Short abstract
Osteoporosis, the most frequent metabolic disorder of bone, is a complex disease with a multifactorial origin that is influenced by genes and environments. However, the pathogenesis of osteoporosis has not been fully elucidated. The theory of “Developmental Origins of Health and Disease” indicates that early life environment exposure determines the risks of cardiometabolic diseases in adulthood. However, investigations into the effects of maternal nutrition and nutrition exposure during early life on the development of osteoporosis are limited. Recently, emerging evidence has strongly suggested that maternal nutrition has long-term influences on bone metabolism in offspring, and epigenetic modifications maybe the underlying mechanisms of this process. This review aimed to address maternal nutrition and its implications for the developmental origins of osteoporosis in offspring. It is novel in providing a theoretical basis for the early prevention of osteoporosis.
Impact statement
Our review aimed to address maternal nutrition and its implications for the developmental origins of osteoporosis in offspring, that can novelly provide a theoretical basis for the early prevention of osteoporosis.
Keywords: Maternal, early life, nutrition, osteoporosis, development, offspring
Introduction
Osteoporosis, as the most frequent bone metabolic disorder, increases morbidity and mortality of humans. The main characteristics of osteoporosis in bone tissue include lower bone mass and abnormal micro-architecture, resulting in increased risks of bone fracture and fragility.1 It is a common bone metabolic disease in humans affecting both sexes and all races. It is estimated that the number of osteoporosis in women who were aged more than 50 years will rise to over 10 million by 2020.2 The prevalence of osteoporosis related fracture is over 1.5 million annually in the United States.3 Mortality and morbidity rates as a result of hip fractures are substantial and the mortality rate within one year of the fracture is between 5% and 20%.4 The number of hip fracture is estimated to increase by 240% in women and 310% in men, with 6.26 million hip fractures worldwide in 2050.5 Vertebral fractures have been called the hallmarks of osteoporosis and tend to occur at younger ages than other fractures. Vertebral fractures can increase the future risks of additional vertebral fractures by 5 to 10 times, and are associated with increased risks of non-vertebral fractures.6 As a global health concern, osteoporosis dramatically increases social and economic burden throughout the world.5
Pathophysiology and etiology of osteoporosis
The skeleton is one of the body's largest organs, composed of mineralized extracellular matrix and bone remodeling unit, with osteocytes, osteoblasts, osteoclasts, and lining cells.2 Osteoblasts and osteoclasts are the critical participants of bone remodeling. Osteoclasts are a type of bone cell that degrade the bone matrix, while osteoblast are cells with single nuclei that build bone.7 The process of bone remodeling cycle is tightly coupled. The rate of bone formation is approximately the same with bone resorption in adulthood. Osteoporosis can occur when bone resorption process is faster than bone formation.8
Osteoporosis is a frequent disease with a complicated origin that is influenced by genes and environments. Kung et al.9 reviewed that 63 genes were associated with bone mineral density (BMD) and several phenotypes related with osteoporosis. Both humans and experimental animals showed certain quantitative trait loci were associated with osteoporosis,10 such as vitamin D receptor (VDR),11 insulin-like growth factor 1 (IGF-1),12 and estrogen receptor α genes.13 However, the role of single gene polymorphism in bone metabolism is less than 1% to 3%.14 Thus, the pathogenesis and etiology of osteoporosis have not been clearly elaborated.
Recently, it is increasingly clear that early life environment determines the develpoment of diseases in adulthood.15 Substantial epidemiological and animal studies showed that early life malnutrition can determine the development of a number of cardiometabolic diseases, such as obesity, insulin resistance, type 2 diabetes, cardiovascular diseases, and stroke.16–19 Environment during early life, especially intrauterine and postnatal nutrition consumption, has long-term metabolic effects in later life. This theory raised interests in the fetal programming of diseases in adulthood and was first proposed in the 1990s, known as “Developmental Origins of Health and Disease (DOHaD).”20,21 It noted that the adaptive responses in infant can impose long-term risk of diseases in adult.22 Growing numbers of studies suggest that early life environment determines the risks of metabolic diseases in adult life. However, the associations between maternal and/or perinatal nutrition and osteoporosis in offspring have not been fully elucidated. This review aimed to address early life nutrition and its implications for the developmental origins of osteoporosis in later life.
Early life nutrition and its implication for osteoporosis
Extensive research is focused on fetal origins hypothesis and this hypothesis proposes that early life environment can affect the development of diseases in adulthood, which was known as DOHaD.23 In recent years, increasing evidence demonstrate that environmental influences during early life can modify the risks of osteoporosis.24 It demonstrated that bone mineral accrual can be affected by environmental exposures during childhood and puberty. The rate of mineral gain is relatively rapid during early life development. Thus, it provides the possibility that environment plays a significant role in bone metabolism during early life.25 Thus, increasing evidence suggests that early life environmental and nutrition exposure determine the susceptibility of osteoporosis in later life.
Evidence from clinical studies about maternal nutrition and osteoporosis in offspring
One epidemiological evidence of early life environment and its implication of osteoporosis indicated that body weight at one year was associated with increased bone mineral content (BMC) at the femoral neck and lumbar spine at about 20 year old.26 Cooper et al.27 showed that the growth rate of infancy was associated with skeletal size in adulthood in a cohort aged about 70 years old. Dennison et al.28 also found that birth weight and body weight at one-year old determined the bone mass when they were aged about 70 years old. A series of clinical studies related to maternal nutrition and the developmental origins of osteoporosis in United States,29 Finland,30 Sweden,31 Norway,32 Australia,33 and the Netherlands34 also demonstrated the same phenomenon. The information of the studis is shown in Table 1.
Table 1.
Human studies of early life nutrition and osteoporosis.
| Study ID | Year | Country | Sample size | Mean age | Primary outcomes |
|---|---|---|---|---|---|
| Cooper et al.26 | 1995 | United Kingdom | 153 women | 21 years | Significant associations between weight at one year and BMC at the lumbar spine and femoral neck; Infant growth and physical activity in childhood are important determinants of peak bone mass in women; |
| Cooper et al.27 | 1997 | United Kingdom | 189 women and 224 men | 63–73 years | Significant associations between weight at one year and BMC at the spine and femoral neck among women, and spine among men; Serum osteocalcin was negatively correlated with BMD; |
| Dennison et al.28 | 2005 | United Kingdom | 498 eight men and 468 women | About 70 years | Birth weight and weight at one year are independent determinants of bone mass in the seventh decade; |
| Yarbrough et al.29 | 2000 | USA | 305 postmenopausal women | 70 years | Birth weight was positively correlated with BMC at the forearm, hip and lumbar spine; |
| Mikkola et al.30 | 2017 | Finland | 178 women | 60.4 years | Birth length and growth in height before seven years of age were positively associated with femoral neck area and growth in height at all age periods studied with spine bone area; |
| Callréus et al.31 | 2013 | Sweden | 1,061 young adult women | 25.00–25.99 years | Significant correlations were observed between birth weight and total body-BMC, femoral neck-BMC, total hip-BMC, lumbar spine L1-L4-BMC, and lean mass; |
| Christoffersen et al.32 | 2017 | Norway | 961 participants | 15–18 years | Birth weight was positively associated with BMD and BMC at all sites among girls, and birth length was positively associated with BMC in boys; |
| Hyde et al.33 | 2017 | Australia | 475 pregnant women | 29.7–30.3 years | Offspring bone area was associated with maternal diet; Birth length, weight and head circumference correlated poorly with all DXA measures at 11 years at both sites; |
| Leunissen et al.34 | 2008 | the Netherlands | 312 young adults | 18–24 years | Adult weight, lean body mass, fat mass and weight gain during childhood were the main positive determinants for BMD of the total body in early adulthood; |
| Antoniades et al.37 | 2003 | London | 4,008 white female twins | 47.5±12.3 years | Significant relationships were found between the intra-pair differences in birth weight and in BMC; |
BMC: bone mineral content; BMD: bone mineral density; DXA: dual energy X-ray absorptiometry.
Vitamin D, an important nutrient, can regulate mineral and bone metabolism. A longitudinal, prospective study in Western Australian found that serum 25(OH)D level of mothers during pregnancy was associated with increased BMC of total body and BMD in their females offspring at 9-year old,36 and even up to about 20-year old.35 Antoniades et al.37 showed that the differences in birth weight between twins were significantly related with BMC in a twin cohort-recruited 4008 female twins aged about 47.5 years old. These data implicate the fetal origins of bone health in later life, with the evidence from genetically identical subjects.
Experimental studies in animals about maternal nutrition and osteoporosis in offspring
In addition to the evidence of human studies, animal models also demonstrated that early life nutrition is associated with osteoporosis in adult life. The evidence of animal models is summarized in Table 2. Maternal protein restriction is a commonly used scheme for malnutrition in animal studies. Mehta et al.38 showed that maternal low-protein diet changed the morphology of growth plate and decreased bone mass in adult rats. Lanham et al.39 indicated that maternal protein restriction during pregnancy prediposed lower serum IGF-1 level in four-week-old female offspring and higher serum osteocalcin concentration in four-week-old male and female offspring. It also decreased serum 25(OH)D concentration in 8, 12, and 20-week-old male offspring.39 Oreffo et al.40 further found that maternal low-protein diet consumption from conception until the end of pregnancy downregulated bone marrow stromal cells proliferation and differentiation in four and eight-week-old offspring.40 -Jahani et al.41 showed maternal low-vitamin D diet (25 IU vitamin D3 /kg diet) during pregnancy and lactation induced lower VDR expression, and increased offspring colon TNF-α and IL-1β genes expressions, which are known to be involved in osteoclastogenesis. Conversely, Suntornsaratoon et al. showed that maternal high dietary vitamin D consumption during pregnancy and lactation period resulted in lower fasting glucose and serum lipopolysaccharide concentrations in male offspring. Maternal vitamin D intake during pregnancy and lactation also improved both femur and lumbar vertebra trabecular bone structure in offspring.42 Interestingly, presuckling calcium supplements with normal chow diet in lactating rats during pregnancy exhibited greater bone elongation, and increased trabecular BMD in offspring even at the age of 27 weeks old.43
Table 2.
Animal models for the developmental origins of osteoporosis.
| Dietary conditions | Species | Period | Age | Main findings | References |
|---|---|---|---|---|---|
| Maternal low-protein diet (9% vs. 18% w/w casein) | Wistar rats | Throughout the 21 days of gestation | 4, 8, 12, and 20 weeks of age | Serum IGF-1 levels were lower in female restricted diet offspring at 4 weeks of age, and serum osteocalcin was significantly higher at 4 weeks of age in male and female offspring from mothers fed the restricted diet, whereas serum 25-OH vitamin D was significantly lower in restricted diet males at 8, 12, and 20 weeks of age; | Mehta et al.38 |
| Maternal low-protein diet (9% vs. 18% w/w casein) | Wistar rats | Throughout the 21 days of gestation | 8, 12, and 20 weeks of age | Lower serum insulin-like growth factor-1 (IGF-1) and 25(OH)D levels; higher serum osteocalcin in offspring rat; | Lanham et al.39 |
| Maternal low-protein diet (9% vs. 18% w/w casein) | Wistar rats | Throughout the 21 days of gestation | 4 and 8 weeks | Downregulated the proliferation and differentiation of bone marrow stromal cells; | Oreffo et al.40 |
| Maternal low-vitamin D diet (25 IU vitamin D3/kg diet vs. 5000 IU vitamin D3/kg diet) | CD1 mice | During pregnancy and lactation | 3 months of age | Predisposed offspring with reduced vitamin D receptor and increased expression of pro-inflammatory genes in colon in offspring; | Jahani et al.41 |
| High dietary vitamin D | C57BL/6J mice | During pregnancy and lactation | 7 months of age | Improved trabecular bone structure at both the lumbar vertebra and femur in male offspring; | Villa et al.42 |
| Calcium supplementation | Sprague-Dawley rats | Presuckling for 14 days | 3 months of age and 27 weeks | Exhibited increases in trabecular bone mineral density; greater bone elongation in offspring; | Suntornsaratoon et al.43 |
| High-fat diet | Sprague-Dawley rats | 10 weeks before mating and during pregnancy | Gestational embryonic day 18.5 | Inhibited bone development, less potential to develop into mature osteoblasts | Chen et al.44 |
| High-fat diet | Sprague-Dawley rats | 12 weeks before mating and during pregnancy | Gestational embryonic day 18.5 | Increased in p53/p21-mediated cell senescence signaling, decreased glucose metabolism and decreased osteoblastic cell differentiation and proliferation. | Chen et al.45 |
| Soy protein isolate diet | Sprague-Dawley rats | continuous diet throughout life | About 6 months of age | Protected against one week post-ovariectomy-associated bone loss, diminished total, trabecular, and cortical bone mineral density loss. | Chen et al.46 |
In addition to vitamin D and calcium supplements, Chen et al.,44 maternal high-fat diet inhibited embryonic day 18.5 embryos bone development and the development into mature osteoblasts in offspring rats.44 They further found that high-fat diet obese dams increased cell senescence in embryonic rat osteogenic calvarial cells, with decreased osteoblastic cell differentiation and proliferation.46One recent animal study indicated that continuous soy protein isolate diet throughout life protected against one week post-ovariectomy-associated bone loss in rats, with diminished total, trabecular, and cortical bone mineral density loss.46 Thus, all these evidence indicate that early life nutrition can impact the develpoment of bone health in offspring in later life.
Potential mechanisms underlying maternal nutrition and osteoporosis in offspring
Critical time windows during fetal stage and neonatal stage can impact the growth and development in adult life. Emerging clinical studies and animal experiments indicated that maternal and postnatal nutrition status determines offspring health in adult life. Recently, increasing evidence has strongly demonstrated that epigenetic modifications maybe the underlying mechanisms of fetal metabolism programming.24 In 1942, the term “epigenetics” was first put forward as a process that can change gene expression and transcription without DNA sequence alteration.47 Epigenetic modifications are inheritable and it can be passed on to the next generation steadily by cell proliferation, differentiation, and division.48 The altered gene expressions may contribute to changes in functions of certain genes and metabolic status, that can persist, and even transmit to the next generation.49 Therefore, epigenetics is supposed to be a potential molecular mechanism of the early life nutrition and the development of osteoporosis in later life.
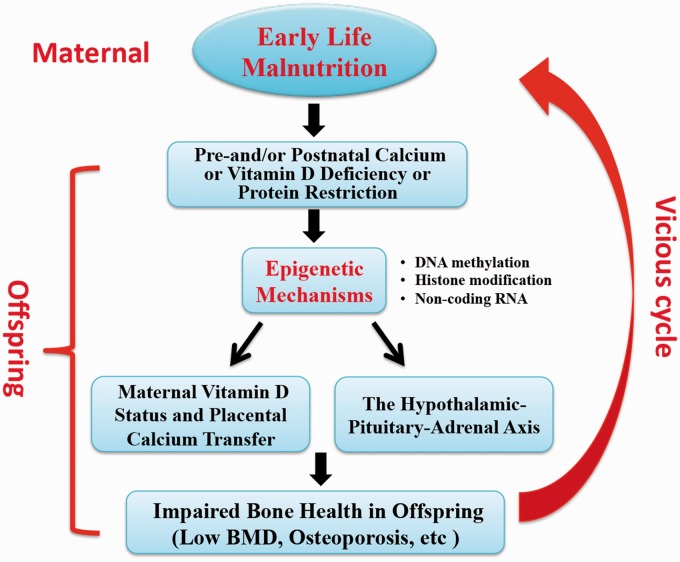
However, investigations into epigenetic mechanisms between early life nutrition consumption and bone metabolic health are are limited. Calcium and vitamin D are known critical nutrients of bone metabolism. Earl et al.50 showed the effects of maternal nutrition regulate DNA methylation of the promoter region of specific genes, such as placental calcium transporters and VDRs can regulate bone mass in offspring. Circulating cortisol level can decrease bone density and increase bone loss rates in adult life. Lillycrop et al.51 showed that maternal protein restriction during pregnancy decreased DNA methylation of glucocorticoid receptor (GR) gene, with increased GR gene expression and hypercortisolism status. Recently, the Southampton Women's Survey reported that higher perinatal cyclin-dependent kinase inhibitor 2A (CDKN2A) methylation was asscociated with lower bone area, BMC, and areal BMD of whole-body minus head. They further found that each 10% increase in CDKN2A DNA methylation was related with BMC decrease (about 4–9 g) at age 4 years in offspring.52 However, Fernandez-Rebollo et al.53 indicate that primary osteoporosis was not associated with DNA methylation or epigenetic modifications in blood obtained from 32 patients. That maybe due to the small sample size, stratification of patients by BMD, and variable clinical characteristics. In summary, the aforementioned evidence demonstrates that epigenetic regulation plays a significant role in the developmental origins of osteoporosis. The proposal of an ‘epigenetic vicious circle’ of maternal nutrition and its implication for bone health in offspring is shown in Figure 1.
Figure 1.
Proposal of an “epigenetic vicious circle” of maternal nutrition and its implication for bone health in offspring. Maternal malnutrition during pregnancy and lactation, including calcium or vitamin D deficiency or protein restriction, can epigenetically regulate gene expressions related with maternal vitamin D status and placental calcium transfer, and the hypothalamic-pituitary-adrenal (HPA) axis. Maternal stress is known to influence the developing HPA axis in the fetus. Thus, epidemiological studies have demonstrated an inverse association between birth weight and fasting plasma cortisol. Indices of the circulating cortisol profile in adult life have also been shown to influence bone density and rates of bone loss. Finally, it can impair bone health in offspring, including low BMD and osteoporosis. If female offspring affected in that way enter reproductive age and become pregnant, they expose their offspring in a similar way to a malnutrition perinatal environment that they were exposed to themselves, thereby closing an epigenetic “vicious intergenerative circle.” BMD: bone mineral density. (A color version of this figure is available in the online journal.)
In addition to epigenetic modifications, we propose that hormonal axis maybe an important mechanism and it should be included, especially for the relationship of parathormone (PTH) to calcium and the disturbance of this axis on the mother and thus the offspring. It indicates that placental calcium transport capacity is both regulated by genes and hormones, such as 1,25 (OH)2 vitamin D3, PTH, PTH-related protein (PTHrP), and calcitonin.54,55 Maternal hyperparathyroidism and hypoparathyroidism appear to be able to increase or decrease the calcium load, and then it can impact the fetus. It shows that lack of fetal parathyroids decreased serum calcium levels and mineralization in mice. Calvi et al.54 further found that PTH and PTHrP affected mineralization of cortical and trabecular bone differentially. Thus, it is proposed that the PTH-calcium hormonal axis maybe an important candidate for fetal bone programming mediation.
Conclusion
In summary, early life stage, especially during perinatal period, is the critical time window for growth and development. Exposure to nutrients during these periods may determine the effects on bone metabolic health in offspring. Emerging evidence has strongly suggested that epigenetic modifications maybe the underlying mechanisms of the developmental origins of osteoporosis. However, the detailed mechanism between epigenetics and osteoporosis has not been fully elucidated yet. Thus, further clinical and basic studies to clarify the potential mechanisms are urgently warranted. We believe that the developmental origins of osteoporosis can novelly provide a theoretical basis for the early prevention of osteoporosis.
Authors’ contributions
JZ and QYF collected data, synthesized data and wrote the manuscript. QY F and SZ reviewed and edited the manuscript. JZ and XHX contributed to the design of this review.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by National Key R&D Program of China (2017YFC1309603), National Key Research and Development Program of China (2016YFA0101002), National Natural Science Foundation of China (No. 81170736, 81570715), Beijing Natural Science Foundation (No. 7184252), the Fund for Fostering Young Scholars of Peking University Health Science Center (No. BMU2017PY008). The funders had no role in study design, data collection and analysis, or preparation of the manuscript.
References
- 1.Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 1993;94:646–50 [DOI] [PubMed]
- 2.Schuiling KD, Robinia K, Nye R. Osteoporosis update. J Midwifery Womens Health 2011; 56: 615–27 [DOI] [PubMed] [Google Scholar]
- 3.Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol 2006; 194: S3–11 [DOI] [PubMed] [Google Scholar]
- 4.Cooper C, Atkinson EJ, Jacobsen SJ, O'Fallon WM, Melton LJ., 3rd Population-based study of survival after osteoporotic fractures. Am J Epidemiol 1993; 137:1001–5 [DOI] [PubMed] [Google Scholar]
- 5.Sambrook P, Cooper C. Osteoporosis. Lancet 2006; 367: 2010–8 [DOI] [PubMed] [Google Scholar]
- 6.Wustrack R, Seeman E, Bucci-Rechtweg C, Burch S, Palermo L, Black DM. Predictors of new and severe vertebral fractures: results from the HORIZON Pivotal Fracture Trial. Osteoporos Int 2012; 23:53–8 [DOI] [PubMed] [Google Scholar]
- 7.Sobacchi C, Schulz A, Coxon FP, Villa A, Helfrich MH. Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol 2013; 9: 522–36 [DOI] [PubMed] [Google Scholar]
- 8.Wu S, Liu Y, Zhang L, Han Y, Lin Y, Deng HW. Genome-wide approaches for identifying genetic risk factors for osteoporosis. Genome Med 2013; 5:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kung AW, Huang QY. Genetic and environmental determinants of osteoporosis. J Musculoskelet Neuronal Interact 2007; 7:26–32 [PubMed] [Google Scholar]
- 10.Ralston SH. Genetic determinants of osteoporosis. Curr Opin Rheumatol 2005; 17:475–9 [DOI] [PubMed] [Google Scholar]
- 11.Thakkinstian A, D'Este C, Eisman J, Nguyen T, Attia J. Meta-analysis of molecular association studies: vitamin D receptor gene polymorphisms and BMD as a case study. J Bone Miner Res 2004; 19:419–28 [DOI] [PubMed] [Google Scholar]
- 12.Rivadeneira F, Houwing-Duistermaat JJ, Vaessen N, Vergeer-Drop JM, Hofman A, Pols HA, Van Duijn CM, Uitterlinden AG. Association between an insulin-like growth factor I gene promoter polymorphism and bone mineral density in the elderly: the Rotterdam Study. J Clin Endocrinol Metab 2003; 88:3878–84 [DOI] [PubMed] [Google Scholar]
- 13.Ioannidis JP, Stavrou I, Trikalinos TA, Zois C, Brandi ML, Gennari L, Albagha O, Ralston SH, Tsatsoulis A. Association of polymorphisms of the estrogen receptor alpha gene with bone mineral density and fracture risk in women: a meta-analysis. J Bone Miner Res 2002; 17:2048–60 [DOI] [PubMed] [Google Scholar]
- 14.Ralston SH. Do genetic markers aid in risk assessment? Osteoporos Int 1998;8:S37–42 [PubMed] [Google Scholar]
- 15.Pentecost M, Ross FC, Macnab A. Beyond the dyad: making Developmental Origins of Health and Disease (DOHaD) interventions more inclusive. J Dev Orig Health Dis 2018;9:10–14. [DOI] [PubMed] [Google Scholar]
- 16.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr 2005; 146:693–700 [DOI] [PubMed] [Google Scholar]
- 17.Rando OJ, Simmons RA. I'm eating for two: parental dietary effects on offspring metabolism. Cell 2015; 161:93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel N, Pasupathy D, Poston L. Determining the consequences of maternal obesity for offspring health. Exp Physiol 2015; 100: 1421–8 [DOI] [PubMed] [Google Scholar]
- 19.Rosendaal NTA, Pirkle CM. Age at first birth and risk of later-life cardiovascular disease: a systematic review of the literature, its limitation, and recommendations for future research. BMC Public Health 2017; 17:627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med 2009; 27:358–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet 1989; 2: 577–80 [DOI] [PubMed] [Google Scholar]
- 22.Hanson M, Gluckman P. Developmental origins of noncommunicable disease: population and public health implications. Am J Clin Nutr 2011; 94: 1754s–8s [DOI] [PubMed] [Google Scholar]
- 23.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986; 1:1077–81 [DOI] [PubMed] [Google Scholar]
- 24.Baird J, Jacob C, Barker M, Fall CH, Hanson M, Harvey NC, Inskip HM, Kumaran K, Cooper C. Developmental origins of health and disease: a lifecourse approach to the prevention of non-communicable diseases. Healthcare 2017; 5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonjour JP, Chevalley T. Pubertal timing, bone acquisition, and risk of fracture throughout life. Endocr Rev 2014; 35: 820–47 [DOI] [PubMed] [Google Scholar]
- 26.Cooper C, Cawley M, Bhalla A, Egger P, Ring F, Morton L, Barker D. Childhood growth, physical activity, and peak bone mass in women. J Bone Miner Res 1995; 10:940–7 [DOI] [PubMed] [Google Scholar]
- 27.Cooper C, Fall C, Egger P, Hobbs R, Eastell R, Barker D. Growth in infancy and bone mass in later life. Ann Rheum Dis 1997; 56:17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dennison EM, Syddall HE, Sayer AA, Gilbody HJ, Cooper C. Birth weight and weight at 1 year are independent determinants of bone mass in the seventh decade: the Hertfordshire cohort study. Pediatr Res 2005; 57:582–6 [DOI] [PubMed] [Google Scholar]
- 29.Yarbrough DE, Barrett-Connor E, Morton DJ. Birth weight as a predictor of adult bone mass in postmenopausal women: the Rancho Bernardo Study. Osteoporos Int 2000; 11:626–30 [DOI] [PubMed] [Google Scholar]
- 30.Mikkola TM, von Bonsdorff MB, Osmond C, Salonen MK, Kajantie E, Cooper C, Valimaki MJ, Eriksson JG. Childhood growth predicts higher bone mass and greater bone area in early old age: findings among a subgroup of women from the Helsinki Birth Cohort Study. Osteoporos Int 2017; 28:2717–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Callreus M, McGuigan F, Akesson K. Birth weight is more important for peak bone mineral content than for bone density: the PEAK-25 study of 1,061 young adult women. Osteoporos Int 2013; 24:1347–55 [DOI] [PubMed] [Google Scholar]
- 32.Christoffersen T, Ahmed LA, Daltveit AK, Dennison EM, Evensen EK, Furberg AS, Gracia-Marco L, Grimnes G, Nilsen OA, Schei B, Tell GS, Vlachopoulos D, Winther A, Emaus N. The influence of birth weight and length on bone mineral density and content in adolescence: the Tromso Study, Fit Futures. Arch Osteoporos 2017; 12:54. [DOI] [PubMed] [Google Scholar]
- 33.Hyde NK, Brennan-Olsen SL, Wark JD, Hosking SM, Pasco JA. Maternal dietary nutrient intake during pregnancy and offspring linear growth and bone: the vitamin D in pregnancy cohort study. Calcif Tissue Int 2017; 100:47–54 [DOI] [PubMed] [Google Scholar]
- 34.Leunissen RW, Stijnen T, Boot AM, Hokken-Koelega AC. Influence of birth size and body composition on bone mineral density in early adulthood: the PROGRAM study. Clin Endocrinol 2008; 69:386–92 [DOI] [PubMed] [Google Scholar]
- 35.Zhu K, Whitehouse AJ, Hart PH, Kusel M, Mountain J, Lye S, Pennell C, Walsh JP. Maternal vitamin D status during pregnancy and bone mass in offspring at 20 years of age: a prospective cohort study. J Bone Miner Res 2014; 29:1088–95 [DOI] [PubMed] [Google Scholar]
- 36.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, Arden NK, Godfrey KM, Cooper C. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 2006; 367:36–43 [DOI] [PubMed] [Google Scholar]
- 37.Antoniades L, MacGregor AJ, Andrew T, Spector TD. Association of birth weight with osteoporosis and osteoarthritis in adult twins. Rheumatology 2003; 42:791–6 [DOI] [PubMed] [Google Scholar]
- 38.Mehta G, Roach HI, Langley-Evans S, Taylor P, Reading I, Oreffo RO, Aihie-Sayer A, Clarke NM, Cooper C. Intrauterine exposure to a maternal low protein diet reduces adult bone mass and alters growth plate morphology in rats. Calcif Tissue Int 2002; 71:493–8 [DOI] [PubMed] [Google Scholar]
- 39.Lanham SA, Roberts C, Cooper C, Oreffo RO. Intrauterine programming of bone. Part 1: alteration of the osteogenic environment. Osteoporos Int 2008; 19:147–56 [DOI] [PubMed] [Google Scholar]
- 40.Oreffo RO, Lashbrooke B, Roach HI, Clarke NM, Cooper C. Maternal protein deficiency affects mesenchymal stem cell activity in the developing offspring. Bone 2003; 33:100–7 [DOI] [PubMed] [Google Scholar]
- 41.Jahani R, Fielding KA, Chen J, Villa CR, Castelli LM, Ward WE, Comelli EM. Low vitamin D status throughout life results in an inflammatory prone status but does not alter bone mineral or strength in healthy 3-month-old CD-1 male mice. Mol Nutr Food Res 2014; 58:1491–501 [DOI] [PubMed] [Google Scholar]
- 42.Villa CR, Chen J, Wen B, Sacco SM, Taibi A, Ward WE, Comelli EM. Maternal vitamin D beneficially programs metabolic, gut and bone health of mouse male offspring in an obesogenic environment. Int J Obes 2016; 40:1875–83 [DOI] [PubMed] [Google Scholar]
- 43.Suntornsaratoon P, Krishnamra N, Charoenphandhu N. Positive long-term outcomes from presuckling calcium supplementation in lactating rats and the offspring. Am J Physiol Endocrinol Metab 2015; 308:E101010–22 [DOI] [PubMed] [Google Scholar]
- 44.Chen JR, Zhang J, Lazarenko OP, Kang P, Blackburn ML, Ronis MJ, Badger TM, Shankar K. Inhibition of fetal bone development through epigenetic down-regulation of HoxA10 in obese rats fed high-fat diet. FASEB J 2012; 26:1131–41 [DOI] [PubMed] [Google Scholar]
- 45.Chen JR, Lazarenko OP, Blackburn ML, Rose S, Frye RE, Badger TM, Andres A, Shankar K. Maternal obesity programs senescence signaling and glucose metabolism in osteo-progenitors from rat and human. Endocrinology 2016; 157:4172–83 [DOI] [PubMed] [Google Scholar]
- 46.Chen JR, Lazarenko OP, Blackburn ML, Shankar K. Dietary factors during early life program bone formation in female rats. FASEB J 2017; 31:376–87 [DOI] [PubMed] [Google Scholar]
- 47.Waddington CH. The epigenotype. 1942. Int J Epidemiol 2012; 41:10–3 [DOI] [PubMed] [Google Scholar]
- 48.Fraga MF. Genetic and epigenetic regulation of aging. Curr Opin Immunol 2009; 21:446–53 [DOI] [PubMed] [Google Scholar]
- 49.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab 2010; 21:214–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Earl SC, Harvey N, Cooper C. The epigenetic regulation of bone mass. IBMS BoneKEy 2010; 7:54–62 [Google Scholar]
- 51.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr 2005; 135:1382–6 [DOI] [PubMed] [Google Scholar]
- 52.Curtis EM, Murray R, Titcombe P, Cook E, Clarke-Harris R, Costello P, Garratt E, Holbrook JD, Barton S, Inskip H, Godfrey KM, Bell CG, Cooper C, Lillycrop KA, Harvey NC. Perinatal DNA Methylation at CDKN2A is associated with offspring bone mass: findings from the Southampton Women's Survey. J Bone Miner Res 2017; 32:2030–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fernandez-Rebollo E, Eipel M, Seefried L, Hoffmann P, Strathmann K, Jakob F, Wagner W. Primary osteoporosis is not reflected by disease-specific DNA methylation or accelerated epigenetic age in blood. J Bone Miner Res 2018; 33:356–61 [DOI] [PubMed] [Google Scholar]
- 54.Calvi LM, Sims NA, Hunzelman JL, Knight MC, Giovannetti A, Saxton JM, Kronenberg HM, Baron R, Schipani E. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest 2001; 107:277–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lanske B, Amling M, Neff L, Guiducci J, Baron R, Kronenberg HM. Ablation of the PTHrP gene or the PTH/PTHrP receptor gene leads to distinct abnormalities in bone development. J Clin Invest 1999; 104:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]