagent (Thermo Scientific). Homogenate was centrifuged at 10,000g for 5 min before 100 µL aliquots of supernatant were stored at −20°C until analysis. Following protein concentration measurement using the DC protein assay (Bio-Rad), all samples were diluted to 2 mg/mL. The Mouse Cytokine/Chemokine and Mouse Th17 Magnetic Bead Panel Milliplex Map Kits (Millipore) were used per the manufacturer’s instructions. The plate was analyzed on a BioPlex 200 (Bio-Rad).
Quantification of metabolites from aromatic amino acids
Fecal samples for targeted metabolomics were processed and run at Integrated Metabolomics Analysis Core at Texas A&M University. Nine metabolites derived from aromatic amino acids were quantified from fecal samples.27 Fecal samples were homogenized in methanol/chloroform and metabolites were extracted as previously described with minor modifications.28 Briefly, metabolites were sequentially extracted twice using 1 mL of cold methanol and 0.5 mL of chloroform using a homogenizer (Omni International). The polar phase was separated and concentrated using a vacufuge (Eppendorf, Hauppauge, NY). The concentrated pellet was re-suspended in methanol/water (1:1 v/v) and metabolites of interest were quantified using a Synergi Fusion-RP 4µ 80 Å 150 × 2.0 mm column (Phenomenex) on a triple Quadrupole Mass Spectrometer (TSQ Quantiva™) coupled to liquid chromatography (Agilent). The solvents used were Water + 0.1% formic acid and methanol, 0.1% formic acid. Pure standards were run for 10 known concentrations (ranging from 0.009 µg/mL to 10 µg/mL) for each metabolite and metabolite concentrations in the samples were determined from the integration of the standard curves.
Statistical analysis
Data were analyzed using JMP 13.0.0 software. Outliers were removed, one-way ANOVA was used to determine significant (P < 0.05) differences between groups, and, once found significant, Student’s t test was used to compare means between specific groups. To determine if survival times were significantly different (P < 0.05) between groups, the log-rank test was performed. Non-parametric, categorical inflammation, injury, and nodularity score data were transformed to achieve normality by assigning an average rank within each sub-group as previously reported.29 One-tailed Student’s t test assuming unequal variances was then used to determine significance (P < 0.05). Metabolome data were normalized to per gram of the starting material and analyzed using KaleidaGraph. Outliers were removed following Grubb’s test and scatterplot analysis, and then data were normalized and analyzed using a one-tailed t test.
Results
Disease activity
In the presence of DSS, BPA co-treatment resulted in earlier and increased mortality compared to control animals (Figure 2). Log-rank test indicated significant differences between survival among all groups (P < 0.001), and DSS and BPA co-treatment resulted in significantly worsened mortality compared to DSS alone (P = 0.0084). DSS alone did not result in significantly increased mortality compared to vehicle control (P = 0.1483). DSS and BPA combination resulted in 67% mortality, with most deaths between five and seven days after initiation of DSS. While DSS alone resulted in 17% mortality, with most deaths occurring between seven and nine days after the start of DSS. No control or BPA alone treated animals died during the course of the study.
Figure 2.
Survival curve. Animals alive at start of day, expressed as percent of total group size at start of experiment. n = 10 to 12 per group at the start of the study and declined over time as shown. Log-rank P < 0.0001. *indicates significant difference; P < 0.05.
Average group body weight did not differ significantly at the start of the study. By day 11, 6 days after initiation of DSS treatment, DSS groups had significantly lower body weight than non-DSS groups, but BPA exposure did not significantly reduce body weight compared to controls in either DSS or non-DSS-treated mice (Figure 3(a)). As expected, DSS worsened fecal consistency scores beginning 24 h after initial exposure. This difference was significant regardless of BPA exposure. After cessation of DSS, DSS alone animals showed improved fecal consistency scores; however, animals co-treated with BPA exhibited significantly worsened scores during the recovery period on days 10–12 (Figure 3(b)). DSS worsened rectal bleeding scores within three days of DSS initiation. BPA exposure significantly worsened macroscopic rectal bleeding beginning four days after initial DSS exposure and throughout the remainder of the study (Figure 3(c)).
Figure 3.
Measures of disease activity. Scoring system adapted from Murthy et al. Dig Dis Sci 1993 and Singh et al. Immunity 2014. n = 10 to 12 per group at the start of the study; n declined over time as shown in the survival curve. Mean ± SEM. Points without a common letter differ on the given day; P < 0.05. (a) Average body weight. (b) Average fecal score. Scoring system: 0 = Normal stool, 1 = Soft but formed pellet, 2 = Very soft pellet, 3 = Diarrhea (no pellet), 4 = Dysenteric diarrhea (blood in diarrhea). (c) Average rectal bleeding score. Scoring system: 0 = No visible blood, 2: presence of visible blood in stool (red/dark pellet), 4: gross macroscopic bleeding (blood around anus). (d) Disease activity index. Average of body weight loss, fecal consistency, and rectal bleeding scores.
DAI was significantly worsened in both DSS-treated groups within 48 h of initiation of DSS (Figure 3(d)). Following cessation of DSS, the DSS group showed score improvement more quickly than the DSS and BPA group. By day 11 of the study, BPA exposure resulted in a significantly worse DAI when compared to the DSS controls. BPA treatment did not significantly alter DAI in groups not treated with DSS. DSS treatment shortened colon length regardless of BPA treatment, and DSS alone significantly increased colon weight/length (Supplementary Figure 1(a) to (c)).
Histological scores
Pathologist scoring of tissues from each group showed an increase in inflammation in the middle portion of the colon in BPA-dosed animals regardless of DSS. This increase was not significant in BPA-treated animals compared to controls (Figure 4(a)). However, inflammation score was significantly increased in the middle colon region in mice exposed to BPA and DSS when compared to DSS-treated controls (P = 0.04; Figure 4(b)). Injury was also scored in these tissues, and, as expected, DSS significantly increased both inflammation and injury scores. BPA did not significantly alter injury score in the presence or absence of DSS (data not shown). Nodularity was assessed in DSS treatment groups to assess pattern of inflammation. Nodularity score was also significantly increased in the middle colon region in BPA and DSS co-treated animals compared to DSS controls (P = 0.02; Figure 4(c)). Representative images of increased inflammation in the middle portion of the colon, colon ulceration and erosion, as well as nodular and diffuse inflammation are shown in Figure 4(d) to (h).
Figure 4.
(a) BPA and colonic inflammation in the absence of DSS. (b) BPA and colonic inflammation in the presence of DSS. (c) Nodularity in the presence of DSS. Mean ± SEM. *indicates significant difference compared to control; P < 0.05. (d) Representative image of increased inflammation in middle portion of colon. Inflamed portion of the middle colon is indicated by black arrows. (e) Representative image of ulceration in the colon. Ulcer is indicated by the black arrow. (f) Representative image of erosion of the colon. Erosion is indicated by the black arrow. (g) Representative image of nodular inflammation. Nodular inflammation is indicated by the black arrow. (h) Representative image of diffuse inflammation. Diffuse inflammation is indicated by the black arrow. (A color version of this figure is available in the online journal.)
Cytokine measurements
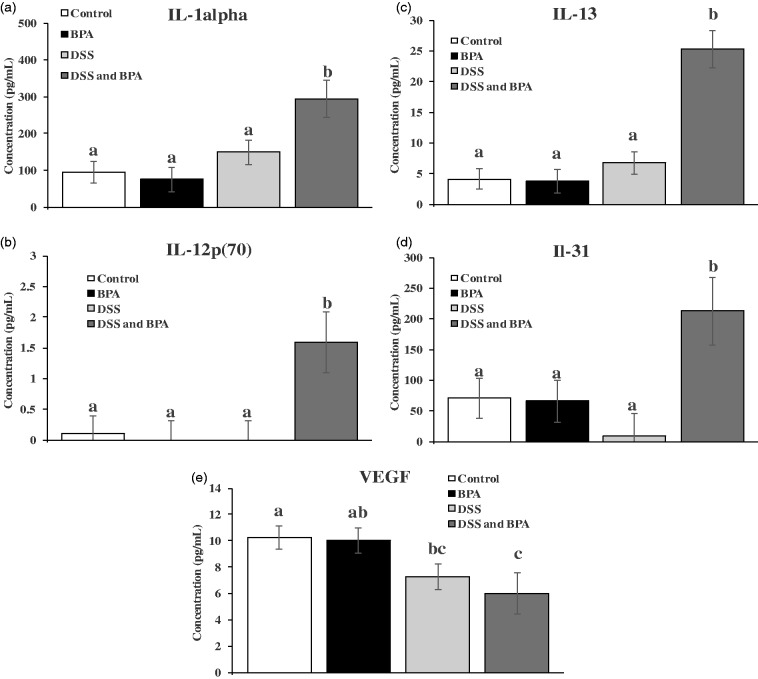
Cytokine protein levels were determined using multiplex magnetic bead assays. Cytokine expression is often used to assess inflammation in DSS models.30,31 In the present study, cytokines were measured in the middle portion of the colon as significant differences in histological inflammation score were observed in this region. As expected, DSS treatment led to an increase in cytokines (e.g. TNF- α and IL-1β) that have been previously reported to be elevated in DSS-treated mice compared to controls (data not shown).30–32 This supports the pathological analysis that DSS induced tissue inflammation in this portion of the colon. However, we chose to focus on cytokines that were changed between the DSS alone and DSS- and BPA-treated mice to explore how BPA may be exacerbating the effects of DSS. DSS and BPA co-treatment significantly increased expression of IL-1α, IL-12p(70), IL-13, and IL-31 compared to all other treatment groups (Figure 5(a) to (d)). VEGF expression was significantly decreased by DSS and BPA treatment compared to control (Figure 5(e)).
Figure 5.
Concentration of cytokines in the middle portion of colon. (a) IL-1α. (b) IL-12p(70). (c) IL-13. (d) IL-31. (e) VEGF. Mean ± SEM. Bars without a common letter differ; P < 0.05.
Targeted metabolomics
The concentration of nine MDAs in fecal pellets collected on day 8 of BPA treatment was analyzed. 5-Hydroxy indole 3-acetic acid (HIAA), serotonin, and Trp concentrations were significantly decreased in the presence of BPA compared to vehicle control without DSS treatment (P < 0.05; Figure 6(a) to (i)). HIAA concentration decreased 66% (P = 0.0004, Figure 6(b)), serotonin concentration decreased 35% (P = 0.003, Figure 6(f)), and Trp concentration in feces decreased 52% (P = 0.006; Figure 6(i)), in BPA-treated animals compared to vehicle-treated controls. No significant changes were found in other MDAs measured, including 3-indole acetic acid (Figure 6(a)), anthranilic acid (Figure 6(c)), indole 3-acetamide (Figure 6(d)), indole 3-carboxaldehyde (Figure 6(e)), shikimic acid (Figure 6(g)), and tryptamine (Figure 6(h)). A heat map shows the changes in the concentrations of the quantified metabolites with or without BPA treatment in the absence of DSS (Supplementary Figure 2(a)).
Figure 6.
Concentration of specific metabolites in feces on day 8 in control compared to BPA-treated animals in the absence of DSS treatment. (a) 3-indole acetic acid. (b) 5-hydroxy indole 3-acetic acid. (c) Anthranilic acid. (d) Indole 3-acetamide. (e) Indole 3-carboxaldehyde. (f) Serotonin. (g) Shikimic Acid. (h) Tryptamine. (i) Tryptophan. Mean ± SEM. *indicates significant difference compared to control; P < 0.05.
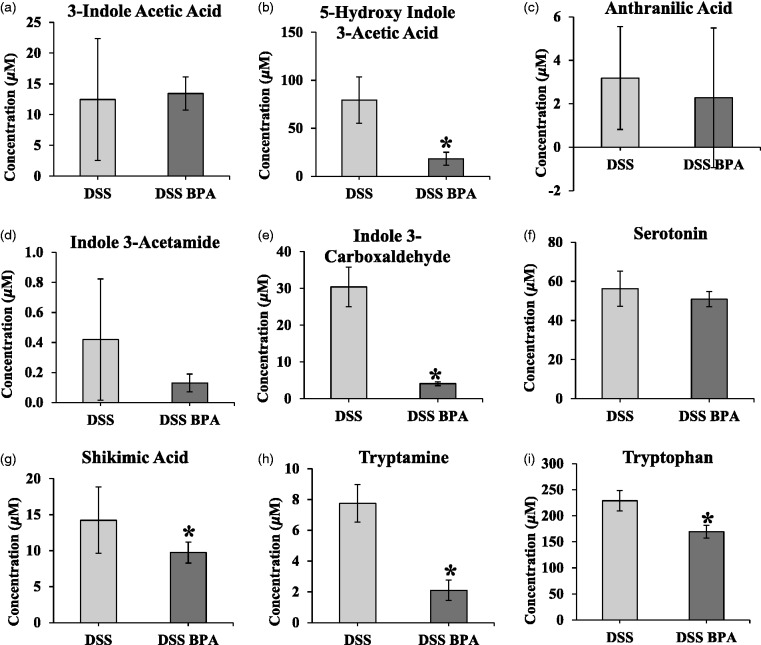
Trp, tryptamine, HIAA, indole 3-carboxaldehyde, and shikimic acid significantly decreased in the presence of BPA and DSS co-treatment compared to DSS alone (P < 0.05; Figure 7(a) to (i)). Metabolite concentration were decreased in the feces of DSS and BPA-treated mice compared with DSS and vehicle control mice as follows: HIAA by 77% (P = 0.02, Figure 7(b)), indole 3-carboxaldehyde by 87% (P = 0.007, Figure 7(e)), shikimic acid by 32% (P = 0.03, Figure 7(g)), tryptamine by 73% (P = 0.003, Figure 7(h)), and Trp by 26% (P = 0.001, Figure 7(i)). Other metabolites measured, including 3-indole acetic acid (Figure 7(a)), anthranilic acid (Figure 7(c)), indole-3-acetamide (Figure 7(d)), and serotonin (Figure 7(f)), did not significantly change. A heat map shows changes in concentrations of the same metabolites with or without BPA treatment in the presence of DSS (Supplementary Figure 2(b)). BPA treatment appears to alter MDAs in the feces more predictably during DSS treatment compared to the absence of DSS treatment, as evidenced by clustering on the heat map of BPA vs. control-treated animals in the DSS-treated groups.
Figure 7.
Concentration of specific metabolites in feces on day 8 in control compared to BPA treated co-treated with DSS. (a) 3-indole acetic acid. (b) 5-hydroxy indole 3-acetic acid. (c) Anthranilic acid. (d) Indole 3-acetamide. (e) Indole 3-carboxaldehyde. (f) Serotonin. (g) Shikimic acid. (h) Tryptamine. (i) Tryptophan. Mean ±SEM. *indicates significant difference compared to DSS alone; P < 0.05.
Discussion
BPA exposure during DSS-induced colitis worsens measures of disease severity. Survival is one method by which degree of colitis can be assessed, and DSS and BPA co-treated animals showed decreased survival compared to DSS alone controls. In addition to survival, severity of experimental colitis is often assessed using a scoring system that accounts for body weight loss, fecal consistency, and rectal bleeding.25,33 These measures were assessed daily in all mice, and, as expected, DSS treatment worsened all disease activity scores, regardless of BPA treatment. Recovery of the scores in animals following cessation of DSS is also used to measure the effects of treatments during experimental colitis. Interestingly, DSS and BPA co-treatment inhibited recovery of animals compared to DSS alone controls following cessation of DSS.
Previous experiments have linked BPA and inflammation. This correlation is most well established between BPA and the low-grade chronic inflammation associated with obesity. For example, several human studies have shown a positive correlation between serum or urinary BPA levels and increased levels of inflammatory markers such as malondialdehyde, 8-hydroxydeoxyguanosine, C-reactive protein, interleukin-6 (IL-6), and tumor necrosis factor-α (TNFα) in serum.34,35 In vitro and in vivo experiments have also demonstrated that BPA exposure results in increases in inflammatory markers in serum (leptin and resistin) and white adipose tissue (IL-6, TNFα, interferon-γ (IFN-γ), and inducible nitric oxide synthase 2) as well as in adipose tissue or differentiated adipocytes (IL-6 and IFN-γ).36,37
Our results demonstrate that BPA exposure at 50 µg/kg/day can exacerbate acute colonic inflammation in the DSS model. This dose is the BPA reference dose set by the Environmental Protection Agency.10 While this dose is in the upper end of what is estimated for human exposures to BPA, it results in circulating BPA concentrations in C57BL/6 mice within the range of that observed in humans.38 The effects of BPA on gut physiology have been investigated in other models. Perinatal exposure to BPA has been shown to decrease gut permeability at relatively low oral doses and in an ERβ-dependent manner, altering gut physiology similar to E2.11 The same group found that BPA treatment protected against measures of TNBS-induced colitis. These conflicting results are mirrored in similar experiments using E2; E2 treatment seems to protect against DNBS or TNBS-induced colitis while exacerbating DSS-induced colitis.22,23 While the effects of estrogenic signaling in intestinal inflammation are clearly complex, these varying results are likely due to the mechanism by which colitis is induced in each model.23 For example, Roy et al.21 used 2,4-dinitrobenzene sulfonic acid (DNBS) for colitis induction while directly exposing animals to 50 µg of BPA/kg/day as in our model. Similarly to TNBS, this chemical acts as a hapenating agent to induce colitis via increased immune activation in the colon that more closely mimics the symptoms of CD.39 DSS chemically damages colonic epithelial cells, leading to inflammation that more closely resembles UC.39 The differing mechanisms of action of these chemicals likely explain the varied results of the Roy et al. study compared to the present study.
Intestinal epithelial cell damage, increased gut permeability, and the resultant migration of colonic bacteria and lipopolysaccharides (LPS) have been implicated in the mechanisms of DSS-induced colitis.23,40 As noted by Verdú et al.,23 E2 has been shown to increase macrophage sensitivity to LPS, providing a possible mechanism by which other estrogenic compounds such as BPA could exacerbate DSS-induced colitis.41,42 BPA exposure in utero has also been shown to alter innate immune responses without affecting adaptive immune responses, possibly explaining the different responses of animals treated with DSS or TNBS.38 E2 has been shown to sensitize immune cells, and coupled with damage caused by DSS, innate immune cells are likely exposed to increased levels of bacteria and their products in BPA and DSS co-treated animals compared to vehicle controls, resulting in a worsening of systemic symptoms.43
To further understand the effects of BPA on inflammation during acute colitis, inflammatory markers were measured. While there is limited information in the literature on cytokine expression following BPA and DSS exposure in vivo, BPA’s effects on cytokine expression have been examined in vitro. BPA significantly increased expression of TNF-α and IL-6 in THP-1 macrophages in vitro, and these changes were attenuated by treatment with an ERα and ERß antagonist, ICI 182,780.44 E2 has also been shown to induce pro-inflammatory cytokine expression in the DSS model; 0.5 mg E2/pellet and 5% DSS co-treated C57BL/6 mice showed a significant increase in TNF-α compared to DSS alone.23 In the present study, TNF-α was significantly increased during DSS treatment, regardless of BPA treatment (data not shown). Significant increases in TNF-α in the DSS and BPA group compared to DSS alone may not have been observed as only the middle portion of the colon was analyzed. At least one report has shown that cytokine expression differs regionally in the colon following acute DSS colitis.30 Several cytokines that were significantly altered by DSS and BPA treatment have been connected to inflammatory responses in the colon, including IL-31, IL-13, and VEGF. IL-31 treatment resulted in STAT, ERK, and Akt phosphorylation in HCT116 and SW480 colon cells in vitro.45 IL-13 has been shown to be increased in UC patients, and this cytokine induces apoptosis in epithelial cells and reduces the ability of epithelial cells to migrate into wounds during repair.46 Increased VEGF levels are commonly found in IBD patients and animal models of colitis, and this increase accompanies an increase in pro-inflammatory cytokines in inflamed tissue.47 However, one study showed that inhibition of VEGF prior to acute DSS colitis resulted in worsened inflammation on day 19 post-DSS, and the authors suggest that VEGF inhibition may impact recovery following an acute bout of colitis.48 Therefore, it is possible that the significant reduction in VEGF expression in DSS and BPA treatment has an impact on delayed recovery observed in the DAI scores of this group compared to mice treated with DSS alone.
Another possible mechanism by which BPA could exacerbate intestinal inflammation could be microbial dysbiosis and a resultant shift in MDAs present in the intestinal lumen. Dietary exposure to BPA resulted in a decrease in gut microbial diversity, similarly to animals fed a high fat diet, as well as an increase in Proteobacteria which is associated with intestinal inflammation.18 Evidence exists that this association may persist in later generations as gut microbial dysbiosis caused by BPA exposure has also been shown to persist in offspring unexposed to BPA.49 Our results indicate that BPA treatment can reduce Trp and MDAs in mouse feces compared to vehicle controls. We have previously used Trp and the metabolites measured to describe phenotypic changes in the intestinal microbiota.27 Trp is the precursor for serotonin synthesis, and HIAA is the metabolic product of serotonin. Our results suggest that BPA affects the metabolism of the essential amino acid Trp itself. This is important since many MDAs like indole are anti-inflammatory and are beneficial to the host, and exposure to BPA might downregulate the metabolism of Trp.50 Low levels of Trp and MDAs have been associated with increased autoimmune disease activity, including IBD.51,52 Human patients with confirmed IBD had decreased the levels of Trp in serum compared to controls.51 Patients with active CD or UC also had reduced Trp serum levels compared to those whose disease was in remission.51 In a mouse model, increased levels of Trp result in increases in the lactobacilli population in the gut, causing increased indole-3-aldehyde production which contributes to interleukin-22 production and anti-inflammatory effects.52
One specific metabolite, serotonin, is significantly reduced in BPA alone treated animals compared to vehicle controls. While serotonin is also decreased in DSS and BPA-treated animals compared to DSS alone controls, this decrease is not significant. Human patients with UC have reduced levels of serotonin in gut mucosa, likely due to alterations in serotonin synthesis, signaling, and reuptake.20 Reduced serotonin reuptake has been suggested to exacerbate colonic inflammation and the symptoms of IBD including diarrhea.20 Conversely, a study by Ghia et al.53 did not find that mice globally lacking tryptophan hydroxylase 1 had decreased levels of serotonin in the GI tract, but that these mice had decreased colitis severity. While this conflicts with our results, tryptophan hydroxylase 1 deficiency could result in increased levels of metabolites produced via other pathways including the kynurenine pathway by indoleamine 2,3-dioxygenase-1 (IDO-1) which is upregulated during colonic inflammation.51,54 For example, tryptophan depletion following host IDO-1 activation reduced microbial proliferation and increased production of the MDA indole-3-alydehyde in IDO-1 knockout mice infected with Candida albicans.52 These mechanisms cannot be separated in the model used by Ghia et al. In the present study, though concentrations of serotonin were similar between the control and DSS alone groups, a variety of reasons can be implicated, including the sample source (feces rather than mucosa), mouse strain, sex, age, and time of sample collection after initial DSS exposure.
Additional MDAs were significantly reduced with BPA treatment. HIAA, a metabolite of serotonin, was also significantly depleted in BPA-treated animals regardless of DSS treatment. Reduction in HIAA level could result from inhibition of the enzymes that converts serotonin to HIAA, monamino-oxidases, or serotonin uptake.55 The reduction in HIAA without a similar reduction in serotonin in the BPA and DSS-treated animals could indicate impairment of serotonin reuptake and metabolism.55 Additional metabolites were found to have decreased concentrations in BPA-treated mice compared to controls in the presence of DSS treatment. These metabolites include tryptamine, indole-3-acetate, and shikimic acid. Tryptamine and indole-3-acetate have both been previously shown to reduce production of pro-inflammatory cytokines in LPS-stimulated murine macrophages in vitro.56 Shikimic acid has been previously shown to reduce measures of disease activity in acetic acid-induced colitis.57 Regardless of DSS treatment, BPA appears to significantly alter MDAs in ways that negatively impact gut physiology.
Concentration differences in MDAs between DSS-treated groups and controls could be the result of microbial dysbiosis caused by DSS treatment. Alterations in gut microbiome by DSS are well reported.43 However, Trp and several MDAs are decreased in BPA-treated animals that were not exposed to DSS. This indicates that BPA is capable of altering Trp concentrations irrespective of microbiome changes resulting from DSS treatment. Though BPA treatment increased inflammation score in non-DSS-treated animals, this increase was not significant. However, significant changes in measured metabolites show that BPA alters MDAs in the colon and may therefore affect intestinal epithelial cell physiology in uninflamed states as well.
In the present study, BPA treatment reduced fecal Trp content, along with that of MDAs including serotonin and its metabolite HIAA, indicating a mechanism by which BPA treatment worsens DSS-induced colitis disease activity and affects recovery after DSS treatment has been halted. The present study is the first to show that BPA treatment alone can alter MDAs in the colon in a way that has been linked with increased colonic inflammation and IBD. Further studies are necessary to determine the mechanisms by which BPA lowers levels of Trp and MDAs in the colon.
Supplemental Material
Supplemental material for Bisphenol-A alters microbiota metabolites derived from aromatic amino acids and worsens disease activity during colitis by Jennifer AA DeLuca, Kimberly F Allred, Rani Menon, Rebekah Riordan, Brad R Weeks, Arul Jayaraman and Clinton D Allred in Experimental Biology and Medicine
Acknowledgments
The authors thank Dr. Gyhye Yoo for experimental design assistance and technical training, as well as Christina Curry for surgery and termination assistance. Dr. Ivan Ivanov’s assistance in analyzing statistics is also appreciated. The opinions and assertions expressed herein are those of the author(s) and do not necessarily reflect the official policy or position of the Uniformed Services University or the Department of Defense.
Authors’ contributions
JD, KA, AJ, and CA designed the study; JD and KA conducted the animal experiments; RM and RR conducted the metabolite experiments; BW conducted the histopathological work; JD, RM, RR, AJ, and CA analyzed statistical data and interpreted the results; JD, RM, AJ, and CA wrote or contributed to writing of the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funding from the National Institutes of Health National Institute of Environmental Health Sciences Center for Translational Environmental Health Research and the National Cancer Institute (1P30ES023512–01 and 1R01 CA202697).
References
- 1.Molodecky N, Kaplan G. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol 2010; 6:339–46 [PMC free article] [PubMed] [Google Scholar]
- 2.Legaki E, Gazouli M. Influence of environmental factors in the development of inflammatory bowel diseases. World J Gastrointest Pharmacol Ther 2016; 7:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin T, Chan S, Hart A. Environmental factors in the relapse and recurrence of inflammatory bowel disease: a review of the literature. Dig Dis Sci 2015; 60:1396–405 [DOI] [PubMed] [Google Scholar]
- 4.Loftus E, MacIntosh D, Fardy J, Simms L, Sandoval J, Hermon TJ, Rhodes G, Pickup R, Hermon TJ, Ward J, Marcy S, Eriksen E, Destefano F, Chen R, Morettini A. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 2004; 126:1504–17 [DOI] [PubMed] [Google Scholar]
- 5.Michałowicz J. Bisphenol A – sources, toxicity and biotransformation. Environ Toxicol Pharmacol 2014; 37:738–58 [DOI] [PubMed] [Google Scholar]
- 6.Geens T, Aerts D, Berthot C, Bourguignon J, Goeyens L, Lecomte P, Maghuin-Rogister G, Pironnet A, Pussemier L, Scippo M, Van Loco J, Covaci A. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol 2012; 50:3725–40 [DOI] [PubMed] [Google Scholar]
- 7.Vandenberg L, Hauser R, Marcus M, Olea N, Welshons W. Human exposure to bisphenol A (BPA). Reprod Toxicol 2007; 24:139–77 [DOI] [PubMed] [Google Scholar]
- 8.Calafat A, Ye X, Wong L, Reidy J, Needham L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect 2007; 116:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konieczna A, Rutkowska A, Rachoń D. Health risk of exposure to bisphenol A (BPA). Rocz Panstw Zakl Hig 2015; 66:5–11 [PubMed] [Google Scholar]
- 10.United States Environmental Protection Agency, Office of Research and Development IRISD. Bisphenol A; CASRN 80-05-7, https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=356 (1988, accessed 1 December 2017)
- 11.Braniste V, Jouault A, Gaultier E, Polizzi A, Buisson-Brenac C, Leveque M, Martin P, Theodorou V, Fioramonti J, Houdeau E. Impact of oral bisphenol A at reference doses on intestinal barrier function and sex differences after perinatal exposure in rats. Proc Natl Acad Sci U S A 2010; 107:448–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willhite C, Ball G, McLellan C. Derivation of a bisphenol A oral reference dose (RfD) and drinking-water equivalent concentration. J Toxicol Environ Heal Part B 2008; 11:69–146 [DOI] [PubMed] [Google Scholar]
- 13.Vandenberg L, Colborn T, Hayes T, Heindel J, Jacobs D, Lee D, Shioda T, Soto A, Vom Saal F, Welshons W, Zoeller R, Myers J. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 2012; 33:378–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acconcia F, Pallottini V, Marino M. Molecular mechanisms of action of BPA. Dose Response 2015; 13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weige C, Allred K, Allred C. Estradiol alters cell growth in nonmalignant colonocytes and reduces the formation of preneoplastic lesions in the colon. Cancer Res 2009; 69:9118–24 [DOI] [PubMed] [Google Scholar]
- 16.Armstrong C, Billimek A, Allred K, Sturino J, Weeks B, Allred C. A novel shift in estrogen receptor expression occurs as estradiol suppresses inflammation-associated colon tumor formation. Endocr Relat Cancer 2013; 20:515–25 [DOI] [PubMed] [Google Scholar]
- 17.Brinkmeyer-Langford C, Rodrigues A, Kochan K, Haney R, Rassu F, Steelman A, Young C, Riggs P, Storts R, Meagher M, Welsh CJ. Consequences of perinatal bisphenol A exposure in a mouse model of multiple sclerosis. Autoimmunity 2014; 47:57–66 [DOI] [PubMed] [Google Scholar]
- 18.Lai K, Chung Y, Li R, Wan H, Wong CK. Bisphenol A alters gut microbiome: comparative metagenomics analysis. Environ Pollut 2016; 218:923–30 [DOI] [PubMed] [Google Scholar]
- 19.Le Gall G, Noor S, Ridgway K, Scovell L, Jamieson C, Johnson I, Colquhoun I, Kemsley EK, Narbad A. Metabolomics of fecal extracts detects altered metabolic activity of gut microbiota in ulcerative colitis and irritable bowel syndrome. J Proteome Res 2011; 10:4208–18 [DOI] [PubMed] [Google Scholar]
- 20.Coates M, Mahoney C, Linden D, Sampson J, Chen J, Blaszyk H, Crowell M, Sharkey K, Gershon M, Mawe G, Moses P. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 2004; 126:1657–64 [DOI] [PubMed] [Google Scholar]
- 21.Roy A, Gaylo A, Cao W, Saubermann L, Lawrence B. Neither direct nor developmental exposure to bisphenol A alters the severity of experimental inflammatory colitis in mice. J Immunotoxicol 2013; 10:334–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong C, Allred K, Weeks B, Chapkin R, Allred C. Estradiol has differential effects on acute colonic inflammation in the presence and absence of estrogen receptor β expression. Dig Dis Sci 2017; 62:1977–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verdú E, Deng Y, Bercik P, Collins S. Modulatory effects of estrogen in two murine models of experimental colitis. Am J Physiol Gastrointest Liver Physiol 2002; 283:G27–36 [DOI] [PubMed] [Google Scholar]
- 24.Cook L, Hillhouse A, Myles M, Lubahn D, Bryda E, Davis J, Franklin C. The role of estrogen signaling in a mouse model of inflammatory bowel disease: a helicobacter hepaticus model. PLoS One 2014; 9:e94209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad P, Manicassamy S, Munn D, Lee J, Offermanns S, Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014; 40:128–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murthy S, Cooper H, Shim H, Shah R, Ibrahim S, Sedergran D. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Digest Dis Sci 1993; 38:1722–34 [DOI] [PubMed] [Google Scholar]
- 27.Sridharan G, Choi K, Klemashevich C, Wu C, Prabakaran D, Pan L, Steinmeyer S, Mueller C, Yousofshahi M, Alaniz R, Lee K, Jayaraman A. Prediction and quantification of bioactive microbiota metabolites in the mouse gut. Nat Commun 2014; 5:5492. [DOI] [PubMed] [Google Scholar]
- 28.Whitfield-Cargile C, Cohen N, Chapkin R, Weeks B, Davidson L, Goldsby J, Hunt C, Steinmeyer S, Menon R, Suchodolski J, Jayaraman A, Alaniz R. The microbiota-derived metabolite indole decreases mucosal inflammation and injury in a murine model of NSAID enteropathy. Gut Microbes 2016; 7:246–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conover W, Iman R. Rank transformations as a bridge between parametric and nonparametric statistics. Am Stat 1981; 35:124 [Google Scholar]
- 30.Yan Y, Kolachala V, Dalmasso G, Nguyen H, Laroui H, Sitaraman S, Merlin D. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS One 2009; 4:6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alex P, Zachos N, Nguyen T, Gonzales L, Chen T, Conklin L, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis 2009; 15:341–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egger B, Bajaj-Elliott M, MacDonald T, Inglin R, Eysselein V, Büchler M. Characterisation of acute murine dextran sodium sulphate colitis: Cytokine profile and dose dependency. Digestion 2000; 62:240–8 [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Shajib S, Manocha M, Khan W. Investigating intestinal inflammation in DSS-induced model of IBD. J Vis Exp 2012; 60:3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Hong Y, Oh S, Park M, Kim H, Leem J, Ha E. Bisphenol A exposure is associated with oxidative stress and inflammation in postmenopausal women. Environ Res 2009; 109:797–801 [DOI] [PubMed] [Google Scholar]
- 35.Savastano S, Tarantino G, D’Esposito V, Passaretti F, Cabaro S, Liotti A, Liguoro D, Perruolo G, Ariemma F, Finelli C, Beguinot F, Formisano P, Valentino R. Bisphenol-A plasma levels are related to inflammatory markers, visceral obesity and insulin-resistance: a cross-sectional study on adult male population. J Transl Med 2015; 13:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valentino R, D’Esposito V, Passaretti F, Liotti A, Cabaro S, Longo M, Perruolo G, Oriente F, Beguinot F, Formisano P. Bisphenol-A impairs insulin action and up-regulates inflammatory pathways in human subcutaneous adipocytes and 3T3-L1 cells. PLoS One 2013; 8:82099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang M, Chen M, Wang J, Xu M, Sun J, Ding L, Lv X, Ma Q, Bi Y, Liu R, Hong J, Ning G. Bisphenol A promotes adiposity and inflammation in a nonmonotonic dose-response way in 5-week-old male and female C57BL/6J mice fed a low-calorie diet. Endocrinology 2016; 157:2333–45 [DOI] [PubMed] [Google Scholar]
- 38.Roy A, Bauer S, Lawrence B. Developmental exposure to bisphenol A modulates innate but not adaptive immune responses to influenza A virus infection. PLoS One 2012; 7:38448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waldner M, Neurath M. Chemically induced mouse models of colitis. In: Current protocols in pharmacology Hoboken, NJ: John Wiley & Sons, Inc., 2009, pp.5.55.1–5.55.15 [DOI] [PubMed]
- 40.Chassaing B, Aitken J, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol 2014; 104:15.25.1–15.25.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ikejima K, Enomoto N, Iimuro Y, Ikejima A, Fang D, Xu J, Forman D, Brenner D, Thurman R. Estrogen increases sensitivity of hepatic Kupffer cells to endotoxin. Am J Physiol 1998; 274:G669–76 [DOI] [PubMed] [Google Scholar]
- 42.Enomoto N, Yamashina S, Schemmer P, Rivera C, Bradford B, Enomoto A, Brenner D, Thurman R. Estriol sensitizes rat Kupffer cells via gut-derived endotoxin. Am J Physiol 1999; 277:G671–7 [DOI] [PubMed] [Google Scholar]
- 43.Gkouskou K, Deligianni C, Tsatsanis C, Eliopoulos A. The gut microbiota in mouse models of inflammatory bowel disease. Front Cell Infect Microbiol 2014; 4:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Mei C, Liu H, Wang H, Zeng G, Lin J, Xu M. Modulation of cytokine expression in human macrophages by endocrine-disrupting chemical bisphenol-A. Biochem Biophys Res Commun 2014; 451:592–8 [DOI] [PubMed] [Google Scholar]
- 45.Dambacher J, Beigel F, Seiderer J, Haller D, Göke B, Auernhammer C, Brand S. Interleukin 31 mediates MAP kinase and STAT1/3 activation in intestinal epithelial cells and its expression is upregulated in inflammatory bowel disease. Gut 2007; 56:1257–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter A, Bürgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke J. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005; 129:550–64 [DOI] [PubMed] [Google Scholar]
- 47.Alkim C, Alkim H, Koksal A, Boga S, Sen I. Angiogenesis in inflammatory bowel disease. Int J Inflam 2015; 2015:970890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chernoguz A, Crawford K, Vandersall A, Rao M, Willson T, Denson L, Frischer J. Pretreatment with anti-VEGF therapy may exacerbate inflammation in experimental acute colitis. J Pediatr Surg 2012; 47:347–54 [DOI] [PubMed] [Google Scholar]
- 49.Javurek A, Spollen W, Johnson S, Bivens N, Bromert K, Givan S, Rosenfeld C. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes 2016; 7:471–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bansal T, Alaniz R, Wood T, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci U S A 2010; 107:228–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nikolaus S, Schulte B, Al-Massad N, Thieme F, Schulte D, Bethge J, Rehman A, Tran F, Aden K, Häsler R, Moll N, Schütze G, Schwarz M, Waetzig G, Rosenstiel P, Krawczak M, Szymczak S, Schreiber S. Increased tryptophan metabolism is associated With activity of inflammatory bowel diseases. Gastroenterology 2017; 153:1504–16 [DOI] [PubMed] [Google Scholar]
- 52.Zelante T, Iannitti R, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, Carvalho A, Puccetti P, Romani L. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013; 39:372–85 [DOI] [PubMed] [Google Scholar]
- 53.Ghia J, Li N, Wang H, Collins M, Deng Y, El–Sharkawy R, Côté F, Mallet J, Khan W. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 2009; 137:1649–60 [DOI] [PubMed] [Google Scholar]
- 54.Ciorba M. Indoleamine 2,3 dioxygenase in intestinal disease. Curr Opin Gastroenterol 2013; 29:146–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spiller R. Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: alterations in 5-HT signalling and metabolism in human disease. Neurogastroenterol Motil 2007; 19:25–31 [DOI] [PubMed] [Google Scholar]
- 56.Krishnan S, Ding Y, Saedi N, Choi M, Sridharan G, Sherr D, Yarmush M, Alaniz R, Jayaraman A, Lee K. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep 2018; 23:1099–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xing J, Sun J, Sun J, Hu S, Guo C, Wang M, Dong Y. Protective effect of shikimic acid on acetic acid induced colitis in rats. J Med Plants Res 2012; 6:2011–8 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Bisphenol-A alters microbiota metabolites derived from aromatic amino acids and worsens disease activity during colitis by Jennifer AA DeLuca, Kimberly F Allred, Rani Menon, Rebekah Riordan, Brad R Weeks, Arul Jayaraman and Clinton D Allred in Experimental Biology and Medicine