Short abstract
MicroRNAs (miRNAs) are a small functional non-coding RNAs that post-transcriptionally regulate gene expression through mRNA degradation or translational repression. miRNAs are key regulatory components of various cellular networks. Current evidence support that multiple mammalian genome-encoded miRNAs impact the cellular biology, including proliferation, apoptosis, differentiation, and tumorigenesis, by targeting specific subsets of mRNAs. This minireview is focused on the current themes underlying the interactions between miRNAs and their mRNA targets and pathways in prostate tumorigenesis and progression, and their potential clinical utility as biomarkers for prostate cancer.
Impact statement
The primary goal of this article was to review recent literature on miRNA biogenesis and further elaborate on the identity of newly discovered miRNAs and their potential functional significance in the complex biological network associated with prostate tumorigenesis and disease progression and as biomarkers for prostate cancer.
Keywords: Prostate cancer, microRNA, onco-miR, tumor suppressor miRNAs, biomarkers
Introduction
Behind lung and colorectal cancer, prostate cancer (PC) is the most common and the third leading cause of cancer-related death among American men.1 According to the 2017 American Cancer Society report, PC is the most common cancer among American males, with about 164,690 newly diagnosed cases and an estimated 29,430 PC-related deaths in 2018.1,2 Family history, age, and African Ancestry are among the well-established risk factors for PC.2 Despite significant advances in disease stratification and functional imaging, challenges in the clinical management exist primarily due to the inherent problems associated with inaccurate disease staging/grading by needle biopsy or prostate-specific antigen (PSA).3 Currently, there are no sensitive and reliable biomarkers that can distinguish patients with less aggressive localized disease from those presenting with an aggressive phenotype.
MicroRNAs (miRNAs) are non-coding RNAs with short sequences (18–22 nucleotides) with the ability to regulate a wide array of cellular functional molecules under physiologic and disease states. MiRNAs repress target-gene expression post-transcriptionally either by inhibiting translation or promoting RNA degradation by binding to the 3′ untranslated region (3′UTR) of mRNA sequences. In cancer, miRNAs target and regulate the expression of multiple target genes, including oncogenic factors and tumor suppressors and their downstream effectors. The stability and existence of circulating free and exosomal miRNAs in biological biofluids (blood, urine, spinal fluids, etc.) made them attractive targets for biomarker discovery and development of non-invasive liquid biopsy clinical tools, including screening, diagnosis, prognosis, monitoring tumor progression, and therapeutic response. Identification and association of unique miRNA signatures with PC tumorigenesis have been established4,5 and their potential clinical utilities as novel diagnostic and prognostic tools for PC have been explored.6,7 The purpose of the current review is to highlight the current knowledge related to miRNAs in prostate tumorigenesis and progression and further discuss their potential clinical utility as biomarkers for PC.
PC: Diagnosis and treatment options
Located below the bladder, the prostate gland is a walnut-sized gland that encircles the urethra.8 Male ejaculate contains about 30% of prostatic fluid.9 From a histological point of view, the prostate gland is a tubuloalveolar exocrine gland of the male reproductive system.10 Prostate epithelial cells consist of three types of cells that can be categorized by morphological and functional significance. First, the secretory luminal cells are the most predominant, differentiated, and androgen-dependent cells that are responsible for the secretion of prostatic proteins. Different biomarkers are expressed by these cells, including androgen receptor (AR), cytokeratin 8 and 18, and cell surface marker CD57.11–13 Second, the basal cells are found between the luminal cells and the basal membrane and represent the second major type of prostate epithelial cell. These cells are characterized by the molecular expression of cytokeratin 5 and 14, as well as CD44 and a low level of AR.11–14 Third, the neuroendocrine cells are a minor population of prostatic cells that confer growth signal to the luminal cells. They are AR-independent cells and express chromogranin A, serotonin, and various neuropeptides.15–17
Androgens and their cognate AR play a pivotal role in prostate gland homeostasis as well as in the development and progression of prostate tumorigenesis.18 Like other steroid hormone groups of nuclear receptors, the AR is a ligand-dependent transcription factor that regulates expression of androgen-regulated genes. AR amplification and mutations have been implicated in the development and progression of PC.19,20 During androgen-dependent PC progression, the ratio between the proliferating cells and the dying cells is maintained by an androgen hormone, which stimulates proliferation and inhibits apoptosis.21 Promiscuous binding and activation of mutant AR by steroid hormones and non-canonical activation by kinases and long non-coding RNAs have been implicated in the development of castration-resistant PC (CRPC).22
Treatment options for the early diagnosed indolent disease include surgery and/or radiotherapy but may have negative effects that adversely affect patients’ quality of life. Nonetheless, recent changes in the grading system and monitoring of high-risk PC have reduced overtreatment and improved disease management and quality of life. This may be attributed to improved risk stratification and advances in functional imaging (e.g. multiparametric magnetic resonance imaging, mpMRI) and emergence of new biomarkers. As a result, incorporation of monitoring modalities, such as active surveillance, emerged as a preferred approach for the management of less-aggressive indolent disease with PSA level of 10 ng/mL. Despite an initial response to various treatment regimens, including first-line androgen-deprivation therapy,23 some patients inevitably progress to CRPC via unknown mechanisms.24 Chemotherapy, vaccines, second-generation hormonal therapeutics, and bone-targeting agents have proven efficacious against metastatic CRPC.
Despite limitations, PSA along with the digital rectal examination remains to be the gold standard approach for PC screening and prognosis.25 The widespread use of PSA has led to the early detection of PC and a reduction of metastatic disease at diagnosis; however, the overall benefit of monitoring serum PSA after treatment remains controversial. Men without elevated PSA may present with clinically significant PC; about 15% of PC cases were reported in men with very low serum PSA levels.26 Additionally, the inconclusive reports from two randomized trials on the benefits and impact of PSA screening and subsequent treatment27,28 prompted the U.S. Preventive Service Task Force (USPSTF) to advise against PSA screening. Accordingly, several new diagnostic markers have been developed for PC screening,26–29 including serum and urine detection of RNA biomarkers (e.g. PCA3), PC tissue protein antibodies (e.g. EPCA), and the TMPRSS2:ERG gene fusion; however, their sensitivities and specificities remain to be established. Such limitations necessitate the development of a new predictive and reliable biomarker(s) that may serve as surrogate end points to improve distinction between indolent and clinical progression of the disease and/or patient survival.
MicroRNA (miRNA/miR): Biogenesis and nomenclature
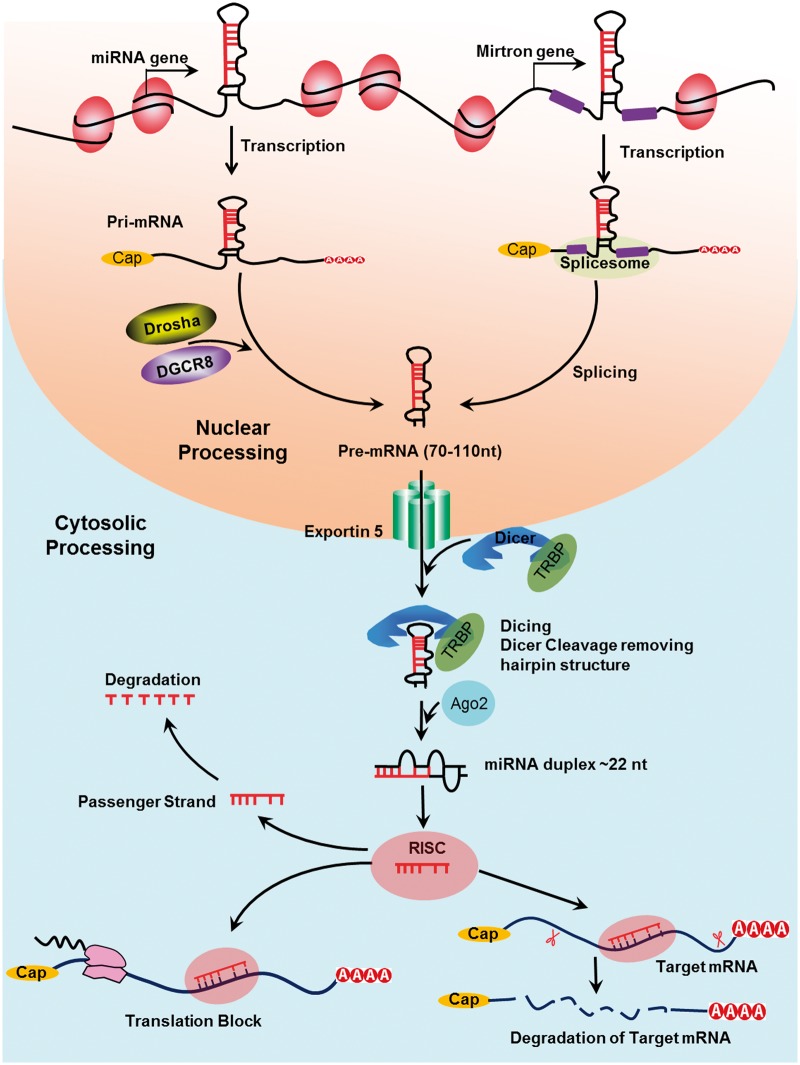
MicroRNAs (miRNAs/miRs) were originally discovered in Caenorhabditis elegans and were later discovered in eukaryotic and human cells.30,31 miRNAs are a class of endogenous, short non-coding, evolutionarily conserved RNA molecule.32,33 This short non-coding RNA molecule regulates its many targets at the post-transcriptional level.32–36 miRNAs are transcribed by RNA polymerase II and III into a primary miRNA transcript (pri-miRNA). Pri-miRNA is characterized by a secondary hairpin structure. A microprocessor complex cleaved the pri-miRNA.37,38 The class 2 ribonuclease III enzyme Drosha cuts the 5'and the 3' arms of the pri-miRNA hairpin, producing 70–110 nucleotides length, known as “precursor miRNA (pre-miRNA).” Following the nuclear processing, exportin 5 (XPO5) export the pre-miRNA to the cytoplasm,39 where Dicer cleaves the pre-miRNA to generate a ∼22-nucleotide miRNA duplex. This duplex binds to the active RNA-induced silencing complex (RISC) that performs gene silencing.
The double miRNA duplex is composed of a functional strand (which is complementary to the target mRNA) and the passenger strand (which is subsequently degraded). According to Chendrimada et al., the mature miRNA functional strand is loaded into the RISC complex and Argonaute 2 (AGO2).40 RISC-AGO2 complex guides the functional strand to target the 3'UTR of the target miRNA, causing translational inhibition or promoting their degradation. Bartel et al. and Hausser et al. reported that the specificity of miRNA can be determined by the base pairing between the seed sequence (a 6–8 nucleotide sequence in the 5' end of the mature miRNA), and the 3'UTR sequence of the target gene41,42 (Figure 1).
Figure 1.
Biogenesis of microRNA: The biogenesis of miRNAs. miRNA is transcribed in the nucleus as a pri-miRNA and then is micro-processed by Drosha, a class 2 ribonuclease III enzyme, and transported to the cytoplasm by exportin 5 (XPO5) where the hairpin structure is removed by Dicer and a single-stranded mature miRNA is produced, which binds to RISC and induces gene silencing.
According to Ambros et al.,43 a special annotation system has been adopted for miRNA nomenclature. Briefly, newly identified miRNA genes have a sequential number that is preceded by a prefix “mir/or MiR,” and then followed by a dash (e.g. mir-19 or miR-19). The pre-miRNA is denoted as lowercase “mir,” while mature miRNA as capitalized “miR.” With lowercase letters, closely related mature miRNA sequences are marked to show their structural similarity (for example, miR-19a and miR-19b). If the mature sequences are expressed from different precursor sequences and genomic loci, an additional number will be added to the miRNA name (for instance, miR-19a1 and miR-19a2). Species annotation should also be mentioned in the miRNA name (for example, has miR-19 in Homo sapiens and mmu-miR-19 in Mus musculus). If the mature miRNA sequence originated from 3' or 5' end, the suffix,-3p' or, -5p' it would be denoted, respectively (e.g. miR-19a-3p and miR-19a-5p).43 A database of miRNA structure and nomenclature is available on the web at http://www.mirbase.org/.44
miRNAs silence their targets by binding to the 3′UTR, inducing mRNA degradation or translational repression.34 Many cellular processes were found to be regulated by miRNAs.45 The potential role of miRNAs in the pathogenesis of different types of cancer has been reported,33,45–47 including initiation, propagation, and metastasis.46,48
Onco-miRNAs in PC
The process of prostate tumorigenesis is a multifactorial and multistep process, during which different cellular and genetic changes cause normal prostate cells to become malignant.49,50 MiRs control many cellular processes and their altered signature was reported in many diseases, including cancer.45,46,51 In cancer, miRNAs may act as “onco-miRNAs” and drive the cells toward cancer progression.44 The first study that described the relationship between miRNA dysregulation and cancer was led by Calin et al.,52 who reported the dysregulation of miR-15 and miR-16 and their role in the pathogenesis of chronic lymphocytic leukemia.
Several studies reported the role of miRs in PC tumorigenesis.27,47 For instance, we reported that exosomal oncogenic miR-125b, miR-130b, and miR-155 are implicated in the oncogenic reprogramming of PC-recruited stem cells.53 Mechanistic studies revealed that exosomal trafficking of miR-125b and miR-155 potentially initiates neoplastic reprogramming in the stem cells by targeted inhibition of the large tumor suppressor homolog2 and programmed cell death protein 4, a neoplastic transformation inhibitor.53 Moreover, miR-125 was found to act as an oncogene by targeting pro-apoptotic genes in PC.54 Furthermore, it has been reported that miR-22 contributes to prostate tumorigenesis by targeting PTEN, the loss of which activates PI3K signaling in PC cells.55 In 2015, Seashole et al. described the relationship between the altered signature of miR-9 and PC progression and metastasis.56 Also, miR-27a was reported to regulate AR processing via targeting prohibition in PC.57 Additionally, miR-18 was reported to be highly expressed in PC tissue and cell lines and also act as an onco-miRNA by targeting the tumor suppressor serine/threonine-protein kinase 4.58
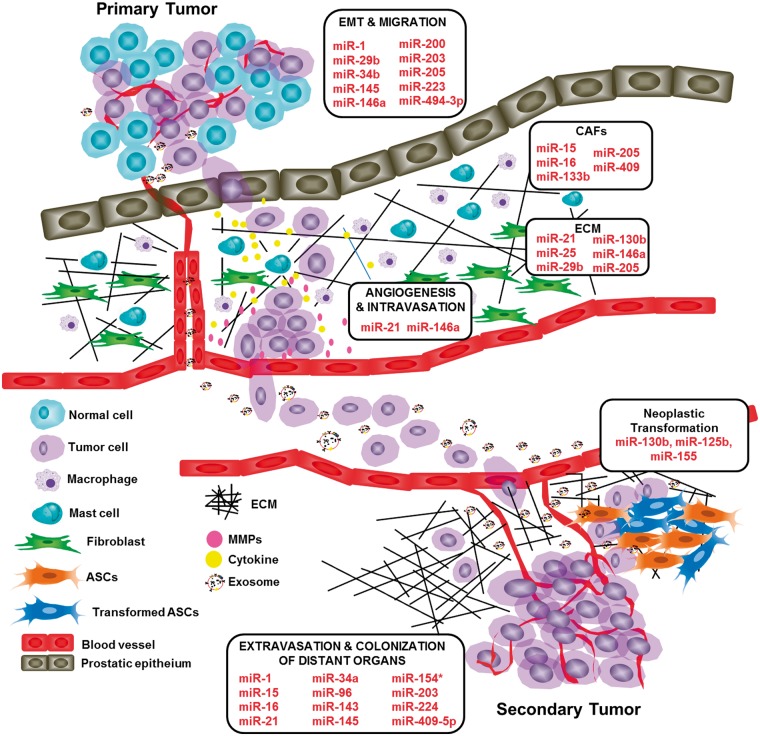
Bone metastasis and skeletal-related events are critical steps in PC progression and portend poorer prognosis than those without metastatic disease.59 The role of miRs in cancer metastasis has been reported in many studies.60–62 MiR-96, miR-154, and miR-409–5p were reported to be upregulated in PC and potentially play a crucial role in bone colonization during PC metastasis.63–65 Likewise, an elevated level of miR-21 induces angiogenesis through AKT and ERK activation in PC.66 Another study reported that in DU-145, a brain metastatic PC cell line, upregulation of miR-21 contributed to PC via targeting matrix metalloproteinase regulator inhibitor RECK.67 PC progression was related to upregulation of miR-133b, miR-409, and miR-210 through targeting key molecules that induce fibroblast activations.68–70 More recently, Bertoli et al. described the relationship between altered miR signature and PC metastasis71 (Figure 2).
Figure 2.
Overview of miRNAs contribution in the metastatic process. The possible miRs function in preventing or promoting PC metastasis through regulation of epithelial-to-mesenchymal transition, regulation of extracellular matrix structure, regulation of anoikis and bone colonization.
Tumor-suppressor miRs in PC
According to Koturbash et al., miRNA may also act as a tumor suppressor gene.35 Several studies reported a relationship between miR downregulation and cancer progression.72–74 In 2015, we showed that dysregulation of miR-212 confers the development of castration-resistant disease via priming hnRNPH1-mediated upregulation of AR and AR-V7.75 MiR-146b was found to be downregulated in prostate tumor tissue and is considered to be a potential tumor suppressor.76 Another study showed downregulation of miR-335 in three PC cell lines (LNCaP/DU145/PC3) and also in PC tissues.77 Zhu et al. reported that miR-30a exerts a tumor suppressor effect and inhibits the proliferation and invasion of PC cells via targeting of sine oculis homeobox homolog 1.78 We recently reported that miRs-595, 4490, -3120–5p, -1299, -21–5p, -3677–3, -let-7b-5p, -5189, 3–121-5p, -4518, -200a-5p, -3682–5p, -3689d, -3149 are downregulated (12–113-fold) in microdissected prostate tumors as compared with adjacent normal glands.47 Subsequent pathway and target prediction analysis showed that several miRs may serve as potential tumor suppressors.47
Additionally, miR-1, miR-29b, and miR-200 were found to be participatory in PC progression via regulation of epithelial–mesenchymal transition signaling pathway (EMT).79,80 Similarly, downregulation of miR-34b, miR-145, miR-146a, miR-200, and miR-205 inhibit PC migration and invasion via targeting of AKT, ZEB2, ROCK1, SNAI2, and centromere protein F, respectively.79 Also, downregulation of miR-29b and miR-130b in PC was found to be related to modulation of the extracellular matrix structure via targeting matrix metalloproteinase 2 (MMP2).80,81 Gandellin et al.82,83 reported that miR-205 downregulation in PC contributed to extracellular matrix structure regulation via targeting laminin-332, integrin-β4, MMP-2in, which in turn inhibits PC progression. Furthermore, downregulation of miR-146a in PC was related to inhibition of angiogenesis via targeting epidermal growth factor receptor pathway.84 Additionally, miR-1 downregulation promotes PC progression and metastasis via targeting Src and TWIST1.85,86 In PC, targeted downregulation of loss of miR-154, miR-203, and miR-224 due to stromal antigen 2 (STAG2), EREG, tumor growth factor-alpha (TGF-α), and Tribbles Pseudokinase 1 (TRIB1), respectively, play a regulatory role in EMT and PC progression.63,87,88
Overall, the diverse role of microRNAs in prostate tumorigenesis has attracted a surge of interest to gain more understanding of their functional significance in suppression and progression of prostate tumorigenesis. Table 1 summarizes the list of selected miRNAs implicated in various processes of prostate tumorigenesis, including tumor suppressor miRs and onco-miRs implicated in tumor growth, neoplastic reprogramming of stem cells, EMT, CAFs, angiogenesis, extravasation, and colonization of distant organs.
Table 1.
List of miRNAs in metastatic PC.
| Function | mRNA | Target | Reference |
|---|---|---|---|
| Tumor growth | miR-125b | p53, Puma, and Bak1 | 54,55 |
| miR-155 | ANX7 | 54,73 | |
| miR-27a | MAP2K4 | 60 | |
| Neoplastic transformation intumor recruited stem cells | miR-125b | p53 | 53 |
| miR-130b | TP53INP1 | 53 | |
| miR-155 | p53 | 53 | |
| EMT and migration | miR-1 | Src, TWIST1, Slug | 79,85,86 |
| miR-29b | Snail | 80 | |
| miR-34b | AKT | 78 | |
| miR-145 | ZEB2 | 80 | |
| miR-146a | EGFR | 84 | |
| miR-200 | Slug | 79 | |
| miR-205 | CENPF, ZEB2 and PKCε | 82,83 | |
| miR-130b | MMP2 | 81 | |
| miR-212 | SOX4 | 75 | |
| CAFs (cancer-associated fibroblasts)and angiogenesis | miR-15 and miR-16 | Bcl-2, Ccnd1, Ccne1, Bmi-1 andWnt family members | 52 |
| miR-21 | RECK | 65,67 | |
| miR-133b | EGFR | 70 | |
| miR-205 | CENPF, ZEB2 and PKCε | 82,83 | |
| miR-409 | RSU1, and PHC3 | 64,69 | |
| Extravasation and colonization of distant organs | miR-1 | Src, TWIST1, Slug | 79,85,86 |
| miR-96 | AKT1S1 | 65 | |
| miR-145 | ZEB2 | 80 | |
| miR-154* | STAG2 | 63 | |
| miR-203 | SRC, SUZ12, API5 , BIRC2, EREG, TRIAP1 and TGF-α | 88 | |
| miR-224 | TRIB1 | 87 | |
| miR-409-5p | STAG2, NPRL2, RSU1 and RBL2 | 64 | |
| Tumor suppressor | miR-9 | E-cadherin and SOCS5 | 56 |
| miR-18 | STK4 | 58 | |
| miR-22 | E-cadherin | 55 | |
| miR-146b | 76 | ||
| miR-30a | SIX1 | 78 | |
| miR-335 | eNOS | 77 |
miRNAs as potential circulating biomarkers for PC
Development of a new biomarker strategy for PC screening is urgently needed due to the limitations of the current tools. Early tumor identification and detection are important elements to informed decision-making in the disease management, prognosis, and overall survival of PC. Exosomes are nanovesicles 50 to 150 nm in size formed within the cells as early endosomes containing miRNAs, mRNA, proteins, and lipids and are particularly important in cell–cell communication and modulation of the biology of recipient cells,89 and indeed are considered to play a fundamental role in many physiological and pathological processes.90 Recently, exosomes have been detected in various body fluids of cancer patients and conditioned media of cultured cells.91 The PC-derived exosomes are detected in the prostatic secretions, seminal fluid,92 urine,93 and blood,94,95 implicating their clinical utility as “liquid biopsies” in the diagnosis and prognosis of PC. The blood-circulating exosomal miRs have been employed as prognostic markers in many types of tumors.96 In 2008, a study conducted by Mitchell et al. demonstrated that elevated expression of miR-141 can be used as a marker to distinguish between PC patients and normal subjects.97 Another study done by Lodes et al. confirmed Mitchell’s and his team’s observation.98 MiR-141 and miR-375 were found to be upregulated in PC patients and their sera levels were associated with disease progression.99 Huang et al. showed that plasma exosomal miR-1290 and miR-375 are promising prognostic biomarkers for CRPC patients.100 The PC-related miRNA in urinary exosomes revealed significant upregulation of miR-574–3p, miR-141–5p, and miR-21–5p associated with PC.101 In a genome-wide serum profiling study by Haldrup et al., several new miR markers for PC and disseminated disease were identified in comparison to benign prostatic hyperplasia and localized disease.102 These and other findings prove the potential clinical utility of miRs as promising biomarkers for PC screening and diagnosis. As with any new biomarker, there are challenges and limitations associated with miRNAs as cancer biomarkers. Before the translation of miRNAs as biomarkers in clinical practice, steps need to be undertaken to remove inaccuracies and inconsistencies due to non-uniform sample choice, handling, and processing, sample preparation and lack of consensus for data normalization. The development and the application of SOPs for whole blood collection to plasma/serum preparation, handling, and banking to RNA extraction and miRNA quantification would keep interlaboratory differences to a minimum and also limit incoherencies among different users. Also, utilization of polymerase chain reaction for miRNA detection may pose a technical challenge, as amplification bias may limit the ability to accurately quantify the relative abundance of specific miRNA targets.103,104 Another caveat in using miRNAs as a biomarker currently is the high costs associated with detection and validation. Cost-effective techniques, such as next-generation sequencing, allow for a faster and more accurate miR profiling. Overcoming such limitations would increase the potential utility of miRNAs as biomarkers in the clinical management of PC.
Conclusions
As their role, in large part, remains elusive, it is premature to determine whether miRs are tipping the balance towards suppression or progression of the disease. Considering the complexity and heterogeneity of the disease, further studies are required to unravel the functional role of miRs in the underlying mechanisms that govern the stepwise transition from an indolent disease into the aggression castration-resistant phenotype. Finally, additional studies are needed to establish the clinical utility of miRs as a multistep screening strategy for early detection and disease progression.
Authors’ Contributions
All authors were involved in writing the manuscript. All authors reviewed, edited, and approved of the final submission.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present work was supported by a grant from the NIH/NCATS (UH2TR000928) to A.B.A.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA 2018; 68:7–30 [DOI] [PubMed] [Google Scholar]
- 2.Society AC. Cancer Facts & Figures 2017. www.cancer.org/cancer/prostate-cancer/about/key-statistics.html (accessed 25 September 2017)
- 3.Turkbey B, Brown AM, Sankineni S, Wood BJ, Pinto PA, Choyke PL. Multiparametric prostate magnetic resonance imaging in the evaluation of prostate cancer. CA 2016; 66:326–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allory Y, Beukers W, Sagrera A, Flández M, Marqués M, Márquez M, van der Keur KA, Dyrskjot L, Lurkin I, Vermeij M, Carrato A, Lloreta J, Lorente JA, Carrillo-de Santa Pau E, Masius RG, Kogevinas M, Steyerberg EW, van Tilborg AA, Abas C, Orntoft TF, Zuiverloon TC, Malats N, Zwarthoff EC, Real FX. Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur Urol 2014; 65:360–6 [DOI] [PubMed] [Google Scholar]
- 5.Ren Q, Liang J, Wei J, Basturk O, Wang J, Daniels G, Gellert LL, Li Y, Shen Y, Osman I, Zhao J, Melamed J, Lee P. Epithelial and stromal expression of miRNAs during prostate cancer progression. Am J Transl Res 2014; 6:329–39 [PMC free article] [PubMed] [Google Scholar]
- 6.Ozen M, Creighton CJ, Ozdemir M, Ittmann M. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene 2008; 27:1788–93 [DOI] [PubMed] [Google Scholar]
- 7.Porkka KP, Pfeiffer MJ, Waltering KK, Vessella RL, Tammela TL, Visakorpi T. MicroRNA expression profiling in prostate cancer. Cancer Res 2007; 67:6130–5 [DOI] [PubMed] [Google Scholar]
- 8.Leissner KH, Tisell LE. The weight of the human prostate. Scand J Urol Nephrol 1979; 13:137–42 [DOI] [PubMed] [Google Scholar]
- 9.Lilja H. Structure and function of prostatic- and seminal vesicle-secreted proteins involved in the gelation and liquefaction of human semen. Scand J Clin Lab Invest Suppl 1988; 191:13–20 [PubMed] [Google Scholar]
- 10.Tsukise A, Yamada K. Complex carbohydrates in the secretory epithelium of the goat prostate. Histochem J 1984; 16:311–9 [DOI] [PubMed] [Google Scholar]
- 11.Brawer MK, Peehl DM, Stamey TA, Bostwick DG. Keratin immunoreactivity in the benign and neoplastic human prostate. Cancer Res 1985; 45:3663–7 [PubMed] [Google Scholar]
- 12.Nagle RB, Ahmann FR, McDaniel KM, Paquin ML, Clark VA, Celniker A. Cytokeratin characterization of human prostatic carcinoma and its derived cell lines. Cancer Res 1987; 47:281–6 [PubMed] [Google Scholar]
- 13.Sherwood ER, Berg LA, Mitchell NJ, McNeal JE, Kozlowski JM, Lee C. Differential cytokeratin expression in normal, hyperplastic and malignant epithelial cells from human prostate. J Urol 1990; 143:167–71 [DOI] [PubMed] [Google Scholar]
- 14.Liu AY, True LD, LaTray L, Nelson PS, Ellis WJ, Vessella RL, Lange PH, Hood L, van den Engh G. Cell-cell interaction in prostate gene regulation and cytodifferentiation. Proc Natl Acad Sci U S A 1997; 94:10705–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abrahamsson PA, Cockett AT, di Sant'Agnese PA. Prognostic significance of neuroendocrine differentiation in clinically localized prostatic carcinoma. Prostate Suppl 1998; 8:37–42 [PubMed] [Google Scholar]
- 16.di Sant'Agnese PA. Neuroendocrine differentiation in carcinoma of the prostate. Diagnostic, prognostic, and therapeutic implications. Cancer 1992; 70:254–68 [DOI] [PubMed] [Google Scholar]
- 17.di SAgnese PA. Neuroendocrine cells of the prostate and neuroendocrine differentiation in prostatic carcinoma: a review of morphologic aspects. Urology 1998; 51:121–4 [DOI] [PubMed] [Google Scholar]
- 18.Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev 2000; 14:2410–34 [DOI] [PubMed] [Google Scholar]
- 19.Gottlieb B, Beitel LK, Nadarajah A, Paliouras M, Trifiro M. The androgen receptor gene mutations database: 2012 update. Hum Mutat 2012; 33:887–94 [DOI] [PubMed] [Google Scholar]
- 20.Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, Trapman J, Cleutjens K, Noordzij A, Visakorpi T, Kallioniemi OP. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res 1997; 57:314–9 [PubMed] [Google Scholar]
- 21.Denmeade SR, Lin XS, Isaacs JT. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate 1996; 28:251–65 [DOI] [PubMed] [Google Scholar]
- 22.Tan MH, Li J, Xu HE, Melcher K, Yong EL. Androgen receptor: structure, role in prostate cancer and drug discovery. Acta Pharmacol Sin 2015; 36:3–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu W, Madan E, Yee M, Zhang H. Progress of molecular targeted therapies for prostate cancers. Biochim Biophys Acta 2012; 1825:140–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masson S, Bahl A. Metastatic castrate-resistant prostate cancer: dawn of a new age of management. BJU Int 2012; 110:1110–4 [DOI] [PubMed] [Google Scholar]
- 25.Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, Gulati R, Feuer E, de Koning H. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst 2009; 101:374–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA., Jr. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med 2004; 350:2239–46 [DOI] [PubMed] [Google Scholar]
- 27.Andriole GL, Crawford ED, Grubb RL, Buys SS, 3rd, Chia D, Church TR, Fouad MN, Isaacs C, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O'Brien B, Ragard LR, Clapp JD, Rathmell JM, Riley TL, Hsing AW, Izmirlian G, Pinsky PF, Kramer BS, Miller AB, Gohagan JK, Prorok PC. Plco Project Team. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst 2012; 104:125–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Määttänen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, Blijenberg BG, Moss SM, de Koning HJ, Auvinen A, Erspc I. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009; 360:1320–8 [DOI] [PubMed] [Google Scholar]
- 29.Wright JL, Lange PH. Newer potential biomarkers in prostate cancer. Rev Urol 2007; 9:207–13 [PMC free article] [PubMed] [Google Scholar]
- 30.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993; 75:843–54 [DOI] [PubMed] [Google Scholar]
- 31.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993; 75:855–62 [DOI] [PubMed] [Google Scholar]
- 32.Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell 2005; 123:631–40 [DOI] [PubMed] [Google Scholar]
- 33.Sassen S, Miska EA, Caldas C. MicroRNA: implications for cancer. Virchows Arch 2008; 452:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krutzfeldt J, Poy MN, Stoffel M. Strategies to determine the biological function of microRNAs. Nat Genet 2006; 38 Suppl:S141–9 [DOI] [PubMed] [Google Scholar]
- 35.Koturbash I, Zemp FJ, Pogribny I, Kovalchuk O. Small molecules with big effects: the role of the microRNAome in cancer and carcinogenesis. Mutat Res 2011; 722:94–105 [DOI] [PubMed] [Google Scholar]
- 36.Macfarlane LA, Murphy PR. MicroRNA: biogenesis, function and role in cancer. Curr Genomics 2010; 11:537–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borchert GM, Lanier W, Davidson BL. RNA polymerase III transcribes human microRNAs. Nat Struct Mol Biol 2006; 13:1097–101 [DOI] [PubMed] [Google Scholar]
- 38.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Rådmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003; 425:415–9 [DOI] [PubMed] [Google Scholar]
- 39.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev 2003; 17:3011–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 2005; 436:740–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136:215–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hausser J, Zavolan M. Identification and consequences of miRNA-target interactions – beyond repression of gene expression. Nat Rev Genet 2014; 15:599–612 [DOI] [PubMed] [Google Scholar]
- 43.Ambros V, Bartel B, Bartel DP, Burge CB, Carrington JC, Chen X, Dreyfuss G, Eddy SR, Griffiths JS, Marshall M, Matzke M, Ruvkun G, Tuschl T. A uniform system for microRNA annotation. RNA 2003; 9:277–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffiths-Jones S, Grocock RJ, van DS, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res 2006; 34:D140–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osada H, Takahashi T. MicroRNAs in biological processes and carcinogenesis. Carcinogenesis 2007; 28:2–12 [DOI] [PubMed] [Google Scholar]
- 46.Cho WC. OncomiRs: the discovery and progress of microRNAs in cancers. Mol Cancer 2007; 6:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moustafa AA, Ziada M, Elshaikh A, Datta A, Kim H, Moroz K, Srivastav S, Thomas R, Silberstein JL, Moparty K, Salem FE, El-Habit OH, Abdel-Mageed AB. Identification of microRNA signature and potential pathway targets in prostate cancer. Exp Biol Med (Maywood) 2017; 242:536–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ho JY, Hsu RJ, Wu CH, Liao GS, Gao HW, Wang TH, Yu CP. Reduced miR-550a-3p leads to breast cancer initiation, growth, and metastasis by increasing levels of ERK1 and 2. Oncotarget 2016; 7:53853–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bosland MC. The role of steroid hormones in prostate carcinogenesis. J Natl Cancer Inst Monograp 2000; 27:39–66 [DOI] [PubMed] [Google Scholar]
- 50.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer 2007; 7:256–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng T, Wang J, Chen X, Liu L. Role of microRNA in anticancer drug resistance. Int J Cancer 2010; 126:2–10 [DOI] [PubMed] [Google Scholar]
- 52.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 2002; 99:15524–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abd Elmageed ZY, Yang Y, Thomas R, Ranjan M, Mondal D, Moroz K, Fang Z, Rezk BM, Moparty K, Sikka SC, Sartor O, Abdel-Mageed AB. Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells 2014; 32:983–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi XB, Xue L, Ma AH, Tepper CG, Kung HJ, White RW. miR-125b promotes growth of prostate cancer xenograft tumor through targeting pro-apoptotic genes. Prostate 2011; 71:538–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Budd WT, Seashols-Williams SJ, Clark GC, Weaver D, Calvert V, Petricoin E, Dragoescu EA, O'Hanlon K, Zehner ZE. Dual action of miR-125b as a tumor suppressor and OncomiR-22 promotes prostate cancer tumorigenesis. PLoS One 2015; 10:e0142373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seashols-Williams SJ, Budd W, Clark GC, Wu Q, Daniel R, Dragoescu E, Zehner ZE. miR-9 acts as an OncomiR in prostate cancer through multiple pathways that drive tumour progression and metastasis. PLoS One 2016; 11:e0159601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fletcher CE, Dart DA, Sita-Lumsden A, Cheng H, Rennie PS, Bevan CL. Androgen-regulated processing of the oncomir miR-27a, which targets prohibitin in prostate cancer. Hum Mol Genet 2012; 21:3112–27 [DOI] [PubMed] [Google Scholar]
- 58.Hsu TI, Hsu CH, Lee KH, Lin JT, Chen CS, Chang KC, Su CY, Hsiao M, Lu PJ. MicroRNA-18a is elevated in prostate cancer and promotes tumorigenesis through suppressing STK4 in vitro and in vivo. Oncogenesis 2014; 3:e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nørgaard M, Jensen AØ, Jacobsen JB, Cetin K, Fryzek JP, Sørensen HT. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J Urol 2010; 184:162–7 [DOI] [PubMed] [Google Scholar]
- 60.Han M, Liu M, Wang Y, Chen X, Xu J, Sun Y, Zhao L, Qu H, Fan Y, Wu C. Antagonism of miR-21 reverses epithelial-mesenchymal transition and cancer stem cell phenotype through AKT/ERK1/2 inactivation by targeting PTEN. PLoS One 2012; 7:e39520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu MH, Huang CC, Pan MR, Chen HH, Hung WC. Prospero homeobox 1 promotes epithelial-mesenchymal transition in colon cancer cells by inhibiting E-cadherin via miR-9. Clin Cancer Res 2012; 18:6416–25 [DOI] [PubMed] [Google Scholar]
- 62.Lou W, Liu J, Gao Y, Zhong G, Chen D, Shen J, Bao C, Xu L, Pan J, Cheng J, Ding B, Fan W. MicroRNAs in cancer metastasis and angiogenesis. Oncotarget 2017; 8:115787–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gururajan M, Josson S, Chu GC, Lu CL, Lu YT, Haga CL, Zhau HE, Liu C, Lichterman J, Duan P, Posadas EM, Chung LW. miR-154* and miR-379 in the DLK1-DIO3 microRNA mega-cluster regulate epithelial to mesenchymal transition and bone metastasis of prostate cancer. Clin Cancer Res 2014; 20:6559–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Josson S, Gururajan M, Hu P, Shao C, Chu GY, Zhau HE, Liu C, Lao K, Lu CL, Lu YT, Lichterman J, Nandana S, Li Q, Rogatko A, Berel D, Posadas EM, Fazli L, Sareen D, Chung LW. miR-409-3p/-5p promotes tumorigenesis, epithelial-to-mesenchymal transition, and bone metastasis of human prostate cancer. Clin Cancer Res 2014; 20:4636–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siu MK, Tsai YC, Chang YS, Yin JJ, Suau F, Chen WY, Liu YN. Transforming growth factor-beta promotes prostate bone metastasis through induction of microRNA-96 and activation of the mTOR pathway. Oncogene 2015; 34:4767–76 [DOI] [PubMed] [Google Scholar]
- 66.Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, Kung HF, Lai L, Jiang BH. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1alpha expression. PLoS One 2011; 6:e19139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reis ST, Pontes-Junior J, Antunes AA, Dall'Oglio MF, Dip N, Passerotti CC, Rossini GA, Morais DR, Nesrallah AJ, Piantino C, Srougi M, Leite KR. miR-21 may acts as an oncomir by targeting RECK, a matrix metalloproteinase regulator, in prostate cancer. BMC Urol 2012; 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doldi V, Callari M, Giannoni E, D'Aiuto F, Maffezzini M, Valdagni R, Chiarugi P, Gandellini P, Zaffaroni N. Integrated gene and miRNA expression analysis of prostate cancer associated fibroblasts supports a prominent role for interleukin-6 in fibroblast activation. Oncotarget 2015; 6:31441–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Josson S, Gururajan M, Sung SY, Hu P, Shao C, Zhau HE, Liu C, Lichterman J, Duan P, Li Q, Rogatko A, Posadas EM, Haga CL, Chung LW. Stromal fibroblast-derived miR-409 promotes epithelial-to-mesenchymal transition and prostate tumorigenesis. Oncogene 2015; 34:2690–9 [DOI] [PubMed] [Google Scholar]
- 70.Tao J, Wu D, Xu B, Qian W, Li P, Lu Q, Yin C, Zhang W. microRNA-133 inhibits cell proliferation, migration and invasion in prostate cancer cells by targeting the epidermal growth factor receptor. Oncol Rep 2012; 27:1967–75 [DOI] [PubMed] [Google Scholar]
- 71.Bertoli G, Cava C, Castiglioni I. MicroRNAs as biomarkers for diagnosis, prognosis and theranostics in prostate cancer. IJMS 2016; 17:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cai ZK, Chen Q, Chen YB, Gu M, Zheng DC, Zhou J, Wang Z. microRNA-155 promotes the proliferation of prostate cancer cells by targeting annexin 7. Mol Med Rep 2015; 11:533–8 [DOI] [PubMed] [Google Scholar]
- 73.Wang Z, Xue Y, Wang P, Zhu J, Ma J. MiR-608 inhibits the migration and invasion of glioma stem cells by targeting macrophage migration inhibitory factor. Oncol Rep 2016; 35:2733–42 [DOI] [PubMed] [Google Scholar]
- 74.Kumar B, Lupold SE. MicroRNA expression and function in prostate cancer: a review of current knowledge and opportunities for discovery. Asian J Androl 2016; 18:559–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Y, Jia D, Kim H, Abd Elmageed ZY, Datta A, Davis R, Srivastav S, Moroz K, Crawford BE, Moparty K, Thomas R, Hudson RS, Ambs S, Abdel-Mageed AB. Dysregulation of miR-212 promotes castration resistance through hnRNPH1-mediated regulation of AR and AR-V7: implications for racial disparity of prostate cancer. Clin Cancer Res 2016; 22:1744–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ding HY, Qian WQ, Xu J. MicroRNA-146b acts as a potential tumor suppressor in human prostate cancer. J BUON 2016; 21:434–43 [PubMed] [Google Scholar]
- 77.Fu Q, Liu X, Liu Y, Yang J, Lv G, Dong S. MicroRNA-335 and -543 suppress bone metastasis in prostate cancer via targeting endothelial nitric oxide synthase. Int J Mol Med 2015; 36:1417–25 [DOI] [PubMed] [Google Scholar]
- 78.Zhu Q, Li H, Li Y, Jiang L. MicroRNA-30a functions as tumor suppressor and inhibits the proliferation and invasion of prostate cancer cells by down-regulation of SIX1. Hum Cell 2017; 30:290–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu YN, Yin JJ, Abou-Kheir W, Hynes PG, Casey OM, Fang L, Yi M, Stephens RM, Seng V, Sheppard-Tillman H, Martin P, Kelly K. MiR-1 and miR-200 inhibit EMT via Slug-dependent and tumorigenesis via slug-independent mechanisms. Oncogene 2013; 32:296–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ren D, Wang M, Guo W, Huang S, Wang Z, Zhao X, Du H, Song L, Peng X. Double-negative feedback loop between ZEB2 and miR-145 regulates epithelial-mesenchymal transition and stem cell properties in prostate cancer cells. Cell Tissue Res 2014; 358:763–78 [DOI] [PubMed] [Google Scholar]
- 81.Chen Q, Zhao X, Zhang H, Yuan H, Zhu M, Sun Q, Lai X, Wang Y, Huang J, Yan J, Yu J. MiR-130b suppresses prostate cancer metastasis through down-regulation of MMP2. Mol Carcinog 2015; 54:1292–300 [DOI] [PubMed] [Google Scholar]
- 82.Gandellini P, Folini M, Longoni N, Pennati M, Binda M, Colecchia M, Salvioni R, Supino R, Moretti R, Limonta P, Valdagni R, Daidone MG, Zaffaroni N. miR-205 Exerts tumor-suppressive functions in human prostate through down-regulation of protein kinase Cepsilon. Cancer Res 2009; 69:2287–95 [DOI] [PubMed] [Google Scholar]
- 83.Gandellini P, Profumo V, Casamichele A, Fenderico N, Borrelli S, Petrovich G, Santilli G, Callari M, Colecchia M, Pozzi S, De Cesare M, Folini M, Valdagni R, Mantovani R, Zaffaroni N. miR-205 regulates basement membrane deposition in human prostate: implications for cancer development. Cell Death Differ 2012; 19:1750–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu B, Wang N, Wang X, Tong N, Shao N, Tao J, Li P, Niu X, Feng N, Zhang L, Hua L, Wang Z, Chen M. MiR-146a suppresses tumor growth and progression by targeting EGFR pathway and in a p-ERK-dependent manner in castration-resistant prostate cancer. Prostate 2012; 72:1171–8 [DOI] [PubMed] [Google Scholar]
- 85.Chang YS, Chen WY, Yin JJ, Sheppard-Tillman H, Huang J, Liu YN. EGF receptor promotes prostate cancer bone metastasis by downregulating miR-1 and activating TWIST1. Cancer Res 2015; 75:3077–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu YN, Yin J, Barrett B, Sheppard-Tillman H, Li D, Casey OM, Fang L, Hynes PG, Ameri AH, Kelly K. Loss of androgen-regulated microRNA 1 activates SRC and promotes prostate cancer bone metastasis. Mol Cell Biol 2015; 35:1940–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin ZY, Huang YQ, Zhang YQ, Han ZD, He HC, Ling XH, Fu X, Dai QS, Cai C, Chen JH, Liang YX, Jiang FN, Zhong WD, Wang F, Wu CL. MicroRNA-224 inhibits progression of human prostate cancer by downregulating TRIB1. Int J Cancer 2014; 135:541–50 [DOI] [PubMed] [Google Scholar]
- 88.Siu MK, Abou-Kheir W, Yin JJ, Chang YS, Barrett B, Suau F, Casey O, Chen WY, Fang L, Hynes P, Hsieh YY, Liu YN, Huang J, Kelly K. Loss of EGFR signaling regulated miR-203 promotes prostate cancer bone metastasis and tyrosine kinase inhibitors resistance. Oncotarget 2014; 5:3770–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Valentino A, Reclusa P, Sirera R, Giallombardo M, Camps C, Pauwels P, Crispi S, Rolfo C. Exosomal microRNAs in liquid biopsies: future biomarkers for prostate cancer. Clin Transl Oncol 2017; 19:651–7 [DOI] [PubMed] [Google Scholar]
- 90.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013; 200:373–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9:654–9 [DOI] [PubMed] [Google Scholar]
- 92.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K. The microRNA spectrum in 12 body fluids. Clin Chem 2010; 56:1733–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rodríguez M, Bajo-Santos C, Hessvik NP, Lorenz S, Fromm B, Berge V, Sandvig K, Linē A, Llorente A. Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Mol Cancer 2017; 16:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Endzeliņš E, Melne V, Kalniņa Z, Lietuvietis V, Riekstiņa U, Llorente A, Linē A. Diagnostic, prognostic and predictive value of cell-free miRNAs in prostate cancer: a systematic review. Mol Cancer 2016; 15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Filella X, Foj L. Prostate cancer detection and prognosis: from prostate specific antigen (PSA) to exosomal biomarkers. Int J Mol Sci 2016; 17:pii: E1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu DC, Li QG, Ding XW, Ding YT. Circulating microRNAs: potential biomarkers for cancer. IJMS 2011; 12:2055–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 2008; 105:10513–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lodes MJ, Caraballo M, Suciu D, Munro S, Kumar A, Anderson B. Detection of cancer with serum miRNAs on an oligonucleotide microarray. PLoS One 2009; 4:e6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brase JC, Johannes M, Schlomm T, Fälth M, Haese A, Steuber T, Beissbarth T, Kuner R, Sültmann H. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int J Cancer 2011; 128:608–16 [DOI] [PubMed] [Google Scholar]
- 100.Huang X, Yuan T, Liang M, Du M, Xia S, Dittmar R, Wang D, See W, Costello BA, Quevedo F, Tan W, Nandy D, Bevan GH, Longenbach S, Sun Z, Lu Y, Wang T, Thibodeau SN, Boardman L, Kohli M, Wang L. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur Urol 2015; 67:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Samsonov R, Shtam T, Burdakov V, Glotov A, Tsyrlina E, Berstein L, Nosov A, Evtushenko V, Filatov M, Malek A. Lectin-induced agglutination method of urinary exosomes isolation followed by mi-RNA analysis: application for prostate cancer diagnostic. Prostate 2016; 76:68–79 [DOI] [PubMed] [Google Scholar]
- 102.Haldrup C, Kosaka N, Ochiya T, Borre M, Høyer S, Orntoft TF, Sorensen KD. Profiling of circulating microRNAs for prostate cancer biomarker discovery. Drug Deliv and Transl Res 2014; 4:19–30 [DOI] [PubMed] [Google Scholar]
- 103.Tiberio P, Callari M, Angeloni V, Daidone MG, Appierto V. Challenges in using circulating miRNAs as cancer biomarkers. Biomed Res Int 2015; 2015:731479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koshiol J, Wang E, Zhao Y, Marincola F, Landi MT. Strengths and limitations of laboratory procedures for microRNA detection. Cancer Epidemiol Biomarkers Prev 2010; 19:907–11 [DOI] [PMC free article] [PubMed] [Google Scholar]