Short abstract
Intermittent fasting may be an effective intervention to protect against age-related metabolic disturbances, although it is still controversial. Here, we investigated the effect of intermittent fasting on the deterioration of the metabolism and cognitive functions in rats with estrogen deficiency and its mechanism was also explored. Ovariectomized rats were infused with β-amyloid (25-35; Alzheimer’s disease) or β-amyloid (35-25, Non-Alzheimer’s disease; normal cognitive function) into the hippocampus. Each group was randomly divided into two sub-groups: one with intermittent fasting and the other fed ad libitum: Alzheimer’s disease-ad libitum, Alzheimer’s disease-intermittent fasting, Non-Alzheimer’s disease-ad libitum, and Non-Alzheimer’s disease-intermittent fasting. Rats in the intermittent fasting groups had a restriction of food consumption to a 3-h period every day. Each group included 10 rats and all rats fed a high-fat diet for four weeks. Interestingly, Alzheimer’s disease increased tail skin temperature more than Non-Alzheimer’s disease and intermittent fasting prevented the increase. Alzheimer’s disease reduced bone mineral density in the spine and femur compared to the Non-Alzheimer’s disease, whereas bone mineral density in the hip and leg was reduced by intermittent fasting. Fat mass only in the abdomen was decreased by intermittent fasting. Intermittent fasting decreased food intake without changing energy expenditure. Alzheimer’s disease increased glucose oxidation, whereas intermittent fasting elevated fat oxidation as a fuel source. Alzheimer’s disease and intermittent fasting deteriorated insulin resistance in the fasting state but intermittent fasting decreased serum glucose levels after oral glucose challenge by increasing insulin secretion. Alzheimer’s disease deteriorated short and spatial memory function compared to the Non-Alzheimer’s disease, whereas intermittent fasting prevented memory loss in comparison to ad libitum. Unexpectedly, cortisol levels were increased by Alzheimer’s disease but decreased by intermittent fasting. Intermittent fasting improved dyslipidemia and liver damage index compared to ad libitum. Alzheimer’s disease lowered low-density lipoprotein cholesterol and serum triglyceride levels compared to Non-Alzheimer’s disease. In conclusion, Alzheimer’s disease impaired not only cognitive function but also disturbed energy, glucose, lipid, and bone metabolism in ovariectomized rats. Intermittent fasting protected against the deterioration of these metabolic parameters, but it exacerbated bone mineral density loss and insulin resistance at fasting in Alzheimer’s disease-induced estrogen-deficient rats.
Impact statement
Intermittent fasting was evaluated for its effects on cognitive function and metabolic disturbances in a rat model of menopause and Alzheimer’s disease. Intermittent fasting decreased skin temperature and fat mass, and improved glucose tolerance with decreasing food intake. Intermittent fasting also prevented memory loss: short-term and special memory loss. Therefore, intermittent fasting may prevent some of the metabolic pathologies associated with menopause and protect against age-related memory decline.
Keywords: Intermittent fasting, memory loss, bone mineral density, glucose tolerance, insulin resistance, dyslipidemia
Introduction
Estrogen plays a crucial role in protecting against the development of metabolic diseases, although women have higher body fat contents than men.1 Estrogen is mainly involved in the accumulation of subcutaneous rather than in visceral fat deposits. After menopause total fat accumulation increases, and moreover, it is redistributed to the visceral fat deposits in the abdomen. Subcutaneous fat deposits have beneficial metabolic activities by secreting adipokines, especially, leptin and adiponectin, that improve energy, glucose, and lipid metabolism and are less pro-inflammatory.1 By contrast, visceral fat has a negative effect on metabolism, it exacerbates insulin resistance and is pro-inflammatory.1 Estrogen deficiency itself acts as a trigger for visceral obesity in the abdomen but its etiology remains unclear. One possible mechanism is the disturbance in the estrogen signaling in the hypothalamus–pituitary–adrenal (HPA) axis2 and in the brain, especially hypothalamus, to regulate energy and glucose metabolism by increasing brain insulin resistance.3
Not only estrogen deficiency per se but also increased abdominal fat is involved in the development of metabolic diseases including Alzheimer’s diseases (AD).4,5 Estrogen deficiency increases body weight by increasing food intake in some studies and has been shown to decrease energy expenditure especially from fat in other human and animal studies.6,7 Estrogen replacement therapy suppresses the increase of body weight and adiposity.8 However, hormonal replacement therapy often utilizes estrogen plus progesterone to prevent uterus cell proliferation and the progesterone treatment antagonizes the reduction of food intake by estrogen replacement therapy.9 Thus, hormonal replacement therapy is not an appropriate treatment for preventing obesity after menopause, although the decrease of abdominal fat storage can alleviate the metabolic diseases. Furthermore, hormone replacement therapy has adverse effects and it is not recommended for treating menopausal symptoms.
Estrogen deficiency increases inflammatory cytokines such as tumor-necrosis factor (TNF)-α, IL-1β, and macrophage inflammatory protein-1 by changing the immune response and it decreases the markers mediated by the estrogen receptors.4 In addition, estrogen deficiency indirectly exacerbates inflammation by increasing abdominal fat which is associated with a low-grade inflammatory state.10 Increased inflammation influences the development and progression of metabolic diseases including dementia. Neuroinflammation and brain insulin resistance are major etiologic factors for AD. Recent studies have shown that obesity and a high-fat diet induce β-amyloid accumulation in the hippocampus by exacerbating brain insulin resistance. Estrogen deficiency directly and/or indirectly increases brain insulin resistance and neuroinflammation. As a result, the prevalence of AD increases after menopause women.4,11 Especially, women with bilateral oophorectomy at a young age have a higher risk for dementia12 but oophorectomy does not alter the risk in women after menopause.13 Thus, it is important to lower central fat storage and inflammation to prevent and/or delay the onset of AD.
AD lowers the quality of life for both the patients themselves and their families. Due to the increase in life expectancy, women will live the second half of their lives with estrogen deficiency.11 Doctors have recommended the reduction of abdominal fat contents as an intervention to prevent metabolic diseases including AD especially after menopause. Abdominal fat loss under well-controlled conditions may be an effective preventive measure for AD with no adverse effects. Caloric restriction has been reported to have beneficial effects on the reduction of body fat, inflammation, and cognitive function.14,15 Furthermore, the caloric restriction method is also an important factor to consider since compliance with weight loss interventions is generally poor. Recently, intermittent fasting (IMF) rather has been shown to have better compliance and equivalent effects compared to continuous energy restriction according to objective measurements of weight, waist and hip circumference, fat mass, and drop-out rates.15 However, IMF reduces body fat without decreasing energy intake but it exacerbates hepatic insulin resistance in young rats.16 The positive effects of IMF are still controversial.17 Therefore, we hypothesized that IMF would modulate cognitive functions in estrogen-deficient animals with or without AD fed a high-fat diet. We tested the hypothesis in Sprague-Dawley ovariectomized (OVX) rats with β-amyloid (25-35) or β-amyloid (35-25) infused into the hippocampus and its mechanism was explored.
Materials and methods
Animals and experimental design
Sprague-Dawley female rats aged 10 weeks (235 ± 17 g) were purchased from DBL (Yeumsung-Kun, Korea) and had a one-week acclimation period in individual stainless steel cages in a controlled environment (23°C and with a 12-h light and dark cycles). All experimental procedures were approved by Hoseo University Animal Care and Use Review Committee (2014-07) and followed NIH Guidelines. Experimental animals consumed water ad libitum (AL) and were assigned to their respective diets for the eight-week experimental period. The high-fat diet was made with a modified semi-purified AIN-93 formulation18 that consisted of 38 energy percent (En%) carbohydrates, 16 En% protein, and 46 En% fats. The major sources of carbohydrate, protein, and fat were starch plus sugar, casein, and lard plus soybean oil (10:1; CJ Co, Seoul).
Eleven-week-old female Sprague-Dawley rats had ovariectomy. After anesthesia by subcutaneously injecting the mixture of ketamine and xylazine (100 and 10 mg/kg body weight), each ovary was separated by ligating the proximal part of each oviduct and each ovary was removed with scissors. The OVX rats had a high-fat diet and they were randomly divided into two groups according to feeding methods: the rats in one group had AL and the rats in the other group had IMF. After two-week feeding diets according to the assigned feeding methods, the rats of one group had ICV infusion with β-amyloid (25-35) and the other group had intracerebroventricular (ICV) infusion with β-amyloid (35-25) in IMF and AL groups. A stainless steel cannula was implanted into the CA1 subregion of the anesthetized rats in a stereotaxic device with the following coordinates: lateral, −3.3 mm from the bregma; posterior, 2.0 mm from the midline; ventral, −2.5 mm from dura.19 The β-amyloid (25-35) and β-amyloid (35-25) were dissolved in sterile saline and each solution was filled in a separate osmotic pump (Alzet Osmotic Pump Company, Cupertino, CA, USA) having the rate of 3.6 nmol/day for 14 days. The stainless steel cannula was connected into an osmotic pump filled with β-amyloid (25-35) for AD and (35-25) for Non-AD. The β-amyloid (35-25) was generated with the reverse sequence of β-amyloid (25-35) and was used as a normal-control since it does not form neurofibrillary tangles or amyloid plaques in the brain. The diets were provided for four weeks after ICV infusion of β-amyloid infusion. As a result, this study included four groups: (1) β-amyloid (25-35) infusion + AL diet (AD-AL), (2) β-amyloid (25-35) infusion + IMF (AD-IMF), (3) β-amyloid (35-25) infusion + AL diet (Non-AD-AL), and (4) β-amyloid (35-25) infusion + IMF (Non-AD-IMF).
We selected a time-restricted type IMF each day since people mainly choose one meal a day for IMF. However, it is difficult to decide the equivalent time for one meal a day for animals since they do not eat at defined meal times. However, previous and preliminary studies16 indicated the restriction to 3-h period for animals would be equivalent to one meal a day for humans. In the intermittent groups, rats had a high-fat diet for 3 h at the beginning of the dark cycle (7–10 PM) which corresponds to the morning for humans, and then food was removed until 7 PM the following day. Overnight-fasted serum glucose levels, food intake, and body weight were measured every Tuesday at 10 AM. After four weeks of providing the assigned diets, an oral glucose tolerance test (OGTT) was conducted in overnight feed-deprived rats by oral administration of 2 g/kg body weight of glucose. Serum glucose levels were measured every 10 min until 90 min and again at 120 min using a Glucometer (Accuchek, Roche Diagnostics, Indianapolis, IN) and serum insulin levels were measured at 0, 20, 40, 60, 90, and 120 min by RIA kit (Linco Research, Billerica, MA).6 Insulin resistance was assessed using the homeostasis model assessment estimate of insulin resistance (HOMA-IR) which was calculated as previously described.6 At the end of the study, rats had an injection of insulin (5 U/kg body weight) into the inferior vena cava after anesthetization. Peri-uterine and retroperitoneal fat pads and uterine were weighed after removal. Blood was collected by cardiac puncture and serum was separated by centrifugation after allowing the blood to coagulate. Serum and tissues were stored at −70°C for biochemical analysis.
Memory impairment measured by passive avoidance and water maze tests
At the third week after β-amyloid infusion, the rats were assessed for short-term memory using a passive avoidance apparatus, a two-compartment dark/light shuttle box, as previously described.20 The short-term memory was measured by the retention latency time to enter the dark chamber at the second trial when electric foot shock was not delivered and the latency time was recorded to a maximum of 600 s. Shorter latency time indicates memory impairment, compared to significantly longer latencies in passive avoidance test.
The acquisition of spatial memory was evaluated with a Morris water maze test, as previously described,20 at two days after passive avoidance test. The water maze test was the latency time to go to zone 5 where the platform was placed, and the period to stay zone 5 to find the platform during the third trial. The shorter latency time and longer staying time indicate better long-term memory and long-term spatial memory which is associated with hippocampal-dependent learning.
Bone mineral density measurement
After calibrating a dual-energy X-ray Absorptiometer (DEXA; Norland pDEXA Sabre; Norland Medical Systems Inc., Fort Atkinson, WI, USA) with a phantom, bone mineral density (BMD), fat mass, and lean body mass (LBM) were measured in an anesthetized rat as previously described.21 Before ovariectomy and after six weeks of treatment, each rat was laid in a prone position and the hip, knee, and ankle articulations in 90° flexion and scanned the body in DEXA equipment. BMD in the right femur, knee, and fat, and lean mass in the abdomen, hip, and leg were calculated by the appropriate software equipped in the DEXA.
Locomotive activity
At three days after the water maze test, locomotive activity was determined using an AM1053 Activity Monitor (Linton Instruments, UK) to measure locomotive activity by breaking a three-dimensional array of infrared beams in a cage. The rat was adapted to the cage for 30 min and activity was measured for 1 h during the dark phase of the light/dark cycle. Total locomotive activity was calculated by the sum of rearing, mobility, and activity measurements.
Gene expression in the hippocampus
The hippocampus from five rats from each group was collected at 28 days after MIA injection. Total RNA was individually extracted from each cartilage with Trizol reagent (Life Technologies, Rockville, MD, USA) according to manufacturer’s instructions. The cDNA was synthesized from 1 µg total RNA extracted from each rat using a superscript III reverse transcriptase kit (Life Science Technology). Specific genes associated with inflammation and degradation of the hippocampus were amplified by mixing the cDNA of each hippocampus, primer for the specific genes and SYBR Green mix (Bio-Rad, Richmond, CA) in duplicate using a real-time PCR instrument (Bio-Rad), as previously described. The primers of TNF-α, interleukin (IL)-1β, and IL-6 genes were used.6,21 Their gene expression was quantitated using the comparative cycle of threshold (CT) method (ΔΔCT method) as previously described.22
Histopathological analysis of hippocampus
At the end of the experimental period rats were perfused with saline and 4% paraformaldehyde solution (pH 7.2). The brains were removed from five rats from each group and postfixed with 4% paraformaldehyde solution overnight at room temperature.20 The fixed brain was stored in 30% sucrose solution to be cryoprotected at 4°C. Frozen tissues were serially sectioned on a cryostat (Leica, Wetzlar, Germany) into 30 µm coronal sections. β-amyloid deposition in the hippocampus was detected with anti-beta-amyloid antibody (Cell Signaling Technology, Danvers, MA, USA) using immunohistochemistry using β-amyloid antibody as previously described.19 The β-amyloid accumulation was presented as the % β-amyloid-positive cells in the hippocampus area.
Immunoblot analysis
The hippocampi from four rats of each group were collected as previously described.20 The hippocampi were lysed with the RIPA buffer containing protease inhibitors, and their protein contents were determined using the Bio-Rad protein assay kit (Hercules, CA, USA). The lysates containing equivalent amounts of protein (30–50 µg) were loaded into sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred into the membrane. The samples in the membrane were reacted with respective antibodies using immunoblotting methods and the antibodies used in this study were following: protein kinase B (PKB or Akt), phosphorylated PKBSer473, glycogen synthase kinase (GSK)-3β, phosphorylated GSK-3βser9, forkhead box protein O-1 (FOXO-1), phosphorylated FOXO-1thr24, cAMP response element binding factor (CREB), phosphorylated CREBser129, phophorylated tauser396, tau (Cell Signaling Technology), and β-actin (Santa Cruz Biotech, Dallas, TX, USA). The intensity of protein expression was measured using Imagequant TL (Amersham Biosciences, Piscataway, NJ).
Statistical analysis
Statistical analysis was conducted with SAS software version 7 (SAS Institute, Cary, NC) and all the results are expressed as a means ± standard deviation. The variables with results from different time points were analyzed with two-way repeated measures ANOVA including with time, group, and interaction terms between time and group. One-cway ANOVA was performed to assess the metabolic effects of IMF and β-amyloid infusion into the hippocampus in OVX rats at a single time point. Multiple comparisons between the groups were identified by Tukey’s test at P<0.05.
Results
β-amyloid deposition and insulin signaling in the hippocampus
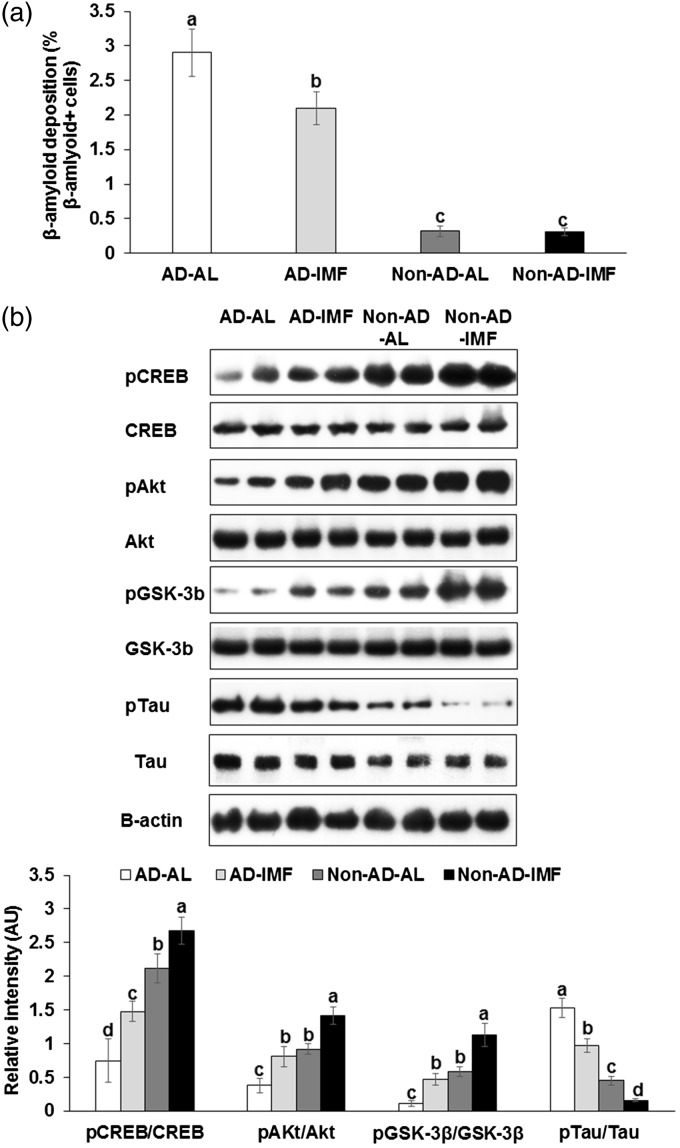
The immunoreactivity of β-amyloid was detected mainly in the hippocampi of AD rats: the rats in the AD-IMF groups exhibited less β-amyloid deposition than those in the AD-AL (P < 0.05) (Figure 1(a)). Its deposition was not altered by IMF in Non-AD rats that had minimal β-amyloid deposits.
Figure 1.
Amyloid-β staining and insulin signaling in the hippocampus of rats infused with β-amyloid and fed high-fat diets with ad libitum (AL) or intermittent (IMF) method. (a) β-amyloid deposition in the hippocampus and (b) insulin signaling in the hippocampus. Each bar indicated mean±SD (n=6). Means without a common alphabet differ at P < 0.05. AD-AL, β-amyloid (25-35) infused plus AL with a high-fat diet; AD-IMF, β-amyloid (25-35) infused plus 3-h feeding per day with a high-fat diet; Non-AD-AL, β-amyloid (35-25) infused plus AL with a high-fat diet; Non-AD-IMF, β-amyloid (35-25) infused plus 3-h feeding per day with a high-fat diet.
Akt: protein kinase B; CREB: cAMP responding element binding protein; GSK-3β: glycogen synthase kinase-3β; pAkt: phosphorylated Akt; pCREB: phosphorylated CREB; pGSK: phosproylated GSK-3β; pTau: phosphorylated Tau protein; Tau: tau protein.
The infusion of β-amyloid into the hippocampus attenuated CREB phosphorylation and IMF protected against its attenuation (Figure 1(b)). In addition, the β-amyloid infusion decreased the phosphorylation of Akt and GSK-3β in the hippocampus, indicating attenuated hippocampal insulin signaling, whereas IMF prevented it. The phosphorylation of tau increased with β-amyloid infusion and IMF reduced the increase (Figure 1(b)). These results suggested that β-amyloid infusion activated its deposition by attenuating CREB phosphorylation and hippocampal insulin signaling and IMF protected against β-amyloid deposition by potentiating CERB phosphorylation and insulin signaling.
Cognitive function
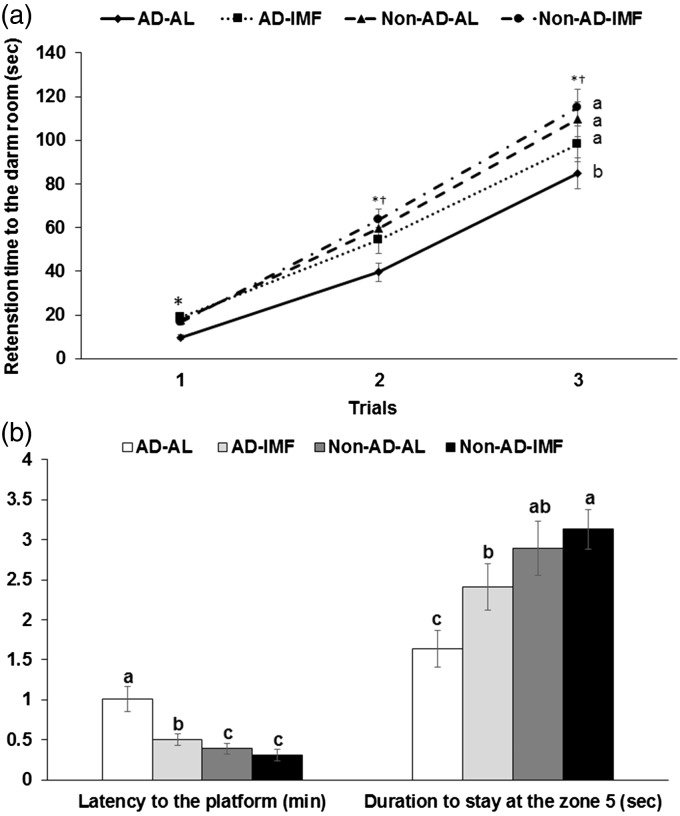
AD increased the time to enter the dark room in the third passive avoidance test in comparison to the Non-AD, and IMF partially prevented the increase in the time compared to AL after entering into the dark room in first and second trials with short electric shock (Figure 2(a)). These results indicated that AD decreased short-term memory to remember the electric shock and IMF prevented the decrease in AD rats.
Figure 2.
Memory, cognitive function, and behavior changes of rats with β-amyloid infusion and fed high-fat diets with or without three different water extracts for 28 days. (a) The entry latency time to enter the dark period in passive avoidance test. (b) The latency to locate zone 5 where the platform existed and the period to stay in the zone 5 during the water maze test on the 4th trial. Dots or bars and error bars indicated means±SD (n=10). Means without a common alphabet differ at P < 0.05. *Significant difference with AD at P < 0.05. †Significant difference with feeding type at P<0.05. AD-AL, β-amyloid (25-35) infused plus AL with a high-fat diet; AD-IMF, β-amyloid (25-35) infused plus 3-h feeding per day with a high-fat diet; Non-AD-AL, β-amyloid (35-25) infused plus AL with a high-fat diet; Non-AD-IMF, β-amyloid (35-25) infused plus 3-h feeding per day with a high-fat diet.
AD rats showed a delayed latency time to locate the zone 5 where the platform was located in the 4th trial and IMF prevented the delay to find the platform (Figure 2(b)). In addition, the duration to stay in the zone 5 was longer in Non-AD groups than AD groups, and IMF made the duration longer in zone 5. The duration to stay in the zone 5 was longer in AD-IMF group than AD-AL group (Figure 2(b)). These results suggested that β-amyloid infusion decreased the spatial memory and IMF partially prevented the decrease.
Estrogen-deficiency symptoms
Serum 17β-estradiol levels and uterine mass were not affected by β-amyloid (25-35) infusion and IMF, which was much lower than the rats with ovaries from the previous studies (about 6.0 pg/mL for serum estradiol levels and 0.58 g for uterine weight; Table 1). Rats infused with β-amyloid (25-35) in the AD groups increased tail skin temperature, an index of hot flushes, in comparison to the Non-AD groups but IMF prevented its increase (Table 1). Therefore, AD exacerbated tail skin temperature and IMF improved it and the changes were not associated with estrogen production. The serum TNF-α levels tended to be higher with AD but it was not significantly different. However, IMF lowered the levels regardless of AD development (Table 1).
Table 1.
Parameters related to estrogen-deficient states.
| AD-AL (n = 10) | AD-IMF (n = 10) | Non-AD-AL (n = 10) | Non-AD-IMF (n = 10) | |
|---|---|---|---|---|
| Serum 17β-estradiol (pg/mL) | 1.5±0.6 | 1.7±0.5 | 1.6±0.5 | 1.8±0.6 |
| Uterine weight (g) | 0.27±0.05 | 0.28±0.04 | 0.28±0.04 | 0.25±0.05 |
| Skin temperature (°C) | 32.0±1.1a | 30.8±1.0b | 30.0±1.0b | 28.9±0.9c |
| Serum TNF-α (pg/mL) | 21.5±2.9a | 17.3±2.3b | 20.6±2.4a | 16.2±2.1b |
AD: Alzheimer’s disease; AL: ad libitum; IMF: intermittent fasting. Values are mean ± SD.
Means without a common alphabet differ at P<0.05.
Energy metabolism
After six-week treatment, body weight gain had been modulated by β-amyloid infusion and IMF. Body weight gain was higher in the ascending order of Non-AD-IMF, Non-AD-AL = AD-IMF, and AD-AL (Table 2). Visceral fat mass measured by retroperitoneal fat and peri-uterine fat were decreased by IMF regardless of cognitive dysfunction: visceral fat mass was lower in the AD-IMF and Non-AD-IMF groups than the other groups (Table 2). This difference was calculated from the balance of the food intake and energy expenditure. Daily food intake was lower during IMF regardless of AD. Daily energy expenditure tended to be higher in the AD groups but it was not significantly different. Non-AD-AL group exhibited lower energy expenditure than the AD-IMF group (P = 0.058). As fuel, carbohydrate oxidation was higher in the ascending order of Non-AD-IMF, Non-AD-AL, AD-IMF, and AD-AL and in contrast fat oxidation was lower in Non-AD-IMF, Non-AD-AL, AD-IMF, and AD-AL. These results inferred that AD increased the use of carbohydrate more than fat as a fuel and that IMF lowered the use of carbohydrate.
Table 2.
Energy metabolism at the end of experimental periods.
| AD-AL (n = 10) | AD-IMF (n = 10) | Non-AD-AL (n = 10) | Non-AD-IMF (n = 10) | |
|---|---|---|---|---|
| Body weight at 6th week (g) | 345±18a | 327±17ab | 331±17a | 313±18b |
| Body weight gain (g) | 82.0±5.1a | 66.6±5.9b | 69.0±5.6b | 51.4±3.2c |
| Retroperitoneal fat (g) | 6.9±0.9a | 5.8±0.8b | 6.2±0.8ab | 5.0±0.7c |
| Peri-uterine fat (g) | 8.9±0.9a | 7.3±0.8b | 8.2±0.8a | 6.5±0.7c |
| Serum ghrelin levels (pg/mL) | 0.72±0.09c | 0.85±0.09b | 0.86±0.09b | 0.97±0.10a |
| Serum cortisol (ng/mL) | 35.7±2.5a | 25.1±2.1b | 32.5±3.1a | 21.8±1.9d |
| Energy expenditure (kcal/bw/day) | 111±16 | 119±18 | 104±13 | 115±17 |
| Food intake (g/day) | 15.0±1.3a | 11.8±1.2b | 14.1±1.5a | 11.4±1.3b |
| Carbohydrate oxidation during dark cycle (mg/kg bw/min) | 7.1±0.9a | 4.2±0.6b | 4.1±0.7b | 2.8±0.4c |
| Fat oxidation during dark cycle (mg/kg bw/min) | 4.7±0.7c | 8.4±1.2ab | 7.0±0.9b | 9.5±1.3a |
AD: Alzheimer’s disease; AL: ad libitum; IMF: intermittent fasting; AD-AL: β-amyloid (25-35) infusion into the hippocampus and fed a high-fat diet ad libitum; AD-IMF: β-amyloid (25-35) infusion into the hippocampus and fed a high-fat diet for 3 h per day; Non-AD-AL: β-amyloid (35-25) infusion into the hippocampus and fed a high-fat diet ad libitum; Non-AD-IMF: β-amyloid (35-25) infusion into the hippocampus and fed a high-fat diet for 3 h per day. Values are mean ± SD.
Means without a common alphabet differ at P<0.05.
Body composition
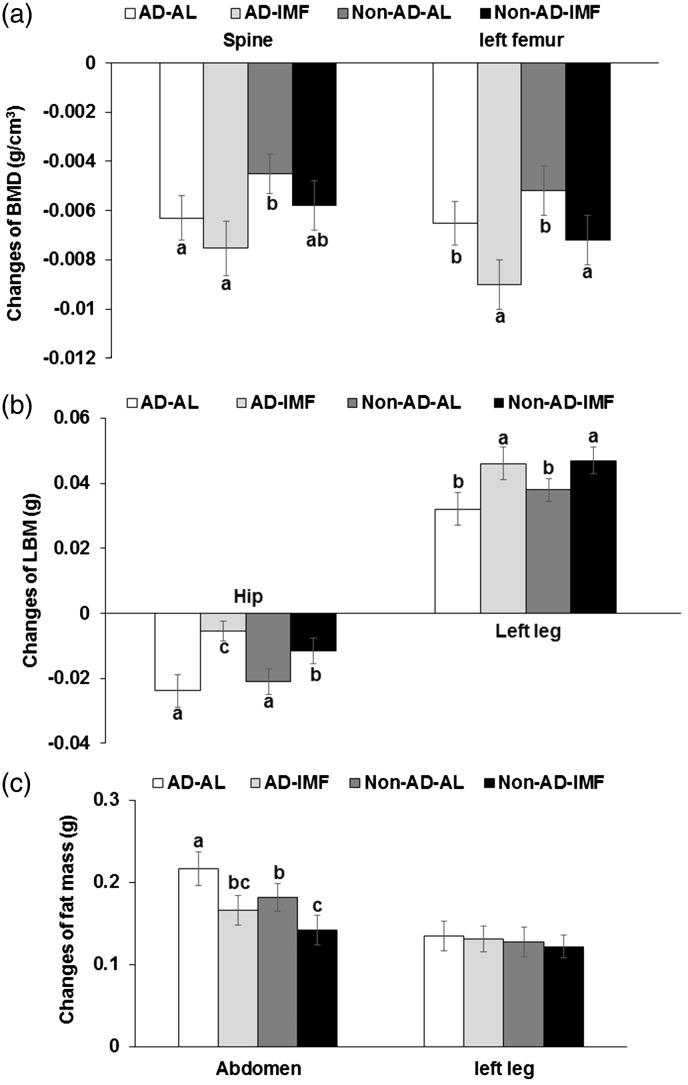
BMD in the spine and femur was lower in all groups at six weeks after ovariectomy. IMF decreased BMD in the spine and femur, whereas AD tended to lower BMD but it was not significantly different (Figure 3(a)). The decrease in spine BMD was lower in Non-AD-AL than AD-IMF. Femur BMD decreased more in the AD-IMF and Non-AD-IMF than in the AD-AL and Non-AD-AL (Figure 3(a)). All rats decreased LBM in the back during the experimental periods, whereas the decrease was lowered with IMF especially in Non-AD rats (Figure 3(b)). IMF increased LBM more than AL regardless of AD (Figure 3(b)). Fat mass in the abdomen was much higher with AD and AL than Non-AD and IMF but fat mass in the left legs did not exhibit the significant differences by AD and IMF (Figure 3(c)).
Figure 3.
Changes of body composition before and after the experimental periods measured by DEXA. (a) The changes of bone mineral density (BMD). (b) The changes of lean body mass (LBM). (c) The changes of fat mass. Bars and error bars indicated means±SD (n=10). Means without a common alphabet differ at P<0.05. AD-AL, β-amyloid (25-35) infused plus AL with a high-fat diet; AD-IMF, β-amyloid (25-35) infused plus 3-h feeding per day with a high-fat diet; Non-AD-AL, β-amyloid (35-25) infused plus AL with a high-fat diet; Non-AD-IMF, β-amyloid (35-25) infused plus 3-h feeding per day with a high-fat diet.
Lipid metabolism and liver damage index
Serum total cholesterol levels were higher in the AD-AL group than the Non-AD-IMF. Serum HDL cholesterol levels were lowered by IMF, whereas AD tended to decrease the levels, but not significantly (Table 3). Serum low-density lipoprotein (LDL) cholesterol levels were higher in the AD groups than the Non-AD groups regardless of feeding methods. Thus, AD and IMF deteriorated cholesterol metabolism and AD increased serum triglyceride levels but IMF decreased the levels. Serum triglyceride levels were lowered in the descending order of AD-AL, Non-AD-AL, AD-IMF, and Non-AD-IMF. Rats with AD exhibited somewhat induced dyslipidemia but IMF partly worsened cholesterol metabolism, although IMF lowered serum triglyceride levels. Serum aspartate transaminase (AST) and alanine transaminase (ALT) levels, indexes of liver damage, were not significantly changed by AD but they were greatly lowered by IMF.
Table 3.
Lipid profiles and glucose and insulin levels in the circulation of overnight-fasted rats.
| AD-AL (n = 10) | AD-IMF (n = 10) | Non-AD-AL (n = 10) | Non-AD-IMF (n = 10) | |
|---|---|---|---|---|
| Total cholesterol (mg/dL) | 80.8±9.5a | 74.4±8.7ab | 74.5±9.3ab | 72.5±8.4b |
| HDL cholesterol (mg/dL) | 31.2±3.5a | 27.0±2.8c | 34.4±3.8a | 30.5±3.6b |
| LDL cholesterol (mg/dL) | 41.4±4.5a | 41.8±4.5a | 33.2±3.6b | 36.5±4.3b |
| Triglyceride levels (mg/dL) | 41.0±3.6a | 28.2±2.4c | 34.3±4.4b | 27.6±3.6c |
| AST (Karmen/mL) | 131±18a | 103±15b | 138±19a | 105±13b |
| ALT (Karmen/mL) | 22.3±4.6a | 15.1±3.9b | 19.4±4.0a | 13.5±2.4b |
| Glucose (mg/dL) | 92.2±9.4b | 112.1±8.3s | 86.4±12.2b | 107.1±12.0a |
| Insulin (ng/mL) | 0.74±0.09b | 1.06±0.17a | 0.57±0.08c | 0.75±0.09b |
| HOMA-IR | 4.9±0.6c | 8.6±1.1a | 3.5±0.5d | 5.8±0.7b |
| Serum β-hydroxybutyrate (mM) | 0.17±0.05 | 0.19±0.06 | 0.16±0.04 | 0.19±0.07 |
AD: Alzheimer’s disease; AL: ad libitum; ALT: alanine transaminase; AST: aspartate transaminase; IMF: intermittent fasting; LDL: low-density lipoprotein; HDL: high-density lipoprotein; AD-AL: β-amyloid (25-35) infusion into the hippocampus and fed a high-fat diet ad libitum; AD-IMF: β-amyloid (25-35) infusion into the hippocampus and fed a high-fat diet for 3 h per day; Non-AD-AL: β-amyloid (35-25) infusion into the hippocampus and fed a high-fat diet ad libitum; Non-AD-IMF: β-amyloid (35-25) infusion into the hippocampus and fed a high-fat diet for 3 h per day. Values are mean ± SD.
Means without a common alphabet differ at P<0.05.
Glucose metabolism
AD increased overnight-fasting serum glucose and insulin levels and IMF also increased both levels. Serum glucose and insulin levels were lower in the descending order of Non-AD-AL, AD-AL, Non-AD-IMF, and AD-IMF. HOMA-IR, an index of insulin resistance, was increased by AD and IMF (Table 3). Serum β-hydroxybutyrate levels were not significantly different among the groups.
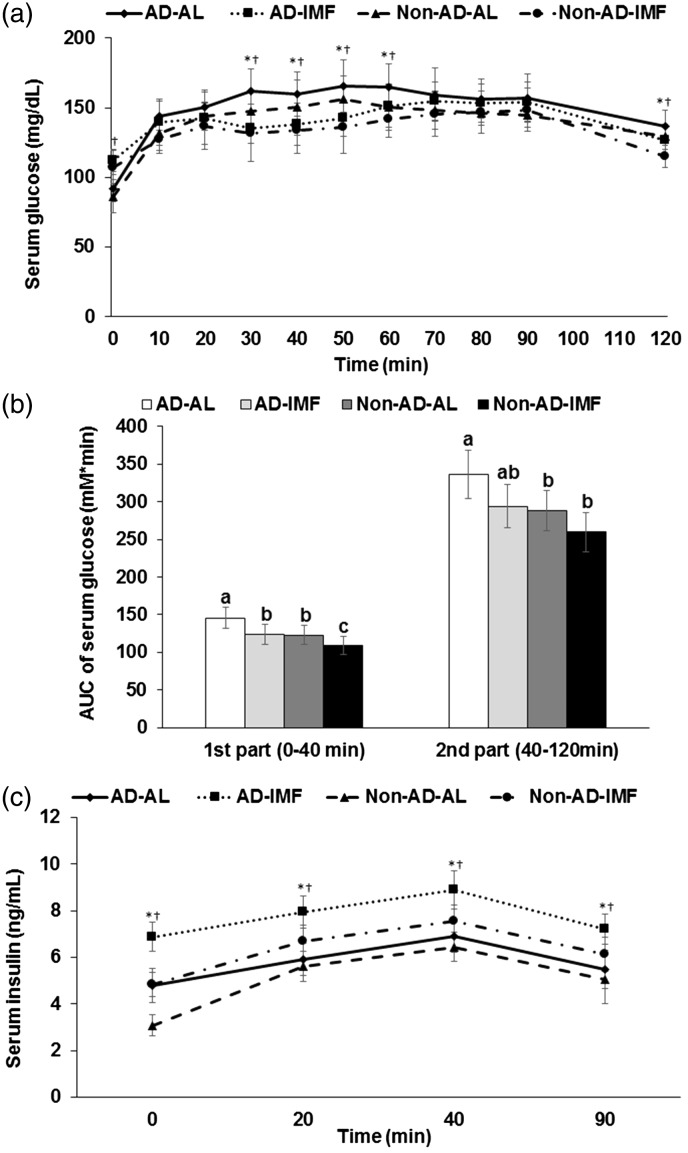
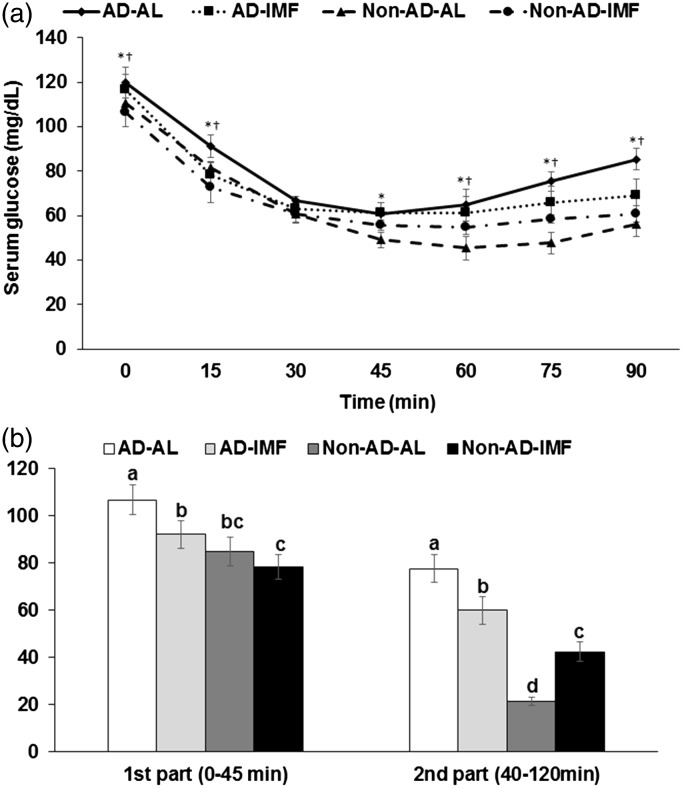
Serum glucose levels were increased by AD but decreased by IMF at 30–50 and 120 min during OGTT (effect of time, P<0.05), although the levels at 0 min were higher in the rats in the IMF groups (Figure 4(a)). Area under the curve (AUC) of serum glucose levels was lower in the descending order of AD-AL, AD-IMF, Non-AD-AL, and Non-AD-IMF in the first and second parts of OGTT (Figure 4(b)). Serum insulin levels increased after glucose challenge until 40 min and then the levels decreased from 40 min in all rats. Serum insulin levels were much higher in the IMF groups than the AL groups and AD increased the levels in comparison to the Non-AD (effect of time, P<0.05, Figure 4(c)). Thus, rats with AD-IMF had much higher serum insulin levels during OGTT in comparison to the Non-AD-AL.
Figure 4.
Serum glucose and insulin levels and area under the curve (AUC) of serum glucose levels. (a) Changes of serum glucose levels in 16-h fasted rats after oral challenge of 2 g glucose/kg body weight. (b) Area under the curve (AUC) of serum glucose calculated in the first (0–40 min) and second phases (40–120 min). (c) Changes of serum insulin levels. Each bar or dot and error bar represented the mean±SD, n=10. Means without a common alphabet differ at P<0.05. *Significant difference with AD at P<0.05. †Significant difference with feeding type at P<0.05. AD-AL, β-amyloid (25-35) infused plus AL with a high-fat diet; AD-IMF, β-amyloid (25-35) infused plus 3-h feeding per day with a high-fat diet; Non-AD-AL, β-amyloid (35-25) infused plus AL with a high-fat diet; Non-AD-IMF, β-amyloid (35-25) infused plus 3-h feeding per day with a high-fat diet.
After subcutaneous insulin injection, serum glucose levels were measured until 45–60 min in all rats with 6-h deprivation of foods (effect of time, P<0.05) (Figure 5(a)). Rats with IMF lowered serum glucose levels quickly until 15 min and the levels were not changed after 30 min of insulin injection in comparison to the rats with AL groups (Figure 5(a)). Rats with AD did not decrease serum glucose levels as fast as the rats with Non-AD until 45 min and the rats with AL had a rebounce of serum glucose levels after 60 min more than the rats with IMF (Figure 5(a) and (b)). The results suggested that AD exacerbated insulin resistance and IMF maintained serum glucose levels at a lower level than Non-AD group after 6-h food deprivation.
Figure 5.
Changes of serum glucose levels during insulin tolerance test. (a) Changes of serum glucose levels after 1 U insulin/kg body weight into subcutaneous injection after 6-h food deprivation. (b) Area under the curve (AUC) of serum glucose levels calculated in the first (0–30 min) and second phases (30–90 min). Each bar or dot and error bar represented the mean±SD, n=10. Means without a common alphabet differ at P<0.05. *Significant difference with AD at P<0.05. †Significant difference with feeding type at P<0.05. AD-AL, β-amyloid (25-35) infused plus AL with a high-fat diet; AD-IMF, β-amyloid (25-35) infused plus 3-h feeding per day with a high-fat diet; Non-AD-AL, β-amyloid (35-25) infused plus AL with a high-fat diet; Non-AD-IMF, β-amyloid (35-25) infused plus 3-h feeding per day with a high-fat diet.
Discussion
After menopause, women often experience impaired energy, glucose and lipid metabolism, and cognitive function.21 IMF is an effective intervention to protect against age-related metabolic disturbance,23,24 although it is still controversial. However, the effects of IMF on estrogen-deficiency symptoms have not been studied. Here, we investigated the effect of IMF on the deterioration of metabolism and cognitive functions in rats with estrogen-deficiency; and memory loss and its mechanism was also explored. Interestingly, estrogen deficiency promoted β-amyloid (25-35) deposition in the hippocampus that exacerbated the menopausal symptoms such as increasing tail skin temperature, BMD loss, visceral fat accumulation, glucose intolerance, insulin resistance, and dyslipidemia in the present study. However, IMF partly protected against menopausal symptoms and cognitive dysfunction and decreased both β-amyloid deposition and also serum cortisol levels. These results suggest that menopausal symptoms are linked to brain dysfunction by not only β-amyloid deposition but also hypothalamus–pituitary gland–adrenal gland axis.
The infusion of β-amyloid proteins including 1-42, 1-45 and 25-35 fractions into the brain initiates AD-like pathologies and induces memory loss.19,25 Estrogen is known to protect against the memory loss by preventing β-amyloid deposits, which may be due to it stimulating the degradation of β-amyloid and down-regulating neuroinflammation and amyloidogenesis.26 Mitochondria estrogen receptor-β deficiency in the brain is reported to contribute to the mitochondrial dysfunction that may be associated with AD pathogenesis.27 In the present study, β-amyloid infusion into the hippocampus of OVX rats caused memory loss which was associated with attenuated insulin signaling, called brain insulin resistance, and increasing inflammation, but IMF protected against memory impairment due to β-amyloid infusion. The decrease in hippocampal insulin signaling is associated with increased tau phosphorylation which accelerates the deposition of β-amyloid.20,28 Obesity also increases not only brain insulin resistance but also inflammation, oxidative stress, and mitochondrial dysfunction.28 Calorie restriction is reported to improve cognitive function in genetically modified mice with tau and β-amyloid deposition.29–31 IMF is one calorie restriction method. In the present study, IMF reduced food intake and fat mass and it protected against memory impairment by decreasing β-amyloid deposition and potentiating hippocampal insulin resistance and decreasing neruroinflammation. Thus, IMF can be an appropriate intervention for improving memory function in estrogen-deficient women.
Estrogen deficiency is well known to cause impairments of the autonomous nervous system, energy, glucose, lipid and bone metabolism, and memory function.32 In addition, the dysfunction of estrogen receptor signaling contributes to dysregulation of innate immune signaling pathways.33 Menopause increases the incidence of metabolic and cardiovascular diseases, neurodegeneration, inflammation related diseases such as osteoarthritis and osteoporosis.34 Especially, osteoporosis is a major disease associated with estrogen deficiency. Estrogen deficiency accelerates cognitive dysfunction by increasing β-amyloid deposition in the brain through attenuating estrogen receptor-β signaling35 and by decreasing serotonin synthesis. Previous studies have reported that the rate of BMD loss and osteoporosis is much greater in patients with AD and the loss of BMD develops before clinical symptoms of cognitive dysfunction appear.36,37 The relationship between BMD and cognitive function may be associated with the hyperphosphorylation of microtubule-associated protein tau in the brain, which increases the deposition of β-amyloids which, in turn, results in cognitive dysfunction. In addition, serotonergic dysfunction is also linked to cognitive dysfunction and BMD loss.38 Therefore, menopausal women are more vulnerable to AD and osteoporosis, which worsens the quality of mid-life. The present study demonstrated that in OVX estrogen-deficient rats, infusions with β-amyloid (25-35) increased β-amyloid deposition which exacerbated elevated tail skin temperature, memory loss in the hippocampus, and decreased BMD in comparison with the OVX rats with β-amyloid (35-25). These results suggested that β-amyloid accumulation exacerbated the effects of estrogen deficiency and worsened menopausal symptoms.
Estrogen plays an important role in regulating body temperature.6 Although the etiology of vasomotor symptoms including hot flashes, sweats, and chills remains unclear, the decline of serum estrogen levels is known to induce hot flushes. The possible mechanism is narrowing the thermoneutral zone by estrogen deficiency. Neurotransmitters including serotonin and norepinephrine are also involved in these processes: estrogen deficiency increases norepinephrine levels and serotonin receptors in the hypothalamus.39,40 The increased serotonin receptors result in lower circulating serotonin levels in the brain, which narrows the thermoneutral zone.39 The known risk factors for hot flashes are obesity, nicotine, and negative moods. Interestingly, β-amyloid deposition exacerbates skin tail temperature; however, in the present study IMF prevented the increase in skin tail temperature in both AD and Non-AD rats along with decreased serum cortisol levels. This suggested that β-amyloid deposition and IMF might be involved in body fat mass and neurotransmitters in the brain. Muralidharan et al.41 have demonstrated that ICV β-amyloid (25-35) infusion decreased memory function and reduced serotonin and dopamine levels in the brain. Gotthardt et al.42 have shown that IMF increases brain hypothalamic norepinephrine and lowers body fat mass. These results might explain that the exacerbation of skin tail temperature, representing hot flushes, might be associated with the changes in neurotransmitters in the brain and visceral fat mass. Therefore, hot flushes might be useful as an early indicator for development of AD and IMF might be an effective way to manage hot flushes in perimenopausal women.
Many studies have demonstrated that both estrogen deficiency and AD impair energy, glucose and lipid metabolisms.6,21 The present study revealed that AD exacerbates the impairments of energy, glucose and lipid metabolism, and that IMF partly protects against the exacerbation without changing serum 17β-estradiol levels and uterine weight. Food intake was slightly lower in the AD groups than the Non-AD groups but it was not significantly different. IMF lowered food intakes more than AL in both AD and Non-AD groups, whereas serum ghrelin levels were higher in IMF groups than AL groups regardless of AD. However, in a previous study, IMF exhibited an insignificant effect on daily energy intake in young male rats with increasing serum ghrelin levels, although it did lower the cumulative energy intake during the experimental period.16 This difference in food intake was associated with daily energy needs since young male rats were allowed to consume as much food as they wanted during the limited time when food was available. However, the female rats in this study could not consume food as much as they wanted during the limited periods. Regardless of the differences in energy intake the effects on energy metabolism were similar between the two studies. The increase in serum ghrelin levels in IMF might have beneficial effects on cognitive function in the present study since the increase of serum ghrelin especially acetyl ghrelin levels have been demonstrated to improve memory function in rats infused with β-amyloid in previous studies.43 Thus, IMF improved energy metabolism and memory function directly by reduction of body weight and visceral fat, and indirectly by modulating serum hormone levels such as cortisol and ghrelin in AD and Non-AD OVX rats.
In addition to energy metabolism, lipid profiles especially LDL cholesterol and triglyceride levels were impaired by AD. IMF decreased serum triglyceride, a benefit which was at least partially offset by decreased HDL cholesterol concentrations with no changes in LDL-cholesterol in this study. IMF also increased insulin resistance at the fasting state. Hennebelle et al.44 showed that energy restriction does not prevent insulin resistance and elevated serum triglycerides. However, in the present study, IMF lowered serum triglyceride levels, although it did exacerbate insulin resistance. Overall, the results suggest that despite the substantial benefits, people need to be aware of potential negative side effects of IMF on cholesterol metabolism and glucose regulation.
Conclusion
AD impaired cognitive function and energy, glucose, lipid, and bone metabolisms in estrogen-deficient rats. IMF protected against both the deterioration of cognitive function and impairment of energy metabolism and dyslipidemia. IMF improved memory function by potentiating hippocampal insulin signaling which in turn inhibited deposition of amyloid-β in AD OVX rats. Most of the metabolism related results were positive for IMF in OVX rats regardless of AD induction. However, serum glucose and insulin concentrations and HOMA-IR were elevated during fasting states in the IMF estrogen-deficient animals regardless of AD induction. On the contrary, serum glucose levels after oral glucose challenge were reduced by elevating serum insulin levels in the IMF groups in comparison to the AL groups in AD and Non-AD OVX rats. Therefore, IMF may not be appropriate in menopausal women with disturbances of glucose metabolism, although more research is needed to fully clarify the effects of IMF on glucose tolerance especially in glucose-intolerant and diabetic women and low BMD women.
Authors’ contributions
BKS and SK contributed to the experimental design, animal study, and interpretation of the data for the manuscript. SP contributed to the conception of the study, experimental design, and interpretation of the data for the manuscript. DSK contributed to biochemical analysis. All authors read and approved the final draft of this manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This study was supported by a grant from the National Research Foundation of Korea (NRF-2015R1D1A3A01019577).
References
- 1.Ma X, Lee P, Chisholm DJ, James DE. Control of adipocyte differentiation in different fat depots; implications for pathophysiology or therapy. Front Endocrinol 2015; 6:1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Law J, Bloor I, Budge H, Symonds ME. The influence of sex steroids on adipose tissue growth and function. Horm Mol Biol Clin Investig 2014; 19:13–24 [DOI] [PubMed] [Google Scholar]
- 3.Nedungadi TP, Clegg DJ. Sexual dimorphism in body fat distribution and risk for cardiovascular diseases. J Cardiovasc Transl Res 2009; 2:321–7 [DOI] [PubMed] [Google Scholar]
- 4.Au A, Feher A, McPhee L, Jessa A, Oh S, Einstein G. Estrogens, inflammation and cognition. Front Neuroendocrinol 2016; 40:87–100 [DOI] [PubMed] [Google Scholar]
- 5.Christensen A, Pike CJ. Menopause, obesity and inflammation: interactive risk factors for Alzheimer’s disease. Front Aging Neurosci 2015; 7:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryuk JA, Ko BS, Lee HW, Kim DS, Kang S, Lee YH, Park S. Tetragonia tetragonioides (Pall.) Kuntze protects estrogen-deficient rats against disturbances of energy and glucose metabolism and decreases proinflammatory cytokines. Exp Biol Med (Maywood) 2017; 242:593–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi TA, Johnson KM. Menopause. Med Clin North Am 2015; 99:521–34 [DOI] [PubMed] [Google Scholar]
- 8.Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, Shah D, Villaseca P; Writing Group of the International Menopause Society for World Menopause Day 2012. Understanding weight gain at menopause. Climacteric 2012; 15:419–29 [DOI] [PubMed] [Google Scholar]
- 9.Butera PC. Estradiol and the control of food intake. Physiol Behav 2010; 99:175-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monteiro R, Teixeira D, Calhau C. Estrogen signaling in metabolic inflammation. Mediators Inflamm 2014; 2014:615917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang HJ, Ko BS, Kwon DY, Lee HW, Kim MJ, Ryuk J, Kang S, Kim DS, Park S. Asian Elm tree inner bark prevents articular cartilage deterioration in ovariectomized obese rats with monoiodoacetate-induced osteoarthritis. Menopause 2016; 23:197–208 [DOI] [PubMed] [Google Scholar]
- 12.Phung TK, Waltoft BL, Laursen TM, Settnes A, Kessing LV, Mortensen PB, Waldemar G. Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dement Geriatr Cogn Disord 2010; 30:43–50 [DOI] [PubMed] [Google Scholar]
- 13.Imtiaz B, Tuppurainen M, Tiihonen M, Kivipelto M, Soininen H, Hartikainen S, Tolppanen AM. Oophorectomy, hysterectomy, and risk of Alzheimer’s disease: a nationwide case-control study. J Alzheimers Dis 2014; 42:575–81 [DOI] [PubMed] [Google Scholar]
- 14.Vasconcelos AR, Yshii LM, Viel TA, Buck HS, Mattson MP, Scavone C, Kawamoto EM. Intermittent fasting attenuates lipopolysaccharide-induced neuroinflammation and memory impairment. J Neuroinflammation 2014; 11:85-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seimon RV, Roekenes JA, Zibellini J, Zhu B, Gibson AA, Hills AP, Wood RE, King NA, Byrne NM, Sainsbury A. Do intermittent diets provide physiological benefits over continuous diets for weight loss? A systematic review of clinical trials. Mol Cell Endocrinol 2015; 418:153–72 [DOI] [PubMed] [Google Scholar]
- 16.Park S, Yoo KM, Hyun JS, Kang S. Intermittent fasting reduces body fat but exacerbates hepatic insulin resistance in young rats regardless of high protein and fat diets. J Nutr Biochem 2017; 40:14–22 [DOI] [PubMed] [Google Scholar]
- 17.McNeill JN, Wu CL, Rabey KN, Schmitt D, Guilak F. Life-long caloric restriction does not alter the severity of age-related osteoarthritis. Age (Dordr) 2014; 36:9669.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993; 123:1939–51 [DOI] [PubMed] [Google Scholar]
- 19.Park S, Kang S, Kim DS, Moon BR. Agrimonia pilosa Ledeb., Cinnamomum cassia Blume, and Lonicera japonica Thunb. protect against cognitive dysfunction and energy and glucose dysregulation by reducing neuroinflammation and hippocampal insulin resistance in beta-amyloid-infused rats. Nutr Neurosci 2017; 20:77–88 [DOI] [PubMed] [Google Scholar]
- 20.Yang HJ, Hwang JT, Kwon DY, Kim MJ, Kang S, Moon NR, Park S. Yuzu extract prevents cognitive decline and impaired glucose homeostasis in beta-amyloid-infused rats. J Nutr 2013; 143:1093–9 [DOI] [PubMed] [Google Scholar]
- 21.Ko BS, Lee HW, Kim DS, Kang S, Ryuk JA, Park S. Supplementing with Opuntia ficus-indica Mill and Dioscorea nipponica Makino extracts synergistically attenuates menopausal symptoms in estrogen-deficient rats. J Ethnopharmacol 2014; 155:267–76 [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402–8 [DOI] [PubMed] [Google Scholar]
- 23.Varady KA. Impact of intermittent fasting on glucose homeostasis. Curr Opin Clin Nutr Metab Care 2016; 19:300–2 [DOI] [PubMed] [Google Scholar]
- 24.Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A, Howell A. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 2011; 35:714–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verdurand M, Chauveau F, Daoust A, Morel AL, Bonnefoi F, Liger F, Bérod A, Zimmer L. Differential effects of amyloid-beta 1-40 and 1-42 fibrils on 5-HT1A serotonin receptors in rat brain. Neurobiol Aging 2016; 40:11–21 [DOI] [PubMed] [Google Scholar]
- 26.Hwang CJ, Yun HM, Park KR, Song JK, Seo HO, Hyun BK, Choi DY, Yoo HS, Oh KW, Hwang DY, Han SB, Hong JT. Memory impairment in estrogen receptor alpha knockout mice through accumulation of amyloid-beta peptides. Mol Neurobiol 2015; 52:176–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long J, He P, Shen Y, Li R. New evidence of mitochondria dysfunction in the female Alzheimer’s disease brain: deficiency of estrogen receptor-beta. J Alzheimers Dis 2012; 30:545–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nuzzo D, Picone P, Baldassano S, Caruana L, Messina E, Marino Gammazza A, Cappello F, Mulè F, Di Carlo M. Insulin resistance as common molecular denominator linking obesity to Alzheimer’s disease. Curr Alzheimer Res 2015; 12:723–35 [DOI] [PubMed] [Google Scholar]
- 29.Brownlow ML, Joly-Amado A, Azam S, Elza M, Selenica ML, Pappas C, Small B, Engelman R, Gordon MN, Morgan D. Partial rescue of memory deficits induced by calorie restriction in a mouse model of tau deposition. Behav Brain Res 2014; 271:79–88 [DOI] [PubMed] [Google Scholar]
- 30.Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, Morgan DG, Morgan TE, Finch CE. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol Aging 2005; 26:995–1000 [DOI] [PubMed] [Google Scholar]
- 31.Mouton PR, Chachich ME, Quigley C, Spangler E, Ingram DK. Caloric restriction attenuates amyloid deposition in middle-aged dtg APP/PS1 mice. Neurosci Lett 2009; 464:184–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia M, Dahlman-Wright K, Gustafsson JA. Estrogen receptor alpha and beta in health and disease. Best Pract Res Clin Endocrinol Metab 2015; 29:557–68 [DOI] [PubMed] [Google Scholar]
- 33.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 2015; 294:63–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakrabarti M, Haque A, Banik NL, Nagarkatti P, Nagarkatti M, Ray SK. Estrogen receptor agonists for attenuation of neuroinflammation and neurodegeneration. Brain Res Bull 2014; 109:22–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarkar S, Jun S, Simpkins JW. Estrogen amelioration of Abeta-induced defects in mitochondria is mediated by mitochondrial signaling pathway involving ERbeta, AKAP and Drp1. Brain Res 2015; 1616:101–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loskutova N, Honea RA, Vidoni ED, Brooks WM, Burns JM. Bone density and brain atrophy in early Alzheimer’s disease. J Alzheimers Dis 2009; 18:777–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loskutova N, Honea RA, Brooks WM, Burns JM. Reduced limbic and hypothalamic volumes correlate with bone density in early Alzheimer’s disease. J Alzheimers Dis 2010; 20:313–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dengler-Crish CM, Smith MA, Wilson GN. Early evidence of low bone density and decreased serotonergic synthesis in the dorsal raphe of a tauopathy model of Alzheimer’s Disease. J Alzheimers Dis 2017; 55:1605–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joffe H, Guthrie KA, LaCroix AZ, Reed SD, Ensrud KE, Manson JE, Newton KM, Freeman EW, Anderson GL, Larson JC, Hunt J, Shifren J, Rexrode KM, Caan B, Sternfeld B, Carpenter JS, Cohen L. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms. Jama Intern Med 2014; 174:1058–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montasser ME, Ziv-Gal A, Brown JP, Flaws JA, Merchenthaler I. A potentially functional variant in the serotonin transporter gene is associated with premenopausal and perimenopausal hot flashes. Menopause 2015; 22:108–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muralidharan P, Kumar VR, Balamurugan G. Protective effect of Morinda citrifolia fruits on beta-amyloid (25-35) induced cognitive dysfunction in mice: an experimental and biochemical study. Phytother Res 2010; 24:252–8 [DOI] [PubMed] [Google Scholar]
- 42.Gotthardt JD, Verpeut JL, Yeomans BL, Yang JA, Yasrebi A, Roepke TA, Bello NT. Intermittent fasting promotes fat loss with lean mass retention, increased hypothalamic norepinephrine content, and increased neuropeptide Y gene expression in diet-induced obese male mice. Endocrinology 2016; 157:679–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang S, Moon NR, Kim DS, Kim SH, Park S. Central acylated ghrelin improves memory function and hippocampal AMPK activation and partly reverses the impairment of energy and glucose metabolism in rats infused with beta-amyloid. Peptides 2015; 71:84–93 [DOI] [PubMed] [Google Scholar]
- 44.Hennebelle M, Roy M, St-Pierre V, Courchesne-Loyer A, Fortier M, Bouzier-Sore AK, Gallis JL, Beauvieux MC, Cunnane SC. Energy restriction does not prevent insulin resistance but does prevent liver steatosis in aging rats on a Western-style diet. Nutrition 2015; 31:523–30 [DOI] [PubMed] [Google Scholar]