Short abstract
The intestinal anastomotic failure is one of the most severe complications in gastrointestinal surgery. Despite the great surgical improvements during the last decade, anastomotic leak rates remain practically the same, with a dramatically high grade of morbidity for patients. Leakages are usually the final consequence of ischemia in the anastomosis, leading to tissue hypoxia. In response to hypoxia, the cell orchestrates a variety of coordinated responses in order to restore oxygen homeostasis. The molecular mechanism of hypoxia sensitivity involves oxygen sensing hydroxylases, prolyl-hydroxylases, orchestrating two main transcription factors related to induction of inflammation and angiogenesis, namely nuclear factor-κB and hypoxia-inducible factors. The immunohistochemical expression of two transcription factors hypoxia-inducible factors-1α and nuclear factor-κB p65 has already been described in several disorders, including wound healing, asthma and chronic obstructive lung disease, rheumatoid arthritis, cancer, inflammatory bowel disease, and acute colitis. In the surgical field, fibrin sealants have been widely used to prevent leaks in lung surgery and they might also be useful as a reinforcement of sutures in intestinal anastomosis. The commercial fibrin sealant patches are hemostatic and adhesive surgical agents mainly derived from human plasma. We herein report the results of a prospective randomized experimental study on pigs. We performed a high-risk leakage model of bowel anastomosis, causing a significant devascularization of 10–15 cm of the bowel wall before performing a conventional colo-ileal anastomosis. We randomized the animals to receive a covering of the anastomosis with a fibrin patch (case group) or not (control group). We report the changes in the immunohistochemical expression of the proteins involved in tissue response to hypoxia in the experimental model. Our results indicate that the fibrin patch delays the healing response, promoting a longer lasting inflammation in the surgical bed. Nevertheless, the fibrin patches effectiveness to reduce dehiscence shown in clinical practice suggests that this delay does not negatively affect patients’ outcome.
Impact statement
The consequences of the anastomotic failure are dramatic for patients. Understanding how the ever-increasing use of fibrin sealant, that seems to have a beneficial effect on the anastomoses, interacts with the tissue and the healing process can help to justify its use and encourage research on how to improve this effect even more. We feel that the present work shows that the patch can improve healing by complex mechanisms other than the mere contention and physical support of the intestine. Furthermore, research is needed to confirm our preliminary findings.
Keywords: Intestine, healing, ischemia, surgery, immunohistochemistry, interactions
Introduction
The intestinal anastomotic failure is one of the most severe complications in gastrointestinal surgery. Despite the great surgical improvements during the last decade, such as the implementation of minimally invasive surgery, anastomotic leak rates remain practically the same, with a dramatically high grade of morbidity for patients. Anastomotic leakage may be as high as 10% following elective colorectal surgery, but it could even reach 20–30% in emergent cases. Once the leakage is present, management options usually require an emergent reoperation with subsequent stoma diversion. The mortality rate of this complication may reach 13–27%.1
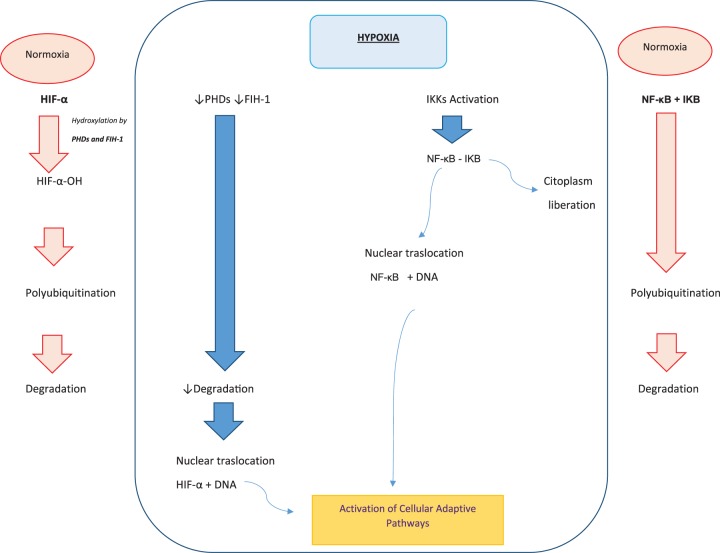
Anastomotic leakages usually are the final consequence of ischemia in the intestinal anastomosis, leading to tissue hypoxia. In response to hypoxia, the cell orchestrates a variety of coordinated responses in order to restore oxygen homeostasis. The molecular mechanism of hypoxia sensitivity involves oxygen sensing hydroxylases, prolyl hydroxylases (PHDs), enzymes orchestrating two main transcription factors related to induction of inflammation and angiogenesis, nuclear factor κB (NF-κB) and hypoxia-inducible factors (HIFs).2 Under normoxia, HIF-α subunits (HIF-1α, HIF-2α, and HIF-3α) are hydroxylated by PHDs and asparaginyl hydroxylase factor (FIH-1), which facilitate the labeling of HIF-α-OH by von Hippel Lindau protein (pVHL), polyubiquitination, and protein degradation of HIF-α by the proteasome. The activity of HIF-1 is oxygen-dependent. When the oxygen levels decrease, the PHD enzymes lose their activity, the hydroxylation of the HIF-α subunit is inhibited and proteasomal degradation fails. The non-hydroxylated-stabilized HIF-α subunits translocate to the nucleus where they dimerize with constitutively expressed HIF-β subunits, bind to DNA, and initiate gene transcription of the adaptive pathways to enable cellular adaptation to a hypoxic environment through promotion of angiogenesis, cell proliferation and migration, increased glucolytic metabolism, and cell survival (Figure 1).3
Figure 1.
Adaptive pathways in cell adaptation to a hypoxic environment. (A color version of this figure is available in the online journal.)
Another important factor involved in tissue response to hypoxia is NF-κB.4–7 NF-κB is a family of transcription factors composed by RelA (NF-κB-p65), RelB, cRel, NF-κB1 (p105/p50), and NF-κB2 (p100/p52). Within a normoxia environment, the inactive NF-κB dimer is present in the cytosol bound to inhibitory proteins κB (IκB). Activation of NF-κB requires the release of IκB from the complex; this release is induced by its phosphorylation by a multiprotein IκB-kinase complex containing IkB-kinase (IKK) and b. This phosphorylation is induced by hypoxia. This is followed by the translocation of the activated NF-κB into the nucleus. There, NF-κB binds to specific base pair sequences (KB sites) to activate a regulatory network in response (Figure 1).
The immunohistochemical (IHC) expression of both transcription factors HIF-1α and NF-κB p65 has already been described in several disorders, including wound healing, asthma and chronic obstructive lung disease, rheumatoid arthritis, cancer, inflammatory bowel disease, and acute colitis.3,8
In the surgical field, fibrin sealants in their various forms have been widely used to prevent leaks in lung surgery and they might also be useful as a reinforcement or replacement of sutures in intestinal anastomosis. The current commercial fibrin sealant patches (Tachosil®, Takeda Company) are hemostatic and adhesive surgical agents mainly derived from human plasma. Their function is to reproduce the final steps in the coagulation cascade to form a fibrin clot that will prevent blood loss and assist in the healing process. The literature, and the EPAR-EMEA report, have assessed their efficacy and safety and show that their clinical use is safe and accepted in humans.9,10
To date, the role of fibrin sealants on preventing anastomotic leakage as well as their behavior under hypoxia conditions is under investigation. In a recent report from our group, we confirmed the efficacy of Tachosil® in decreasing anastomotic leakage rates in an experimental model of ischemic sutures in pigs. Overall, we observed a significant reduction of the rate and clinical consequences of anastomotic failures.11 However, a higher rate of local abscess was observed due to the patch employment.11
Therefore, the present study aims to first, validate whether our experimental model of ischemic intestinal anastomotic sutures cause hypoxia; second, to define the patterns of IHC expression of markers of response to hypoxia (HIF-1α and NF-kB-p65) caused by the experimental model and third, to analyze potential histological or IHC changes when a Tachosil® patch is employed to cover the anastomotic sutures.
Material and methods
A prospective randomized experimental study was proposed using large white pigs. We included 30 male pigs (between 27 kg and 32 kg) in which we performed a high-risk leakage model of bowel anastomosis. The surgeons obtained a significant devascularization of 10 cm of the bowel wall on each side, before performing the sutures, and then created a colo-ileal anastomosis in the same way as performed in the regular clinical practice. Thereafter, we randomized the animals to receive a Tachosil® covering of the anastomosis (case group) versus no covering (control group). After fifth postoperative days, the animals were reoperated and the anastomotic specimens were sent to the Pathology Department. A description of our protocol was published in detail in our previous study.11 All the animals were cared for as per requirements of the regional Animal Experiments Inspectorate (Registered as number: 10/129734.9/14). As required, the study obtained the Institutional Review Board (IRB) approval at our institution (IIS-FJD).
The pathologists performed a standard gross analysis of the specimens and selected samples for paraffin-embedding. In all the cases, we selected at least five representative areas from the anastomosis and performed routine hematoxylin–eosin stains. In these slides, we measured inflammation, fibrosis, and neovascularization, following standard grading systems for this type of ischemia model.10
Neovascularization was counted manually on D2–40 stained slides.
Thereafter, we chose a representative paraffin block from each case and performed IHC for NF-κB-p65 and HIF-1α. All the IHC techniques were performed in the Human Histology and Pathology Department, University Rey Juan Carlos (Alcorcón, Spain). Antigen retrieval was performed for 20 min at 95°C in high pH-buffered solution. Endogenous peroxidase was blocked, by immersing the sections in 0.03% hydrogen peroxide for 5 min. Slides were washed for 5 min with Tris-buffered saline solution containing Tween 20 at pH 7.6 and incubated with the primary antibodies anti-NF-κB-p65 (NF-κB-p65 antibody, bs-0465R, Bioss, 1/100) and anti-HIF-1α (anti-HIF1-α antibody, GTX30105, Gene Tex®, 1/1000) for 20 min at room temperature, followed by incubation with the appropriate anti-Ig horseradish peroxidase-conjugated polymer to detect antigen–antibody (MasVision Universal, Master Diagnostica, Ref. MAD-041880QK-U). Sections were then visualized with 3,3″-diaminobenzidine as a chromogen for 5 min and counterstained with hematoxylin. All IHC stains were performed manually.
Immunohistochemistry was evaluated and measured by two independent pathologists blinded to the group to which the animal belonged. We both measured the percentage of cells expressing the marker and described the location of the expression (endothelium, inflammatory cells, crypt base, fibroblasts) and also the cell location of the staining. HIF-α is expressed in the cell cytoplasm as a response to mild hypoxemia, and when hypoxemia worsens, expression becomes both cytoplasmic and nuclear. In advanced stages, expression can become limited to the nuclei. On the other hand, NF-κB is only nuclear and it becomes positive under hypoxemic conditions.
Concordance between observers was established with Kappa correlation coefficient.
Data were analyzed with SPSS for Windows 20.0 statistical package (IBMÒ corporation). Association between IHC expression and histopathological variables was evaluated by chi-squared (or Fisher’s exact test). The level of statistical significance was defined as a P value less than 0.05.
Results
We have analyzed the pattern of IHC expression of HIF-1α and NF-κB-p65, both in covered and uncovered anastomosis. Results are shown on Table 1, describing the percentage, location, and intensity of IHC expression of the markers in both groups.
Table 1.
Percentage of expression and intracellular location of HIF-1α and NF-κB-p65 in the surgical bed and the surrounding normal tissues.
|
HIF-1α |
NF-κB-p65 |
|||||
|---|---|---|---|---|---|---|
|
Control group |
Tachosil group |
Control group |
Tachosil group |
|||
| % Expression | Intracellular location | % Expression | Intracellular location | % Expression | % Expression | |
| Crypts | ||||||
| Basal cells | 20%* | Citopl. 46.1%*C + N38.4%Nuclear 15.38% | 41.6% | Citopl. 60%*C + N 10%Nuclear 30% | 13.3% | 12.5% |
| Lamina propria | ||||||
| Fibroblasts | 80%* | Citopl. 0%*C + N 100%Nuclear 0% | 50% | Citopl. 8.3%*C + N 91.6%Nuclear 0% | 46.6% | 63.6% |
| Endothelium | 80%* | Citopl. 0%C + N 100%Nuclear 0% | 50% | Citopl.C + NNuclear | 46.6% | 45.5% |
| Surgical bed | ||||||
| Fibroblasts | 100%* | Citopl. 0%*C + N 100%Nuclear 0% | 83.3% | Citopl. 25%*C + N 75%Nuclear 0% | 80% | 90.9% |
| Endothelium | 100%* | Citopl. 0%*C + N 100%Nuclear 0% | 75% | Citopl. 25%*C + N 75%Nuclear 0% | 33% | 72.7%* |
HIF: hypoxia-inducible factor; NF: nuclear factor.
*Statistically significant.
We first analyzed the IHC pattern in uncovered anastomosis and found a significant increase of HIF-1α and NF-kB-p65 when comparing the samples from the surgical bed to normal nearby tissue. Of note, we have found exclusively nuclear positivity for HIF-1α in the epithelial cells from the basal crypts adjacent the surgical bed as opposed to cytoplasmic and nuclear expression in the other compartments of the sample (endothelia, fibroblasts, Figures 2 and 3). This pattern of expression was not found in the epithelial cells located far from the anastomotic bed. As for NF-κB, we have only found an increased expression in the fibroblasts of the surgical bed, which is significantly higher as in normal tissue fibroblasts and endothelium (Figure 4).
Figure 2.
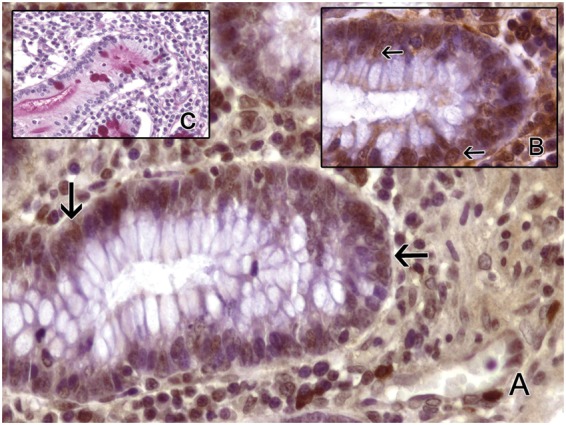
Bowel mucosa stained for HIF-1α in control group. Only nuclear positivity is found (arrow). PAS stain highlights goblet cells. (a, b) Immunohistochemistry with anti-HIF-1α. (a) 400×. (b) 1000×. (c) PAS 400×. (A color version of this figure is available in the online journal.)
Figure 3.

Bowel mucosa stained for HIF-1α in Tachosil group. Nuclear (black arrow) and cytoplasmic positivity (white arrow) is found. PAS stain confirms that the number of goblet cells is similar in control and Tachosil groups. (a, b) Immunohistochemistry with anti-HIF-1α. (a) 400×. (b) 1000×. (c) PAS 400×. (A color version of this figure is available in the online journal.)
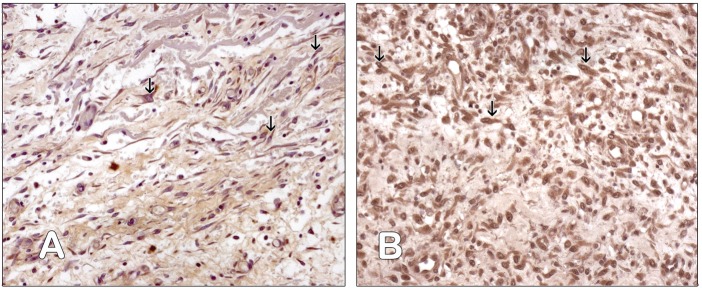
Figure 4.
Surgical field of Tachosil Group. (a) Fibroblasts cytoplasm is positive for anti-HIF-1α antibody (arrow). Immunohistochemistry anti-HIF-1α, PAP, 200×. (b) The cytoplasm and the nuclei of the fibroblasts are positive for anti-NF-kB-p65 antibody (arrow). Immunohistochemistry anti-NF-kB-p65, PAP, 200×. (A color version of this figure is available in the online journal.)
Second, we compared the pattern of expression in animals receiving the Tachosil® patch and control animals. The most relevant finding is HIF-1α expression becomes cytoplasmic in all the compartments, with a significant reduction of nuclear expression in the basal compartment (35% to 10%; P = 0.04). We have found no significant differences in the expression of NF-κB due to the patch.
As for the association with other histopathologic parameters (Table 2), we have found a statistically significant association between HIF-1α cytoplasmic expression and more intense inflammatory infiltrates (P = 0.04). Expression of NF-κB-p65 has been significantly more intense in the endothelial cells of the surgical bed.
Table 2.
Histopathologic parameters in the surgical bed and the surrounding normal tissues.
|
HIF-1α |
NF-κB-p65 |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Inflammatory reaction |
Neovascularizationa |
Inflammatory reaction |
Neovascularizationa |
|||||
| Control group | Tachosil group | Control group | Tachosil group | Control group | Tachosil group | Control group | Tachosil group | |
| Crypts | ||||||||
| Basal cells | 16.6% | 14.2% | 33.3% | 40% | 0% | 0% | 16.6% | 20% |
| Lamina propia | ||||||||
| Fibroblasts | 66.6% | 57.1%* | 83.3% | 60% | 33.3% | 42.8% | 33.3% | 60% |
| Endothelium | 100% | 28.5%* | 100% | 60% | 50% | 28.5% | 16.6% | 40% |
| Surgical bed | ||||||||
| Fibroblasts | 100% | 57.1%* | 100% | 60% | 50% | 57.1% | 66.6% | 60% |
| Endothelium | 100% | 42.8%* | 100% | 60% | 33.3% | 57.1% | 50% | 60% |
HIF: hypoxia-inducible factor; NF: nuclear factor.
aCounted manually on D2–40 stained slides.
*Statistically significant.
Discussion
The present study shows HIF-1α expression in control tissue, therefore validating the utility of this experimental model for the study of ischemia. Hypoxia is a very important event in many human diseases, including tumor development and growth, but it is an important complication following surgery that can lead to anastomotic leakage which clearly increases the morbidity and mortality after surgery.12 The discovery of HIF-1α has shed much light into the molecular pathways that underlie tissue response to hypoxia. It has now become clear that HIF-1 can activate many downstream genes that are collectively known as hypoxia-responsive genes (HRGs) and are involved in the cellular proliferation aimed to heal the injuries caused by hypoxia and restore normal tissue structure.13
However, HIF-1α is not the only factor involved in these pathways and it cross-reacts with other molecules, like NF-κB. NF-κB increases HIF-1α gene translation as shown in many experimental models and cell lines, but it also seems HIF-1α can increase NF-κB-p65 in a reciprocal relationship. NF-κB is a large family of molecules, of which RelA (p65), p50, and c-Rel are the best understood. Besides it seems clear that the interaction between these complex pathways can change depending on the cell or tissue type, to gain the desired biological effect in each case.2,7
For the present study, we have chosen an experimental model in pigs that mimics gastrointestinal surgery under hypoxic conditions and it is considered one of the best models to test strategies to improve surgery in human cases, including the effects of fibrin patchs.14 This kind of fibrin sealants is being increasingly used in human practice to improve sealing and prevent dehiscence. There have been previous reports describing the histopathological changes in the hypoxic intestine in this model and some groups, including ours,11 have shown that significantly more inflammation is associated to the presence of the patch, but the number of dehiscences is significantly decreased, therefore confirming the expected clinical effects.3,8 To the best of our knowledge, no previous report has analyzed the molecular pathways controlling hypoxia in this model. To highlight this issue, we have chosen p65 (part of the NF-κB complex) and HIF-1α IHC expression to see whether the patch determines significant changes in the molecular pathways underlying tissue response to hypoxia.
In the first part of the work, we have analyzed the normal expression of these molecules in the control surgeries, without any fibrin patch. As expected we have found the HIF-1α is translocated into the nuclei in the basal crypts. Previous experimental work, mainly in tumor development models, has shown that HIF-1α accumulates in the cytoplasm under hypoxic conditions and then it is translocated to the nucleus, where it binds to HIF-1β and promotes the transcription of downstream genes that mediate cell proliferation.13
Our results are in accordance with these findings, for we have also shown nuclear expression in the epithelial crypt cells of the surgical bed, aimed to heal the damaged area. Besides we have seen a high expression of NF-κB in the fibroblasts of the surgical bed, also in accordance with a local healing response.
When comparing the changes in the control model and the model covered by Tachosil®, we have noticed a significant decrease in nuclear expression of HIF-1α and a higher expression of NF-κB-p65 in endothelial cells of the surgical bed. The reduction in nuclear HIF-1α seems to indicate a delay in the activation of the nuclear downstream signals regulating cell proliferation and could be a sign of a certain delay in the healing proliferative response in the surgical bed. In a previous report,11 we showed that the patch induced more inflammation with abscess formation in the surgical site and this inflammatory reaction is associated to more vascularization, as expected, and could be molecularly reflected in the higher expression of NF-κB-p65.
We can consider this translocation of HIF-1α into the nuclei as a sign of cell cycle activation aimed to regenerate the injured tissues. We also feel NF-κB-p65 expression in fibroblasts speaks of a tissue reaction to facilitate healing. In summary, we feel the presence of the patch is associated to a more intense inflammatory reaction that delays the tissue response to hypoxia as shown by the lack of translocation of HIF-1α into the cell nuclei at the crypts base. On the other hand, the patch enhances neovascularization mediated by NF-κB-p65, in an attempt to heal the tissue after inflammatory reaction. However, these findings are not contradictory with the clinical effect we have already reported of a significant reduction of anastomotic leakage.11 We feel the delay in the molecular events of healing is counterbalanced with the scaffold effect of the fibrin patch, that limits inflammatory reaction to the area of the patch, therefore reducing the risk of widespread inflammation of the surgical bed with subsequent negative effects on suture stability. Scaffold effect can partially explain the reduction of leakage, despite delayed healing and could explain the positive effect of the patch, already shown by many authors.15
In summary, we herein report the changes in the IHC expression of the proteins involved in tissue response to hypoxia in a pig experimental model of high-risk suture in gastrointestinal surgery. Our results indicate that the fibrin patch delays the healing response, promoting a longer lasting inflammation in the surgical bed. However, as said before, fibrin patches are effective in clinical practice to reduce dehiscence, as also confirmed by our group, and it does not seem this delay negatively affect patients’ outcome.
Acknowledgments
Authors want to thank Mr David de Pablo for his work preparing the samples and Patricia Tejedor, MD for helping on the edition of the manuscript.
Authors’ contributions
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Furthermore, each author certifies that this material or similar material has not been and will not be submitted to or published in any other publication.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funds were provided by Takeda Pharmaceutical; however, we do not have any contractual relation with the company and had complete liberty on the study design and elaboration. We do not have any other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Buchs NC, Gervaz P, Secic M, Bucher P, Mugnier-Konrad B, Morel P. Incidence, consequences and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Colorectal Dis 2008; 23:265–70 [DOI] [PubMed] [Google Scholar]
- 2.Szade A, Grochot-Przeczek A, Florczyk U, Jozkowicz A, Dulak J. Cellular and molecular mechanisms of inflammation-induced angiogenesis. IUBMB Life 2015; 67:145–59 [DOI] [PubMed] [Google Scholar]
- 3.D'Ignazio L, Rocha S. Hypoxia induced NF-κB. Cells 2016; 5:10–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015; 3:83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darby IA, Hewitson TD. Hypoxia in tissue repair and fibrosis. Cell Tissue Res 2016; 365:553–62 [DOI] [PubMed] [Google Scholar]
- 6.Kumar H, Choi D-K. Hypoxia inducible factor pathway and physiological adaptation: a cell survival pathway? Mediators Inflamm 2015; 2015:584758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Ignazio L, Bandarra D, Rocha S. NF-kB and HIF crosstalk in immune responses. Febs J 2016; 283:413–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogler G, Brand K, Vogl D, Page S, Hofmeister R, Andus T, Knuechel R, Baeuerle PA, Schölmerich J, Gross V. Nuclear factor kB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology 1998; 115:357–69 [DOI] [PubMed] [Google Scholar]
- 9.EPAR EMEA. TC-014-IN, TC-016-IN y TC-013-IN, http://www.emea.eu.int/humandocs/Humans/EPAR/tachosil/tachosil.htm. (accessed August 2015).
- 10.Tallón-Aguilar L, Lopez-Bernal FA, Muntane–Relat J, García-Martínez JA, Castillo–Sanchez E, Padillo–Ruiz J. The use of TachoSil as sealant in an experimental model of colonic perforation. Surg Innov 2015; 22:54–60 [DOI] [PubMed] [Google Scholar]
- 11.García-Vásquez C, Gómez García de Las Heras S, Pastor Idoate C, De Pablo D, Fernández-Aceñero MJ. Histopathological changes associated to an absorbable fibrin patch (Tachosil®) covering in an experimental model of high-risk colonic anastomoses. Histol Histopathol 2018; 33:299–306 [DOI] [PubMed] [Google Scholar]
- 12.Walker KG, Bell SW, Rickard MJ, Mehanna D, Dent OF, Chapuis PH, Bokey EL. Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg 2004; 240:255–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudhry H, Harris AL. Advances in hypoxia-inducible factor biology. Cell Metab 2018; 27:281–98 [DOI] [PubMed] [Google Scholar]
- 14.Suárez-Grau JM, Bernardos García C, Cepeda Franco C, Mendez García C, García Ruiz S, Docobo Durantez F, Morales-Conde S, Padillo Ruiz J. Fibrinogen-thrombin collagen patch reinforcement of high-risk colonic anastomoses in rats. World J Gastrointest Surg 2016; 8:627–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pantelis D, Beissel A, Kahl P, Wehner S, Vilz TO, Kalff JC. The effect of sealing with a fixed combination of collagen matrix-bound coagulation factors on the healing of colonic anastomoses in experimental high-risk mice models. Langenbecks Arch Surg 2010; 395:1039. [DOI] [PubMed] [Google Scholar]